Genome-Wide Identification and Analysis Uncovers the Potential Role of JAZ and MYC Families in Potato under Abiotic Stress
Abstract
1. Introduction
2. Results
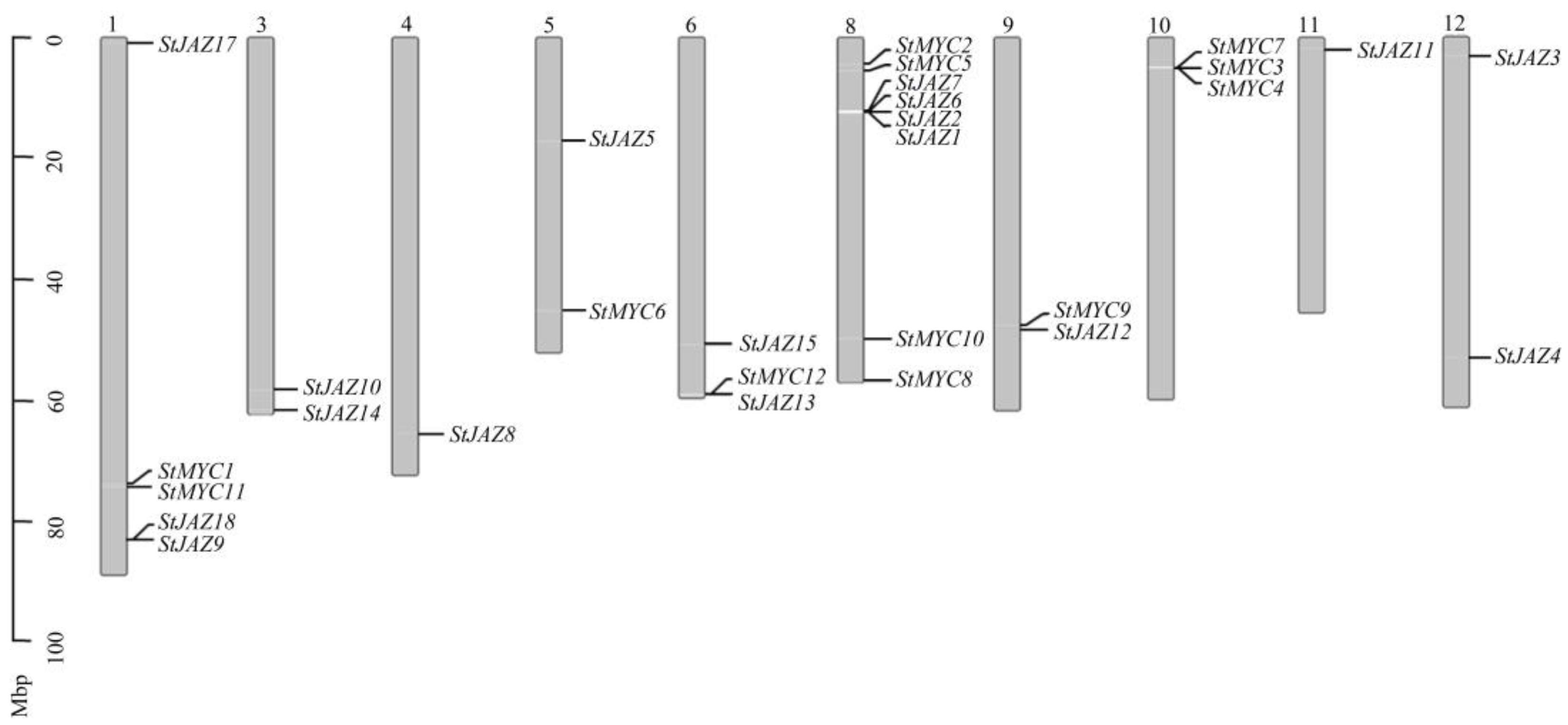
2.1. Identification and Chromosome Mapping of StJAZ and Members of Family in Potato
2.2. Phylogenetic Analysis of the JAZ and MYC Gene Families
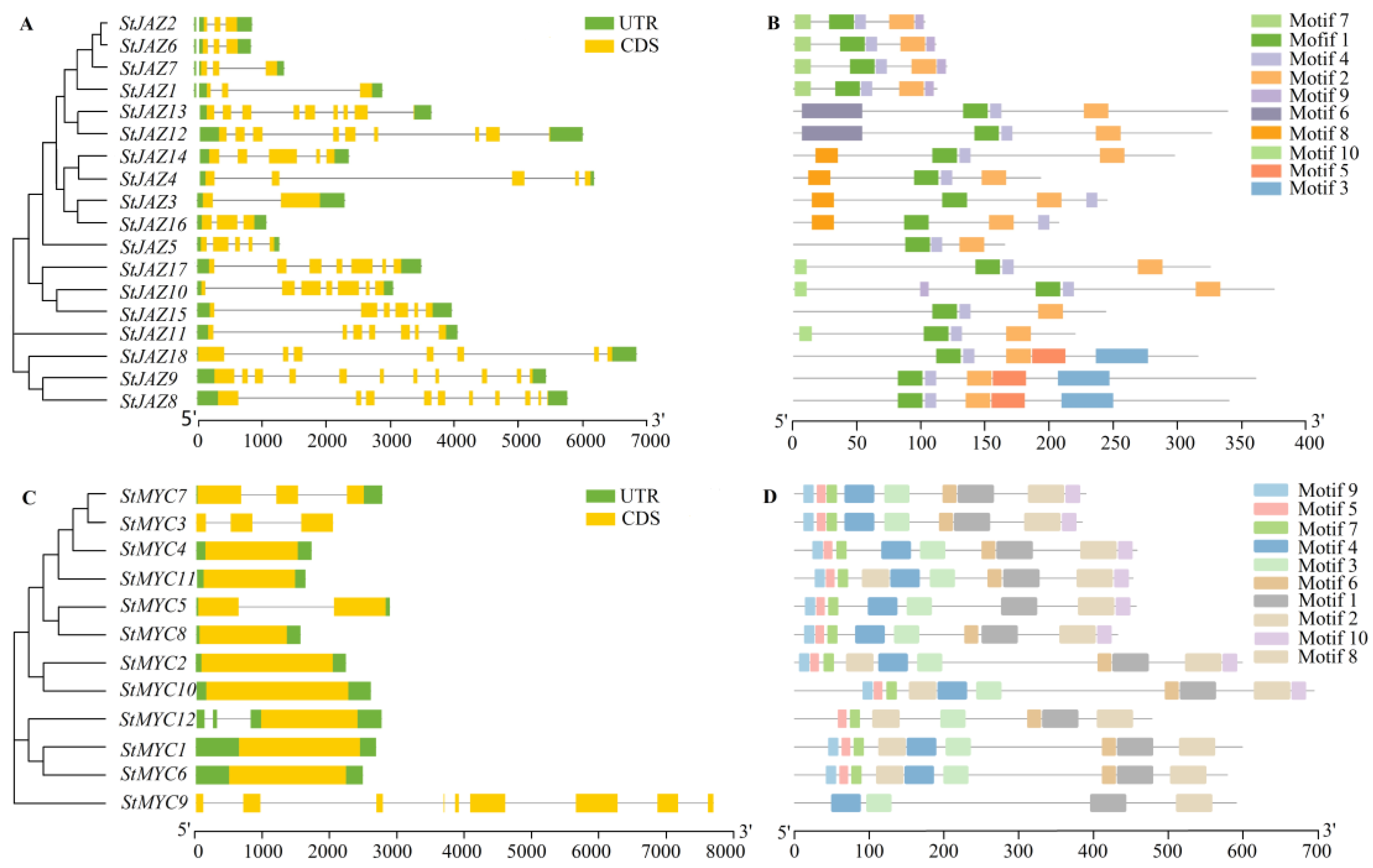
2.3. Gene Structure and Protein Motif Analysis of StJAZ and StMYC
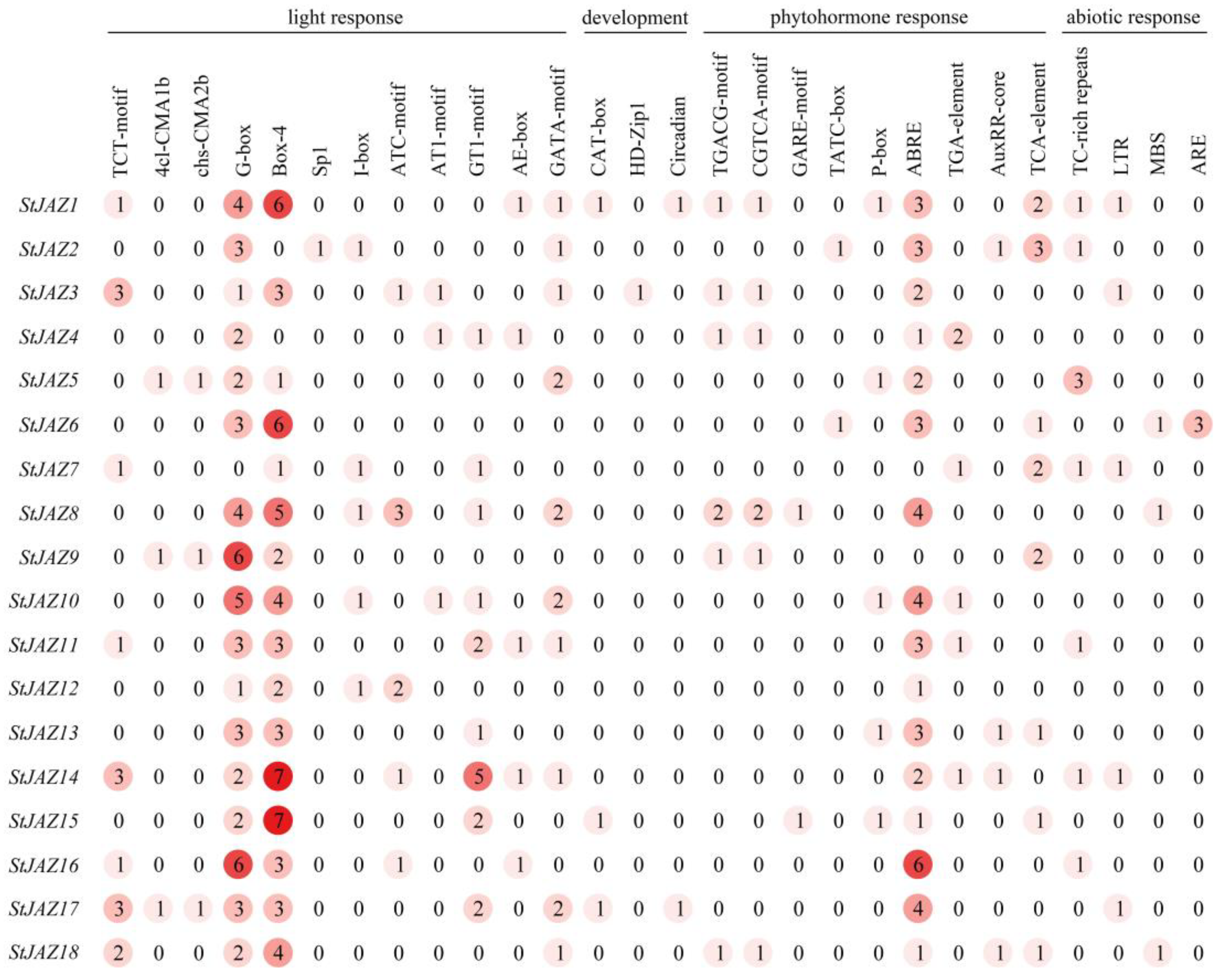
2.4. Promoter Cis-Acting Analysis of StJAZ Genes
2.5. Interaction Networks of StJAZ and StMYC Proteins
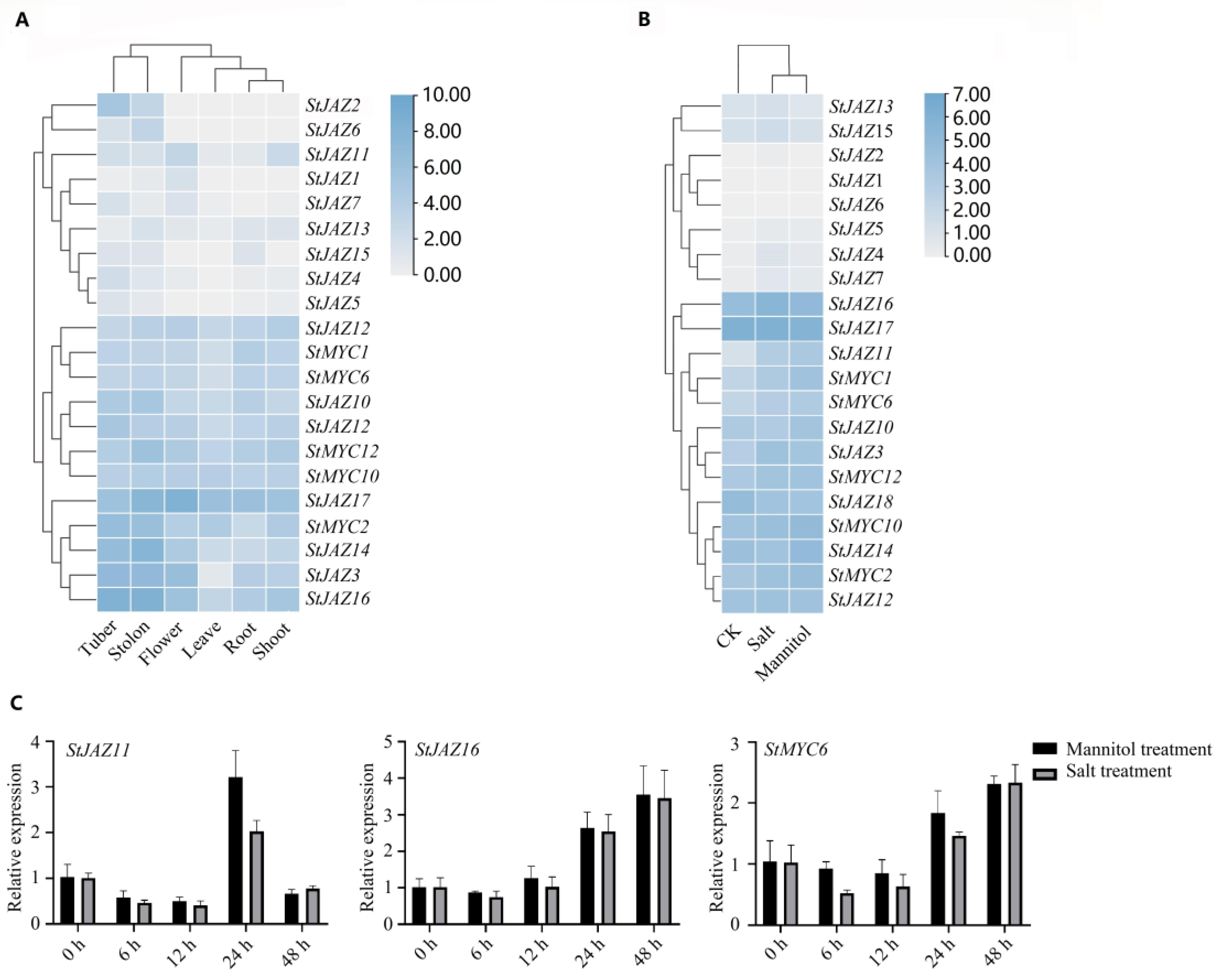
2.6. Expression Analysis of StJAZ and StMYC Genes with Interactions and Their Responses to Abiotic Stress
3. Discussion
4. Materials and Methods
4.1. Identification of the Potato JAZs and MYCs
4.2. Chromosomal Distribution of StJAZs and StMYCs
4.3. Analysis of Conserved Motif and Gene Structure
4.4. Phylogenetic Analysis
4.5. Analysis of StJAZs Gene Promoter Cis-Acting Element
4.6. StJAZ and StMYC PPI Network Analysis
4.7. Self-Activation Detection and Yeast Two-Hybrid Assay
4.8. Expression Analysis from RNA-Seq Data
4.9. Plant Treatments and RT-qPCR Analysis of StJAZ and StMYC Genes of Potatoes under Abiotic Stress
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Devaux, A.; Goffart, J.P.; Kromann, P.; Andrade-Piedra, J.; Polar, V.; Hareau, G. The Potato of the Future: Opportunities and Challenges in Sustainable Agri-food Systems. Potato Res. 2021, 64, 681–720. [Google Scholar] [CrossRef] [PubMed]
- Pelacho, A.M.; Mingo-Castel, A.M. Jasmonic Acid induces tuberization of potato stolons cultured in vitro. Plant Physiol. 1991, 97, 1253–1255. [Google Scholar] [CrossRef] [PubMed]
- Cenzano, A.; Vigliocco, A.; Kraus, T.; Abdala, G. Exogenously applied jasmonic acid induces changes in apical meristem morphology of potato stolons. Ann. Bot. 2003, 91, 915–919. [Google Scholar] [CrossRef]
- Cenzano, A.; Abdala, G.; Hause, B. Cytochemical immuno-localization of allene oxide cyclase, a jasmonic acid biosynthetic enzyme, in developing potato stolons. J. Plant Physiol. 2007, 164, 1449–1456. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Cheng, L.; Li, H.; An, C.; Wang, Y.; Zhang, F. Physiological and protein profiling analysis provides insight into the underlying molecular mechanism of potato tuber development regulated by jasmonic acid in vitro. BMC Plant Biol. 2022, 22, 481. [Google Scholar] [CrossRef]
- Efimova, M.V.; Mukhamatdinova, E.A.; Kovtun, I.S.; Kabil, F.F.; Medvedeva, Y.V.; Kuznetsov, V.V. Jasmonic Acid Enhances the Potato Plant Resistance to the Salt Stress In Vitro. Dokl. Biol. Sci. 2019, 488, 149–152. [Google Scholar] [CrossRef]
- Miyawaki, K.; Inoue, S.; Kitaoka, N.; Matsuura, H. Potato tuber-inducing activities of jasmonic acid and related-compounds (II). Biosci. Biotechnol. Biochem. 2021, 85, 2378–2382. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Liu, B.; Liu, L.; Song, S. Jasmonate action in plant growth and development. J. Exp. Bot. 2017, 68, 1349–1359. [Google Scholar] [CrossRef]
- Chico, J.M.; Chini, A.; Fonseca, S.; Solano, R. JAZ repressors set the rhythm in jasmonate signaling. Curr. Opin. Plant Biol. 2008, 11, 486–494. [Google Scholar] [CrossRef]
- Pauwels, L.; Goossens, A. The JAZ proteins: A crucial interface in the jasmonate signaling cascade. Plant Cell 2011, 23, 3089–3100. [Google Scholar] [CrossRef]
- Chini, A.; Boter, M.; Solano, R. Plant oxylipins: COI1/JAZs/MYC2 as the core jasmonic acid-signaling module. FEBS J. 2009, 276, 4682–4692. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Yao, J.; Ke, J.; Zhang, L.; Lam, V.Q.; Xin, X.F.; Zhou, X.E.; Chen, J.; Brunzelle, J.; Griffin, P.R.; et al. Structural basis of JAZ repression of MYC transcription factors in jasmonate signaling. Nature 2015, 525, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Dombrecht, B.; Xue, G.P.; Sprague, S.J.; Kirkegaard, J.A.; Ross, J.J.; Reid, J.B.; Fitt, G.P.; Sewelam, N.; Schenk, P.M.; Manners, J.M.; et al. MYC2 differentially modulates diverse jasmonate-dependent functions in Arabidopsis. Plant Cell 2007, 19, 2225–2245. [Google Scholar] [CrossRef]
- Chini, A.; Fonseca, S.; Fernández, G.; Adie, B.; Chico, J.M.; Lorenzo, O.; García-Casado, G.; López-Vidriero, I.; Lozano, F.M.; Ponce, M.R.; et al. The JAZ family of repressors is the missing link in jasmonate signaling. Nature 2007, 448, 666–671. [Google Scholar] [CrossRef]
- Fernández-Calvo, P.; Chini, A.; Fernández-Barbero, G.; Chico, J.M.; Gimenez-Ibanez, S.; Geerinck, J.; Eeckhout, D.; Schweizer, F.; Godoy, M.; Franco-Zorrilla, J.M.; et al. The Arabidopsis bHLH transcription factors MYC3 and MYC4 are targets of JAZ repressors and act additively with MYC2 in the activation of jasmonate responses. Plant Cell 2011, 23, 701–715. [Google Scholar] [CrossRef]
- Schweizer, F.; Fernández-Calvo, P.; Zander, M.; Diez-Diaz, M.; Fonseca, S.; Glauser, G.; Lewsey, M.G.; Ecker, J.R.; Solano, R.; Reymond, P. Arabidopsis basic helix-loop-helix transcription factors MYC2, MYC3, and MYC4 regulate glucosinolate biosynthesis, insect performance, and feeding behavior. Plant Cell 2013, 25, 3117–3132. [Google Scholar] [CrossRef]
- Gao, C.; Qi, S.; Liu, K.; Li, D.; Jin, C.; Li, Z.; Huang, G.; Hai, J.; Zhang, M.; Chen, M. MYC2, MYC3, and MYC4 function redundantly in seed storage protein accumulation in Arabidopsis. Plant Physiol. Biochem. 2016, 108, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, Y.; Pan, J.; Lou, D.; Hu, Y.; Yu, D. The bHLH Transcription Factors MYC2, MYC3, and MYC4 Are Required for Jasmonate-Mediated Inhibition of Flowering in Arabidopsis. Mol. Plant. 2017, 10, 1461–1464. [Google Scholar] [CrossRef]
- Wang, D.D.; Li, P.; Chen, Q.Y.; Chen, X.Y.; Yan, Z.W.; Wang, M.Y.; Mao, Y.B. Differential Contributions of MYCs to Insect Defense Reveals Flavonoids Alleviating Growth Inhibition Caused by Wounding in Arabidopsis. Front. Plant Sci. 2021, 12, 700555. [Google Scholar] [CrossRef]
- Oblessuc, P.R.; Obulareddy, N.; DeMott, L.; Matiolli, C.C.; Thompson, B.K.; Melotto, M. JAZ4 is involved in plant defense, growth, and development in Arabidopsis. Plant J. 2020, 101, 371–383. [Google Scholar] [CrossRef]
- Major, I.T.; Yoshida, Y.; Campos, M.L.; Kapali, G.; Xin, X.F.; Sugimoto, K.; de Oliveira Ferreira, D.; He, S.Y.; Howe, G.A. Regulation of growth-defense balance by the JASMONATE ZIM-DOMAIN (JAZ)-MYC transcriptional module. New Phytol. 2017, 215, 1533–1547. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Seong, K.; Pang, S.; Song, J.; Gao, H.; Wang, C.; Zhai, J.; Zhang, Y.; Gao, S.; Li, X.; et al. Functional specificity, diversity, and redundancy of Arabidopsis JAZ family repressors in jasmonate and COI1-regulated growth, development, and defense. New Phytol. 2021, 231, 1525–1545. [Google Scholar] [CrossRef]
- Han, Y.; Luthe, D. Identification and evolution analysis of the JAZ gene family in maize. BMC Genom. 2021, 22, 256. [Google Scholar] [CrossRef] [PubMed]
- Jia, K.; Yan, C.; Zhang, J.; Cheng, Y.; Li, W.; Yan, H.; Gao, J. Genome-wide identification and expression analysis of the JAZ gene family in turnip. Sci. Rep. 2021, 11, 21330. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, C.; Liu, Z.; Zhao, T.; Jiang, J.; Li, J.; Xu, X.; Yang, H. Genome-Wide Identification, Characterization, and Expression Analysis of the JAZ Gene Family in Resistance to Gray Leaf Spots in Tomato. Int. J. Mol. Sci. 2021, 22, 9974. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.F.; Wang, Y.K.; Guo, L.P.; Guo, X.M.; Guo, H.Y.; Yuan, S.H.; Duan, W.J.; Liu, Z.; Zhao, C.P.; Zhang, F.T.; et al. Genomic identification and characterization of MYC family genes in wheat (Triticum aestivum L.). BMC Genom. 2019, 20, 1032. [Google Scholar] [CrossRef]
- Chen, S.; Kong, Y.; Zhang, X.; Liao, Z.; He, Y.; Li, L.; Liang, Z.; Sheng, Q.; Hong, G. Structural and functional organization of the MYC transcriptional factors in Camellia sinensis. Planta 2021, 253, 93. [Google Scholar] [CrossRef]
- Li, L.; Zhang, H.; Chai, X.; Lv, J.; Hu, L.; Wang, J.; Li, Z.; Yu, J.; Liu, Z. Genome-wide identification and expression analysis of the MYC transcription factor family and its response to sulfur stress in cabbage (Brassica oleracea L.). Gene 2022, 814, 146116. [Google Scholar] [CrossRef]
- Wu, H.; Ye, H.; Yao, R.; Zhang, T.; Xiong, L. OsJAZ9 acts as a transcriptional regulator in jasmonate signaling and modulates salt stress tolerance in rice. Plant Sci. 2015, 232, 1–12. [Google Scholar] [CrossRef]
- Fu, J.; Wu, H.; Ma, S.; Xiang, D.; Liu, R.; Xiong, L. OsJAZ1 Attenuates Drought Resistance by Regulating JA and ABA Signaling in Rice. Front. Plant Sci. 2017, 8, 2108. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Vande-Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Gable, A.L.; Nastou, K.C.; Lyon, D.; Kirsch, R.; Pyysalo, S.; Doncheva, N.T.; Legeay, M.; Fang, T.; Bork, P.; et al. The STRING database in 2021: Customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021, 49, D605–D612. [Google Scholar] [CrossRef]
- Kohl, M.; Wiese, S.; Warscheid, B. Cytoscape: Software for visualization and analysis of biological networks. In Data Mining in Proteomics; Methods in Molecular Biology; Humana Press: New York, NY, USA, 2011; Volume 696, pp. 291–303. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]


| Gene ID | CDS Lengths (bp) | Protein Lengths (aa) | PI | MW | Localization |
|---|---|---|---|---|---|
| StJAZ1 | 351 | 117 | 9.3 | 13,350.27 | Nucleus |
| StJAZ2 | 321 | 107 | 9.81 | 12,530.24 | Nucleus |
| StJAZ3 | 765 | 255 | 9.24 | 28,431.98 | Nucleus |
| StJAZ4 | 604 | 201 | 9.3 | 22,397.20 | Nucleus |
| StJAZ5 | 531 | 177 | 9.01 | 19,473.24 | Nucleus |
| StJAZ6 | 348 | 116 | 9.74 | 13,198.17 | Nucleus |
| StJAZ7 | 375 | 125 | 9.17 | 14,341.22 | Nucleus |
| StJAZ8 | 1023 | 341 | 5.11 | 38,583.98 | Nucleus |
| StJAZ9 | 1128 | 376 | 4.93 | 40,849.43 | Nucleus |
| StJAZ10 | 1173 | 391 | 9.43 | 41,118.75 | Nucleus |
| StJAZ11 | 687 | 229 | 6.4 | 26,127.31 | Nucleus |
| StJAZ12 | 1020 | 340 | 8.23 | 37,486.27 | Nucleus |
| StJAZ13 | 984 | 328 | 8.45 | 39,257.33 | Nucleus |
| StJAZ14 | 930 | 310 | 8.51 | 33,698.76 | Nucleus |
| StJAZ15 | 777 | 259 | 9.71 | 27,460.62 | Nucleus |
| StJAZ16 | 648 | 216 | 8.84 | 23,905.17 | Nucleus |
| StJAZ17 | 1017 | 339 | 8.93 | 35,760.7 | Nucleus |
| StJAZ18 | 987 | 329 | 6.13 | 34,998.6 | Nucleus |
| Gene ID | CDS Lengths (bp) | Protein Lengths (aa) | PI | MW | Localization |
|---|---|---|---|---|---|
| StMYC1 | 1797 | 599 | 6.86 | 66,369.87 | Nucleus |
| StMYC2 | 1953 | 651 | 5.92 | 65,258.62 | Nucleus |
| StMYC3 | 432 | 144 | 6.03 | 44,083.76 | Nucleus |
| StMYC4 | 1374 | 458 | 5.99 | 50,898.24 | Nucleus |
| StMYC5 | 1371 | 457 | 6.22 | 51,791.64 | Nucleus |
| StMYC6 | 1737 | 579 | 7.61 | 64,285.91 | Nucleus |
| StMYC7 | 1224 | 408 | 5.88 | 44,514.29 | Nucleus |
| StMYC8 | 1296 | 432 | 6.71 | 48,790.06 | Nucleus |
| StMYC9 | 2064 | 688 | 5.81 | 64,760.08 | Nucleus |
| StMYC10 | 2109 | 703 | 5.47 | 75,939.94 | Nucleus |
| StMYC11 | 1359 | 453 | 5.61 | 49,967.36 | Nucleus |
| StMYC12 | 1434 | 478 | 6.59 | 52,903.87 | Nucleus |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Wang, Y.; Yang, R.; Cai, W.; Liu, Y.; Zhou, D.; Meng, L.; Wang, P.; Huang, B. Genome-Wide Identification and Analysis Uncovers the Potential Role of JAZ and MYC Families in Potato under Abiotic Stress. Int. J. Mol. Sci. 2023, 24, 6706. https://doi.org/10.3390/ijms24076706
Wang S, Wang Y, Yang R, Cai W, Liu Y, Zhou D, Meng L, Wang P, Huang B. Genome-Wide Identification and Analysis Uncovers the Potential Role of JAZ and MYC Families in Potato under Abiotic Stress. International Journal of Molecular Sciences. 2023; 24(7):6706. https://doi.org/10.3390/ijms24076706
Chicago/Turabian StyleWang, Shan, Yongbin Wang, Rui Yang, Wanhua Cai, Yaning Liu, Duanrong Zhou, Li Meng, Ping Wang, and Binquan Huang. 2023. "Genome-Wide Identification and Analysis Uncovers the Potential Role of JAZ and MYC Families in Potato under Abiotic Stress" International Journal of Molecular Sciences 24, no. 7: 6706. https://doi.org/10.3390/ijms24076706
APA StyleWang, S., Wang, Y., Yang, R., Cai, W., Liu, Y., Zhou, D., Meng, L., Wang, P., & Huang, B. (2023). Genome-Wide Identification and Analysis Uncovers the Potential Role of JAZ and MYC Families in Potato under Abiotic Stress. International Journal of Molecular Sciences, 24(7), 6706. https://doi.org/10.3390/ijms24076706




