Integration of mRNA and miRNA Analysis Reveals the Post-Transcriptional Regulation of Salt Stress Response in Hemerocallis fulva
Abstract
1. Introduction
2. Results
2.1. Identification of Conserved and Novel miRNAs in Hemerocallis
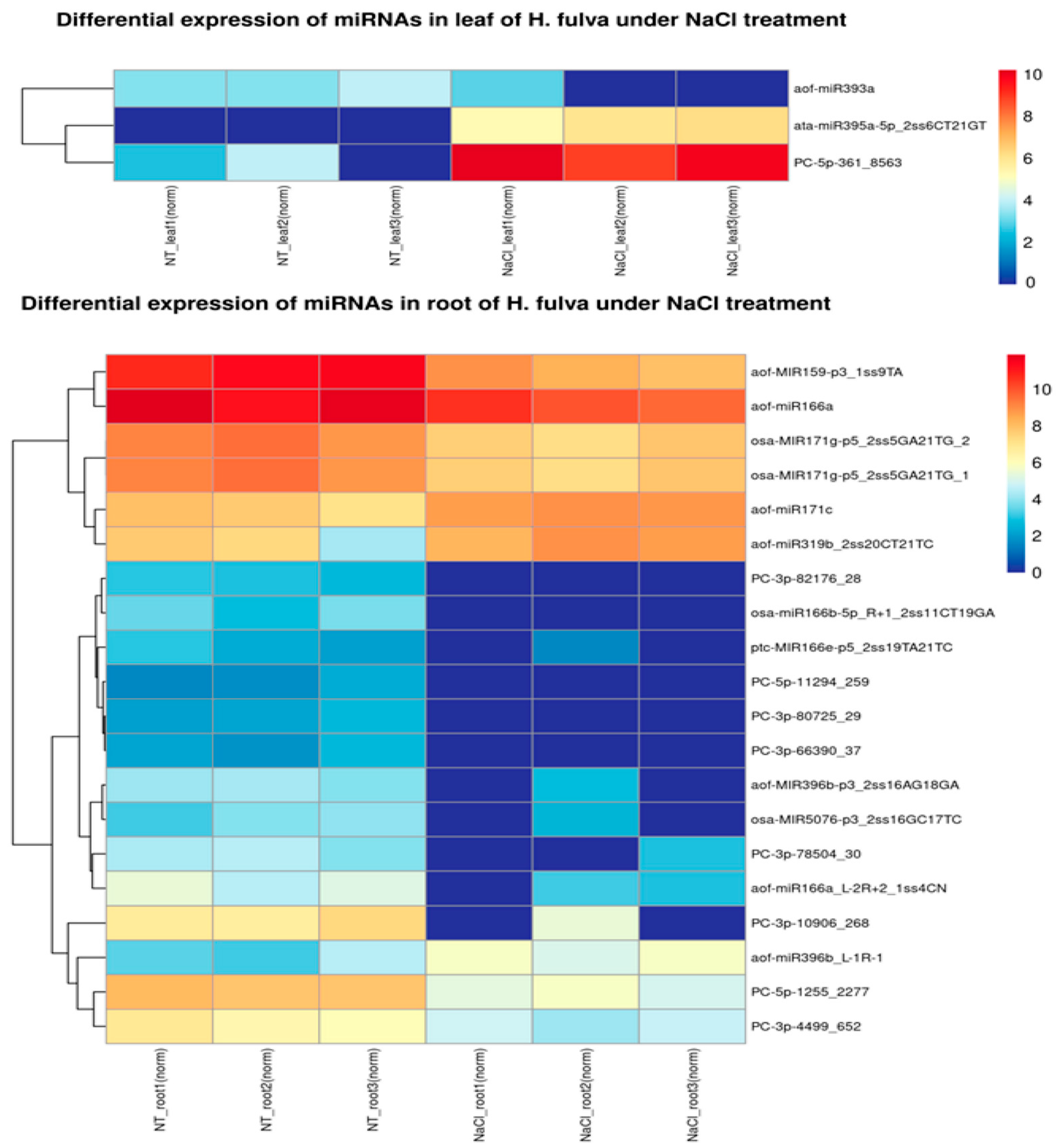
2.2. Differential Analysis of Candidate miRNAs in Roots and Leaves under NaCl Treatment
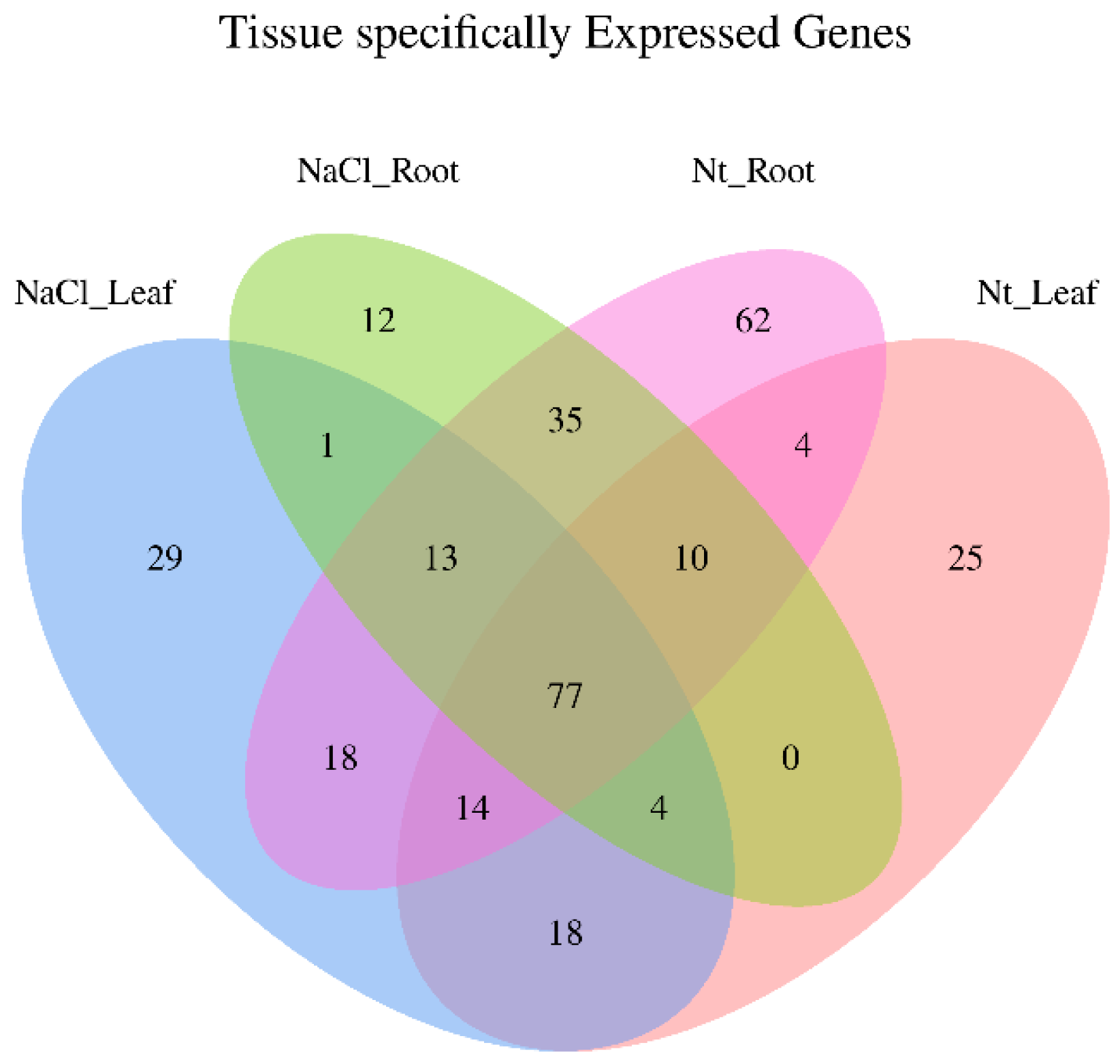
2.3. Identification of Differential NaCl-Responsive Genes by RNA-Seq Analysis
2.4. Identification of Cleaved miRNA Targets by Degradome Sequencing
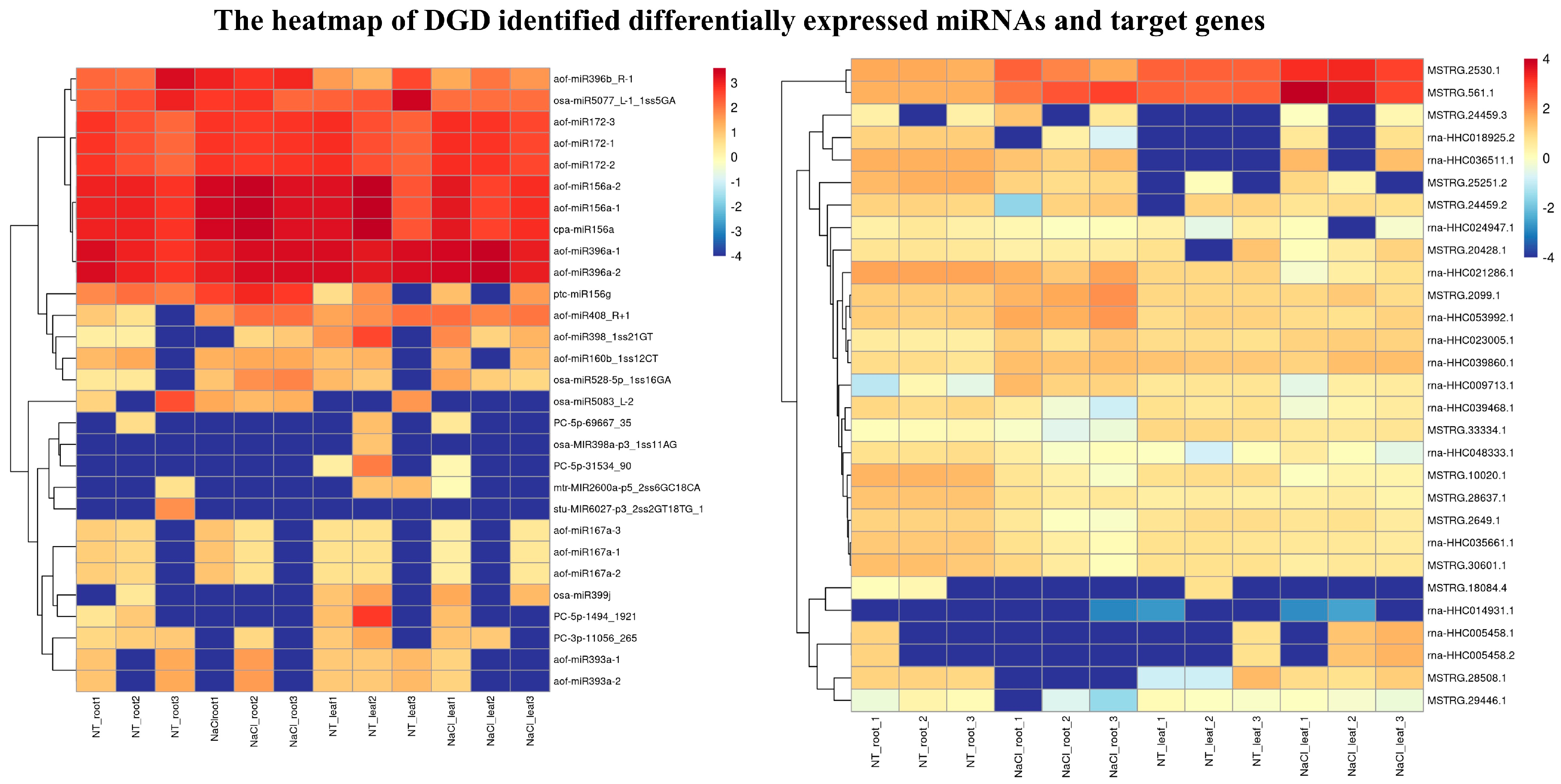
2.5. Transcriptome Profiles of miRNAs and Their Target Genes under NaCl Stress
2.6. qRT-PCR Validation of NaCl Stress Response-miRNAs and -Target Genes in H. fulva
2.7. miRNA-Mediated Gene Regulatory Network in Response to NaCl Stress in H. fulva
3. Discussion
3.1. Analysis of Conserved and Novel miRNAs and Their Targets in H. fulva
3.2. Differential Expression of the Identified miRNAs and Their Target Genes
3.3. miRNAs Regulate NaCl Stress Response Partially via Phytohormone Signaling, Ca2+ Signaling, and Oxidative Defense Signaling Pathways
4. Materials and Methods
4.1. Plant Materials and Salt Stress Treatment
4.2. Small RNA, RNA-Seq, and Degradome Library Construction and Sequencing
4.3. Identification of miRNAs
4.4. RNA-Seq, Transcriptome Assembly, and Annotation
4.5. Degradome Sequencing Data Analysis and miRNA Targets Identification
4.6. Differential Expression Analysis of miRNAs–mRNAs in Different Tissues of H. fulva
4.7. Validation of the Identified miRNAs and Targets by Quantitative Real-Time PCR
4.8. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, J.K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Wan, Q.; Zhang, K.; Zhang, X.; Guo, R.; Wang, C.; Zheng, C.; Liu, F.; Ding, Z.; Wan, Y. AhABI4s Negatively Regulate Salt-Stress Response in Peanut. Front. Plant Sci. 2021, 12, 741641. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, J. Communications Between the Endoplasmic Reticulum and Other Organelles during Abiotic Stress Response in Plants. Front. Plant Sci. 2019, 10, 749. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J.K. Abiotic stress responses in plants. Nat. Rev. Genet. 2022, 23, 104–119. [Google Scholar] [CrossRef]
- Mushtaq, Z.; Faizan, S.; Gulzar, B. Salt stress, its impacts on plants and the strategies plants are employing against it: A review. J. Appl. Biol. Biotechnol. 2020, 8, 81–91. [Google Scholar]
- Zhang, B.; Pan, X.; Cobb, G.P.; Anderson, T.A. Plant microRNA: A small regulatory molecule with big impact. Dev. Biol. 2006, 289, 3–16. [Google Scholar] [CrossRef]
- Zhan, J.; Diao, Y.; Yin, G.; Sajjad, M.; Wei, X.; Lu, Z.; Wang, Y. Integration of mRNA and miRNA Analysis Reveals the Molecular Mechanism of Cotton Response to Salt Stress. Front. Plant Sci. 2021, 12, 767984. [Google Scholar] [CrossRef]
- Liu, J.N.; Ma, X.; Yan, L.; Liang, Q.; Fang, H.; Wang, C.; Dong, Y.; Chai, Z.; Zhou, R.; Bao, Y.; et al. MicroRNA and Degradome Profiling Uncover Defense Response of Fraxinus velutina Torr. to Salt Stress. Front. Plant Sci. 2022, 13, 847853. [Google Scholar] [CrossRef]
- Ma, Y.; Xue, H.; Zhang, F.; Jiang, Q.; Yang, S.; Yue, P.; Wang, F.; Zhang, Y.; Li, L.; He, P.; et al. The miR156/SPL module regulates apple salt stress tolerance by activating MdWRKY100 expression. Plant Biotechnol. J. 2021, 19, 311–323. [Google Scholar] [CrossRef]
- Liu, Y.; Li, D.; Yan, J.; Wang, K.; Luo, H.; Zhang, W. MiR319 mediated salt tolerance by ethylene. Plant Biotechnol. J. 2019, 17, 2370–2383. [Google Scholar] [CrossRef]
- Denver, J.B.; Ullah, H. miR393s regulate salt stress response pathway in Arabidopsis thaliana through scaffold protein RACK1A mediated ABA signaling pathways. Plant Signal. Behav. 2019, 14, 1600394. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ji, F.; Hou, F.; Cui, H.; Shi, Q.; Xing, G.; Weng, Y.; Kang, X. Characterization of Hemerocallis citrina Transcriptome and Development of EST-SSR Markers for Evaluation of Genetic Diversity and Population Structure of Hemerocallis Collection. Front. Plant Sci. 2020, 11, 686. [Google Scholar] [CrossRef] [PubMed]
- Qing, Z.; Liu, J.; Yi, X.; Liu, X.; Hu, G.; Lao, J.; He, W.; Yang, Z.; Zou, X.; Sun, M.; et al. The chromosome-level Hemerocallis citrina Borani genome provides new insights into the rutin biosynthesis and the lack of colchicine. Hortic. Res. 2021, 8, 89. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.O. Chapter 54—Lilies. In Small Animal Toxicology, 3rd ed.; Peterson, M.E., Talcott, P.A., Eds.; W.B. Saunders: Saint Louis, MO, USA, 2013; pp. 617–620. [Google Scholar]
- Bao, Y.; Wang, J.; Chen, C.; Yu, X. Effects of NaCl and NaHCO3 Stress on Photosynthesis and Chlorophyll Fluorescence Characteristics of Hemerocallis fulva ‘Golden Doll’. J. Agric. Biotechnol. 2020, 9, 18–25+28. [Google Scholar]
- Yu, Y.; Zhang, Y.; Chen, X.; Chen, Y. Plant Noncoding RNAs: Hidden Players in Development and Stress Responses. Annu. Rev. Cell Dev. Biol. 2019, 35, 407–431. [Google Scholar] [CrossRef]
- Ma, X.; Zhao, F.; Zhou, B. The Characters of Non-Coding RNAs and Their Biological Roles in Plant Development and Abiotic Stress Response. Int. J. Mol. Sci. 2022, 23, 4124. [Google Scholar] [CrossRef]
- An, F.; Liang, Y.; Li, J.; Chen, X.; Han, H.; Li, F. Construction and significance analysis of the MicroRNA expression profile of Hemerocallis fulva at low temperature. Biosci. Biotechnol. Biochem. 2014, 78, 378–383. [Google Scholar] [CrossRef]
- Huang, C.; Jiang, C.; Jin l Zhang, H. Identification of cold stress responsive microRNAs in cold tolerant and susceptible Hemerocallis fulva by high throughput sequencing. Res. Sq. 2020. [Google Scholar] [CrossRef]
- Addo-Quaye, C.; Miller, W.; Axtell, M.J. CleaveLand: A pipeline for using degradome data to find cleaved small RNA targets. Bioinformatics 2009, 25, 130–131. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, Y.; Yuan, L.; Zhang, Q. Functional characterization and spatial interaction of TERMINAL FLOWER 1 in Hemerocallis. Sci. Hortic. 2019, 253, 154–162. [Google Scholar] [CrossRef]
- Panavas, T.; Pikula, A.; Reid, P.D.; Rubinstein, B.; Walker, E.L. Identification of senescence-associated genes from daylily petals. Plant Mol. Biol. 1999, 40, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.M.; Chen, Y.; Liu, X.; Ni, D.A.; Bai, L.; Qin, Q.P. Genome-wide identification and expression analysis of the SWEET gene family in daylily (Hemerocallis fulva) and functional analysis of HfSWEET17 in response to cold stress. BMC Plant Biol. 2022, 22, 211. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liu, Y.; Teng, F.; Cen, H.; Yan, J.; Lin, S.; Li, D.; Zhang, W. Heterogeneous expression of Osa-MIR156bc increases abiotic stress resistance and forage quality of alfalfa. Crop J. 2021, 9, 1135–1144. [Google Scholar] [CrossRef]
- Reyes, J.L.; Chua, N.H. ABA induction of miR159 controls transcript levels of two MYB factors during Arabidopsis seed germination. Plant J. 2007, 49, 592–606. [Google Scholar] [CrossRef]
- Guo, C.; Jiang, Y.; Shi, M.; Wu, X.; Wu, G. ABI5 acts downstream of miR159 to delay vegetative phase change in Arabidopsis. New Phytol. 2021, 231, 339–350. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, Q.; Liu, M.; Zhou, H.; Ma, C.; Wang, P. Regulation of Plant Responses to Salt Stress. Int. J. Mol. Sci. 2021, 22, 4609. [Google Scholar] [CrossRef]
- Shen, W.; Li, H.; Teng, R.; Wang, Y.; Wang, W.; Zhuang, J. Genomic and transcriptomic analyses of HD-Zip family transcription factors and their responses to abiotic stress in tea plant (Camellia sinensis). Genomics 2019, 111, 1142–1151. [Google Scholar] [CrossRef]
- Sharma, A.; Ruiz-Manriquez, L.M.; Serrano-Cano, F.I.; Reyes-Pérez, P.R.; Tovar Alfaro, C.K.; Barrón Andrade, Y.E.; Hernández Aros, A.K.; Srivastava, A.; Paul, S. Identification of microRNAs and Their Expression in Leaf Tissues of Guava (Psidium guajava L.) under Salinity Stress. Agronomy 2020, 10, 1920. [Google Scholar] [CrossRef]
- Caruana, J.C.; Dhar, N.; Raina, R. Overexpression of Arabidopsis microRNA167 induces salicylic acid-dependent defense against Pseudomonas syringae through the regulation of its targets ARF6 and ARF8. Plant Direct 2020, 4, e00270. [Google Scholar] [CrossRef]
- Ye, Y.; Wang, J.; Wang, W.; Xu, L.A. ARF family identification in Tamarix chinensis reveals the salt responsive expression of TcARF6 targeted by miR167. PeerJ 2020, 8, e8829. [Google Scholar] [CrossRef]
- Li, W.; Cui, X.; Meng, Z.; Huang, X.; Xie, Q.; Wu, H.; Jin, H.; Zhang, D.; Liang, W. Transcriptional regulation of Arabidopsis MIR168a and argonaute1 homeostasis in abscisic acid and abiotic stress responses. Plant Physiol. 2012, 158, 1279–1292. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Fan, T.; Hu, X.; Cheng, T.; Zhang, M. Overexpressing osa-miR171c decreases salt stress tolerance in rice. J. Plant Biol. 2017, 60, 485–492. [Google Scholar] [CrossRef]
- Gao, Z.; Ma, C.; Zheng, C.; Yao, Y.; Du, Y. Advances in the regulation of plant salt-stress tolerance by miRNA. Mol. Biol. Rep. 2022, 49, 5041–5055. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Zhao, J.; Li, Z.; Hu, Q.; Yuan, N.; Zhou, M.; Xia, X.; Noorai, R.; Saski, C.; Li, S.; et al. MicroRNA396-mediated alteration in plant development and salinity stress response in creeping bentgrass. Hortic. Res. 2019, 6, 48. [Google Scholar] [CrossRef]
- Liebsch, D.; Palatnik, J.F. MicroRNA miR396, GRF transcription factors and GIF co-regulators: A conserved plant growth regulatory module with potential for breeding and biotechnology. Curr. Opin. Plant Biol. 2020, 53, 31–42. [Google Scholar] [CrossRef]
- Sun, X.; Xu, L.; Wang, Y.; Yu, R.; Zhu, X.; Luo, X.; Gong, Y.; Wang, R.; Limera, C.; Zhang, K.; et al. Identification of novel and salt-responsive miRNAs to explore miRNA-mediated regulatory network of salt stress response in radish (Raphanus sativus L.). BMC Genom. 2015, 16, 197. [Google Scholar] [CrossRef]
- Liu, H.H.; Tian, X.; Li, Y.J.; Wu, C.A.; Zheng, C.C. Microarray-based analysis of stress-regulated microRNAs in Arabidopsis thaliana. RNA 2008, 14, 836–843. [Google Scholar] [CrossRef]
- Ding, D.; Zhang, L.; Wang, H.; Liu, Z.; Zhang, Z.; Zheng, Y. Differential expression of miRNAs in response to salt stress in maize roots. Ann. Bot. 2009, 103, 29–38. [Google Scholar] [CrossRef]
- Liu, C.; Peang, H.; Li, X.; Liu, C.; Lv, X.; Wei, X.; Zou, A.; Zhang, J.; Fan, G.; Ma, G.; et al. Genome-wide analysis of NDR1/HIN1-like genes in pepper (Capsicum annuum L.) and functional characterization of CaNHL4 under biotic and abiotic stresses. Hortic. Res. 2020, 7, 93. [Google Scholar] [CrossRef]
- Schuck, S.; Camehl, I.; Gilardoni, P.A.; Oelmueller, R.; Baldwin, I.T.; Bonaventure, G. HSPRO controls early Nicotiana attenuata seedling growth during interaction with the fungus Piriformospora indica. Plant Physiol. 2012, 160, 929–943. [Google Scholar] [CrossRef]
- Wang, S.; Lv, X.; Zhang, J.; Chen, D.; Chen, S.; Fan, G.; Ma, C.; Wang, Y. Roles of E3 Ubiquitin Ligases in Plant Responses to Abiotic Stresses. Int. J. Mol. Sci. 2022, 23, 2308. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Saxena, S.; Sinha, A.; Nandi, A.K. DORMANCY/AUXIN ASSOCIATED FAMILY PROTEIN 2 of Arabidopsis thaliana is a negative regulator of local and systemic acquired resistance. J. Plant Res. 2020, 133, 409–417. [Google Scholar] [CrossRef]
- Shin, D.; Moon, S.-J.; Park, S.R.; Kim, B.-G.; Byun, M.-O. Elongation factor 1α from A. thaliana functions as molecular chaperone and confers resistance to salt stress in yeast and plants. Plant Sci. 2009, 177, 156–160. [Google Scholar] [CrossRef]
- Garcia de la Garma, J.; Fernandez-Garcia, N.; Bardisi, E.; Pallol, B.; Asensio-Rubio, J.S.; Bru, R.; Olmos, E. New insights into plant salt acclimation: The roles of vesicle trafficking and reactive oxygen species signalling in mitochondria and the endomembrane system. New Phytol. 2015, 205, 216–239. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.; Su, Y.C.F.; Saunders, R.M.K.; Chye, M.L. The rice acyl-CoA-binding protein gene family: Phylogeny, expression and functional analysis. New Phytol. 2011, 189, 1170–1184. [Google Scholar] [CrossRef] [PubMed]
- Park, H.Y.; Seok, H.Y.; Woo, D.H.; Lee, S.Y.; Tarte, V.N.; Lee, E.H.; Lee, C.H.; Moon, Y.H. AtERF71/HRE2 transcription factor mediates osmotic stress response as well as hypoxia response in Arabidopsis. Biochem. Biophys. Res. Commun. 2011, 414, 135–141. [Google Scholar] [CrossRef]
- Li, A.L.; Wen, Z.; Yang, K.; Wen, X.P. Conserved miR396b-GRF Regulation Is Involved in Abiotic Stress Responses in Pitaya (Hylocereus polyrhizus). Int. J. Mol. Sci. 2019, 20, 2501. [Google Scholar] [CrossRef]
- Li, S.; Tian, Y.; Wu, K.; Ye, Y.; Yu, J.; Zhang, J.; Liu, Q.; Hu, M.; Li, H.; Tong, Y.; et al. Modulating plant growth-metabolism coordination for sustainable agriculture. Nature 2018, 560, 595–600. [Google Scholar] [CrossRef]
- Lu, Y.; Zeng, J.; Liu, Q. The Rice miR396-GRF-GIF-SWI/SNF Module: A Player in GA Signaling. Front. Plant Sci. 2021, 12, 786641. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Guo, Q.; Zhang, X.; Zhou, L.; Zhang, Y.; Zhang, C. Conservation and Diversity of miR166 Family Members From Highbush Blueberry (Vaccinium corymbosum) and Their Potential Functions in Abiotic Stress. Front. Genet. 2022, 13, 919856. [Google Scholar] [CrossRef]
- Zhao, C.; Ma, J.; Zhang, Y.; Yang, S.; Feng, X.; Yan, J. The miR166 mediated regulatory module controls plant height by regulating gibberellic acid biosynthesis and catabolism in soybean. J. Integr. Plant Biol. 2022, 64, 995–1006. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Liu, W.; Li, W.; Liu, Y.; Wang, W.; Xie, P.; Kang, Y.; Liao, L.; Qian, L.; Liu, Z.; et al. Genome-wide identification and expression analysis of CaM/CML genes in Brassica napus under abiotic stress. J. Plant Physiol. 2020, 255, 153251. [Google Scholar] [CrossRef]
- Perochon, A.; Aldon, D.; Galaud, J.P.; Ranty, B. Calmodulin and calmodulin-like proteins in plant calcium signaling. Biochimie 2011, 93, 2048–2053. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Y.; Zhang, J.; Ren, Y.; Li, M.; Tian, S.; Yu, Y.; Zuo, Y.; Gong, G.; Zhang, H.; et al. The NAC transcription factor ClNAC68 positively regulates sugar content and seed development in watermelon by repressing ClINV and ClGH3.6. Hortic. Res. 2021, 8, 214. [Google Scholar] [CrossRef]
- Negi, S.; Tak, H.; Ganapathi, T.R. Expression analysis of MusaNAC68 transcription factor and its functional analysis by overexpression in transgenic banana plants. Plant Cell Tissue Organ Cult. (PCTOC) 2016, 125, 59–70. [Google Scholar] [CrossRef]
- Wang, J.; Ye, Y.; Xu, M.; Feng, L.; Xu, L.A. Roles of the SPL gene family and miR156 in the salt stress responses of tamarisk (Tamarix chinensis). BMC Plant Biol. 2019, 19, 370. [Google Scholar] [CrossRef]
- Skodra, C.; Michailidis, M.; Moysiadis, T.; Stamatakis, G.; Ganopoulou, M.; Adamakis, I.S.; Angelis, L.; Ganopoulos, I.; Tanou, G.; Samiotaki, M.; et al. Disclosing the molecular basis of salinity priming in olive trees using proteogenomic model discovery. Plant Physiol. 2023, 191, 1913–1933. [Google Scholar] [CrossRef]
- Khatun, K.; Robin, A.H.K.; Park, J.I.; Nath, U.K.; Kim, C.K.; Lim, K.B.; Nou, I.S.; Chung, M.Y. Molecular Characterization and Expression Profiling of Tomato GRF Transcription Factor Family Genes in Response to Abiotic Stresses and Phytohormones. Int. J. Mol. Sci. 2017, 18, 1056. [Google Scholar] [CrossRef]
- Omidbakhshfard, M.A.; Proost, S.; Fujikura, U.; Mueller-Roeber, B. Growth-Regulating Factors (GRFs): A Small Transcription Factor Family with Important Functions in Plant Biology. Mol. Plant 2015, 8, 998–1010. [Google Scholar] [CrossRef]
- Gao, S.; Chu, C. Gibberellin Metabolism and Signaling: Targets for Improving Agronomic Performance of Crops. Plant Cell Physiol. 2020, 61, 1902–1911. [Google Scholar] [CrossRef]
- Li, S.; Gao, F.; Xie, K.; Zeng, X.; Cao, Y.; Zeng, J.; He, Z.; Ren, Y.; Li, W.; Deng, Q.; et al. The OsmiR396c-OsGRF4-OsGIF1 regulatory module determines grain size and yield in rice. Plant Biotechnol. J. 2016, 14, 2134–2146. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, A.; Zhang, Z.; Huang, Z.; Lu, P.; Zhang, D.; Liu, X.; Zhang, Z.F.; Huang, R. Ethylene Response Factor TERF1, Regulated by ETHYLENE-INSENSITIVE3-like Factors, Functions in Reactive Oxygen Species (ROS) Scavenging in Tobacco (Nicotiana tabacum L.). Sci. Rep. 2016, 6, 29948. [Google Scholar] [CrossRef]
- Dey, S.; Corina Vlot, A. Ethylene responsive factors in the orchestration of stress responses in monocotyledonous plants. Front. Plant Sci. 2015, 6, 640. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Hu, L.; Han, N.; Hu, J.; Yang, Y.; Xiang, T.; Zhang, X.; Wang, L. Overexpression of a miR393-resistant form of transport inhibitor response protein 1 (mTIR1) enhances salt tolerance by increased osmoregulation and Na+ exclusion in Arabidopsis thaliana. Plant Cell Physiol. 2015, 56, 73–83. [Google Scholar] [CrossRef]
- Dharmasiri, N.; Dharmasiri, S.; Estelle, M. The F-box protein TIR1 is an auxin receptor. Nature 2005, 435, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Ribba, T.; Garrido-Vargas, F.; O’Brien, J.A. Auxin-mediated responses under salt stress: From developmental regulation to biotechnological applications. J. Exp. Bot. 2020, 71, 3843–3853. [Google Scholar] [CrossRef]
- Verma, S.; Negi, N.P.; Pareek, S.; Mudgal, G.; Kumar, D. Auxin response factors in plant adaptation to drought and salinity stress. Physiol. Plant 2022, 174, e13714. [Google Scholar] [CrossRef]
- Sun, Q.; Yu, S.; Guo, Z. Calmodulin-Like (CML) Gene Family in Medicago truncatula: Genome-Wide Identification, Characterization and Expression Analysis. Int. J. Mol. Sci. 2020, 21, 7142. [Google Scholar] [CrossRef]
- Munir, S.; Liu, H.; Xing, Y.; Hussain, S.; Ouyang, B.; Zhang, Y.; Li, H.; Ye, Z. Overexpression of calmodulin-like (ShCML44) stress-responsive gene from Solanum habrochaites enhances tolerance to multiple abiotic stresses. Sci. Rep. 2016, 6, 31772. [Google Scholar] [CrossRef]
- Zeng, H.; Xu, L.; Singh, A.; Wang, H.; Du, L.; Poovaiah, B.W. Involvement of calmodulin and calmodulin-like proteins in plant responses to abiotic stresses. Front. Plant Sci. 2015, 6, 600. [Google Scholar] [CrossRef]
- He, F.; Long, R.; Wei, C.; Zhang, Y.; Li, M.; Kang, J.; Yang, Q.; Wang, Z.; Chen, L. Genome-wide identification, phylogeny and expression analysis of the SPL gene family and its important role in salt stress in Medicago sativa L. BMC Plant Biol. 2022, 22, 295. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.X.; Zhu, W.C.; Feng, X.H.; Jin, J.H.; Wei, A.M.; Gong, Z.H. Transcription Factor CaSBP12 Negatively Regulates Salt Stress Tolerance in Pepper (Capsicum annuum L.). Int. J. Mol. Sci. 2020, 21, 444. [Google Scholar] [CrossRef] [PubMed]
- Caverzan, A.; Passaia, G.; Rosa, S.B.; Ribeiro, C.W.; Lazzarotto, F.; Margis-Pinheiro, M. Plant responses to stresses: Role of ascorbate peroxidase in the antioxidant protection. Genet. Mol. Biol. 2012, 35 (Suppl. 4), 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Ul Haq, S.; Khan, A.; Ali, M.; Khattak, A.M.; Gai, W.X.; Zhang, H.X.; Wei, A.M.; Gong, Z.H. Heat Shock Proteins: Dynamic Biomolecules to Counter Plant Biotic and Abiotic Stresses. Int. J. Mol. Sci. 2019, 20, 5321. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef]
- Pertea, G.; Pertea, M. GFF Utilities: GffRead and GffCompare. F1000Res 2020, 9, 304. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene ontology analysis for RNA-seq: Accounting for selection bias. Genome Biol. 2010, 11, R14. [Google Scholar] [CrossRef]
- Kanehisa, M.; Araki, M.; Goto, S.; Hattori, M.; Hirakawa, M.; Itoh, M.; Katayama, T.; Kawashima, S.; Okuda, S.; Tokimatsu, T.; et al. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 2008, 36, D480–D484. [Google Scholar] [CrossRef]
- Berninger, P.; Gaidatzis, D.; van Nimwegen, E.; Zavolan, M. Computational analysis of small RNA cloning data. Methods 2008, 44, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Needleman, S.B.; Wunsch, C.D. A general method applicable to the search for similarities in the amino acid sequence of two proteins. J. Mol. Biol. 1970, 48, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Allen, E.; Xie, Z.; Gustafson, A.M.; Carrington, J.C. microRNA-directed phasing during trans-acting siRNA biogenesis in plants. Cell 2005, 121, 207–221. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
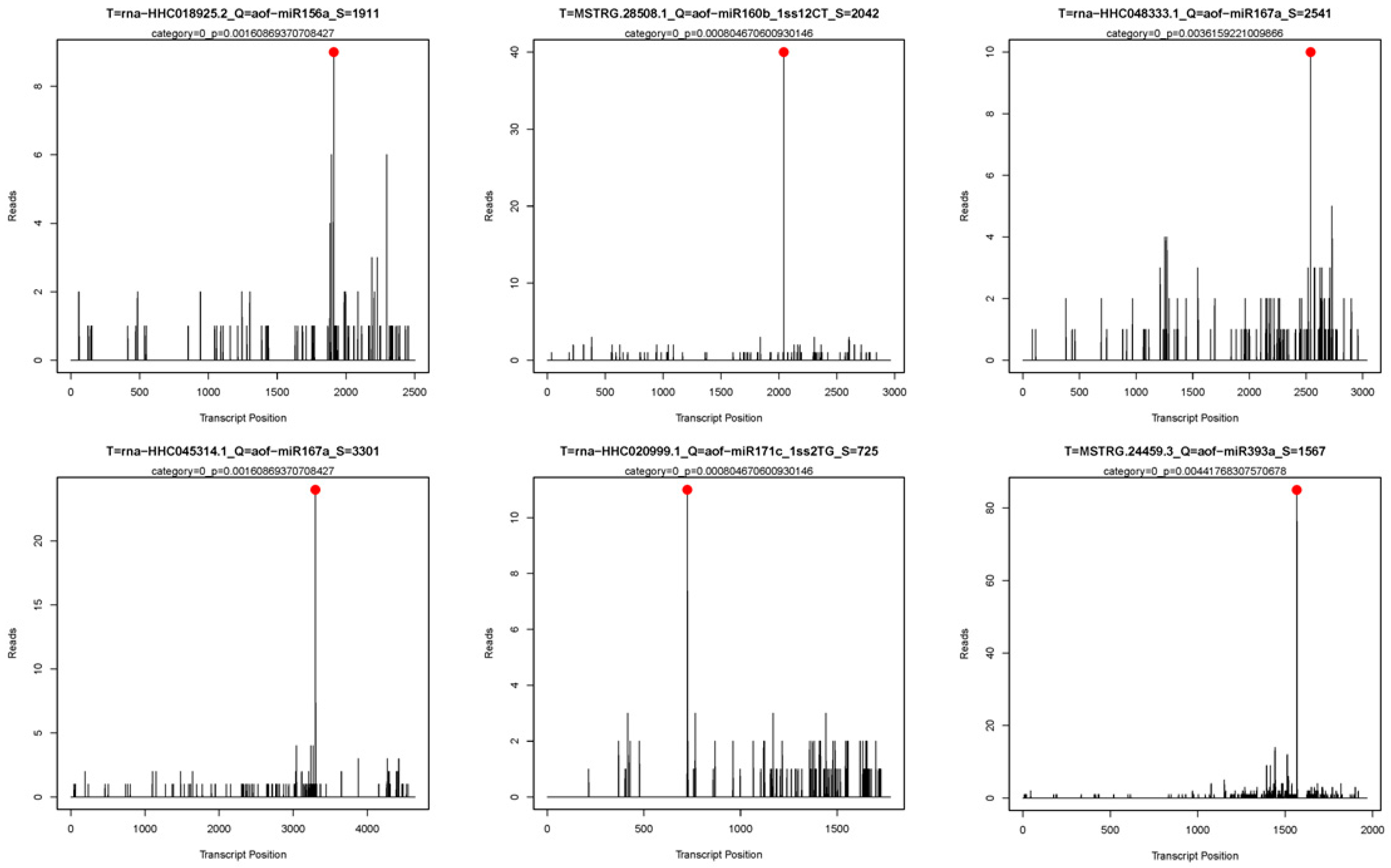
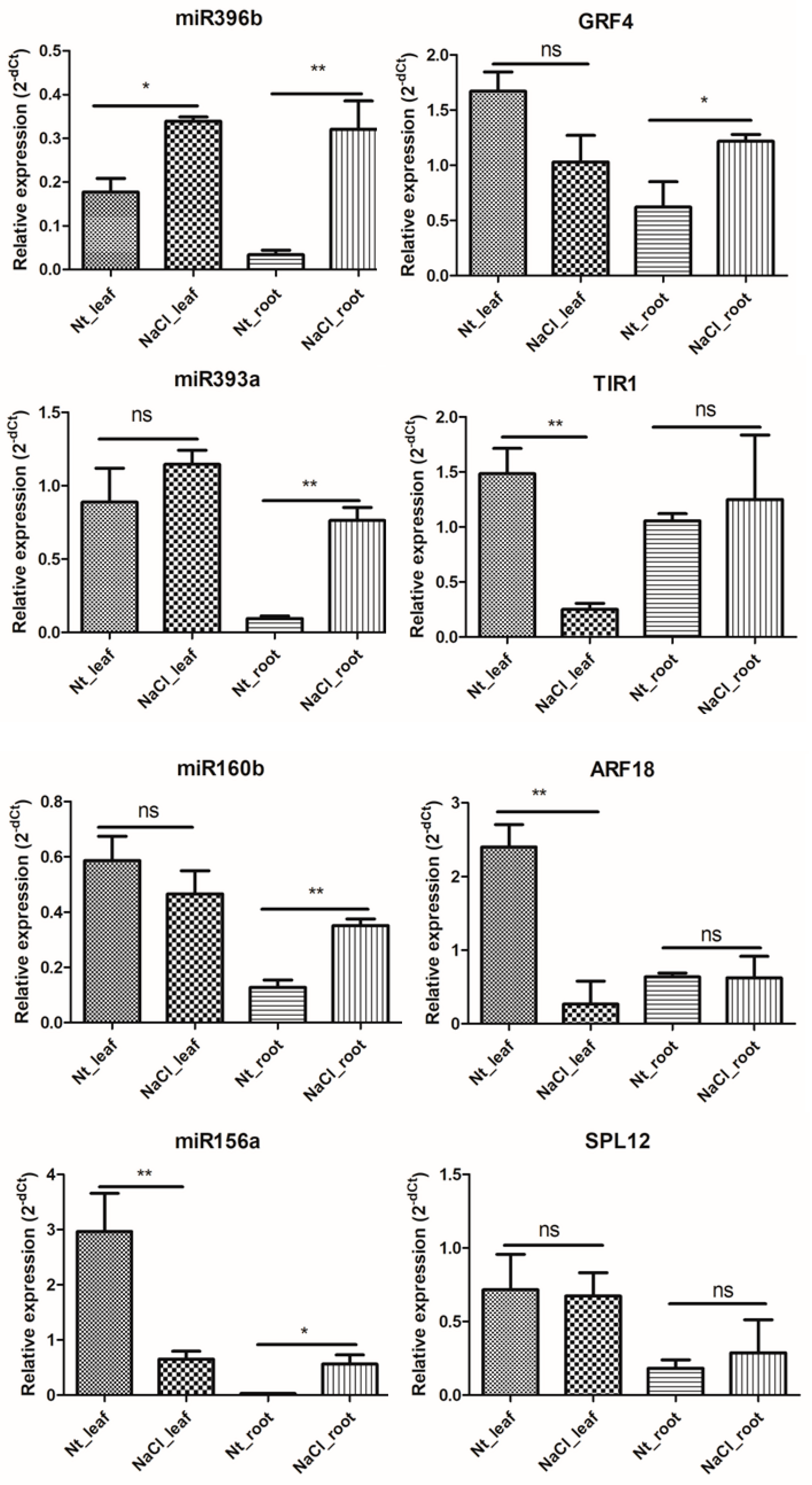
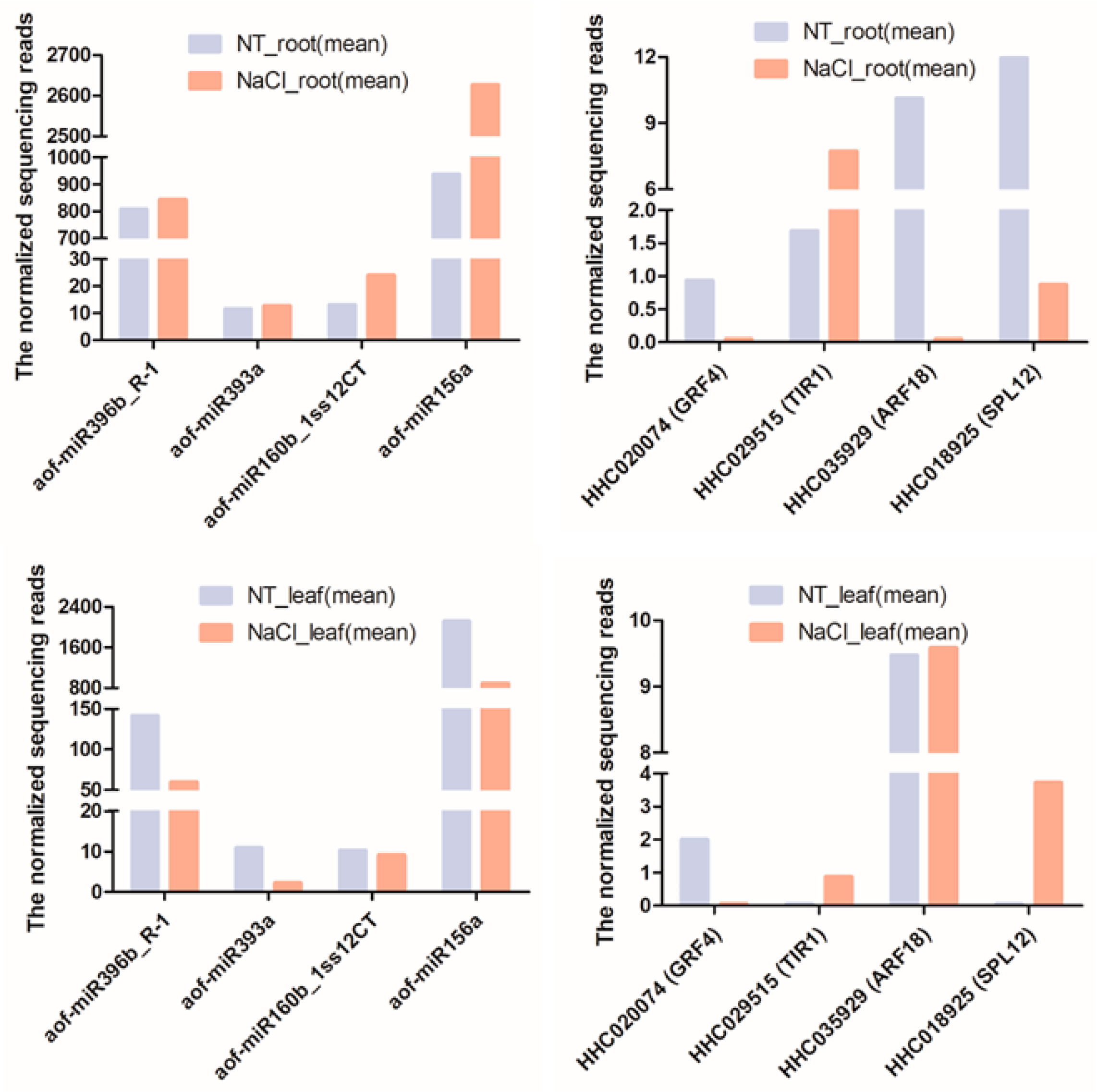
| miRNA ID | Gene ID | Target Gene | Gene Annotation |
|---|---|---|---|
| aof-miR156a | rna-HHC018925.2 | SPL12 | squamosa promoter-binding-like protein 12 [Asparagus officinalis] |
| aof-miR160b_1ss12CT | MSTRG.28508.1 | ARF18-like | auxin response factor 18-like isoform X1 [Asparagus officinalis] |
| aof-miR167a | rna-HHC035661.1 | ARF12-like | auxin response factor 12-like isoform X1 [Phoenix dactylifera] |
| aof-miR167a | rna-HHC048333.1 | ARF12-like | auxin response factor 12-like isoform X1 [Phoenix dactylifera] |
| aof-miR167a | MSTRG.28637.1 | ARF12-like | auxin response factor 12-like isoform X1 [Phoenix dactylifera] |
| aof-miR172 | MSTRG.29446.1 | A4U43_C07F3620 | uncharacterized protein A4U43_C07F3620 [Asparagus officinalis] |
| aof-miR172 | MSTRG.2649.1 | A4U43_C07F3620 | uncharacterized protein A4U43_C07F3620 [Asparagus officinalis] |
| aof-miR172 | MSTRG.10020.1 | A4U43_C07F3620 | uncharacterized protein A4U43_C07F3620 [Asparagus officinalis] |
| aof-miR396a | MSTRG.30601.1 | GRF4-like | growth-regulating factor 4-like [Asparagus officinalis] |
| aof-miR396a | rna-HHC039468.1 | GRF4-like | growth-regulating factor 4-like protein [Cinnamomum micranthum f. kanehirae] |
| aof-miR396b_R-1 | MSTRG.18084.4 | GRF10-like | PREDICTED: growth-regulating factor 10-like [Elaeis guineensis] |
| aof-miR398_1ss21GT | rna-HHC005458.2 | SOD | copper/zinc superoxide dismutase, partial [Allium sativum] |
| cpa-miR156a | rna-HHC024947.1 | LOC109823482 | uncharacterized protein LOC109823482 [Asparagus officinalis] |
| mtr-MIR2600a-p5_2ss6GC18CA | rna-HHC036511.1 | DED37-like | DEAD-box ATP-dependent RNA helicase 37-like [Asparagus officinalis] |
| osa-MIR398a-p3_1ss11AG | rna-HHC005458.2 | SOD | copper/zinc superoxide dismutase, partial [Allium sativum] |
| osa-miR399j | MSTRG.2099.1 | UBE2 | probable ubiquitin-conjugating enzyme E2 24 [Asparagus officinalis] |
| osa-miR5077_L-1_1ss5GA | rna-HHC009713.1 | AXF42_Ash001226 | hypothetical protein AXF42_Ash001226 [Apostasia shenzhenica] |
| osa-miR5083_L-2 | rna-HHC023005.1 | LOC110098070 | uncharacterized protein LOC110098070 [Dendrobium catenatum] |
| osa-miR528-5p_1ss16GA | MSTRG.25251.2 | PPO | PREDICTED: polyphenol oxidase, chloroplastic-like [Elaeis guineensis] |
| PC-3p-11056_265 | rna-HHC014931.1 | SNAT6 | probable sodium-coupled neutral amino acid transporter 6 isoform X1 [Asparagus officinalis] |
| PC-5p-1494_1921 | MSTRG.2530.1 | TanjilG_28990 | hypothetical protein TanjilG_28990 [Lupinus angustifolius] |
| PC-5p-69667_35 | rna-HHC053992.1 | CKAN_02569300 | hypothetical protein CKAN_02569300 [Cinnamomum micranthum f. kanehirae] |
| ptc-miR156g | MSTRG.20428.1 | SPL12 | squamosa promoter-binding-like protein 12 [Asparagus officinalis] |
| stu-MIR6027-p3_2ss2GT18TG_1 | rna-HHC039860.1 | MADS box protein 5 | MADS box protein 5, partial [Agave tequilana] |
| aof-miR408_R+1 | rna-HHC021286.1 | BCB-like | PREDICTED: blue copper protein-like [Elaeis guineensis] |
| aof-miR156a | MSTRG.33334.1 | A4U43_C04F14680 | uncharacterized protein A4U43_C04F14680 [Asparagus officinalis] |
| aof-miR393a | MSTRG.24459.3 | TIR1-like | transport inhibitor response 1-like protein Os04g0395600 [Phoenix dactylifera] |
| aof-miR393a | MSTRG.24459.2 | TIR1-like | transport inhibitor response 1-like protein Os04g0395600 [Phoenix dactylifera] |
| PC-5p-31534_90 | MSTRG.561.1 | MT-3a | Metallothionein 3a [Dracaena cambodiana] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, B.; Gao, X.; Zhao, F. Integration of mRNA and miRNA Analysis Reveals the Post-Transcriptional Regulation of Salt Stress Response in Hemerocallis fulva. Int. J. Mol. Sci. 2023, 24, 7290. https://doi.org/10.3390/ijms24087290
Zhou B, Gao X, Zhao F. Integration of mRNA and miRNA Analysis Reveals the Post-Transcriptional Regulation of Salt Stress Response in Hemerocallis fulva. International Journal of Molecular Sciences. 2023; 24(8):7290. https://doi.org/10.3390/ijms24087290
Chicago/Turabian StyleZhou, Bo, Xiang Gao, and Fei Zhao. 2023. "Integration of mRNA and miRNA Analysis Reveals the Post-Transcriptional Regulation of Salt Stress Response in Hemerocallis fulva" International Journal of Molecular Sciences 24, no. 8: 7290. https://doi.org/10.3390/ijms24087290
APA StyleZhou, B., Gao, X., & Zhao, F. (2023). Integration of mRNA and miRNA Analysis Reveals the Post-Transcriptional Regulation of Salt Stress Response in Hemerocallis fulva. International Journal of Molecular Sciences, 24(8), 7290. https://doi.org/10.3390/ijms24087290




