Revisiting Alpha-Synuclein Pathways to Inflammation
Abstract
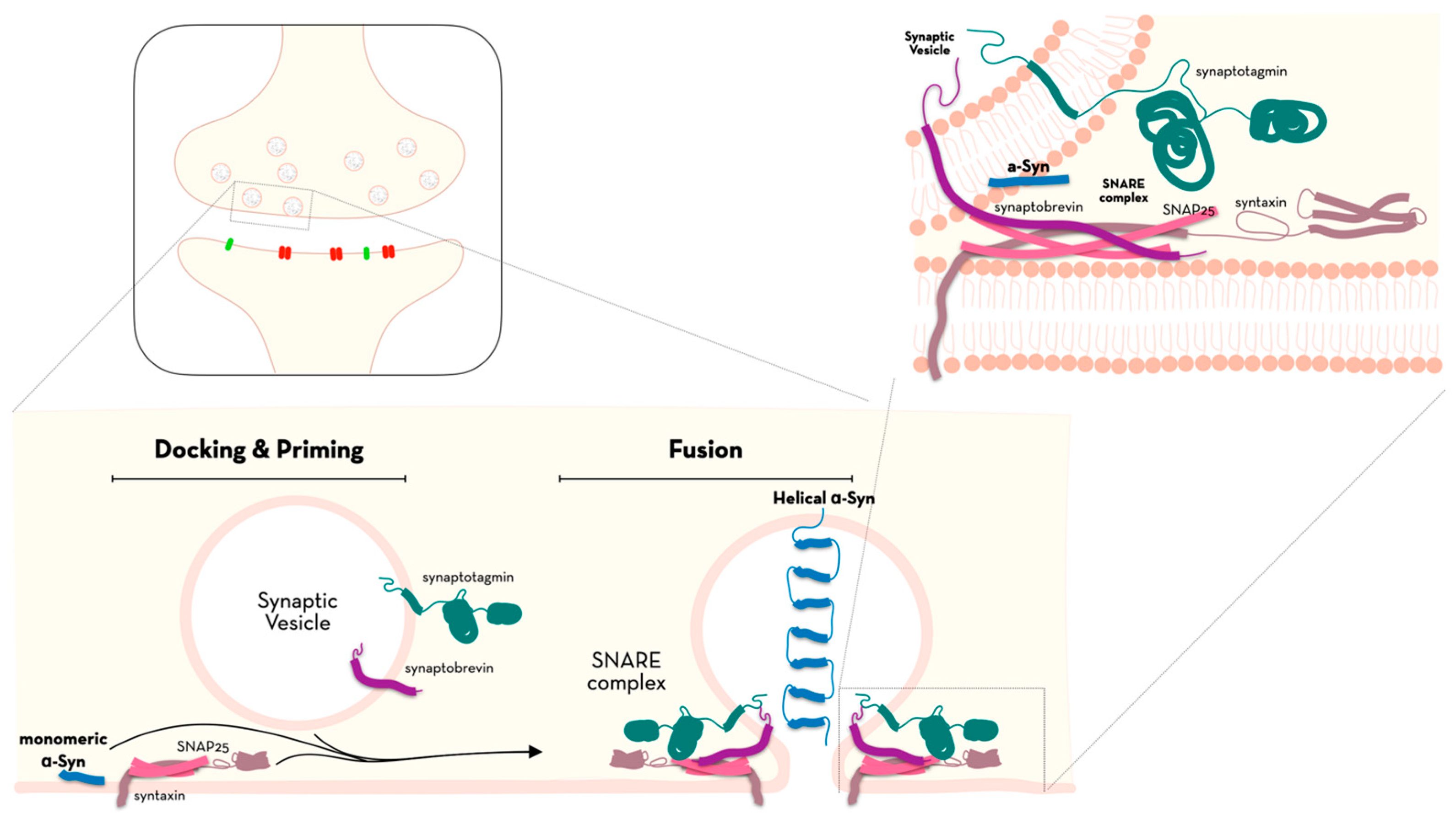
1. Introduction
2. α-Syn and Inflammation
3. Microbial Dysbiosis and α-Syn
3.1. Gut Microbiota and α-Syn
3.2. Oral Microbiota and α-Syn
4. Inflammatory Mitigation and α-Syn
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kramer, M.L.; Schulz-Schaeffer, W.J. Presynaptic α-Synuclein Aggregates, Not Lewy Bodies, Cause Neurodegeneration in Dementia with Lewy Bodies. J. Neurosci. 2007, 27, 1405–1410. [Google Scholar] [CrossRef] [PubMed]
- Ait Wahmane, S.; Achbani, A.; Ouhaz, Z.; Elatiqi, M.; Belmouden, A.; Nejmeddine, M. The Possible Protective Role of α-Synuclein Against Severe Acute Respiratory Syndrome Coronavirus 2 Infections in Patients with Parkinson’s Disease. Mov. Disord. 2020, 35, 1293–1294. [Google Scholar] [CrossRef] [PubMed]
- Devi, L.; Anandatheerthavarada, H.K. Mitochondrial Trafficking of APP and Alpha Synuclein: Relevance to Mitochondrial Dysfunction in Alzheimer’s and Parkinson’s Diseases. Biochim. Biophys. Acta Mol. Basis Dis. 2010, 1802, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Goers, J.; Manning-Bog, A.B.; McCormack, A.L.; Millett, I.S.; Doniach, S.; Di Monte, D.A.; Uversky, V.N.; Fink, A.L. Nuclear Localization of α-Synuclein and Its Interaction with Histones. Biochemistry 2003, 42, 8465–8471. [Google Scholar] [CrossRef] [PubMed]
- Gosavi, N.; Lee, H.J.; Lee, J.S.; Patel, S.; Lee, S.J. Golgi Fragmentation Occurs in the Cells with Prefibrillar α-Synuclein Aggregates and Precedes the Formation of Fibrillar Inclusion. J. Biol. Chem. 2002, 277, 48984–48992. [Google Scholar] [CrossRef] [PubMed]
- Guardia-Laguarta, C.; Area-Gomez, E.; Rüb, C.; Liu, Y.; Magrané, J.; Becker, D.; Voos, W.; Schon, E.A.; Przedborski, S. α-Synuclein Is Localized to Mitochondria-Associated ER Membranes. J. Neurosci. 2014, 34, 249–259. [Google Scholar] [CrossRef]
- Lee, H.J.; Khoshaghideh, F.; Patel, S.; Lee, S.J. Clearance of α-Synuclein Oligomeric Intermediates via the Lysosomal Degradation Pathway. J. Neurosci. 2004, 24, 1888–1896. [Google Scholar] [CrossRef]
- Li, W.W.; Yang, R.; Guo, J.C.; Ren, H.M.; Zha, X.L.; Cheng, J.S.; Cai, D.F. Localization of α-Synuclein to Mitochondria within Midbrain of Mice. Neuroreport 2007, 18, 1543–1546. [Google Scholar] [CrossRef]
- Mavroeidi, P.; Xilouri, M. Neurons and Glia Interplay in α-Synucleinopathies. Int. J. Mol. Sci. 2021, 22, 4994. [Google Scholar] [CrossRef]
- McLean, P.J.; Kawamata, H.; Ribich, S.; Hyman, B.T. Membrane Association and Protein Conformation of α-Synuclein in Intact Neurons. Effect of Parkinson’s Disease-Linked Mutations. J. Biol. Chem. 2000, 275, 8812–8816. [Google Scholar] [CrossRef]
- Pinho, R.; Paiva, I.; Jerčić, K.G.; Fonseca-Ornelas, L.; Gerhardt, E.; Fahlbusch, C.; Garcia-Esparcia, P.; Kerimoglu, C.; Pavlou, M.A.S.; Villar-Piqué, A.; et al. Nuclear Localization and Phosphorylation Modulate Pathological Effects of Alpha-Synuclein. Hum. Mol. Genet. 2019, 28, 31–50. [Google Scholar] [CrossRef] [PubMed]
- Polymeropoulos, M.H.; Lavedan, C.; Leroy, E.; Ide, S.E.; Dehejia, A.; Dutra, A.; Pike, B.; Root, H.; Rubenstein, J.; Boyer, R.; et al. Mutation in the α-Synuclein Gene Identified in Families with Parkinson’s Disease. Science 1997, 276, 2045–2047. [Google Scholar] [CrossRef] [PubMed]
- Brás, I.C.; Xylaki, M.; Outeiro, T.F. Mechanisms of Alpha-Synuclein Toxicity: An Update and Outlook. Prog. Brain Res. 2020, 252, 91–129. [Google Scholar] [CrossRef] [PubMed]
- Calo, L.; Wegrzynowicz, M.; Santivañez-Perez, J.; Grazia Spillantini, M. Synaptic Failure and α-Synuclein. Mov. Disord. 2016, 31, 169–177. [Google Scholar] [CrossRef]
- Logan, T.; Bendor, J.; Toupin, C.; Thorn, K.; Edwards, R.H. α-Synuclein Promotes Dilation of the Exocytotic Fusion Pore. Nat. Neurosci. 2017, 20, 681–689. [Google Scholar] [CrossRef]
- Huang, C.C.; Chiu, T.Y.; Lee, T.Y.; Hsieh, H.J.; Lin, C.C.; Kao, L. Sen Soluble α-Synuclein Facilitates Priming and Fusion by Releasing Ca2+ from the Thapsigargin-Sensitive Ca2+ Pool in PC12 Cells. J. Cell Sci. 2018, 131, jcs213017. [Google Scholar] [CrossRef]
- Butler, B.; Saha, K.; Rana, T.; Becker, J.P.; Sambo, D.; Davari, P.; Goodwin, J.S.; Khoshbouei, H. Dopamine Transporter Activity Is Modulated by α-Synuclein. J. Biol. Chem. 2015, 290, 29542–29554. [Google Scholar] [CrossRef]
- Burré, J.; Sharma, M.; Tsetsenis, T.; Buchman, V.; Etherton, M.R.; Südhof, T.C. α-Synuclein Promotes SNARE-Complex Assembly in Vivo and in Vitro. Science 2010, 329, 1663–1667. [Google Scholar] [CrossRef]
- Xiao, W.; Shameli, A.; Harding, C.V.; Meyerson, H.J.; Maitta, R.W. Late Stages of Hematopoiesis and B Cell Lymphopoiesis Are Regulated by α-Synuclein, a Key Player in Parkinson’s Disease. Immunobiology 2014, 219, 836–844. [Google Scholar] [CrossRef]
- Lesteberg, K.E.; Beckham, J.D. Immunology of West Nile Virus Infection and the Role of Alpha-Synuclein as a Viral Restriction Factor. Viral Immunol. 2019, 32, 38–47. [Google Scholar] [CrossRef]
- Ip, C.W.; Klaus, L.C.; Karikari, A.A.; Visanji, N.P.; Brotchie, J.M.; Lang, A.E.; Volkmann, J.; Koprich, J.B. AAV1/2-Induced Overexpression of A53T-α-Synuclein in the Substantia Nigra Results in Degeneration of the Nigrostriatal System with Lewy-like Pathology and Motor Impairment: A New Mouse Model for Parkinson’s Disease. Acta Neuropathol. Commun. 2017, 5, 11. [Google Scholar] [CrossRef]
- Cardinale, A.; Calabrese, V.; de Iure, A.; Picconi, B. Alpha-Synuclein as a Prominent Actor in the Inflammatory Synaptopathy of Parkinson’s Disease. Int. J. Mol. Sci. 2021, 22, 6517. [Google Scholar] [CrossRef] [PubMed]
- Ingelsson, M. Alpha-Synuclein Oligomers—Neurotoxic Molecules in Parkinson’s Disease and Other Lewy Body Disorders. Front. Neurosci. 2016, 10, 38–48. [Google Scholar] [CrossRef]
- Lashuel, H.A.; Overk, C.R.; Oueslati, A.; Masliah, E. The Many Faces of α-Synuclein: From Structure and Toxicity to Therapeutic Target. Nat. Rev. Neurosci. 2013, 14, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Bengoa-Vergniory, N.; Roberts, R.F.; Wade-Martins, R.; Alegre-Abarrategui, J. Alpha-Synuclein Oligomers: A New Hope. Acta Neuropathol. 2017, 134, 819–838. [Google Scholar] [CrossRef] [PubMed]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.-E.; Lang, A.E. Parkinson Disease. Nat. Rev. Dis. Prim. 2017, 3, 17013. [Google Scholar] [CrossRef]
- La Vitola, P.; Balducci, C.; Baroni, M.; Artioli, L.; Santamaria, G.; Castiglioni, M.; Cerovic, M.; Colombo, L.; Caldinelli, L.; Pollegioni, L.; et al. Peripheral Inflammation Exacerbates α-Synuclein Toxicity and Neuropathology in Parkinson’s Models. Neuropathol. Appl. Neurobiol. 2021, 47, 43–60. [Google Scholar] [CrossRef]
- Hijaz, B.A.; Volpicelli-Daley, L.A. Initiation and Propagation of α-Synuclein Aggregation in the Nervous System. Mol. Neurodegener. 2020, 15, 19. [Google Scholar] [CrossRef]
- Nussbaum, R.L.; Ellis, C.E. Alzheimer’s Disease and Parkinson’s Disease. N. Engl. J. Med. 2003, 348, 1356–1364. [Google Scholar] [CrossRef]
- Peelaerts, W.; Bousset, L.; Van Der Perren, A.; Moskalyuk, A.; Pulizzi, R.; Giugliano, M.; Van Den Haute, C.; Melki, R.; Baekelandt, V. α-Synuclein Strains Cause Distinct Synucleinopathies after Local and Systemic Administration. Nature 2015, 522, 340–344. [Google Scholar] [CrossRef]
- Pacheco, C.R.; Morales, C.N.; Ramírez, A.E.; Muñoz, F.J.; Gallegos, S.S.; Caviedes, P.A.; Aguayo, L.G.; Opazo, C.M. Extracellular α-Synuclein Alters Synaptic Transmission in Brain Neurons by Perforating the Neuronal Plasma Membrane. J. Neurochem. 2015, 132, 731–741. [Google Scholar] [CrossRef]
- Del Tredici, K.; Braak, H. Review: Sporadic Parkinson’s Disease: Development and Distribution of α-Synuclein Pathology. Neuropathol. Appl. Neurobiol. 2016, 42, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Bougea, A.; Koros, C.; Stefanis, L. Salivary Alpha-Synuclein as a Biomarker for Parkinson’s Disease: A Systematic Review. J. Neural Transm. 2019, 126, 1373–1382. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; Chen, W.; Yang, Q.; Zhang, L.; Zhang, L.; Wang, X.; Dong, F.; Zhao, Y.; Chen, S.; Quinn, T.J.; et al. Salivary Total α-Synuclein, Oligomeric α-Synuclein and SNCA Variants in Parkinson’s Disease Patients. Sci. Rep. 2016, 6, 28143. [Google Scholar] [CrossRef]
- Miranda, H.V.; Cássio, R.; Correia-Guedes, L.; Gomes, M.A.; Chegão, A.; Miranda, E.; Soares, T.; Coelho, M.; Rosa, M.M.; Ferreira, J.J.; et al. Posttranslational Modifications of Blood-Derived Alpha-Synuclein as Biochemical Markers for Parkinson’s Disease. Sci. Rep. 2017, 7, 13713. [Google Scholar] [CrossRef]
- Vivacqua, G.; Latorre, A.; Suppa, A.; Nardi, M.; Pietracupa, S.; Mancinelli, R.; Fabbrini, G.; Colosimo, C.; Gaudio, E.; Berardelli, A. Abnormal Salivary Total and Oligomeric Alpha-Synuclein in Parkinson’s Disease. PLoS ONE 2016, 11, e0151156. [Google Scholar] [CrossRef]
- Chang, C.W.; Yang, S.Y.; Yang, C.C.; Chang, C.W.; Wu, Y.R. Plasma and Serum Alpha-Synuclein as a Biomarker of Diagnosis in Patients With Parkinson’s Disease. Front. Neurol. 2020, 10, 1388. [Google Scholar] [CrossRef] [PubMed]
- Dorsey, E.R.; Bloem, B.R. The Parkinson Pandemic—A Call to Action. JAMA Neurol. 2018, 75, 9–10. [Google Scholar] [CrossRef]
- Malek, N.; Swallow, D.; Grosset, K.A.; Anichtchik, O.; Spillantini, M.; Grosset, D.G. Alpha-Synuclein in Peripheral Tissues and Body Fluids as a Biomarker for Parkinson’s Disease—A Systematic Review. Acta Neurol. Scand. 2014, 130, 59–72. [Google Scholar] [CrossRef]
- Pasanen, P.; Myllykangas, L.; Siitonen, M.; Raunio, A.; Kaakkola, S.; Lyytinen, J.; Tienari, P.J.; Pöyhönen, M.; Paetau, A. A Novel α-Synuclein Mutation A53E Associated with Atypical Multiple System Atrophy and Parkinson’s Disease-Type Pathology. Neurobiol. Aging 2014, 35, 2180.e1–2180.e5. [Google Scholar] [CrossRef]
- Tysnes, O.-B.; Storstein, A. Epidemiology of Parkinson’s Disease. J. Neural Transm. 2017, 124, 901–905. [Google Scholar] [CrossRef]
- Guan, L.; Han, Y.; Yang, C.; Lu, S.; Du, J.; Li, H.; Lin, J. CRISPR-Cas9-Mediated Gene Therapy in Neurological Disorders. Mol. Neurobiol. 2022, 59, 968–982. [Google Scholar] [CrossRef]
- Safari, F.; Hatam, G.; Behbahani, A.B.; Rezaei, V.; Barekati-Mowahed, M.; Petramfar, P.; Khademi, F. CRISPR System: A High-Throughput Toolbox for Research and Treatment of Parkinson’s Disease. Cell. Mol. Neurobiol. 2020, 40, 477–493. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, P.; Öhrfelt, A.; Lashley, T.; Blennow, K.; Brinkmalm, A.; Zetterberg, H. Mass Spectrometric Analysis of Lewy Body-Enriched α-Synuclein in Parkinson’s Disease. J. Proteome Res. 2019, 18, 2109–2120. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, E.; Murphy, S.; Martinson, H.A. Alpha-Synuclein Pathology and the Role of the Microbiota in Parkinson’s Disease. Front. Neurosci. 2019, 13, 369. [Google Scholar] [CrossRef] [PubMed]
- Picconi, B.; Hernández, L.F.; Obeso, J.A.; Calabresi, P. Motor Complications in Parkinson’s Disease: Striatal Molecular and Electrophysiological Mechanisms of Dyskinesias. Mov. Disord. 2018, 33, 867–876. [Google Scholar] [CrossRef]
- Martinez-Martin, P.; Jeukens-Visser, M.; Lyons, K.E.; Rodriguez-Blazquez, C.; Selai, C.; Siderowf, A.; Welsh, M.; Poewe, W.; Rascol, O.; Sampaio, C.; et al. Health-Related Quality-of-Life Scales in Parkinson’s Disease: Critique and Recommendations. Mov. Disord. 2011, 26, 2371–2380. [Google Scholar] [CrossRef]
- Kalia, L.V.; Lang, A.E. Parkinson’s Disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Jankovic, J. Parkinson’s Disease: Clinical Features and Diagnosis. J. Neurol. Neurosurg. Psychiatry 2008, 79, 368–376. [Google Scholar] [CrossRef]
- Chaudhuri, K.R.; Healy, D.G.; Schapira, A.H. Non-Motor Symptoms of Parkinson’s Disease: Diagnosis and Management. Lancet Neurol. 2006, 5, 235–245. [Google Scholar] [CrossRef]
- Heintz-Buschart, A.; Pandey, U.; Wicke, T.; Sixel-Döring, F.; Janzen, A.; Sittig-Wiegand, E.; Trenkwalder, C.; Oertel, W.H.; Mollenhauer, B.; Wilmes, P. The Nasal and Gut Microbiome in Parkinson’s Disease and Idiopathic Rapid Eye Movement Sleep Behavior Disorder. Mov. Disord. 2018, 33, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Obeso, J.A.; Rodriguez-Oroz, M.C.; Goetz, C.G.; Marin, C.; Kordower, J.H.; Rodriguez, M.; Hirsch, E.C.; Farrer, M.; Schapira, A.H.V.; Halliday, G. Missing Pieces in the Parkinson’s Disease Puzzle. Nat. Med. 2010, 16, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.Y.; Zhang, S.P.; Cao, C.; Loh, Y.P.; Cheng, Y. Aberrations in Peripheral Inflammatory Cytokine Levels in Parkinson Disease: A Systematic Review and Meta-Analysis. JAMA Neurol. 2016, 73, 1316–1324. [Google Scholar] [CrossRef] [PubMed]
- van Vuuren, M.J.; Nell, T.A.; Carr, J.A.; Kell, D.B.; Pretorius, E. Iron Dysregulation and Inflammagens Related to Oral and Gut Health Are Central to the Development of Parkinson’s Disease. Biomolecules 2021, 11, 30. [Google Scholar] [CrossRef]
- Dominy, S.S.; Lynch, C.; Ermini, F.; Benedyk, M.; Marczyk, A.; Konradi, A.; Nguyen, M.; Haditsch, U.; Raha, D.; Griffin, C.; et al. Porphyromonas Gingivalis in Alzheimer’s Disease Brains: Evidence for Disease Causation and Treatment with Small-Molecule Inhibitors. Sci. Adv. 2019, 5, eaau3333. [Google Scholar] [CrossRef]
- Gelders, G.; Baekelandt, V.; Van der Perren, A. Linking Neuroinflammation and Neurodegeneration in Parkinson’s Disease. J. Immunol. Res. 2018, 2018, 4784268. [Google Scholar] [CrossRef]
- Hirsch, E.C.; Vyas, S.; Hunot, S. Neuroinflammation in Parkinson’s Disease. Park. Relat. Disord. 2012, 18, 210–212. [Google Scholar] [CrossRef]
- Krashia, P.; Cordella, A.; Nobili, A.; La Barbera, L.; Federici, M.; Leuti, A.; Campanelli, F.; Natale, G.; Marino, G.; Calabrese, V.; et al. Blunting Neuroinflammation with Resolvin D1 Prevents Early Pathology in a Rat Model of Parkinson’s Disease. Nat. Commun. 2019, 10, 3945. [Google Scholar] [CrossRef]
- Watson, M.B.; Richter, F.; Lee, S.K.; Gabby, L.; Wu, J.; Masliah, E.; Effros, R.B.; Chesselet, M.F. Regionally-Specific Microglial Activation in Young Mice over-Expressing Human Wildtype Alpha-Synuclein. Exp. Neurol. 2012, 237, 318–334. [Google Scholar] [CrossRef]
- Ascherio, A.; Schwarzschild, M.A. The Epidemiology of Parkinson’s Disease: Risk Factors and Prevention. Lancet Neurol. 2016, 15, 1257–1272. [Google Scholar] [CrossRef]
- Lema Tomé, C.M.; Tyson, T.; Rey, N.L.; Grathwohl, S.; Britschgi, M.; Brundin, P. Inflammation and α-Synuclein’s Prion-like Behavior in Parkinson’s Disease—Is There a Link? Mol. Neurobiol. 2013, 47, 561–574. [Google Scholar] [CrossRef] [PubMed]
- Gustot, A.; Gallea, J.I.; Sarroukh, R.; Celej, M.S.; Ruysschaert, J.M.; Raussens, V. Amyloid Fibrils Are the Molecular Trigger of Inflammation in Parkinson’s Disease. Biochem. J. 2015, 471, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.D.; Glanzer, J.G.; Kadiu, I.; Ricardo-Dukelow, M.; Chaudhuri, A.; Ciborowski, P.; Cerny, R.; Gelman, B.; Thomas, M.P.; Mosley, R.L.; et al. Nitrated Alpha-Synuclein-Activated Microglial Profiling for Parkinson’s Disease. J. Neurochem. 2008, 104, 1504–1525. [Google Scholar] [CrossRef] [PubMed]
- Brochard, V.; Combadière, B.; Prigent, A.; Laouar, Y.; Perrin, A.; Beray-Berthat, V.; Bonduelle, O.; Alvarez-Fischer, D.; Callebert, J.; Launay, J.M.; et al. Infiltration of CD4+ Lymphocytes into the Brain Contributes to Neurodegeneration in a Mouse Model of Parkinson Disease. J. Clin. Investig. 2009, 119, 182–192. [Google Scholar] [CrossRef]
- Kannarkat, G.T.; Boss, J.M.; Tansey, M.G. The Role of Innate and Adaptive Immunity in Parkinson’s Disease. J. Park. Dis. 2013, 3, 493–514. [Google Scholar] [CrossRef]
- Chatterjee, K.; Roy, A.; Banerjee, R.; Choudhury, S.; Mondal, B.; Halder, S.; Basu, P.; Shubham, S.; Dey, S.; Kumar, H. Inflammasome and α-Synuclein in Parkinson’s Disease: A Cross-Sectional Study. J. Neuroimmunol. 2020, 338, 577089. [Google Scholar] [CrossRef]
- Zhou, Y.; Lu, M.; Du, R.H.; Qiao, C.; Jiang, C.Y.; Zhang, K.Z.; Ding, J.H.; Hu, G. MicroRNA-7 Targets Nod-like Receptor Protein 3 Inflammasome to Modulate Neuroinflammation in the Pathogenesis of Parkinson’s Disease. Mol. Neurodegener. 2016, 11, 28. [Google Scholar] [CrossRef]
- Anderson, F.L.; von Herrmann, K.M.; Andrew, A.S.; Kuras, Y.I.; Young, A.L.; Scherzer, C.R.; Hickey, W.F.; Lee, S.L.; Havrda, M.C. Plasma-Borne Indicators of Inflammasome Activity in Parkinson’s Disease Patients. NPJ Park. Dis. 2021, 7, 2. [Google Scholar] [CrossRef]
- Codolo, G.; Plotegher, N.; Pozzobon, T.; Brucale, M.; Tessari, I.; Bubacco, L.; de Bernard, M. Triggering of Inflammasome by Aggregated α-Synuclein, an Inflammatory Response in Synucleinopathies. PLoS ONE 2013, 8, e55375. [Google Scholar] [CrossRef]
- Schroder, K.; Tschopp, J. The Inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef]
- Baroja-Mazo, A.; Martín-Sánchez, F.; Gomez, A.I.; Martínez, C.M.; Amores-Iniesta, J.; Compan, V.; Barberà-Cremades, M.; Yagüe, J.; Ruiz-Ortiz, E.; Antón, J.; et al. The NLRP3 Inflammasome Is Released as a Particulate Danger Signal That Amplifies the Inflammatory Response. Nat. Immunol. 2014, 15, 738–748. [Google Scholar] [CrossRef] [PubMed]
- von Herrmann, K.M.; Salas, L.A.; Martinez, E.M.; Young, A.L.; Howard, J.M.; Feldman, M.S.; Christensen, B.C.; Wilkins, O.M.; Lee, S.L.; Hickey, W.F.; et al. NLRP3 Expression in Mesencephalic Neurons and Characterization of a Rare NLRP3 Polymorphism Associated with Decreased Risk of Parkinson’s Disease. NPJ Park. Dis. 2018, 4, 24. [Google Scholar] [CrossRef]
- Reale, M.; Iarlori, C.; Thomas, A.; Gambi, D.; Perfetti, B.; Di Nicola, M.; Onofrj, M. Peripheral Cytokines Profile in Parkinson’s Disease. Brain Behav. Immun. 2009, 23, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Karpenko, M.N.; Vasilishina, A.A.; Gromova, E.A.; Muruzheva, Z.M.; Bernadotte, A. Interleukin-1β, Interleukin-1 Receptor Antagonist, Interleukin-6, Interleukin-10, and Tumor Necrosis Factor-α Levels in CSF and Serum in Relation to the Clinical Diversity of Parkinson’s Disease. Cell. Immunol. 2018, 327, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.; Kolodziejczyk, A.A.; Thaiss, C.A.; Elinav, E. Dysbiosis and the Immune System. Nat. Rev. Immunol. 2017, 17, 219–232. [Google Scholar] [CrossRef]
- Falony, G.; Joossens, M.; Vieira-Silva, S.; Wang, J.; Darzi, Y.; Faust, K.; Kurilshikov, A.; Bonder, M.J.; Valles-Colomer, M.; Vandeputte, D.; et al. Population-Level Analysis of Gut Microbiome Variation. Science 2016, 352, 560–564. [Google Scholar] [CrossRef]
- Goodrich, J.K.; Davenport, E.R.; Beaumont, M.; Jackson, M.A.; Knight, R.; Ober, C.; Spector, T.D.; Bell, J.T.; Clark, A.G.; Ley, R.E. Genetic Determinants of the Gut Microbiome in UK Twins. Cell Host Microbe 2016, 19, 731–743. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Pedersen, O. Gut Microbiota in Human Metabolic Health and Disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Dekkers, K.F.; Sayols-Baixeras, S.; Baldanzi, G.; Nowak, C.; Hammar, U.; Nguyen, D.; Varotsis, G.; Brunkwall, L.; Nielsen, N.; Eklund, A.C.; et al. An Online Atlas of Human Plasma Metabolite Signatures of Gut Microbiome Composition. Nat. Commun. 2022, 13, 5370. [Google Scholar] [CrossRef]
- Diener, C.; Dai, C.L.; Wilmanski, T.; Baloni, P.; Smith, B.; Rappaport, N.; Hood, L.; Magis, A.T.; Gibbons, S.M. Genome-Microbiome Interplay Provides Insight into the Determinants of the Human Blood Metabolome. Nat. Metab. 2022, 4, 1560–1572. [Google Scholar] [CrossRef]
- Darveau, R.P. Periodontitis: A Polymicrobial Disruption of Host Homeostasis. Nat. Rev. Microbiol. 2010, 8, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Schippa, S.; Conte, M.P. Dysbiotic Events in Gut Microbiota: Impact on Human Health. Nutrients 2014, 6, 5786–5805. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.M.; Zhang, F.; Zhou, H.; Kam, W.; Wilson, B.; Hong, J.S. Neuroinflammation and α-Synuclein Dysfunction Potentiate Each Other, Driving Chronic Progression of Neurodegeneration in a Mouse Model of Parkinson’s Disease. Environ. Health Perspect. 2011, 119, 807–814. [Google Scholar] [CrossRef]
- Kim, C.; Lv, G.; Lee, J.S.; Jung, B.C.; Masuda-Suzukake, M.; Hong, C.S.; Valera, E.; Lee, H.J.; Paik, S.R.; Hasegawa, M.; et al. Exposure to Bacterial Endotoxin Generates a Distinct Strain of α-Synuclein Fibril. Sci. Rep. 2016, 6, 30891. [Google Scholar] [CrossRef]
- Stolzenberg, E.; Berry, D.; Yang, D.; Lee, E.Y.; Kroemer, A.; Kaufman, S.; Wong, G.C.L.; Oppenheim, J.J.; Sen, S.; Fishbein, T.; et al. A Role for Neuronal Alpha-Synuclein in Gastrointestinal Immunity. J. Innate Immun. 2017, 9, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Honda, K.; Littman, D.R. The Microbiota in Adaptive Immune Homeostasis and Disease. Nature 2016, 535, 75–84. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, S.; Chang, S.C.; Lee, J. Significant Roles of Neuroinflammation in Parkinson’s Disease: Therapeutic Targets for PD Prevention. Arch. Pharmacal Res. 2019, 42, 416–425. [Google Scholar] [CrossRef]
- Leow-Dyke, S.; Allen, C.; Denes, A.; Nilsson, O.; Maysami, S.; Bowie, A.G.; Rothwell, N.J.; Pinteaux, E. Neuronal Toll-like Receptor 4 Signaling Induces Brain Endothelial Activation and Neutrophil Transmigration in Vitro. J. Neuroinflamm. 2012, 9, 230. [Google Scholar] [CrossRef]
- Popichak, K.A.; Hammond, S.L.; Moreno, J.A.; Afzali, M.F.; Backos, D.S.; Slayden, R.D.; Safe, S.; Tjalkens, R.B. Compensatory Expression of NuR77 and NURR1 Regulates NF-KB–Dependent Inflammatory Signaling in Astrocytes. Mol. Pharmacol. 2018, 94, 1174–1186. [Google Scholar] [CrossRef]
- Shi, J.; Wang, Y.; Chen, D.; Xu, X.; Li, W.; Li, K.; He, J.; Su, W.; Luo, Q. The Alteration of Intestinal Mucosal α-Synuclein Expression and Mucosal Microbiota in Parkinson’s Disease. Appl. Microbiol. Biotechnol. 2023, 107, 1917–1929. [Google Scholar] [CrossRef]
- Ribeiro, G.R.; Campos, C.H.; Rodrigues Garcia, R.C.M. Parkinson’s Disease Impairs Masticatory Function. Clin. Oral Investig. 2017, 21, 1149–1156. [Google Scholar] [CrossRef]
- van Stiphout, M.A.E.; Marinus, J.; van Hilten, J.J.; Lobbezoo, F.; de Baat, C. Oral Health of Parkinson’s Disease Patients: A Case-Control Study. Park. Dis. 2018, 2018, 9315285. [Google Scholar] [CrossRef]
- Kaur, T.; Uppoor, A.; Naik, D. Parkinson’s Disease and Periodontitis—The Missing Link? A Review. Gerodontology 2016, 33, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Botelho, J.; Mascarenhas, P.; Mendes, J.J.; Machado, V. Network Protein Interaction in Parkinson’s Disease and Periodontitis Interplay: A Preliminary Bioinformatic Analysis. Genes 2020, 11, 1385. [Google Scholar] [CrossRef]
- Hu, S.; Li, S.; Ning, W.; Huang, X.; Liu, X.; Deng, Y.; Franceschi, D.; Ogbuehi, A.C.; Lethaus, B.; Savkovic, V.; et al. Identifying Crosstalk Genetic Biomarkers Linking a Neurodegenerative Disease, Parkinson’s Disease, and Periodontitis Using Integrated Bioinformatics Analyses. Front. Aging Neurosci. 2022, 14, 1032401. [Google Scholar] [CrossRef]
- Armingohar, Z.; Jørgensen, J.J.; Kristoffersen, A.K.; Abesha-Belay, E.; Olsen, I. Bacteria and Bacterial DNA in Atherosclerotic Plaque and Aneurysmal Wall Biopsies from Patients with and without Periodontitis. J. Oral Microbiol. 2014, 6, 23408. [Google Scholar] [CrossRef]
- Könönen, E.; Gursoy, M.; Gursoy, U. Periodontitis: A Multifaceted Disease of Tooth-Supporting Tissues. J. Clin. Med. 2019, 8, 1135. [Google Scholar] [CrossRef]
- Mougeot, J.-L.C.; Stevens, C.B.; Paster, B.J.; Brennan, M.T.; Lockhart, P.B.; Mougeot, F.K.B. Porphyromonas Gingivalis Is the Most Abundant Species Detected in Coronary and Femoral Arteries. J. Oral Microbiol. 2017, 9, 1281562. [Google Scholar] [CrossRef]
- Zhao, D.; Zhen, Z.; Pelekos, G.; Yiu, K.H.; Jin, L. Periodontal Disease Increases the Risk for Onset of Systemic Comorbidities in Dental Hospital Attendees: An 18-Year Retrospective Cohort Study. J. Periodontol. 2019, 90, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Botelho, J.; Leira, Y.; Viana, J.; Machado, V.; Lyra, P.; Aldrey, J.M.; Pías-Peleteiro, J.M.; Blanco, J.; Sobrino, T.; Mendes, J.J. The Role of Inflammatory Diet and Vitamin D on the Link between Periodontitis and Cognitive Function: A Mediation Analysis in Older Adults. Nutrients 2021, 13, 924. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Chen, Y.; van der Sluis, L.W.M.; Schuller, A.A.; Tjakkes, G.H. White Blood Cell Count Mediates the Association between Periodontal Inflammation and Cognitive Performance Measured by Digit Symbol Substitution Test among Older U.S. Adults. J. Gerontol. Ser. A 2021, 76, 1309–1315. [Google Scholar] [CrossRef]
- Leira, Y.; Domínguez, C.; Seoane, J.; Seoane-Romero, J.; Pías-Peleteiro, J.M.; Takkouche, B.; Blanco, J.; Aldrey, J.M. Is Periodontal Disease Associated with Alzheimer’s Disease? A Systematic Review with Meta-Analysis. Neuroepidemiology 2017, 48, 21–31. [Google Scholar] [CrossRef]
- Lyra, P.; Machado, V.; Proença, L.; Domingos, J.; Godinho, C.; Mendes, J.J.; Botelho, J. Parkinson’s Disease, Periodontitis and Patient-Related Outcomes: A Cross-Sectional Study. Medicina 2020, 56, 383. [Google Scholar] [CrossRef]
- Chen, C.-K.; Wu, Y.-T.; Chang, Y.-C. Periodontal Inflammatory Disease Is Associated with the Risk of Parkinson’s Disease: A Population-Based Retrospective Matched-Cohort Study. PeerJ 2017, 5, e3647. [Google Scholar] [CrossRef] [PubMed]
- Ishida, N.; Ishihara, Y.; Ishida, K.; Tada, H.; Funaki-Kato, Y.; Hagiwara, M.; Ferdous, T.; Abdullah, M.; Mitani, A.; Michikawa, M.; et al. Periodontitis Induced by Bacterial Infection Exacerbates Features of Alzheimer’s Disease in Transgenic Mice. NPJ Aging Mech. Dis. 2017, 3, 15. [Google Scholar] [CrossRef]
- Botelho, J.; Lyra, P.; Proença, L.; Godinho, C.; Mendes, J.J.; Machado, V. Relationship between Blood and Standard Biochemistry Levels with Periodontitis in Parkinson’s Disease Patients: Data from the NHANES 2011–2012. J. Pers. Med. 2020, 10, 69. [Google Scholar] [CrossRef]
- Lyra, P.; Botelho, J.; Machado, V.; Rota, S.; Walker, R.; Staunton, J.; Proença, L.; Chaudhuri, K.R.; Mendes, J.J. Self-Reported Periodontitis and C-Reactive Protein in Parkinson’s Disease: A Cross-Sectional Study of Two American Cohorts. NPJ Park. Dis. 2022, 8, 40. [Google Scholar] [CrossRef]
- Pereira, P.A.B.; Aho, V.T.E.; Paulin, L.; Pekkonen, E.; Auvinen, P.; Scheperjans, F. Oral and Nasal Microbiota in Parkinson’s Disease. Park. Relat. Disord. 2017, 38, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Rozas, N.S.; Tribble, G.D.; Jeter, C.B. Oral Factors That Impact the Oral Microbiota in Parkinson’s Disease. Microorganisms 2021, 9, 1616. [Google Scholar] [CrossRef]
- Zapała, B.; Stefura, T.; Milewicz, T.; Wątor, J.; Piwowar, M.; Wójcik-Pędziwiatr, M.; Doręgowska, M.; Dudek, A.; Jania, Z.; Rudzińska-Bar, M. The Role of the Western Diet and Oral Microbiota in Parkinson’s Disease. Nutrients 2022, 14, 355. [Google Scholar] [CrossRef] [PubMed]
- Fleury, V.; Zekeridou, A.; Lazarevic, V.; Gaïa, N.; Giannopoulou, C.; Genton, L.; Cancela, J.; Girard, M.; Goldstein, R.; Bally, J.F.; et al. Oral Dysbiosis and Inflammation in Parkinson’s Disease. J. Park. Dis. 2021, 11, 619–631. [Google Scholar] [CrossRef]
- Zheng, Y.; Yu, Z.; Zhao, J.; Cai, H.; Wang, Z.; Wang, X.; Feng, T. Oral Mucosa Derived A—Synuclein as a Potential Diagnostic Biomarker for Parkinson′s Disease. Front. Aging Neurosci. 2022, 14, 867528. [Google Scholar] [CrossRef]
- Jo, S.; Kang, W.; Hwang, Y.S.; Lee, S.H.; Park, K.W.; Kim, M.S.; Lee, H.; Yoon, H.J.; Park, Y.K.; Chalita, M.; et al. Oral and Gut Dysbiosis Leads to Functional Alterations in Parkinson’s Disease. NPJ Park. Dis. 2022, 8, 87. [Google Scholar] [CrossRef] [PubMed]
- Manocha, G.D.; Floden, A.M.; Puig, K.L.; Nagamoto-Combs, K.; Scherzer, C.R.; Combs, C.K. Defining the Contribution of Neuroinflammation to Parkinson’s Disease in Humanized Immune System Mice. Mol. Neurodegener. 2017, 12, 17. [Google Scholar] [CrossRef]
- Chen, C.K.; Huang, J.Y.; Wu, Y.T.; Chang, Y.C. Dental Scaling Decreases the Risk of Parkinson’s Disease: A Nationwide Population-Based Nested Case-Control Study. Int. J. Environ. Res. Public Health 2018, 15, 1587. [Google Scholar] [CrossRef] [PubMed]
- Kuai, X.Y.; Yao, X.H.; Xu, L.J.; Zhou, Y.Q.; Zhang, L.P.; Liu, Y.; Pei, S.F.; Zhou, C.L. Evaluation of Fecal Microbiota Transplantation in Parkinson’s Disease Patients with Constipation. Microb. Cell Factories 2021, 20, 98. [Google Scholar] [CrossRef] [PubMed]
- Borzabadi, S.; Oryan, S.; Eidi, A.; Aghadavod, E.; Kakhaki, R.D.; Tamtaji, O.R.; Taghizadeh, M.; Asemi, Z. The Effects of Probiotic Supplementation on Gene Expression Related to Inflammation, Insulin and Lipid in Patients with Parkinson’s Disease: A Randomized, Double-Blind, Placebo-Controlled Trial. Arch. Iran. Med. 2018, 21, 289–295. [Google Scholar]
- Hsieh, T.H.; Kuo, C.W.; Hsieh, K.H.; Shieh, M.J.; Peng, C.W.; Chen, Y.C.; Chang, Y.L.; Huang, Y.Z.; Chen, C.C.; Chang, P.K.; et al. Probiotics Alleviate the Progressive Deterioration of Motor Functions in a Mouse Model of Parkinson’s Disease. Brain Sci. 2020, 10, 206. [Google Scholar] [CrossRef]
- Ibrahim, A.; Raja Ali, R.A.; Abdul Manaf, M.R.; Ahmad, N.; Tajurruddin, F.W.; Qin, W.Z.; Md Desa, S.H.; Ibrahim, N.M. Multi-Strain Probiotics (Hexbio) Containing MCP BCMC Strains Improved Constipation and Gut Motility in Parkinson’s Disease: A Randomised Controlled Trial. PLoS ONE 2020, 15, e0244680. [Google Scholar] [CrossRef]
- Srivastav, S.; Neupane, S.; Bhurtel, S.; Katila, N.; Maharjan, S.; Choi, H.; Hong, J.T.; Choi, D.Y. Probiotics Mixture Increases Butyrate, and Subsequently Rescues the Nigral Dopaminergic Neurons from MPTP and Rotenone-Induced Neurotoxicity. J. Nutr. Biochem. 2019, 69, 73–86. [Google Scholar] [CrossRef]
- Tamtaji, O.R.; Taghizadeh, M.; Daneshvar Kakhaki, R.; Kouchaki, E.; Bahmani, F.; Borzabadi, S.; Oryan, S.; Mafi, A.; Asemi, Z. Clinical and Metabolic Response to Probiotic Administration in People with Parkinson’s Disease: A Randomized, Double-Blind, Placebo-Controlled Trial. Clin. Nutr. 2019, 38, 1031–1035. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.H.; Lim, S.Y.; Chong, K.K.; Manap, M.A.A.A.; Hor, J.W.; Lim, J.L.; Low, S.C.; Chong, C.W.; Mahadeva, S.; Lang, A.E. Probiotics for Constipation in Parkinson Disease: A Randomized Placebo-Controlled Study. Neurology 2021, 96, e772–e782. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, J.; Wu, C. Modulation of Gut Microbiota and Immune System by Probiotics, Pre-Biotics, and Post-Biotics. Front. Nutr. 2022, 8, 634897. [Google Scholar] [CrossRef]
- Bortolanza, M.; Nascimento, G.C.; Socias, S.B.; Ploper, D.; Chehín, R.N.; Raisman-Vozari, R.; Del-Bel, E. Tetracycline Repurposing in Neurodegeneration: Focus on Parkinson’s Disease. J. Neural Transm. 2018, 125, 1403–1415. [Google Scholar] [CrossRef]
- Cankaya, S.; Cankaya, B.; Kilic, U.; Kilic, E.; Yulug, B. The Therapeutic Role of Minocycline in Parkinson’s Disease. Drugs Context 2019, 8, 212553. [Google Scholar] [CrossRef]
- Koutzoumis, D.N.; Vergara, M.; Pino, J.; Buddendorff, J.; Khoshbouei, H.; Mandel, R.J.; Torres, G.E. Alterations of the Gut Microbiota with Antibiotics Protects Dopamine Neuron Loss and Improve Motor Deficits in a Pharmacological Rodent Model of Parkinson’s Disease. Exp. Neurol. 2020, 325, 113159. [Google Scholar] [CrossRef]
- Pu, Y.; Chang, L.; Qu, Y.; Wang, S.; Zhang, K.; Hashimoto, K. Antibiotic-Induced Microbiome Depletion Protects against MPTP-Induced Dopaminergic Neurotoxicity in the Brain. Aging 2019, 11, 6915–6929. [Google Scholar] [CrossRef]
- Svensson, E.; Horváth-Puhó, E.; Thomsen, R.W.; Djurhuus, J.C.; Pedersen, L.; Borghammer, P.; Sørensen, H.T. Vagotomy and Subsequent Risk of Parkinson’s Disease. Ann. Neurol. 2015, 78, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Lei, Q.; Wu, T.; Wu, J.; Hu, X.; Guan, Y.; Wang, Y.; Yan, J.; Shi, G. Roles of A-synuclein in Gastrointestinal Microbiome Dysbiosis-related Parkinson’s Disease Progression (Review). Mol. Med. Rep. 2021, 24, 734. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyra, P.; Machado, V.; Rota, S.; Chaudhuri, K.R.; Botelho, J.; Mendes, J.J. Revisiting Alpha-Synuclein Pathways to Inflammation. Int. J. Mol. Sci. 2023, 24, 7137. https://doi.org/10.3390/ijms24087137
Lyra P, Machado V, Rota S, Chaudhuri KR, Botelho J, Mendes JJ. Revisiting Alpha-Synuclein Pathways to Inflammation. International Journal of Molecular Sciences. 2023; 24(8):7137. https://doi.org/10.3390/ijms24087137
Chicago/Turabian StyleLyra, Patrícia, Vanessa Machado, Silvia Rota, Kallol Ray Chaudhuri, João Botelho, and José João Mendes. 2023. "Revisiting Alpha-Synuclein Pathways to Inflammation" International Journal of Molecular Sciences 24, no. 8: 7137. https://doi.org/10.3390/ijms24087137
APA StyleLyra, P., Machado, V., Rota, S., Chaudhuri, K. R., Botelho, J., & Mendes, J. J. (2023). Revisiting Alpha-Synuclein Pathways to Inflammation. International Journal of Molecular Sciences, 24(8), 7137. https://doi.org/10.3390/ijms24087137






_Chaudhuri_also_Ray-Chaudhuri.png)


