Membrane Lesions and Reduced Life Span of Red Blood Cells in Preeclampsia as Evidenced by Atomic Force Microscopy
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
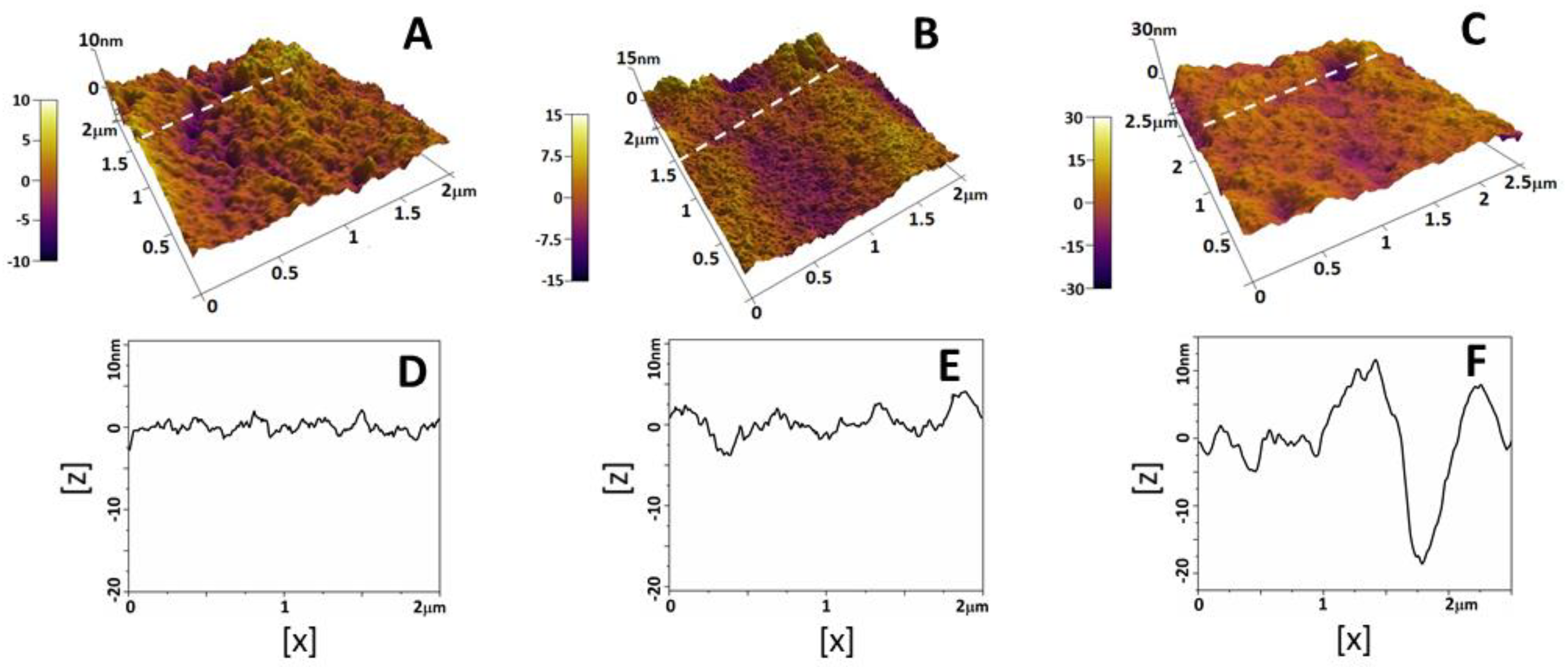
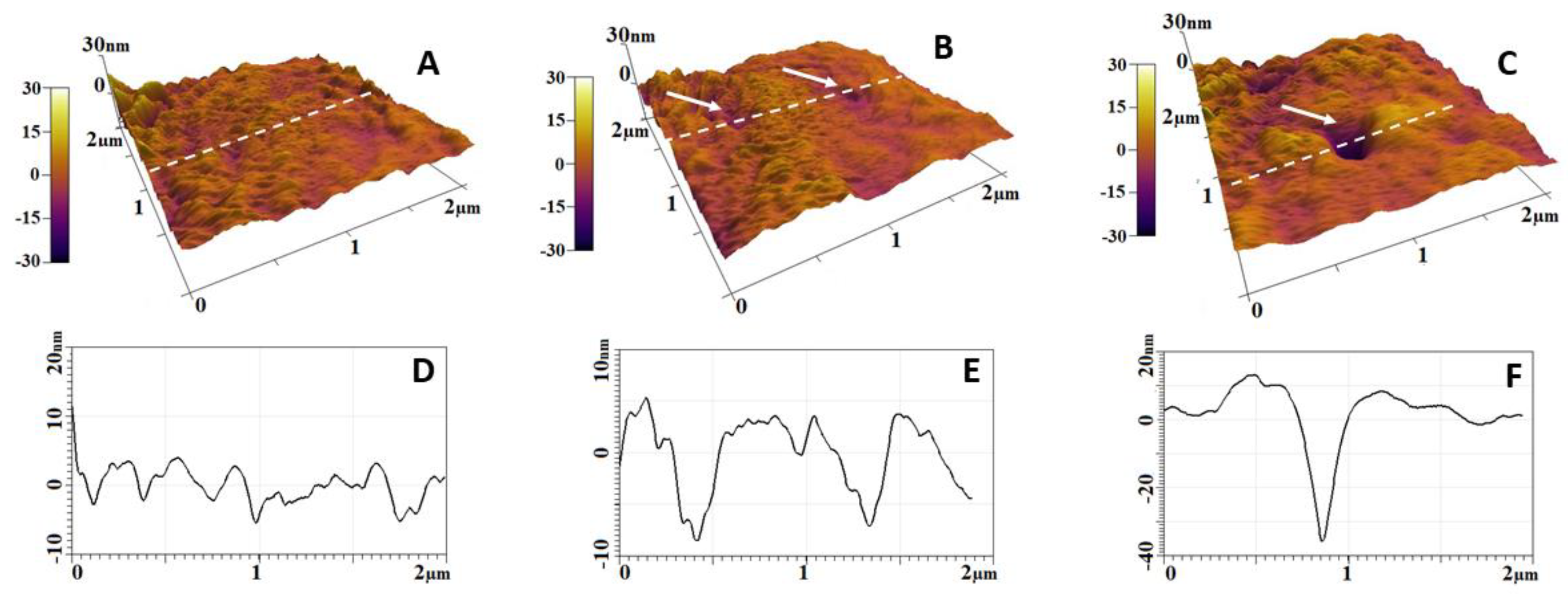
2.2. Morphology and Nanostructure of Freshly Isolated RBCs
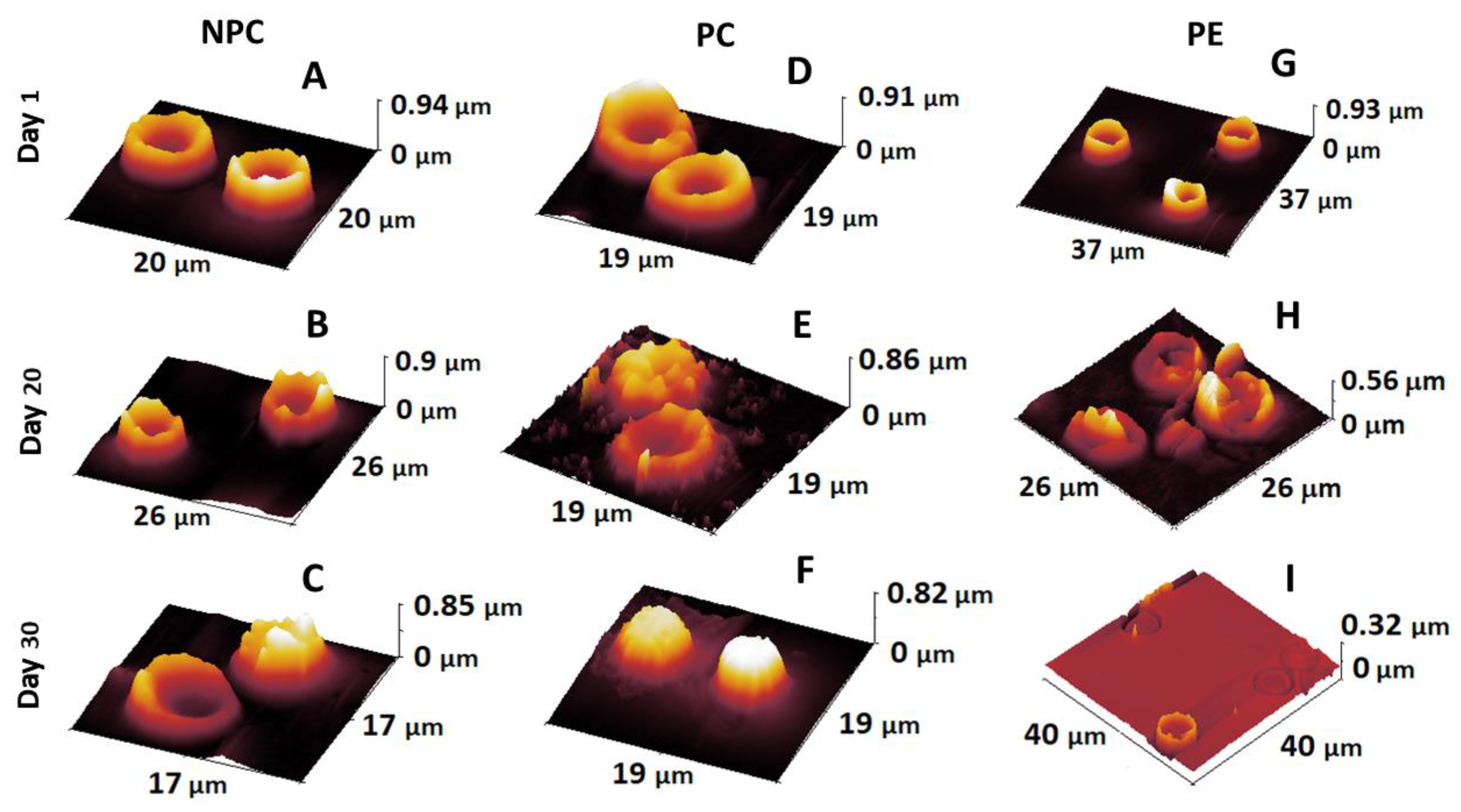
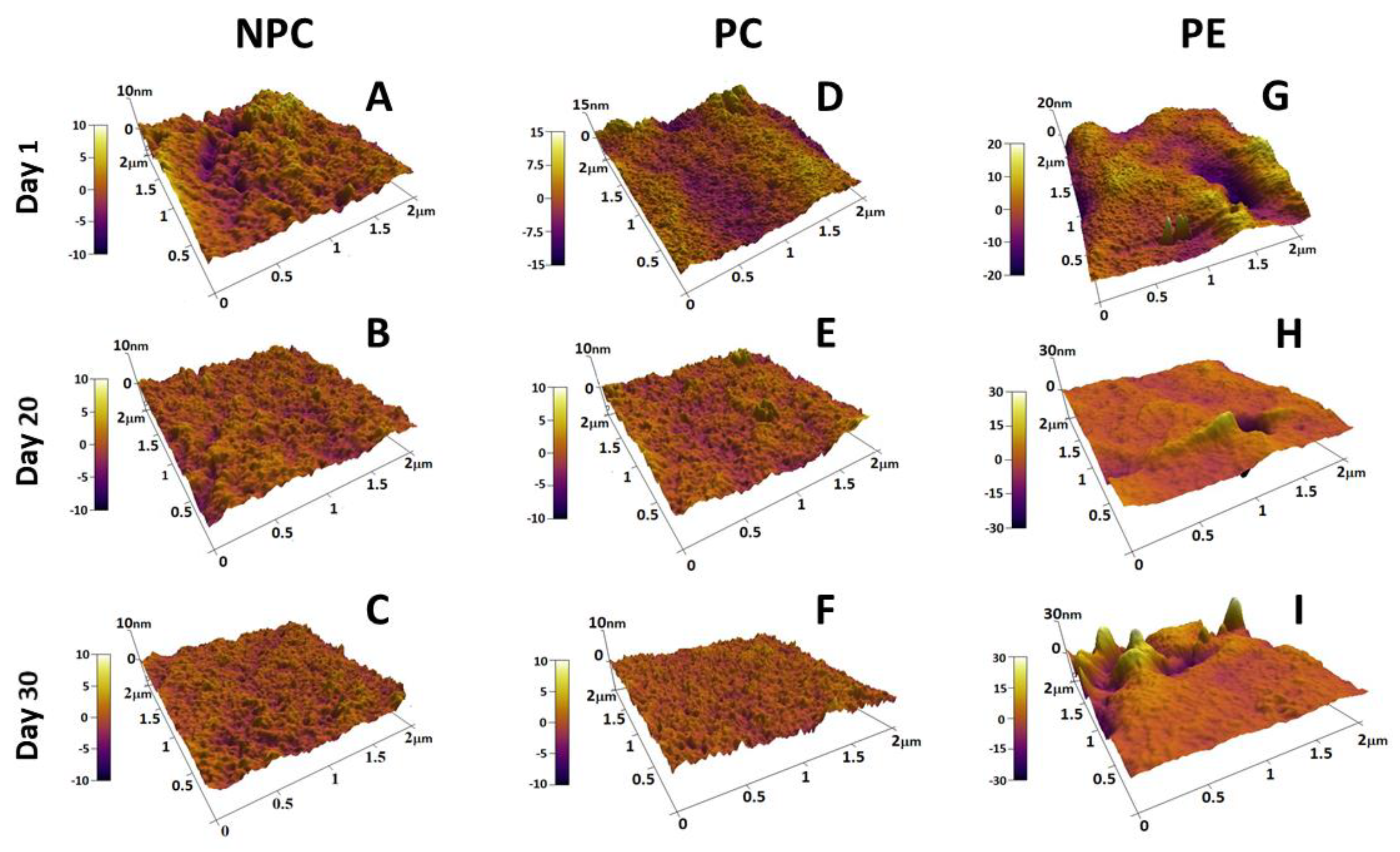
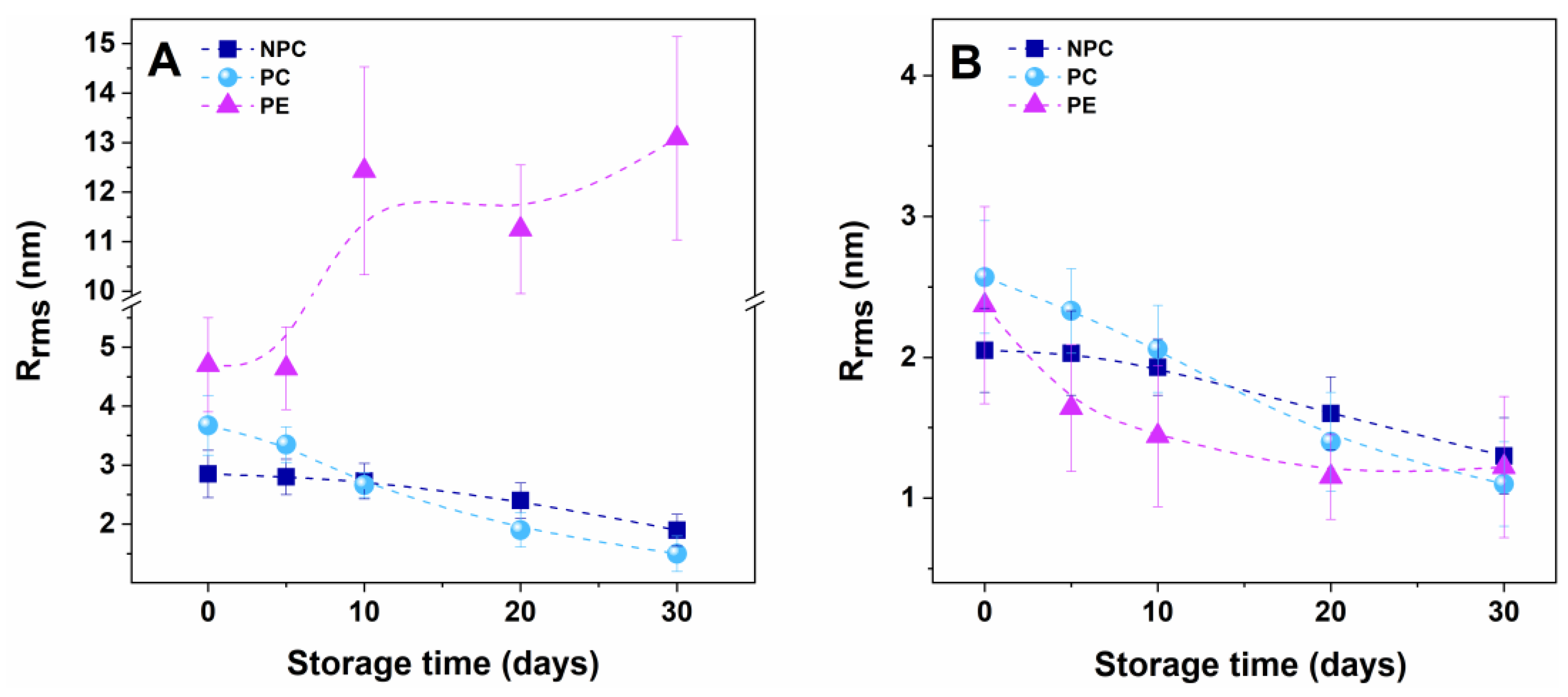
2.3. Alteration of Morphology and Nanostructure along the Aging of RBCs
2.4. Simulation of Oxidative Stress
3. Discussion
3.1. Membrane Lesions and Vesiculation of PE Erythrocytes
3.2. Oxidative Stress as a Component of PE Development
3.3. Accelerated Aging of PE Erythrocytes
4. Materials and Methods
4.1. Study Groups and Ethic Statement
4.2. Sample Preparations
4.3. Preparation of Spread Cells
4.4. Optical Microscopy
4.5. AFM Measurements
4.6. Preparation of Oxidized RBCs
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Echeverria, C.; Eltit, F.; Santibanez, J.F.; Gatica, S.; Cabello-Verrugio, C.; Simon, F. Endothelial dysfunction in pregnancy metabolic disorders. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2020, 1866, 165414. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, N.; Nahid, S.; Ummunnisa, F.; Fatima, I.; Hilani, M.; Gul, A.; Basha, A.A.; Yahia, W.; Hail, F.A.; Elfil, H.; et al. Preeclampsia: From Etiopathology to Organ Dysfunction. In Preeclampsia; Abduljabbar, H., Ed.; IntechOpen: Jeddah, Saudi Arabia, 2021. [Google Scholar]
- Erez, O.; Romero, R.; Jung, E.; Chaemsaithong, P.; Bosco, M.; Suksai, M.; Gallo, D.M.; Gotsch, F. Preeclampsia and eclampsia: The conceptual evolution of a syndrome. Am. J. Obstet. Gynecol. 2022, 226, S786–S803. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, F.G.; Lowe, T.; Guss, S.; Mason, R. Erythrocyte morphology in women with severe preeclampsia and eclampsia. Preliminary observations with scanning electron microscopy. Am. J. Obstet. Gynecol. 1985, 153, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Spickett, C.M.; Reglinski, J.W.; Smith, E.; Wilson, R.; Walker, J.J.; McKillop, J. Erythrocyte Glutathione Balance and Membrane Stability During Preeclampsia. Free Radic. Biol. Med. 1998, 24, 1049–1055. [Google Scholar] [CrossRef]
- Huisjes, R.; Bogdanova, A.; Van Solinge, W.W.; Schiffelers, R.M.; Kaestner, L.; van Wijk, R. Squeezing for life—Properties of red blood cell deformability. Front. Physiol. 2018, 9, 656. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Lykotrafitis, G. Erythrocyte membrane model with explicit description of the lipid bilayer and the spectrin network. Biophys. J. 2014, 107, 642–653. [Google Scholar] [CrossRef]
- Gilligan, D.M.; Bennett, V. The junctional complex of the membrane skeleton. Semin. Hematol. 1993, 30, 74–83. [Google Scholar]
- Bennett, V. The spectrin-actin junction of erythrocyte membrane skeletons. Biochim. Biophys. Acta (BBA)-Rev. Biomembr. 1989, 988, 107–121. [Google Scholar] [CrossRef]
- Tse, W.T.; Lux, S.E. Red blood cell membrane disorders. Br. J. Haematol. 1999, 104, 2–13. [Google Scholar] [CrossRef]
- Chiarello, D.I.; Abad, C.; Rojas, D.; Toledo, F.; Vázquez, C.M.; Mate, A.; Sobrevia, L.; Marín, R. Oxidative stress: Normal pregnancy versus preeclampsia. Biochim. Et Biophys. Acta (BBA)-Mol. Basis Dis. 2020, 1866, 165354. [Google Scholar] [CrossRef]
- Bell, M.J. A historical overview of preeclampsia-eclampsia. J. Obstet. Gynecol. Neonatal Nurs. 2010, 39, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Huppertz, B. Placental origins of preeclampsia: Challenging the current hypothesis. Hypertension 2008, 51, 970–975. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.M.; Cooper, D.W. Pathogenesis and genetics of pre-eclampsia. Lancet. 2001, 357, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Satchwell, T.J.; Toye, A.M. Band 3, an essential red blood cell hub of activity. Haematologica 2021, 106, 2792. [Google Scholar] [CrossRef]
- Elgari, M.M.; Khabour, O.F.; Alhag, S.M. Correlations between changes in hematological indices of mothers with preeclampsia and umbilical cord blood of newborns. Clin. Exp. Hypertens. 2019, 41, 58–61. [Google Scholar] [CrossRef]
- Aires Rodrigues de Freitas, M.; Vieira da Costa, A.; Alves de Medeiros, L.; da Silva Garrote Filho, M.; Lemos Debs Diniz, A.; Penha-Silva, N. Are There Differences in the Anthropometric, Hemodynamic, Hematologic, and Biochemical Profiles between Late- and Early-Onset Preeclampsia? Obstet. Gynecol. Int. 2018, 2018, 9628726. [Google Scholar] [CrossRef]
- Girasole, M.; Dinarelli, S.; Boumis, G. Structure and function in native and pathological erythrocytes: A quantitative view from the nanoscale. Micron 2012, 43, 1273–1286. [Google Scholar] [CrossRef]
- Yeow, N.; Tabor, R.F.; Garnier, G. Atomic force microscopy: From red blood cells to immunohaematology. Adv. Colloid Interface Sci. 2017, 249, 149–162. [Google Scholar] [CrossRef]
- Sergunova, V.; Leesment, S.; Kozlov, A.; Inozemtsev, V.; Platitsina, P.; Lyapunova, S.; Onufrievich, A.; Polyakov, V.; Sherstyukova, E. Investigation of Red Blood Cells by Atomic Force Microscopy. Sensors (Basel) 2022, 22, 2055. [Google Scholar] [CrossRef]
- Kiio, T.M.; Park, S. Nano-scientific Application of Atomic Force Microscopy in Pathology: From Molecules to Tissues. Int. J. Med. Sci. 2020, 17, 844–858. [Google Scholar] [CrossRef]
- Mukherjee, R.; Chaudhury, K.; Chakraborty, C. Topological features of erythrocytes in thalassemic patients: Quantitative characterization by scanning electron and atomic force microscopy. Anal. Quant. Cytopathol. Histopathol. 2014, 36, 91–99. [Google Scholar]
- Girasole, M.; Pompeo, G.; Cricenti, A.; Longo, G.; Boumis, G.; Bellelli, A.; Amiconi, S. The how, when, and why of the aging signals appearing on the human erythrocyte membrane: An atomic force microscopy study of surface roughness. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Maciaszek, J.L.; Andemariam, B.; Lykotrafitis, G. Microelasticity of red blood cells in sickle cell disease. J. Strain Anal. Eng. Design. 2011, 46, 368–379. [Google Scholar] [CrossRef]
- Zuk, A.; Targosz-Korecka, M.; Szymonski, M. Effect of selected drugs used in asthma treatment on morphology and elastic properties of red blood cells. Int. J. Nanomed. 2011, 6, 249–257. [Google Scholar] [CrossRef]
- Langari, A.; Strijkova, V.; Komsa-Penkova, R.; Danailova, A.; Krumova, S.; Taneva, S.G.; Giosheva, I.; Gartchev, E.; Kercheva, K.; Savov, A.; et al. Morphometric and Nanomechanical Features of Erythrocytes Characteristic of Early Pregnancy Loss. Int. J. Mol. Sci. 2022, 19, 4512. [Google Scholar] [CrossRef]
- Ford, J. Red blood cell morphology. Int. J. Lab. Hematol. 2013, 35, 351–357. [Google Scholar] [CrossRef]
- Loyola-Leyva, A.; Loyola-Rodríguez, J.P.; González, F.J.; Camacho-Lopez, S.; Terán-Figueroa, Y. Differences in erythrocytes size and shape in prediabetes and diabetes assessed by two microscopy techniques and its association with dietary patterns. Pilot study. Microsc. Res. Tech. 2022, 85, 3726–3735. [Google Scholar] [CrossRef]
- Girasole, M.; Pompeo, G.; Cricenti, A.; Congiu-Castellano, A.; Andreola, F.; Serafino, A.; Frazer, B.H.; Boumis, G.; Amiconi, G. Roughness of the plasma membrane as an independent morphological parameter to study RBCs: A quantitative atomic force microscopy investigation. Biochim. Biophys. Acta (BBA)-Biomembr. 2007, 1768, 1268–1276. [Google Scholar] [CrossRef]
- Barcellini, W.; Bianchi, P.; Fermo, E.; Imperiali, F.G.; Marcello, A.P.; Vercellati, C.; Zaninoni, A.; Zanella, A. Hereditary red cell membrane defects: Diagnostic and clinical aspects. Blood Transfus. 2011, 9, 274. [Google Scholar]
- López-Canizales, A.M.; Angulo-Molina, A.; Garibay-Escobar, A.; Silva-Campa, E.; Mendez-Rojas, M.A.; Santacruz-Gómez, K.; Acosta-Elías, M.; Castañeda-Medina, B.; Soto-Puebla, D.; Álvarez-Bajo, O.; et al. Nanoscale Changes on RBC Membrane Induced by Storage and Ionizing Radiation: A Mini-Review. Front. Physiol. 2021, 4, 669455. [Google Scholar] [CrossRef]
- Csiszar, B.; Galos, G.; Funke, S.; Kevey, D.K.; Meggyes, M.; Szereday, L.; Kenyeres, P.; Toth, K.; Sandor, B. Peripartum Investigation of Red Blood Cell Properties in Women Diagnosed with Early-Onset Preeclampsia. Cells 2021, 10, 2714. [Google Scholar] [CrossRef]
- Schauf, B.; Lang, U.; Stute, P.; Schneider, S.; Dietz, K.; Aydeniz, B.; Wallwiener, D. Reduced red blood cell deformability, an indicator for high fetal or maternal risk, is found in preeclampsia and IUGR. Hypertens. Pregnancy 2002, 21, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Lykotrafitis, G. Vesiculation of healthy and defective red blood cells. Phys. Rev. E 2015, 92, 012715. [Google Scholar] [CrossRef]
- Burton, G.J.; Redman, C.W.; Roberts, J.M.; Moffett, A. Pre-eclampsia: Pathophysiology and clinical implications. BMJ 2019, 366, l2381. [Google Scholar] [CrossRef] [PubMed]
- Hansson, S.R.; Nääv, Å.; Erlandsson, L. Oxidative stress in preeclampsia and the role of free fetal hemoglobin. Front. Physiol. 2015, 5, 516. [Google Scholar] [CrossRef] [PubMed]
- Badior, K.E.; Casey, J.R. Molecular mechanism for the red blood cell senescence clock. IUBMB Life 2018, 70, 32–40. [Google Scholar] [CrossRef]
- Aris, A.; Benali, S.; Ouellet, A.; Moutquin, J.M.; Leblanc, S. Potential biomarkers of preeclampsia: Inverse correlation between hydrogen peroxide and nitric oxide early in maternal circulation and at term in placenta of women with preeclampsia. Placenta 2009, 30, 342–347. [Google Scholar] [CrossRef]
- Dordević, N.Z.; Babić, G.M.; Marković, S.D.; Ognjanović, B.I.; Stajn, A.S.; Zikić, R.V.; Saicić, Z.S. Oxidative stress and changes in antioxidative defense system in erythrocytes of preeclampsia in women. Reprod. Toxicol. 2008, 25, 213–218. [Google Scholar] [CrossRef]
- Diez-Silva, M.; Dao, M.; Han, J.; Lim, C.T.; Suresh, S. Shape and Biomechanical Characteristics of Human Red Blood Cells in Health and Disease. MRS Bull. 2010, 35, 382–388. [Google Scholar] [CrossRef]
- Park, Y.K.; Best, C.A.; Auth, T.; Gov, N.; Safran, G.; Popescu, G.; Suresh, S.; Feld, M.S. Metabolic remodeling of the human red blood cell membrane. Proc. Natl. Acad. Sci. USA 2010, 107, 1289–1294. [Google Scholar] [CrossRef]
- Lurie, S.; Mamet, Y. Red blood cell survival and kinetics during pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2000, 93, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Reinhart, W.H. Peculiar red cell shapes: Fahraeus Lecture 2011. Clin. Hemorheol. Microcirc. 2011, 49, 11–27. [Google Scholar] [CrossRef] [PubMed]
- Tachev, K.D.; Danov, K.D.; Kralchevsky, P.A. On the mechanism of stomatocyte–echinocyte transformations of red blood cells: Experiment and theoretical model. Colloids Surf. B Biointerfaces 2004, 34, 123–140. [Google Scholar] [CrossRef] [PubMed]
- Li, J.H.; Luo, J.F.; Jiang, Y.; Ma, Y.J.; Ji, Y.Q.; Zhu, G.L.; Zhou, C.; Chu, H.W.; Zhang, H.D. Red Blood Cell Lifespan Shortening in Patients with Early-Stage Chronic Kidney Disease. Kidney Blood Press. Res. 2019, 44, 1158–1165. [Google Scholar] [CrossRef] [PubMed]
- Ostafiichuk, S.O.; Heraschenko, S.B.; Ostafiichuk, A.R. The Morphometric Study of Red Blood Cells in Pregnant Women with Preeclampsia. Galician Med. J. 2015, 22, 41–45. [Google Scholar]
- Hernández Hernández, J.D.; Villaseñor, O.R.; Del Rio Alvarado, J.; Lucach, R.O.; Zárate, A.; Saucedo, R.; Hernández-Valencia, M. Morphological changes of red blood cells in peripheral blood smear of patients with pregnancy-related hypertensive disorders. Arch. Med. Res. 2015, 46, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Antonio, P.D.; Lasalvia, M.; Perna, G.; Capozzi, V. Scale-independent roughness value of cell membranes studied by means of AFM technique. Biochim. Biophys. Acta (BBA)-Biomembr. 2012, 1818, 3141–3148. [Google Scholar] [CrossRef]
- Buys, A.V.; Van Rooy, M.J.; Soma, P.; Van Papendorp, D.; Lipinski, B.; Pretorius, E. Changes in red blood cell membrane structure in type 2 diabetes: A scanning electron and atomic force microscopy study. Cardiovasc. Diabetol. 2013, 12, 25. [Google Scholar] [CrossRef]
- Bosman, G.J.; Lasonder, E.; Luten, M.; Roerdinkholder-Stoelwinder, B.; Novotný, V.M.; Bos, H.; De Grip, W.J. The proteome of red cell membranes and vesicles during storage in blood bank conditions. Transfusion 2008, 48, 827–835. [Google Scholar]
- Almizraq, R.J.; Holovati, J.L.; Acker, J.P. Characteristics of extracellular vesicles in red blood concentrates change with storage time and blood manufacturing method. Transfus. Med. Hemother. 2018, 45, 185–193. [Google Scholar] [CrossRef]
- Hypertension in Pregnancy: Diagnosis and Management|NICE. Available online: https://www.nice.org.uk/guidance/ng133 (accessed on 25 June 2019).
- Dinarelli, S.; Longo, G.; Dietler, G.; Francioso, A.; Mosca, L.; Pannitteri, G.; Boumis, G.; Bellelli, A.; Girasole, M. Erythrocyte’s aging in microgravity highlights how environmental stimuli shape metabolism and morphology. Sci. Rep. 2018, 8, 5277. [Google Scholar] [CrossRef] [PubMed]


| Characteristic | Reference Values | Reference Values for Pregnant Women | NPC (n = 11) | PC (n = 12) | PE (n = 19) |
|---|---|---|---|---|---|
| Maternal age (years) | – | 29.8 ± 5.5 | 27.3 ± 3.4 | 25.8 ± 2.8 | |
| Gestational week (interval) | – | – | 24–38 | 24–36 | |
| Mean BP (systolic/diastolic) | 111 ± 5/76 ± 4 | 104 ± 6/72 ± 3 | 155 ± 11/104 ± 7 | ||
| GA at diagnosis of PE | - | - | 29.3 ± 3.2 | ||
| GA at delivery | - | 39.2 ± 0.4 | 32.6 ± 2.4 | ||
| Body weight (kg) | 87 ± 7 | 93 ± 5 | |||
| RBC count (T/L) | 3.6–5.1 | 4.0–6.2 | 4.2 ± 0.5 | 3.8 ± 0.4 | 3.9 ± 0.5 |
| Hb (g/L) | 110–148 | 120–160 | 127.0 ± 12 | 117.3 ± 7.7 | 112.6 ± 10 |
| Ht (L/L) | 0.30–0.46 | 0.36–0.54 | 0.376 ± 0.05 | 0.354 ± 0.02 | 0.364 ± 0.04 |
| MCV (mmol/L) | 82–98 | 82–100 | 96 ± 6.0 | 92.1 ± 6.2 | 95.2 ± 8.4 |
| MCH (Pg/L) | 26.5–32.0 | 29.0–45.0 | 39.2 ± 2.0 | 31.1 ± 2.1 | 31.4 ± 2.9 |
| MCHC (g/L) | 295–360 | 240–360 | 331.0 ± 14.0 | 335.4 ± 5.0 | 335.4 ± 5.0 |
| RDW (%) | 11.5–14.5 | 11.5–14.5 | 12.8 ± 1.5 | 13.8 ± 1.4 | 13.8 ± 1.6 |
| Platelet count ×109/L | 150–400 | 146–429 | 301 ± 62 | 248.6 ± 51.6 | 242.7 ± 97 |
| ASAT (U/L) | 12–38 | 4–32 | 18.4 ± 2.8 | 22.5 ± 3.1 | 19.7 ± 7.0 |
| ALAT (U/L) | 7–41 | 3–30 | 17.9 ± 3.3 | 14.6 ± 1.7 | 16.3 ± 7.6 |
| Study Group | Morphological Classes (%) | ||
|---|---|---|---|
| Biconcave | Spiculated | Spherocytes | |
| NPC | 84 ± 8 | 16 ± 6 | 0 |
| PC | 79 ± 5 | 21 ± 4 | 0 |
| PE | 80 ± 9 | 13 ± 5 | 7 ± 3 |
| Group | D (µm) | H (µm) | V (µm3) | h2 (µm) | H/h2 | Rrms (nm) |
|---|---|---|---|---|---|---|
| NPC | 8.1 ± 0.37 | 0.57 ± 0.05 | 24.64 ± 3.29 | 0.50 ± 0.03 | 1.14 ± 0.11 | 2.9 ± 0.4 |
| PC | 8.2 ± 0.36 | 0.60 ± 0.07 | 26.87 ± 3.78 | 0.51 ± 0.06 | 1.18 ± 0.09 | 3.8 ± 0.5 |
| PE | 8.5 ± 0.38 * | 0.61 ± 0.09 | 29.82 ± 4.19 * | 0.39 ± 0.11 * | 2.42 ± 0.82 * | 4.7 ± 0.8 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giosheva, I.; Strijkova, V.; Komsa-Penkova, R.; Krumova, S.; Langari, A.; Danailova, A.; Taneva, S.G.; Stoyanova, T.; Topalova, L.; Gartchev, E.; et al. Membrane Lesions and Reduced Life Span of Red Blood Cells in Preeclampsia as Evidenced by Atomic Force Microscopy. Int. J. Mol. Sci. 2023, 24, 7100. https://doi.org/10.3390/ijms24087100
Giosheva I, Strijkova V, Komsa-Penkova R, Krumova S, Langari A, Danailova A, Taneva SG, Stoyanova T, Topalova L, Gartchev E, et al. Membrane Lesions and Reduced Life Span of Red Blood Cells in Preeclampsia as Evidenced by Atomic Force Microscopy. International Journal of Molecular Sciences. 2023; 24(8):7100. https://doi.org/10.3390/ijms24087100
Chicago/Turabian StyleGiosheva, Ina, Velichka Strijkova, Regina Komsa-Penkova, Sashka Krumova, Ariana Langari, Avgustina Danailova, Stefka G. Taneva, Tanya Stoyanova, Lora Topalova, Emil Gartchev, and et al. 2023. "Membrane Lesions and Reduced Life Span of Red Blood Cells in Preeclampsia as Evidenced by Atomic Force Microscopy" International Journal of Molecular Sciences 24, no. 8: 7100. https://doi.org/10.3390/ijms24087100
APA StyleGiosheva, I., Strijkova, V., Komsa-Penkova, R., Krumova, S., Langari, A., Danailova, A., Taneva, S. G., Stoyanova, T., Topalova, L., Gartchev, E., Georgieva, G., & Todinova, S. (2023). Membrane Lesions and Reduced Life Span of Red Blood Cells in Preeclampsia as Evidenced by Atomic Force Microscopy. International Journal of Molecular Sciences, 24(8), 7100. https://doi.org/10.3390/ijms24087100







