Synergistic Effects of Silicate-Platelet Supporting Ag and ZnO, Offering High Antibacterial Activity and Low Cytotoxicity
Abstract
1. Introduction
2. Results and Discussion
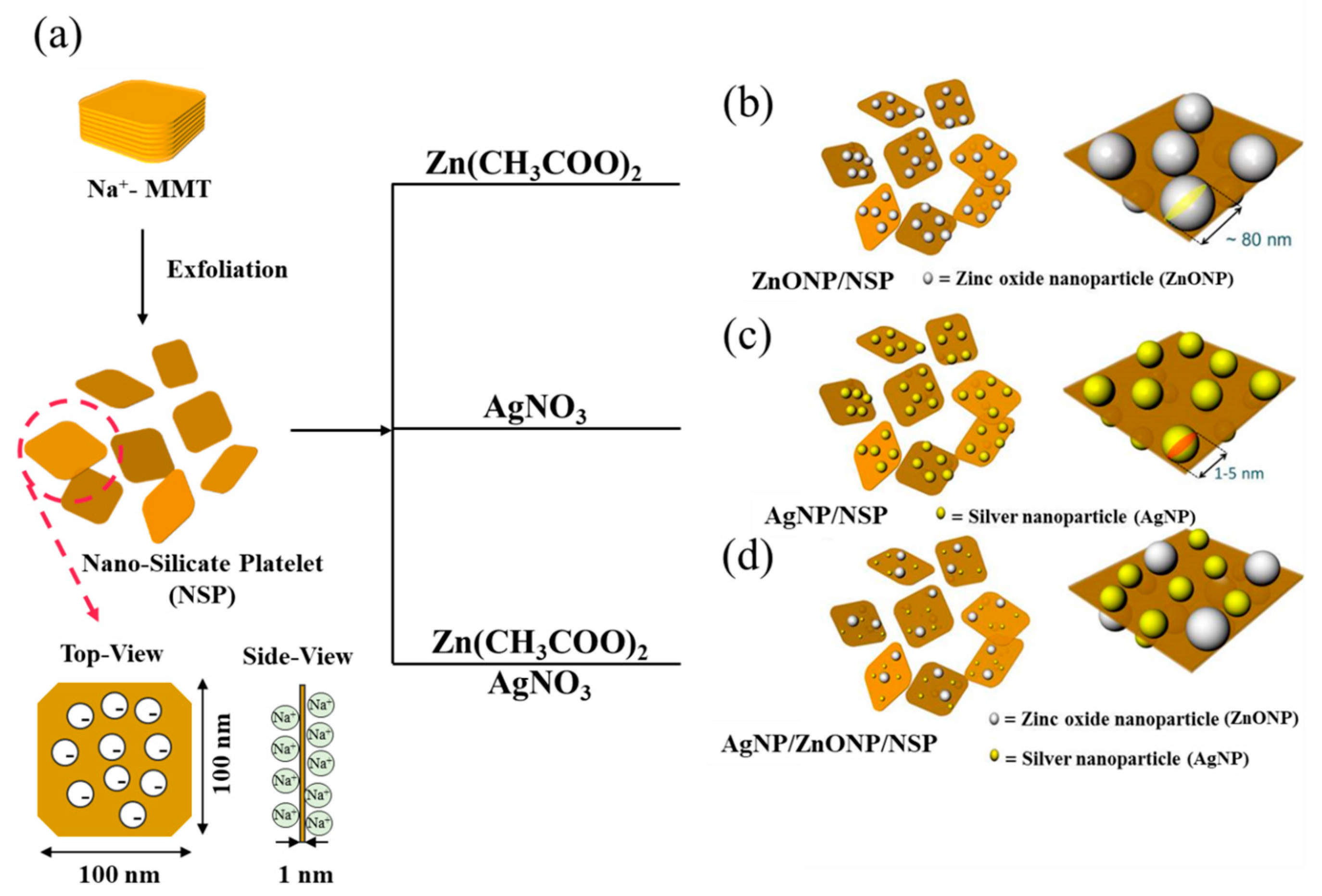
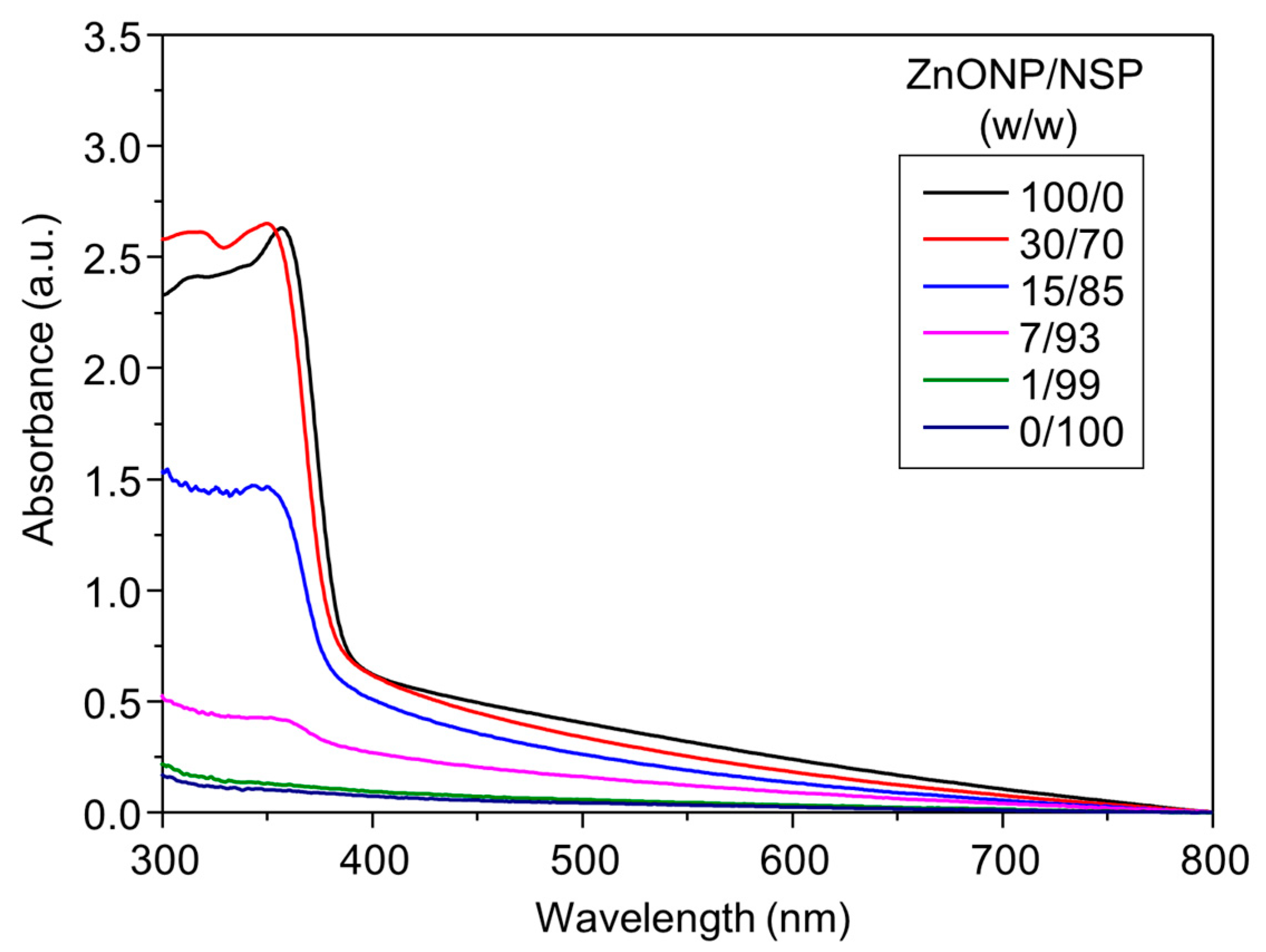
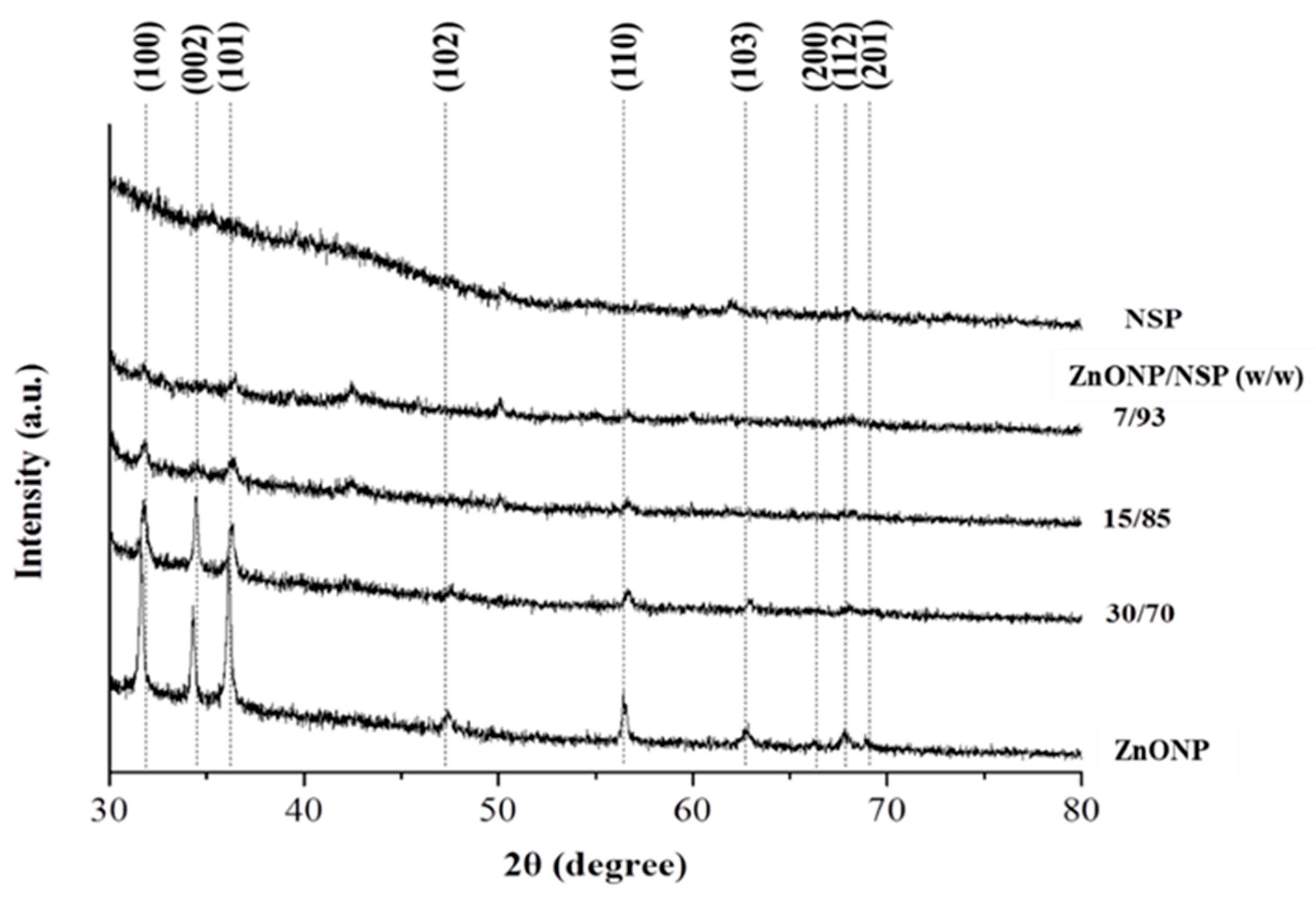
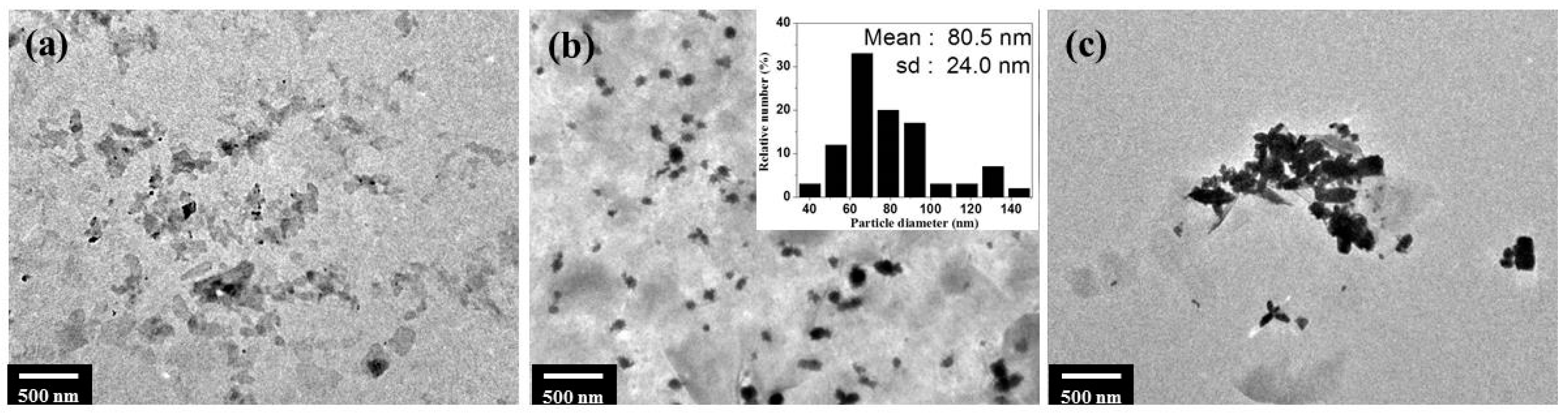
2.1. Synthesis of ZnONP/NSP Hybrids
2.2. Synthesis of AgNP/NSP and AgNP/ZnONP/NSP Hybrids
2.3. Cytotoxicity of the Hybrids
2.4. Antibacterial Efficacies of the Hybrids
3. Materials and Methods
3.1. Materials
3.2. Synthesis of ZnONP/NSP Hybrids
3.3. Synthesis of AgNP/NSP and AgNP/ZnONP/NSP Hybrids
3.4. Instrumentation and Analyses
3.5. Cytotoxicity Tests
3.6. Antibacterial Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Service, R.F. Nanotechnology takes aim at cancer. Science 2005, 310, 1132–1134. [Google Scholar] [CrossRef] [PubMed]
- Goodman, T.T.; Ng, C.P.; Pun, S.H. 3-D tissue culture systems for the evaluation and optimization of nanoparticle-based drug carriers. Bioconjug. Chem. 2008, 19, 1951–1959. [Google Scholar] [CrossRef]
- Shipway, A.N.; Katz, E.; Willner, I. Nanoparticle arrays on surfaces for electronic, optical, and sensor applications. ChemPhysChem 2000, 1, 18–52. [Google Scholar] [CrossRef] [PubMed]
- Mallick, K.; Witcomb, M.; Scurrell, M. Silver nanoparticle catalysed redox reaction: An electron relay effect. Mater. Chem. Phys. 2006, 97, 283–287. [Google Scholar] [CrossRef]
- Masunaga, T. Nanomaterials in Cosmetics-Present Situation and Future. Yakugaku Zasshi/J. Pharm. Soc. Jpn. 2014, 134, 39–43. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Lotfipour, F.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater. Sci. Eng. C—Mater. Biol. Appl. 2014, 44, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Li, X.H.; Xing, Y.; Jiang, Y.; Ding, Y.; Li, W. Antimicrobial activities of ZnO powder-coated PVC film to inactivate food pathogens. Int. J. Food Sci. Technol. 2009, 44, 2161–2168. [Google Scholar] [CrossRef]
- Xiong, H.M. ZnO Nanoparticles Applied to Bioimaging and Drug Delivery. Adv. Mater. 2013, 25, 5329–5335. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.L.; Morrin, A.; Killard, A.J.; Smyth, M.R. Application of nanoparticles in electrochemical sensors and biosensors. Electroanalysis 2006, 18, 319–326. [Google Scholar] [CrossRef]
- DeVasConCellos, P.; Bose, S.; Beyenal, H.; Bandyopadhyay, A.; Zirkle, L.G. Antimicrobial particulate silver coatings on stainless steel implants for fracture management. Mater. Sci. Eng. C—Mater. Biol. Appl. 2012, 32, 1112–1120. [Google Scholar] [CrossRef]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef]
- Noritomi, H.; Igari, N.; Kagitani, K.; Umezawa, Y.; Muratsubaki, Y.; Kato, S. Synthesis and size control of silver nanoparticles using reverse micelles of sucrose fatty acid esters. Colloid Polym. Sci. 2010, 288, 887–891. [Google Scholar] [CrossRef]
- Lin, X.Z.; Teng, X.W.; Yang, H. Direct synthesis of narrowly dispersed silver nanoparticles using a single-source precursor. Langmuir 2003, 19, 10081–10085. [Google Scholar] [CrossRef]
- Kvitek, L.; Panacek, A.; Soukupová, J.; Kolář, M.; Večeřová, R.; Prucek, R.; Holecová, M.; Zbořil, R. Effect of surfactants and polymers on stability and antibacterial activity of silver nanoparticles (NPs). J. Phys. Chem. C 2008, 112, 5825–5834. [Google Scholar] [CrossRef]
- Luo, L.B.; Yu, S.H.; Qian, H.-S.; Zhou, T. Large-scale fabrication of flexible silver/cross-linked poly(vinyl alcohol) coaxial nanocables by a facile solution approach. J. Am. Chem. Soc. 2005, 127, 2822–2823. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.W.; Yin, L.Y.; Lin, S.; Wiesner, M.; Bernhardt, E.; Liu, J. Toxicity Reduction of Polymer-Stabilized Silver Nanoparticles by Sunlight. J. Phys. Chem. C 2011, 115, 4425–4432. [Google Scholar] [CrossRef]
- Guo, L.; Yang, S.H.; Yang, C.-S.; Yu, P.; Wang, J.; Ge, W.; Wong, G.K.L. Highly monodisperse polymer-capped ZnO nanoparticles: Preparation and optical properties. Appl. Phys. Lett. 2000, 76, 2901–2903. [Google Scholar] [CrossRef]
- Singh, A.K.; Viswanath, V.; Janu, V.C. Synthesis, effect of capping agents, structural, optical and photoluminescence properties of ZnO nanoparticles. J. Lumin. 2009, 129, 874–878. [Google Scholar] [CrossRef]
- Garbin, V.; Crocker, J.C.; Stebe, K.J. Nanoparticles at fluid interfaces: Exploiting capping ligands to control adsorption, stability and dynamics. J. Colloid Interface Sci. 2012, 387, 1–11. [Google Scholar] [CrossRef]
- Dutta, R.K.; Nenavathu, B.P.; Gangishetty, M.K. Correlation between defects in capped ZnO nanoparticles and their antibacterial activity. J. Photochem. Photobiol. B Biol. 2013, 126, 105–111. [Google Scholar] [CrossRef] [PubMed]
- De Lima, R.; Seabra, A.B.; Durán, N. Silver nanoparticles: A brief review of cytotoxicity and genotoxicity of chemically and biogenically synthesized nanoparticles. J. Appl. Toxicol. 2012, 32, 867–879. [Google Scholar] [CrossRef]
- Singh, R.; Shedbalkar, U.U.; Wadhwani, S.A.; Chopade, B.A. Bacteriagenic silver nanoparticles: Synthesis, mechanism, and applications. Appl. Microbiol. Biotechnol. 2015, 99, 4579–4593. [Google Scholar] [CrossRef] [PubMed]
- Magana, S.M.; Quintana, P.; Aguilar, D.H.; Toledo, J.A.; Ángeles-Chávez, C.; Cortés, M.A.; León, L.; Freile-Pelegrín, Y.; López, T.; Sánchez, R.M.T. Antibacterial activity of montmorillonites modified with silver. J. Mol. Catal. A Chem. 2008, 281, 192–199. [Google Scholar] [CrossRef]
- Shameli, K.; Bin Ahmad, M.; Zargar, M.; Yunus, W.M.; Rustaiyan, A.; Ibrahim, N.A. Synthesis of silver nanoparticles in montmorillonite and their antibacterial behavior. Int. J. Nanomed. 2011, 6, 581–590. [Google Scholar] [CrossRef]
- Kiransan, M.; Khataee, A.; Karaca, S.; Sheydaei, M. Artificial neural network modeling of photocatalytic removal of a disperse dye using synthesized of ZnO nanoparticles on montmorillonite. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 140, 465–473. [Google Scholar] [CrossRef]
- Su, H.L.; Chou, C.C.; Hung, D.-J.; Lin, S.-H.; Pao, I.C.; Lin, J.-H.; Huang, F.-L.; Dong, R.-X.; Lin, J.-J. The disruption of bacterial membrane integrity through ROS generation induced by nanohybrids of silver and clay. Biomaterials 2009, 30, 5979–5987. [Google Scholar] [CrossRef]
- Alexandre, M.; Dubois, P. Polymer-layered silicate nanocomposites: Preparation, properties and uses of a new class of materials. Mater. Sci. Eng. R—Rep. 2000, 28, 1–63. [Google Scholar] [CrossRef]
- Zhang, D.; Zhou, C.H.; Lin, C.-X.; Tong, D.-S.; Yu, W.-H. Synthesis of clay minerals. Appl. Clay Sci. 2010, 50, 1–11. [Google Scholar] [CrossRef]
- Chu, C.C.; Chiang, M.L.; Tsai, C.-M.; Lin, J.-J. Exfoliation of montmorillonite clay by Mannich polyamines with multiple quaternary salts. Macromolecules 2005, 38, 6240–6243. [Google Scholar] [CrossRef]
- Lin, J.J.; Chu, C.C.; Chiang, M.-L.; Tsai, W.-C. First isolation of individual silicate platelets from clay exfoliation and their unique self-assembly into fibrous arrays. J. Phys. Chem. B 2006, 110, 18115–18120. [Google Scholar] [CrossRef]
- Hsu, S.H.; Tseng, H.J.; Hung, H.-S.; Wang, M.-C.; Hung, C.-H.; Li, P.-R.; Lin, J.-J. Antimicrobial Activities and Cellular Responses to Natural Silicate Clays and Derivatives Modified by Cationic Alkylamine Salts. ACS Appl. Mater. Interfaces 2009, 1, 2556–2564. [Google Scholar] [CrossRef]
- Wei, J.C.; Yen, Y.T.; Su, H.-L.; Lin, J.-J. Inhibition of Bacterial Growth by the Exfoliated Clays and Observation of Physical Capturing Mechanism. J. Phys. Chem. C 2011, 115, 18770–18775. [Google Scholar] [CrossRef]
- Liang, J.J.; Wei, J.C.; Lee, Y.L.; Hsu, S.H.; Lin, J.J.; Lin, Y.L. Surfactant-modified nanoclay exhibits an antiviral activity with high potency and broad spectrum. J. Virol. 2014, 88, 4218–4228. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.J.; Chang, P.-F.L.; Huang, J.; Lin, J.; Chung, W. Effect of Nanoscale Silicate Platelets on Azoxystrobin-resistant Isolates of Botrytis cinerea from Strawberry In Vitro and In Vivo. J. Plant Pathol. Microbiol. 2016, 7, 345. [Google Scholar] [CrossRef]
- Li, P.R.; Wei, J.C.; Chiu, Y.-F.; Su, H.-L.; Peng, F.-C.; Lin, J.-J. Evaluation on Cytotoxicity and Genotoxicity of the Exfoliated Silicate Nanoclay. ACS Appl. Mater. Interfaces 2010, 2, 1608–1613. [Google Scholar] [CrossRef]
- Chiao, S.H.; Lin, S.H.; Shen, C.I.; Liao, J.W.; Bau, I.J.; Wei, J.C.; Tseng, L.P.; Hsu, S.H.; Lai, P.S.; Lin, S.Z.; et al. Efficacy and safety of nanohybrids comprising silver nanoparticles and silicate clay for controlling Salmonella infection. Int. J. Nanomed. 2012, 7, 2421–2432. [Google Scholar]
- Chu, C.Y.; Peng, F.C.; Chiu, Y.-F.; Lee, H.-C.; Chen, C.-W.; Wei, J.-C.; Lin, J.-J. Nanohybrids of Silver Particles Immobilized on Silicate Platelet for Infected Wound Healing. PLoS ONE 2012, 7, e38360. [Google Scholar] [CrossRef]
- Su, H.L.; Lin, S.H.; Wei, J.-C.; Pao, I.C.; Chiao, S.-H.; Huang, C.-C.; Lin, S.-Z.; Lin, J.-J. Novel Nanohybrids of Silver Particles on Clay Platelets for Inhibiting Silver-Resistant Bacteria. PLoS ONE 2011, 6, e21125. [Google Scholar] [CrossRef]
- Liu, T.Y.; Chen, C.L.; Lee, Y.-C.; Chan, T.-Y.; Wang, Y.-L.; Lin, J.-J. First Observation of Physically Capturing and Maneuvering Bacteria using Magnetic Clays. ACS Appl. Mater. Interfaces 2016, 8, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Kokate, M.; Garadkar, K.; Gole, A. Zinc-oxide-silica-silver nanocomposite: Unique one-pot synthesis and enhanced catalytic and anti-bacterial performance. J. Colloid Interface Sci. 2016, 483, 249–260. [Google Scholar] [CrossRef]
- Motshekga, S.C.; Ray, S.S.; Onyango, M.S.; Momba, M.N.B. Microwave-assisted synthesis, characterization and antibacterial activity of Ag/ZnO nanoparticles supported bentonite clay. J. Hazard. Mater. 2013, 262, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Stankic, S.; Suman, S.; Haque, F.; Vidic, J. Pure and multi metal oxide nanoparticles: Synthesis, antibacterial and cytotoxic properties. J. Nanobiotechnol. 2016, 14, 73. [Google Scholar] [CrossRef] [PubMed]
- Istiqola, A.; Syafiuddin, A. A review of silver nanoparticles in food packaging technologies: Regulation, methods, properties, migration, and future challenges. J. Chin. Chem. Soc. 2020, 67, 1942–1956. [Google Scholar] [CrossRef]
- Lansdown, A.B. A review of the use of silver in wound care: Facts and fallacies. Br. J. Nurs. 2004, 13 (Suppl. S6), S6–S19. [Google Scholar] [CrossRef] [PubMed]
- Ghiuta, I.; Croitoru, C.; Kost, J.; Wenkert, R.; Munteanu, D. Bacteria-Mediated Synthesis of Silver and Silver Chloride Nanoparticles and Their Antimicrobial Activity. Appl. Sci. 2021, 11, 3134. [Google Scholar] [CrossRef]
- Dharmaraj, D.; Krishnamoorthy, M.; Rajendran, K.; Karuppiah, K.; Annamalai, J.; Durairaj, K.R.; Santhiyagu, P.; Ethiraj, K. Antibacterial and cytotoxicity activities of biosynthesized silver oxide (Ag2O) nanoparticles using Bacillus paramycoides. J. Drug Deliv. Sci. Technol. 2021, 61, 102111. [Google Scholar] [CrossRef]
- Moyer, C.A.; Brentano, L.; Gravens, D.L.; Margraf, H.W.; Monafo, W.W., Jr. Treatment of Large Human Burns With 0.5 Per Cent Silver Nitrate Solution. Arch. Surg. 1965, 90, 812–867. [Google Scholar] [CrossRef]
- AshaRani, P.V.; Low Kah Mun, G.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and Genotoxicity of Silver Nanoparticles in Human Cells. ACS Nano 2009, 3, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.W.; Hong, P.D.; Lin, J.-J. Clay-Mediated Synthesis of Silver Nanoparticles Exhibiting Low-Temperature Melting. Langmuir 2011, 27, 11690–11696. [Google Scholar] [CrossRef]
- Wei, J.C.; Yen, Y.T.; Wang, Y.-T.; Hsu, S.-H.; Lin, J.-J. Enhancing silver nanoparticle and antimicrobial efficacy by the exfoliated clay nanoplatelets. RSC Adv. 2013, 3, 7392–7397. [Google Scholar] [CrossRef]
- Lin, J.J.; Lin, W.C.; Li, S.-D.; Lin, C.-Y.; Hsu, S.-H. Evaluation of the Antibacterial Activity and Biocompatibility for Silver Nanoparticles Immobilized on Nano Silicate Platelets. ACS Appl. Mater. Interfaces 2013, 5, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.J.; Lin, W.C.; Dong, R.X.; Hsu, S.H. The cellular responses and antibacterial activities of silver nanoparticles stabilized by different polymers. Nanotechnology 2012, 23, 065102. [Google Scholar] [CrossRef] [PubMed]
- Marambio-Jones, C.; Hoek, E.M.V. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J. Nanoparticle Res. 2010, 12, 1531–1551. [Google Scholar] [CrossRef]
- Bondarenko, O.; Juganson, K.; Ivask, A.; Kasemets, K.; Mortimer, M.; Kahru, A. Toxicity of Ag, CuO and ZnO nanoparticles to selected environmentally relevant test organisms and mammalian cells in vitro: A critical review. Arch. Toxicol. 2013, 87, 1181–1200. [Google Scholar] [CrossRef] [PubMed]
- Ivask, A.; Juganson, K.; Bondarenko, O.; Mortimer, M.; Aruoja, V.; Kasemets, K.; Blinova, I.; Heinlaan, M.; Slaveykova, V.; Kahru, A. Mechanisms of toxic action of Ag, ZnO and CuO nanoparticles to selected ecotoxicological test organisms and mammalian cells in vitro: A comparative review. Nanotoxicology 2014, 8, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Azam, A.; Ahmed, A.S.; Oves, M.; Khan, M.S.; Habib, S.S.; Memic, A. Antimicrobial activity of metal oxide nanoparticles against Gram-positive and Gram-negative bacteria: A comparative study. Int. J. Nanomed. 2012, 7, 6003–6009. [Google Scholar] [CrossRef] [PubMed]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Sawada, H.; Wang, R.P.; Sleight, A.W. An electron density residual study of zinc oxide. J. Solid State Chem. 1996, 122, 148–150. [Google Scholar] [CrossRef]
- Park, M.V.D.Z.; Neigh, A.M.; Vermeulen, J.P.; de la Fonteyne, L.J.; Verharen, H.W.; Briedé, J.J.; van Loveren, H.; de Jong, W.H. The effect of particle size on the cytotoxicity, inflammation, developmental toxicity and genotoxicity of silver nanoparticles. Biomaterials 2011, 32, 9810–9817. [Google Scholar] [CrossRef] [PubMed]
- Roy, N.; Gaur, A.; Jain, A.; Bhattacharya, S.; Rani, V. Green synthesis of silver nanoparticles: An approach to overcome toxicity. Environ. Toxicol. Pharmacol. 2013, 36, 807–812. [Google Scholar] [CrossRef]
- Djurisic, A.B.; Leung, Y.H.; Ng, A.M.; Xu, X.Y.; Lee, P.K.; Degger, N.; Wu, R.S. Toxicity of Metal Oxide Nanoparticles: Mechanisms, Characterization, and Avoiding Experimental Artefacts. Small 2015, 11, 26–44. [Google Scholar] [CrossRef] [PubMed]
- Vimbela, G.V.; Ngo, S.M.; Fraze, C.; Yang, L.; Stout, D.A. Antibacterial properties and toxicity from metallic nanomaterials. Int. J. Nanomed. 2017, 12, 3941–3965. [Google Scholar] [CrossRef]
- Agnihotri, S.; Bajaj, G.; Mukherji, S.; Mukherji, S. Arginine-assisted immobilization of silver nanoparticles on ZnO nanorods: An enhanced and reusable antibacterial substrate without human cell cytotoxicity. Nanoscale 2015, 7, 7415–7429. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gao, X.J.; Zhi, L.; Liu, X.; Jiang, W.; Sun, Y.; Yang, J. The synergetic antibacterial activity of Ag islands on ZnO (Ag/ZnO) heterostructure nanoparticles and its mode of action. J. Inorg. Biochem. 2014, 130, 74–83. [Google Scholar] [CrossRef]
- Matai, I.; Sachdev, A.; Dubey, P.; Kumar, S.U.; Bhushan, B.; Gopinath, P. Antibacterial activity and mechanism of Ag-ZnO nanocomposite on S. aureus and GFP-expressing antibiotic resistant E-coli. Colloids Surf. B Biointerfaces 2014, 115, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.S.; Amna, T.; Sheikh, F.A.; Al-Deyab, S.S.; Eun Choi, K.; Hwang, I.H.; Khil, M.-S. Bimetallic Zn/Ag doped polyurethane spider net composite nanofibers: A novel multipurpose electrospun mat. Ceram. Int. 2013, 39, 2503–2510. [Google Scholar] [CrossRef]
- Karunakaran, C.; Rajeswari, V.; Gomathisankar, P. Enhanced photocatalytic and antibacterial activities of sol-gel synthesized ZnO and Ag-ZnO. Mater. Sci. Semicond. Process. 2011, 14, 133–138. [Google Scholar] [CrossRef]
- Lu, W.W.; Liu, G.S.; Gao, S.; Xing, S.; Wang, J. Tyrosine-assisted preparation of Ag/ZnO nanocomposites with enhanced photocatalytic performance and synergistic antibacterial activities. Nanotechnology 2008, 19, 445711. [Google Scholar] [CrossRef]
- Xiang, Y.M.; Li, J.; Liu, X.; Cui, Z.; Yang, X.; Yeung, K.W.K.; Pan, H.; Wu, S. Construction of poly(lactic-co-glycolic acid)/ZnO nanorods/Ag nanoparticles hybrid coating on Ti implants for enhanced antibacterial activity and biocompatibility. Mater. Sci. Eng. C—Mater. Biol. Appl. 2017, 79, 629–637. [Google Scholar] [CrossRef]
- Lu, Z.; Gao, J.T.; He, Q.; Wu, J.; Liang, D.; Yang, H.; Chen, R. Enhanced antibacterial and wound healing activities of microporous chitosan-Ag/ZnO composite dressing. Carbohydr. Polym. 2017, 156, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.S.; Patil, R.H.; Kale, S.B.; Tamboli, M.S.; Ambekar, J.D.; Gade, W.N.; Kolekar, S.S.; Kale, B.B. Nanostructured microspheres of silver @ zinc oxide: An excellent impeder of bacterial growth and biofilm. J. Nanoparticle Res. 2014, 16, 2717. [Google Scholar] [CrossRef]
- Jain, A.; Bhargava, R.; Poddar, P. Probing interaction of Gram-positive and Gram-negative bacterial cells with ZnO nanorods. Mater. Sci. Eng. C—Mater. Biol. Appl. 2013, 33, 1247–1253. [Google Scholar] [CrossRef]
- Reddy, K.M.; Feris, K.; Bell, J.; Wingett, D.G.; Hanley, C.; Punnoose, A. Selective toxicity of zinc oxide nanoparticles to prokaryotic and eukaryotic systems. Appl. Phys. Lett. 2007, 90, 213902. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, S.; Nazam, N.; Rizvi, S.M.D.; Ahmad, K.; Baig, M.H.; Lee, E.J.; Choi, I. Mechanistic Insights into the Antimicrobial Actions of Metallic Nanoparticles and Their Implications for Multidrug Resistance. Int. J. Mol. Sci. 2019, 20, 2468. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.F.; Lin, J.J. Observation of carbon nanotube and clay micellelike microstructures with dual dispersion property. J. Phys. Chem. A 2009, 113, 8654–8659. [Google Scholar] [CrossRef] [PubMed]
- Pai, Y.H.; Ke, J.-H.; Chou, C.-C.; Lin, J.-J.; Zen, J.-M.; Shieu, F.-S. Clay as a dispersion agent in anode catalyst layer for PEMFC. J. Power Sources 2006, 163, 398–402. [Google Scholar] [CrossRef]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic Basis of Antimicrobial Actions of Silver Nanoparticles. Front. Microbiol. 2016, 7, 1831. [Google Scholar] [CrossRef]


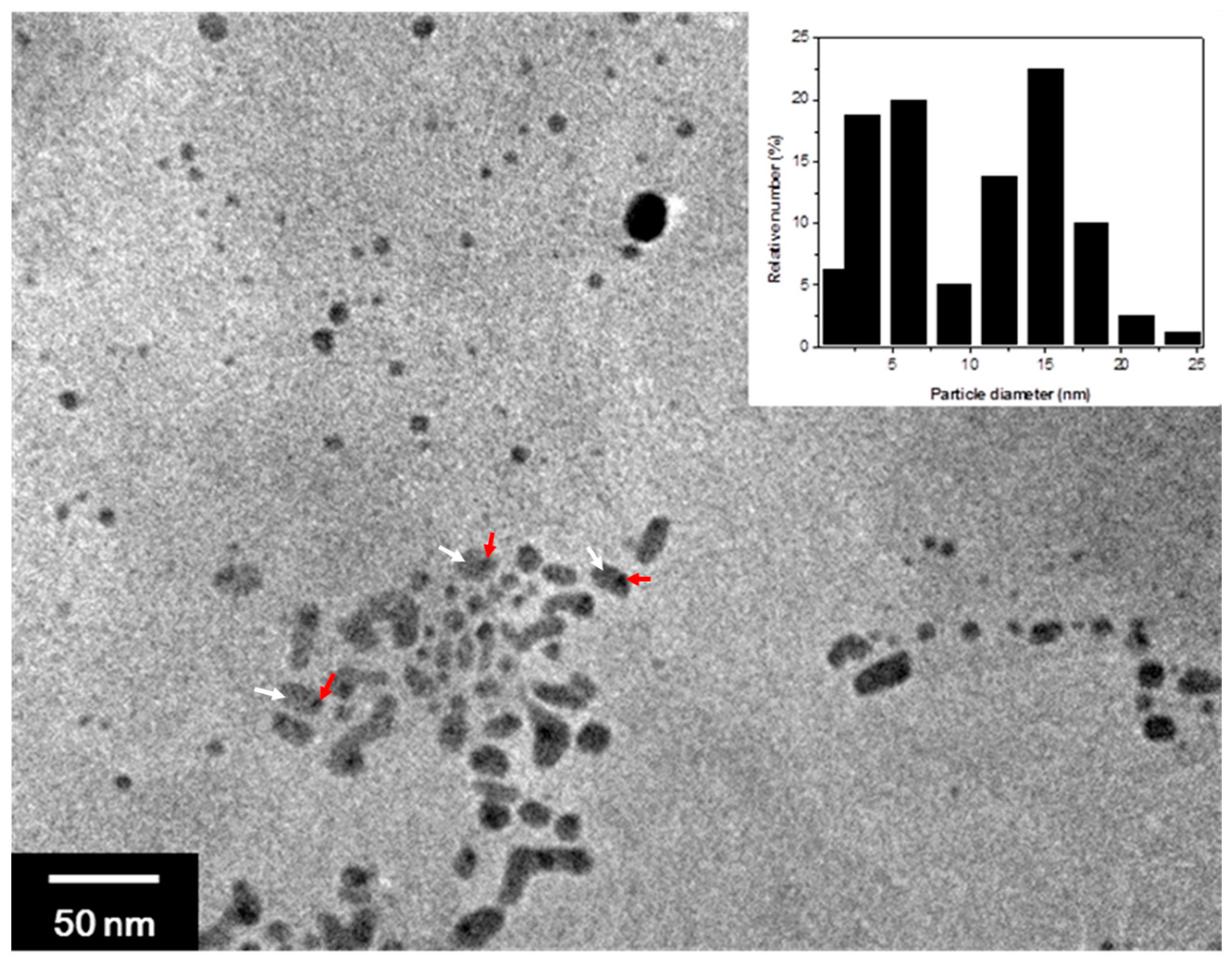
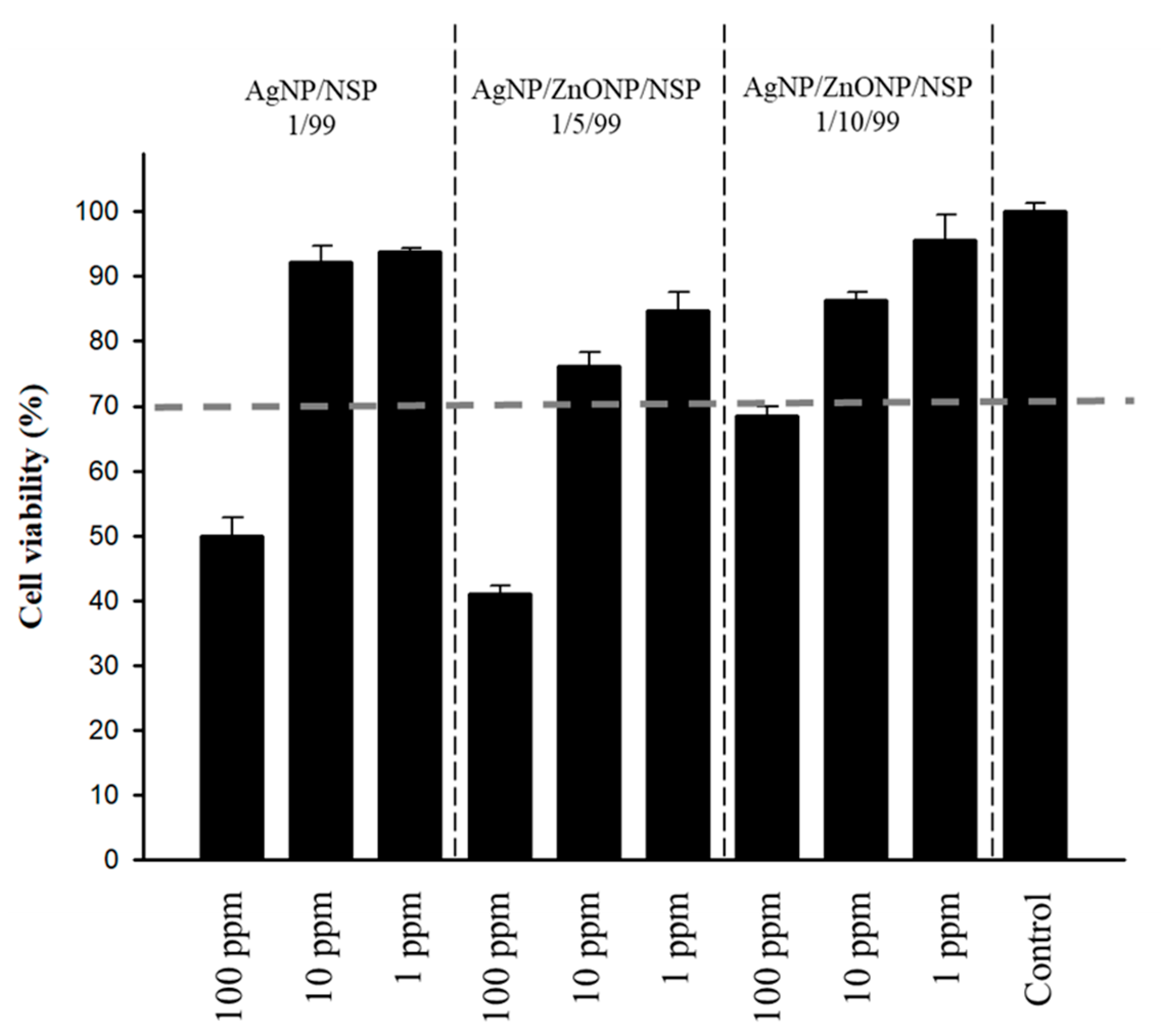
| Material | Weight Ratio (w/w/w) | Bacteria [MBC a (ppm)] | |
|---|---|---|---|
| S. aureus | E. coli | ||
| NSP | - | 7000 | 5000 |
| ZnONP/NSP | 15/85 | 4300 | 6000 |
| AgNP/NSP | 1/99 | 1500 (15) b | 10.0 (0.1) |
| AgNP/ZnONP/NSP | 1/5/99 | 420 (4) | 10.5 (0.1) |
| 1/10/99 | 440 (4) | 11.0 (0.1) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, C.-H.; Tsai, L.-H.; Lee, Y.-C.; Yao, W.-C.; Lin, J.-J. Synergistic Effects of Silicate-Platelet Supporting Ag and ZnO, Offering High Antibacterial Activity and Low Cytotoxicity. Int. J. Mol. Sci. 2023, 24, 7024. https://doi.org/10.3390/ijms24087024
Chang C-H, Tsai L-H, Lee Y-C, Yao W-C, Lin J-J. Synergistic Effects of Silicate-Platelet Supporting Ag and ZnO, Offering High Antibacterial Activity and Low Cytotoxicity. International Journal of Molecular Sciences. 2023; 24(8):7024. https://doi.org/10.3390/ijms24087024
Chicago/Turabian StyleChang, Chih-Hao, Li-Hui Tsai, Yi-Chen Lee, Wei-Cheng Yao, and Jiang-Jen Lin. 2023. "Synergistic Effects of Silicate-Platelet Supporting Ag and ZnO, Offering High Antibacterial Activity and Low Cytotoxicity" International Journal of Molecular Sciences 24, no. 8: 7024. https://doi.org/10.3390/ijms24087024
APA StyleChang, C.-H., Tsai, L.-H., Lee, Y.-C., Yao, W.-C., & Lin, J.-J. (2023). Synergistic Effects of Silicate-Platelet Supporting Ag and ZnO, Offering High Antibacterial Activity and Low Cytotoxicity. International Journal of Molecular Sciences, 24(8), 7024. https://doi.org/10.3390/ijms24087024







