Assessment of Interleukin-15 (IL-15) Concentration in Children with Idiopathic Nephrotic Syndrome
Abstract
1. Introduction
1.1. The Idiopathic Nephrotic Syndrome
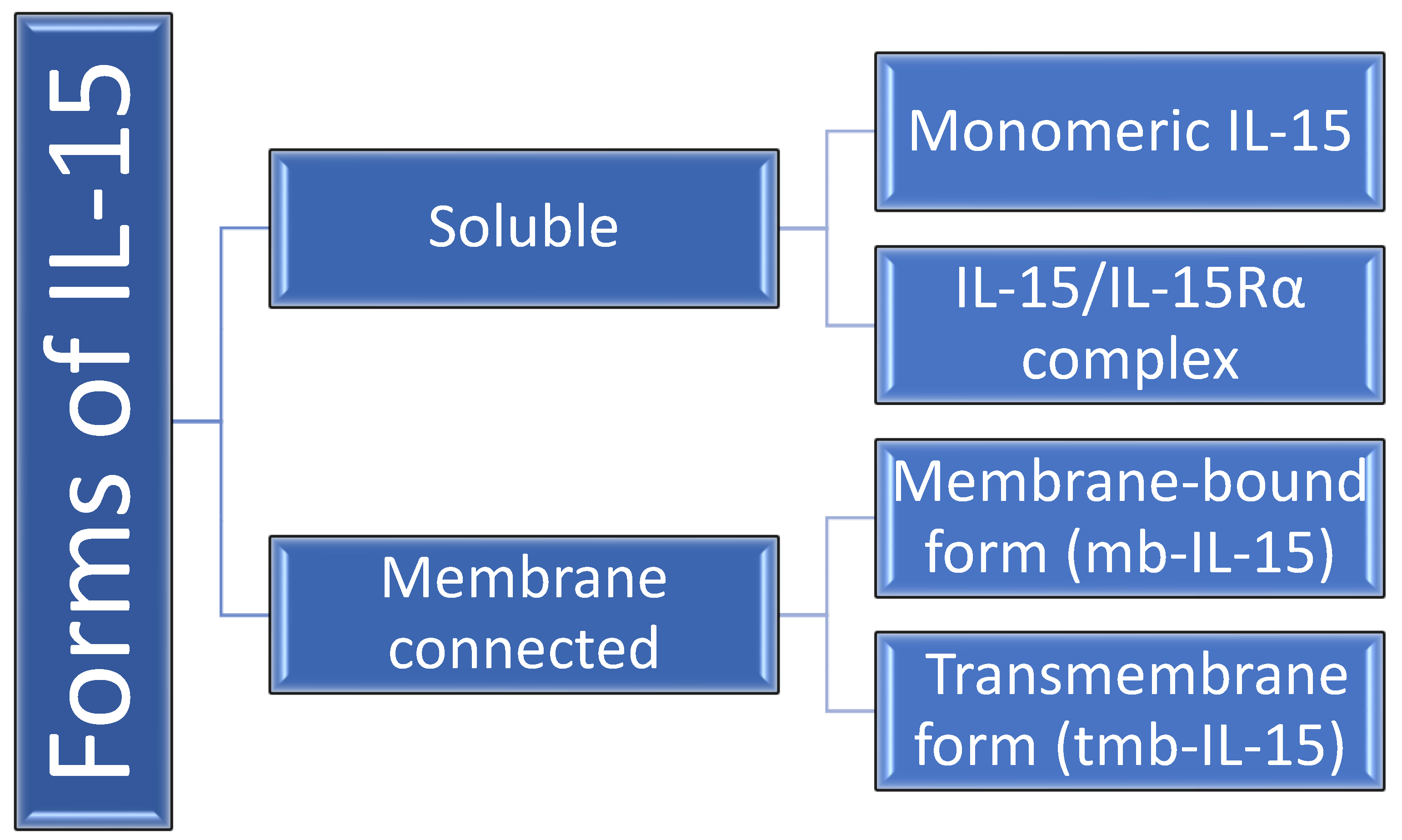
1.2. Interleukin-15
2. Results
2.1. Descriptive Analysis and Comparison—INS Patients and Control Group
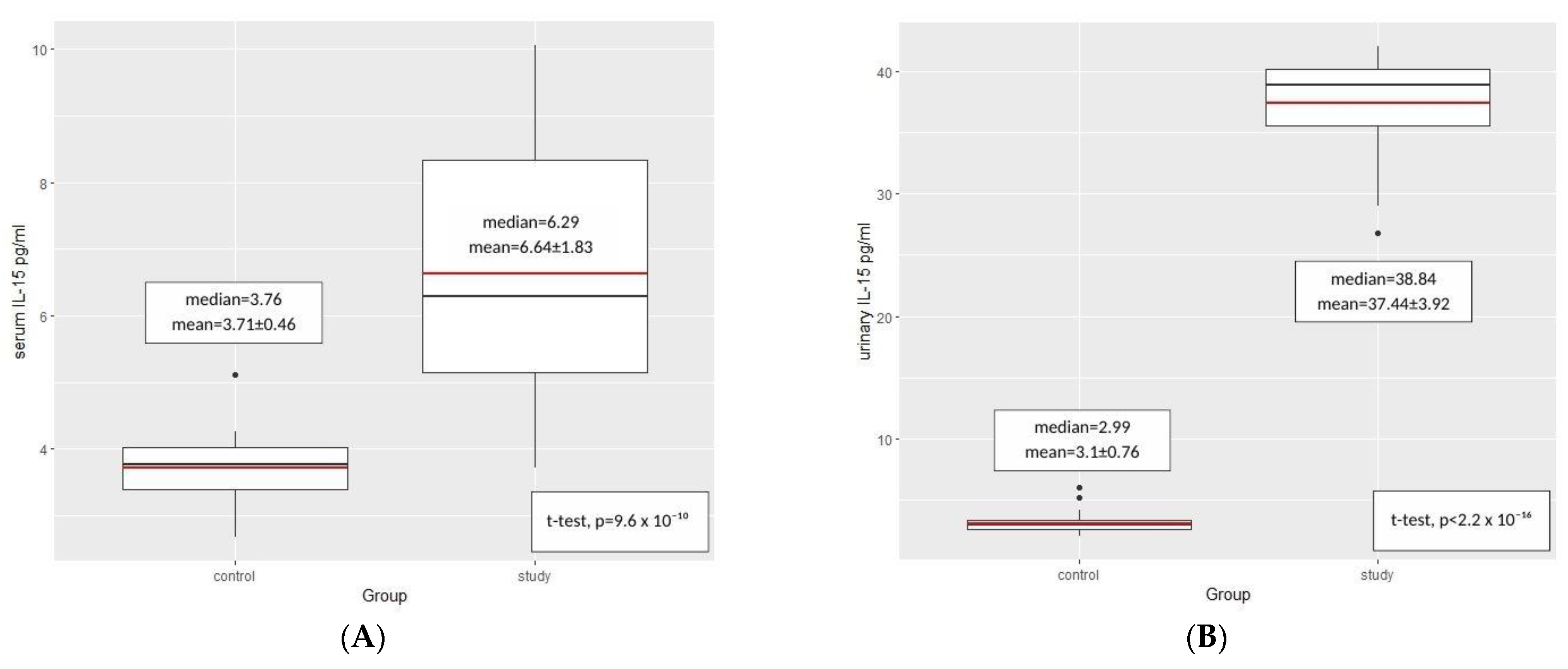
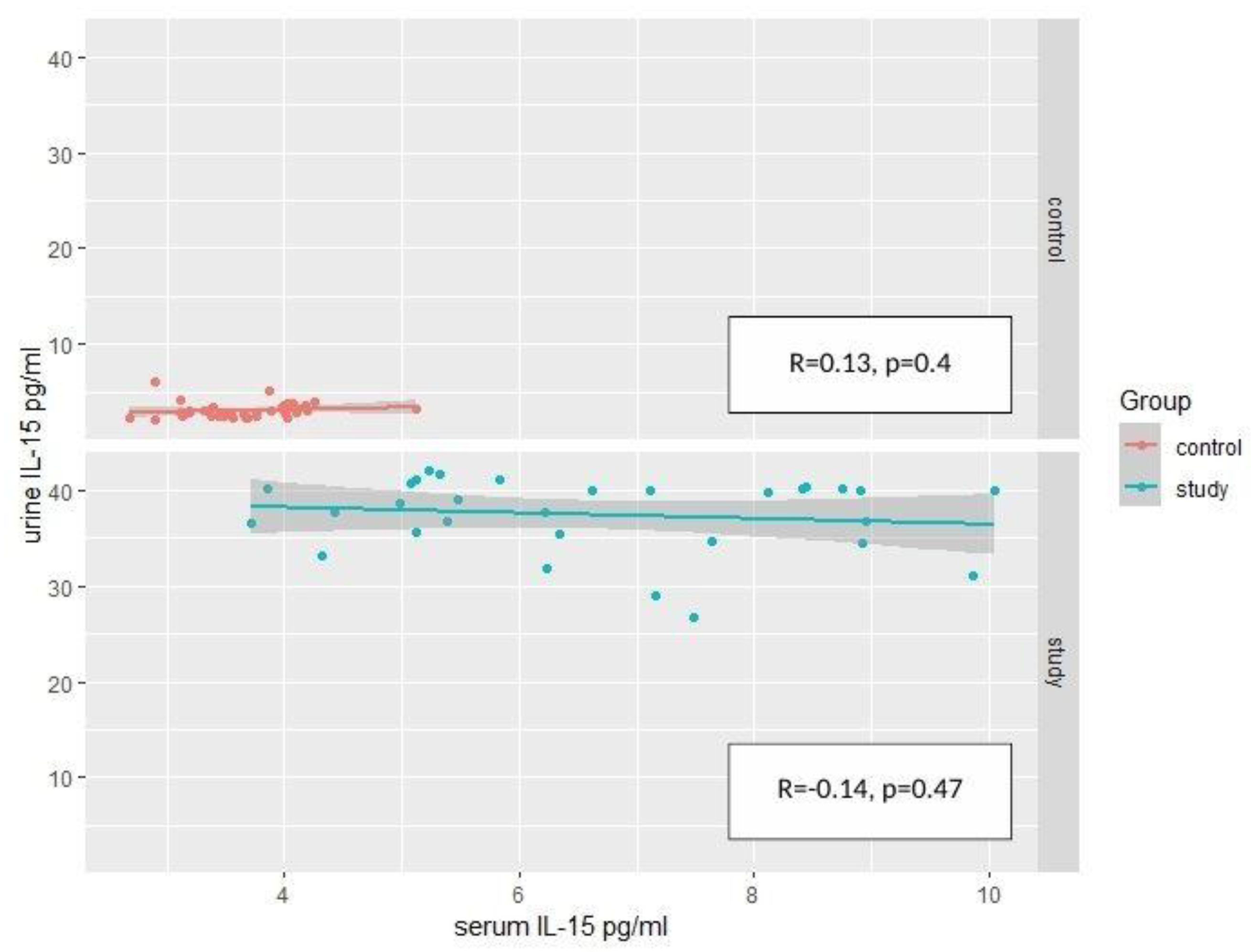
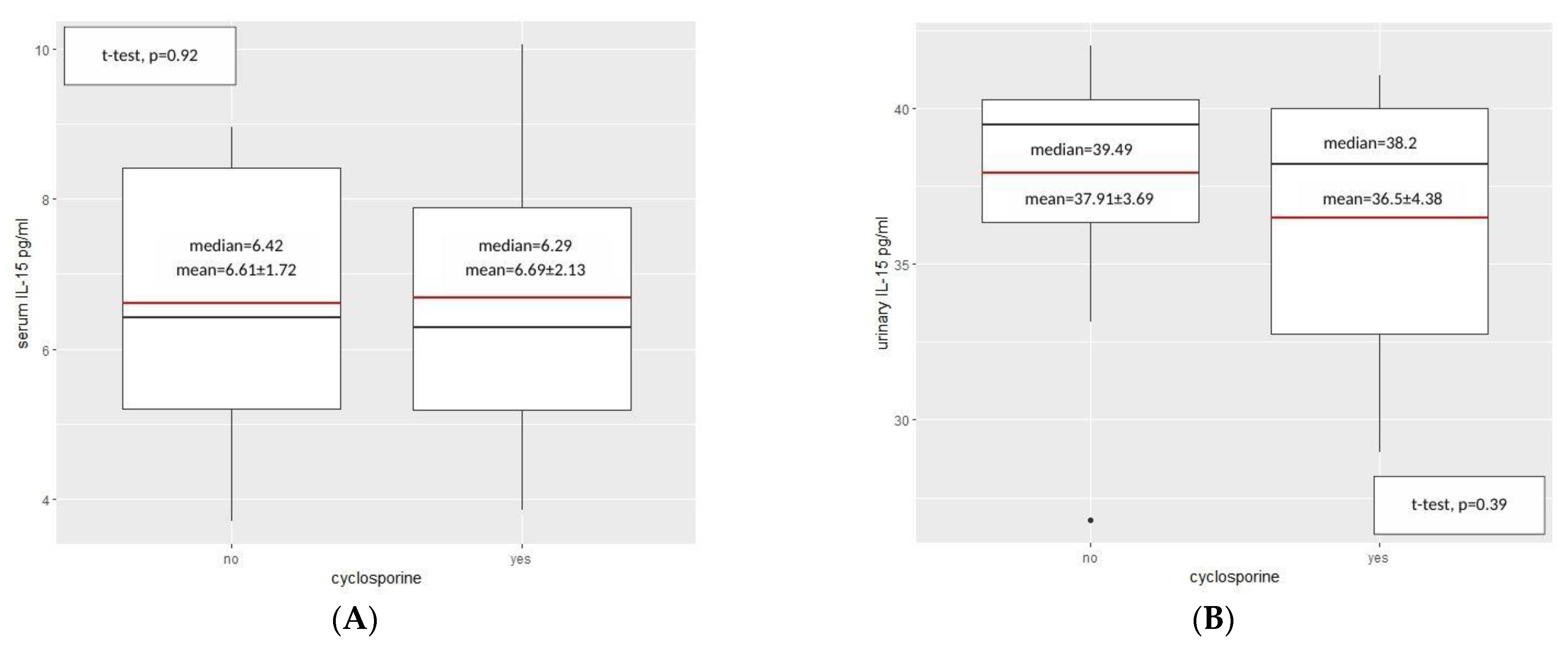
2.2. IL-15—Results
3. Discussion
Limitations of the Study
4. Materials and Methods
4.1. Studied Groups
4.2. Laboratory Tests
4.3. IL-15 Concentration
4.4. Anthropometric Measurements
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Noone, D.G.; Iijima, K.; Parekh, R. Idiopathic nephrotic syndrome in children. Lancet 2018, 392, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Anders, H.J.; Kitching, A.R.; Leung, N.; Romagnani, P. Glomerulonephritis: Immunopathogenesis and immunotherapy. Nat. Rev. Immunol. 2023, 12, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Vivarelli, M.; Massella, L.; Ruggiero, B.; Emma, F. Minimal change disease. Clin. J. Am. Soc. Nephrol. 2017, 12, 332–345. [Google Scholar] [CrossRef]
- Lombel, R.M.; Gipson, D.S.; Hodson, E.M. Treatment of steroid-sensitive nephrotic syndrome: New guidelines from KDIGO. Pediatr. Nephrol. 2013, 28, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Tullus, K.; Webb, H.; Bagga, A. Management of steroid-resistant nephrotic syndrome in children and adolescents. Lancet Child Adolesc. Health 2018, 2, 880–890. [Google Scholar] [CrossRef]
- Fehniger, T.A.; Caligiuri, M.A. Interleukin 15: Biology and relevance to human disease. Blood 2001, 97, 14–32. [Google Scholar] [CrossRef]
- Grabstein, K.H.; Eisenman, J.; Shanebeck, K.; Rauch, C.; Srinivasan, S.; Fung, V.; Beers, C.; Richardson, J.; Schoenborn, M.A.; Ahdieh, M.; et al. Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. Science 1994, 264, 965–968. [Google Scholar] [CrossRef]
- Bulfone-Pau, S.S.; Bulanova, E.; Pohl, T.; Budagian, V.; Durkop, H.; Ruckert, R.; Kunzendorf, U.; Paus, R.; Krause, H. Death deflected: IL-15 inhibits TNF-alpha-mediated apoptosis in fibroblasts by TRAF2 recruitment to the IL-15Ralpha chain. FASEB J. 1999, 13, 1575–1585. [Google Scholar]
- Masuda, A.; Matsuguchi, T.; Yamaki, K.; Hayakawa, T.; Yoshikai, Y. Interleukin-15 prevents mouse mast cell apoptosis through STAT6-mediated Bcl-xL expression. J. Biol. Chem. 2001, 276, 26107–26113. [Google Scholar] [CrossRef]
- Ruckert, R.; Asadullah, K.; Seifert, M.; Budagian, V.M.; Arnold, R.; Trombotto, C.; Paus, R.; Bulfone-Paus, S. Inhibition of keratinocyte apoptosis by IL-15: A new parameter in the pathogenesis of psoriasis? J. Immunol. 2000, 165, 2240–2250. [Google Scholar] [CrossRef]
- Bergamaschi, C.; Bear, J.; Rosati, M.; Beach, R.K.; Alicea, C.; Sowder, R.; Chertova, E.; Rosenberg, S.A.; Felber, B.K.; Pavlakis, G.N. Circulating IL-15 exists as heterodimeric complex with soluble IL-15Rα in human and mouse serum. Blood 2012, 120, e1–e8. [Google Scholar] [CrossRef]
- Giron-Michel, J.; Giuliani, M.; Fogli, M.; Brouty-Boye, D.; Ferrini, S.; Baychelier, F.; Eid, P.; Lebousse-Kerdiles, C.; Durali, D.; Biassoni, R.; et al. Membrane-bound and soluble IL-15/IL-15Rα complexes display differential signaling and functions on human hematopoietic progenitors. Blood 2005, 106, 2302–2310. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, A.; Xiao, X.; Degauque, N.; Edtinger, K.; Wei, H.; Demirci, G.; Li, X.C. The innate NK cells, allograft rejection, and a key role for IL-15. J. Immunol. 2008, 180, 7818–7826. [Google Scholar] [CrossRef] [PubMed]
- Stoklasek, T.A.; Schluns, K.S.; Lefrancois, L. Combined IL-15/IL-15Rα immunotherapy maximizes IL-15 activity in vivo. J. Immunol. 2006, 177, 6072–6080. [Google Scholar] [CrossRef] [PubMed]
- Dubois, S.; Mariner, J.; Waldmann, T.A.; Tagaya, Y. IL-15Rα recycles and presents IL-15 In trans to neighboring cells. Immunity 2002, 17, 537–547. [Google Scholar] [CrossRef]
- Bo, H.; Wei, X.Q.; Dong, H.; Zhang, Y.; Lv, P.; Liu, W.; Koutoulaki, A.; Gao, X.M. Elevated expression of transmembrane IL-15 in immune cells correlates with the development of murine lupus: A potential target for immunotherapy against SLE. Scand. J. Immunol. 2009, 69, 119–129. [Google Scholar] [CrossRef]
- Khawam, K.; Giron-Michel, J.; Gu, Y.; Perier, A.; Giuliani, M.; Caignard, A.; Devocelle, A.; Ferrini, S.; Fabbi, M.; Charpentier, B.; et al. Human renal cancer cells express a novel membrane-bound interleukin-15 that induces, in response to the soluble interleukin-15 receptor alpha chain, epithelial-to-mesenchymal transition. Cancer Res. 2009, 69, 1561–1569. [Google Scholar] [CrossRef]
- Waldmann, T.A. The biology of interleukin-2 and interleukin-15: Implications for cancer therapy and vaccine design. Nat. Rev. Immunol. 2006, 6, 595–601. [Google Scholar] [CrossRef]
- Koka, R.; Burkett, P.; Chien, M.; Chai, S.; Boone, D.L.; Ma, A. Cutting edge: Murine dendritic cells require IL-15Rα to prime NK cells. J. Immunol. 2004, 173, 3594–3598. [Google Scholar] [CrossRef]
- Lodolce, J.P.; Burkett, P.R.; Boone, D.L.; Chien, M.; Ma, A. T cell-independent interleukin 15Ralpha signals are required for bystander proliferation. J. Exp. Med. 2001, 194, 1187–1194. [Google Scholar] [CrossRef]
- Cole, D.J.; Rubinstein, M.P. Soluble IL-15/IL-15Rα complexes in human serum. Blood 2012, 120, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Badeński, A.; Badeńska, M.; Świętochowska, E.; Didyk, A.; Morawiec-Knysak, A.; Szczepańska, M. Assessment of Brain-Derived Neurotrophic Factor (BDNF) Concentration in Children with Idiopathic Nephrotic Syndrome. Int. J. Mol. Sci. 2022, 23, 12312. [Google Scholar] [CrossRef]
- Azzi, S.; Gallerne, C.; Romei, C.; Le Coz, V.; Gangemi, R.; Khawam, K.; Devocelle, A.; Gu, Y.; Bruno, S.; Ferrini, S.; et al. Human Renal Normal, Tumoral, and Cancer Stem Cells Express Membrane-Bound Interleukin-15 Isoforms Displaying Different Functions. Neoplasia 2015, 17, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, M.; Hirahashi, J.; Lebedeva, T.; Liew, F.Y.; Salant, D.J.; Maron, R.; Kelley, V.R. IL-15, a survival factor for kidney epithelial cells, counteracts apoptosis and inflammation during nephritis. J. Clin. Investig. 2002, 109, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Gerritsma, J.S.; Gerritsen, A.F.; van Kooten, C.; van Es, L.A.; Daha, M.R. Expression of the IL-2 receptor on human renal proximal tubular epithelial cells. J. Am. Soc. Nephrol. 1997, 8, 1510–1516. [Google Scholar] [CrossRef] [PubMed]
- Giron-Michel, J.; Azzi, S.; Khawam, K.; Mortier, E.; Caignard, A.; Devocelle, A.; Ferrini, S.; Croce, M.; Francois, H.; Lecru, L.; et al. Interleukin-15 plays a central role in human kidney physiology and cancer through the γc signaling pathway. PLoS ONE 2012, 7, e31624. [Google Scholar] [CrossRef]
- Giron-Michel, J.; Azzi, S.; Ferrini, S.; Chouaib, S.; Camussi, G.; Eid, P.; Azzarone, B. Interleukin-15 is a major regulator of the cell-microenvironment interactions in human renal homeostasis. Cytokine Growth Factor Rev. 2013, 24, 13–22. [Google Scholar] [CrossRef]
- Eini, H.; Tejman-Yarden, N.; Lewis, E.C.; Chaimovitz, C.; Zlotnik, M.; Douvdevani, A. Association between renal injury and reduced interleukin-15 and interleukin-15 receptor levels in acute kidney injury. J. Interferon Cytokine Res. 2010, 30, 1–8. [Google Scholar] [CrossRef]
- Tejman-Yarden, N.; Zlotnik, M.; Lewis, E.; Etzion, O.; Chaimovitz, C.; Douvdevani, A. Renal cells express a functional interleukin-15 receptor. Nephrol. Dial. Transplant. 2005, 20, 516–523. [Google Scholar] [CrossRef]
- Devocelle, A.; Lecru, L.; François, H.; Desterke, C.; Gallerne, C.; Eid, P.; Estelle, O.; Azzarone, B.; Giron-Michel, J. Inhibition of TGF- β1 Signaling by IL-15: A Novel Role for IL-15 in the Control of Renal Epithelial-Mesenchymal Transition: IL-15 Counteracts TGF- β1-Induced EMT in Renal Fibrosis. Int. J. Cell Biol. 2019, 2019, 9151394. [Google Scholar] [CrossRef]
- Mooslechner, A.A.; Schuller, M.; Artinger, K.; Kirsch, A.H.; Schabhüttl, C.; Eller, P.; Rosenkranz, A.R.; Eller, K. Low-Dose rIL-15 Protects from Nephrotoxic Serum Nephritis via CD8+ T Cells. Cells 2022, 11, 3656. [Google Scholar] [CrossRef] [PubMed]
- Strehlau, J.; Pavlakis, M.; Lipman, M.; Shapiro, M.; Vasconcellos, L.; Harmon, W.; Strom, T.B. Quantitative detection of immune activation transcripts as a diagnostic tool in kidney transplantation. Proc. Natl. Acad. Sci. USA 1997, 94, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Hingorani, S.; Gooley, T.; Pao, E.; Sandmaier, B.; McDonald, G. Urinary cytokines after HCT: Evidence for renal inflammation in the pathogenesis of proteinuria and kidney disease. Bone Marrow. Trans. 2014, 49, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.J.; Russell, R.C.; Roche, O.; Burry, T.N.; Fish, J.E.; Chow, V.W.; Kim, W.Y.; Saravanan, A.; Maynard, M.A.; Gervais, M.L.; et al. VHL promotes E2 box-dependent E-cadherin transcription by HIF-mediated regulation of SIP1 and snail. Mol. Cell. Biol. 2007, 27, 157–169. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, X.; Zhao, Y.; Xu, X.; Liu, Y.; Wu, X. Excessive IL-15 promotes cytotoxic CD4+ CD28− T cell-mediated renal injury in lupus nephritis. Immun. Ageing 2022, 19, 50. [Google Scholar] [CrossRef]
- Pacheco-Lugo, L.; Sáenz-García, J.; Navarro Quiroz, E.; González Torres, H.; Fang, L.; Díaz-Olmos, Y.; Garavito de Egea, G.; Egea Bermejo, E.; Aroca Martínez, G. Plasma cytokines as potential biomarkers of kidney damage in patients with systemic lupus erythematosus. Lupus 2019, 28, 34–43. [Google Scholar] [CrossRef]
- Li, L.; Tang, W.; Zhang, Y.; Jia, M.; Wang, L.; Li, Q.; Han, Q.; Peng, X.; Xie, Y.; Wu, J.; et al. Targeting tissue-resident memory CD8+ T cells in the kidney is a potential therapeutic strategy to ameliorate podocyte injury and glomerulosclerosis. Mol. Ther. 2022, 30, 2746–2759. [Google Scholar] [CrossRef] [PubMed]
- Ziółkowska, H.; Bałasz-Chmielewska, I.; Grenda, R.; Musiał, K.; Ogarek, I.; Szczepańska, M.; Żurowska, A. The Polish Society for Pediatric Nephrology (PTNFD) recommendations on the management of children with nephrotic syndrome. Ren. Dis. Transplant. Forum 2015, 8, 238–256. [Google Scholar]
- De Souza, V.C.; Rabilloud, M.; Cochat, P.; Selistre, L.; Hadj-Aissa, A.; Kassai, B.; Ranchin, B.; Berg, U.; Herthelius, M.; Dubourg, L. Schwartz formula: Is one k-coefficient adequate for all children? PLoS ONE 2012, 7, e53439. [Google Scholar] [CrossRef]
- Kułaga, Z.; Różdżyńska, A.; Palczewska, I.; Grajda, A.; Gurzkowska, B.; Napieralska, E.; Litwin, M. Percentile charts of height, body mass and body mass index in children and adolescents in Poland—Results of the OLAF study. Stand. Med. Pediatr. 2010, 7, 690–700. [Google Scholar]
- Kułaga, Z.; Litwin, M.; Grajda, A.; Gurzkowska, B.; Napieralska, E.; Kułaga, K. Distribution of blood pressure in school-aged children and adolescents reference population. Stand. Med. Pediatr. 2010, 7, 100–111. [Google Scholar]
| Parameter | Children with Idiopathic Nephrotic Syndrome (n = 30) | Control Group (n = 44) | ||||
|---|---|---|---|---|---|---|
| Whole Group | Female | Male | Whole Group | Female | Male | |
| Age (year) | 7.50 ± 4.14 (2.00–17.00) | 6.14 ± 3.35 (2.00–13.00) | 9.00 ± 4.40 (2.00–17.00) | 7.75 ± 4.10 (2.00–17.50) | 8.33 ± 3.95 (2.00–17.00) | 9.85 ± 4.17 (4.50–17.50) |
| Height (cm) | 125 ± 25.00 (92.00–179.00) | 115.46 ± 19.24 (92.00–158.00) | 134.41 ± 26.70 (92.00–179.00) | 127 ± 24.90 (82.00–197.00) | 127.50 ± 21.59 (82.00–170.00) | 142.17 ± 25.60 (109.00–197.00) |
| SDS for height | 0.06 ± 1.36 (−2.80–2.29) | −00.7 ± 1.20 (−2.80–2.20) | 0.08 ± 1.53 (−2.80–2.29) | 0.09 ± 1.10 (1.53–3.00) | −0.14 ± 1.08 (−1.53–2.35) | 0.35 ± 1.08 (−1.34–3.00) |
| BW (kg) | 29.80 ± 21.60 (12.10–88.90) | 26.54 ± 13.55 (12.10–59.80) | 43.26 ± 24.49 (14.50–88.90) | 27.10 ± 18.80 (9.70–87.50) | 30.35 ± 15.03 (9.70–71.00) | 38.38 ± 20.68 (15.00–87.50) |
| SDS for BW | 1.15 ± 2.1 (−1.83–9.58) | 1.12 ± 1.58 (−1.83–3.80) | 1.75 ± 2.49 (−1.63–9.58) | 0.07 ± 1.11 (−2.34–2.12) | 0.08 ± 1.23 (−1.87–2.11) | 0.04 ± 1.04 (−2.34–2.12) |
| BMI (kg/m2) | 18.90 ± 4.07 (13.50–30.90) | 18.41 ± 3.20 (13.82–23.98) | 20.92 ± 4.48 (13.50–30.90) | 16.60 ± 3.45 (12.40–26.50) | 17.52 ± 3.12 (13.90–24.60) | 17.52 ± 3.72 (12.40–26.50) |
| SDS for BMI | 0.83 ± 2.10 (−2.18–8.17) | 0.07 ± 1.18 (−1.47–2.35) | 2.20 ± 2.26 (−2.18–8.17) | −0.06 ± 1.18 (−2.97–2.29) | 0.25 ± 1.05 (−1.38–2.29) | −0.25 ± 1.25 (−2.97–2.02) |
| SYS (mmHg) | 115 ± 14.6 (81.00–152.00) | 108.21 ± 14.87 (81.00–138.00) | 117.88 ± 13.17 (102.00–152.00) | 110.00 ± 11.30 (85.00–134.00) | 107.17 ± 8.54 (89.00–122.00) | 113.96 ± 12.25 (85.00–134.00) |
| DIA (mmHg) | 67.50 ± 10.90 (50.00–90.00) | 64.71 ± 9.51 (50.00–88.00) | 73.44 ± 10.66 (56.00–90.00) | 70.00 ± 12.00 (40.00–107.00) | 66.83 ± 11.45 (45.00–96.00) | 69.96 ± 12.44 (40.00–107.00) |
| MAP (mmHg) | 82.50 ± 11.30 (67.00–111.00) | 79.21 ± 9.97 (67.00–100) | 88.25 ± 11.03 (71.33–111.00) | 82.50 ± 10.70 (58.70–116.00) | 80.28 ± 9.54 (62.33–102.33) | 84.63 ± 11.23 (58.67–115.70) |
| Parameter | Children with Idiopathic Nephrotic Syndrome (n = 30) | ||
|---|---|---|---|
| Whole Group | Female | Male | |
| C-reactive protein (mg/L) | 2.88 ± 7.13 (0.12–31.69) | 2.92 ± 6.60 (0.12–24.60) | 2.84 ± 7.78 (0.2–31.69) |
| Total serum protein (g/L) | 49.84 ± 9.15 (33.60–67.00) | 50.29 ± 8.02 (39.50–66.80) | 49.45 ± 10.28 (33.60–67.00) |
| Serum albumin (mg/mL) | 26.42 ± 9.21 (11.24–41.4) | 27.47 ± 9.39 (11.95–41.40) | 25.51 ± 9.26 (11.24–40.31) |
| Urea concentration (mmol/L) | 4.40 ± 1.90 (2.30–10.00) | 3.65 ± 1.27 (2.40–5.90) | 5.06 ± 2.14 (2.30–10.00) |
| Uric acid level (mg/dL) | 284.37 ± 83.49 (139.00–453.00) | 233.14 ± 61.33 (139.00–337.00) | 329.19 ± 75.01 (163.00–453.00) |
| Serum creatinine (μmol/L) | 34.10 ± 14.64 (14.00 ± 75.00) | 27.36 ± 9.59 (15.00–47.00) | 40.00 ± 15.97 (14.00–75.00) |
| Total cholesterol (mmol/L) | 7.97 ± 2.97 (2.81–14.33) | 7.44 ± 3.12 (2.81–14.33) | 8.44 ± 2.84 (5.11–14.15) |
| Triglyceride level (mmol/L) | 2.63 ± 1.88 (0.74–8.46) | 2.40 ± 2.28 (0.74–8.46) | 2.81 ± 1.54 (1.56–7.73) |
| Urine protein (g/L) | 4.45 ± 8.78 (0.67–35.60) | 6.04 ± 6.15 (0.88–23.70) | 10.28 ± 10.35 (0.67–35.60) |
| Parameter | Control group (n = 44) | Study group (n = 30) |
| Serum IL-15/serum creatinine | 0.08 ± 0.02 (0.04–0.13) | 0.30 ± 0.33 (0.05–1.88) * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badeński, A.; Badeńska, M.; Świętochowska, E.; Janek, A.; Gliwińska, A.; Morawiec-Knysak, A.; Szczepańska, M. Assessment of Interleukin-15 (IL-15) Concentration in Children with Idiopathic Nephrotic Syndrome. Int. J. Mol. Sci. 2023, 24, 6993. https://doi.org/10.3390/ijms24086993
Badeński A, Badeńska M, Świętochowska E, Janek A, Gliwińska A, Morawiec-Knysak A, Szczepańska M. Assessment of Interleukin-15 (IL-15) Concentration in Children with Idiopathic Nephrotic Syndrome. International Journal of Molecular Sciences. 2023; 24(8):6993. https://doi.org/10.3390/ijms24086993
Chicago/Turabian StyleBadeński, Andrzej, Marta Badeńska, Elżbieta Świętochowska, Artur Janek, Aleksandra Gliwińska, Aurelia Morawiec-Knysak, and Maria Szczepańska. 2023. "Assessment of Interleukin-15 (IL-15) Concentration in Children with Idiopathic Nephrotic Syndrome" International Journal of Molecular Sciences 24, no. 8: 6993. https://doi.org/10.3390/ijms24086993
APA StyleBadeński, A., Badeńska, M., Świętochowska, E., Janek, A., Gliwińska, A., Morawiec-Knysak, A., & Szczepańska, M. (2023). Assessment of Interleukin-15 (IL-15) Concentration in Children with Idiopathic Nephrotic Syndrome. International Journal of Molecular Sciences, 24(8), 6993. https://doi.org/10.3390/ijms24086993






