Transcriptome, Biochemical and Phenotypic Analysis of the Effects of a Precision Engineered Biostimulant for Inducing Salinity Stress Tolerance in Tomato
Abstract
1. Introduction
2. Results
2.1. Effect of Salinity and PSI-475 Rate on Tomato Phenotypic and Fruit Yield Parameters
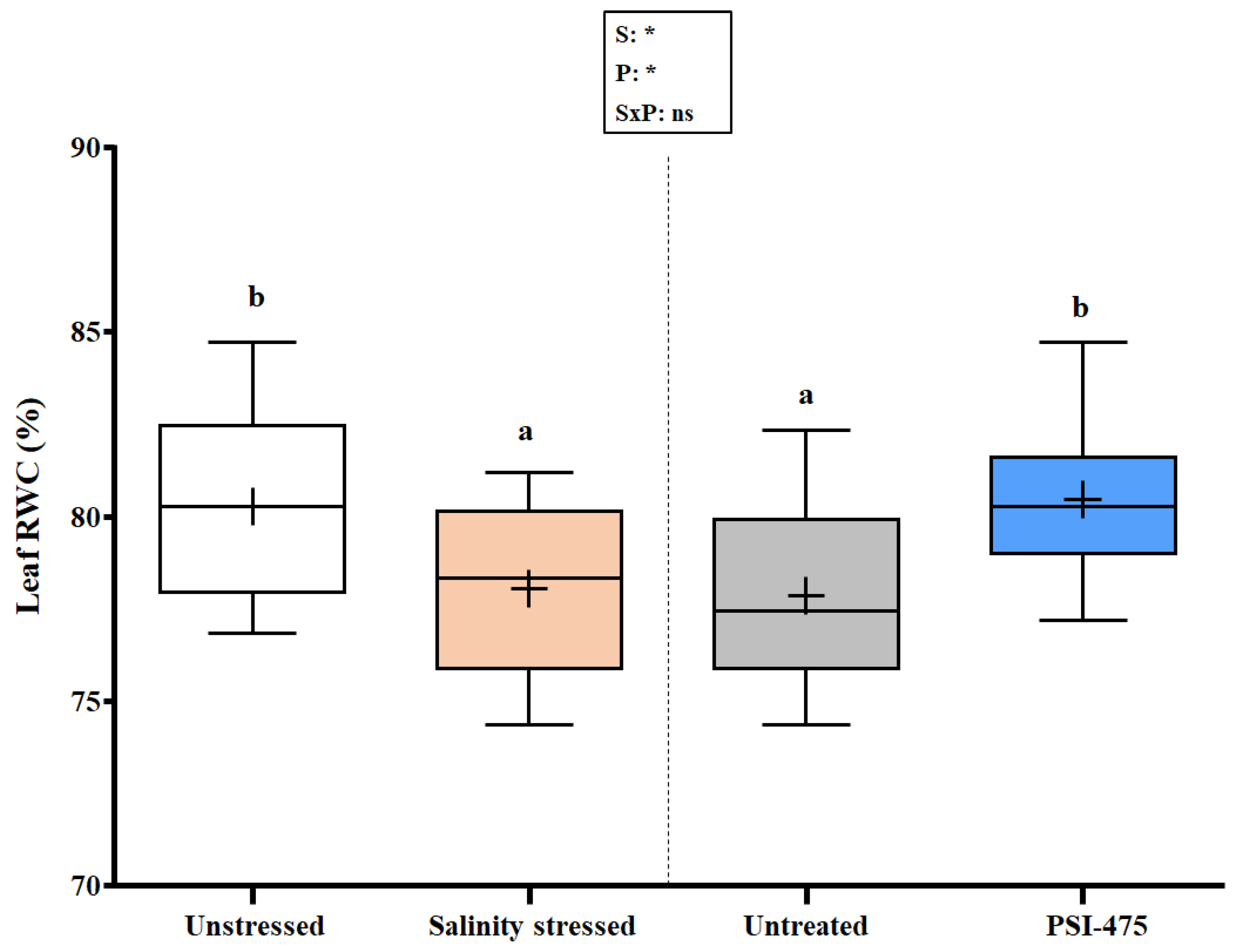
2.2. Effect of Salinity and PSI-475 on Tomato Leaf RWC
2.3. Effect of Salinity and PSI-475 on Sodium and Potassium Content in Different Tomato Tissues
2.4. Effect of Salinity and PSI-475 on Proline and Soluble Sugars Content in Different Tomato Tissues
2.5. Effect of Salinity and PSI-475 on Tomato Soluble Protein and Photosynthetic Pigments
2.6. RNA Sample Characterisation, RNA Sequencing and Mapping
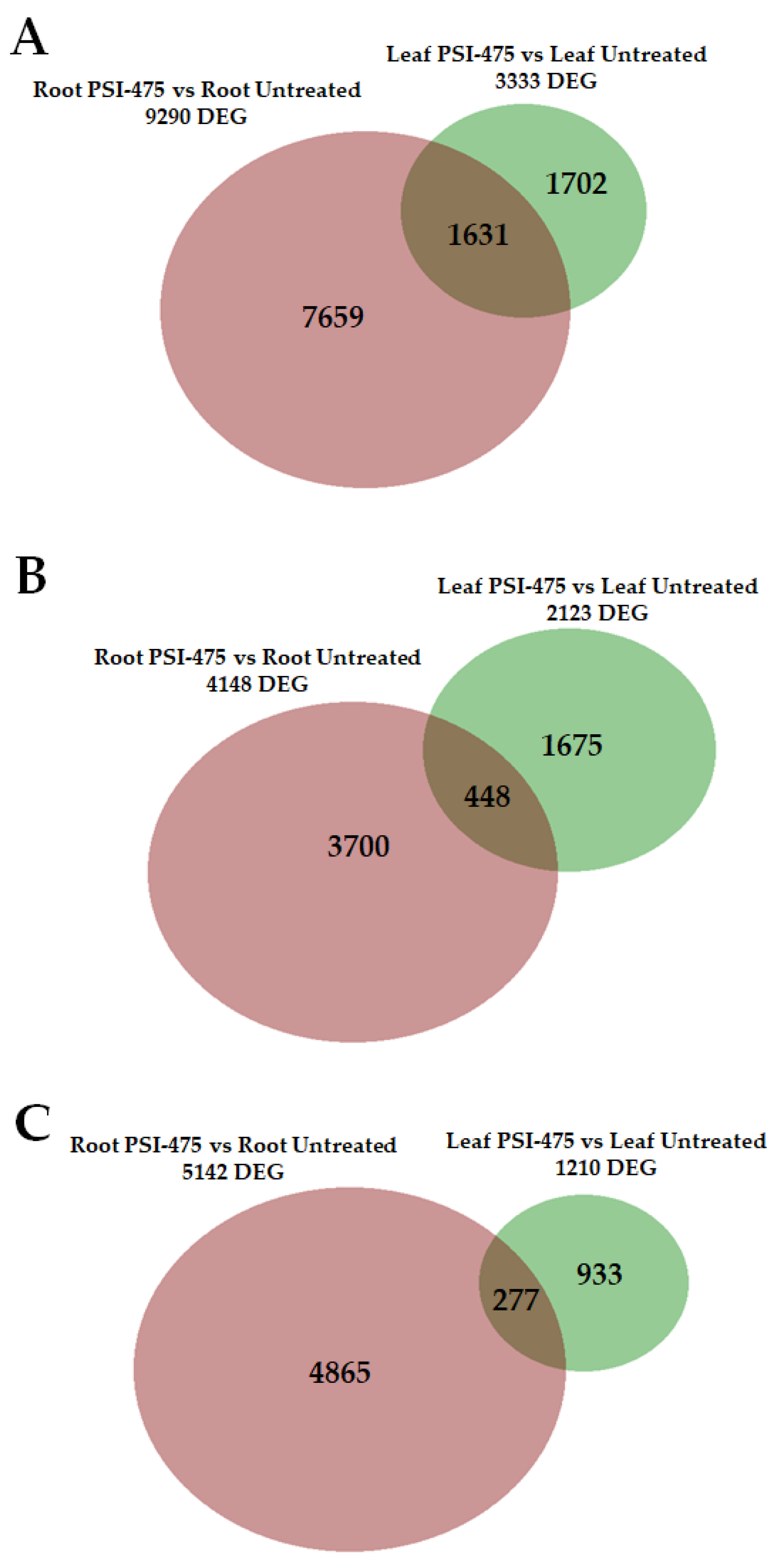
2.7. Differentially Expressed Genes (DEGs) in Young Leaf and Root Tomato Tissues
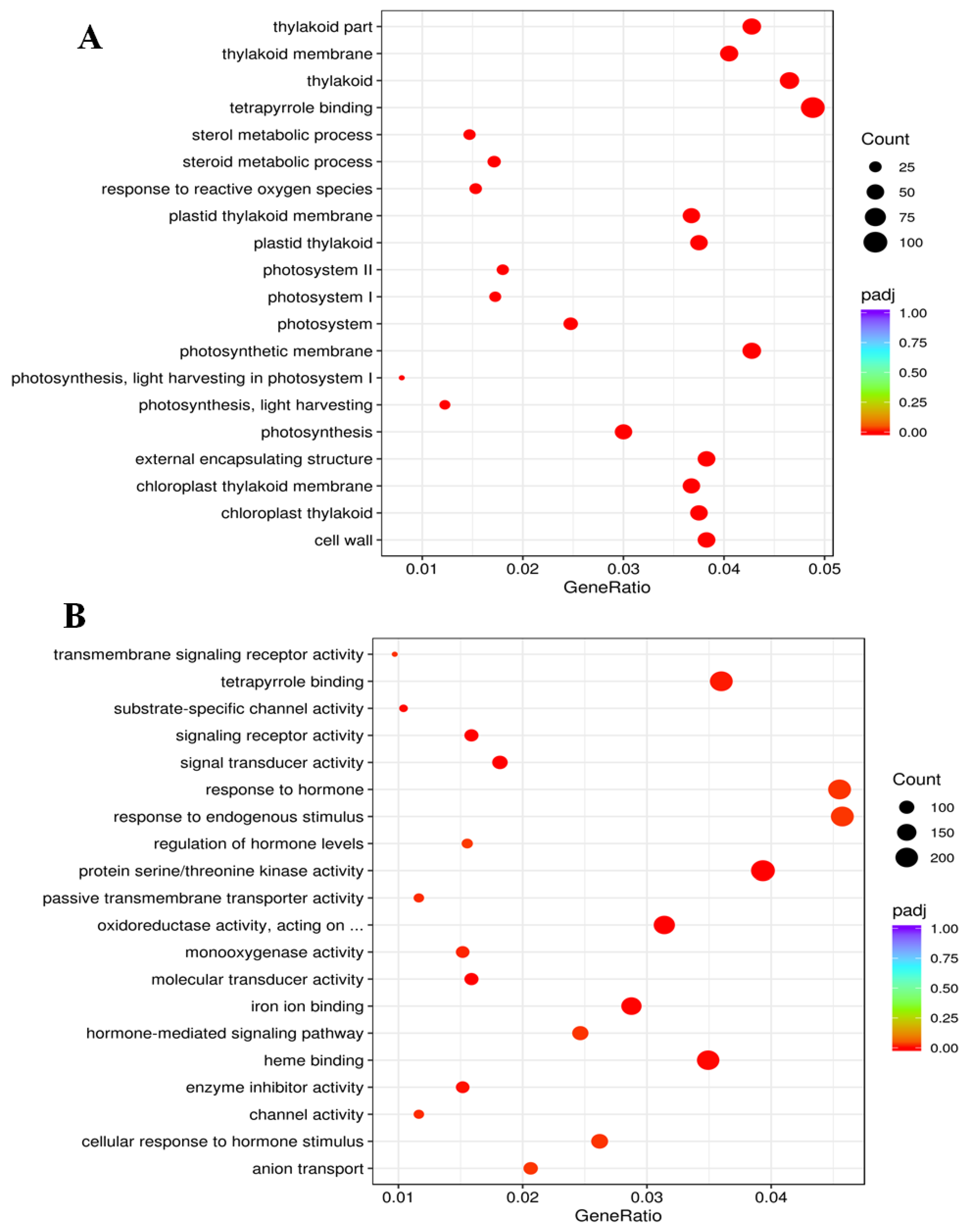
2.8. Functional Classification of Gene Expression Patterns
2.9. Modulation of Genes Related to Metabolic Pathways
2.10. Validation of RNA-Seq Data Using RT-qPCR
3. Discussion
3.1. Impact of PSI-475 on Phenotypic and Yield Related Markers (Dose)
3.2. Effect of PSI-475 and Salinity Stress on Ion Homeostasis Metabolic Pathways
3.3. Effect of PSI-475 and Salinity Stress on Osmotic Adjustment Metabolic Pathways
3.4. Effect of PSI-475 and Salinity Stress on Photosynthetic and N Metabolic Pathways
3.5. Effect of PSI-475 and Salinity Stress on Other Stress Response Related Pathways
3.6. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Salinity Stress Tolerance Experimental Design in Tomato
4.3. Phenotypic Evaluation of Tomato Plants and Fruit Yield Assessment
4.4. Tomato Leaf RWC
4.5. Biochemical Characterisation of the Collected Plant Tissues
4.6. RNA Extraction
4.7. RNA-Seq Analysis
4.8. Validation of RNA-Seq Analysis by qRT-PCR
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Machado, R.; Serralheiro, R. Soil salinity: Effect on vegetable crop growth. Management practices to prevent and mitigate soil salinization. Horticulturae 2017, 3, 30. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Van Zelm, E.; Zhang, Y.; Testerink, C. Salt tolerance mechanisms of plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Motos, J.; Ortuño, M.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.; Hernandez, J. Plant responses to salt stress: Adaptive mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef]
- Shahid, M.A.; Sarkhosh, A.; Khan, N.; Balal, R.M.; Ali, S.; Rossi, L.; Gómez, C.; Mattson, N.; Nasim, W.; Garcia-Sanchez, F. Insights into the physiological and biochemical impacts of salt stress on plant growth and development. Agronomy 2020, 10, 938. [Google Scholar] [CrossRef]
- Yaaran, A.; Moshelion, M. Role of aquaporins in a composite model of water transport in the leaf. Int. J. Mol. Sci. 2016, 17, 1045. [Google Scholar] [CrossRef] [PubMed]
- Shekoofa, A.; Sinclair, T.R. Aquaporin activity to improve crop drought tolerance. Cells 2018, 7, 123. [Google Scholar] [CrossRef] [PubMed]
- Isayenkov, S.; Maathuis, F.J.M. Plant Salinity Stress; Many Unanswered Questions Remain. Front. Plant Sci. 2019, 10, 80. [Google Scholar] [CrossRef]
- Raddatz, N.; Morales De Los Ríos, L.; Lindahl, A.M.; Quintero, F.J.; Pardo, J.M. Coordinated Transport of Nitrate, Potassium and Sodium. Front. Plant Sci. 2020, 11, 247. [Google Scholar] [CrossRef]
- Muñoz-Mayor, A.; Pineda, B.; Garcia-Abellán, J.O.; Antón, T.; Garcia-Sogo, B.; Sanchez-Bel, P.; Flores, F.B.; Atarés, A.; Angosto, T.; Pintor-Toro, J.A.; et al. Overexpression of dehydrin tas14 gene improves the osmotic stress imposed by drought and salinity in tomato. J. Plant Physiol. 2012, 169, 459–468. [Google Scholar] [CrossRef]
- Mishra, D.; Shekhar, S.; Singh, D.; Chakraborty, S.; Chakraborty, N. Heat shock proteins and abiotic stress tolerance in plants. In Regulation of Heat Shock Protein Responses; Asea, A., Kaur, P., Eds.; Springer: Cham, Switzerland, 2018; Volume 13, pp. 41–69. [Google Scholar] [CrossRef]
- Thalmann, M.; Pazmino, D.; Seung, D.; Horrer, D.; Nigro, A.; Meier, T.; Kölling, K.; Pfeifhofer, H.W.; Zeeman, S.C.; Santelia, D. Regulation of leaf starch degradation by abscisic acid is important for osmotic stress tolerance in plants. Plant Cell 2016, 28, 1860–1878. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Mao, C.; Shi, Z.; Kou, X. The amino acid metabolic and carbohydrate metabolic pathway play important roles during salt-stress response in tomato. Front. Plant Sci. 2017, 8, 1231. [Google Scholar] [CrossRef] [PubMed]
- Rady, M.M.; Kuşvuran, A.; Alharby, H.F.; Alzahrani, Y.; Kuşvuran, S. Pretreatment with proline or an organic bio-stimulant induces salt tolerance in wheat plants by improving antioxidant redox state and enzymatic activities and reducing the oxidative stress. J. Plant Growth Regul. 2019, 38, 449–462. [Google Scholar] [CrossRef]
- Wanichthanarak, K.; Boonchai, C.; Kojonna, T.; Chadchawan, S.; Sangwongchai, W.; Thitisaksakul, M. Deciphering rice metabolic flux reprograming under salinity stress via in silico metabolic modeling. Comput. Struct. Biotechnol. J. 2020, 18, 3555–3566. [Google Scholar] [CrossRef]
- Khan, S.A.; Li, M.Z.; Wang, S.M.; Yin, H.J. Revisiting the role of plant transcription factors in the battle against abiotic stress. Int. J. Mol. Sci. 2018, 19, 1634. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Duan, X.; Luo, L.; Dai, S.; Ding, Z.; Xia, G. How plant hormones mediate salt stress responses. Trends Plant Sci. 2020, 25, 1117–1130. [Google Scholar] [CrossRef] [PubMed]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in plant science: A global perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Biostimulants in agriculture. Front. Plant Sci. 2020, 11, 40. [Google Scholar] [CrossRef]
- Drobek, M.; Frąc, M.; Cybulska, J. Plant biostimulants: Importance of the quality and yield of horticultural crops and the improvement of plant tolerance to abiotic stress—A review. Agronomy 2019, 9, 335. [Google Scholar] [CrossRef]
- Deolu-Ajayi, A.O.; van der Meer, I.M.; Van der Werf, A.; Karlova, R. The power of seaweeds as plant biostimulants to boost crop production under abiotic stress. Plant Cell Environ. 2022, 45, 2537–2553. [Google Scholar] [CrossRef]
- Ertani, A.; Schiavon, M.; Nardi, S. Transcriptome-wide identification of differentially expressed genes in Solanum lycopersicon L. in response to an alfalfa-protein hydrolysate using microarrays. Front. Plant Sci. 2017, 8, 1159. [Google Scholar] [CrossRef] [PubMed]
- Santi, C.; Zamboni, A.; Varanini, Z.; Pandolfini, T. Growth stimulatory effects and genome-wide transcriptional changes produced by protein hydrolysates in maize seedlings. Front. Plant Sci. 2017, 8, 433. [Google Scholar] [CrossRef]
- Trevisan, S.; Manoli, A.; Ravazzolo, L.; Franceschi, C.; Quaggiotti, S. mRNA-sequencing analysis reveals transcriptional changes in root of maize seedlings treated with two increasing concentrations of a new biostimulant. J. Agric. Food Chem. 2017, 65, 9956–9969. [Google Scholar] [CrossRef] [PubMed]
- Jithesh, M.N.; Shukla, P.S.; Kant, P.; Joshi, J.; Critchley, A.T.; Prithiviraj, B. Physiological and transcriptomics analyses reveal that Ascophyllum nodosum extracts induce salinity tolerance in Arabidopsis by regulating the expression of stress responsive genes. J. Plant Growth Regul. 2019, 38, 463–478. [Google Scholar] [CrossRef]
- Ebinezer, L.B.; Franchin, C.; Trentin, A.R.; Carletti, P.; Trevisan, S.; Agrawal, G.K.; Rakwal, R.; Quaggiotti, S.; Arrigoni, G.; Masi, A. Quantitative Proteomics of Maize Roots Treated with a Protein Hydrolysate: A Comparative Study with Transcriptomics Highlights the Molecular Mechanisms Responsive to Biostimulants. J. Agric. Food Chem. 2020, 68, 7541–7553. [Google Scholar] [CrossRef]
- Ali, O.; Ramsubhag, A.; Benn, D., Jr.; Ramnarine, S.; Jayaraman, J. Transcriptomic changes induced by applications of a commercial extract of Ascophyllum nodosum on tomato plants. Sci. Rep. 2022, 12, 8042. [Google Scholar] [CrossRef] [PubMed]
- González-Morales, S.; Solís-Gaona, S.; Valdés-Caballero, M.V.; Juárez-Maldonado, A.; Loredo-Treviño, A.; Benavides-Mendoza, A. Transcriptomics of biostimulation of plants under abiotic stress. Front. Genet. 2021, 12, 583888. [Google Scholar] [CrossRef]
- Ikuyinminu, E.; Goñi, O.; O’Connell, S. Enhancing Irrigation Salinity Stress Tolerance and Increasing Yield in Tomato Using a Precision Engineered Protein Hydrolysate and Ascophyllum nodosum-Derived Biostimulant. Agronomy 2022, 12, 809. [Google Scholar] [CrossRef]
- Negrão, S.; Schmöckel, S.M.; Tester, M. Evaluating physiological responses of plants to salinity stress. Ann. Bot. 2017, 119, 1091–1096. [Google Scholar] [CrossRef]
- Munns, R.; Passioura, J.B.; Colmer, T.D.; Byrt, C.S. Osmotic adjustment and energy limitations to plant growth in saline soil. New Phytol. 2020, 225, 1091–1096. [Google Scholar] [CrossRef]
- Ertani, A.; Cavani, L.; Pizzeghello, D.; Brandellero, E.; Altissimo, A.; Ciavatta, C.; Nardi, S. Biostimulant activity of two protein hydrolyzates in the growth and nitrogen metabolism of maize seedlings. J. Plant Nutr. Soil Sci. 2009, 172, 237–244. [Google Scholar] [CrossRef]
- Trevisan, S.; Manoli, A.; Quaggiotti, S. A Novel Biostimulant, Belonging to Protein Hydrolysates, Mitigates Abiotic Stress Effects on Maize Seedlings Grown in Hydroponics. Agronomy 2019, 9, 28. [Google Scholar] [CrossRef]
- De Saeger, J.; Van Praet, S.; Vereecke, D.; Park, J.; Jacques, S.; Han, T.; Depuydt, S. Toward the molecular understanding of the action mechanism of Ascophyllum nodosum extracts on plants. J. Appl. Phycol. 2020, 32, 573–597. [Google Scholar] [CrossRef]
- Cerdán, M.; Sánchez-Sánchez, A.; Oliver, M.; Juárez, M.; Sánchez-Andreu, J.J. Effect of foliar and root applications of amino acids on iron uptake by tomato plants. In Proceedings of the IV Balkan Symposium on Vegetables and Potatoes, Plovdiv, Bulgaria, 17 June 2009; Volume 830, pp. 481–488. [Google Scholar] [CrossRef]
- Rouphael, Y.; Carillo, P.; Cristofano, F.; Cardarelli, M.; Colla, G. Effects of vegetal-versus animal-derived protein hydrolysate on sweet basil morpho-physiological and metabolic traits. Sci. Hortic. 2021, 284, 110123. [Google Scholar] [CrossRef]
- Maathuis, F.J.; Amtmann, A.N.N.A. K+ nutrition and Na+ toxicity: The basis of cellular K+/Na+ ratios. Ann. Bot. 1999, 84, 123–133. [Google Scholar] [CrossRef]
- Shabala, S.; Cuin, T.A. Potassium transport and plant salt tolerance. Physiol. Plant. 2008, 133, 651–669. [Google Scholar] [CrossRef]
- Møller, I.S.; Gilliham, M.; Jha, D.; Mayo, G.M.; Roy, S.J.; Coates, J.C.; Haseloff, J.; Tester, M. Shoot Na+ exclusion and increased salinity tolerance engineered by cell type–specific alteration of Na+ transport in Arabidopsis. Plant Cell 2009, 21, 2163–2178. [Google Scholar] [CrossRef]
- Cuartero, J.; Fernández-Muñoz, R. Tomato and salinity. Sci. Hortic. 1998, 78, 83–125. [Google Scholar] [CrossRef]
- Picchioni, G.A.; Miyamoto, S.; Storey, J.B. Salt effects on growth and ion uptake of pistachio rootstock seedlings. J. Am. Soc. Hortic. Sci. 1990, 115, 647–653. [Google Scholar] [CrossRef]
- Gucci, R.; Tattini, M. Salinity tolerance in olive. In Horticultural Reviews; Janick, J., Ed.; John Wiley & Sons, Inc.: Oxford, UK, 2020; Volume 21, pp. 177–214. [Google Scholar]
- Yasar, F.; Uzal, O.; Tufenkci, S.; Yildiz, K. Ion accumulation in different organs of green bean genotypes grown under salt stress. Plant Soil Environ. 2006, 52, 476. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, M.; Guo, R.; Shi, D.; Liu, B.; Lin, X.; Yang, C. Effects of salt stress on ion balance and nitrogen metabolism of old and young leaves in rice (Oryza sativa L.). BMC Plant Biol. 2012, 12, 194. [Google Scholar] [CrossRef] [PubMed]
- Horie, T.; Hauser, F.; Schroeder, J.I. HKT transporter-mediated salinity resistance mechanisms in Arabidopsis and monocot crop plants. Trends Plant Sci. 2009, 14, 660–668. [Google Scholar] [CrossRef] [PubMed]
- Jaime-Pérez, N.; Pineda, B.; García-Sogo, B.; Atares, A.; Athman, A.; Byrt, C.S.; Olías, R.; Asins, M.J.; Gilliham, M.; Moreno, V.; et al. The sodium transporter encoded by the HKT1; 2 gene modulates sodium/potassium homeostasis in tomato shoots under salinity. Plant Cell Environ. 2017, 40, 658–671. [Google Scholar] [CrossRef] [PubMed]
- Almeida, P.; de Boer, G.J.; de Boer, A.H. Differences in shoot Na+ accumulation between two tomato species are due to differences in ion affinity of HKT1; 2. J. Plant Physiol. 2014, 171, 438–447. [Google Scholar] [CrossRef]
- Ali, A.; Khan, I.U.; Jan, M.; Khan, H.A.; Hussain, S.; Nisar, M.; Chung, W.S.; Yun, D.J. The high-affinity potassium transporter EpHKT1; 2 from the extremophile Eutrema parvula mediates salt tolerance. Front. Plant Sci. 2018, 9, 1108. [Google Scholar] [CrossRef]
- Maach, M.; Baghour, M.; Akodad, M.; Gálvez, F.J.; Sánchez, M.E.; Aranda, M.N.; Venema, K.; Rodríguez-Rosales, M.P. Overexpression of LeNHX4 improved yield, fruit quality and salt tolerance in tomato plants (Solanum lycopersicum L.). Mol. Biol. Rep. 2020, 47, 4145–4153. [Google Scholar] [CrossRef]
- Adams, E.; Shin, R. Transport, signaling, and homeostasis of potassium and sodium in plants. J. Integr. Plant Biol. 2014, 56, 231–249. [Google Scholar] [CrossRef]
- Gobert, A.; Isayenkov, S.; Voelker, C.; Czempinski, K.; Maathuis, F.J. The two-pore channel TPK1 gene encodes the vacuolar K+ conductance and plays a role in K+ homeostasis. Proc. Natl. Acad. Sci. USA 2007, 104, 10726–10731. [Google Scholar] [CrossRef]
- Sze, H.; Chanroj, S. Plant endomembrane dynamics: Studies of K+/H+ antiporters provide insights on the effects of pH and ion homeostasis. Plant Physiol. 2018, 177, 875–895. [Google Scholar] [CrossRef]
- Padmanaban, S.; Chanroj, S.; Kwak, J.M.; Li, X.; Ward, J.M.; Sze, H. Participation of endomembrane cation/H+ exchanger AtCHX20 in osmoregulation of guard cells. Plant Physiol. 2007, 144, 82–93. [Google Scholar] [CrossRef]
- Giuliani, M.M.; Nardella, E.; Gagliardi, A.; Gatta, G. Deficit irrigation and partial root-zone drying techniques in processing tomato cultivated under Mediterranean climate conditions. Sustainability 2017, 9, 2197. [Google Scholar] [CrossRef]
- Li, R.; Wang, J.; Li, S.; Zhang, L.; Qi, C.; Weeda, S.; Zhao, B.; Ren, S.; Guo, Y.D. Plasma membrane intrinsic proteins SlPIP2; 1, SlPIP2; 7 and SlPIP2; 5 conferring enhanced drought stress tolerance in tomato. Sci. Rep. 2016, 6, 31814. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.P.; Neves, D.M.; Cidade, L.C.; Mendes, A.F.; Silva, D.C.; Almeida, A.A.F.; Coelho-Filho, M.A.; Gesteira, A.S.; Soares-Filho, W.S.; Costa, M.G. Expression of the citrus CsTIP2; 1 gene improves tobacco plant growth, antioxidant capacity and physiological adaptation under stress conditions. Planta 2017, 245, 951–963. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, L.; Natarajan, S.K.; Becker, D.F. Proline mechanisms of stress survival. Antioxid. Redox Signal. 2013, 19, 998–1011. [Google Scholar] [CrossRef] [PubMed]
- El Moukhtari, A.; Cabassa-Hourton, C.; Farissi, M.; Savouré, A. How does proline treatment promote salt stress tolerance during crop plant development? Front. Plant Sci. 2020, 11, 1127. [Google Scholar] [CrossRef] [PubMed]
- Lucini, L.; Rouphael, Y.; Cardarelli, M.; Canaguier, R.; Kumar, P.; Colla, G. The effect of a plant-derived biostimulant on metabolic profiling and crop performance of lettuce grown under saline conditions. Sci. Hortic. 2015, 182, 124–133. [Google Scholar] [CrossRef]
- Rouphael, Y.; Cardarelli, M.; Bonini, P.; Colla, G. Synergistic action of a microbial-based biostimulant and a plant derived-protein hydrolysate enhances lettuce tolerance to alkalinity and salinity. Front. Plant Sci. 2017, 8, 131. [Google Scholar] [CrossRef] [PubMed]
- Sitohy, M.Z.; Desoky, E.S.M.; Osman, A.; Rady, M.M. Pumpkin seed protein hydrolysate treatment alleviates salt stress effects on Phaseolus vulgaris by elevating antioxidant capacity and recovering ion homeostasis. Sci. Hortic. 2020, 271, 109495. [Google Scholar] [CrossRef]
- Di Stasio, E.; Van Oosten, M.J.; Silletti, S.; Raimondi, G.; dell’Aversana, E.; Carillo, P.; Maggio, A. Ascophyllum nodosum-based algal extracts act as enhancers of growth, fruit quality, and adaptation to stress in salinized tomato plants. J. Appl. Phycol. 2018, 30, 2675–2686. [Google Scholar] [CrossRef]
- Dell’Aversana, E.; Cirillo, V.; Van Oosten, M.J.; Di Stasio, E.; Saiano, K.; Woodrow, P.; Ciarmiello, L.F.; Maggio, A.; Carillo, P. Ascophyllum nodosum based extracts counteract salinity stress in tomato by remodeling leaf nitrogen metabolism. Plants 2021, 10, 1044. [Google Scholar] [CrossRef]
- Deuschle, K.; Funck, D.; Hellmann, H.; Däschner, K.; Binder, S.; Frommer, W.B. A nuclear gene encoding mitochondrial Δ1-pyrroline-5-carboxylate dehydrogenase and its potential role in protection from proline toxicity. Plant J. 2001, 27, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.L.; Zhao, L.; Wu, C.W.; Shen, B.; Zhu, A.Y. Exogenous proline reduces NaCl-induced damage by mediating ionic and osmotic adjustment and enhancing antioxidant defense in Eurya emarginata. Acta Physiol. Plant. 2015, 37, 181. [Google Scholar] [CrossRef]
- Koch, K. Sucrose metabolism: Regulatory mechanisms and pivotal roles in sugar sensing and plant development. Curr. Opin. Plant Biol. 2004, 7, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Galtier, N.; Foyer, C.H.; Huber, J.; Voelker, T.A.; Huber, S.C. Effects of elevated sucrose-phosphate synthase activity on photosynthesis, assimilate partitioning, and growth in tomato (Lycopersicon esculentum var UC82B). Plant Physiol. 1993, 101, 535–543. [Google Scholar] [CrossRef]
- Taffouo, V.D.; Wamba, O.F.; Youmbi, E.; Nono, G.V.; Akoa, A. Growth, yield, water status and ionic distribution response of three bambara groundnut (Vigna subterranea (L.) Verdc.) landraces grown under saline conditions. Int. J. Bot. 2010, 6, 53–58. [Google Scholar] [CrossRef]
- Sayyad-Amin, P.; Jahansooz, M.R.; Borzouei, A.; Ajili, F. Changes in photosynthetic pigments and chlorophyll-a fluorescence attributes of sweet-forage and grain sorghum cultivars under salt stress. J. Biol. Phys. 2016, 42, 601–620. [Google Scholar] [CrossRef]
- Yang, Z.; Li, J.L.; Liu, L.N.; Xie, Q.; Sui, N. Photosynthetic regulation under salt stress and salt-tolerance mechanism of sweet sorghum. Front. Plant Sci. 2020, 10, 1722. [Google Scholar] [CrossRef]
- Al-Ghamdi, A.A.; Elansary, H.O. Synergetic effects of 5-aminolevulinic acid and Ascophyllum nodosum seaweed extracts on Asparagus phenolics and stress related genes under saline irrigation. Plant Physiol. Biochem. 2018, 129, 273–284. [Google Scholar] [CrossRef]
- Gabruk, M.; Mysliwa-Kurdziel, B. The origin, evolution and diversification of multiple isoforms of light-dependent protochlorophyllide oxidoreductase (LPOR): Focus on angiosperms. Biochem. J. 2020, 477, 2221–2236. [Google Scholar] [CrossRef]
- Tanaka, R.; Oster, U.; Kruse, E.; Rüdiger, W.; Grimm, B. Reduced activity of geranylgeranyl reductase leads to loss of chlorophyll and tocopherol and to partially geranylgeranylated chlorophyll in transgenic tobacco plants expressing antisense RNA for geranylgeranyl reductase. Plant Physiol. 1999, 120, 695–704. [Google Scholar] [CrossRef]
- Wang, P.; Richter, A.S.; Kleeberg, J.R.; Geimer, S.; Grimm, B. Post-translational coordination of chlorophyll biosynthesis and breakdown by BCMs maintains chlorophyll homeostasis during leaf development. Nat. Commun. 2020, 11, 1254. [Google Scholar] [CrossRef] [PubMed]
- Ertani, A.; Francioso, O.; Tugnoli, V.; Righi, V.; Nardi, S. Effect of commercial lignosulfonate-humate on Zea mays L. metabolism. J. Agric. Food Chem. 2011, 59, 11940–11948. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Ali, M.; Khattak, A.M.; Gai, W.X.; Zhang, H.X.; Wei, A.M.; Gong, Z.H. Heat shock proteins: Dynamic biomolecules to counter plant biotic and abiotic stresses. Int. J. Mol. Sci. 2019, 20, 5321. [Google Scholar] [CrossRef]
- Carmody, N.; Goñi, O.; Łangowski, Ł.; O’Connell, S. Ascophyllum nodosum extract biostimulant processing and its impact on enhancing heat stress tolerance during tomato fruit set. Front. Plant Sci. 2020, 11, 807. [Google Scholar] [CrossRef]
- Goñi, O.; Quille, P.; O’Connell, S. Ascophyllum nodosum extract biostimulants and their role in enhancing tolerance to drought stress in tomato plants. Plant Physiol. Biochem. 2018, 126, 63–73. [Google Scholar] [CrossRef]
- Imai, R.; Chang, L.; Ohta, A.; Bray, E.A.; Takagi, M. A lea-class gene of tomato confers salt and freezing tolerance when expressed in Saccharomyces cerevisiae. Gene 1996, 170, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Wallace, P.A.; Teakle, N.L.; Colmer, T.D. Measuring soluble ion concentrations (Na+, K+, Cl−) in salt-treated plants. In Plant Stress Tolerance; Humana Press: Totowa, NJ, USA, 2010; pp. 371–382. [Google Scholar] [CrossRef]
- Carillo, P.; Gibon, Y. PrometheusWiki Contributors (2011) Extraction and Determination of Proline. Available online: http://prometheuswiki.publish.csiro.au/tikiindex.php?page=Extraction+and+determination+of+proline (accessed on 20 March 2023).
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Expósito-Rodríguez, M.; Borges, A.A.; Borges-Pérez, A.; Pérez, J.A. Selection of internal control genes for quantitative real-time RT-PCR studies during tomato development process. BMC Plant Biol. 2008, 8, 131. [Google Scholar] [CrossRef]
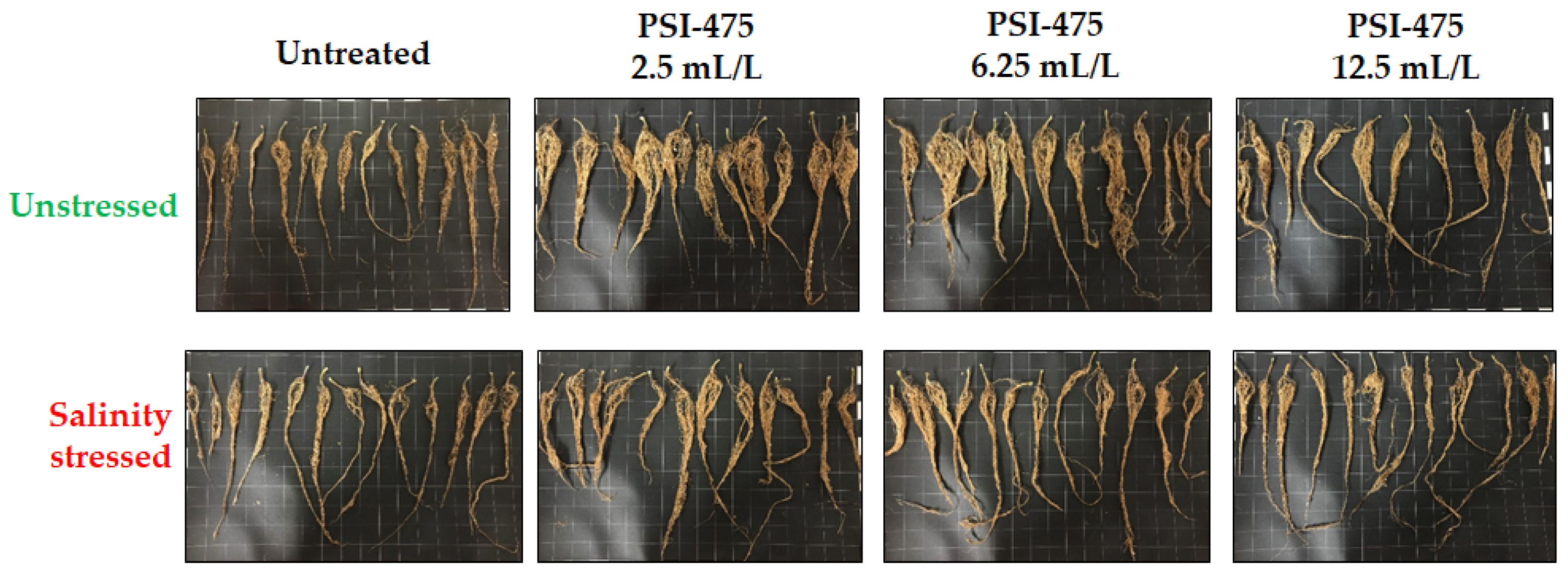

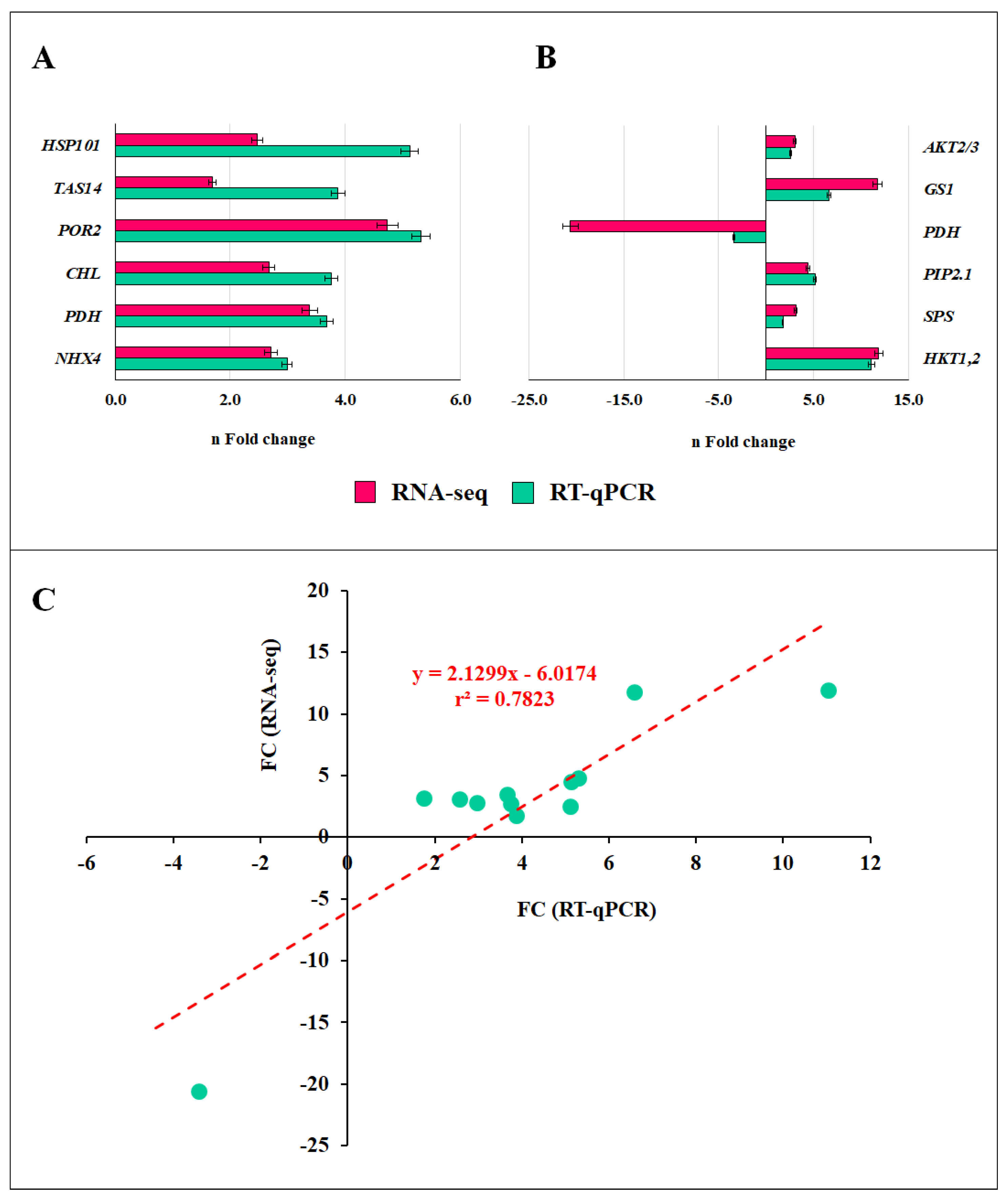
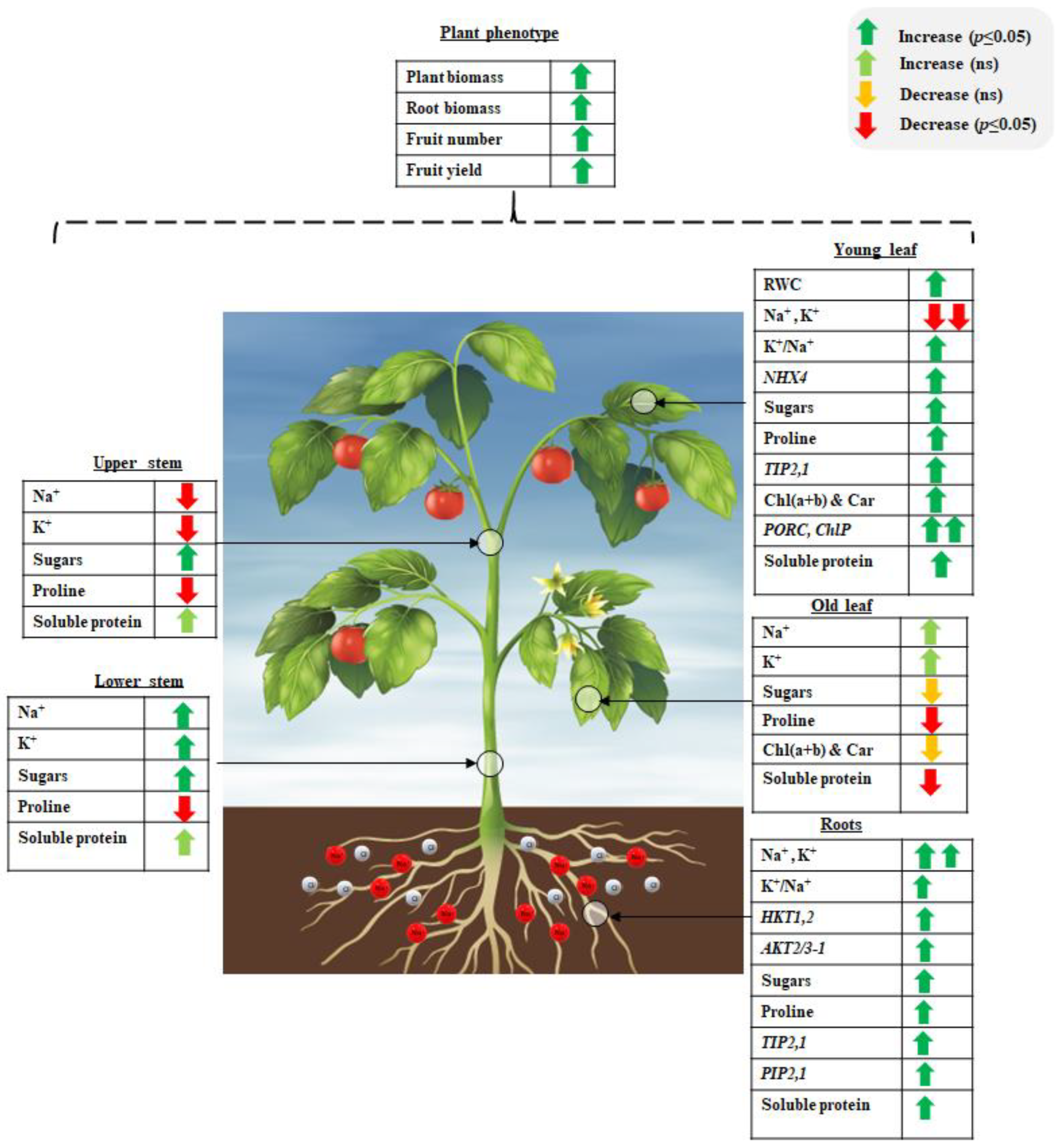
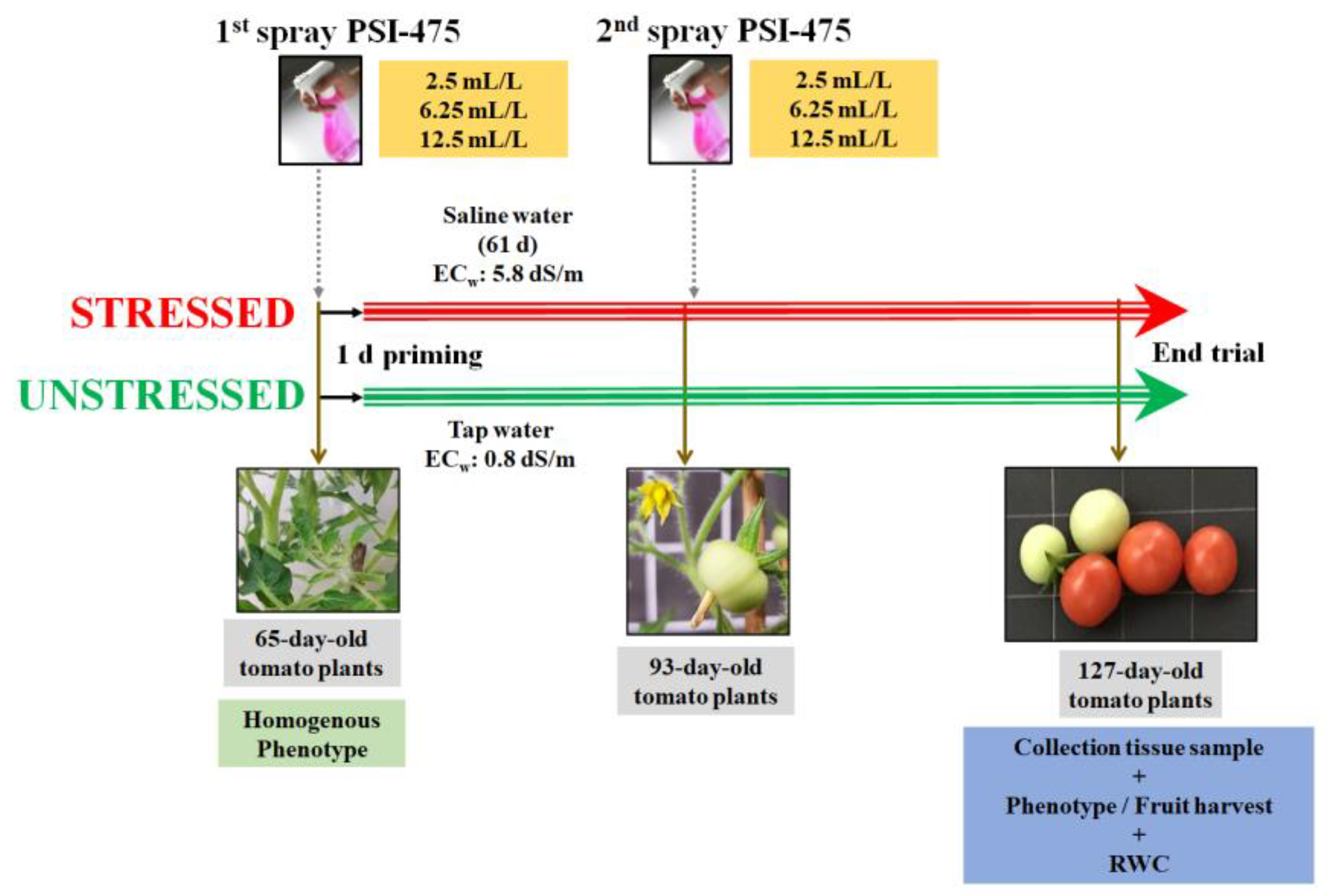
| Source of Variance | Plant Biomass (g) | Root Biomass (mg) | Fruit Number | Yield (g) |
|---|---|---|---|---|
| Salinity (S) | ||||
| Unstressed | 18.02 b | 127.88 b | 9.07 b | 12.86 b |
| Salinity stressed | 13.81 a | 108.71 a | 7.89 a | 9.47 b |
| PSI-475 (P) | ||||
| Untreated | 15.05 a | 74.57 a | 7.31 a | 8.45 a |
| PSI-475 2.5 mL/L | 17.00 b | 146.59 b | 9.69 b | 12.47 b |
| PSI-475 6.25 mL/L | 16.96 b | 136.50 b | 8.57 b | 12.35 b |
| PSI-475 12.5 mL/L | 14.64 ab | 115.52 b | 8.36 ab | 11.43 ab |
| S × P | ||||
| Unstressed × Untreated | 17.52 | 76.02 | 8.00 | 9.47 |
| Unstressed × PSI-475 2.5 | 19.63 | 171.92 | 10.24 | 13.91 |
| Unstressed × PSI-475 6.25 | 18.26 | 143.59 | 8.57 | 14.67 |
| Unstressed × PSI-475 12.5 | 16.66 | 119.99 | 9.48 | 13.41 |
| Salinity stressed × Untreated | 12.59 | 73.11 | 6.62 | 7.43 |
| Salinity stressed × PSI-475 2.5 | 14.37 | 121.27 | 9.14 | 11.04 |
| Salinity stressed × PSI-475 6.25 | 15.66 | 129.4 | 8.57 | 10.03 |
| Salinity stressed × PSI-475 12.5 | 12.62 | 111.05 | 7.24 | 9.45 |
| Statistical significance | ||||
| Salinity (S) | *** | * | * | *** |
| PSI-475 (P) | * | *** | * | * |
| S × P | ns | ns | ns | ns |
| Source of Variance | Root (mg/g DW) | Lower Stem (mg/g DW) | Old Leaf (mg/g DW) | Upper Stem (mg/g DW) | Young Leaf (mg/g DW) |
|---|---|---|---|---|---|
| Salinity (S) | |||||
| Unstressed | 31.21 a | 9.90 a | 3.46 a | 10.16 a | 1.43 a |
| Salinity stressed | 73.11 b | 91.78 b | 66.19 b | 90.09 b | 70.26 b |
| PSI-475 (P) | |||||
| Untreated | 49.41 | 45.93 a | 34.30 | 55.11 b | 41.46 b |
| PSI-475 | 59.92 | 55.75 b | 35.35 | 45.14 a | 30.23 a |
| S × P | |||||
| Unstressed × Untreated | 35.73 a | 10.55 a | 3.29 | 10.67 a | 2.05 a |
| Unstressed × PSI-475 | 26.70 a | 9.25 a | 3.64 | 9.65 a | 0.80 a |
| Salinity stressed × Untreated | 63.10 b | 81.31 b | 65.32 | 99.55 c | 80.87 c |
| Salinity stressed × PSI-475 | 83.13 c | 102.25 c | 67.07 | 80.63 b | 59.65 b |
| Statistical significance | |||||
| Salinity (S) | *** | *** | *** | *** | *** |
| PSI-475 (P) | ns | ** | ns | *** | *** |
| S × P | *** | *** | ns | *** | *** |
| Source of Variance | Root (mg/g DW) | Lower Stem (mg/g DW) | Old Leaf (mg/g DW) | Upper Stem (mg/g DW) | Young Leaf (mg/g DW) |
|---|---|---|---|---|---|
| Salinity (S) | |||||
| Unstressed | 48.01 b | 290.23 b | 134.12 b | 240.02 | 101.66 b |
| Salinity stressed | 37.35 a | 259.41 a | 115.78 a | 199.26 | 91.70 a |
| PSI-475 (P) | |||||
| Untreated | 27.78 a | 280.05 | 124.17 | 231.47 b | 101.45 b |
| PSI-475 | 57.58 b | 269.59 | 125.73 | 207.81 a | 91.91 a |
| S × P | |||||
| Unstressed × Untreated | 28.79 a | 314.12 c | 136.02 | 242.24 b | 104.84 |
| Unstressed × PSI-475 | 62.24 c | 266.35 ab | 132.23 | 237.79 b | 98.48 |
| Salinity stressed × Untreated | 26.77 a | 245.98 a | 112.32 | 220.70 b | 98.06 |
| Salinity stressed × PSI-475 | 47.92 b | 272.84 b | 119.23 | 117.83 a | 85.34 |
| Statistical significance | |||||
| Salinity (S) | *** | *** | *** | *** | *** |
| PSI-475 (P) | *** | ns | ns | *** | *** |
| S × P | *** | *** | ns | ** | ns |
| Source of Variance | Root (mg/g DW) | Lower Stem (mg/g DW) | Old Leaf (mg/g DW) | Upper Stem (mg/g DW) | Young Leaf (mg/g DW) |
|---|---|---|---|---|---|
| Salinity (S) | |||||
| Unstressed | 1.12 a | 1.07 a | 1.43 a | 0.67 a | 1.01 a |
| Salinity stressed | 2.24 b | 6.05 b | 12.72 b | 3.76 b | 13.21 b |
| PSI-475 (P) | |||||
| Untreated | 1.58 a | 3.84 b | 7.51 b | 2.59 b | 6.86 a |
| PSI-475 | 1.78 b | 3.29 a | 6.64 a | 1.83 a | 7.36 b |
| S × P | |||||
| Unstressed × Untreated | 1.05 | 1.05 a | 1.55 a | 0.72 a | 1.05 a |
| Unstressed × PSI-475 | 1.19 | 1.10 a | 1.31 a | 0.61 a | 0.97 a |
| Salinity stressed × Untreated | 2.11 | 6.63 c | 13.48 c | 4.46 c | 12.67 b |
| Salinity stressed × PSI-475 | 2.37 | 5.48 b | 11.96 b | 3.06 b | 13.75 c |
| Statistical significance | |||||
| Salinity (S) | *** | *** | *** | *** | *** |
| PSI-475 (P) | *** | *** | *** | *** | *** |
| S × P | ns | *** | *** | *** | *** |
| Source of Variance | Root (mg/g DW) | Lower Stem (mg/g DW) | Old Leaf (mg/g DW) | Upper Stem (mg/g DW) | Young Leaf (mg/g DW) |
|---|---|---|---|---|---|
| Salinity (S) | |||||
| Unstressed | 1.95 a | 3.07 | 1.30 a | 3.22 | 1.12 a |
| Salinity stressed | 3.47 b | 2.65 | 2.27 b | 3.23 | 2.45 b |
| PSI-475 (P) | |||||
| Untreated | 1.87 a | 2.16 a | 1.91 a | 2.93 a | 1.56 a |
| PSI-475 | 3.55 b | 3.56 b | 1.66 a | 3.51 b | 2.01 b |
| S × P | |||||
| Unstressed × Untreated | 1.39 a | 2.42 | 1.33 | 2.93 | 0.99 |
| Unstressed × PSI-475 | 2.52 b | 3.72 | 1.28 | 3.50 | 1.25 |
| Salinity stressed × Untreated | 2.35 b | 1.91 | 2.50 | 2.94 | 2.12 |
| Salinity stressed × PSI-475 | 4.59 c | 3.40 | 2.04 | 3.52 | 2.77 |
| Statistical significance | |||||
| Salinity (S) | *** | ns | ** | ns | *** |
| PSI-475 (P) | *** | *** | * | ** | ** |
| S × P | *** | ns | ns | ns | ns |
| Source of Variance | Old Leaf Chl (a + b) (mg/g DW) | Old Leaf Carotenoids (mg/g DW) | Young Leaf Chl (a + b) (mg/g DW) | Young Leaf Carotenoids (mg/g DW) |
|---|---|---|---|---|
| Salinity (S) | ||||
| Unstressed | 10.16 b | 1.75 b | 13.27 b | 2.31 b |
| Salinity stressed | 6.48 a | 1.19 a | 10.85 a | 1.94 a |
| PSI-475 (P) | ||||
| Untreated | 8.55 b | 1.46 | 11.73 | 2.07 |
| PSI-475 | 8.09 a | 1.48 | 12.39 | 2.18 |
| S × P | ||||
| Unstressed × Untreated | 10.50 | 1.76 | 13.91 c | 2.37 b |
| Unstressed × PSI-475 | 9.83 | 1.74 | 12.63 b | 2.26 b |
| Salinity stressed × Untreated | 6.60 | 1.16 | 9.54 a | 1.76 a |
| Salinity stressed × PSI-475 | 6.36 | 1.22 | 12.16 b | 2.11 b |
| Statistical significance | ||||
| Salinity (S) | *** | *** | *** | *** |
| PSI-475 (P) | * | ns | ns | ns |
| S × P | ns | ns | *** | ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikuyinminu, E.; Goñi, O.; Łangowski, Ł.; O’Connell, S. Transcriptome, Biochemical and Phenotypic Analysis of the Effects of a Precision Engineered Biostimulant for Inducing Salinity Stress Tolerance in Tomato. Int. J. Mol. Sci. 2023, 24, 6988. https://doi.org/10.3390/ijms24086988
Ikuyinminu E, Goñi O, Łangowski Ł, O’Connell S. Transcriptome, Biochemical and Phenotypic Analysis of the Effects of a Precision Engineered Biostimulant for Inducing Salinity Stress Tolerance in Tomato. International Journal of Molecular Sciences. 2023; 24(8):6988. https://doi.org/10.3390/ijms24086988
Chicago/Turabian StyleIkuyinminu, Elomofe, Oscar Goñi, Łukasz Łangowski, and Shane O’Connell. 2023. "Transcriptome, Biochemical and Phenotypic Analysis of the Effects of a Precision Engineered Biostimulant for Inducing Salinity Stress Tolerance in Tomato" International Journal of Molecular Sciences 24, no. 8: 6988. https://doi.org/10.3390/ijms24086988
APA StyleIkuyinminu, E., Goñi, O., Łangowski, Ł., & O’Connell, S. (2023). Transcriptome, Biochemical and Phenotypic Analysis of the Effects of a Precision Engineered Biostimulant for Inducing Salinity Stress Tolerance in Tomato. International Journal of Molecular Sciences, 24(8), 6988. https://doi.org/10.3390/ijms24086988







