Abstract
Terpenes, especially volatile terpenes, are important components of tea aroma due to their unique scents. They are also widely used in the cosmetic and medical industries. In addition, terpene emission can be induced by herbivory, wounding, light, low temperature, and other stress conditions, leading to plant defense responses and plant–plant interactions. The transcriptional levels of important core genes (including HMGR, DXS, and TPS) involved in terpenoid biosynthesis are up- or downregulated by the MYB, MYC, NAC, ERF, WRKY, and bHLH transcription factors. These regulators can bind to corresponding cis-elements in the promoter regions of the corresponding genes, and some of them interact with other transcription factors to form a complex. Recently, several key terpene synthesis genes and important transcription factors involved in terpene biosynthesis have been isolated and functionally identified from tea plants. In this work, we focus on the research progress on the transcriptional regulation of terpenes in tea plants (Camellia sinensis) and thoroughly detail the biosynthesis of terpene compounds, the terpene biosynthesis-related genes, the transcription factors involved in terpene biosynthesis, and their importance. Furthermore, we review the potential strategies used in studying the specific transcriptional regulation functions of candidate transcription factors that have been discriminated to date.
1. Introduction
Terpenoids are the most abundant secondary metabolites of plants with diverse structures and aromatic scents [1,2,3]. The chemical structures of terpenoids are extremely variable yet share the common feature of biosynthesis, and the number of five-carbon isoprene units in the skeleton structure is the key for the classification of terpenoids [4,5,6,7]. Isoprene (C5), monoterpenes (C10), sesquiterpenes (C15), diterpenoids (C20), sesterterpenes (C25), triterpenes (C30), and tetraterpenes (C40) are important terpenoid substances [8,9,10]. It is well known that terpenoids play an important role in plant life through direct and indirect plant defense, pollinator attraction, and different interactions between plants and their environment [11,12,13,14]. Monoterpenes (linalool, limonene, myrcene, and trans-β-ocimene) and some sesquiterpenes (farnesene, nerolidol, and caryophyllene) are common constituents of floral scents [4,15,16,17]. In addition, these terpenoid substances might serve as important signals mediating interactions between plants and herbivores, and they have potential applications in the pharmaceutical, food, and cosmetic industries [6,18,19,20,21].
The mevalonic acid (MVA) and methylerythritol phosphate (MEP) pathways are two independent, compartmentally separated pathways for terpenoid formation [22,23,24,25]. The MEP pathway is mainly responsible for the biosynthesis of mono- and diterpenes, while the MVA pathway mainly produces sesquiterpenes, sterols, and triterpenes in plants [1,26]. Linear prenyl diphosphates are the basic building blocks for C5 and can be produced from the condensation of IPP and DMAPP by prenyltransferases [1]. The precursor of monoterpenes, geranyl pyrophosphate (GPP), is formed from one DMAPP and one IPP molecule by GPP synthase (GPPS) in plastids [27]. Moreover, the precursor of sesquiterpenes, farnesyl diphosphate (FPP), is synthesized from two IPP molecules and one DMAPP molecule by farnesyl diphosphate (FPP) synthase in the cytosol [28]. The tremendous diversity of volatile terpenoids in plants is mainly ascribed to the ample catalytic versatility of terpene synthases (TPSs), many of which have the distinctive ability to synthesize multiple products from prenyl diphosphate precursors, GPP, FPP, and geranylgeranyl diphosphate (GGPP) [29,30]. Since the isolation and purification of S-linalool synthase (LIS) from the flower fragrance in Clarkia breweri by Pichersky [31], some TPS genes related to floral scent biosynthesis have been gradually reported, such as in citrus (Citrus sinensis) [32], carrot (Daucus carota) [33], tea (Camellia sinensis) [34], and sweet pea (Lathyrus odoratus) [35]. The TPS family genes constitute a medium-sized to large family. There are approximately 20–150 functional members in the genomes of almost all plant species [36], and a total of 80 TPS-like genes have been identified in tea plants to date [34].
The biosynthesis of terpenoids is reported to be regulated by a variety of transcription factors. For example, MYB and MYC were found to work cooperatively in controlling the expression of terpene synthase genes. In the myb21 mutant of Arabidopsis, it was observed that the emission of sesquiterpenes catalyzed by AtTPS11 and AtTPS21 was decreased, but whether AtMYB21 could regulate AtTPS11 and AtTPS21 directly or not was unclear. Moreover, AtMYB21 and AtMYC2 might form the MYB–bHLH complex to regulate the biosynthesis of sesquiterpenes [37]. Recently, the highly transactive MYB transcription factor in Freesia hybrida, FhMYB21L2, was demonstrated to activate the expression of FhTPS1 via binding to the MYBCORE motif (CAACCG) in its promoter region. On the other hand, FhMYC2 exhibited a negative effect on the expression of FhTPS1 by trapping the FhMYB21L2, which might be the proposed regulatory mechanism for the dynamic change of linalool emission at different developmental stages of the Freesia flowers. In addition, the MYC2 transcription factor was found to interact with RERJ1 (a bHLH transcription factor) [38] or DELLA proteins to regulate the expression of sesquiterpene synthase genes [39]. Other transcription factors, such as NAC, EIN3-like [40], HY5 (bZIP transcription factor) [41], bHLH [42], and scarecrow-like (SCL) [43], have also been found to participate in regulating the expression of TPSs.
Tea plants are evergreen perennial plants that originated in southwest China [44]. Although the TPS gene family has been identified and classified in tea plants [34], the functions of most CsTPSs in tea plants remain unknown [45]. Studies based on the integration of metabolomics and transcriptomics have suggested that the MYB, MYC, bHLH, NAC, ERF, and WRKY transcription factors may regulate tea aroma through the transcriptional regulation of the MVA pathway- and MEP pathway-related genes, thereby causing changes in terpenoid volatiles in tea leaves in different seasons [46,47,48]. This article discusses the recent advances in understanding the functions and molecular mechanisms of terpenoid biosynthesis and the transcriptional regulation of terpenoid production by different types of transcription factors in tea plants.
2. Terpenoid Compounds in Tea Plants
Terpenes, or terpenoids, include more than 50,000–80,000 compounds with different structures, which are important resources for building essential isoprenoid compounds, such as sterols, brassinosteroids, cytokinins, quinones, chlorophyll, tocopherols, carotenoids, abscisic acid (ABA), and gibberellins [49,50]. Although some terpenes are primarily responsible for floral scents, including their aromas and physiological effects, most members of the terpenes are defensive toxins and herbivore deterrents [51]. This article summarizes the main functions of terpenoids in tea plants according to the research published in recent years (Figure 1). From the perspective of defense responses, it is well known that volatile terpenes play essential roles in communication between plants and in the communication between plants and other organisms, thus improving plant fitness (for example, through preventing herbivory and improving the success rate of pollination) [52]. Because of their special floral scents, some terpenes are widely used in industrial applications, such as in the pharmaceutical and cosmetic industries [6,18,53].
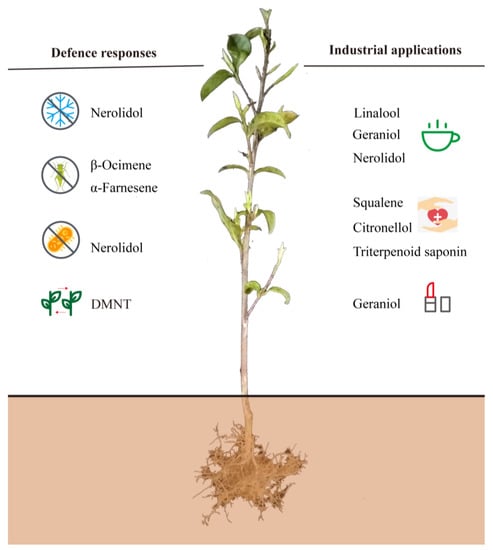
Figure 1.
Important functions of main terpenoids in tea plants. DMNT, (3E)-4,8-Dimethyl-1,3,7-nonatriene.
2.1. Terpenoids in Tea Aroma
Tea is the most popular beverage worldwide apart from water due to its sensory qualities and health benefits [54]. As an important component of tea quality, aroma has received great attention in tea production [55]. Terpenoids, along with lipids, carotenoids, phenylpropanoids, and their glycoside derivatives, constitute the main volatile organic compounds (VOCs) in tea aroma [56,57]. Although terpenoid volatiles have a relatively low detection threshold, they have been reported as key aroma compounds for the sensory quality of tea [46]. There are more than 100 types of terpenoid tea aroma substances, most of which are volatile monoterpenoids (such as geraniol and linalool) and sesquiterpenes (such as nerolidol) [34,58]. Studies have shown that volatile terpenes dominate the aroma (more than 60%) in tea products [59,60].
Generally speaking, β-myrcene, α-phellandrene, (Z)-β-ocimene, (Z)-furan linalool oxide, (E)-furan linalool oxide, γ-terpinene, DMNT, cosmene, (E)-pyranoid linalool oxide, linalool oxide pyranoside, linalyl formate, α-cubebene, γ-elemene, humulene, (E)-β-famesene, (Z, E)-α-farnesene, α-muurolene, α-farnesene, T-muurolol, α-cadinol, α-terpineol, geraniol, β-caryophyllen, δ-cadinene, (E)-nerolidol, linalool, D-limonene, and β-pinene are reported to be important terpene compounds in tea aroma [48,59,61]. Among them, linalool and its furan oxides, (Z)-furan linalool oxide and (E)-furan linalool oxide, are important components of sweet and floral aromas, while (E)-pyranoid linalool oxide and linalool oxide pyranoside have earthy aromas [62]. Geraniol and linalool oxides present pleasant floral scents and may be the principal contributors to the floral and green odor of Longjing tea [63,64,65]. The compound (E)-nerolidol has floral green, citrus woody, and waxy odors [59], which are among the characteristic aromas of oolong tea [66]. The content of these terpenes varies among different tea plant species and might affect the suitability of tea cultivars, indicating that they may play a major role in the formation of floral fragrance [59].
Although many aroma compounds are found in tea, only a small number of components whose concentration exceeds their odor threshold contribute to the aroma characteristics of tea. Different fragrances have different threshold concentrations because the detection limits of the human olfactory system are different. In some cases, the threshold concentration between the two different aroma components differs by a thousand times [67]. Linalool, geraniol, and nerolidol are potent odorants of tea, with flavor dilution (FD) factors of 64, 64, and 32, respectively. These compounds impart tea products with a creamy, rose-like floral odor [68,69]. Three terpenoids, β-myrcene, D-limonene, and (E)-α-farnesene, are considered aroma-active compounds and have been detected in Rougui Wuyi rock tea, especially β-myrcene, which has a spicy aroma that might contribute to herbal or woody odors [69]. In white tea, volatile terpenes, including β-myrcene, linalool, and geraniol, are the key potent odorants, and geraniol and linalool mainly contribute a strong floral aroma when tea leaves are withered under sunlight [70]. In addition, some terpene substances that do not contribute to human sensory perception are also involved in sensory formation by indirectly affecting the formation of other aroma components or in response to adverse processes [59].
2.2. Responses of Terpenoids to Stress
Plants synthesize and release many types of VOCs for reproduction, defense, and communication between plants [71,72,73]. The formation of volatile terpenoids can be influenced by various stresses, including biotic stress (such as tea green leafhopper herbivory) and abiotic stress (such as light, temperature, and wounding) during the tea plant’s growth process and the manufacture of tea products [67]. At present, little is known about the specific mechanism through which plants sense volatiles sent by other plants [52]. Some modern strategies, such as E. coli, yeast expression systems, and plant transgenic systems, can be applied to the investigation of the relationships between characteristic aroma compounds and plants [67].
The direct defenses of plants include physical structures (trichomes and thorns) and the accumulation of chemical or biochemical compounds induced by herbivore feeding, most of which exhibit antibiotic activities or toxicity [74]. Some of the sesquiterpenoids provide direct protection through the formation of phytoalexins, which are produced as part of the plant’s defense system [75]. Volatiles emitted from damaged vegetative tissues after herbivore feeding have been reported to exhibit the function of protecting plants by deterring herbivores and by attracting the enemies of herbivores [5]. It has been established that herbivory-induced volatile terpenoids may serve as both indirect and direct defenses [76,77]. After egg deposition or feeding by herbivorous arthropods, plants can be induced to emit volatile terpenoids [78,79], such as geraniol, farnesene, ocimenes, linalool, and nerolidol [80]. These volatile blends can be exploited by natural enemies of the herbivores to locate infested plants (predators and parasitoids), and the release of these terpenoids is considered to be an important mechanism for plants to indirectly defend themselves [81]. Herbivore-specific compounds from the oral secretions of feeding insects might be the underlying cause of a rapid change in the green leaf volatiles emitted from plants [79]. DMNT, a common volatile released in response to herbivore attacks and a floral odor constituent, was identified as an effective compound used by herbivorous insects to find their host plants for feeding and egg deposition [82]. After being attacked by the geometrid Ectropis obliqua, the emission of DMNT was significantly increased, and it seems to have interacted with jasmonic acid (JA) to promote the resistance of neighboring intact plants to herbivorous insects [83].
In addition to biotic stresses, abiotic stresses have also been found to increase the concentration of most volatile terpenes significantly [84]. Recent studies have demonstrated that light can activate the formation of plant volatiles, while the metabolite levels of tea leaves require a relatively long time to respond to light treatment. In contrast to natural light or dark treatment, blue light (470 nm) and red light (660 nm) significantly increased most endogenous volatile terpenes via significantly upregulating the expression levels of key genes involved in volatile terpene formation [84]. However, green light irradiation could markedly damage the aroma and taste of the tea, leading to a strong greenish flavor and an astringent taste, probably because green light irradiation decreased the contents of chemical compounds in black tea [85]. Furthermore, UV-B treatment application on one-year-old potted plants of C. sinensis cv. “Longjing-43” differentially altered the metabolism of terpenoids with significant effects at 8 h of treatment, demonstrating the strong potential for UV-B application in flavor improvement in tea [86]. During the processing of tea, the combination of a low-temperature application and wounding damage has been demonstrated to improve tea aroma [87]. For example, (E)-nerolidol can be induced during the turn over stage with the mechanical damage at a relatively low temperature, which is not detected in intact tea plants [88]. In addition, drought and wounding caused by long-term withering (36–48 h) significantly induced the upregulated expression of monoterpenes, such as linalool and geraniol, while floral and fruit flavor compounds ((E)-nerolidol and cis-jasmone) showed a significant decrease in content concomitantly [58].
2.3. Potential Uses of Terpenoids in Industrial Applications
In addition to distinguishing the importance of some terpenes in the tea aroma quality due to their unique floral scents as discussed in Section 2.1. of the present work, terpenoids in tea plants have been widely applied in the medical and cosmetic industries, including squalene, citronellol, triterpenoid saponin, and geraniol [89,90,91]. Squalene (C30H50) is an intermediate hydrocarbon in the biosynthesis of terpenes that was first found in shark liver oil. Since its discovery, squalene has been widely used in biological applications due to its beneficial properties, including its anticarcinogenic, antioxidant, and skin-hydrating properties, among others [92,93,94]. Due to the health properties of squalene and its rising demand in industrial uses, alternative sources from plants or microorganisms need to be identified given the recent restrictions on harvesting sharks [93,95,96]. The essential oils in medicinal plants have profound applications in treating central nervous system disorders and diseases of inflammatory etiology. Some of the medicinal plants are rich in secondary metabolites with antihyperalgesic activity, such as citronellal with antihyperalgesic activity [97]. Two enantiomers of citronellol, namely (R)-(+)-β-citronellol and (S)-(−)-β-citronellol, are distributed in many medicinal and aromatic plants [98]. Triterpenoid saponins are recognized for their medicinal benefits, such as acylated oleanane-type triterpene oligoglycosides and florathea saponins A–C in tea plants [99,100]. Oleanane-type triterpenoid saponins exhibit antiproliferative activity against digestive carcinoma human cell lines, indicating that triterpenoid saponins may be a valuable tool to improve the efficacy of cytostatics [101,102]. Geraniol is known as an important ingredient in many highly valued essential oils for its rose-like aroma and is widely applied in cosmetic products [103]. Fragrance is an integral character of cosmetics, and some essential oils of citrus, lavender, eucalyptus, and tea tree are often used as fragrances in the cosmetic industry to stimulate the interest among consumers [104,105].
3. Biosynthesis Pathways of Terpenoids
Terpenoids contain the basic building block units for IPP and its isomer DMAPP, from two relatively separate pathways, namely the MEP and MVA pathways [27], in plastids and the cytosol, respectively (Figure 2). The MEP pathway is mainly responsible for the biosynthesis of mono- and diterpenes (~53 and ~1% of total floral terpenoids, respectively), and the MVA pathway is mainly responsible for the biosynthesis of sesquiterpenes (~28% of total floral terpenoids) [1,106]. Although these isoprenoid biosynthetic pathways are separated, they are connected by metabolic “cross-talk”, which is mediated by unrecognized transporters [1]. The MEP pathway begins with the condensation of D-glyceraldehyde 3-phosphate and pyruvate and involves seven enzymatic reactions, while the MVA pathway starts from the stepwise condensation of three molecules of acetyl-CoA and consists of six enzymatic reactions [107]. Then, two IPP molecules and one DMAPP molecule are synthesized to form FPP by FPP synthase in the cytosol, and one DMAPP with one IPP molecule in the plastids results in the formation of GPP, the precursor of monoterpenes, and is catalyzed by the GPPS [107]. TPS is responsible for the final steps in terpenoid biosynthesis (Figure 2) through catalyzing complex carbocation-driven cyclization, rearrangement, and elimination reactions [108]. Increasing evidence has shown that the TPS family has profound plastic functions in variable family sizes and evolving new enzymes with new functions [108,109,110]. The TPS family is classified into class I and class II TPSs [111]. Each group of TPSs has specific motifs that enable their distinctive functions, such as class I TPSs with the DDxx (D, E) motif and metal-binding NSE/DTE motif and class II TPSs with the DxDD motif [111,112,113].
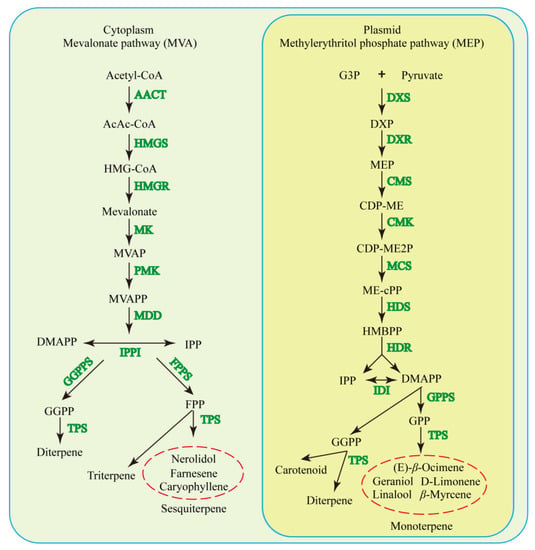
Figure 2.
Terpenoid biosynthesis pathways in plants. MVA, mevalonic acid; AACT, acetyl-CoA acetyltransferase; HMG, 3-hy-droxy-3-methylglutaryl-CoA; HMGS, hydroxy methylglutaryl-CoA synthase; HMGR, hydroxy methylglutaryl-CoA reductase; MK, mevalonate kinase; MVAP, mevalonate-5-phosphate; PMK, phosphomevalonate kinase; MVAPP, mevalonate-5-diphosphate; MDD, mevalonate-5-diphosphate decarboxylase; IPP, isopentenyl diphosphate; IPPI, isopentenyl diphosphate isomerase; DMAPP, dimethylallyl pyrophosphate; GGPPS, GGPP synthase; FPPS, farnesyl pyrophosphate synthase; FPP, farnesyl pyrophosphate; GGPP, geranylgeranyl pyrophosphate; TPS, terpene synthase/cyclase; MEP, methylerythritol phosphate; DXS, 1-deoxy-D-xylulose-5-phosphate synthase; DXP, 1-deoxy-D-xylulose-5-phosphate; DXR, 1-deoxy-D-xylulose-5-phosphate reductoisomerase; CMS, CDP-ME synthase; CDP-ME, 4-diphosphocytidyl-2-C-methyl-D-erythritol; CMK, 4-diphosphocytidyl-2-C-methyl-D-erythritol kinase; CDP-ME2P, 4-(cytidine-5′ -diphospho)-2-C-methyl-D-erythritol-2-phosphate; MCS, 2-C-methyl-D-erythritol-2,4-cyclodiphosphate synthase; ME-cPP, 2-C-methyl-d-erythritol 2, 4-cyclodiphosphate; HDS, 4-hydroxy-3-methylbut-2-enyl diphosphate synthase; HMBPP, 4-hydroxy-3-methylbut-2-enyl diphosphate; HDR, 4-hydroxy-3-methylbut-2-enyl diphosphate reductase; DMAPP, dimethylallyl diphosphate; IDI, IPP/DMAPP isomerase; GPPS, GPP synthase; and GPP, geranyl pyrophosphate.
It is generally believed that both class I and class II TPSs are responsible for the formation of hemiterpenes, monoterpenes, sesquiterpenes, diterpenes, sesterterpenes, and terpenes, while triterpenes and tetraterpenes are mainly synthesized by class II TPSs [114]. To date, few class I TPSs were also charactered to catalyze the formation of large-terpene, such as C25, C30, and sesquarterpenes (C35) terpenes [114,115,116]. Isoprene (2-methyl-1,3-butadiene) is the most common form of hemiterpene and has the smallest and simplest C5 basic building block [117]. Isoprene emissions from plants play important roles in plant defenses against biotic and abiotic stresses, and they can also improve photosynthetic performance at high temperatures [118,119,120]. Monoterpenes are essential substances in the aromatic oil, cosmetic, perfume, food, and pharmaceutical industries for their unique odors [121,122,123]. While monoterpene synthases (mTPSs) are considered to catalyze GPP for monoterpenes, the GPP transporter has not yet been discovered [111]. The sesquiterpenes are constituents of floral scents and have health-promoting properties (such as antioxidant, anti-inflammatory, and anticancer properties), which might be mainly induced by ambient temperatures [124,125,126,127,128]. Sesquiterpenes are mainly synthesized in the cytosol through the MVA pathway with FPP as a substrate. In addition, the formation of small amounts of sesquiterpenes has been detected in plasmids [129,130]. Diterpene biosynthesis is well known to be initiated in plastids from GGPP in the MEP pathway [131,132,133]. Diterpene synthases (diTPSs) can be characterized as class I diTPSs and class II diTPSs, with the functions of forming additional rings, double bonds, or hydroxyl groups [134,135]. In addition, diTPS and cytochrome P450 monooxygenase (P450) enzymes have been found to work in combination to produce novel diterpene compounds [136,137,138]. Taxol, a famous diterpenoid secondary metabolite, is considered one of the most effective anticancer agents originally extracted from the bark of Taxus brevifolia [139]. In the taxol biosynthetic pathway, taxadiene synthase (TS), taxadiene-5α-hydroxy-lase (T5αOH), and specialized Taxus BAHD family acyltransferases (ACTs) are responsible for the cyclization of taxadiene and the modification of the taxane skeleton [140]. In addition, sclareol (labdane diterpenoid) is a natural starting material for ambrox and related ambroxide synthesis, because of its delicate odor and fixative properties [141]. In clary sage (Salvia sclarea), the main plant species for sclareol production, functional modification of labdane and labdane-related diterpenoids mainly involved the addition of hydroxy groups, which can be catalyzed by diTPSs and P450s [141,142]. Sesterterpenes consist of relatively rare terpenoid substances that are formed by geranylfarnesyl diphosphate synthase (GFPPS) using GFPP as the precursor in the plastid MEP pathway [143,144,145]. Triterpenes are C30 compounds derived from two molecules of FPP to generate the squalene catalyzed by squalene synthase (SQS) via the MVA pathway in the cytoplasm [26,146,147]. Although triterpenoids are not necessary for plant growth and development, the substances in this group have a wide range of biological activities and widespread commercial applications [148,149,150,151]. Tetraterpenes are derived from the phytoene condensation of isopentenyl diphosphate (IDP) and dimethylallyl diphosphate (DMADP) by phytoene synthase (PYS) [152]. Carotenoids are the most representative tetraterpenes and are famous natural functional pigments and photoprotectors that have demonstrated efficiency in preventing human health disorders [153,154,155,156].
4. Transcriptional Regulation of Volatile Terpenoids
4.1. Terpenoid Biosynthesis-Related Genes
To date, the terpene biosynthesis pathways (MEP and MVA) and many genes (such as HMGR, DXS, and TPS) related to terpenoid biosynthesis in tea plants have been extensively studied, and it has been demonstrated that TPSs of different classes catalyze the rate-limiting step of converting terpenoid precursors into monoterpenes, sesquiterpenes, and diterpenes [46,54]. The plant TPS gene family can be classified into the following subgroups: a (TPS-a, monofunctional class I sesqui- and di-TPSs), b (monofunctional class I mono-TPSs), c (TPS-c, monofunctional class II diTPSs), d (TPS-d, gymnosperm-specific class I mono-, sesqui-, di-TPSs, and bifunctional class II/I diTPSs), g (TPS-g, monofunctional class I mono-, sesqui-, and di-TPSs), e/f (TPS-e/f, monofunctional class I diTPSs), and h (TPS-h, Selaginella-specific bifunctional class II/I diterpene synthases (diTPSs)) [108]. Among them, TPS-a, TPS-b, and TPS-g are angiosperm-specific subgroups, while TPS-d is a gymnosperm-specific subgroup [36,108]. Few plants contain all the TPS subfamilies. Usually, the TPS family of each plant contains two or more subfamilies. A recent study identified 80 TPS-like genes in the C. sinensis cv. ‘Shuchazao’ (SCZ) genome, including TPS-a, TPS-b, TPS-c, TPS-g and TPS-e/f subgroups [34]. Interestingly, most sesquiterpene synthetized CsTPSs gained high transcriptional levels in flowers and leaves, while limited monoterpene synthase genes maintained substantial transcript levels in many tested organs. It was noted that the functions of the most-recognized CsTPSs remained unclear due to the limitation of sequence precision, indicating that more strategies need to be developed to obtain the full sequences of these genes for their functional validation in vitro and in plants. However, through the comprehensive comparison of several different tea plant genomes, it was found that the CsTPS family varied among different cultivars. For example, CsTPS08 is only annotated in the genome of C. sinensis cv. ‘Huangdan’ (HD) and C. sinensis cv. ‘Tieguanyin’ (TGY) of the oolong tea species with higher terpene aroma, but not completely annotated in some tea species suitable for green tea processing [45].
Linalool is one of the most abundant and scent-determining constituents, including two isomers (R)-linalool and (S)-linalool, existing in plants [157,158,159]. Notably, (R)-linalool has been found to have a woodier and lavender-like aroma, while (S)-linalool has a sweet, floral, and petitgrain-like smell [158]. Linalool synthase (LIS) in tea plants specifically catalyzes the formation of linalool [160]. The transcript levels of CsLISs (CsLIS1, KF006849; CsLIS2, KR873396) were increased in tea leaves under single-wounding treatment and continuous-wounding treatment compared to that in fresh tea leaves, indicating that mechanical damage may promote the release of linalool from tea leaves [61]. Similarly, wounding stress during the turn over process was presumed to be the main factor to activate some key genes involved in the formation of volatiles, such as the linalool synthase gene CsTPS2 (KR873395) [66]. CsTPS42 (CSS0000049), a bifunctional enzyme defined as CsLIS/NES (NES, nerolidol synthase), can generate linalool with GPP as a substrate, and the upregulated expression levels of CsTPS42 might lead to the corresponding release of linalool [48]. In addition, CsTPS2 (KR873395) was also recognized as a linalool synthase gene that was significantly upregulated during the ‘withering’ step of oolong tea manufacture [66]. In conclusion, these previous studies indicate that the fact that the linalool synthase gene in tea plants might be proposed as a wounding stress-response gene. However, few studies have focused on the specific linalool stereoisomer-producing genes in tea plants. Zhou et al. [160] cloned two (R)-linalool synthase candidate genes, CsRLIS (MT178265) and CsTPS (XM_028210969), which specifically catalyzed the formation of (R)-linalool and caused the accumulation of internal (R)-linalool during oolong tea manufacture. Moreover, the relative expression levels of (S)-linalool synthase and (R)-linalool synthase genes might cause the dynamic levels of the proportions between two isomers of linalool among different C. sinensis cultivars.
The genes responsible for the biosynthesis of other essential terpene substances, such as nerolidol, α-farnesene, and β-ocimene, have also been studied in tea plants [46,48,60]. Nerolidol, a sesquiterpenoid alcohol, is the most effective allelopathic compound showing effective applications in agricultural practices [161,162,163,164]. In terms of health-promoting properties and floral scents, nerolidol is also known for its pharmaceutical and medicinal values [165,166,167]. NES is a key enzyme responsible for nerolidol biosynthesis, and some bifunctional linalool/nerolidol synthases severed by TPS can also produce nerolidol [46,165,168,169]. CsTPS4 (KY033151), an NES gene, is significantly downregulated along with the withering degrees of white tea [60]. Based on the transcriptome analysis of the green tea spreading process, it was found that the expression level of CsTPS35 (CSS0012706) was generally increased in different tea plant varieties, which was indicated to help produce (E)-nerolidol from FPP [48].The farnesyl pyrophosphate synthase (FPS) gene is a key enzyme gene in the terpenoid metabolism pathway that is crucial to the formation of tea quality and flavor [46]. The expression of CsFPS increases with the aggravation of drought, and the expression of CsFPS is upregulated under light conditions during the withering process of tea [170]. Ocimene (3,7-dimethyl-1,3,6-octatriene), a ubiquitous floral volatile compound in plants, can be emitted from flowers or vegetative tissues and can be used to attract pollinators or as an antiaphrodisiac pheromone in plant defense [171,172,173]. The FPKM-based gene expression profile of CsOCS2 (OCS, β-ocimene synthase, and TEA004606.1) showed a high correlation not only with the accumulation of (E)-β-ocimene, but also with the other three monoterpenes (geraniol, β-myrcene, and D-limonene) and one sesquiterpene ((E)-β-fanesene) [46]. A new CsOCS gene (MN135992) that shared a low similarity to the previously characterized tea ocimene synthase genes (CsOCS1, TEA031457.1; CsOCS2, TEA004606.1) was isolated in ‘TGY’ tea plants [174]. The in vitro enzymatic reaction experiment indicted that CsOCS protein was a key enzyme responsible for a large amount of (E)-β-ocimene and a small amount of (Z)-β-ocimene using GPP as the substrate [174]. In addition, a plastid-located β-ocimene synthase gene CsBOS1 (TRINITY_DN105425_c1_g2), was determined to be involved in the synthesis of β-ocimene in tea plants, which is especially sensitive to light treatment and the attack of tea geometrids [175]. Intriguingly, a CsAFS gene (GFMV01032657) was found that converted GPP to β-ocimene in vitro, revealing the bifunctional enzyme activity that is common in the TPS gene family [176]. Meanwhile, the diterpenoid-related genes in tea plants have recently been reported, including one CPS (ent-copalyl diphosphate synthase, CPS) and two highly similar KSs (ent-kaurene synthase, KS) [142]. In fast-growing tissues, such as tender stems and roots, the relative expression levels of CsCPS (MN961684) and CsKSs (MN961685; MN961686) exhibited highly coordinated patterns [142]. However, it seems that the 1% differential amino acids between CsKS1 and CsKS2 led to their functional divergence, according to the functional characterization experimental results [142]. In conclusion, the identification and definition of these structural genes have provided extended profiles for future transcriptional investigations.
4.2. Regulation of Transcription Factors Affects Terpenoid Biosynthesis
Many transcription factors have been confirmed to be involved in the regulation of plant terpenoids (Table 1). Among them, transcription factors that are involved in the secondary metabolism of plants (ERF and MYB) have been studied extensively. In maize (Zea mays), TPS10 mainly forms (E)-α- bergamotene and (E)-β-farnesene in leaves damaged by lepidopteran larvae. These compounds are highly attractive to the natural enemies of the herbivores [177]. EREB58, an AP2/ERF family transcription factor, was found to be a positive regulator of TPS10 expression and hence stimulated the emission of two major TPS10 products [178]. Similarly, PpERF61 in peach (Prunus persica) could activate both PpTPS1 and PpTPS3 transcriptions simultaneously, leading to the accumulation of linalool during fruit ripening [179]. In addition to the ERF transcription factor, the expression of PpbHLH1 in Prunus persica was observed to be significantly positively correlated with flavor-related linalool production, and PpbHLH1 could directly bind to the E-box (CACATG) in the PpTPS3 promoter and activate its expression [42]. In tomato (Solanum lycopersicum), the downregulation of SlMYB75 can moderately increase the sesquiterpene accumulation (δ-elemene, β-caryophyllene, and α-humulene) through targeting the SlTPS12, SlTPS31, and SlTPS35 genes [180]. SlSCL3, the SCL transcription factor, modulates the expression of terpene biosynthetic pathway genes via transcriptional activation and has similar expression patterns to those of SlTPS12, while neither direct protein–DNA binding nor interaction with known regulators has been observed [43]. More information about the biosynthesis of volatile terpenoids is presented in Table 1. However, it should be noted that the synthesis of volatile terpenoids is also associated with interactive transcription factor complexes and epigenetic modifications involving DNA methylation. It is interesting to further examine the multiple layers of transcriptional regulation for volatile terpenoids.

Table 1.
Transcription factors regulating terpene synthesis-related genes in plants.
In C. sinensis, the miR171b-3p_2-DELLA-MYC2 and miR166d-5p_1-ABCG2-MYC2 modules were demonstrated to correlate with the terpenoid content, and these modules could enhance terpenoid biosynthesis in sun-withered leaves [184]. Xu et al. found that CsMYB13 and CsbHLH10 played possible crucial roles in the regulation of terpene metabolism based on metabolomic and transcriptome profiles [46]. Four CsMYBs, CsMYB193, CsMYB148, CsMYB147, and CsMYB68, showed high homology to the terpenoid regulator MYBs, and the synergistic functions of the MYB, TPS, and MYC2 genes in terpenoid biosynthesis were confirmed based on the research of Ectropis oblique (EA) attacking tea leaves [47]. Based on transposase-accessible chromatin with sequencing (ATAC-seq) and DNA affinity purification sequencing (DAP-seq) analyses of the artificial hybrids of oolong tea, it was then speculated that MYB83, MYB58, MYB30, MYB81, ARF25, ARF27, and NAC45 might be the core transcription factors regulating the rate-limiting enzyme genes (CsDXS, CsHMGS, and CsHMGR) in terpenoid metabolic pathways [185]. More importantly, it was found that CsMYB83 contained a binding peak in the accessible chromatin region of CsDXS, which provided important evidence for studying its specific transcriptional regulation function [185]. Additionally, the metabolic phenotypes and gene expression profiles of tea leaves revealed a highly significant correlation between the expression of the NAC, ERF, WRKY, and bHLH transcription factors and key genes in the terpenoid biosynthesis pathway, suggesting the crucial roles of these transcription factors in aroma synthesis [48]. Although the comprehensive analysis of metabolomics and high-throughput sequencing technology has been used to identify many key transcription factors that might be involved in the biosynthesis of terpenes in tea plants, due to the lack of relevant evidence on the specific regulatory functions of these transcription factors, research on the transcription regulation of tea plant terpenes is still at the beginning stage. For example, it remains unknown whether these candidate transcription factors can directly bind to the structural genes in the terpene synthesis pathways, whether their transcriptional regulation functions are positive or negative, and whether there are interactive transcription factors involved in the regulatory network, and so on. Collectively, in-depth research on terpene biosynthesis in other horticultural crops has provided good examples for future investigations in finding answers to the above questions. Through conducting further in vivo and in vitro experiments on regulatory proteins, researchers can expect to extend and enrich the current understanding of the field.
5. Conclusions
In recent years, significant progress has been made in the study of terpene biochemistry in plants, which has promoted the transcriptional regulation of the terpene biosynthesis pathway in C. sinensis. Based on metabolomic and transcriptomic data, many terpene-related structural genes and transcriptional regulatory genes have been identified. The biotic and abiotic stress factors suffered by tea plants stimulate the production of volatile terpenes and have significant impacts on the quality of tea. Additionally, transcription factors including MYB, MYC, bHLH, NAC, ERF, and WRKY play important roles in the biosynthesis of tea plant terpenes. Despite the current knowledge of the close associations between the expression of individual or multiple genes involved in terpene biosynthesis, the transcriptional regulation research of tea terpene compounds remains limited. Overall, identifying additional transcription factors and structural genes would make it easier and more comprehensive to manipulate the transcription factors involved in pathway-level regulation. In the future, advanced biotechnologies and metabolic engineering technologies should be applied to explore the specific functions and binding sites of structural genes related to the MEP and MVA pathways.
Author Contributions
Conceptualization, B.W. and M.L.; writing-original draft preparation, J.W., Y.Y., Y.P., S.W., J.Z., X.L. and J.L.; writing-review and editing, J.W., Y.Y., Y.P., S.W., J.Z., X.L., J.L., B.W. and M.L.; visualization, B.W. and M.L.; supervision, B.W. and M.L.; funding acquisition, B.W., M.L. and J.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Guizhou Provincial Science and Technology Projects (ZK [2022]-YB115), Laboratory Opening Projects of Guizhou University (SYSKF2023-017), Science Research Project for Introducing Talents from Guizhou University (GDRJHZ [2021]23, GDRJHZ [2021]02), National Key Research and Development Plan (2022YFD1600801, 2022YFD1600802), and Regional Science Foundation Project of National Science Foundation (32260786, 32060701).
Conflicts of Interest
The authors have no conflict of interest to declare.
References
- Muhlemann, J.K.; Klempien, A.; Dudareva, N. Floral volatiles: From biosynthesis to function. Plant Cell Environ. 2014, 37, 1936–1949. [Google Scholar] [CrossRef]
- Silva, E.A.P.; Carvalho, J.S.; Guimaraes, A.G.; Barreto, R.D.S.; Santos, M.R.V.; Barreto, A.S.; Quintans, L.J. The use of terpenes and derivatives as a new perspective for cardiovascular disease treatment: A patent review (2008–2018). Expert. Opin. Ther. Pat. 2019, 29, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Vecerova, K.; Klem, K.; Vesela, B.; Holub, P.; Grace, J.; Urban, O. Combined effect of altitude, season and light on the accumulation of extractable terpenes in norway spruce needles. Forests 2021, 12, 1737. [Google Scholar] [CrossRef]
- Dudareva, N.; Pichersky, E. Biochemical and molecular genetic aspects of floral scents. Plant Physiol. 2000, 122, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Pichersky, E.; Gershenzon, J. The formation and function of plant volatiles perfumes for pollinator attraction and defense. Curr. Opin. Plant Biol. 2002, 5, 237–243. [Google Scholar] [CrossRef]
- Abbas, F.; Ke, Y.; Yu, R.; Yue, Y.; Amanullah, S.; Jahangir, M.M.; Fan, Y. Volatile terpenoids: Multiple functions, biosynthesis, modulation and manipulation by genetic engineering. Planta 2017, 246, 803–816. [Google Scholar] [CrossRef] [PubMed]
- Abbas, F.; Rothenberg, D.O.; Zhou, Y.W.; Ke, Y.G.; Wang, H.C. Volatile organic compounds as mediators of plant communication and adaptation to climate change. Physiol. Plant. 2022, 174, e13840. [Google Scholar] [CrossRef]
- Tholl, D.; Sohrabi, R.; Huh, J.H.; Lee, S. The biochemistry of homoterpenes-common constituents of floral and herbivore-induced plant volatile bouquets. Phytochemistry 2011, 72, 1635–1646. [Google Scholar] [CrossRef]
- Dudareva, N.; Negre, F.; Nagegowda, D.A.; Orlova, I. Plant volatiles: Recent advances and future perspectives. Crit. Rev. Plant Sci. 2006, 25, 417–440. [Google Scholar] [CrossRef]
- Miranda, R.D.; de Jesus, B.D.M.; Luiz, S.R.D.; Viana, C.B.; Malafaia, C.R.A.; Figueiredo, F.D.; Carvalho, T.D.C.; Silva, M.L.; Londero, V.S.; da Costa-Silva, T.A.; et al. Antiinflammatory activity of natural triterpenes—An overview from 2006 to 2021. Phytother. Res. 2022, 36, 1459–1506. [Google Scholar] [CrossRef]
- Tetali, S.D. Terpenes and isoprenoids: A wealth of compounds for global use. Planta 2019, 249, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hammerbacher, A.; Coutinho, T.A.; Gershenzon, J. Roles of plant volatiles in defence against microbial pathogens and microbial exploitation of volatiles. Plant Cell Environ. 2019, 42, 2827–2843. [Google Scholar] [CrossRef]
- Block, A.K.; Vaughan, M.M.; Schmelz, E.A.; Christensen, S.A. Biosynthesis and function of terpenoid defense compounds in maize (Zea mays). Planta 2019, 249, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.V.; Berryman, D.L.; Mendoza, J.; Yactayo-Chang, J.P.; Li, Q.B.; Christensen, S.A.; Hunter, C.T.; Best, N.; Soubeyrand, E.; Akhtar, T.A.; et al. Dedicated farnesyl diphosphate synthases circumvent isoprenoid-derived growth-defense tradeoffs in Zea mays. Plant J. 2022, 112, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Ramya, M.; Jang, S.; An, H.R.; Lee, S.Y.; Park, P.M.; Park, P.H. Volatile organic compounds from orchids: From synthesis and function to gene regulation. Int. J. Mol. Sci. 2020, 21, 1160. [Google Scholar] [CrossRef]
- Ghissing, U.; Jayanthan, K.; Bera, P.; Bimolata, W.; Mitra, A. Targeted profiling and temporal expression of a few key genes revealed an apparent coordination among the metabolites contributing to the volatiles internal pool in Jasminum sambac (L.) Aiton flowers. Braz. J. Bot. 2022, 45, 587–597. [Google Scholar] [CrossRef]
- Abbas, F.; Ke, Y.G.; Zhou, Y.W.; Yu, R.C.; Imran, M.; Amanullah, S.; Rothenberg, D.O.; Wang, Q.; Wang, L.; Fan, Y.P. Functional characterization of Hedychium coronarium J. Koenig MYB132 confers the potential role in floral aroma synthesis. Plants 2021, 10, 2014. [Google Scholar] [CrossRef]
- Garms, S.; Boland, W.; Arimura, G. Early herbivore-elicited events in terpenoid biosynthesis. Plant Signal Behav. 2008, 3, 418–419. [Google Scholar] [CrossRef]
- Brosset, A.; Blande, J.D. Volatile-mediated plant-plant interactions: Volatile organic compounds as modulators of receiver plant defence, growth, and reproduction. J. Exp. Bot. 2022, 73, 511–528. [Google Scholar] [CrossRef]
- Mundim, F.M.; Vieira-Neto, E.H.M.; Alborn, H.; Bruna, E.M. Disentangling the influence of water limitation and simultaneous above and belowground herbivory on plant tolerance and resistance to stress. J. Ecol. 2021, 109, 2729–2739. [Google Scholar] [CrossRef]
- Clancy, M.V.; Haberer, G.; Jud, W.; Niederbacher, B.; Niederbacher, S.; Senft, M.; Zytynska, S.E.; Weisser, W.W.; Schnitzler, J.P. Under fire-simultaneous volatilome and transcriptome analysis unravels fine-scale responses of tansy chemotypes to dual herbivore attack. BMC Plant Biol. 2020, 20, 551. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.N.A.; Utsumi, R. Diversity, regulation, and genetic manipulation of plant mono- and sesquiterpenoid biosynthesis. Cell Mol. Life Sci. 2009, 66, 3043–3052. [Google Scholar] [CrossRef] [PubMed]
- Movahedi, A.; Wei, H.; Pucker, B.; Ghaderi-Zefrehei, M.; Rasouli, F.; Kiani-Pouya, A.; Jiang, T.B.; Zhuge, Q.; Yang, L.M.; Zhou, X.H. Isoprenoid biosynthesis regulation in poplars by methylerythritol phosphate and mevalonic acid pathways. Front. Plant Sci. 2022, 13, 968780. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.R.; Divakaran, K.; Pillai, P. Evidence for methylerythritol pathway (MEP) contributions to zerumbone biosynthesis as revealed by expression analysis of regulatory genes and metabolic inhibitors studies. Plant Mol. Biol. Rep. 2020, 38, 370–379. [Google Scholar] [CrossRef]
- Pathak, G.; Dudhagi, S.S.; Raizada, S.; Singh, R.K.; Sane, A.P.; Sane, V.A. Phosphomevalonate kinase regulates the MVA/MEP pathway in mango during ripening. Plant Physiol. Biochem. 2023, 196, 174–185. [Google Scholar] [CrossRef]
- Kempinski, C.; Jiang, Z.; Zinck, G.; Sato, S.J.; Ge, Z.; Clemente, T.E.; Chappell, J. Engineering linear, branched-chain triterpene metabolism in monocots. Plant Biotechnol. J. 2019, 17, 373–385. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. The 1-deoxy-D-xylulose-5-phosphate pathway of isoprenoid biosynthesis in plants. Plant Mol. Biol. 1999, 50, 47–65. [Google Scholar] [CrossRef]
- McCaskill, D.; Croteau, R. Some caveats for bioengineering terpenoid metabolism in plants. Trends Biotechnol. 1998, 16, 349–355. [Google Scholar] [CrossRef]
- Rohmer, M. Monoterpene biosynthesis. In Comprehensive Natural Products Chemistry: Isoprenoids Including Carotenoids and Steroids; Cane, D.D., Ed.; Elsevier: Amsterdam, The Netherlands, 1999. [Google Scholar]
- Rohmer, M. Sesquiterpene biosynthesis: Cyclization mechanisms. In Comprehensive Natural Products Chemistry: Isoprenoids Including Carotenoids and Steroids; Cane, D.D., Ed.; Elsevier: Amsterdam, The Netherlands, 1999. [Google Scholar]
- Pichersky, E.; Lewinsohn, E.; Croteaut, R. Purification and characterization of S-linalool synthase, an enzyme involved in the production of floral scent in Clarkia breweri. Arch. Biochem. Biophys. 1995, 316, 803–807. [Google Scholar] [CrossRef]
- Dornelas, M.C.; Mazzafera, P. A genomic approach to characterization of the Citrus terpene synthase gene family. Genet. Mol. Biol. 2007, 30, 832–840. [Google Scholar] [CrossRef]
- Yahyaa, M.; Tholl, D.; Cormier, G.; Jensen, R.; Simon, P.W.; Ibdah, M. Identification and characterization of terpene synthases potentially involved in the formation of volatile terpenes in carrot (Daucus carota L.) roots. J. Agric. Food Chem. 2015, 63, 4870–4878. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.C.; Shamala, L.F.; Yi, X.K.; Yan, Z.; Wei, S. Analysis of terpene synthase family genes in Camellia sinensis with an emphasis on abiotic stress conditions. Sci. Rep. 2020, 10, 933. [Google Scholar] [CrossRef] [PubMed]
- Bao, T.T.; Shadrack, K.; Yang, S.; Xue, X.X.; Li, S.Y.; Wang, N.; Wang, Q.Y.; Wang, L.; Gao, X.; Cronk, Q. Functional characterization of terpene synthases accounting for the volatilized-terpene heterogeneity in Lathyrus odoratus cultivar flowers. Plant Cell Physiol. 2020, 61, 1733–1749. [Google Scholar] [CrossRef]
- Chen, F.; Tholl, D.; Bohlmann, J.; Pichersky, E. The family of terpene synthases in plants: A mid-size family of genes for specialized metabolism that is highly diversified throughout the kingdom. Plant J. 2011, 66, 212–229. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Li, Y.; Gao, F.; Jin, W.; Li, S.; Kimani, S.; Yang, S.; Bao, T.; Gao, X.; Wang, L. MYB21 interacts with MYC2 to control the expression of terpene synthase genes in flowers of Freesia hybrida and Arabidopsis thaliana. J. Exp. Bot. 2020, 71, 4140–4158. [Google Scholar] [CrossRef]
- Valea, I.; Motegi, A.; Kawamura, N.; Kawamoto, K.; Miyao, A.; Ozawa, R.; Takabayashi, J.; Gomi, K.; Nemoto, K.; Nozawa, A.; et al. The rice wound-inducible transcription factor RERJ1 sharing same signal transduction pathway with OsMYC2 is necessary for defense response to herbivory and bacterial blight. Plant Mol. Biol. 2022, 109, 651–666. [Google Scholar] [CrossRef]
- Hong, G.J.; Xue, X.Y.; Mao, Y.B.; Wang, L.J.; Chen, X.Y. Arabidopsis MYC2 interacts with DELLA proteins in regulating sesquiterpene synthase gene expression. Plant Cell 2012, 24, 2635–2648. [Google Scholar] [CrossRef]
- Nieuwenhuizen, N.J.; Chen, X.; Wang, M.Y.; Matich, A.J.; Perez, R.L.; Allan, A.C.; Green, S.A.; Atkinson, R.G. Natural variation in monoterpene synthesis in kiwifruit: Transcriptional regulation of terpene synthases by NAC and ETHYLENE-INSENSITIVE3-like transcription factors. Plant Physiol. 2015, 167, 1243–1258. [Google Scholar] [CrossRef] [PubMed]
- Michael, R.; Ranjan, A.; Kumar, R.S.; Pathak, P.K.; Trivedi, P.K. Light-regulated expression of terpene synthase gene, AtTPS03, is controlled by the bZIP transcription factor, HY5, in Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 2020, 529, 437–443. [Google Scholar] [CrossRef]
- Wei, C.; Liu, H.; Cao, X.; Zhang, M.; Li, X.; Chen, K.; Zhang, B. Synthesis of flavour-related linalool is regulated by PpbHLH1 and associated with changes in DNA methylation during peach fruit ripening. Plant Biotechnol. J. 2021, 19, 2082–2096. [Google Scholar] [CrossRef]
- Yang, C.; Marillonnet, S.; Tissier, A. The scarecrow-like transcription factor SlSCL3 regulates volatile terpene biosynthesis and glandular trichome size in tomato (Solanum lycopersicum). Plant J. 2021, 107, 1102–1118. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.L.; Yang, H.; Wang, S.B.; Zhao, J.; Liu, C.; Gao, L.P.; Xia, E.H.; Lu, Y.; Tai, Y.L.; She, G.B.; et al. Draft genome sequence of Camellia sinensis var. sinensis provides insights into the evolution of the tea genome and tea quality. Proc. Natl. Acad. Sci. USA 2018, 115, E4151–E4158. [Google Scholar] [CrossRef] [PubMed]
- Qiao, D.; Tang, M.; Jin, L.; Mi, X.; Chen, H.; Zhu, J.; Liu, S.; Wei, C. A monoterpene synthase gene cluster of tea plant (Camellia sinensis) potentially involved in constitutive and herbivore-induced terpene formation. Plant Physiol. Biochem. 2022, 184, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; He, Y.; Yan, X.; Zhao, S.; Zhu, J.; Wei, C. Unraveling a crosstalk regulatory network of temporal aroma accumulation in tea plant (Camellia sinensis) leaves by integration of metabolomics and transcriptomics. Environ. Exp. Bot. 2018, 149, 81–94. [Google Scholar] [CrossRef]
- Li, P.; Xia, E.; Fu, J.; Xu, Y.; Zhao, X.; Tong, W.; Tang, Q.; Tadege, M.; Fernie, A.R.; Zhao, J. Diverse roles of MYB transcription factors in regulating secondary metabolite biosynthesis, shoot development, and stress responses in tea plants (Camellia sinensis). Plant J. 2022, 110, 1144–1165. [Google Scholar] [CrossRef]
- Qiao, D.; Mi, X.; An, Y.; Xie, H.; Cao, K.; Chen, H.; Chen, M.; Liu, S.; Chen, J.; Wei, C. Integrated metabolic phenotypes and gene expression profiles revealed the effect of spreading on aroma volatiles formation in postharvest leaves of green tea. Food Res. Int. 2021, 149, 110680. [Google Scholar] [CrossRef]
- Vranova, E.; Coman, D.; Gruissem, W. Structure and dynamics of the isoprenoid pathway network. Mol. Plant 2012, 5, 318–333. [Google Scholar] [CrossRef]
- Hilgers, F.; Habash, S.S.; Loeschcke, A.; Ackermann, Y.S.; Neumann, S.; Heck, A.; Klaus, O.; Hage-Hulsmann, J.; Grundler, F.M.W.; Jaeger, K.E.; et al. Heterologous production of β-caryophyllene and evaluation of its activity against plant pathogenic fungi. Microorganisms 2021, 9, 168. [Google Scholar] [CrossRef]
- Jan, R.; Asaf, S.; Numan, M.; Lubna; Kim, K.-M. Plant secondary metabolite biosynthesis and transcriptional regulation in response to biotic and abiotic stress conditions. Agronomy 2021, 11, 968. [Google Scholar] [CrossRef]
- Loreto, F.; D’Auria, S. How do plants sense volatiles sent by other plants? Trends Plant Sci. 2022, 27, 29–38. [Google Scholar] [CrossRef]
- Zhao, S.; Cheng, H.; Xu, P.; Wang, Y. Regulation of biosynthesis of the main flavor-contributing metabolites in tea plant (Camellia sinensis): A review. Crit. Rev. Food Sci. Nutr. 2022, 1–16. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, X.; Yan, X.; Guo, L.; Mi, X.; Xu, Q.; Zhu, J.; Wu, A.; Liu, L.; Wei, C. Revealing of microRNA involved regulatory gene networks on terpenoid biosynthesis in Camellia sinensis in different growing time points. J. Agric. Food Chem. 2018, 66, 12604–12616. [Google Scholar] [CrossRef]
- Yang, Z.; Baldermann, S.; Watanabe, N. Recent studies of the volatile compounds in tea. Food Res. Int. 2013, 53, 585–599. [Google Scholar] [CrossRef]
- Zeng, L.; Jin, S.; Xu, Y.Q.; Granato, D.; Fu, Y.Q.; Sun, W.J.; Yin, J.F.; Xu, Y.Q. Exogenous stimulation-induced biosynthesis of volatile compounds: Aroma formation of oolong tea at postharvest stage. Crit. Rev. Food Sci. Nutr. 2022, 8, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Picazo-Aragones, J.; Terrab, A.; Balao, F. Plant volatile organic compounds evolution: Transcriptional regulation, epigenetics and polyploidy. Int. J. Mol. Sci. 2020, 21, 8956. [Google Scholar] [CrossRef]
- Cui, J.; Katsuno, T.; Totsuka, K.; Ohnishi, T.; Takemoto, H.; Mase, N.; Toda, M.; Narumi, T.; Sato, K.; Matsuo, T.; et al. Characteristic fluctuations in glycosidically bound volatiles during tea processing and identification of their unstable derivatives. J. Agric. Food Chem. 2016, 64, 1151–1157. [Google Scholar] [CrossRef]
- He, C.; Li, Y.; Zhou, J.; Yu, X.; Zhang, D.; Chen, Y.; Ni, D.; Yu, Z. Study on the suitability of tea cultivars for processing oolong tea from the perspective of aroma based on olfactory sensory, electronic nose, and GC-MS data correlation analysis. Foods 2022, 11, 2880. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhu, Y.; Dai, W.; Lv, H.; Mu, B.; Li, P.; Tan, J.; Ni, D.; Lin, Z. Aroma formation and dynamic changes during white tea processing. Food Chem. 2018, 274, 915–924. [Google Scholar] [CrossRef]
- Wang, M.; Li, J.; Liu, X.; Liu, C.; Qian, J.; Yang, J.; Zhou, X.; Jia, Y.; Tang, J.; Zeng, L. Characterization of key odorants in Lingtou Dancong oolong tea and their differences induced by environmental conditions from different altitudes. Metabolites 2022, 12, 1063. [Google Scholar] [CrossRef]
- Ho, C.-T.; Zheng, X.; Li, S. Tea aroma formation. Food Sci. Hum. Wellness 2015, 4, 9–27. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, F.; Wang, L.; Niu, Y.; Yu, D.; Shu, C.; Chen, H.; Wang, H.; Xiao, Z. Comparison of aroma-active volatiles in oolong tea infusions using GC-olfactometry, GC-FPD, and GC-MS. J. Agric. Food Chem. 2015, 63, 7499–7510. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Ruan, J. Analysis of chemical components in green tea in relation with perceived quality, a case study with Longjing teas. Int. J. Food Sci. Technol. 2009, 44, 2476–2484. [Google Scholar] [CrossRef]
- Wang, K.; Liu, F.; Liu, Z.; Huang, J.; Xu, Z.; Li, Y.; Chen, J.; Gong, Y.; Yang, X. Comparison of catechins and volatile compounds among different types of tea using high performance liquid chromatograph and gas chromatograph mass spectrometer. Int. J. Food Sci. Technol. 2011, 46, 1406–1412. [Google Scholar] [CrossRef]
- Gui, J.; Fu, X.; Zhou, Y.; Katsuno, T.; Mei, X.; Deng, R.; Xu, X.; Zhang, L.; Dong, F.; Watanabe, N.; et al. Does enzymatic hydrolysis of glycosidically bound volatile compounds really contribute to the formation of volatile compounds during the oolong tea manufacturing process? J. Agric. Food Chem. 2015, 63, 6905–6914. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Watanabe, N.; Yang, Z. Understanding the biosyntheses and stress response mechanisms of aroma compounds in tea (Camellia sinensis) to safely and effectively improve tea aroma. Crit. Rev. Food Sci. Nutr. 2019, 59, 2321–2334. [Google Scholar] [CrossRef] [PubMed]
- Schuh, C.; Schieberle, P. Characterization of the key aroma compounds in the beverage prepared from darjeeling black tea: Quantitative differences between tea leaves and infusion. J. Agric. Food Chem. 2006, 54, 916–924. [Google Scholar] [CrossRef]
- Yang, P.; Yu, M.; Song, H.; Xu, Y.; Lin, Y.; Granvogl, M. Characterization of key aroma-active compounds in rough and moderate fire Rougui Wuyi Rock tea (Camellia sinensis) by sensory-directed flavor analysis and elucidation of the influences of roasting on aroma. J. Agric. Food Chem. 2021, 70, 267–278. [Google Scholar] [CrossRef]
- Wu, H.; Chen, Y.; Feng, W.; Shen, S.; Wei, Y.; Jia, H.; Wang, Y.; Deng, W.; Ning, J. Effects of three different withering treatments on the aroma of white tea. Foods 2022, 11, 2502. [Google Scholar] [CrossRef]
- Boachon, B.; Lynch, J.H.; Ray, S.; Yuan, J.; Caldo, K.M.P.; Junker, R.R.; Kessler, S.A.; Morgan, J.A.; Dudareva, N. Natural fumigation as a mechanism for volatile transport between flower organs. Nat. Chem. Biol. 2019, 15, 583. [Google Scholar] [CrossRef]
- Landi, M.; Araniti, F.; Flamini, G.; Lo Piccolo, E.; Trivellini, A.; Abenavoli, M.R.; Guidi, L. “Help is in the air”: Volatiles from salt-stressed plants increase the reproductive success of receivers under salinity. Planta 2020, 251, 48. [Google Scholar] [CrossRef]
- Lo, M.M.; Benfodda, Z.; Benimelis, D.; Fontaine, J.X.; Molinie, R.; Meffre, P. Extraction and identification of volatile organic compounds emitted by fragrant flowers of three Tillandsia species by HS-SPME/GC-MS. Metabolites 2021, 11, 594. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.; Lou, Y.; Mao, Y.; Lu, S.; Wang, L.; Chen, X. Plant terpenoids: Biosynthesis and ecological functions. J. Integr. Plant Biol. 2007, 49, 179–186. [Google Scholar] [CrossRef]
- Phillips, M.; Croteau, R. Resin-based defenses in conifers. Trends Plant Sci. 1999, 4, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Paré, P.W.; Tumlinson, J.H. Plant volatiles as a defense against insect herbivores. Plant Physiol. 1999, 121, 325–331. [Google Scholar] [CrossRef]
- Mumm, R.; Posthumus, M.A.; Dicke, M. Significance of terpenoids in induced indirect plant defence against herbivorous arthropods. Plant Cell Environ. 2008, 31, 575–585. [Google Scholar] [CrossRef]
- Mei, X.; Liu, X.; Zhou, Y.; Wang, X.; Zeng, L.; Fu, X.; Li, J.; Tang, J.; Dong, F.; Yang, Z. Formation and emission of linalool in tea (Camellia sinensis) leaves infested by tea green leafhopper (Empoasca (Matsumurasca) onukii Matsuda). Food Chem. 2017, 237, 356–363. [Google Scholar] [CrossRef]
- Dong, F.; Yang, Z.; Baldermann, S.; Sato, Y.; Asai, T.; Watanabe, N. Herbivore-induced volatiles from tea (Camellia sinensis) plants and their involvement in intraplant communication and changes in endogenous nonvolatile metabolites. J. Agric. Food Chem. 2011, 59, 13131–13135. [Google Scholar] [CrossRef]
- Han, B.Y.; Chen, Z.M. Composition of the volatiles from intact and mechanically pierced tea aphid-tea shoot complexes and their attraction to natural enemies of the tea aphid. J. Agric. Food Chem. 2002, 50, 2571–2575. [Google Scholar] [CrossRef]
- Hilker, M.; Meiners, T. Early herbivore alert: Insect eggs induce plant defense. J. Chem. Ecol. 2006, 32, 1379–1397. [Google Scholar] [CrossRef]
- Gharaei, A.M.; Ziaaddini, M.; Frerot, B.; Ebrahimi, S.N.; Jalali, M.A.; Reddy, G.V.P. Identification and evaluation of four cucurbitaceous host plant volatiles attractive to Diaphania indica (Saunders) (Lep.: Pyralidae). Chemoecology 2020, 30, 173–182. [Google Scholar] [CrossRef]
- Jing, T.T.; Du, W.K.; Gao, T.; Wu, Y.; Zhang, N.; Zhao, M.Y.; Jin, J.Y.; Wang, J.M.; Schwab, W.; Wan, X.C.; et al. Herbivore-induced DMNT catalyzed by CYP82D47 plays an important role in the induction of JA-dependent herbivore resistance of neighboring tea plants. Plant Cell Environ. 2021, 44, 1178–1191. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Chen, Y.; Mei, X.; Katsuno, T.; Kobayashi, E.; Dong, F.; Watanabe, N.; Yang, Z. Regulation of formation of volatile compounds of tea (Camellia sinensis) leaves by single light wavelength. Sci. Rep. 2015, 5, 16858. [Google Scholar] [CrossRef] [PubMed]
- Ai, Z.; Zhang, B.; Chen, Y.; Yu, Z.; Chen, H.; Ni, D. Impact of light irradiation on black tea quality during withering. J. Food Sci. Technol. 2017, 54, 1212–1227. [Google Scholar] [CrossRef]
- Shamala, L.F.; Zhou, H.C.; Han, Z.X.; Wei, S. UV-B induces distinct transcriptional re-programing in UVR8-signal transduction, flavonoid, and terpenoids pathways in Camellia sinensis. Front. Plant Sci. 2020, 11, 234. [Google Scholar] [CrossRef]
- Shao, C.; Zhang, C.; Lv, Z.; Shen, C. Pre- and post-harvest exposure to stress influence quality-related metabolites in fresh tea leaves (Camellia sinensis). Sci. Hortic. 2021, 281, 109984. [Google Scholar] [CrossRef]
- Zhou, Y.; Zeng, L.; Liu, X.; Gui, J.; Mei, X.; Fu, X.; Dong, F.; Tang, J.; Zhang, L.; Yang, Z. Formation of (E)-nerolidol in tea (Camellia sinensis) leaves exposed to multiple stresses during tea manufacturing. Food Chem. 2017, 231, 78–86. [Google Scholar] [CrossRef]
- Caser, M.; Chitarra, W.; D’Angiolillo, F.; Perrone, I.; Demasi, S.; Lovisolo, C.; Pistelli, L.; Pistelli, L.; Scariot, V. Drought stress adaptation modulates plant secondary metabolite production in Salvia dolomitica Codd. Ind. Crop. Prod. 2019, 129, 85–96. [Google Scholar] [CrossRef]
- Takshak, S.; Agrawal, S.B. Defense potential of secondary metabolites in medicinal plants under UV-B stress. J. Photochem. Photobiol. B 2019, 193, 51–88. [Google Scholar] [CrossRef]
- Kaur, G.; Arya, S.K.; Singh, B.; Singh, S.; Dhar, Y.V.; Verma, P.C.; Ganjewala, D. Transcriptome analysis of the palmarosa Cymbopogon martinii inflorescence with emphasis on genes involved in essential oil biosynthesis. Ind. Crop. Prod. 2019, 140, 111602. [Google Scholar] [CrossRef]
- Mateos, R.; Sarria, B.; Bravo, L. Nutritional and other health properties of olive pomace oil. Crit. Rev. Food Sci. Nutr. 2020, 60, 3506–3521. [Google Scholar] [CrossRef]
- Martinez-Beamonte, R.; Sanclemente, T.; Surra, J.C.; Osada, J. Could squalene be an added value to use olive by-products? J. Sci. Food Agric. 2020, 100, 915–925. [Google Scholar] [CrossRef]
- Romani, A.; Ieri, F.; Urciuoli, S.; Noce, A.; Marrone, G.; Nediani, C.; Bernini, R. Health effects of phenolic compounds found in extra-virgin olive oil, by-products, and leaf of Olea europaea L. Nutrients 2019, 11, 1776. [Google Scholar] [CrossRef]
- Yarkent, C.; Oncel, S.S. Recent progress in microalgal squalene production and its cosmetic application. Biotechnol. Bioproc. E 2022, 27, 295–305. [Google Scholar] [CrossRef]
- Mendes, A.; Azevedo-Silva, J.; Fernandes, J.C. From sharks to yeasts: Squalene in the development of vaccine adjuvants. Pharmaceuticals 2022, 15, 265. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.L.; Matos, J.; Picot, L.; Almeida, J.; Quintans, J.S.S.; Quintans-Junior, L.J. Citronellol, a monoterpene alcohol with promising pharmacological activities—A systematic review. Food Chem. Toxicol. 2019, 123, 459–469. [Google Scholar] [CrossRef]
- Silva, D.; Diniz-Neto, H.; Cordeiro, L.; Silva-Neta, M.; Silva, S.; Andrade-Junior, F.; Leite, M.; Nobrega, J.; Morais, M.; Souza, J.; et al. (R)-(+)-β-citronellol and (S)-(−)-β-citronellol in combination with amphotericin B against Candida spp. Int. J. Mol. Sci. 2020, 21, 1785. [Google Scholar] [CrossRef]
- Sochacki, M.; Vogt, O. Triterpenoid saponins from washnut (Sapindus mukorossi Gaertn.)-A source of natural surfactants and other active components. Plants 2022, 11, 2355. [Google Scholar] [CrossRef]
- Yates, P.S.; Roberson, J.; Ramsue, L.K.; Song, B.H. Bridging the gaps between plant and human health: A systematic review of soyasaponins. J. Agric. Food Chem. 2021, 69, 14387–14401. [Google Scholar] [CrossRef] [PubMed]
- Messi, L.M.; Note, O.P.; Mbing, J.N.; Lavedan, P.; Vedrenne, M.; Ouedraogo, N.; Carraz, M.; Bourgeade-Delmas, S.; Pegnyemb, D.E.; Haddad, M. Triterpenoid saponins from Calliandra calothyrsus Meisn. and their antiproliferative activity against two digestive carcinoma human cell lines. Fitoterapia 2020, 146, 104669. [Google Scholar] [CrossRef]
- Gevrenova, R.; Zaharieva, M.M.; Kroumov, A.D.; Voutquenne-Nazabadioko, L.; Zheleva-Dimitrova, D.; Balabanova, V.; Hajdenski, H.M.; Konstantinov, S. Gypsophila saponins enhance the cytotoxicity of etoposide in HD-MY-Z lymphoma cells. Food Chem. Toxicol. 2019, 133, 110777. [Google Scholar] [CrossRef] [PubMed]
- Maczka, W.; Winska, K.; Grabarczyk, M. One hundred faces of geraniol. Molecules 2020, 25, 3303. [Google Scholar] [CrossRef]
- Sharmeen, J.B.; Mahomoodally, F.M.; Zengin, G.; Maggi, F. Essential oils as natural sources of fragrance compounds for cosmetics and cosmeceuticals. Molecules 2021, 26, 666. [Google Scholar] [CrossRef]
- Panico, A.; Serio, F.; Bagordo, F.; Grassi, T.; Idolo, A.; De Giorgi, M.; Guido, M.; Congedo, M.; De Donno, M. Skin safety and health prevention: An overview of chemicals in cosmetic products. J. Prev. Med. Hyg. 2019, 60, E50–E57. [Google Scholar] [CrossRef] [PubMed]
- Simkin, A.J.; Underwood, B.A.; Auldridge, M.; Loucas, H.M.; Shibuya, K.; Schmelz, E.; Clark, D.G.; Klee, H.J. Circadian regulation of the PhCCD1 carotenoid cleavage dioxygenase controls emission of β-ionone, a fragrance volatile of petunia flowers. Plant Physiol. 2004, 136, 3504–3514. [Google Scholar] [CrossRef] [PubMed]
- Dudareva, N.; Anderssont, S.; Orlova, I.; Gattot, N.; Reicheltt, M.; Rhodes, D.; Bolandt, W.; Gershenzont, J. The nonmevalonate pathway supports both monoterpene and sesquiterpene formation in snapdragon flowers. Proc. Natl. Acad. Sci. USA 2005, 102, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Karunanithi, P.S.; Zerbe, P. Terpene Synthases as Metabolic Gatekeepers in the Evolution of Plant Terpenoid Chemical Diversity. Front. Plant Sci. 2019, 10, 1166. [Google Scholar] [CrossRef]
- Muchlinski, A.; Chen, X.; Lovell, J.T.; Kollner, T.G.; Pelot, K.A.; Zerbe, P.; Ruggiero, M.; Callaway, L., III; Laliberte, S.; Chen, F.; et al. Biosynthesis and emission of stress-induced volatile terpenes in roots and leaves of switchgrass (Panicum virgatum L.). Front. Plant Sci. 2019, 10, 1144. [Google Scholar] [CrossRef]
- Mele, M.A.; Kang, H.M.; Lee, Y.T.; Islam, M.Z. Grape terpenoids: Flavor importance, genetic regulation, and future potential. Crit. Rev. Food Sci. Nutr. 2021, 61, 1429–1447. [Google Scholar] [CrossRef] [PubMed]
- Nagegowda, D.A.; Gupta, P. Advances in biosynthesis, regulation, and metabolic engineering of plant specialized terpenoids. Plant Sci. 2020, 294, 110457. [Google Scholar] [CrossRef]
- Trindade, H.; Pedro, L.G.; Figueiredo, A.C.; Barroso, J.G. Chemotypes and terpene synthase genes in Thymus genus: State of the art. Ind. Crop. Prod. 2018, 124, 530–547. [Google Scholar] [CrossRef]
- Tohidi, B.; Rahimmalek, M.; Arzani, A.; Trindade, H. Sequencing and variation of terpene synthase gene (TPS2) as the major gene in biosynthesis of thymol in different Thymus species. Phytochemistry 2020, 169, 112126. [Google Scholar] [CrossRef] [PubMed]
- Fujihashi, M.; Sato, T.; Tanaka, Y.; Yamamoto, D.; Nishi, T.; Ueda, D.; Murakami, M.; Yasuno, Y.; Sekihara, A.; Fuku, K.; et al. Crystal structure and functional analysis of large-terpene synthases belonging to a newly found subclass. Chem. Sci. 2018, 9, 3754–3758. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Lauterbach, L.; Bian, G.K.; Chen, R.; Hou, A.W.; Mori, T.; Cheng, S.; Hu, B.; Lu, L.; Mu, X.; et al. Discovery of non-squalene triterpenes. Nature 2022, 606, 414. [Google Scholar] [CrossRef]
- Stepanova, R.; Inagi, H.; Sugawara, K.; Asada, K.; Nishi, T.; Ueda, D.; Yasuno, Y.; Shinada, T.; Miki, K.; Fujihashi, M.; et al. Characterization of class IB terpene synthase: The first crystal structure bound with a substrate surrogate. ACS Chem. Biol. 2020, 15, 1517–1525. [Google Scholar] [CrossRef]
- Lantz, A.T.; Allman, J.; Weraduwage, S.M.; Sharkey, T.D. Isoprene: New insights into the control of emission and mediation of stress tolerance by gene expression. Plant Cell Environ. 2019, 42, 2808–2826. [Google Scholar] [CrossRef]
- Fernandez-Martinez, M.; Llusia, J.; Filella, I.; Niinemets, U.; Arneth, A.; Wright, I.J.; Loreto, F.; Penuelas, J. Nutrient-rich plants emit a less intense blend of volatile isoprenoids. New Phytol. 2018, 220, 773–784. [Google Scholar] [CrossRef]
- Monson, R.K.; Winkler, B.; Rosenstiel, T.N.; Block, K.; Merl-Pham, J.; Strauss, S.H.; Ault, K.; Maxfield, J.; Moore, D.J.P.; Trahan, N.A.; et al. High productivity in hybrid-poplar plantations without isoprene emission to the atmosphere. Proc. Natl. Acad. Sci. USA 2020, 117, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Carrion, O.; Gibson, L.; Elias, D.M.O.; McNamara, N.P.; van Alen, T.A.; Op den Camp, H.J.M.; Supramaniam, C.V.; McGenity, T.J.; Murrell, J.C. Diversity of isoprene-degrading bacteria in phyllosphere and soil communities from a high isoprene-emitting environment: A malaysian oil palm plantation. Microbiome 2020, 8, 81. [Google Scholar] [CrossRef]
- Sprea, R.M.; Fernandes, A.; Calhelha, R.C.; Pereira, C.; Pires, T.C.; Alves, M.J.; Canan, C.; Barros, L.; Amaral, J.S.; Ferreira, I.C.F.R. Chemical and bioactive characterization of the aromatic plant Levisticum officinale WDJ Koch: A comprehensive study. Food Funct. 2020, 11, 1292–1303. [Google Scholar] [CrossRef]
- Soares-Castro, P.; Soares, F.; Santos, P.M. Current advances in the bacterial toolbox for the biotechnological production of monoterpene-based aroma compounds. Molecules 2021, 26, 91. [Google Scholar] [CrossRef]
- Paulino, B.N.; Silva, G.N.S.; Araujo, F.F.; Neri-Numa, I.A.; Pastore, G.M.; Bicas, J.L.; Molina, G. Beyond natural aromas: The bioactive and technological potential of monoterpenes. Trends Food Sci. Tech. 2022, 128, 188–201. [Google Scholar] [CrossRef]
- Farre-Armengol, G.; Fernandez-Martinez, M.; Filella, I.; Junker, R.R.; Penuelas, J. Deciphering the biotic and climatic factors that influence floral scents: A systematic review of floral volatile emissions. Front. Plant Sci. 2020, 11, 1154. [Google Scholar] [CrossRef] [PubMed]
- Larsson, M.C.; Madjidian, J.A.; Lankinen, A. Floral scent and pollinator visitation in relation to floral colour morph in the mixed-mating annual herb Collinsia heterophylla. Nord. J. Bot. 2021, 39, 1–11. [Google Scholar] [CrossRef]
- Bora, H.; Kamle, M.; Mahato, D.K.; Tiwari, P.; Kumar, P. Citrus essential oils (CEOs) and their applications in food: An overview. Plants 2020, 9, 357. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Iriti, M.; Vitalini, S.; Antolak, H.; Pawlikowska, E.; Kregiel, D.; Sharifi-Rad, J.; Oyeleye, S.I.; Ademiluyi, A.O.; Czopek, K.; et al. Euphorbia-derived natural products with potential for use in health maintenance. Biomolecules 2019, 9, 337. [Google Scholar] [CrossRef]
- Abu-Izneid, T.; Rauf, A.; Shariati, M.A.; Khalil, A.A.; Imran, M.; Rebezov, M.; Uddin, M.S.; Mahomoodally, M.F.; Rengasamy, K.R.R. Sesquiterpenes and their derivatives-natural anticancer compounds: An update. Pharmacol. Res. 2020, 161, 105165. [Google Scholar] [CrossRef]
- Park, S.; Mani, V.; Kim, J.A.; Lee, S.I.; Lee, K. Combinatorial transient gene expression strategies to enhance terpenoid production in plants. Front. Plant Sci. 2022, 13, 1034893. [Google Scholar] [CrossRef]
- Therezan, R.; Kortbeek, R.; Vendemiatti, E.; Legarrea, S.; de Alencar, S.M.; Schuurink, R.C.; Bleeker, P.; Peres, L.E.P. Introgression of the sesquiterpene biosynthesis from Solanum habrochaites to cultivated tomato offers insights into trichome morphology and arthropod resistance. Planta 2021, 254, 11. [Google Scholar] [CrossRef]
- Su, P.; Gao, L.; Tong, Y.; Guan, H.; Liu, S.; Zhang, Y.; Zhao, Y.; Wang, J.; Hu, T.; Tu, L.; et al. Analysis of the role of geranylgeranyl diphosphate synthase 8 from Tripterygium wilfordii in diterpenoids biosynthesis. Plant Sci. 2019, 285, 184–192. [Google Scholar] [CrossRef]
- Vaccaro, M.; Ocampo Bernal, V.; Malafronte, N.; De Tommasi, N.; Leone, A. High yield of bioactive abietane diterpenes in Salvia sclarea hairy roots by overexpressing cyanobacterial DXS or DXR genes. Planta Med. 2019, 85, 973–980. [Google Scholar] [CrossRef]
- Vaccaro, M.C.; Alfieri, M.; De Tommasi, N.; Moses, T.; Goossens, A.; Leone, A. Boosting the synthesis of pharmaceutically active abietane diterpenes in S. sclarea hairy roots by engineering the GGPPS and CPPS genes. Front. Plant Sci. 2020, 11, 924. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.R.; Bhat, W.W.; Bibik, J.; Turmo, A.; Hamberger, B.; Hamberger, B.; Genomics, E.M. A database-driven approach identifies additional diterpene synthase activities in the mint family (Lamiaceae). J. Biol. Chem. 2019, 294, 1349–1362. [Google Scholar] [CrossRef]
- Tasnim, S.; Gries, R.; Mattsson, J. Identification of three monofunctional diterpene synthases with specific enzyme activities expressed during heartwood formation in western redcedar (Thuja plicata) trees. Plants 2020, 9, 1018. [Google Scholar] [CrossRef]
- Murphy, K.M.; Chung, S.; Fogla, S.; Minsky, H.B.; Zhu, K.Y.; Zerbe, P. A customizable approach for the enzymatic production and purification of diterpenoid natural products. J. Vis. Exp. 2019, 152, e59992. [Google Scholar] [CrossRef]
- Bathe, U.; Tissier, A. Cytochrome P450 enzymes: A driving force of plant diterpene diversity. Phytochemistry 2019, 161, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Mitu, S.A.; Ogbourne, S.M.; Klein, A.H.; Tran, T.D.; Reddell, P.W.; Cummins, S.F. The P450 multigene family of Fontainea and insights into diterpenoid synthesis. BMC Plant Biol. 2021, 21, 191. [Google Scholar] [CrossRef]
- Sharma, A.; Bhatia, S.K.; Banyal, A.; Chanana, I.; Kumar, A.; Chand, D.; Kulshrestha, S.; Kumar, P. An overview on taxol production technology and its applications as anticancer agent. Biotechnol. Bioproc. E 2022, 27, 680–702. [Google Scholar] [CrossRef]
- Wang, T.; Li, L.; Zhuang, W.; Zhang, F.; Shu, X.; Wang, N.; Wang, Z. Recent research progress in taxol biosynthetic pathway and acylation reactions mediated by Taxus acyltransferases. Molecules 2021, 26, 2855. [Google Scholar] [CrossRef]
- Caniard, A.; Zerbe, P.; Legrand, S.; Cohade, A.; Valot, N.; Magnard, J.L.; Bohlmann, J.; Legendre, L. Discovery and functional characterization of two diterpene synthases for sclareol biosynthesis in Salvia sclarea (L.) and their relevance for perfume manufacture. BMC Plant Biol. 2012, 12, 119. [Google Scholar] [CrossRef]
- Trikka, F.A.; Nikolaidis, A.; Ignea, C.; Tsaballa, A.; Tziveleka, L.A.; Ioannou, E.; Roussis, V.; Stea, E.A.; Bozic, D.; Argiriou, A.; et al. Combined metabolome and transcriptome profiling provides new insights into diterpene biosynthesis in S. pomifera glandular trichomes. BMC Genom. 2015, 16, 935. [Google Scholar] [CrossRef]
- Okada, M.; Matsuda, Y.; Mitsuhashi, T.; Hoshino, S.; Mori, T.; Nakagawa, K.; Quan, Z.Y.; Qin, B.; Zhang, H.P.; Hayashi, F.; et al. Genome-based discovery of an unprecedented cyclization mode in fungal sesterterpenoid biosynthesis. J. Am. Chem. Soc. 2016, 138, 10011–10018. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Chen, Q.; Wang, Y.; Zhang, F.; Wang, C.; Wang, G. Heteromerization of short-chain trans-prenyltransferase controls precursor allocation within a plastidial terpenoid network. J. Integr. Plant Biol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Rinkel, J.; Steiner, S.T.; Bian, G.K.; Chen, R.; Liu, T.G.; Dickschat, J.S. A family of related fungal and bacterial di- and sesterterpenes: Studies on fusaterpenol and variediene. Chembiochem 2020, 21, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Gastaldo, C.; Lipko, A.; Motsch, E.; Adam, P.; Schaeffer, P.; Rohmer, M. Biosynthesis of isoprene units in Euphorbia lathyris Laticifers vs. other tissues: MVA and MEP pathways, compartmentation and putative endophytic fungi contribution. Molecules 2019, 24, 4322. [Google Scholar] [CrossRef]
- Hage-Hulsmann, J.; Klaus, O.; Linke, K.; Troost, K.; Gora, L.; Hilgers, F.; Wirtz, A.; Santiago-Schubel, B.; Loeschcke, A.; Jaeger, K.E.; et al. Production of C20, C30 and C40 terpenes in the engineered phototrophic bacterium Rhodobacter capsulatus. J. Biotechnol. 2021, 338, 20–30. [Google Scholar] [CrossRef]
- Miclea, I. Secondary metabolites with biomedical applications from plants of the Sarraceniaceae family. Int. J. Mol. Sci. 2022, 23, 9877. [Google Scholar] [CrossRef]
- Bittner, M.; Schenk, R.; Springer, A.; Melzig, M.F. Profiles of phenolic acids and triterpene glycosides in commercial and cultivated black cohosh. Planta Med. 2019, 85, 1160–1167. [Google Scholar] [CrossRef]
- Parepalli, Y.; Chavali, M.; Sami, R.; Khojah, E.; Elhakem, A.; El Askary, A.; Singh, M.; Sinha, S.; El-Chaghaby, G. Evaluation of some active nutrients, biological compounds and health benefits of reishi mushroom (Ganoderma lucidum). Int. J. Pharmacol. 2021, 17, 243–250. [Google Scholar] [CrossRef]
- Kempinski, C.; Chappell, J. Engineering triterpene metabolism in the oilseed of Arabidopsis thaliana. Plant Biotechnol. J. 2019, 17, 386–396. [Google Scholar] [CrossRef]
- Shin, J.H.; Yoo, H.J.; Yeam, I.; Lee, J.M. Distinguishing two genetic factors that control yellow fruit color in tomato. Hortic. Environ. Biotechnol. 2018, 60, 59–67. [Google Scholar] [CrossRef]
- Maoka, T. Carotenoids as natural functional pigments. J. Nat. Med. 2020, 74, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Ghorat, F.; Ul-Haq, I.; Ur-Rehman, H.; Aslam, F.; Heydari, M.; Shariati, M.A.; Okuskhanova, E.; Yessimbekov, Z.; Thiruvengadam, M.; et al. Lycopene as a natural antioxidant used to prevent human health disorders. Antioxidants 2020, 9, 706. [Google Scholar] [CrossRef] [PubMed]
- Almagro, L.; Correa-Sabater, J.M.; Sabater-Jara, A.B.; Pedreno, M.A. Biotechnological production of β-carotene using plant in vitro cultures. Planta 2022, 256, 41. [Google Scholar] [CrossRef] [PubMed]
- Maj, A.; Dziewit, L.; Drewniak, L.; Garstka, M.; Krucon, T.; Piatkowska, K.; Gieczewska, K.; Czarnecki, J.; Furmanczyk, E.; Lasek, R.; et al. In vivo creation of plasmid pCRT01 and its use for the construction of carotenoid-producing Paracoccus spp. strains that grow efficiently on industrial wastes. Microb. Cell Fact. 2020, 19, 141. [Google Scholar] [CrossRef] [PubMed]
- Adal, A.M.; Sarker, L.S.; Malli, R.P.N.; Liang, P.; Mahmoud, S.S. RNA-Seq in the discovery of a sparsely expressed scent-determining monoterpene synthase in lavender (Lavandula). Planta 2019, 249, 271–290. [Google Scholar] [CrossRef]
- Hoshino, Y.; Moriya, M.; Matsudaira, A.; Katashkina, J.I.; Nitta, N.; Nishio, Y.; Usuda, Y. Stereospecific linalool production utilizing two-phase cultivation system in Pantoea ananatis. J. Biotechnol. 2020, 324, 21–27. [Google Scholar] [CrossRef]
- He, J.; Fandino, R.A.; Halitschke, R.; Luck, K.; Kollner, T.G.; Murdock, M.H.; Ray, R.; Gase, K.; Knaden, M.; Baldwin, I.T.; et al. An unbiased approach elucidates variation in (S)-(+)-linalool, a context-specific mediator of a tri-trophic interaction in wild tobacco. Proc. Natl. Acad. Sci. USA 2019, 116, 14651–14660. [Google Scholar] [CrossRef]
- Zhou, Y.; Deng, R.; Xu, X.; Yang, Z. Enzyme catalytic efficiencies and relative gene expression levels of (R)-linalool synthase and (S)-linalool synthase determine the proportion of linalool enantiomers in Camellia sinensis var. sinensis. J. Agric. Food Chem. 2020, 68, 10109–10117. [Google Scholar] [CrossRef]
- Wroblewska-Kurdyk, A.; Dancewicz, K.; Gliszczynska, A.; Gabrys, B. New insight into the behaviour modifying activity of two natural sesquiterpenoids farnesol and nerolidol towards Myzus persicae (Sulzer) (Homoptera: Aphididae). Bull. Entomol. Res. 2020, 110, 249–258. [Google Scholar] [CrossRef]
- Naskar, S.; Roy, C.; Ghosh, S.; Mukhopadhyay, A.; Hazarika, L.K.; Chaudhuri, R.K.; Roy, S.; Chakraborti, D. Elicitation of biomolecules as host defense arsenals during insect attacks on tea plants (Camellia sinensis (L.) Kuntze). Appl. Microbiol. Biotechnol. 2021, 105, 7187–7199. [Google Scholar] [CrossRef]
- Favaris, A.P.; Tuler, A.C.; Silva, W.D.; Rodrigues, S.R.; Leal, W.S.; Bento, J.M.S. (3S,6E)-nerolidol-mediated rendezvous of Cyclocephala paraguayensis beetles in bottle gourd flowers. PLoS ONE 2020, 15, e0235028. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, T.; Svensson, G.P.; Goto, R.; Kawakita, A.; Kato, M. Nocturnal emission and post-pollination change of floral scent in the leafflower tree, Glochidion rubrum, exclusively pollinated by seed-parasitic leafflower moths. Plant Spec. Biol. 2022, 37, 197–208. [Google Scholar] [CrossRef]
- Ibdah, M.; Hino, S.; Nawade, B.; Yahyaa, M.; Bosamia, T.C.; Shaltiel-Harpaz, L. Identification and characterization of three nearly identical linalool/ nerolidol synthase from Acorus calamus. Phytochemistry 2022, 202, 113318. [Google Scholar] [CrossRef] [PubMed]
- Bihani, T. Plumeria rubra L.—A review on its ethnopharmacological, morphological, phytochemical, pharmacological and toxicological studies. J. Ethnopharmacol. 2021, 264, 113291. [Google Scholar] [CrossRef]
- de Oliveira, J.C.; Pinto, A.D.; de Medeiros, C.A.C.; Ponte, H.A.S.; Pereira, F.D. The sensitivity modifying activity of nerolidol and alpha-bisabolol against Trichophyton spp. Indian. J. Microbiol. 2020, 60, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Ashaari, N.S.; Ab Rahim, M.H.; Sabri, S.; Lai, K.S.; Song, A.A.L.; Rahim, R.A.; Abdullah, W.M.A.N.W.; Abdullah, J.O. Functional characterization of a new terpene synthase from Plectranthus amboinicus. PLoS ONE 2020, 15, e0235416. [Google Scholar] [CrossRef] [PubMed]
- Zager, J.J.; Lange, I.; Srividya, N.; Smith, A.; Lange, B.M. Gene networks underlying cannabinoid and terpenoid accumulation in cannabis. Plant Physiol. 2019, 180, 1877–1897. [Google Scholar] [CrossRef]
- Caiyun, T.; Chengzhe, Z.; Chen, Z.; Xiaozhen, L.; Haifeng, F.; Lan, C.; Zhongxiong, L.; Yuqiong, G. Cloning of CsFPS gene in Camellia sinensis and expression analysis under drought stress and the tea withering process. Chin. J. Appl. Environ. Biol. 2022, 28, 40–49. (In Chinese) [Google Scholar] [CrossRef]
- Darragh, K.; Orteu, A.; Black, D.; Byers, K.J.R.P.; Szczerbowski, D.; Warren, I.A.; Rastas, P.; Pinharanda, A.; Davey, J.W.; Garza, S.F.; et al. A novel terpene synthase controls differences in anti-aphrodisiac pheromone production between closely related Heliconius butterflies. PLoS Biol. 2021, 19, e3001022. [Google Scholar] [CrossRef]
- Abbas, F.; Ke, Y.G.; Zhou, Y.W.; Yu, Y.Y.; Waseem, M.; Ashraf, U.; Li, X.Y.; Yu, R.C.; Fan, Y.P. Genome-wide analysis of ARF transcription factors reveals HcARF5 expression profile associated with the biosynthesis of β-ocimene synthase in Hedychium coronarium. Plant Cell Rep. 2021, 40, 1269–1284. [Google Scholar] [CrossRef]
- Abbas, F.; Nian, X.X.; Zhou, Y.W.; Ke, Y.G.; Liu, L.; Yu, R.C.; Fan, Y.P. Putative regulatory role of hexokinase and fructokinase in terpenoid aroma biosynthesis in Lilium ‘Siberia’. Plant Physiol. Bioch. 2021, 167, 619–629. [Google Scholar] [CrossRef]
- Chen, S.; Xie, P.; Li, Y.; Wang, X.; Liu, H.; Wang, S.; Han, W.; Wu, R.; Li, X.; Guan, Y.; et al. New insights into stress-induced β-ocimene biosynthesis in tea (Camellia sinensis) leaves during oolong tea processing. J. Agric. Food Chem. 2021, 69, 11656–11664. [Google Scholar] [CrossRef]
- Jian, G.; Jia, Y.; Li, J.; Zhou, X.; Liao, Y.; Dai, G.; Zhou, Y.; Tang, J.; Zeng, L. Elucidation of the regular emission mechanism of volatile β-ocimene with anti-insect function from tea plants (Camellia sinensis) exposed to herbivore attack. J. Agric. Food Chem. 2021, 69, 11204–11215. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zeng, L.; Liao, Y.; Li, J.; Tang, J.; Yang, Z. Formation of alpha-farnesene in tea (Camellia sinensis) leaves Induced by herbivore-derived wounding and its effect on neighboring tea plants. Int. J. Mol. Sci. 2019, 20, 4151. [Google Scholar] [CrossRef] [PubMed]
- Schnee, C.; Kollner, T.G.; Held, M.; Turlings, T.C.; Gershenzon, J.; Degenhardt, J. The products of a single maize sesquiterpene synthase form a volatile defense signal that attracts natural enemies of maize herbivores. Proc. Natl. Acad. Sci. USA 2006, 103, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, H.; Li, F.; Chen, Z.; Li, X.; Zhu, L.; Wang, G.; Yu, J.; Huang, D.; Lang, Z. The maize transcription factor EREB58 mediates the jasmonate-induced production of sesquiterpene volatiles. Plant J. 2015, 84, 296–308. [Google Scholar] [CrossRef]
- Wei, C.; Li, M.; Cao, X.; Jin, Z.; Zhang, C.; Xu, M.; Chen, K.; Zhang, B. Linalool synthesis related PpTPS1 and PpTPS3 are activated by transcription factor PpERF61 whose expression is associated with DNA methylation during peach fruit ripening. Plant Sci. 2022, 317, 111200. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Luo, Y.; Zhang, W.; Jian, W.; Zhang, L.; Gao, X.; Hu, X.; Yuan, Y.; Wu, M.; Xu, X.; et al. A SlMYB75-centred transcriptional cascade regulates trichome formation and sesquiterpene accumulation in tomato. J. Exp. Bot. 2021, 72, 3806–3820. [Google Scholar] [CrossRef]
- Kiferle, C.; Fantini, E.; Bassolino, L.; Povero, G.; Spelt, C.; Buti, S.; Giuliano, G.; Quattrocchio, F.; Koes, R.; Perata, P.; et al. Tomato R2R3-MYB proteins SlANT1 and SlAN2: Same protein activity, different roles. PLoS ONE 2015, 10, e0136365. [Google Scholar] [CrossRef]
- Faldt, J.; Arimura, G.; Gershenzon, J.; Takabayashi, J.; Bohlmann, J. Functional identification of AtTPS03 as (E)-β-ocimene synthase: A monoterpene synthase catalyzing jasmonate- and wound-induced volatile formation in Arabidopsis thaliana. Planta 2003, 216, 745–751. [Google Scholar] [CrossRef]
- Xu, Y.H.; Liao, Y.C.; Lv, F.F.; Zhang, Z.; Sun, P.W.; Gao, Z.H.; Hu, K.P.; Sui, C.; Jin, Y.; Wei, J.H. Transcription factor AsMYC2 controls the jasmonate-responsive expression of ASS1 regulating sesquiterpene biosynthesis in Aquilaria sinensis (Lour.) Gilg. Plant Cell Physiol. 2017, 58, 1924–1933. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Zhang, S.; Zhou, C.; Chen, L.; Zaripov, T.; Zhan, D.; Weng, J.; Lin, Y.; Lai, Z.; Guo, Y. Integrated transcriptome, microRNA, and phytochemical analyses reveal roles of phytohormone signal transduction and ABC transporters in flavor formation of oolong tea (Camellia sinensis) during solar withering. J. Agric. Food Chem. 2020, 68, 12749–12767. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Gu, M.; Yu, X.; Shao, S.; Du, J.; Wang, Y.; Wang, F.; Chen, S.; Liao, Z.; Ye, N.; et al. Allele-specific expression and chromatin accessibility contribute to heterosis in tea plants (Camellia sinensis). Plant J. 2022, 112, 1194–1211. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).