Unveiling the Genomic Basis of Chemosensitivity in Sarcomas of the Extremities: An Integrated Approach for an Unmet Clinical Need
Abstract
1. Introduction
2. Results
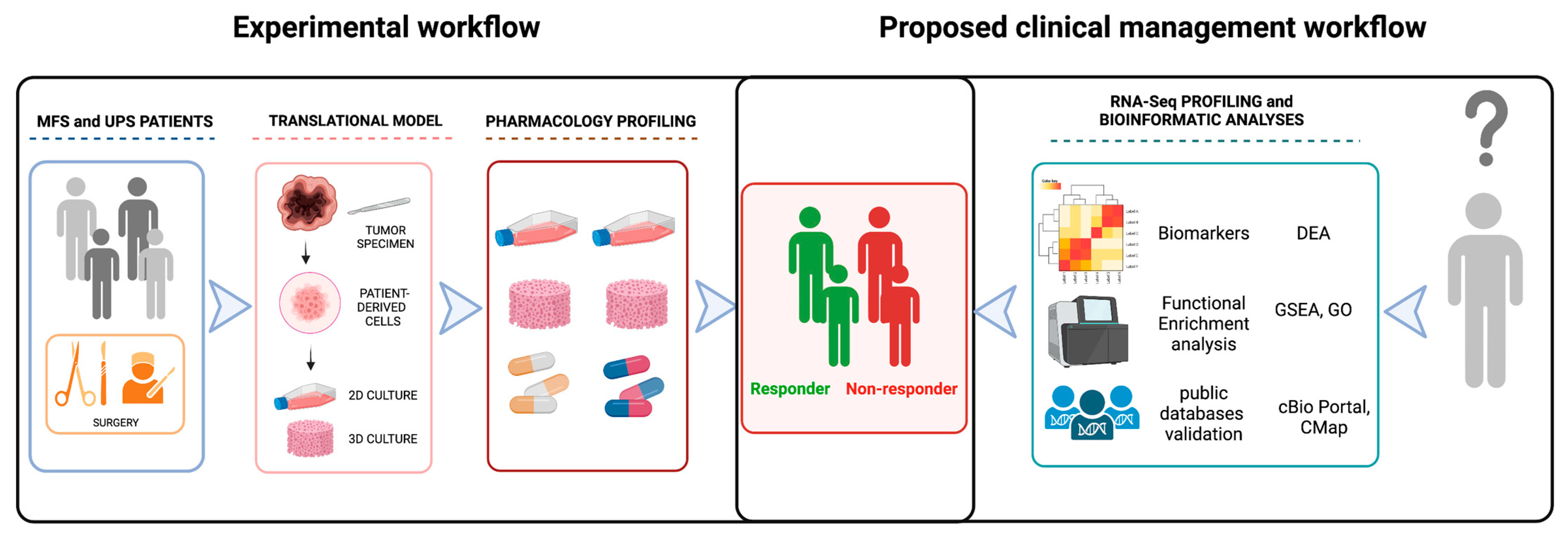
2.1. Patients
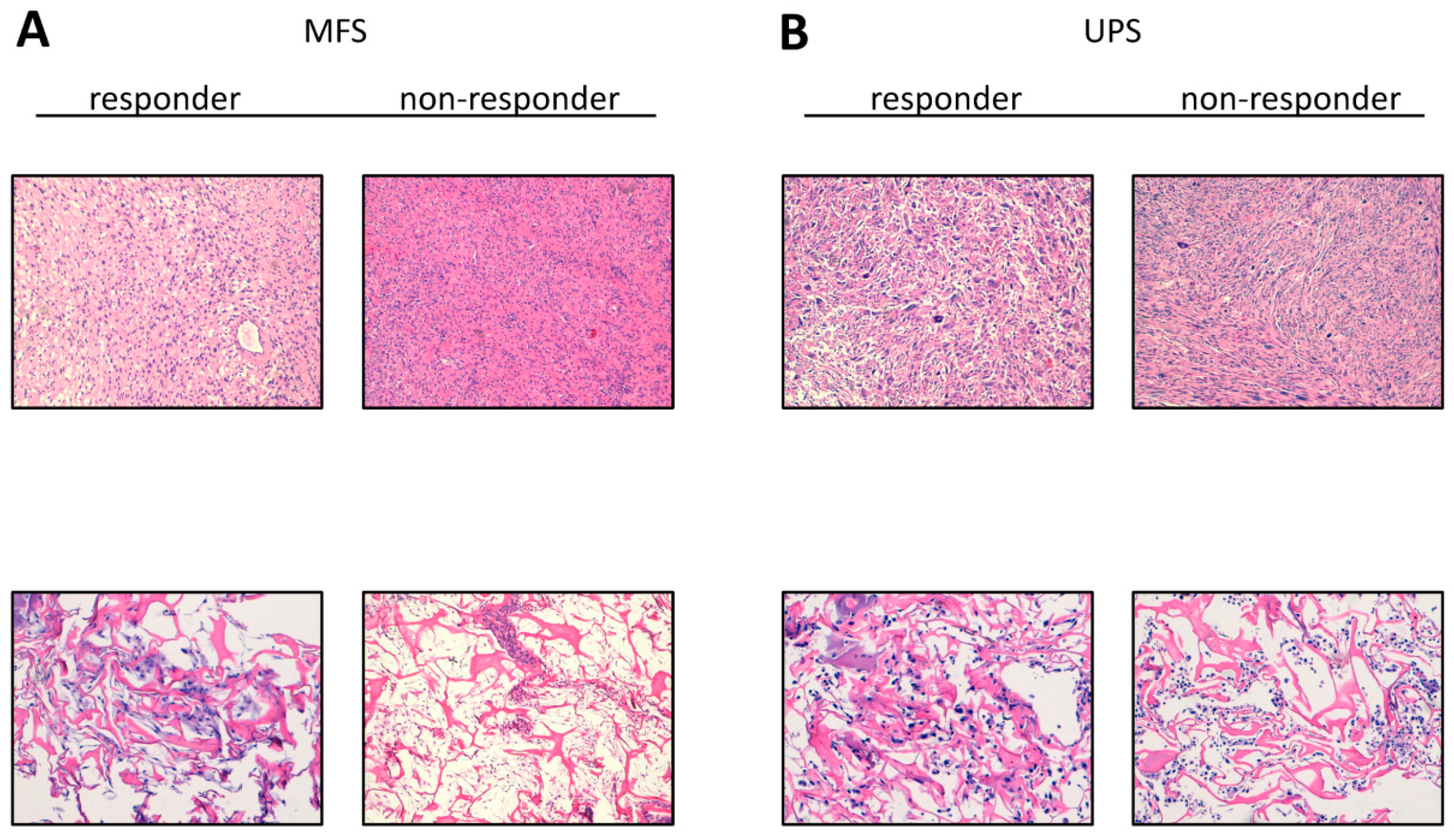
2.2. Establishment of Patient-Derived MFS and UPS Primary Cell Lines
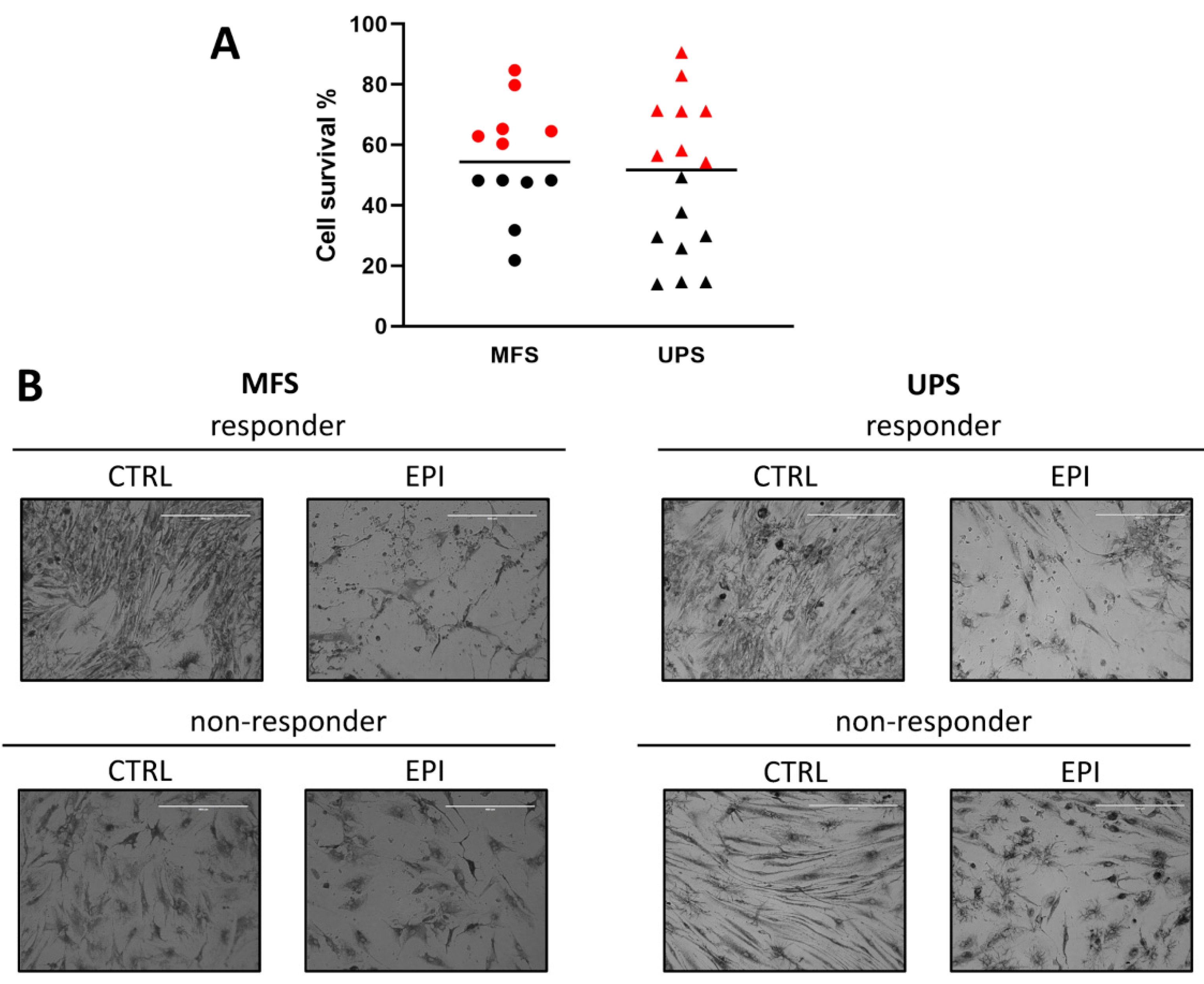
2.3. Chemobiogram Assessment
2.4. RNA Sequencing Profiling
2.5. Analyses of Public Databases
3. Discussion
4. Materials and Methods
4.1. Case Series
4.2. Collagen-Based Scaffold 3D Culture Model Synthesis
4.3. Histologic Characterization of Tumor Specimens and Derived Primary Cell Lines
4.4. Isolation of Patient-Derived UPS and MFS Cell Lines
4.5. Pharmacological Profiling
4.6. Tissues and RNA Extraction
4.7. Next Generation Sequencing Analysis
4.8. Bioinformatic NGS Data Analysis
4.9. In Silico Analysis on Public Clinical Dataset
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO Classification of Tumors Editorial Board. Soft Tissue and Bone Tumors: WHO Classification of Tumours, 5th ed.; IARC Publications: Geneva, Switzerland, 2020; Volume 3. [Google Scholar]
- Pasquali, S.; Gronchi, A. Neoadjuvant chemotherapy in soft tissue sarcomas: Latest evidence and clinical implications. Ther. Adv. Med. Oncol. 2017, 9, 415–429. [Google Scholar] [CrossRef]
- Widemann, B.C.; Italiano, A. Biology and management of undifferentiated pleomorphic sarcoma, myxofibrosarcoma, and malignant peripheral nerve sheath tumors: State of the art and perspectives. J. Clin. Oncol. 2018, 36, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Sbaraglia, M.; Bellan, E.; Dei Tos, A.P. The 2020 WHO Classification of Soft Tissue Tumours: News and perspectives. Pathologica 2021, 113, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Vanni, S.; De Vita, A.; Gurrieri, L.; Fausti, V.; Miserocchi, G.; Spadazzi, C.; Liverani, C.; Cocchi, C.; Calabrese, C.; Bongiovanni, A.; et al. Myxofibrosarcoma landscape: Diagnostic pitfalls, clinical management and future perspectives. Ther. Adv. Med. Oncol. 2022, 14, 17588359221093973. [Google Scholar] [CrossRef]
- Fausti, V.; De Vita, A.; Vanni, S.; Ghini, V.; Gurrieri, L.; Riva, N.; Casadei, R.; Maraldi, M.; Ercolani, G.; Cavaliere, D.; et al. Systemic Inflammatory Indices in Second-Line Soft Tissue Sarcoma Patients: Focus on Lymphocyte/Monocyte Ratio and Trabectedin. Cancers 2023, 15, 1080. [Google Scholar] [CrossRef] [PubMed]
- De Vita, A.; Recine, F.; Mercatali, L.; Miserocchi, G.; Spadazzi, C.; Liverani, C.; Bongiovanni, A.; Pieri, F.; Casadei, R.; Riva, N.; et al. Primary Culture of Undifferentiated Pleomorphic Sarcoma: Molecular Characterization and Response to Anticancer Agents. Int. J. Mol. Sci. 2017, 18, 2662. [Google Scholar] [CrossRef]
- Kawai, A.; Yonemori, K.; Takahashi, S.; Araki, N.; Ueda, T. Systemic Therapy for Soft Tissue Sarcoma: Proposals for the Optimal Use of Pazopanib, Trabectedin, and Eribulin. Adv. Ther. 2017, 34, 1556–1571. [Google Scholar] [CrossRef]
- Grünewald, T.G.; Alonso, M.; Avnet, S.; Banito, A.; Burdach, S.; Cidre-Aranaz, F.; Di Pompo, G.; Distel, M.; Dorado-Garcia, H.; Garcia-Castro, J.; et al. Sarcoma treatment in the era of molecular medicine. EMBO Mol. Med. 2020, 12, e11131. [Google Scholar] [CrossRef]
- Vyse, S.; Thway, K.; Huang, P.H.; Jones, R.L. Next-generation sequencing for the management of sarcomas with no known driver mutations. Curr. Opin. Oncol. 2021, 33, 315–322. [Google Scholar] [CrossRef]
- Yoshimoto, M.; Yamada, Y.; Ishihara, S.; Kohashi, K.; Toda, Y.; Ito, Y.; Yamamoto, H.; Furue, M.; Nakashima, Y.; Oda, Y. Comparative Study of Myxofibrosarcoma With Undifferentiated Pleomorphic Sarcoma: Histopathologic and Clinicopathologic Review. Am. J. Surg. Pathol. 2020, 44, 87–97. [Google Scholar] [CrossRef]
- Crago, A.M.; Cardona, K.; Koseła-Paterczyk, H.; Rutkowski, P. Management of Myxofibrosarcoma and Undifferentiated Pleomorphic Sarcoma. Surg. Oncol. Clin. N. Am. 2022, 31, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Gounder, M.M.; Agaram, N.P.; Trabucco, S.E.; Robinson, V.; Ferraro, R.A.; Millis, S.Z.; Krishnan, A.; Lee, J.; Attia, S.; Abida, W.; et al. Clinical genomic profiling in the management of patients with soft tissue and bone sarcoma. Nat. Commun. 2022, 13, 3406. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Huang, W.; Luo, P.; Cai, W.; Yang, L.; Sun, Z.; Zheng, B.; Yan, W.; Wang, C. Undifferentiated Pleomorphic Sarcoma: Long-Term Follow-Up from a Large Institution. Cancer Manag. Res. 2019, 11, 10001–10009. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhao, M.; Tian, A.; Zhang, X.; Yao, Z.; Ma, X. Aberrant activation of Wnt/β-catenin signaling drives proliferation of bone sarcoma cells. Oncotarget 2015, 6, 17570–17583. [Google Scholar] [CrossRef]
- Tian, H.; Zhou, T.; Chen, H.; Li, C.; Jiang, Z.; Lao, L.; Kahn, S.A.; Duarte, M.E.L.; Zhao, J.; Daubs, M.D.; et al. Bone morphogenetic protein-2 promotes osteosarcoma growth by promoting epithelial-mesenchymal transition (EMT) through the Wnt/β-catenin signaling pathway. J. Orthop. Res. 2019, 37, 1638–1648. [Google Scholar] [CrossRef]
- Martinez-Font, E.; Pérez-Capó, M.; Vögler, O.; Martín-Broto, J.; Alemany, R.; Obrador-Hevia, A. WNT/β-Catenin Pathway in Soft Tissue Sarcomas: New Therapeutic Opportunities? Cancers 2021, 13, 5521. [Google Scholar] [CrossRef]
- Xu, S.; Xu, H.; Wang, W.; Li, S.; Li, H.; Li, T.; Zhang, W.; Yu, X.; Liu, L. The role of collagen in cancer: From bench to bedside. J. Transl. Med. 2019, 17, 309. [Google Scholar] [CrossRef]
- Scheid, A.D.; Beadnell, T.C.; Welch, D.R. Roles of mitochondria in the hallmarks of metastasis. Br. J. Cancer 2021, 124, 124–135. [Google Scholar] [CrossRef]
- Recine, F.; De Vita, A.; Fausti, V.; Pieri, F.; Bongiovanni, A.; Franchini, E.; Casadei, R.; Falasconi, M.C.; Oboldi, D.; Matteucci, F.; et al. Case Report: Adult NTRK-Rearranged Spindle Cell Neoplasm: Early Tumor Shrinkage in a Case With Bone and Visceral Metastases Treated With Targeted Therapy. Front. Oncol. 2022, 11, 740676. [Google Scholar] [CrossRef]
- Plebuch, M.; Soldan, M.; Hungerer, C.; Koch, L.; Maser, E. Increased resistance of tumor cells to daunorubicin after transfection of cDNAs coding for anthracycline inactivating enzymes. Cancer Lett. 2007, 255, 49–56. [Google Scholar] [CrossRef]
- Zhong, T.; Xu, F.; Xu, J.; Liu, L.; Chen, Y. Aldo-keto reductase 1C3 (AKR1C3) is associated with the doxorubicin resistance in human breast cancer via PTEN loss. Biomed. Pharmacother. 2015, 69, 317–325. [Google Scholar] [CrossRef] [PubMed]
- De Larco, J.E.; Wuertz, B.R.; Furcht, L.T. The potential role of neutrophils in promoting the metastatic phenotype of tumors releasing interleukin-8. Clin. Cancer Res. 2004, 10, 4895–4900. [Google Scholar] [CrossRef]
- Kitamura, T.; Qian, B.Z.; Pollard, J.W. Immune cell promotion of metastasis. Nat. Rev. Immunol. 2015, 15, 73–86. [Google Scholar] [CrossRef]
- Uhlén, M.; Fagerberg, L.; Hallström, B.; Lindskog, C.; Oksvold, P.; Mardinoğlu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Proteomics. Tissue-based map of the human proteome. Science (New York N.Y.) 2015, 347, 1260419. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Narayan, R.; Corsello, S.M.; Peck, D.; Natoli, T.E.; Lu, X.; Gould, J.; Davis, J.F.; Tubelli, A.A.; Asiedu, J.K.; et al. A Next Generation Connectivity Map: L1000 Platform and the First 1,000,000 Profiles. Cell 2017, 171, 1437–1452.e17. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-Y.; Chiao, C.-C.; Phan, N.; Li, C.-Y.; Sun, Z.; Jiang, J.-Z.; Hung, J.-H.; Chen, Y.-L.; Yen, M.; Weng, T.-Y.; et al. Gene signatures and potential therapeutic targets of amino acid metabolism in estrogen receptor-positive breast cancer. Am. J. Cancer Res. 2020, 10, 95–113. [Google Scholar]
- De Vita, A.; Vanni, S.; Fausti, V.; Cocchi, C.; Recine, F.; Miserocchi, G.; Liverani, C.; Spadazzi, C.; Bassi, M.; Gessaroli, M.; et al. Deciphering the Genomic Landscape and Pharmacological Profile of Uncommon Entities of Adult Rhabdomyosarcomas. Int. J. Mol. Sci. 2021, 22, 11564. [Google Scholar] [CrossRef]
- De Vita, A.; Recine, F.; Miserocchi, G.; Pieri, F.; Spadazzi, C.; Cocchi, C.; Vanni, S.; Liverani, C.; Farnedi, A.; Fabbri, F.; et al. The potential role of the extracellular matrix in the activity of trabectedin in UPS and L-sarcoma: Evidences from a patient-derived primary culture case series in tridimensional and zebrafish models. J. Exp. Clin. Cancer Res. 2021, 40, 165. [Google Scholar] [CrossRef]
- Fogli, S.; Danesi, R.; Gennari, A.; Donati, S.; Conte, P.F.; del Tacca, M. Gemcitabine, epirubicin and paclitaxel: Pharmacokinetic and pharmacodynamic interactions in advanced breast cancer. Ann. Oncol. 2002, 13, 919–927. [Google Scholar] [CrossRef]
- De Vita, A.; Recine, F.; Mercatali, L.; Miserocchi, G.; Liverani, C.; Spadazzi, C.; Casadei, R.; Bongiovanni, A.; Pieri, F.; Riva, N.; et al. Myxofibrosarcoma primary cultures: Molecular and pharmacological profile. Ther. Adv. Med. Oncol. 2017, 9, 755–767. [Google Scholar] [CrossRef]
- Bray, N.L.; Pimentel, H.; Melsted, P.; Pachter, L. Near-optimal probabilistic RNA-seq quantification. Nat. Biotechnol. 2016, 34, 525–527. [Google Scholar] [CrossRef] [PubMed]
- Soneson, C.; Love, M.I.; Robinson, M.D. Differential analyses for RNA-seq: Transcript-level estimates improve gene-level inferences. F1000Research 2015, 4, 1521. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Waskom, M.L. Seaborn: Statistical data visualization. JOSS 2021, 6, 3021. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Liu, X.; Peltz, G. GSEApy: A comprehensive package for performing gene set enrichment analysis in Python. Bioinformatics 2023, 39, btac757. [Google Scholar] [CrossRef]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 2013, 6, pl1. [Google Scholar] [CrossRef]



| Sex Male 20 (MFS 10, UPS 10) (51.3%) |
| Female 19 (MFS 11, UPS 9) (48.7%) |
| Age at treatment |
| Median ± SD (range) 69.19 ± 13.58 (44–90) |
| Histotype |
| Myxofibrosarcoma 19 (46.7%) |
| Undifferentiated Pleomorphic Sarcoma 20 (53.3%) |
| Chemotherapy |
| Anthracycline-based regimen (4) (10.2%) Radiotherapy Adjuvant RT (9) (23.1%) |
| Tumor burden |
| Recurrence (15) (38.5%) |
| Primitive (24) (61.5%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vanni, S.; Fausti, V.; Fonzi, E.; Liverani, C.; Miserocchi, G.; Spadazzi, C.; Cocchi, C.; Calabrese, C.; Gurrieri, L.; Riva, N.; et al. Unveiling the Genomic Basis of Chemosensitivity in Sarcomas of the Extremities: An Integrated Approach for an Unmet Clinical Need. Int. J. Mol. Sci. 2023, 24, 6926. https://doi.org/10.3390/ijms24086926
Vanni S, Fausti V, Fonzi E, Liverani C, Miserocchi G, Spadazzi C, Cocchi C, Calabrese C, Gurrieri L, Riva N, et al. Unveiling the Genomic Basis of Chemosensitivity in Sarcomas of the Extremities: An Integrated Approach for an Unmet Clinical Need. International Journal of Molecular Sciences. 2023; 24(8):6926. https://doi.org/10.3390/ijms24086926
Chicago/Turabian StyleVanni, Silvia, Valentina Fausti, Eugenio Fonzi, Chiara Liverani, Giacomo Miserocchi, Chiara Spadazzi, Claudia Cocchi, Chiara Calabrese, Lorena Gurrieri, Nada Riva, and et al. 2023. "Unveiling the Genomic Basis of Chemosensitivity in Sarcomas of the Extremities: An Integrated Approach for an Unmet Clinical Need" International Journal of Molecular Sciences 24, no. 8: 6926. https://doi.org/10.3390/ijms24086926
APA StyleVanni, S., Fausti, V., Fonzi, E., Liverani, C., Miserocchi, G., Spadazzi, C., Cocchi, C., Calabrese, C., Gurrieri, L., Riva, N., Recine, F., Casadei, R., Pieri, F., Guerrieri, A. N., Serra, M., Ibrahim, T., Mercatali, L., & De Vita, A. (2023). Unveiling the Genomic Basis of Chemosensitivity in Sarcomas of the Extremities: An Integrated Approach for an Unmet Clinical Need. International Journal of Molecular Sciences, 24(8), 6926. https://doi.org/10.3390/ijms24086926











