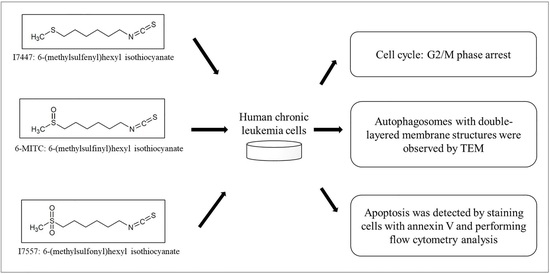
Anti-Cancer Effects of Oxygen-Atom-Modified Derivatives of Wasabi Components on Human Leukemia Cells
Abstract
1. Introduction
2. Results
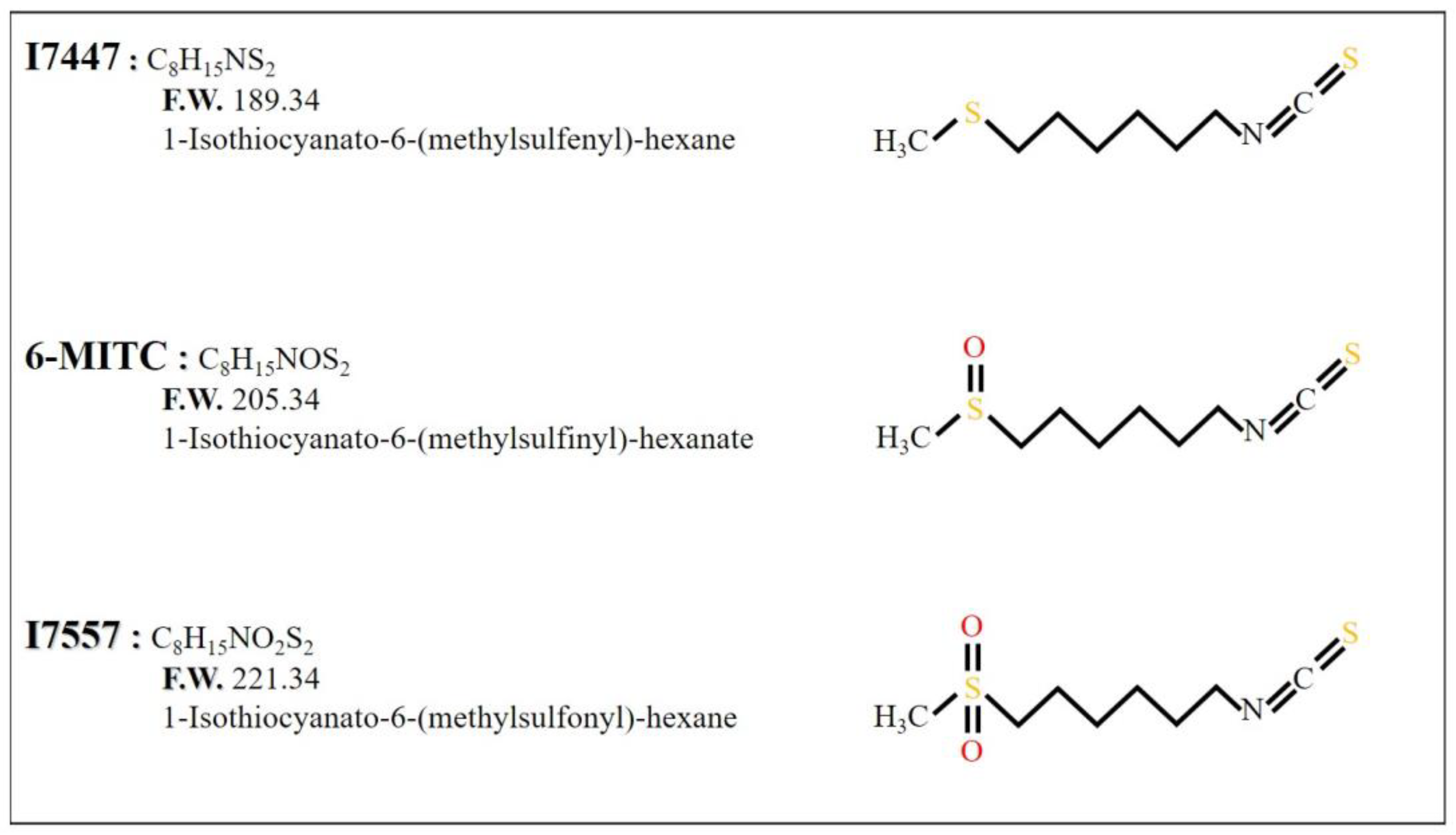
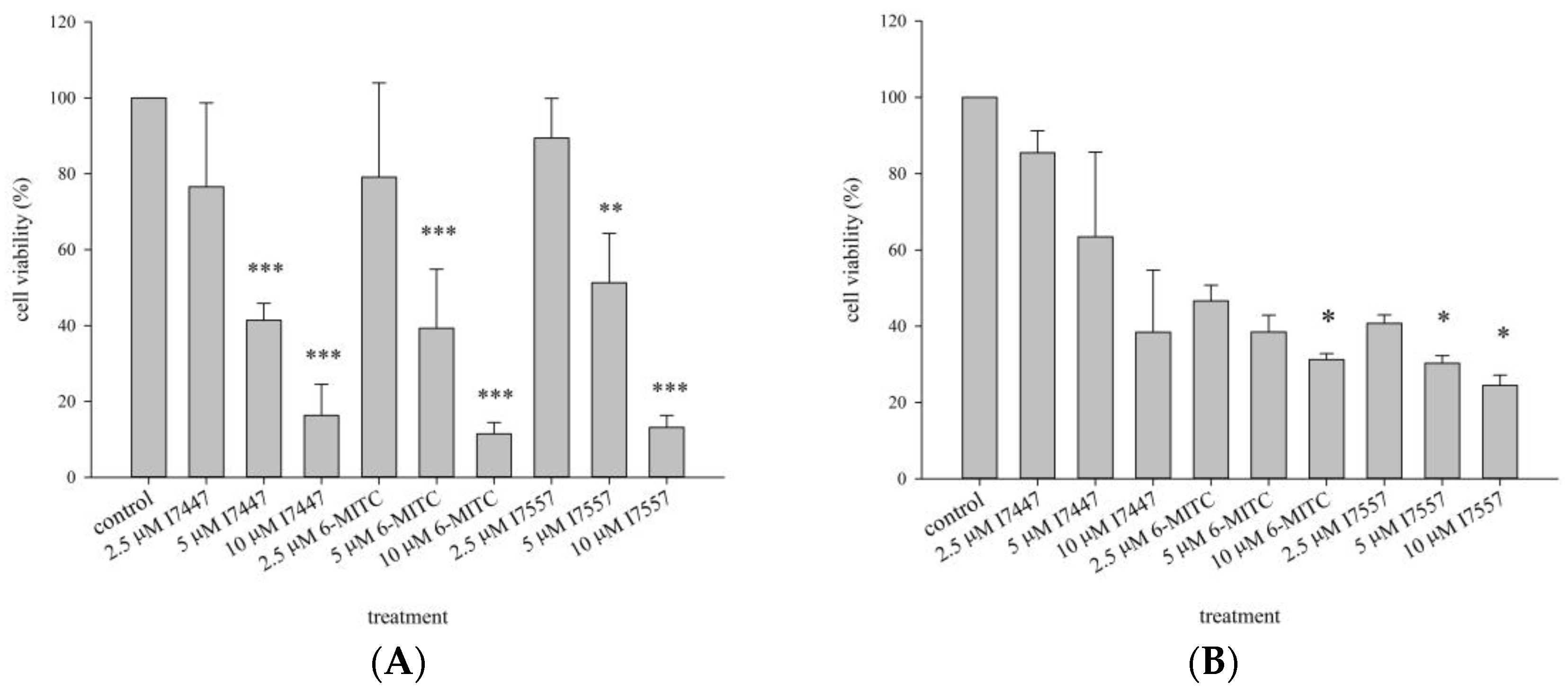
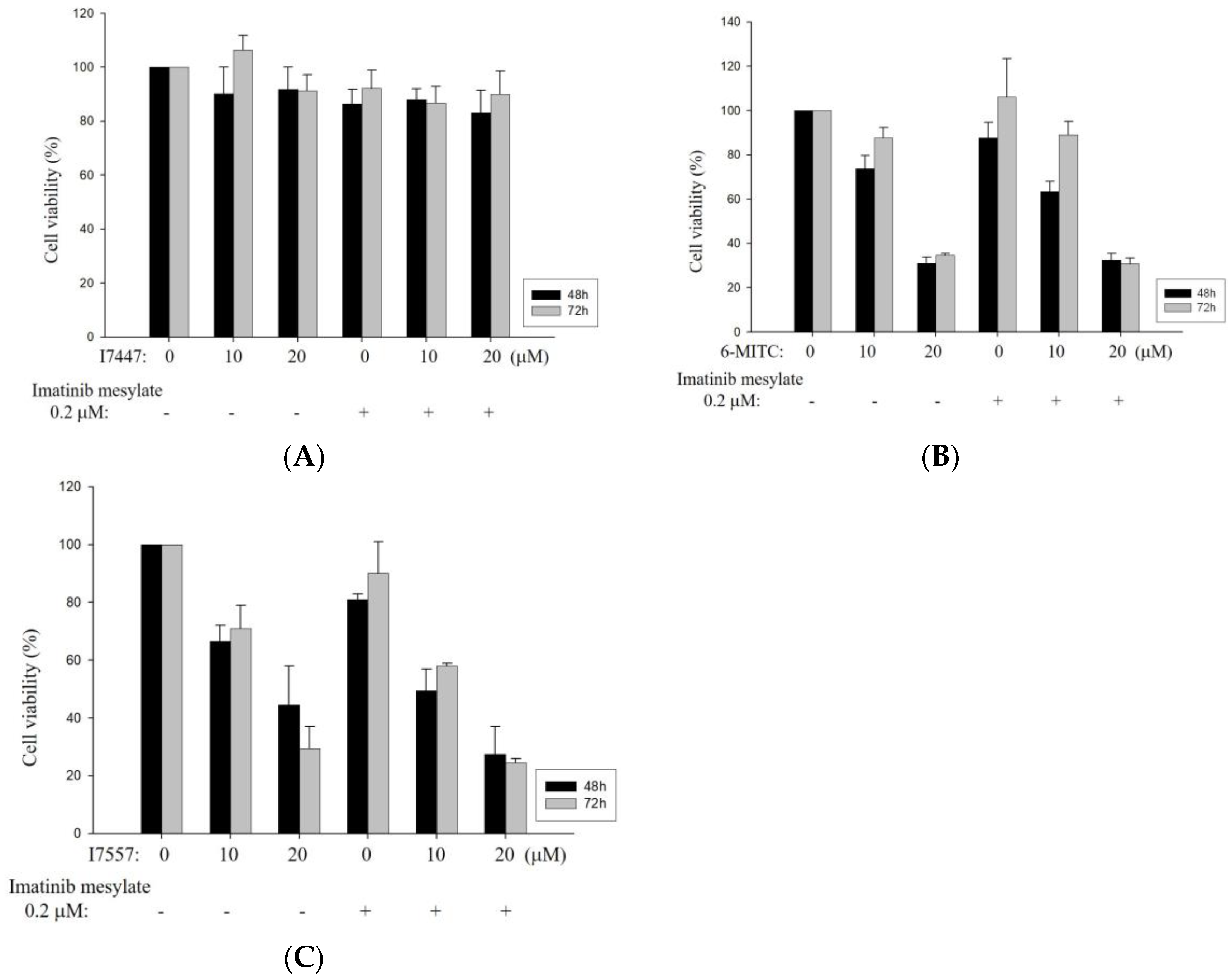
2.1. Growth Inhibition of I7447-, 6-MITC-, and I7557-Treated Human K562 and HEL Cells
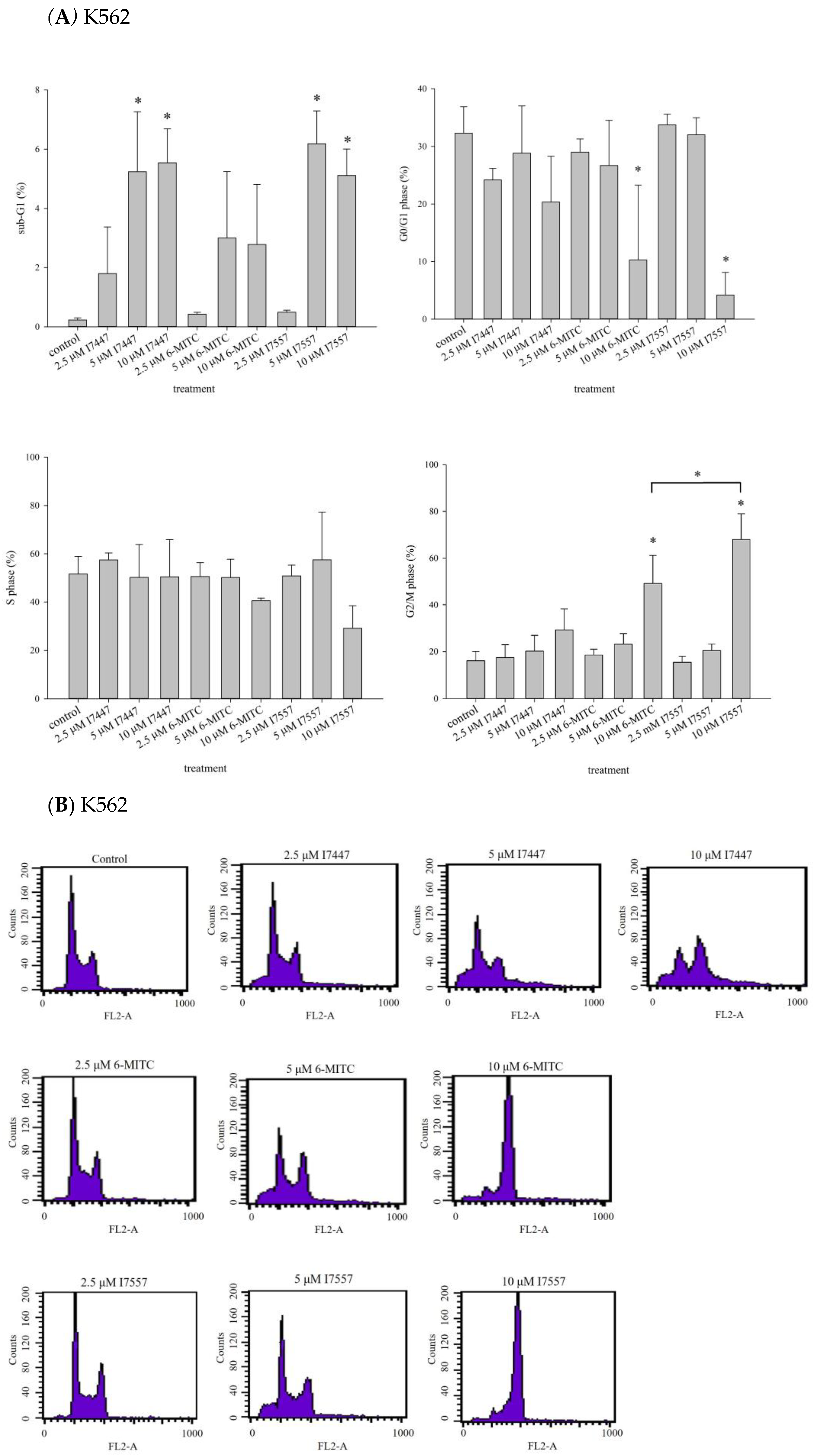
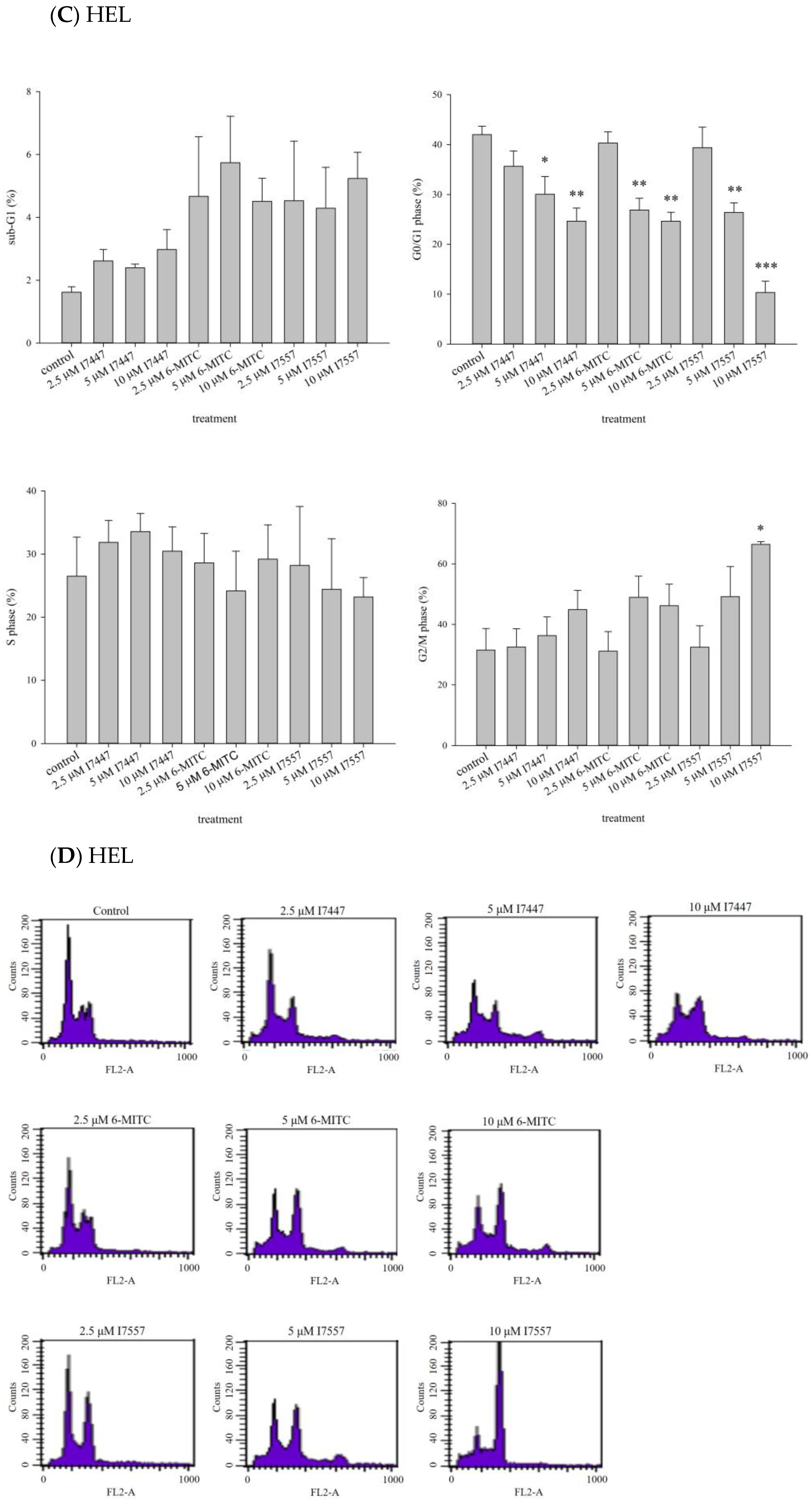
2.2. Impact of I7447, 6-MITC, and I7557 on the Cell Cycle
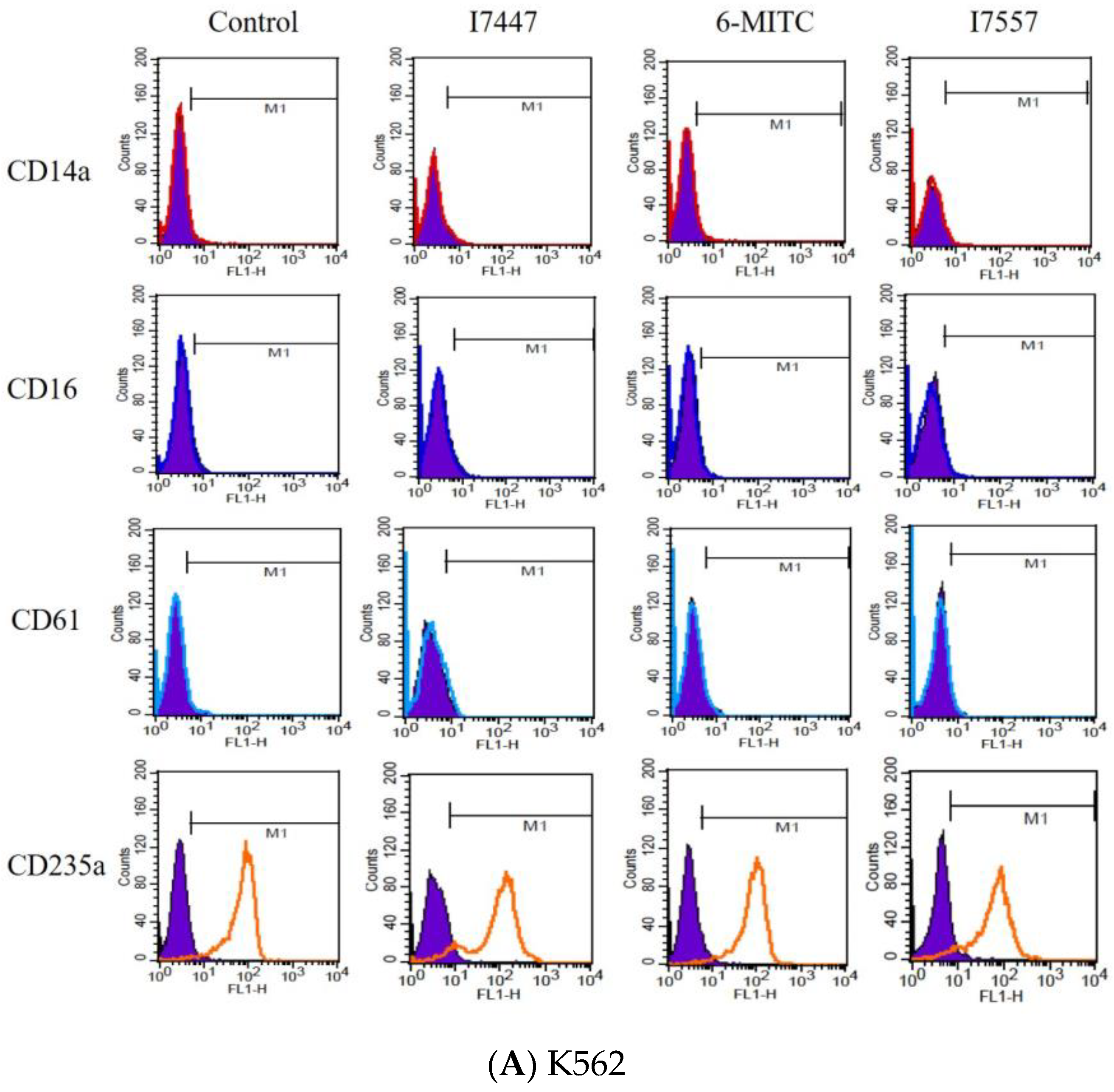
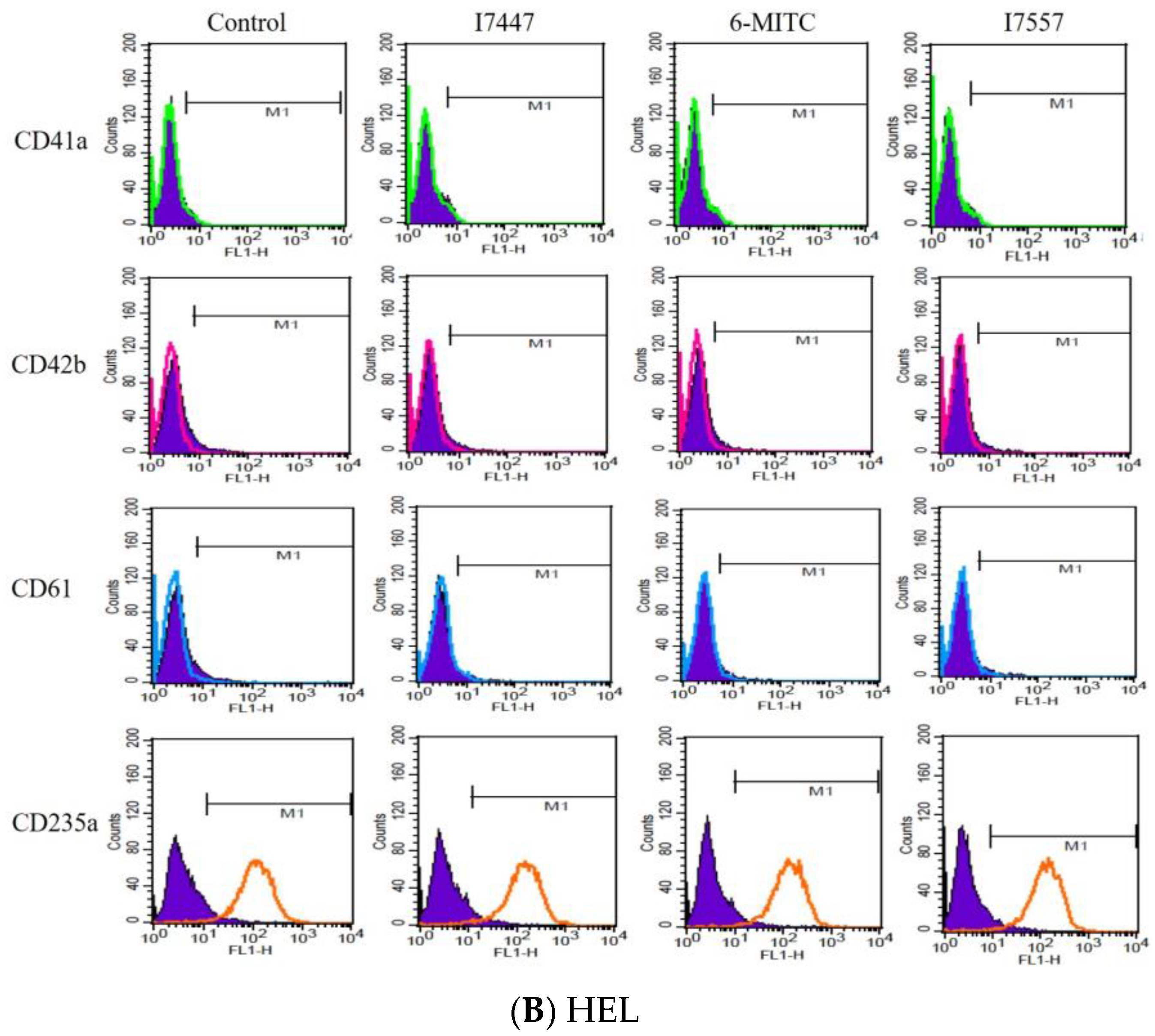
2.3. Exclusion of Cell Differentiation by I7447, 6-MITC, and I7557
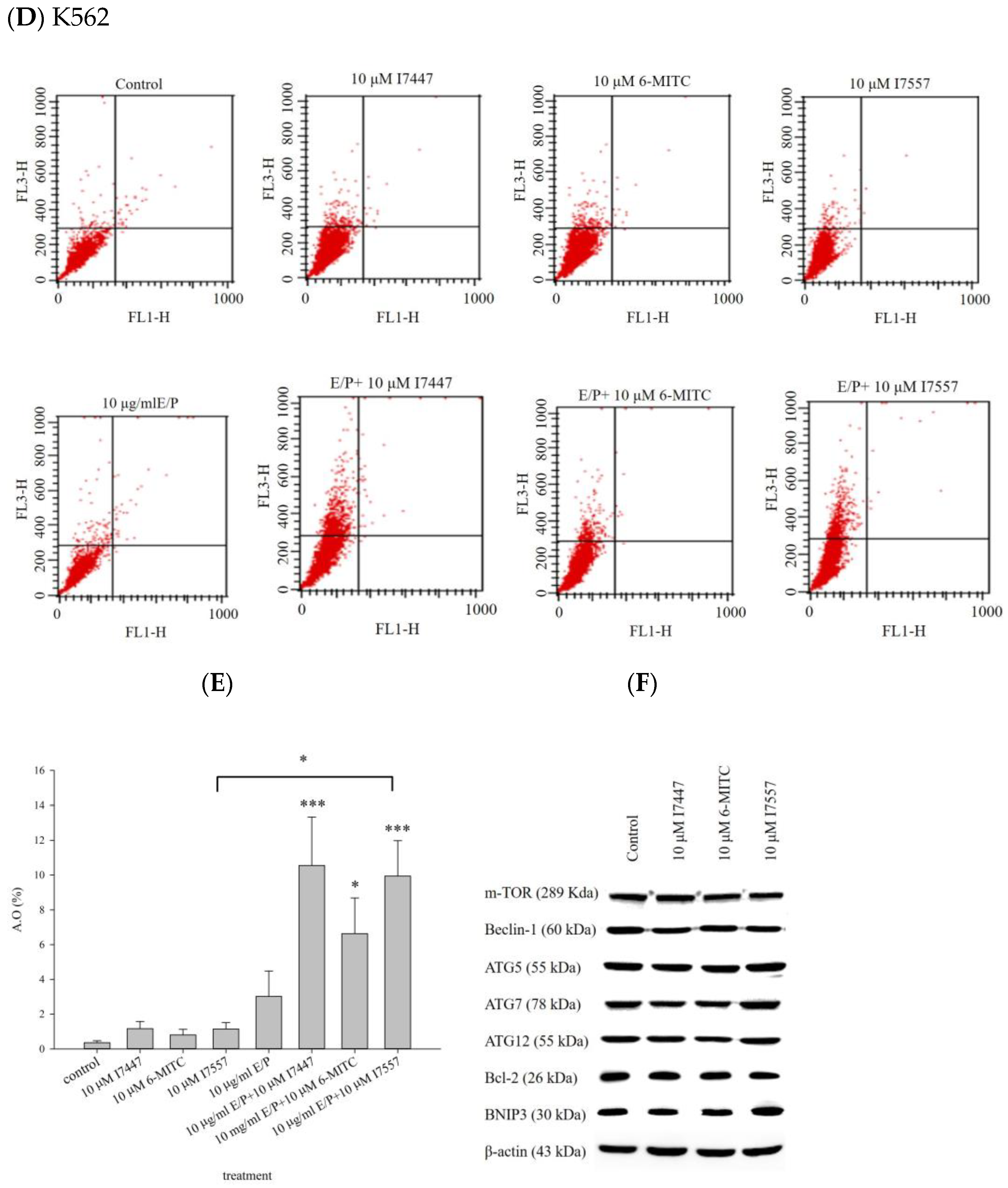
2.4. Autophagy in I7447-, 6-MITC-, and I7557-Treated Human K562 Cells
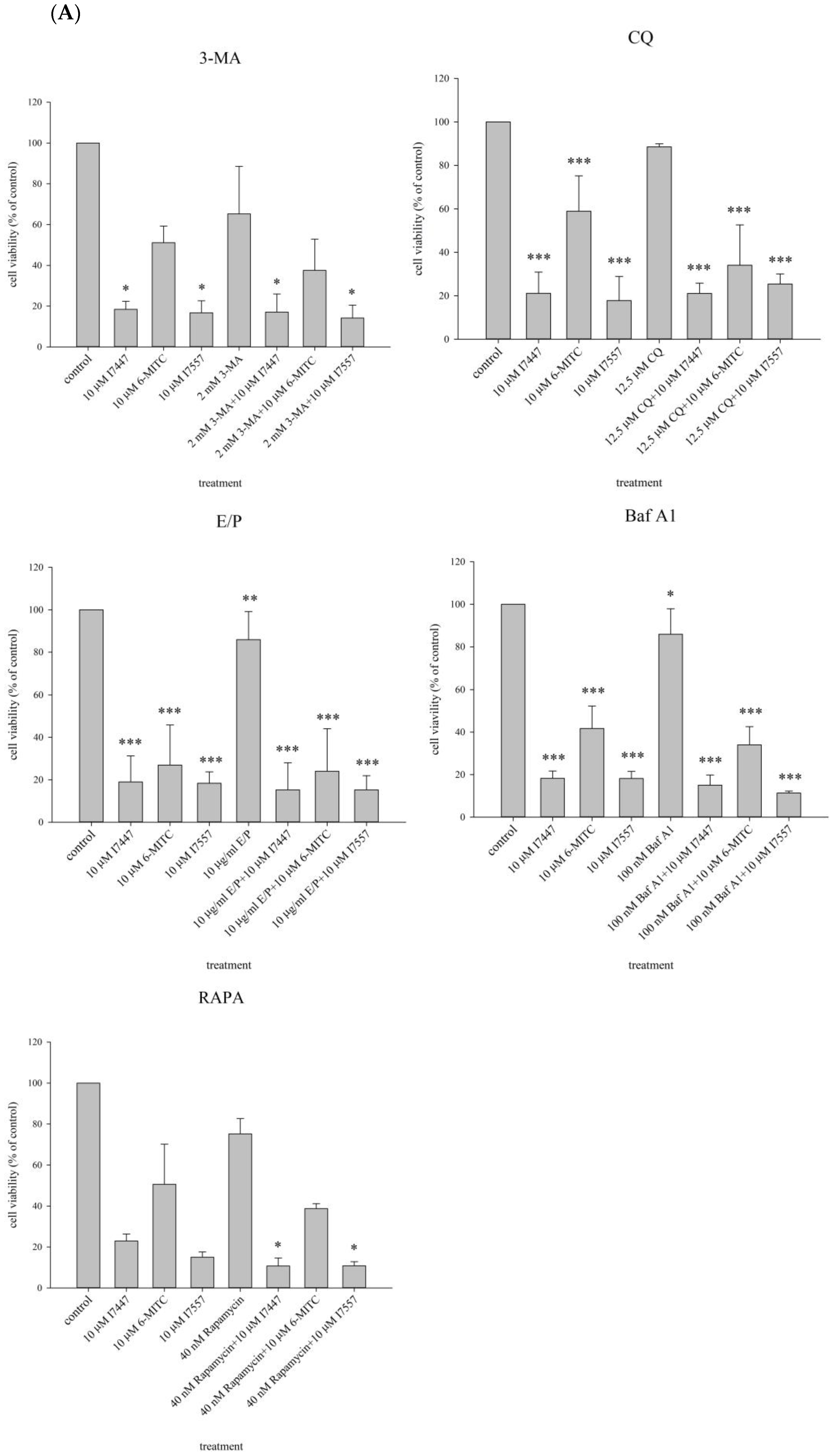
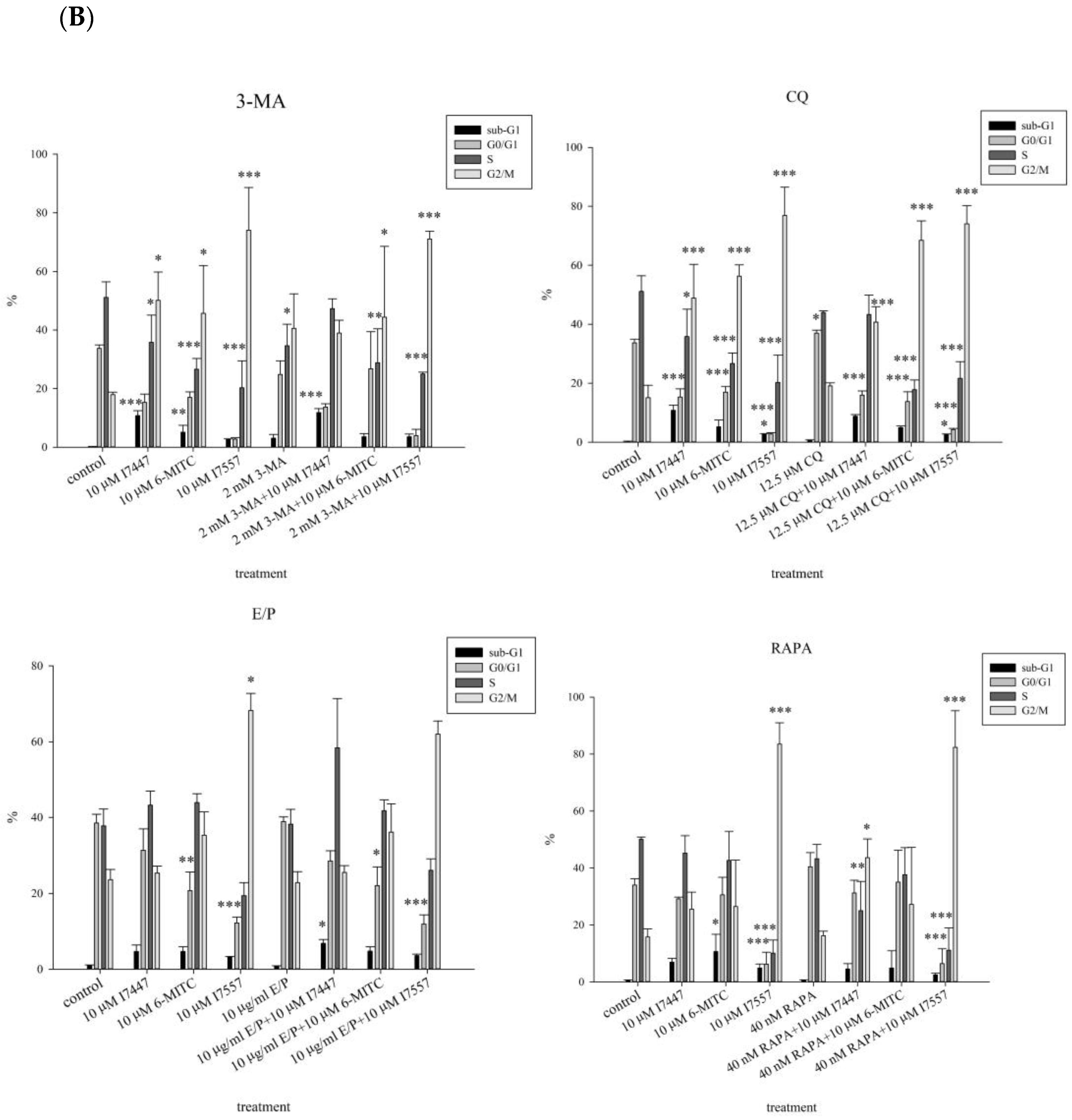
2.5. Cell Viability and Cell Cycle Analysis after Treatment with an Autophagy Inhibitor
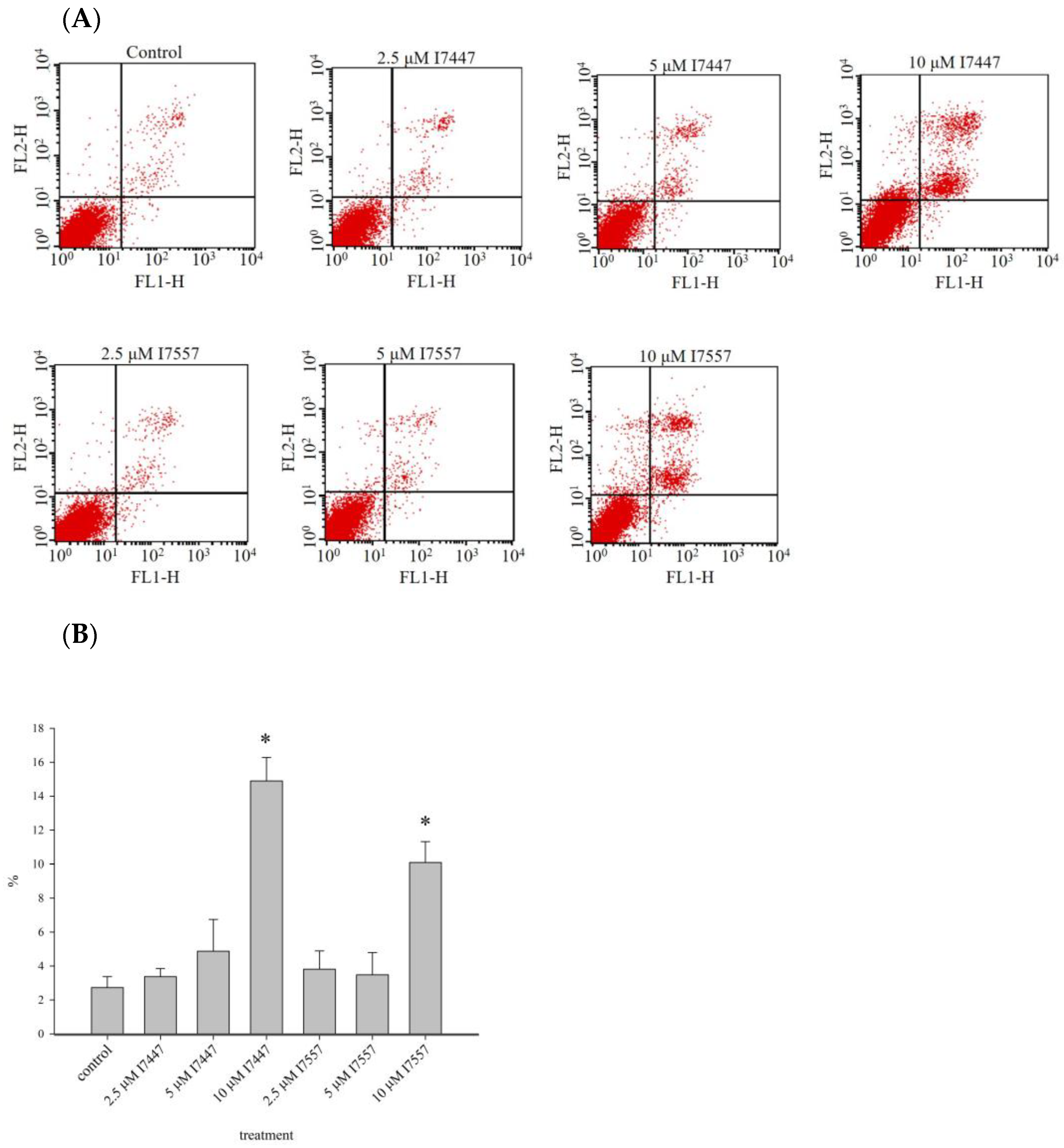
2.6. Apoptosis Induction by I7447 and I7557
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Cell Lines and Culture
4.3. Cell Viability
4.4. Morphology Based on Light and Transmission Electron Microscopy
4.5. Cell Cycle Analysis Using a DNA Histogram
4.6. Surface Antigen Assay for Differentiation
4.7. Acridine Orange Stain
4.8. Western Blot Analysis
4.9. Detection of Apoptosis with Annexin V Staining
4.10. Immunofluorescent Staining
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rowley, J.D. Letter: A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature 1973, 243, 290–293. [Google Scholar] [CrossRef] [PubMed]
- Goldman, J.M.; Melo, J.V. Chronic myeloid leukemia--advances in biology and new approaches to treatment. N. Engl. J. Med. 2003, 349, 1451–1464. [Google Scholar] [CrossRef] [PubMed]
- Kaleem, B.; Shahab, S.; Ahmed, N.; Shamsi, T.S. Chronic Myeloid Leukemia--Prognostic Value of Mutations. Asian Pac. J. Cancer Prev. 2015, 16, 7415–7423. [Google Scholar] [CrossRef]
- Sell, S. Leukemia. Stem Cell Rev. 2005, 1, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Hochhaus, A.; Baccarani, M.; Silver, R.T.; Schiffer, C.; Apperley, J.F.; Cervantes, F.; Clark, R.E.; Cortes, J.E.; Deininger, M.W.; Guilhot, F.; et al. European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia 2020, 34, 966–984. [Google Scholar] [CrossRef]
- Guilhot, F.; Roy, L.; Guilhot, J.; Millot, F. Interferon therapy in chronic myelogenous leukemia. Hematol. Oncol. Clin. N. Am. 2004, 18, 585–603. [Google Scholar] [CrossRef]
- Barrett, A.J.; Ito, S. The role of stem cell transplantation for chronic myelogenous leukemia in the 21st century. Blood 2015, 125, 3230–3235. [Google Scholar] [CrossRef]
- Horowitz, M.M.; Rowlings, P.A.; Passweg, J.R. Allogeneic bone marrow transplantation for CML: A report from the International Bone Marrow Transplant Registry. Bone Marrow Transplant. 1996, 17 (Suppl. S3), S5–S6. [Google Scholar]
- Hehlmann, R.; Heimpel, H.; Hasford, J.; Kolb, H.J.; Pralle, H.; Hossfeld, D.K.; Queiβer, W.; Löffler, H.; Heinze, B.; Georgii, A.; et al. Randomized comparison of busulfan and hydroxyurea in chronic myelogenous leukemia: Prolongation of survival by hydroxyurea. The German CML Study Group. Blood 1993, 82, 398–407. [Google Scholar] [CrossRef]
- Zuo, S.; Sun, L.; Wang, Y.; Chen, B.; Wang, J.; Ge, X.; Lu, Y.; Yang, N.; Shen, P. Establishment of a novel mesenchymal stem cell-based regimen for chronic myeloid leukemia differentiation therapy. Cell Death Dis. 2021, 12, 208. [Google Scholar] [CrossRef]
- Melo, J.V.; Gordon, D.E.; Cross, N.C.; Goldman, J.M. The ABL-BCR fusion gene is expressed in chronic myeloid leukemia. Blood 1993, 81, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Burslem, G.M.; Schultz, A.R.; Bondeson, D.P.; Eide, C.A.; Savage Stevens, S.L.; Druker, B.J.; Crews, C.M. Targeting BCR-ABL1 in Chronic Myeloid Leukemia by PROTAC-Mediated Targeted Protein Degradation. Cancer Res. 2019, 79, 4744–4753. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Chattopadhyay, S.; Das, N.; Shree, S.; Patel, D.; Mohapatra, J.; Gurjar, A.; Kushwaha, S.; Singh, A.K.; Dubey, S.; et al. Leprosy drug clofazimine activates peroxisome proliferator-activated receptor-γ and synergizes with imatinib to inhibit chronic myeloid leukemia cells. Haematologica 2020, 105, 971–986. [Google Scholar] [CrossRef]
- Hochhaus, A.; Larson, R.A.; Guilhot, F.; Radich, J.P.; Branford, S.; Hughes, T.P.; Baccarani, M.; Deininger, M.W.; Cervantes, F.; Fujihara, S.; et al. Long-Term Outcomes of Imatinib Treatment for Chronic Myeloid Leukemia. N. Engl. J. Med. 2017, 376, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Hochhaus, A.; O’Brien, S.G.; Guilhot, F.; Druker, B.J.; Branford, S.; Foroni, L.; Goldman, J.M.; Müller, M.C.; Radich, J.P.; Rudoltz, M.; et al. Six-year follow-up of patients receiving imatinib for the first-line treatment of chronic myeloid leukemia. Leukemia 2009, 23, 1054–1061. [Google Scholar] [CrossRef] [PubMed]
- Kantarjian, H.; Shah, N.P.; Hochhaus, A.; Cortes, J.; Shah, S.; Ayala, M.; Moiraghi, B.; Shen, Z.; Mayer, J.; Pasquini, R.; et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N. Engl. J. Med. 2010, 362, 2260–2270. [Google Scholar] [CrossRef]
- Olivieri, A.; Manzione, L. Dasatinib: A new step in molecular target therapy. Ann. Oncol. 2007, 18 (Suppl. S6), vi42–vi46. [Google Scholar] [CrossRef]
- Kantarjian, H.M.; Cortes, J.E.; O’Brien, S.; Luthra, R.; Giles, F.; Verstovsek, S.; Faderl, S.; Thomas, D.; Garcia-Manero, G.; Rios, M.B.; et al. Long-term survival benefit and improved complete cytogenetic and molecular response rates with imatinib mesylate in Philadelphia chromosome-positive chronic-phase chronic myeloid leukemia after failure of interferon-alpha. Blood 2004, 104, 1979–1988. [Google Scholar] [CrossRef]
- Quintás-Cardama, A.; Kantarjian, H.; O’Brien, S.; Jabbour, E.; Borthakur, G.; Ravandi, F.; Verstovsek, S.; Shan, J.; Cortes, J. Outcome of patients with chronic myeloid leukemia with multiple ABL1 kinase domain mutations receiving tyrosine kinase inhibitor therapy. Haematologica 2011, 96, 918–921. [Google Scholar] [CrossRef]
- Breccia, M.; Colafigli, G.; Scalzulli, E.; Martelli, M. Asciminib: An investigational agent for the treatment of chronic myeloid leukemia. Expert. Opin. Investig. Drugs 2021, 30, 803–811. [Google Scholar] [CrossRef]
- Hughes, T.P.; Mauro, M.J.; Cortes, J.E.; Minami, H.; Rea, D.; DeAngelo, D.J.; Breccia, M.; Goh, Y.T.; Talpaz, M.; Hochhaus, A.; et al. Asciminib in Chronic Myeloid Leukemia after ABL Kinase Inhibitor Failure. N. Engl. J. Med. 2019, 381, 2315–2326. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Bai, B.; Tian, C.; Li, D.; Yu, H.; Song, B.; Li, B.; Chu, X. The Molecular Mechanisms of Cardiotoxicity Induced by HER2, VEGF, and Tyrosine Kinase Inhibitors: An Updated Review. Cardiovasc. Drugs Ther. 2022, 36, 511–524. [Google Scholar] [CrossRef]
- Lavoie, D.C.T.; Robinson, M.E.; Johnston, D.; Pagé, M.; Konji, V.N.; Rauch, F.; Ward, L.M. The Bone Phenotype and Pain Response to Pamidronate in Tyrosine Kinase Inhibitor-Treated Chronic Myelogenous Leukemia. J. Endocr. Soc. 2019, 3, 857–864. [Google Scholar] [CrossRef]
- Fuke, Y.; Shinoda, S.; Nagata, I.; Sawaki, S.; Murata, M.; Ryoyama, K.; Koizumi, K.; Saiki, I.; Nomura, T. Preventive effect of oral administration of 6-(methylsulfinyl)hexyl isothiocyanate derived from wasabi (Wasabia japonica Matsum) against pulmonary metastasis of B16-BL6 mouse melanoma cells. Cancer Detect. Prev. 2006, 30, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Nomura, T.; Shinoda, S.; Yamori, T.; Sawaki, S.; Nagata, I.; Ryoyama, K.; Fuke, Y. Selective sensitivity to wasabi-derived 6-(methylsulfinyl)hexyl isothiocyanate of human breast cancer and melanoma cell lines studied in vitro. Cancer Detect. Prev. 2005, 29, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M. Regulatory mechanism of food factors in bone metabolism and prevention of osteoporosis. Yakugaku Zasshi 2006, 126, 1117–1137. [Google Scholar] [CrossRef]
- Kitakaze, T.; Yuan, S.; Inoue, M.; Yoshioka, Y.; Yamashita, Y.; Ashida, H. 6-(Methylsulfinyl)hexyl isothiocyanate protects acetaldehyde-caused cytotoxicity through the induction of aldehyde dehydrogenase in hepatocytes. Arch. Biochem. Biophys. 2020, 686, 108329. [Google Scholar] [CrossRef]
- Wu, K.M.; Liao, H.F.; Chi, C.W.; Kou, Y.R.; Chen, Y.J. Wasabi Compound 6-(Methylsulfinyl) Hexyl Isothiocyanate Induces Cell Death with Coexisting Mitotic Arrest and Autophagy in Human Chronic Myelogenous Leukemia K562 Cells. Biomolecules 2019, 9, 774. [Google Scholar] [CrossRef]
- Lee, M.J.; Tseng, W.S.; Lai, J.C.; Shieh, H.R.; Chi, C.W.; Chen, Y.J. Differential Pharmacological Activities of Oxygen Numbers on the Sulfoxide Moiety of Wasabi Compound 6-(Methylsulfinyl) Hexyl Isothiocyanate in Human Oral Cancer Cells. Molecules 2018, 23, 2427. [Google Scholar] [CrossRef]
- Lakshmanan, I.; Batra, S.K. Protocol for Apoptosis Assay by Flow Cytometry Using Annexin V Staining Method. Bio Protoc. 2013, 3, e374. [Google Scholar] [CrossRef]
- Kroemer, G.; Levine, B. Autophagic cell death: The story of a misnomer. Nat. Rev. Mol. Cell Biol. 2008, 9, 1004–1010. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.; Kroemer, G. Autophagy in the pathogenesis of disease. Cell 2008, 132, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Hsuan, S.W.; Chyau, C.C.; Hung, H.Y.; Chen, J.H.; Chou, F.P. The induction of apoptosis and autophagy by Wasabia japonica extract in colon cancer. Eur. J. Nutr. 2016, 55, 491–503. [Google Scholar] [CrossRef] [PubMed]
- Milkereit, R.; Persaud, A.; Vanoaica, L.; Guetg, A.; Verrey, F.; Rotin, D. LAPTM4b recruits the LAT1-4F2hc Leu transporter to lysosomes and promotes mTORC1 activation. Nat. Commun. 2015, 6, 7250. [Google Scholar] [CrossRef]
- Settembre, C.; Fraldi, A.; Medina, D.L.; Ballabio, A. Signals from the lysosome: A control centre for cellular clearance and energy metabolism. Nat. Rev. Mol. Cell Biol. 2013, 14, 283–296. [Google Scholar] [CrossRef]
- Cui, L.; Zhao, L.P.; Ye, J.Y.; Yang, L.; Huang, Y.; Jiang, X.P.; Zhang, Q.; Jia, J.Z.; Zhang, D.X.; Huang, Y. The Lysosomal Membrane Protein Lamp2 Alleviates Lysosomal Cell Death by Promoting Autophagic Flux in Ischemic Cardiomyocytes. Front. Cell Dev. Biol. 2020, 8, 31. [Google Scholar] [CrossRef]
- Kontar, S.; Imrichova, D.; Bertova, A.; Mackova, K.; Poturnayova, A.; Sulova, Z.; Breier, A. Cell Death Effects Induced by Sulforaphane and Allyl Isothiocyanate on P-Glycoprotein Positive and Negative Variants in L1210 Cells. Molecules 2020, 25, 2093. [Google Scholar] [CrossRef]
- Fuke, Y.; Hishinuma, M.; Namikawa, M.; Oishi, Y.; Matsuzaki, T. Wasabi-derived 6-(methylsulfinyl)hexyl isothiocyanate induces apoptosis in human breast cancer by possible involvement of the NF-κB pathways. Nutr. Cancer 2014, 66, 879–887. [Google Scholar] [CrossRef]
- Yano, S.; Wu, S.; Sakao, K.; Hou, D.X. Wasabi 6-(methylsulfinyl)hexyl isothiocyanate induces apoptosis in human colorectal cancer cells through p53-independent mitochondrial dysfunction pathway. Biofactors 2018, 44, 361–368. [Google Scholar] [CrossRef]
- Chen, Y.J.; Huang, Y.C.; Tsai, T.H.; Liao, H.F. Effect of Wasabi Component 6-(Methylsulfinyl)hexyl Isothiocyanate and Derivatives on Human Pancreatic Cancer Cells. Evid. Based Complement. Alternat. Med. 2014, 2014, 494739. [Google Scholar] [CrossRef]
- Yano, S.; Wu, S.; Sakao, K.; Hou, D.X. Involvement of ERK1/2-mediated ELK1/CHOP/DR5 pathway in 6-(methylsulfinyl)hexyl isothiocyanate-induced apoptosis of colorectal cancer cells. Biosci. Biotechnol. Biochem. 2019, 83, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jiang, S.; Deng, Z.; Dedon, P.C.; Chen, S. DNA phosphorothioate modification-a new multi-functional epigenetic system in bacteria. FEMS Microbiol. Rev. 2019, 43, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.F.; Su, Y.C.; Zheng, Z.Y.; Jhih Cai, C.; Hou, M.H.; Chao, K.S.; Chen, Y.J. Sonic hedgehog signaling regulates Bcr-Abl expression in human chronic myeloid leukemia cells. Biomed. Pharmacother. 2012, 66, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Wu, Y.; Zhao, G.L.; Ye, Z.Y.; Xing, C.G.; Yang, X.D. Inhibition of autophagy by 3-MA promotes hypoxia-induced apoptosis in human colorectal cancer cells. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Cheng, X.; Kuang, J.; Chen, L.; Yuen, S.; Shi, M.; Liang, J.; Shen, B.; Jin, Z.; Yan, J.; et al. Apatinib-induced protective autophagy and apoptosis through the AKT-mTOR pathway in anaplastic thyroid cancer. Cell Death Dis. 2018, 9, 1030. [Google Scholar] [CrossRef]
- Choi, S.E.; Lee, S.M.; Lee, Y.J.; Li, L.J.; Lee, S.J.; Lee, J.H.; Kim, Y.; Jun, H.S.; Lee, K.W.; Kang, Y. Protective role of autophagy in palmitate-induced INS-1 beta-cell death. Endocrinology 2009, 150, 126–134. [Google Scholar] [CrossRef]
- Mauvezin, C.; Neufeld, T.P. Bafilomycin A1 disrupts autophagic flux by inhibiting both V-ATPase-dependent acidification and Ca-P60A/SERCA-dependent autophagosome-lysosome fusion. Autophagy 2015, 11, 1437–1438. [Google Scholar] [CrossRef]
- Bao, J.; Chen, Z.; Xu, L.; Wu, L.; Xiong, Y. Rapamycin protects chondrocytes against IL-18-induced apoptosis and ameliorates rat osteoarthritis. Aging 2020, 12, 5152–5167. [Google Scholar] [CrossRef]
- Strober, W. Trypan Blue Exclusion Test of Cell Viability. Curr. Protoc. Immunol. 2015, 111, A3.B.1–A3.B.3. [Google Scholar] [CrossRef]
- Kim, D.; Kim, J.Y. Anti-CD14 antibody reduces LPS responsiveness via TLR4 internalization in human monocytes. Mol. Immunol. 2014, 57, 210–215. [Google Scholar] [CrossRef]
- Salmon, J.E.; Edberg, J.C.; Kimberly, R.P. Fc gamma receptor III on human neutrophils. Allelic variants have functionally distinct capacities. J. Clin. Investig. 1990, 85, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Bany-Łaszewicz, U.; Kamińska, J.; Klimczak-Jajor, E.; Kościelak, J. The activity of alpha1,6-fucosyltransferase during human megakaryocytic differentiation. Cell. Mol. Biol. Lett. 2004, 9, 145–152. [Google Scholar] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, J.-F.; Chi, C.-W.; Huang, Y.-C.; Tsai, T.-H.; Chen, Y.-J. Anti-Cancer Effects of Oxygen-Atom-Modified Derivatives of Wasabi Components on Human Leukemia Cells. Int. J. Mol. Sci. 2023, 24, 6823. https://doi.org/10.3390/ijms24076823
Lin J-F, Chi C-W, Huang Y-C, Tsai T-H, Chen Y-J. Anti-Cancer Effects of Oxygen-Atom-Modified Derivatives of Wasabi Components on Human Leukemia Cells. International Journal of Molecular Sciences. 2023; 24(7):6823. https://doi.org/10.3390/ijms24076823
Chicago/Turabian StyleLin, Jui-Feng, Chih-Wen Chi, Yu-Chuen Huang, Tung-Hu Tsai, and Yu-Jen Chen. 2023. "Anti-Cancer Effects of Oxygen-Atom-Modified Derivatives of Wasabi Components on Human Leukemia Cells" International Journal of Molecular Sciences 24, no. 7: 6823. https://doi.org/10.3390/ijms24076823
APA StyleLin, J.-F., Chi, C.-W., Huang, Y.-C., Tsai, T.-H., & Chen, Y.-J. (2023). Anti-Cancer Effects of Oxygen-Atom-Modified Derivatives of Wasabi Components on Human Leukemia Cells. International Journal of Molecular Sciences, 24(7), 6823. https://doi.org/10.3390/ijms24076823