Donor Pericardial Interleukin and Apolipoprotein Levels May Predict the Outcome after Human Orthotopic Heart Transplantation
Abstract
1. Introduction
2. Results
2.1. Donor Characteristics
2.2. Relationship between Donor Risk Scores and Immune Parameters
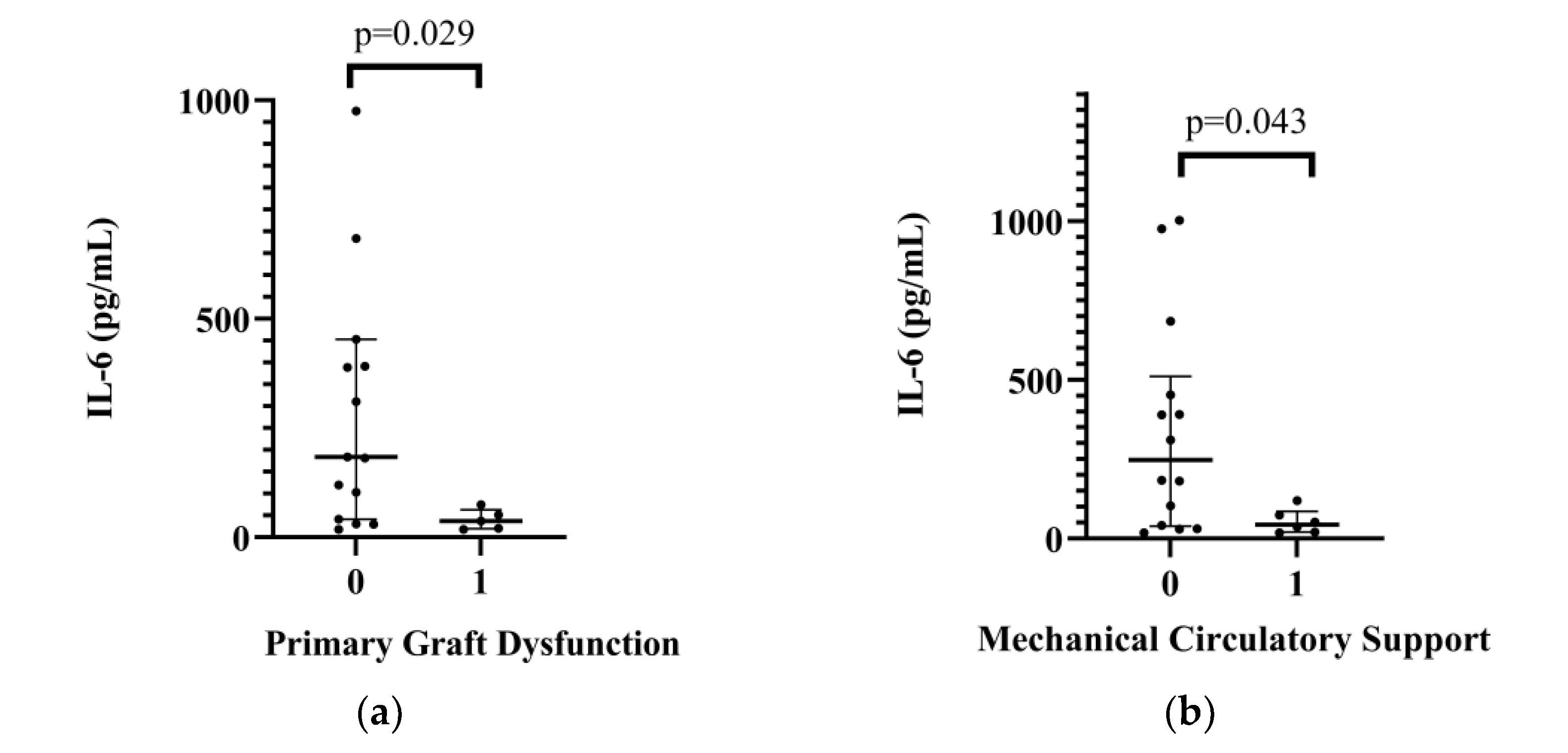
2.3. Relationship between Donor Interleukin Levels and Postoperative Complications
2.4. Effects of Hormone Replacement Therapy on Interleukin Levels
2.5. Laboratory and Echocardiography Parameters
3. Discussion
Limitations of the Study
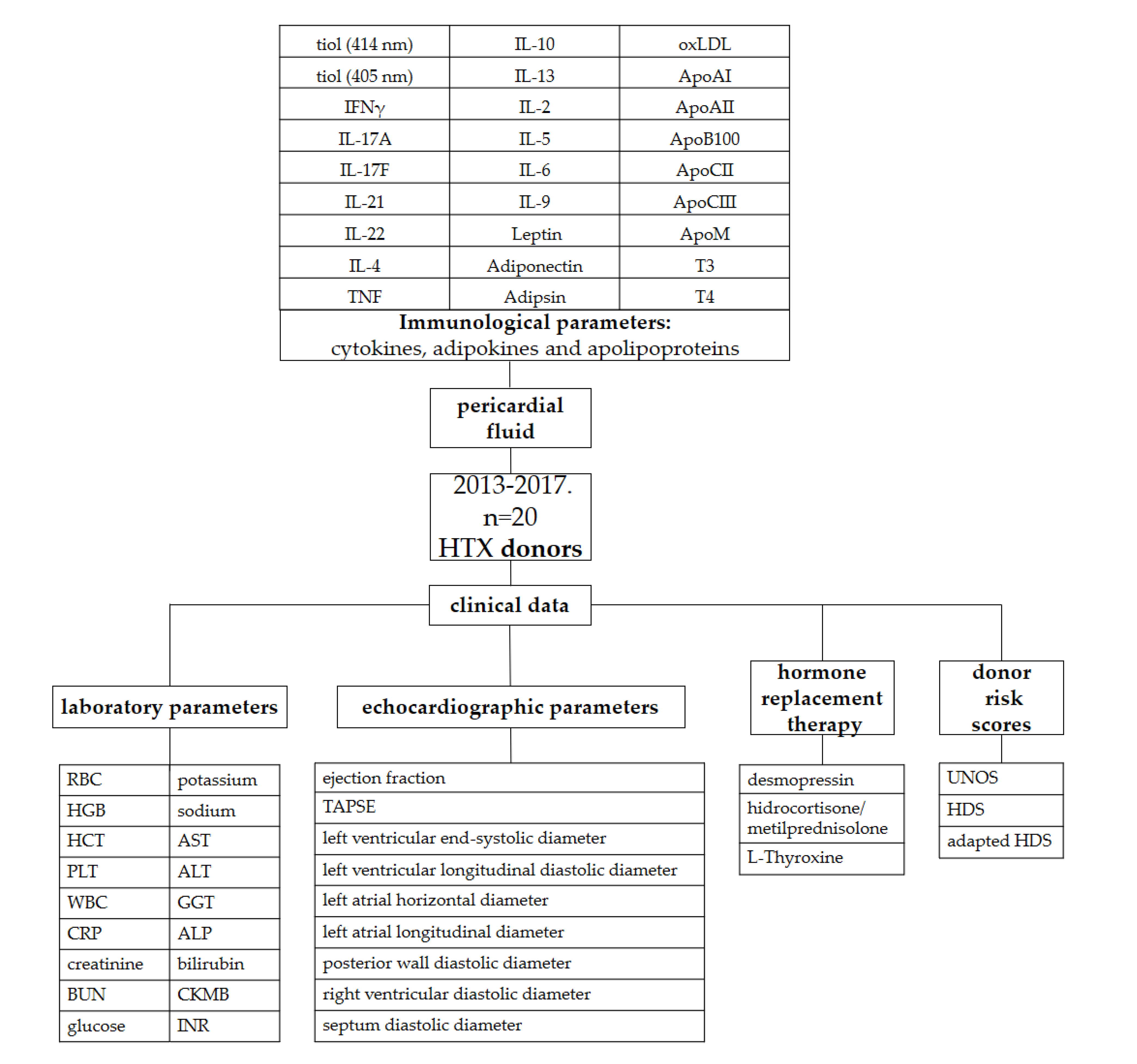
4. Materials and Methods
4.1. Study Design, Setting and Participants
4.2. Local Protocols, and Donor Management
4.3. Definitions and Measurements (Variables, Data Sources and Grouping)
The Heart Donor Score and the Adapted Heart Donor Score
4.4. Sample Collection and Preparation
4.5. Flow Cytometric Multiplexed Bead-Based Immunoassays
4.6. Enzyme-Linked Immunosorbent Assays (ELISA)
4.7. Outcomes
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Copeland, H.; Knezevic, I.; Baran, D.A.; Rao, V.; Pham, M.; Gustafsson, F.; Pinney, S.; Lima, B.; Masetti, M.; Ciarka, A.; et al. Donor heart selection: Evidence-based guidelines for providers. J. Heart Lung Transplant. 2022, 42, 7–29. [Google Scholar] [CrossRef]
- Baran, D.A.; Long, A.; Lansinger, J.; Copeland, J.G.; Copeland, H. Donor Utilization in the Recent Era: Effect of Sex, Drugs, and Increased Risk. Circ Heart Fail. 2022, 15, e009547. [Google Scholar] [CrossRef] [PubMed]
- Resch, T.; Cardini, B.; Oberhuber, R.; Weissenbacher, A.; Dumfarth, J.; Krapf, C.; Boesmueller, C.; Oefner, D.; Grimm, M.; Schneeberger, S. Transplanting Marginal Organs in the Era of Modern Machine Perfusion and Advanced Organ Monitoring. Front. Immunol. 2020, 11, 631. [Google Scholar] [CrossRef]
- Tanim Anwar, A.S.M.; Lee, J.-M. Medical Management of Brain-Dead Organ Donors. Acute Crit. Care 2019, 34, 14–29. [Google Scholar] [CrossRef]
- Ludhwani, D.; Abraham, J.; Kanmanthareddy, A. Heart Transplantation Rejection; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Smits, J.M.; De Pauw, M.; de Vries, E.; Rahmel, A.; Meiser, B.; Laufer, G.; Zuckermann, A. Donor scoring system for heart transplantation and the impact on patient survival. J. Heart Lung Transplant. 2012, 31, 387–397. [Google Scholar] [CrossRef]
- Trivedi, J.R.; Cheng, A.; Ising, M.; Lenneman, A.; Birks, E.; Slaughter, M.S. Heart Transplant Survival Based on Recipient and Donor Risk Scoring: A UNOS Database Analysis. ASAIO J. 2016, 62, 297–301. [Google Scholar] [CrossRef]
- Sayour, A.A.; Oláh, A.; Ruppert, M.; Barta, B.A.; Horváth, E.M.; Benke, K.; Pólos, M.; Hartyánszky, I.; Merkely, B.; Radovits, T. Characterization of left ventricular myocardial sodium-glucose cotransporter 1 expression in patients with end-stage heart failure. Cardiovasc. Diabetol. 2020, 19, 159. [Google Scholar] [CrossRef] [PubMed]
- Donation-Transplantation Baseline Data 2021, National Organ Donation and Transplantation Follow-Up Register. Available online: https://www.ovsz.hu/hu/oco/adatok (accessed on 8 January 2023).
- Angleitner, P.; Kaider, A.; Smits, J.M.; Aliabadi-Zuckermann, A.Z.; Osorio-Jaramillo, E.; Laufer, G.; Zuckermann, A.O. The adapted Heart Donor Score. Transpl. Int. 2021, 34, 546–560. [Google Scholar] [CrossRef] [PubMed]
- Kobashigawa, J.; Khush, K.; Colvin, M.; Acker, M.; Van Bakel, A.; Eisen, H.; Naka, Y.; Patel, J.; Baran, D.A.; Daun, T.; et al. Report From the American Society of Transplantation Conference on Donor Heart Selection in Adult Cardiac Transplantation in the United States. Am. J. Transplant. 2017, 17, 2559–2566. [Google Scholar] [CrossRef]
- Khush, K.K.; Potena, L.; Cherikh, W.S.; Chambers, D.C.; Harhay, M.O.; Hayes, D., Jr.; Hsich, E.; Sadavarte, A.; Singh, T.P.; Zuckermann, A.; et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: 37th adult heart transplantation report-2020, focus on deceased donor characteristics. J. Heart Lung Transplant. 2020, 39, 1003–1015. [Google Scholar] [CrossRef]
- Chambers, D.C.; Cherikh, W.S.; Goldfarb, S.B.; Hayes, D., Jr.; Kucheryavaya, A.Y.; Toll, A.E.; Khush, K.K.; Levvey, B.J.; Meiser, B.; Rossano, J.W. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-fifth adult lung and heart-lung transplant report-2018, Focus theme: Multiorgan Transplantation. J. Heart Lung Transplant. 2018, 37, 1169–1183. [Google Scholar] [CrossRef] [PubMed]
- Kobashigawa, J.; Zuckermann, A.; Macdonald, P.; Leprince, P.; Esmailian, F.; Luu, M.; Mancini, D.; Patel, J.; Razi, R.; Reichenspurner, H.; et al. Report from a consensus conference on primary graft dysfunction after cardiac transplantation. J. Heart Lung Transplant. 2014, 33, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Batchelor, R.J.; Wong, N.; Liu, D.H.; Chua, C.; William, J.; Tee, S.L.; Sata, Y.; Bergin, P.; Hare, J.; Leet, A.; et al. Vasoplegia Following Orthotopic Heart Transplantation: Prevalence, Predictors and Clinical Outcomes. J. Card. Fail. 2021, 28, 617–626. [Google Scholar] [CrossRef]
- Wilhelm, M.J.; Pratschke, J.; Beato, F.; Taal, M.; Kusaka, M.; Hancock, W.W.; Tilney, N.L. Activation of the Heart by Donor Brain Death Accelerates Acute Rejection After Transplantation. Circulation 2000, 102, 2426–2433. [Google Scholar] [CrossRef] [PubMed]
- Plenz, G.; Eschert, H.; Erren, M.; Wichter, T.; Böhm, M.; Flesch, M.; Scheld, H.H.; Deng, M.C. The interleukin-6/interleukin-6-receptor system is activated in donor hearts. J. Am. Coll. Cardiol. 2002, 39, 1508–1512. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, P.; Custódio, G.; Rheinheimer, J.; Crispim, D.; Leitão, C.B.; Rech, T.H. Brain Death-Induced Inflammatory Activity is Similar to Sepsis-Induced Cytokine Release. Cell Transplant. 2018, 27, 1417–1424. [Google Scholar] [CrossRef] [PubMed]
- Diakos, N.A.; Taleb, I.; Kyriakopoulos, C.P.; Shah, K.S.; Javan, H.; Richins, T.J.; Yin, M.Y.; Yen, C.-G.; Dranow, E.; Bonios, M.J.; et al. Circulating and Myocardial Cytokines Predict Cardiac Structural and Functional Improvement in Patients with Heart Failure Undergoing Mechanical Circulatory Support. J. Am. Heart Assoc. 2021, 10, e020238. [Google Scholar] [CrossRef]
- Buchan, T.A.; Moayedi, Y.; Truby, L.K.; Guyatt, G.; Posada, J.D.; Ross, H.J.; Khush, K.K.; Alba, A.C.; Foroutan, F. Incidence and impact of primary graft dysfunction in adult heart transplant recipients: A systematic review and meta-analysis. J. Heart Lung Transplant. 2021, 40, 642–651. [Google Scholar] [CrossRef]
- Palani, H.; Balasubramani, G. Donor Left Ventricular Function Assessed by Echocardiographic Strain is a Novel Predictor of Primary Graft Failure After Orthotopic Heart Transplantation. J. Cardiothorac. Vasc. Anesth. 2021, 35, 3010–3020. [Google Scholar] [CrossRef]
- Nicoara, A.; Ruffin, D.; Cooter, M.; Patel, C.B.; Thompson, A.; Schroder, J.N.; Daneshmand, M.A.; Hernandez, A.F.; Rogers, J.G.; Podgoreanu, M.V.; et al. Primary graft dysfunction after heart transplantation: Incidence, trends, and associated risk factors. Am. J. Transplant. 2017, 18, 1461–1470. [Google Scholar] [CrossRef]
- D’Ancona, G.; Santise, G.; Falletta, C.; Pirone, F.; Sciacca, S.; Turrisi, M.; Biondo, D.; Pilato, M. Primary graft failure after heart transplantation: The importance of donor pharmacological management. Transplant Proc. 2010, 42, 710–712. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.A. Directing Transition from Innate to Acquired Immunity: Defining a Role for IL-6. J. Immunol. 2005, 175, 3463–3468. [Google Scholar] [CrossRef] [PubMed]
- Fontes, J.A.; Rose, N.R.; Čiháková, D. The varying faces of IL-6: From cardiac protection to cardiac failure. Cytokine 2015, 74, 62–68. [Google Scholar] [CrossRef]
- Dawn, B.; Xuan, Y.T.; Guo, Y.; Rezazadeh, A.; Stein, A.B.; Hunt, G.; Wu, W.-J.; Tan, W.; Bolli, R. IL-6 plays an obligatory role in late preconditioning via JAK-STAT signaling and upregulation of iNOS and COX-2. Cardiovasc Res. 2004, 64, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Sook Lee, E.; Park, S.S.; Kim, E.; Sook Yoon, Y.; Ahn, H.Y.; Park, C.Y.; Yun, Y.H.; Woo Oh, S. Association between adiponectin levels and coronary heart disease and mortality: A systematic review and meta-analysis. Int. J. Epidemiol. 2013, 42, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Copeland, H.; Hayanga, J.A.; Neyrinck, A.; MacDonald, P.; Dellgren, G.; Bertolotti, A.; Khuu, T.; Burrows, F.; Copeland, J.G.; Gooch, D.; et al. Donor heart and lung procurement: A consensus statement. J. Heart Lung Transplant. 2020, 39, 501–517. [Google Scholar] [CrossRef]
- Dhar, R.; Cotton, C.; Coleman, J.; Brockmeier, D.; Kappel, D.; Marklin, G.; Wright, R. Comparison of high- and low-dose corticosteroid regimens for organ donor management. J. Crit. Care 2013, 28, 111-e1. [Google Scholar] [CrossRef] [PubMed]
- Nagy, A.; Szécsi, B.; Eke, C.; Szabó, A.; Mihály, S.; Fazekas, L.; Hartyánszky, I.; Párkányi, B.; Holndonner-Kirst, E.; Lex, D.; et al. Endocrine Management and Hormone Replacement Therapy in Cardiac Donor Management: A Retrospective Observational Study. Transplant. Proc. 2021, 53, 2807–2815. [Google Scholar] [CrossRef] [PubMed]
- Peled, Y.; Lavee, J.; Kassif, Y.; Arad, M.; Kogan, A.; Peled, A.; Tirosh, A.; Sternik, L.; Ram, E. Donor thyroid hormone therapy is associated with an increased risk of graft dysfunction after heart transplantation. Clin. Transplant. 2020, 34, e13887. [Google Scholar] [CrossRef]
- Macdonald, P.S.; Aneman, A.; Bhonagiri, D.; Jones, D.; O’Callaghan, G.; Silvester, W.; Watson, A.; Dobb, G. A systematic review and meta-analysis of clinical trials of thyroid hormone administration to brain dead potential organ donors. Crit. Care Med. 2012, 40, 1635–1644. [Google Scholar] [CrossRef]
- Tsuchiya, M.; Tsuchiya, K.; Yasuda, K.; Fujita, M.; Takinishi, A.; Furukawa, M.; Nitta, K.; Maeda, A. MafA is a Key Molecule in Glucose and Energy Balance in the Central Nervous System and Peripheral Organs. Int. J. Biomed. Sci. IJBS 2011, 7, 19–26. [Google Scholar]
- Udden, J.; Bjorntorp, P.; Arner, P.; Barkeling, B.; Meurling, L.; Rossner, S. Effects of glucocorticoids on leptin levels and eating behaviour in women. J. Intern. Med. 2003, 253, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Lund, L.H.; Khush, K.K.; Cherikh, W.S.; Goldfarb, S.; Kucheryavaya, A.Y.; Levvey, B.J.; Meiser, B.; Rossano, J.W.; Chambers, D.C.; Yusen, R.D.; et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-fourth Adult Heart Transplantation Report-2017, Focus Theme: Allograft ischemic time. J. Heart Lung Transplant. 2017, 36, 1037–1046. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, M.E.; Chen, T.; LeBlanc, J.F.; Wei, X.; Gjertson, D.W.; Li, K.C.; Khalighi, M.A.; Lassman, C.R.; Veale, J.L.; Gritsch, H.A.; et al. Apolipoprotein A1 and C-terminal fragment of alpha-1 antichymotrypsin are candidate plasma biomarkers associated with acute renal allograft rejection. Transplantation 2011, 92, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Millan, O.; Brunet, M. Cytokine-based immune monitoring. Clin Biochem. 2016, 49, 338–346. [Google Scholar] [CrossRef]

| Factor | n Median | % (IQR 25–75) |
|---|---|---|
| BMI (kg/m2) | 25.40 | (22.05–27.78) |
| Age (year) | 30.00 | (23.25–37.00) |
| Cause of death | ||
| Head trauma | 16 | 80 |
| Subarachnoid hemorrhage | 2 | 10 |
| Cardiac arrest | 1 | 5 |
| Intoxication | 1 | 5 |
| UNOS donor score | 0 | (0–0.75) |
| UNOS donor risk group | ||
| low (0) | 15 | 75 |
| intermediate (1.2) | 5 | 25 |
| high (≥3) | 0 | 0 |
| HDS | 16.00 | (15.00–21.00) |
| Adapted HDS | 3.77 | (3.69–4.38) |
| Donor treatment | ||
| Desmopressin | 12 | 60 |
| Hydrocortisone/Methylprednisolone | 8 | 40 |
| L-Thyroxine | 6 | 30 |
| Noradrenalin dose (µg/kg/min) | 0.17 | (0.09–0.26) |
| Dopamin/dobutamine dose (µg/kg/min) | 0.00 | (0.00–0.00) |
| Laboratory values | ||
| Sodium (mmol/L) | 144.00 | (140.00–153.00) |
| Potassium (mmol/L) | 4.00 | (3.86–4.20) |
| Creatinine (µmol/L) | 76.50 | (63.25–96.25) |
| BUN (mmol/L) | 4.40 | (3.00–7.20) |
| Glucose (mmol/L) | 8.30 | (6.20–9.50) |
| CRP (mg/L) | 133.70 | (48.58–170.43) |
| CKMB (UI/L) | 36.00 | (11.00–97.00) |
| AST (UI/L) | 56.00 | (37.00–194.00) |
| ALT (UI/L) | 44.00 | (23.00–90.00) |
| GGT (UI/L) | 28.00 | (15.50–53.00) |
| ALP (UI/L) | 140.00 | (96.50–176.00) |
| Echocardiography parameters | ||
| Ejection Fraction | 62.00 | (59.00–65.00) |
| TAPSE | 22.00 | (21.00–26.50) |
| Posterior Wall Diastolic Diameter | 11.00 | (10.00–11.50) |
| LVLDD | 44.50 | (42.00–48.00) |
| LVLSD | 30.00 | (26.75–33.50) |
| Left Atrial Longitudinal Diameter | 33.50 | (29.75–37.25) |
| Left Atrial Horizontal Diameter | 37.00 | (32.75–40.75) |
| Abnormal Valve Function | 3.00 | 15.00 |
| Transplant characteristics | ||
| Total ischemic time (min) | 185.00 | (128.50–213.25) |
| Sex mismatch | 3 | 15 |
| Donor cardiac arrest | 5 | 25 |
| Median | IQR (25–75) | UNOS-D p Value | HDS p Value | aHDS p Value | |
|---|---|---|---|---|---|
| IL-17A (pg/mL) | 8.56 | (8.49–8.72) | 0.004 | <0.001 | 0.009 |
| IL-5 (pg/mL) | 1.04 | (0.93–1.31) | 0.004 | <0.001 | <0.001 |
| Adiponectin (ng/mL) | 16.07 | (12.18–23.55) | 0.006 | 0.030 | |
| Leptin (ng/mL) | 5.14 | (4.58–5.39) | 0.014 | ||
| ApoAI (ug/mL) | 51.85 | (41.52–94.00) | 0.005 | ||
| ApoAII (ug/mL) | 194.83 | (108.36–230.30) | 0.030 | ||
| ApoCII (ug/mL) | 9.28 | (8.40–10.06) | 0.022 | ||
| T4 (ug/dL) | 3.26 | (2.84–3.78) | 0.002 |
| No PGF | PGF | No MCS | MCS | |||||
| IL-6 (pg/mL) | Median | IQR (25–75) | Median | IQR (25–75) | Median | IQR (25–75) | Median | IQR (25–75) |
| 183.67 | (41.21–452.56) | 36.72 | (19.47–62.90) | 247.13 | (38.51–510.38) | 44.12 | (20.12–85.70) | |
| p value | 0.029 | 0.043 | ||||||
| No Rejection | Rejection | ||||
|---|---|---|---|---|---|
| Median | IQR (25–75) | Median | IQR (25–75) | p Value | |
| ApoAII (ug/mL) | 177.35 | (101.55–212.41) | 339.08 | (180.50–371.39) | 0.021 |
| ApoB100 (ug/mL) | 65.01 | (47.00–183.16) | 358.92 | (95.16–1570.34) | 0.032 |
| ApoM (ug/mL) | 10.44 | (0.00–17.91) | 34.07 | (18.57–180.63) | 0.025 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pállinger, É.; Székely, A.; Töreki, E.; Bencsáth, E.Z.; Szécsi, B.; Losoncz, E.; Oleszka, M.; Hüttl, T.; Kosztin, A.; Buzas, E.I.; et al. Donor Pericardial Interleukin and Apolipoprotein Levels May Predict the Outcome after Human Orthotopic Heart Transplantation. Int. J. Mol. Sci. 2023, 24, 6780. https://doi.org/10.3390/ijms24076780
Pállinger É, Székely A, Töreki E, Bencsáth EZ, Szécsi B, Losoncz E, Oleszka M, Hüttl T, Kosztin A, Buzas EI, et al. Donor Pericardial Interleukin and Apolipoprotein Levels May Predict the Outcome after Human Orthotopic Heart Transplantation. International Journal of Molecular Sciences. 2023; 24(7):6780. https://doi.org/10.3390/ijms24076780
Chicago/Turabian StylePállinger, Éva, Andrea Székely, Evelin Töreki, Erzsébet Zsófia Bencsáth, Balázs Szécsi, Eszter Losoncz, Máté Oleszka, Tivadar Hüttl, Annamária Kosztin, Edit I. Buzas, and et al. 2023. "Donor Pericardial Interleukin and Apolipoprotein Levels May Predict the Outcome after Human Orthotopic Heart Transplantation" International Journal of Molecular Sciences 24, no. 7: 6780. https://doi.org/10.3390/ijms24076780
APA StylePállinger, É., Székely, A., Töreki, E., Bencsáth, E. Z., Szécsi, B., Losoncz, E., Oleszka, M., Hüttl, T., Kosztin, A., Buzas, E. I., Radovits, T., & Merkely, B. (2023). Donor Pericardial Interleukin and Apolipoprotein Levels May Predict the Outcome after Human Orthotopic Heart Transplantation. International Journal of Molecular Sciences, 24(7), 6780. https://doi.org/10.3390/ijms24076780







