Abstract
Myogenic differentiation is a complex biological process that is regulated by multiple factors, among which long noncoding RNAs (lncRNAs) play an essential role. However, in-depth studies on the regulatory mechanisms of long noncoding RNAs (lncRNAs) in myogenic differentiation are limited. In this study, we characterized the role of the novel lncRNA TCONS_00323213, which is upregulated during porcine skeletal muscle satellite cell (PSC) differentiation in myogenesis. We found that TCONS_00323213 affected the proliferation and differentiation of PSC in vitro. We performed quantitative polymerase chain reaction (qPCR), 5-ethynyl-20-deoxyuridine (EdU), western blotting, immunofluorescence staining, pull-down assays, and cleavage under targets and tagmentation (CUT and Tag) assays to clarify the effects and action mechanisms of TCONS_00323213. LncRNA TCONS_00323213 inhibited myoblast proliferation based on analyses of cell survival rates during PSC proliferation. Functional analyses revealed that TCONS_00323213 promotes cell differentiation and enhances myogenin (MyoG), myosin heavy chain (MyHC), and myocyte enhancer factor 2 (MEF2C) during myoblast differentiation. As determined by pull-down and RNA immunoprecipitation (RIP) assays, the lncRNA TCONS_00323213 interacted with PBX/Knotted Homeobox 2 (PKNOX2). CUT and Tag assays showed that PKNOX2 was significantly enriched on the MyoG promoter after lncRNA TCONS_00323213 knockdown. Our findings demonstrate that the interaction between lncRNA TCONS_00323213 and PKNOX2 relieves the inhibitory effect of PKNOX2 on the MyoG promoter, increases its expression, and promotes PSC differentiation. This novel role of lncRNA TCONS_00323213 sheds light on the molecular mechanisms by which lncRNAs regulate porcine myogenesis.
1. Introduction
Skeletal muscle growth and development are regulated by a variety of genes and epigenetic factors [1]. Porcine skeletal muscle satellite cells (PSC) are the fundamental unit for skeletal muscle formation [2,3,4]. The growth, development, and regeneration of skeletal muscle depends on the activation state of skeletal muscle satellite cells, which proliferate and differentiate for repair when skeletal muscle is stimulated by exercise or injury and exhibits self-renewal [3,5,6]. Owing to these crucial roles in the growth, development, and regeneration of skeletal muscle, satellite cells are an ideal material to study myogenic differentiation.
Long noncoding RNAs (lncRNAs) are important regulators of skeletal muscle growth and development [7,8]. There is increasing evidence that lncRNAs participate in the regulation of skeletal muscle development, myoblast proliferation and differentiation, and related biological processes at transcriptional, post-transcriptional, translational, and epigenetic levels. H19, a well-known imprinted lncRNA [9], is expressed in animal skeletal muscle and heart tissues and exerts important functions [10]. H19 can inhibit insulin-like growth factor 2 (IGF2) transcription by binding to polycomb repressive complex 2 (PRC2) [11] and recruit TAR DNA-binding protein 43 (TDP43) and drebrin 1 (DBN1) to induce differentiation of PSCs [12,13]. The lncRNA metastasis-associated lung adenocarcinoma transcript 1 (Malat1) functions via the suppressor of variation 39 homolog 1 (Suv39h1) to repress the expression of the target gene myogenic differentiation factor (MyoD) [14]. Developmental pluripotency-associated 2 (Dppa2) upstream binding muscle (Dum) is a lncRNA expressed in myogenic cells. During MyoD-induced differentiation of myogenic cells, Dum recruits large amounts of DNA methyltransferases (DNMTs) to the Dppa2 promoter, thereby silencing Dppa2 expression and promoting myogenesis [15]. Maternally expressed gene 3 (Meg3) is a homologous heterozygous lncRNA of Gtl2 in humans and promotes PSCs by interacting with miR-423-5p to relieve the inhibitory effect on serum response factor (SRF) [16]. It was shown that synaptopodin-2 (SYNPO2) intron sense-overlapping lncRNA (lncSYISL) and muscle growth-promoting factor (lncMGPF) are directly regulated by MyoD [17,18]. lncSYISL can repress target gene expression and promote cell proliferation by recruiting PRC2, leading to H3K27 in the target gene promoter regions [17]. lncMGPF competes with myocyte enhancer factor 2 (MEF2C) to bind miR-135a-5p and promote myogenic differentiation. Furthermore, lncMGPF enhances the binding ability of human antigen R (HuR) proteins to myogenic regulatory mRNAs while enhancing the stability of these mRNAs, thereby promoting myoblast differentiation [18]. In skeletal muscle, the lncRNA taurine-upregulated gene 1 (Tug1) interacts with the peroxisome proliferator-activated receptor-γ co-activator-1α (PGC-1α) in the regulation of transcriptional responses to exercise [19]. The novel lncRNA Gm10561 was confirmed to sponge miR-432 to modulate MEF2C and E2F transcription factor 3 (E2F3) expression to regulate myoblast proliferation and differentiation [20]. These findings indicate the important contributions of lncRNAs to myogenesis and skeletal muscle development.
The lncRNA TCONS_00323213 on chromosome 15 is an intergenic lncRNA [21]. A recent report used published RNA-Seq data to perform transcriptome analysis of PSCs differentiated 24 and 36 h after TCONS_00323213 knockdown, the results showed that TCONS_00323213 participates in the regulation of PSC differentiation by regulated gene expression and alternative splicing events [22]. Therefore, we speculate that TCONS_00323213 is essential and required for PSC differentiation. In this study, we further explored the function and molecular mechanism of action of TCONS_00323213 in myogenic PSCs. In particular, we conducted pull-down assays and mass spectrometry (MS) analysis to identify the protein that binds to TCONS_00323213, followed by additional analyses of the role of this binding protein in the myogenic differentiation of PSCs. The results improve our understanding of the functions of lncRNA TCONS_00323213 and its regulatory axis in the differentiation of PSCs.
2. Results
2.1. TCONS_00323213 Inhibits Myoblast Proliferation
We knocked down and overexpressed TCONS_00323213 in PSCs in the proliferation phase and performed EdU staining and CCK-8 assays. We used three phosphorothioate-modified antisense oligonucleotide (ASO) sequences (ASO-3213-1, ASO-3213-2, and ASO-3213-3) to knockdown TCONS_00323213. The most effective ASO sequence was ASO-3213-1 (Figure 1A) (Table S1), and we used it for subsequent experiments. The ASO group had higher EdU incorporation than the control group (Figure 1C), suggesting that knocking down TCONS_00323213 can increase mitotic activity. We cloned TCONS_00323213 into the PZW1 plasmid and successfully constructed the overexpression vector PZW1-TCONS_00323213 (Figure 1B). Overexpressing TCONS_00323213 decreased EdU binding efficiency and mitotic activity (Figure 1D). A CCK-8 assay revealed that TCONS_00323213 knockdown significantly accelerated cell proliferation (Figure 1E). In contrast, TCONS_00323213 overexpression significantly diminished the proliferation of PSCs compared with that in the negative control group (Figure 1F).
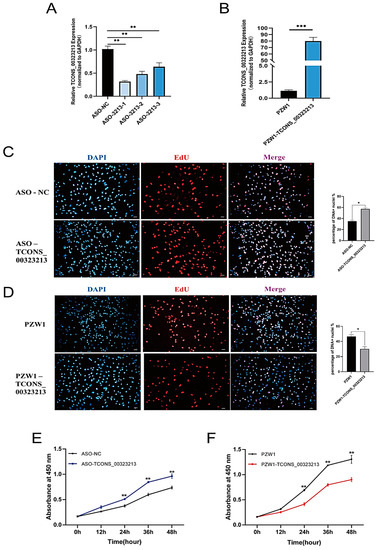
Figure 1.
LncRNA TCONS_00323213 inhibits myoblast proliferation. (A) TCONS_00323213 knockdown efficiency detection. (B) TCONS_00323213 overexpression efficiency detection. The PZW1 plasmid was used to construct the overexpression vector. (C) EdU staining assays after TCONS_00323213 knockdown. (D) EdU staining assays after TCONS_00323213 overexpression. Scale bar: 50 µm. (E,F) CCK-8 assays of PSCs suggested that TCONS_00323213 knockdown significantly promoted myoblast proliferation after transfection with ASO-3213-1 for 12, 24, 36, and 48 h compared with proliferation in the NC group (E), while TCONS_00323213 overexpression inhibited myoblast proliferation (F). Mean values ± SD, n = 3. Statistical significance was assessed by Student’s t-test. * p < 0.05, ** p < 0.01, and *** p < 0.001.
2.2. TCONS_00323213 Promotes Myoblast Differentiation
To further investigate the effect of TCONS_00323213 on the differentiation of PSCs. We have detected the expression of TCONS_00323213 at the different tissue and different time points of proliferation and differentiation of PSCs. The result showed TCONS_00323213 is specific expression in skeletal muscle and heart (Figure S1A), and it increases gradually during myogenic differentiation (Figure S1B). Additionally, qPCR results demonstrated that the mRNA expression levels of myogenic differentiation-related genes were significantly down regulated under TCONS_00323213 knockdown (Figure S2). These results implied that TCONS_00323213 plays an important role in myogenic differentiation. Then we detected the expression of MyoD, MyoG, and MEF2C at the protein level after knockdown and overexpression of TCONS_00323213. As determined by western blot, the protein expression levels of MyoG and MEF2C decreased significantly after TCONS_00323213 knockdown (Figure 2A) and increased significantly after TCONS_00323213 overexpression (Figure 2B). Immunofluorescence results demonstrated that MyoG expression was significantly downregulated after TCONS_00323213 knockdown (Figure 2C) and significantly upregulated after TCONS_00323213 overexpression compared with levels in the control group (Figure 2D).
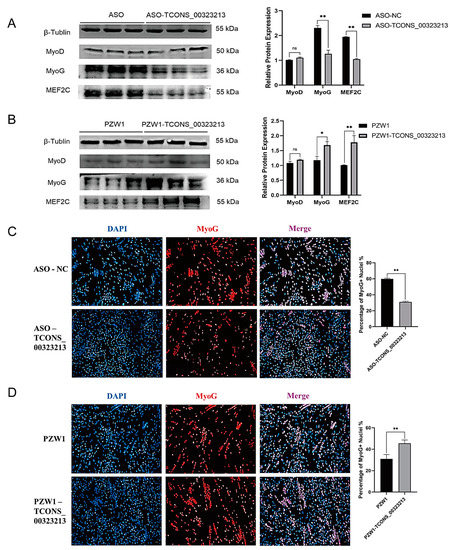
Figure 2.
TCONS_00323213 promotes myoblast differentiation. (A) Western blot analysis of MyoD, MyoG, and MEF2C expression levels after TCONS_00323213 knockdown. (B) Western blot analysis of MyoD, MyoG, and MEF2C expression levels after TCONS_00323213 overexpression. The western blot results contained three biological replicates in each group. (C) In PSCs differentiated for 36 h, a knowckdown of TCONS_00323213 significantly decreased MyoG expression. (D) Immunofluorescence staining in PSCs differentiated for 36 h showed that TCONS_00323213 overexpression significantly increased the MyoG expression level. Mean values ± SD, n = 3. * p < 0.05, ** p < 0.01. ns indicates no significant difference.
2.3. TCONS_00323213 Interacts Directly with PBX/Knotted Homeobox 2
To explore the function of TCONS_00323213, an RNA pull-down assay was performed to identify the interacting proteins of TCONS_00323213 (Figure 3A). Given that the TCONS_00323213 full-length sequence is excessively long, we truncated TCONS_00323213 into 5 biotinylated fragments (Figure 3B) and incubated these fragments with cell lysates from differentiated PSCs for 36 h. RNA-binding proteins were captured with streptavidin magnetic beads. Among the differentially expressed proteins detected by MS, the transcription factor PBX/Knotted Homeobox 2 (PKNOX2) was of particular interest. PKNOX2, a member of the PREP family, is highly expressed in skeletal muscle tissue [23]. The western blot showed that PKNOX2 was captured by biotinylated TCONS_00323213 fragments 1, 2, and 3 (Figure 3C). The results indicated that PKNOX2 was precipitated by biotinylated TCONS_00323213. We then performed the RIP assay to confirm this interaction (Figure 3D). As expected, the PKNOX2 protein captured TCONS_00323213 in the PSC lysate (Figure 3E,F), indicating that PKNOX2 interacts with TCONS_00323213 in vivo. PKNOX2 is a member of the triple amino acid loop extension (TALE) superfamily of homology domains and is highly similar to its homologous protein PKNOX1 in terms of structural domains. It specifically recognizes the TGACAG motif [23]. We subsequently analyzed the sequence characteristics of TCONS_00323213 and found 2 TGACAG motifs located at bases 44–49 and 4381–4386 in TCONS_00323213 (Figure 3B), so we speculated that PKNOX2 can bind to TCONS_00323213 at the TGACAG sequence.
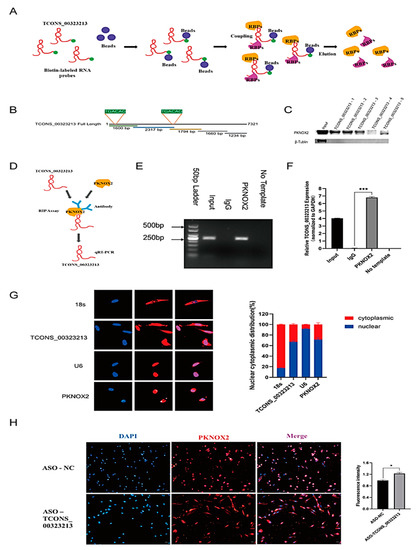
Figure 3.
TCONS_00323213 physically interacts with PKNOX2. (A) A schematic representation of the RNA pull-down assays. (B) Location of the TCONS_00323213-3 mutant fragments. (C) Interactions between a series of TCONS_00323213 mutant fragments (TCONS_00323213-1, TCONS_00323213-2, TCONS_00323213-3, TCONS_00323213-4, and TCONS_00323213-5) were assessed by RNA pull-down and WB assays. (D) Schematic representation of the RIP assay. (E) RIP assays were performed to validate the interaction between TCONS_00323213 and PKNOX2. (F) qPCR analysis of RIP assay results shows that TCONS_00323213’s relative expression is normalized to GAPDH. (G) Localization detection of TCONS_00323213 and PKNOX2 in PSCs. The 18s was used as an internal reference for cytoplasmic localization, while U6 was used as an internal reference for nuclear localization. (H) Localization analysis of PKNOX2 expression in PSCs after knockdown of TCONS_00323213. Mean values ± SD, n = 3. * p < 0.05, *** p < 0.001.
We further performed fluorescence in situ hybridization (FISH) and IF to further investigate the regulatory mechanisms of TCONS_00323213 and PKNOX2 in PSCs. The results showed that TCONS_00323213 was mainly expressed in the nucleus and present at low levels in the cytoplasm, and PKNOX2 was also mainly localized in the nucleus (Figure 3G). These findings indicate that TCONS_00323213 and PKNOX2 may interact mainly in the nucleus. In addition, we examined the expression of PKNOX2 in PSCs after the TCONS_00323213 knockdown. The IF results showed that the expression of PKNOX2 in the cytoplasm of PSCs increased after TCONS_00323213 knockdown, and the fluorescence expression intensity increased significantly as well (Figure 3H). This result suggests that TCONS_00323213 may interact with PKNOX2 to affect the nuclear retention of PKNOX2 and thus participate in the regulation of PSC differentiation.
2.4. PKNOX2 Affects PSCs Differentiation
To explore the function of PKNOX2 in PSCs, we detected the expression pattern of PKNOX2 in PSCs at different proliferation and differentiation time points. The results showed that the PKNOX2 expression level in the proliferation periods was higher than in the differentiation periods (Figure 4A). This result indicates that PKNOX2 functions in the differentiation of PSCs. Then, we designed and synthesized two siRNA sequences targeting PKNOX2 (si-PKNOX2-1 and si-PKNOX2-2) (Table S1). After transfection, si-PKNOX2-2 had the highest knockdown efficiency (Figure 4B). Therefore, si-PKNOX2-2 was used in all subsequent experiments for PKNOX2 knockdown. We also constructed an effective overexpression vector for PKNOX2 via homologous recombination (Figure 4D). Subsequently, skeletal muscle satellite cells were transfected with si-PKNOX2-2, and the overexpression vector PZW1-PKNOX2 and the expression of related marker genes were examined after 36 h of PSC differentiation. The qPCR results confirmed that the mRNA expression levels of TCONS_00323213 did not significantly change by knockdown and overexpression of PKNOX2 (Figure 4C,E). However, MyoG and MEF2C were significantly upregulated by PKNOX2 knockdown (Figure 4C) and significantly downregulated by overexpression of PKNOX2 (Figure 4E). The western blot illustrated that the knockdown of PKNOX2 significantly upregulated the protein expression levels of MyoG, MEF2C, and MyHC (Figure 4F,G). In contrast, overexpression of PKNOX2 significantly downregulated the protein expression levels of MyoG, MEF2C, and MyHC (Figure 4H,I). Immunofluorescence results demonstrated that MyHC expression was significantly upregulated after PKNOX2 knockdown (Figure 4J) and downregulated by PKNOX2 overexpression (Figure 4K). These results suggest that PKNOX2 knockdown promotes the differentiation of PSCs.
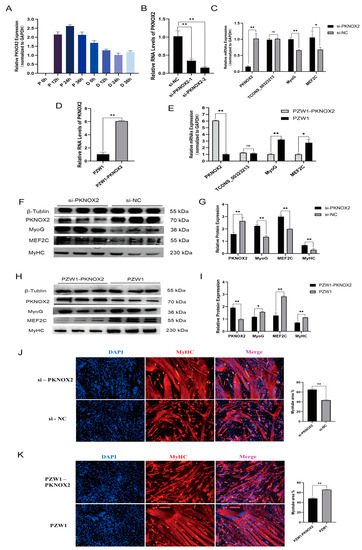
Figure 4.
Role of PKNOX2 in the differentiation of PSCs. (A) Real-time PCR analysis of PKNOX2 expression in PSCs during the period of proliferation and differentiation. There was a 12 h interval between each period. (B) A screening assay of siRNAs targeting PKNOX2 showed that si-PKNOX2-2 had the highest interference efficiency. (C) The knockdown of PKNOX2 increased the mRNA levels of MyoG and MEF2C. (D) Overexpression vector effect testing. (E) Overexpression of PKNOX2 decreased the mRNA levels of MyoG and MEF2C. (F,G) Knockdown of PKNOX2 increased the protein levels of MyoG, MEF2C, and MyHC. (H,I) Overexpression of PKNOX2 decreased MyoG, MEF2C, and MyHC protein expression levels. (J) IF in PSCs differentiated for 36 h, showing that the MyHC expression level was significantly increased by PKNOX2 knockdown. (K) IF in PSCs differentiated for 36 h showing that PKNOX2 overexpression significantly decreased the MyHC expression level. Mean values ± SD, n = 3. * p < 0.05, ** p < 0.01. ns indicates no significant difference.
2.5. LncRNA TCONS_00323213 Decreased the Enrichment of PKNOX2 on the MyoG Promoter
Our experimental results demonstrated that TCONS_00323213 interacts with PKNOX2. Next, we identified the downstream target sites of PKNOX2 after the knockdown of TCONS_00323213 by CUT and Tag experiments. We transfected PSCs with ASO to knock down TCONS_00323213 and conducted the CUT and Tag assay after 36 h of induced differentiation to obtain a genome-wide binding site map for PKNOX2 (Figure 5A). The genome-wide analysis of binding sites showed that PKNOX2 was mainly enriched in promoter regions (<2 kb, ASO-NC: 35.27%, ASO-TCONS_00323213: 33.80%) (Figure 5C). A motif analysis showed that PKNOX2 was significantly enriched in TGACAG motifs (Figure 5B), indicating that the TCONS_00323213–PKNOX2 complex participates in the regulation of gene expression.
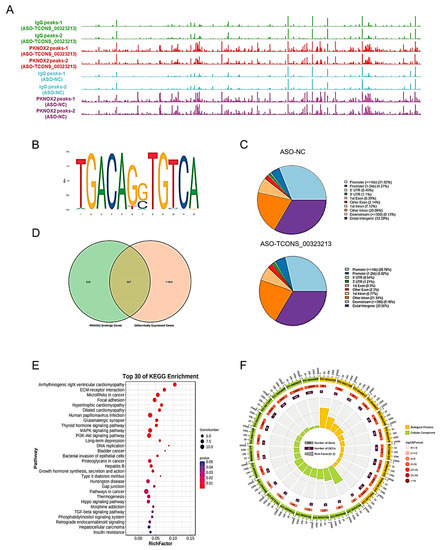
Figure 5.
CUT and Tag Analysis of PKNOX2. (A) PKNOX2 enrichment analysis. (B) PKNOX2 significant enrichment motif sequence. (C). Distribution of PKNOX2-enriched regions on the genome. (D) Venn diagram of PKNOX2-binding genes versus differentially expressed genes in PSCs with TCONS_00323213 knockdown. (E). KEGG pathway enrichment analysis of the 307 genes in panel (D). (F). GO enrichment analysis of the 307 genes in panel (D). The RNA-seq experiment had three biological replicates, and the CUT and Tag experiment had two biological replicates.
To investigate how the TCONS_00323213–PKNOX2 complex regulates the expression of genes, we first analyzed the RNA-seq of PSCs for 36 h of differentiation after knockdown of TCONS_00323213 (accession number: SRP186451) and obtained 12,111 differentially expressed genes (Table S2). Then, we compared PKNOX2’s enriched peaks in the control and TCONS_00323213 knockdown groups and obtained 833 target genes (Table S3) of PKNOX2 by a CUT and Tag analysis. A total of 307 significantly differentially expressed genes (Table S4) were obtained by combining the target genes of PKNOX2 with differentially expressed genes identified by the RNA-seq of PSCs for 36 h of differentiation after knockdown of TCONS_00323213 (Figure 5D). These genes were significantly enriched in KEGG pathways and biological processes related to skeletal muscle growth and regeneration, such as ECM–receptor interaction, focal adhesion, thyroid hormone, PI3K–Akt, MAPK, Hippo, and TGF-β signaling pathways (Figure 5E,F).
Combined with Integrative Genomics Viewer (IGV) analysis, we found that MyoG and MYC, which are myogenic-related genes, had significantly different PKNOX2 enrichment peaks in their promoter regions (Figure 6A,B). Therefore, we hypothesized that TCONS_00323213 regulates gene expression through binding to PKNOX2, which in turn affects PSC differentiation. Given that enrichment of PKNOX2 peaks on the MyoG promoter increased significantly after the TCONS_00323213 knockdown, a luciferase report assay was performed to further verify this relationship. We found that the overexpression of PKNOX2 reduced the luciferase activity of the wild-type MyoG promoter and that luciferase activity increased upon TCONS_00323213 overexpression after PKNOX2 and the MyoG promoter plasmid had been co-transfected with the MyoG promoter (Figure 6C). Overexpression of PKNOX2 did not affect the luciferase activity of the MyoG promoter mutant (Figure 6D). Altogether, these results showed that the lncRNA TCONS_00323213 interacted with PKNOX2 to relieve the inhibitory effect of PKNOX2 on MyoG, thereby promoting PSC differentiation (Figure 7).
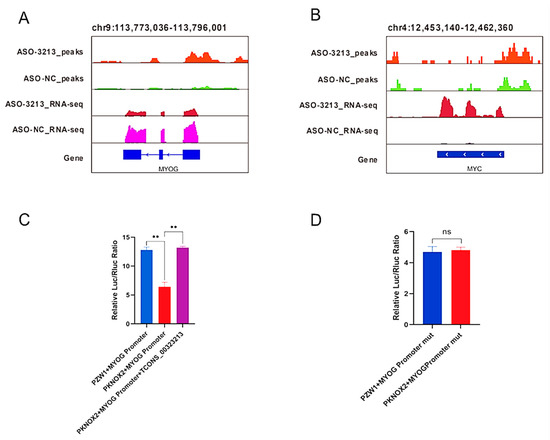
Figure 6.
IGV visualization demonstrates the RNA-seq and CUT and Tag signatures of myogenic differentiation-related genes. (A) Enrichment signal of PKNOX2 on MyoG and differential expression of MyoG by RNA-Seq. (B) The enrichment signal of PKNOX2 on MYC and differential expression of MYC by RNA-Seq. (C) PKNOX2 overexpression reduced the luciferase activity of the wild-type MyoG promoter construct, and luciferase activity increased upon TCONS_00323213 overexpression after PKNOX2 and the MyoG promoter plasmid had been co-transfected with the MyoG promoter. (D) Overexpression of PKNOX2 does not affect the luciferase activity of the MyoG promoter mutant. Mean values ± SD, n = 3. ** p < 0.01. ns indicates no significant difference.
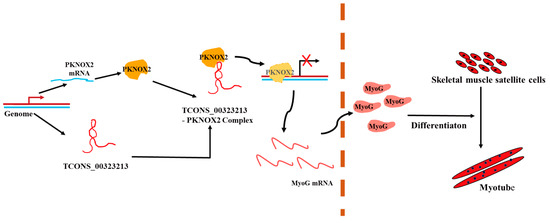
Figure 7.
A schematic diagram depicting the functions of TCONS_00323213 during skeletal muscle satellite cell differentiation. In differentiating PSCs, TCONS_00323213 binds to PKNOX2 to relieve the inhibitory effect of PKNOX2 on MyoG, thereby increasing MyoG expression and promoting PSC differentiation.
3. Discussion
Skeletal muscle growth is a complex and precise process that is tightly linked to gene expression regulation. Numerous transcription factors, such as Pax7, Myf5, MyoD, MyoG, and MEF2C, are involved in the regulation of myogenesis [24,25,26]. Moreover, a growing number of lncRNAs and related binding proteins involved in this process have been identified [27,28,29]. In our study, we found TCONS_00323213 to be specifically expressed in porcine skeletal muscle and cardiac tissues and regularly expressed in the differentiation period of PSCs. A recent report used public RNA-seq data and performed a LivestockExp transcriptome analysis of PSC differentiation at 24 and 36 h after the TCONS_00323213 knockdown. This research reported 18,436 differentially expressed genes and 2036 differential alternative splicing events in PSC differentiation at 24 h and 14,635 differentially expressed genes and 2193 differential alternative splicing events in PSC differentiation at 36 h. This report implies that TCONS_00323213 participates in the regulation of PSC differentiation through regulated gene expression and alternative splicing events [22]. In our study, we analyzed RNA-seq data only 36 h after TCONS_00323213 knockdown, and we obtained 12,111 differentially expressed genes. The reason for the different number of differentially expressed genes obtained may be due to the different analysis methods and screening conditions. Our results also demonstrate that TCONS_00323213 can be involved in satellite cell differentiation by regulating gene expression. In addition, our study explored the regulatory mechanism of TCONS_00323213 on PSCs in more detail through a series of molecular biology experiments. This further indicates that TCONS_00323213 plays a very important role in the regulation of myogenic cell differentiation.
PBX/knotted 1 homeobox 2 (PKNOX2) was first discovered in 2001 and lies on the chromosomal region 11q24 in humans. It is a nuclear transcription factor belonging to the TALE class of homologous domain proteins. PKNOX2, which is similar to PKNOX1, specifically recognizes the TGACAG motif and plays key roles in cell growth, differentiation, and apoptosis [23,30]. In this study, localization experiments of the PKNOX2 protein showed that it is mainly present in the nucleus, which is consistent with the results of previous studies. Research on PKNOX2 has focused on its roles in diseases, such as inhibition of tumor cell migration, proliferation by PKNOX2 promoter demethylation, and induction of apoptosis [31]. Studies have shown that PKNOX2 knockdown can accelerate the death of myofibroblasts and proximal tubular epithelial cells [32]. LINC02489 interacts with PKNOX2 through the PTEN/mTOR axis to reduce the migration and invasion of chemotherapy-resistant SKOV3 cells, thereby increasing the sensitivity of ovarian cancer to paclitaxel [33]. However, there is a scarcity of research on the role of PKNOX2 in pig muscle development. In the present study, a series of experimental assays demonstrated that the PKNOX2 protein interacts with TCONS_00323213 to affect PSC differentiation. This provides new insights into the regulation of cell differentiation by PKNOX2.
Recent studies revealed that lncRNAs have diverse functions in muscle development and myoblast-directed differentiation [34,35,36,37]. As with most noncoding RNAs with regulatory functions, the majority of lncRNAs exert functions in the nucleus by directing RNA complexes to specific RNA, DNA, or protein targets [38,39,40,41]. For example, lncSYISL can recruit the enhancer of the zeste homolog 2 (EZH2) protein from the PRC2 complex to the promoter regions of muscle development-related target genes, such as cyclin-dependent kinase inhibitor 1A (p21) and MyoG, thereby repressing the transcription of these genes and inhibiting cell differentiation [17]. LncRNA muscle atrophy-associated transcript (lncMAAT) negatively regulates transcription of miR-29b through the sex-determining region Y (SRY) related high-mobility group box 6 (SOX6) by a trans-regulatory module and increases the expression of the neighboring gene Mbnl1 by a cis-regulatory module [42]. The lncRNA OIP5 antisense RNA 1 (OIP5-AS1) is expressed in muscle tissues and acts as an interactive scaffold, recruiting RNA binding protein (RBP) HuR to bind to the MEF2C 3′ UTR region, stabilizing MEF2C mRNA, and promoting myogenic differentiation [43]. By influencing the transcriptional regulation of its neighboring gene, filamin A-interacting protein 1 (Filip1), and by specifically binding to the TDP-43 protein, muscle-enriched lncRNA (lncMyolinc) promotes the expression of α-actin (Acta1) and MyoD and thus contributes to muscle development [44]. Myoparr is a nuclear retention protein that acts as a protein scaffold, enhancing the binding of the DEAD-box 17/P300-CBP-associated factor (Ddx17/PCAF) protein to the MyoG promoter, which in turn activates MyoG expression and promotes myogenic differentiation [45]. LncRNA Irm regulates the expression of myogenic genes by directly binding to myocyte enhancer factor 2D (MEF2D), which in turn promotes the assembly of MyoD/MEF2D on the regulatory elements of target genes [27]. In our study, we demonstrate that the interaction between lncRNA TCONS_00323213 and PKNOX2 relieves the inhibitory effect of PKNOX2 on the MyoG promoter and promotes PSC differentiation. This provides new evidence for how lncRNA and proteins interact to regulate myogenic differentiation.
In reviewing the results of this study, some potential limitations should be con-sidered. First, we more focused on the function of TCONS_00323213 on PSCs differen-tiation, but failed to explored deeply into the molecular mechanism of TCONS_00323213 in regulating PSCs proliferation. Second, we found TCONS_00323213 affects the locali-zation of PKNOX2 protein in PSCs, but specific mechanism has not to be clearly. In addition, the fact that TCONS_00323213 was also detected in the cytoplasm implies that it can regulate myogenesis though other mechanisms. All these limitations deserve further investigation in the future.
4. Materials and Methods
4.1. Ethics Statement
In this study, animal care and all experiments were carried out in accordance with the pre-approved guidelines of Regulation Proclamation No. 5 of the Standing Committee of the Hubei People’s Congress. All experimental protocols were approved by the Ethics Committee (HZAUSW2015-0003) of Huazhong Agricultural University, Wuhan City, Hubei Province, China.
4.2. PSCs Culture and Transfection
Fresh PSCs were isolated from the hind legs of male Yorkshire piglets less than 1 week of age. In accordance with the methods of Wang et al. [46], tissue block digestion was used to isolate skeletal muscle satellite cells in a sterile environment. Muscle tissue was cut into pieces and then digested with 320 U/mL collagenase type II (Gibco, LA, CA, USA) in a 37 °C water bath with shaking for 2 h. After termination with DMEM (Gibco, LA, CA, USA) that contained 10% FBS (Gibco, LA, CA, USA), the cell suspension was filtered through 400-, 200-, 100-, and 50-µm filters to remove tissue debris. The filtrate was retained. Afterwards, the resulting cell pellet was resuspended and cultured in PM+ containing an RPMI-1640 medium supplemented with 20% FBS, 0.5% GlutaMax, 0.5% nonessential amino acids, 0.5% Anti-Anti (Gibco, LA, CA, USA), 0.25% chicken embryo extract (Gemini, Woodland, CA, USA), and 2.5 ng/mL basic fibroblast growth factor (Invitrogen, Grand Island, NY, USA). When the PSCs grew to 90% confluence, they were transferred into DMEM supplemented with 5% horse serum (Gibco, LA, CA, USA) to induce differentiation. All cells described above were incubated at 37 °C under 5% CO2.
When cell confluence reached 80%, cells were transfected with 200 nM (final concentration) of ASO-negative control (NC)/ASO-TCONS_00323213 (Genepharma, Suzhou, China), 100 nM of si-NC/si-PKNOX2 (Genepharma, Suzhou, China), or 2.5 μg of plasmid per well (6-well culture plate) using jetPRIME (Polyplus, Illkirch, France) according to the user manual. Transfection efficiency was measured using a PZW1 vector expressing GFP or an NC after 36 h of transfection, and qPCR assays were performed.
4.3. EdU Assay
EdU, a thymidine nucleoside analog, can be used to detect cellular DNA replication activity during DNA replication in living cells through its specific reaction with Apollo® fluorescent dyes. When the PSCs grew to approximately 80% density in 10-cm culture plates, they were transferred to 12-well plates. TCONS_00323213 knockdown and overexpression were performed when the cell density reached 40–50%. The EdU (RiboBio, Guangzhou, China) reagent was added to each well at a final concentration of 50 μM for 1.5–2 h. Then, the cells were fixed with 4% paraformaldehyde solution and 0.5% Triton X-100 and subsequently incubated with apollo staining solution for 30 min at 25 °C and protected from light. Nuclei were lightproof-stained with 4′,6-diamidino-2-phenylindole (DAPI) for 10 min and finally examined and photographed under a Leica SP8 fluorescent microscope.
4.4. CCK-8 Assay
PSCs were seeded into 96-well plates at approximately 2500 cells per well with 100 µL of PM+. Then, cell proliferation was detected by the CCK-8 assay kit (Vazyme, Nanjing, China) in accordance with the manufacturer’s instructions. Absorbance at 450 nm was measured using a spectrophotometer after 0, 12, 24, 36, and 48 h of transfection.
4.5. RNA Extraction and RT-qPCR Analysis
Total RNA was extracted from cultured PSCs using a StayPure RNA Extraction Kit (Accurate, Changsha, China). The total RNA from the pull-down and RIP assays was isolated using TRIzol reagent (Vazyme, Nanjing, China) in accordance with the manufacturer’s protocol. For cDNA synthesis, RNA was reverse-transcribed using ABScript III RT Master Mix for qPCR with gDNA Remover (ABconal, Wuhan, China). qPCR for RNA was carried out with 2 × Universal SYBR Green Mix Fast qPCR Mix (ABconal, Wuhan, China) on a Bio-Rad CFX96 Real-Time Detection System, following the manufacturers’ instructions. The 2−∆∆CT method was used to analyze qPCR data. The primers used for qPCR in this study were designed using Primer 5.0 (Table S5).
4.6. Western Blot Analysis
The total protein of cells was lysed in a RIPA buffer containing 1% protease inhibitors (PMSF) and collected. After SDS-PAGE, the proteins were transferred onto polyvinylidene fluoride membranes. The primary antibodies were anti-PKNOX2 (Proteintech, Wuhan, China), anti-MyoG (Abcam, Cambridge, UK), anti-MEF2C (Proteintech, Wuhan, China), anti-MyHC (Millipore, Billerica, MA, USA), and anti-β-tubulin (Servicebio, Wuhan, China). The HRP-conjugated secondary antibodies HRP-labeled goat anti-mouse IgG (Servicebio, Wuhan, China) and HRP-labeled goat anti-rabbit IgG (Servicebio, Wuhan, China) were used.
4.7. Immunofluorescence Assay
PSCs were washed 2 times with phosphate-buffered saline (PBS) and fixed with ice-cold 4% paraformaldehyde for 15 min and subsequently incubated in 0.3% Triton X-100 at room temperature for 10 min. Then, the PSCs were blocked with a blocking solution (3% bovine serum albumin (BSA), 0.3% Triton X-100, and 10% FBS complemented with PBS) at 37 °C for 0.5 h and washed 2–3 times with PBS. Next, the PSCs were incubated with anti-MyoG (Abcam, Cambridge, MA, USA) and MyHC antibodies (Millipore, Billerica, MA, USA) at 4 °C for 12 h. After they had been washed three times with PBS, the PSCs were incubated with CY3-labeled (red fluorescence) goat anti-mouse IgG antibodies (ABconal, Wuhan, China) at 37 °C for 1 h. The cells were kept from light, stained with DAPI (blue fluorescence) for 10 min, and washed with PBS three times. Images were captured using a Leica SP8 microscope.
4.8. RNA FISH
A lncRNA FISH kit (RiboBio, Guangzhou, China) was used for an RNA FISH analysis of PSCs in accordance with the operating procedures. Images were captured using a confocal laser scanning microscope. The lncRNA TCONS_00323213 FISH probe was synthesized by RiboBio, a biotechnology company(RiboBio, Guangzhou, China).
4.9. RNA Pull-Down and RIP Assays
For the RNA pull-down assay, T7 RNA polymerase (Roche, Mannheim, Germany) and biotin RNA labeling mix (Roche, Mannheim, Germany) were used to synthesize the five TCONS_00323213 truncated fragments. A total of 2 µg of in vitro biotinylated RNAs were incubated with PSC protein lysis solution for 16 h. Then, the complex was pulled down using DynabeadsTM M-280 Streptavidin (Invitrogen, New York, NY, USA). The beads were washed at least three times. Subsequently, the lncRNA–protein complexes associated with streptavidin magnetic beads were subjected to MS and western blot.
RIP assays were performed using an EZ-Magna RIP kit (Millipore, Billerica, MA, USA) following the operating procedures. Approximately 108 PSCs were collected at 36 h of differentiation; cells were lysed with RIP lysis buffer supplemented with PMSF (Beyotime, Shanghai, China), and the lysates were incubated with 10 µg of the antibody to PKNOX2 (Proteintech, Wuhan, China) or IgG (ABconal, Wuhan, China; control) at 4 °C for at least 16–20 h. Then, DynabeadsTM protein G (Invitrogen, New York, NY, USA) beads were added to capture the protein–RNA complexes.
4.10. CUT and Tag and RNA-Seq Analysis
A CUT and Tag Assay Kit for Illumina (Vazyme, Nanjing, China) was used to construct a CUT and Tag library for PKNOX2 in PSCs in accordance with the operating instructions, followed by Illumina sequencing. CUT and Tag analyses were performed according to the transcription factor processing chip-seq (https://github.com/nf-core/chipseq/releases) (accessed on 22 April 2021). The pig reference genome sequence file that we used was Sus_scrofa 11.1 (https://ftp.ensembl.org/pub/release-108/fasta/sus_scrofa/dna/Sus_scrofa.Sscrofa11.1.dna.toplevel.fa.gz) (accessed on 22 April 2021), and the genomic annotation file was downloaded from Ensembl (https://ftp.ensembl.org/pub/release108/gtf/sus_scrofa/us_scrofa.Sscrofa11.1.108.chr.gtf.gz) (accessed on 22 April 2021). The R package ChIPseeker [47] was used to identify the nearest genes around the peak and annotate genomic regions of peaks. All alignment results were then converted to coverage bigwig files and normalized to the corresponding input using deepTools [48]. The bigwig formats were visualized using the Integrative Genomics Viewer (IGV) [49] software. Motif analysis was performed using the Hommer software (v4.11) [50]. We analyzed RNA-seq of PSCs for 36 h of differentiation after the knockdown of TCONS_00323213 (accession number: SRP186451). The reference genome and reference genome annotation file were similar to those used in the CUT and Tag analysis. RNA-seq read mapping efficiency is supplied in Supplementary Table S6. DEseq2 [51] was used to identify differentially expressed genes. Annotated genes showing |log2FoldChange|(|log2FC|) ≥ 1 and a p-value < 0.05 were considered to be differentially expressed.
4.11. Dual-Luciferase Reporter Assay
When cell confluence reached approximately 80%, the wild-type and mutant dual-luciferase reporter vectors of the MyoG promoter and PZW1, PZW1-PKNOX2, and PZW1-TCONS_00323213 were separately co-transfected into C2C12 cells with the TK normalizing reporter plasmid. After incubation for 36 h, the firefly and renilla luciferase activities were measured using the Dual-Luciferase® Reporter Assay System (Promega, Fitchburg, WI, USA).
4.12. Statistical Analysis
The results are presented as the mean ± standard deviation (SD). A one-way analysis of variance with post hoc Student–Newman–Keuls tests for multigroup comparisons of means was carried out. Two-tailed Student’s t-tests were performed using SPSS. Values of p < 0.05 indicated significant differences.
5. Conclusions
In summary, TCONS_00323213 is a vital regulator that affects proliferation and differentiation in PSCs. We present a molecular model to elucidate the function of TCONS_00323213 in regulating PSC differentiation through interactions with tran-scription factor PKNOX2, while relieves its inhibitory effect on the MyoG promoter. Our research provides new insights into the molecular mechanisms of lncRNA in porcine myogenesis.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms24076773/s1. Table S1: TCONS_00323213 ASO sequence and si-PKNOX2 sequence. Table S2: The differentially expressed genes list from RNA-seq. Table S3: PKNOX2-enriched genes in CUT and Tag data. Table S4: The genes list of combined CUT and Tag and RNA-seq analysis. Table S5: Primer information. Table S6: RNA-seq reads information. Figure S1: TCONS_00323213 is a muscle-specific expression closely related to muscle differentiation lncRNA. Figure S2: Real-time quantitative PCR analysis of marker gene expression level in differentiated PSC 36 h after ASO-TCONS_00323213 was transfected.
Author Contributions
Conceptualization, M.L. and C.L.; methodology, Q.L.; software, X.L.; validation, M.L., Q.L. and S.X.; formal analysis, C.F.; investigation, J.L.; resources, C.T.; writing—original draft preparation, M.L.; writing—review and editing, M.L. and C.L.; supervision, C.L.; project administration, C.L.; funding acquisition, C.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (NSFC, 31872322 and 32172707).
Institutional Review Board Statement
The animal study protocol was approved by the Ethics Committee of Huazhong Agricultural University (protocol code HZAUSW2015-0003), Wuhan City, Hubei Province, China.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding authors.
Acknowledgments
We thank Wenzhe Luo, Jingxun Li, Xiaofang Cheng, and Sheng Wang for their guidance and assistance on the isolation of PSCs, and acknowledge Lu Zhang for her help with the CUT and Tag bioinformatics analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Baghdadi, M.B.; Tajbakhsh, S. Regulation and phylogeny of skeletal muscle regeneration. Dev. Biol. 2018, 433, 200–209. [Google Scholar] [CrossRef]
- Anderson, D.M.; Anderson, K.M.; Chang, C.L.; Makarewich, C.A.; Nelson, B.R. A micropeptide encoded by a putative long noncoding RNA regulates muscle performance. Cell 2015, 160, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Verdijk, L.B.; Snijders, T.; Drost, M.; Delhaas, T.; Kadi, F.; van Loon, L.J. Satellite cells in human skeletal muscle; from birth to old age. Age 2014, 36, 545–547. [Google Scholar] [CrossRef] [PubMed]
- Verdijk, L.B. Satellite cell activation as a critical step in skeletal muscle plasticity. Exp. Physiol. 2014, 99, 1449–1450. [Google Scholar] [CrossRef] [PubMed]
- Cornelison, D. “Known Unknowns”: Current Questions in Muscle Satellite Cell Biology. Curr. Top. Dev. Biol. 2018, 126, 205–233. [Google Scholar]
- Ciciliot, S.; Schiaffino, S. Regeneration of mammalian skeletal muscle. Basic mechanisms and clinical implications. Curr. Pharm. Des. 2010, 16, 906–914. [Google Scholar] [CrossRef]
- Wei, C.H.; Wu, M.M.; Liu, R.Z. Research Progress in Muscular Growth and Development of Long Noncoding RNAs. Sci. Agr. Sin. 2014, 47, 4078–4085. [Google Scholar]
- Li, Y.; Chen, M.M.; Zhang, J.X. Effects of Bovine LncRNA-133a on Proliferation and Differentiation of Skeletal Muscle Satellite Cells. Sci. Agr. Sin. 2019, 52, 143–153. [Google Scholar]
- Brannan, C.I.; Dees, E.C.; Ingram, R.S.; Tilghman, S.M. The product of the H19 gene may function as an RNA. Mol. Cell. Biol. 1990, 10, 28–36. [Google Scholar]
- Poirier, F.; Chan, C.T.; Timmons, P.M.; Robertson, E.J.; Evans, M.J.; Rigby, P.W. The murine H19 gene is activated during embryonic stem cell differentiation in vitro and at the time of implantation in the developing embryo. Development. 1991, 113, 1105–1114. [Google Scholar] [CrossRef]
- Dey, B.K.; Pfeifer, K.; Dutta, A. The H19 long noncoding RNA gives rise to microRNAs miR-675–3p and miR-675–5p to promote skeletal muscle differentiation and re-generation. Genes. Dev. 2014, 28, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Su, T.; Zou, C.; Luo, W.; Shi, G.; Chen, L.; Fang, C.; Li, C. Long Non-coding RNA H19 Regulates Porcine Satellite Cell Differentiation through miR-140-5p/SOX4 and DBN1. Front. Cell. Dev. Biol. 2020, 8, 518724. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, W.; Li, Q.; Huang, Z.; Shi, G.; Li, C. Long Non-Coding RNA H19 Promotes Porcine Satellite Cell Differentiation by Interacting with TDP43. Genes. 2020, 11, 259. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; He, L.; Zhao, Y.; Li, Y.; Zhang, S. Malat1 regulates myogenic differentiation and muscle regeneration through modulating MyoD transcriptional activity. Cell Discov. 2017, 3, 17002. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, Y.; Bao, X. LncRNA Dum interacts with Dnmts to regulate Dppa2 expression during myogenic differentiation and muscle regeneration. Cell Res. 2015, 25, 335–350. [Google Scholar] [CrossRef]
- Cheng, X.; Li, L.; Shi, G. MEG3 Promotes Differentiation of Porcine Satellite Cells by Sponging miR-423-5p to Relieve Inhibiting Effect on SRF. Cells 2020, 9, 449. [Google Scholar] [CrossRef]
- Jin, J.J.; Lv, W.; Xia, P.; Xu, Z.Y.; Zheng, A.D.; Wang, X.J.; Wang, S.S.; Zeng, R.; Luo, H.M.; Li, G.L.; et al. Long noncoding RNA SYISL regulates myogenesis by interacting with polycomb repressive complex 2. Proc. Natl. Acad. Sci. USA 2018, 115, E9802–E9811. [Google Scholar] [CrossRef]
- Lv, W.; Jin, J.; Xu, Z. lncMGPF is a novel positive regulator of muscle growth and regeneration. J. Cachexia Sarcopenia Muscle 2020, 11, 1723–1746. [Google Scholar] [CrossRef]
- Trewin, A.J.; Silver, J.; Dillon, H.T. Long non-coding RNA Tug1 modulates mitochondrial and myogenic responses to exercise in skeletal muscle. BMC Bio. 2022, 20, 164. [Google Scholar] [CrossRef]
- Shanshan, W.; Baohua, T.; Liyao, X.; Jiekang, Z.; Xinming, Z. Long non-coding RNA Gm10561 promotes myogenesis by sponging miR-432. Epigenetics 2022, 17, 2039–2055. [Google Scholar]
- Tang, Z.L.; Wu, Y.; Yang, Y.l. Comprehensive analysis of long non-coding RNAs highlights their spatio-temporal expression patterns and evolutional conservation in Sus scrofa. Sci. Rep. 2017, 7, 43166. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lang, K.; Tan, S.; Jie, W.; Zhu, Y.; Huang, S.; Huang, W. A web-based database server using 43,710 public RNA-seq samples for the analysis of gene expression and alternative splicing in livestock animals. BMC Genom. 2022, 23, 706. [Google Scholar] [CrossRef] [PubMed]
- Imoto, I.; Sonoda, I.; Yuki, Y.; Inazawa, J. Identification and characterization of human PKNOX2, a novel homeobox-containing gene. Biochem Biophys. Res. Commun. 2001, 287, 270–276. [Google Scholar]
- Robinson, D.C.L.; Dilworth, F.J. Epigenetic Regulation of Adult Myogenesis. Curr. Top. Dev. Biol. 2018, 126, 235–284. [Google Scholar]
- Hutcheson, D.A.; Zhao, J.; Merrell, A.; Haldar, M.; Kardon, G. Embryonic and fetal limb myogenic cells are derived from developmentally distinct progenitors and have different requirements for beta-catenin. Genes. Dev. 2009, 23, 997–1013. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.; Rudnicki, M.A. Satellite cells, the engines of muscle repair. Nat. Rev. Mol. Cell Biol. 2011, 13, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.; Han, Y.; Zhao, X.; Li, D.; Li, G. Long non-coding RNA Irm enhances myogenic differentiation by interacting with MEF2D. Cell Death Dis. 2019, 10, 181. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, Y.; Li, T.; Ma, Z.; Jia, H.; Chen, Q.; Zhao, Y.; Zhai, L. Long non-coding RNA Linc-RAM enhances myogenic differentiation by interacting with MyoD. Nat. Commun. 2017, 8, 14016. [Google Scholar] [CrossRef]
- Dykes, I.M.; Emanueli, C. Transcriptional and Post-transcriptional Gene Regulation by Long Non-coding RNA. Genom. Proteom. Bioinform. 2017, 15, 177–186. [Google Scholar] [CrossRef]
- Moens, C.B.; Selleri, L. Hox cofactors in vertebrate development. Dev. Biol. 2006, 291, 193–206. [Google Scholar] [CrossRef]
- Zhang, L.; Li, W.; Cao, L.; Xu, J.; Qian, Y.; Chen, H.; Zhang, Y.; Kang, W.; Gou, H.; Wong, C.C.; et al. PKNOX2 suppresses gastric cancer through the transcriptional activation of IGFBP5 and p53. Oncogene 2019, 38, 4590–4604. [Google Scholar] [CrossRef] [PubMed]
- Miyake, Y.; Obana, M.; Nakae, T. PKNOX2 regulates myofibroblast functions and tubular cell survival during kidney fibrosis. Biochem. Biophys. Res. Commun. 2021, 571, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Wang, L.; Luo, Y.; Chen, H.; Yang, Y.; Shen, Q.; Cao, G. LINC02489 with m6a modification increase paclitaxel sensitivity by inhibiting migration and invasion of ovarian cancer cells. Biotechnol. Genet. Eng. Rev. 2023, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.T.; Bartolomei, M.S. X-inactivation, imprinting, and long noncoding RNAs in health and disease. Cell 2013, 152, 1308–1323. [Google Scholar] [CrossRef]
- Gupta, R.A.; Shah, N.; Wang, K.C.; Kim, J.; Horlings, H.M.; Wong, D.J.; Tsai, M.C. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010, 464, 1071–1076. [Google Scholar] [CrossRef]
- Tripathi, V.; Ellis, J.D.; Shen, Z.; Song, D.Y.; Pan, Q.; Watt, A.T.; Freier, S.M. The nuclear-retained noncoding RNA MALAT1 regulates alternative splicing by modulating SR splicing factor phosphorylation. Mol. Cell 2010, 39, 925–938. [Google Scholar] [CrossRef]
- Li, X.; Zhou, B.; Chen, L.; Gou, L.T.; Li, H.; Fu, X.D. GRID-seq reveals the global RNA-chromatin interactome. Nat. Biotechnol. 2017, 35, 940–950. [Google Scholar] [CrossRef]
- Rinn, J.L.; Chang, H.Y. Genome regulation by long noncoding RNAs. Annu. Rev. Biochem. 2012, 81, 145–166. [Google Scholar] [CrossRef]
- Hanshan, H.; Ge, S. LncRNAs in Stem Cells. Stem Cells Int. 2016, 2681925. [Google Scholar]
- Luisa, S.; Marco, M.; Elena, G. Identification of RNA-binding proteins in exosomes capable of interacting with different types of RNA: RBP-facilitated transport of RNAs into exosomes. PLoS ONE 2018, 13, e0195969. [Google Scholar]
- Bonetti, A.; Agostini, F.; Suzuki, A.M. RADICL-seq identifies general and celltype–specific principles of genome-wide RNA-chromatin interactions. Nat. Commun. 2020, 11, 1018. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, T.; Tang, H.; Sha, Z.; Chen, R.; Chen, L.; Yu, Y.; Rowe, G.C.; Das, S.; Xiao, J. Inhibition of lncRNA MAAT Controls Multiple Types of Muscle Atrophy by cis- and trans-Regulatory Actions. Mol. Ther. 2021, 29, 1102–1119. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Chang, M.W.; Pandey, P.R.; Tsitsipatis, D.; Yang, X.; Martindale, J.L. Interaction of OIP5-AS1 with MEF2C mRNA promotes myogenic gene expression. Nucleic Acids Res. 2020, 48, 12943–12956. [Google Scholar] [CrossRef]
- Militello, G.; Hosen, M.R.; Ponomareva, Y.; Gellert, P.; Weirick, T.; John, D. A novel long non-coding RNA Myolinc regulates myogenesis through TDP-43 and Filip1. J. Mol. Cell Biol. 2018, 10, 102–117. [Google Scholar] [CrossRef]
- Hitachi, K.; Nakatani, M.; Takasaki, A.; Ouchi, Y.; Uezumi, A.; Ageta, H.; Inagaki, H.; Kurahashi, H.; Tsuchida, K. Myogenin promoter-associated lncRNA Myoparr is essential for myogenic differentiation. EMBO Rep. 2019, 20, e47468. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Sun, Y.; Ren, R.; Xie, J.; Tian, X.; Zhao, S.; Li, X.; Cao, J. H3K27me3 Depletion during Differentiation Promotes Myogenic Transcription in Porcine Satellite Cells. Genes 2019, 10, 231. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.G.; He, Q.Y. ChIPseeker: An R/Bioconductor package for ChIP peak annotation, comparison and visualization. Bioinformatics 2015, 31, 2382–2383. [Google Scholar] [CrossRef]
- Ramírez, F.; Dündar, F.; Diehl, S.; Grüning, B.A.; Manke, T. deepTools: A flexible platform for exploring deep-sequencing data. Nucleic Acids Res. 2014, 42, W187–W191. [Google Scholar] [CrossRef]
- Thorvaldsdóttir, H.; Robinson, J.T.; Mesirov, J.P. Integrative Genomics Viewer (IGV): High-performance genomics data visualization and exploration. Brief. Bioinform. 2013, 14, 178–192. [Google Scholar] [CrossRef]
- Heinz, S.; Benner, C.; Spann, N.; Bertolino, E.; Lin, Y.C.; Laslo, P.; Cheng, J.X.; Murre, C.; Singh, H.; Glass, C.K. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell 2010, 38, 576–589. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).