The Role of Vitamin C and Vitamin D in the Pathogenesis and Therapy of Periodontitis—Narrative Review
Abstract
1. Introduction
2. Vitamin C in Periodontitis
3. Vitamin D in Periodontitis
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal diseases. Nat. Rev. Dis. Primers 2017, 3, 17038. [Google Scholar] [CrossRef]
- Sedghi, L.M.; Bacino, M.; Kapila, Y.L. Periodontal Disease: The Good, The Bad, and The Unknown. Front. Cell Infect. Microbiol. 2021, 11, 766944. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Lambris, J.D. Complement and dysbiosis in periodontal disease. Immunobiology 2012, 217, 1111–1116. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Yoneda, M.; Hirofuji, T. Mixed red-complex bacterial infection in periodontitis. Int. J. Dent. 2013, 2013, 587279. [Google Scholar] [CrossRef] [PubMed]
- Rai, B.; Kaur, J.; Anand, S.C.; Jacobs, R. Salivary stress markers, stress, and periodontitis: A pilot study. J. Periodontol. 2011, 82, 287–292. [Google Scholar] [CrossRef]
- Woelber, J.P.; Gärtner, M.; Breuninger, L.; Anderson, A.; König, D.; Hellwig, E.; Al-Ahmad, A.; Vach, K.; Dötsch, A.; Ratka-Krüger, P.; et al. The influence of an anti-inflammatory diet on gingivitis. A randomized controlled trial. J. Clin. Periodontol. 2019, 46, 481–490. [Google Scholar] [CrossRef]
- Padayatty, S.J.; Levine, M. Vitamin C: The known and the unknown and Goldilocks. Oral Dis. 2016, 22, 463–493. [Google Scholar] [CrossRef]
- Lykkesfeldt, J.; Michels, A.J.; Frei, B. Vitamin C. Adv. Nutr. 2014, 5, 16–18. [Google Scholar] [CrossRef] [PubMed]
- Chambial, S.; Dwivedi, S.; Shukla, K.K.; John, P.J.; Sharma, P. Vitamin C in disease prevention and cure: An overview. Indian J. Clin. Biochem. 2013, 28, 314–328. [Google Scholar] [CrossRef]
- Hemilä, H. Vitamin C and Infections. Nutrients 2017, 9, 339. [Google Scholar] [CrossRef] [PubMed]
- Peterkofsky, B. Ascorbate requirement for hydroxylation and secretion of procollagen: Relationship to inhibition of collagen synthesis in scurvy. Am. J. Clin. Nutr. 1991, 54, 1135S–1140S. [Google Scholar] [CrossRef] [PubMed]
- Doseděl, M.; Jirkovský, E.; Macáková, K.; Krčmová, L.K.; Javorská, L.; Pourová, J.; Mercolini, L.; Remião, F.; Nováková, L.; Mladěnka, P.; et al. Vitamin C-Sources, Physiological Role, Kinetics, Deficiency, Use, Toxicity, and Determination. Nutrients 2021, 13, 615. [Google Scholar] [CrossRef]
- Englard, S.; Seifter, S. The biochemical functions of ascorbic acid. Annu. Rev. Nutr. 1986, 6, 365–406. [Google Scholar] [CrossRef]
- Carr, A.; Frei, B. Does vitamin C act as a pro-oxidant under physiological conditions? FASEB J. 1999, 13, 1007–1024. [Google Scholar] [CrossRef]
- Carr, A.C.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.C.; Shaw, G.M.; Fowler, A.A.; Natarajan, R. Ascorbate-dependent vasopressor synthesis: A rationale for vitamin C administration in severe sepsis and septic shock? Crit. Care 2015, 19, 418. [Google Scholar] [CrossRef]
- Holick, M.F.; Chen, T.C. Vitamin D deficiency: A worldwide problem with health consequences. Am. J. Clin. Nutr. 2008, 87, 1080S–1086S. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.; Christakos, S. New aspects of vitamin D metabolism and action—addressing the skin as source and target. Nat. Rev. Endocrinol. 2020, 16, 234–252. [Google Scholar] [CrossRef]
- Panda, D.K.; Miao, D.; Bolivar, I.; Li, J.; Huo, R.; Hendy, G.N.; Goltzman, D. Inactivation of the 25-hydroxyvitamin D 1alpha-hydroxylase and vitamin D receptor demonstrates independent and interdependent effects of calcium and vitamin D on skeletal and mineral homeostasis. J. Biol. Chem. 2004, 279, 16754–16766. [Google Scholar] [CrossRef]
- Xue, Y.; Fleet, J.C. Intestinal vitamin D receptor is required for normal calcium and bone metabolism in mice. Gastroenterology 2009, 136, 1317–1327, e1311-1312. [Google Scholar] [CrossRef]
- Bischoff-Ferrari, H.A.; Willett, W.C.; Wong, J.B.; Stuck, A.E.; Staehelin, H.B.; Orav, E.J.; Thoma, A.; Kiel, D.P.; Henschkowski, J. Prevention of nonvertebral fractures with oral vitamin D and dose dependency: A meta-analysis of randomized controlled trials. Arch. Intern. Med. 2009, 169, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Prietl, B.; Treiber, G.; Pieber, T.R.; Amrein, K. Vitamin D and immune function. Nutrients 2013, 5, 2502–2521. [Google Scholar] [CrossRef] [PubMed]
- Mitri, J.; Dawson-Hughes, B.; Hu, F.B.; Pittas, A.G. Effects of vitamin D and calcium supplementation on pancreatic β cell function, insulin sensitivity, and glycemia in adults at high risk of diabetes: The Calcium and Vitamin D for Diabetes Mellitus (CaDDM) randomized controlled trial. Am. J. Clin. Nutr. 2011, 94, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Pittas, A.G.; Lau, J.; Hu, F.B.; Dawson-Hughes, B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2007, 92, 2017–2029. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Norman, A.W.; Okamura, W.H.; Sen, A.; Zemel, M.B. 1alpha,25-Dihydroxyvitamin D3 modulates human adipocyte metabolism via nongenomic action. FASEB J. 2001, 15, 2751–2753. [Google Scholar] [CrossRef]
- Uysal, S.; Kalayci, A.G.; Baysal, K. Cardiac functions in children with vitamin D deficiency rickets. Pediatr. Cardiol. 1999, 20, 283–286. [Google Scholar] [CrossRef]
- Brøndum-Jacobsen, P.; Benn, M.; Jensen, G.B.; Nordestgaard, B.G. 25-hydroxyvitamin d levels and risk of ischemic heart disease, myocardial infarction, and early death: Population-based study and meta-analyses of 18 and 17 studies. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2794–2802. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D and skin cancer. J. Nutr. 2004, 134, 3472S–3478S. [Google Scholar] [CrossRef]
- Botelho, J.; Machado, V.; Proença, L.; Delgado, A.S.; Mendes, J.J. Vitamin D Deficiency and Oral Health: A Comprehensive Review. Nutrients 2020, 12, 1471. [Google Scholar] [CrossRef]
- Saeed, R.W.; Peng, T.; Metz, C.N. Ascorbic acid blocks the growth inhibitory effect of tumor necrosis factor-alpha on endothelial cells. Exp. Biol. Med. 2003, 228, 855–865. [Google Scholar] [CrossRef]
- Schor, A.M.; Schor, S.L.; Allen, T.D. Effects of culture conditions on the proliferation, morphology and migration of bovine aortic endothelial cells. J. Cell Sci. 1983, 62, 267–285. [Google Scholar] [CrossRef] [PubMed]
- Shekhonin, B.V.; Domogatsky, S.P.; Idelson, G.L.; Koteliansky, V.E.; Rukosuev, V.S. Relative distribution of fibronectin and type I, III, IV, V collagens in normal and atherosclerotic intima of human arteries. Atherosclerosis 1987, 67, 9–16. [Google Scholar] [CrossRef]
- Mikirova, N.; Casciari, J.; Rogers, A.; Taylor, P. Effect of high-dose intravenous vitamin C on inflammation in cancer patients. J. Transl. Med. 2012, 10, 189. [Google Scholar] [CrossRef] [PubMed]
- Helmersson, J.; Arnlöv, J.; Larsson, A.; Basu, S. Low dietary intake of beta-carotene, alpha-tocopherol and ascorbic acid is associated with increased inflammatory and oxidative stress status in a Swedish cohort. Br. J. Nutr. 2009, 101, 1775–1782. [Google Scholar] [CrossRef]
- Uetaki, M.; Tabata, S.; Nakasuka, F.; Soga, T.; Tomita, M. Metabolomic alterations in human cancer cells by vitamin C-induced oxidative stress. Sci. Rep. 2015, 5, 13896. [Google Scholar] [CrossRef]
- Rembe, J.D.; Fromm-Dornieden, C.; Stuermer, E.K. Effects of Vitamin B Complex and Vitamin C on Human Skin Cells: Is the Perceived Effect Measurable? Adv. Skin Wound Care 2018, 31, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Pullar, J.M.; Carr, A.C.; Vissers, M.C.M. The Roles of Vitamin C in Skin Health. Nutrients 2017, 9, 866. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Yang, N.; Feng, X.; Sun, T.; Shen, P.; Sun, W. Effect of vitamin C administration on hydrogen peroxide-induced cytotoxicity in periodontal ligament cells. Mol. Med. Rep. 2015, 11, 242–248. [Google Scholar] [CrossRef]
- Ahuja, A.; Minz, R.S.M.; Ahuja, V.; Mishra, A.; Kumari, S. Evaluation of Regenerative Potential of Locally Delivered Vitamin C along with Microneedling in the Treatment of Deficient Interdental Papilla: A Clinical Study. J. Contemp. Dent. Pract. 2022, 23, 503–507. [Google Scholar] [CrossRef]
- Gokhale, N.H.; Acharya, A.B.; Patil, V.S.; Trivedi, D.J.; Thakur, S.L. A short-term evaluation of the relationship between plasma ascorbic acid levels and periodontal disease in systemically healthy and type 2 diabetes mellitus subjects. J. Diet. Suppl. 2013, 10, 93–104. [Google Scholar] [CrossRef]
- Lee, J.H.; Shin, M.S.; Kim, E.J.; Ahn, Y.B.; Kim, H.D. The association of dietary vitamin C intake with periodontitis among Korean adults: Results from KNHANES Ⅳ. PLoS ONE 2017, 12, e0177074. [Google Scholar] [CrossRef]
- Park, J.A.; Lee, J.H.; Lee, H.J.; Jin, B.H.; Bae, K.H. Association of Some Vitamins and Minerals with Periodontitis in a Nationally Representative Sample of Korean Young Adults. Biol. Trace Elem. Res. 2017, 178, 171–179. [Google Scholar] [CrossRef]
- Tada, A.; Miura, H. The Relationship between Vitamin C and Periodontal Diseases: A Systematic Review. Int. J. Environ. Res. Public Health 2019, 16, 2472. [Google Scholar] [CrossRef]
- Chapple, I.L.; Milward, M.R.; Dietrich, T. The prevalence of inflammatory periodontitis is negatively associated with serum antioxidant concentrations. J. Nutr. 2007, 137, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Munday, M.R.; Rodricks, R.; Fitzpatrick, M.; Flood, V.M.; Gunton, J.E. A Pilot Study Examining Vitamin C Levels in Periodontal Patients. Nutrients 2020, 12, 2255. [Google Scholar] [CrossRef] [PubMed]
- Assaf, M.; Rabi, H. Assessment of Vitamin C Levels in Periodontal Patients: A Cross-Sectional Study in Palestine. J. Pharm. Bioallied. Sci. 2022, 14, S903–S906. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tang, L.; Lin, Y.F.; Xie, G.F. Role of vitamin C in wound healing after dental implant surgery in patients treated with bone grafts and patients with chronic periodontitis. Clin. Implant Dent. Relat. Res. 2018, 20, 793–798. [Google Scholar] [CrossRef]
- Lang, F.; Ma, K.; Leibrock, C.B. 1,25(OH). Neurosignals 2019, 27, 40–49. [Google Scholar] [CrossRef]
- Latic, N.; Erben, R.G. Vitamin D and Cardiovascular Disease, with Emphasis on Hypertension, Atherosclerosis, and Heart Failure. Int. J. Mol. Sci. 2020, 21, 6483. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, S.; Yuan, X.; Wang, Y.; Liu, Y.; Zhou, J. The role of vitamin D deficiency in the development of paediatric diseases. Ann. Med. 2023, 55, 127–135. [Google Scholar] [CrossRef]
- Lu, E.M. The role of vitamin D in periodontal health and disease. J. Periodontal. Res. 2022, 58, 213–224. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, M.; Xu, W.; Li, F.; Ma, C.; Tang, X. Calcitriol-enhanced autophagy in gingival epithelium attenuates periodontal inflammation in rats with type 2 diabetes mellitus. Front. Endocrinol. 2022, 13, 1051374. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D and the immune system: Role in protection against bacterial infection. Curr. Opin. Nephrol. Hypertens. 2008, 17, 348–352. [Google Scholar] [CrossRef]
- Girgis, C.M.; Clifton-Bligh, R.J.; Hamrick, M.W.; Holick, M.F.; Gunton, J.E. The roles of vitamin D in skeletal muscle: Form, function, and metabolism. Endocr. Rev. 2013, 34, 33–83. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Quesada Gomez, J.M. Comparison of calcifediol with vitamin D for prevention or cure of vitamin D deficiency. J. Steroid. Biochem. Mol. Biol. 2023, 228, 106248. [Google Scholar] [CrossRef] [PubMed]
- Woelber, J.P.; Tennert, C. Chapter 13: Diet and Periodontal Diseases. Monogr. Oral Sci. 2020, 28, 125–133. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Chavakis, T. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat. Rev. Immunol. 2021, 21, 426–440. [Google Scholar] [CrossRef]
- Costantini, E.; Sinjari, B.; Piscopo, F.; Porreca, A.; Reale, M.; Caputi, S.; Murmura, G. Evaluation of Salivary Cytokines and Vitamin D Levels in Periodontopathic Patients. Int. J. Mol. Sci. 2020, 21, 2669. [Google Scholar] [CrossRef] [PubMed]
- Taskan, M.M.; Gevrek, F. PPAR-γ, RXR, VDR, and COX-2 Expressions in gingival tissue samples of healthy individuals, periodontitis and peri-implantitis patients. Niger. J. Clin. Pract. 2020, 23, 46–53. [Google Scholar] [CrossRef]
- Machado, V.; Lobo, S.; Proença, L.; Mendes, J.J.; Botelho, J. Vitamin D and Periodontitis: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 2177. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Niu, L.; Ma, C.; Huang, Y.; Yang, X.; Shi, Y.; Pan, C.; Liu, J.; Wang, H.; Li, Q.; et al. Calcitriol decreases live Porphyromonas gingivalis internalized into epithelial cells and monocytes by promoting autophagy. J. Periodontol. 2020, 91, 956–966. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Cheng, C.; Zhu, Z.; Lin, M.; Zhang, D.X.; Wang, Z.M.; Wang, S. Vitamin D reduces the serum levels of inflammatory cytokines in rat models of periodontitis and chronic obstructive pulmonary disease. J. Oral Sci. 2019, 61, 53–60. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, X.; Zhang, P.; Zhao, P.; Nie, L.; Ji, N.; Ding, Y. 25-Hydroxyvitamin D. Steroids 2020, 156, 108570. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhong, X.; Li, W.; Wang, Q. Effects of 1,25-dihydroxyvitamin D3 on experimental periodontitis and AhR/NF-κB/NLRP3 inflammasome pathway in a mouse model. J. Appl. Oral Sci. 2019, 27, e20180713. [Google Scholar] [CrossRef]
- Anand, A.; Singh, S.; Sonkar, A.A.; Husain, N.; Singh, K.R.; Kushwaha, J.K. Expression of vitamin D receptor and vitamin D status in patients with oral neoplasms and effect of vitamin D supplementation on quality of life in advanced cancer treatment. Contemp. Oncol. 2017, 21, 145–151. [Google Scholar] [CrossRef]
- Jagelavičienė, E.; Vaitkevičienė, I.; Šilingaitė, D.; Šinkūnaitė, E.; Daugėlaitė, G. The Relationship between Vitamin D and Periodontal Pathology. Medicina 2018, 54, 45. [Google Scholar] [CrossRef]
- Uwitonze, A.M.; Murererehe, J.; Ineza, M.C.; Harelimana, E.I.; Nsabimana, U.; Uwambaye, P.; Gatarayiha, A.; Haq, A.; Razzaque, M.S. Effects of vitamin D status on oral health. J. Steroid Biochem. Mol. Biol. 2018, 175, 190–194. [Google Scholar] [CrossRef]
- Zhou, T.; Xu, W.; Wang, Q.; Jiang, C.; Li, H.; Chao, Y.; Sun, Y.; Lan, A. The effect of the “Oral-Gut” axis on periodontitis in inflammatory bowel disease: A review of microbe and immune mechanism associations. Front. Cell Infect. Microbiol. 2023, 13, 1132420. [Google Scholar] [CrossRef]
- Butera, A.; Maiorani, C.; Gallo, S.; Pascadopoli, M.; Venugopal, A.; Marya, A.; Scribante, A. Evaluation of Adjuvant Systems in Non-Surgical Peri-Implant Treatment: A Literature Review. Healthcare 2022, 10, 886. [Google Scholar] [CrossRef]
- Teughels, W.; Newman, M.G.; Coucke, W.; Haffajee, A.D.; Van Der Mei, H.C.; Haake, S.K.; Schepers, E.; Cassiman, J.J.; Van Eldere, J.; van Steenberghe, D.; et al. Guiding periodontal pocket recolonization: A proof of concept. J. Dent. Res. 2007, 86, 1078–1082. [Google Scholar] [CrossRef]
- Haas, A.N.; Furlaneto, F.; Gaio, E.J.; Gomes, S.C.; Palioto, D.B.; Castilho, R.M.; Sanz, M.; Messora, M.R. New tendencies in non-surgical periodontal therapy. Braz. Oral Res. 2021, 35, e095. [Google Scholar] [CrossRef]
- Looijer-van Langen, M.A.; Dieleman, L.A. Prebiotics in chronic intestinal inflammation. Inflamm. Bowel Dis. 2009, 15, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Patil, V.S.; Mali, R.S.; Moghe, A.S. Evaluation and comparison of Vitamin D receptors in periodontal ligament tissue of Vitamin D-deficient chronic periodontitis patients before and after supplementation of Vitamin D3. J. Indian Soc. Periodontol. 2019, 23, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Tang, H.; Wang, D.; Zhou, X.; Song, Y.; Wang, Z. Effect of short-term vitamin D supplementation after nonsurgical periodontal treatment: A randomized, double-masked, placebo-controlled clinical trial. J. Periodontal. Res. 2020, 55, 354–362. [Google Scholar] [CrossRef]
- Dietrich, T.; Joshipura, K.J.; Dawson-Hughes, B.; Bischoff-Ferrari, H.A. Association between serum concentrations of 25-hydroxyvitamin D3 and periodontal disease in the US population. Am. J. Clin. Nutr. 2004, 80, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Isola, G.; Alibrandi, A.; Rapisarda, E.; Matarese, G.; Williams, R.C.; Leonardi, R. Association of vitamin D in patients with periodontitis: A cross-sectional study. J. Periodontal. Res. 2020, 55, 602–612. [Google Scholar] [CrossRef]
- Bashutski, J.D.; Eber, R.M.; Kinney, J.S.; Benavides, E.; Maitra, S.; Braun, T.M.; Giannobile, W.V.; McCauley, L.K. The impact of vitamin D status on periodontal surgery outcomes. J. Dent. Res. 2011, 90, 1007–1012. [Google Scholar] [CrossRef]
- Millen, A.E.; Hovey, K.M.; LaMonte, M.J.; Swanson, M.; Andrews, C.A.; Kluczynski, M.A.; Genco, R.J.; Wactawski-Wende, J. Plasma 25-hydroxyvitamin D concentrations and periodontal disease in postmenopausal women. J. Periodontol. 2013, 84, 1243–1256. [Google Scholar] [CrossRef]
- Antonoglou, G.N.; Knuuttila, M.; Niemelä, O.; Raunio, T.; Karttunen, R.; Vainio, O.; Hedberg, P.; Ylöstalo, P.; Tervonen, T. Low serum level of 1,25(OH)2 D is associated with chronic periodontitis. J. Periodontal. Res. 2015, 50, 274–280. [Google Scholar] [CrossRef]
- Ebersole, J.L.; Lambert, J.; Bush, H.; Huja, P.E.; Basu, A. Serum Nutrient Levels and Aging Effects on Periodontitis. Nutrients 2018, 10, 1986. [Google Scholar] [CrossRef] [PubMed]
- Boggess, K.A.; Espinola, J.A.; Moss, K.; Beck, J.; Offenbacher, S.; Camargo, C.A. Vitamin D status and periodontal disease among pregnant women. J. Periodontol. 2011, 82, 195–200. [Google Scholar] [CrossRef]
- Sablok, A.; Batra, A.; Thariani, K.; Bharti, R.; Aggarwal, A.R.; Kabi, B.C.; Chellani, H. Supplementation of vitamin D in pregnancy and its correlation with feto-maternal outcome. Clin. Endocrinol. 2015, 83, 536–541. [Google Scholar] [CrossRef]
- Khan, F.R.; Ahmad, T.; Hussain, R.; Bhutta, Z.A. Relationship among Hypovitaminosis D, Maternal Periodontal Disease, and Low Birth Weight. J. Coll. Physicians Surg. Pak. 2018, 28, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.Q.; Qi, H.P.; Luo, Z.C.; Fraser, W.D. Maternal vitamin D status and adverse pregnancy outcomes: A systematic review and meta-analysis. J. Matern. Fetal. Neonatal. Med. 2013, 26, 889–899. [Google Scholar] [CrossRef]
- Albandar, J.M.; Susin, C.; Hughes, F.J. Manifestations of systemic diseases and conditions that affect the periodontal attachment apparatus: Case definitions and diagnostic considerations. J. Clin. Periodontol. 2018, 45 (Suppl. 20), S171–S189. [Google Scholar] [CrossRef]
- Sabharwal, A.; Gomes-Filho, I.S.; Stellrecht, E.; Scannapieco, F.A. Role of periodontal therapy in management of common complex systemic diseases and conditions: An update. Periodontol 2000 2018, 78, 212–226. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zong, X.; Pan, Y. Associations between vitamin D receptor genetic variants and periodontitis: A meta-analysis. Acta Odontol. Scand. 2019, 77, 484–494. [Google Scholar] [CrossRef]
- Wan, Q.S.; Li, L.; Yang, S.K.; Liu, Z.L.; Song, N. Role of Vitamin D Receptor Gene Polymorphisms on the Susceptibility to Periodontitis: A Meta-Analysis of a Controversial Issue. Genet. Test Mol. Biomark. 2019, 23, 618–633. [Google Scholar] [CrossRef]
- Ege, F.; Sarıkaya, S. FokI polymorphism in the vitamin D receptor gene in patients with hip osteoarthritis: A case-control study. Turk. J. Phys. Med. Rehabil. 2022, 68, 532–537. [Google Scholar] [CrossRef]
- Isola, G. The Impact of Diet, Nutrition and Nutraceuticals on Oral and Periodontal Health. Nutrients 2020, 12, 2724. [Google Scholar] [CrossRef] [PubMed]
- Santonocito, S.; Giudice, A.; Polizzi, A.; Troiano, G.; Merlo, E.M.; Sclafani, R.; Grosso, G.; Isola, G. A Cross-Talk between Diet and the Oral Microbiome: Balance of Nutrition on Inflammation and Immune System’s Response during Periodontitis. Nutrients 2022, 14, 2426. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, B.M.; Fisher, B.J.; Kraskauskas, D.; Ward, S.; Wayne, J.S.; Brophy, D.F.; Fowler, A.A.; Yager, D.R.; Natarajan, R. Vitamin C promotes wound healing through novel pleiotropic mechanisms. Int. Wound J. 2016, 13, 572–584. [Google Scholar] [CrossRef] [PubMed]
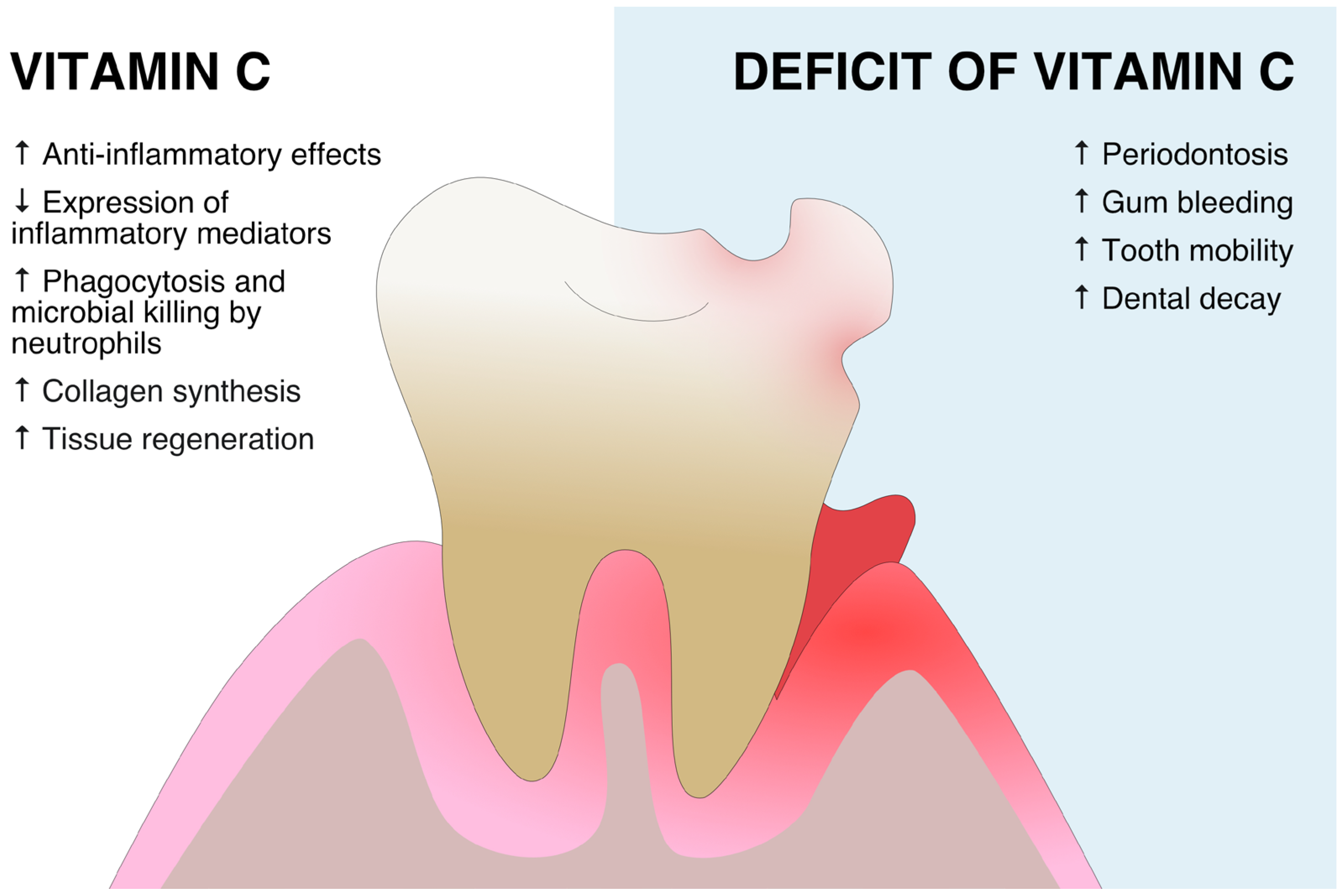
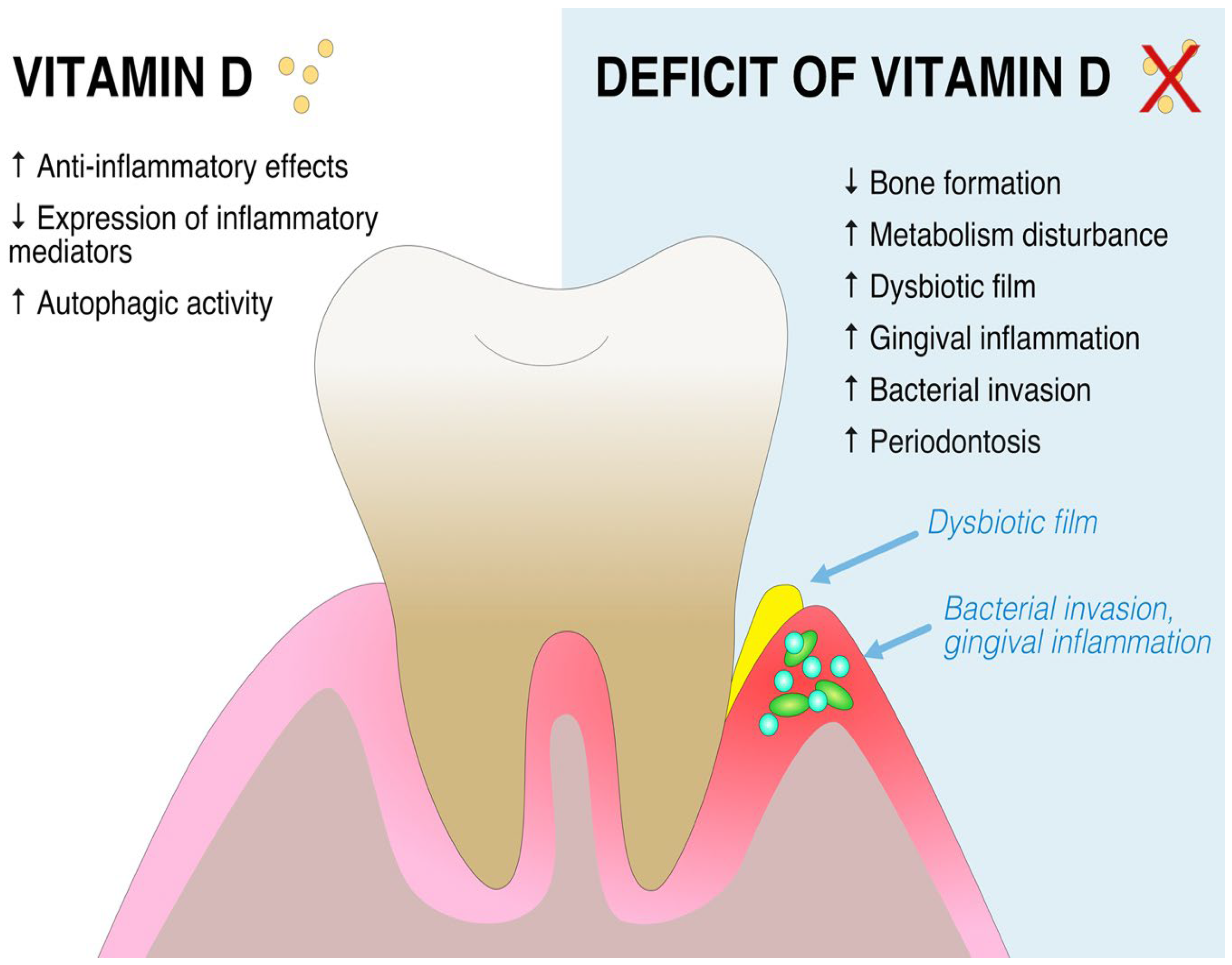
| Study | Sample | Control | Measured Variable | Assessment Method | Results |
|---|---|---|---|---|---|
| N.H. Gokhale, A.B. Acharya, V.S. Patil, D.J. Trivedi, S.L. Thakur (2013) [40] | 90 cases with different stages of periodontitis | 30 healthy individuals | Serum VitC levels with sample groups receiving VitC supplementation after first clinical examination | Plaque index (PlI), sulcus bleeding index (SBI), and probing pocket depths | Serum VitC levels were lower in sample groups compared to the control group. Subsequent supplementation of VitC reduces the SBI score. |
| J.A. Park, J.H. Lee, H.J. Lee, B.H. Jin, K.H. Bae (2017) [42] | 2049 young adults aged 19–39 years divided into groups based on clinical examination and nutrition survey | Vitamin and mineral intakes | Community Periodontal Index (CPI) | There were significant associations of periodontitis with lower intake of vitamin C in women (OR 1.66; 95% CI 1.04–2.64) and in current non-smokers (OR 1.49; 95% CI 1.04–2.14). | |
| I.L. Chapple, M.R. Milward, T. Dietrich (2007) [44] | Analysis of 11,480 NHANES III adult participants (>20 y of age). | Serum VitC level | Severity of periodontal disease | Increased serum antioxidant concentrations are associated with a reduced relative risk of periodontitis of 0.53 (95% CI, 0.42, 0.68) for vitamin C. | |
| M. Assaf, H. Rabi (2022) [46] | 25 patients with different stages of periodontitis | Serum VitC level | Clinical examination | Severity of periodontitis was inversely proportional to VitC serum levels (p < 0.05). | |
| X. Li, L. Tang, Y.F. Lin, G.F. Xie (2018) [47] | 128 patients requiring dental implants divided into an experimental subgroup, who received vitamin C, and a control subgroup | Soft tissue healing and pain response scores | Clinical examination | The experimental subgroups had significantly higher healing indices than the controls (p < 0.05). | |
| M.R. Munday, R. Rodricks, M. Fitzpatrick, V.M. Flood, J.E. Gunton (2020) [45] | 20 patients with periodontitis | Serum VitC and C-reactive protein (CRP) levels | Clinical examination | Low VitC was associated with higher periodontal disease stage (p = 0.03). Elevated CRP was found in 2/3 of people with low VitC, and CRP was negatively correlated with VitC (p < 0.01). | |
| Study | Sample | Control | Measured Variable | Assessment Method | Results |
|---|---|---|---|---|---|
| G.N. Antonoglou et al. (2015) [80] | 55 cases of chronic periodontitis | 30 healthy individuals | Serum VitD level | Plaque index | Low 1,25(OH)2D was correlated with a risk |
| K.A. Boggess et al. (2011) [82] | 117 cases of maternal periodontitis | 118 healthy pregnant women | Serum VitD level | Clinical symptoms of moderate to severe periodontitis | Pregnant women with periodontitis had a median serum VitD concentration of 59 vs. 100 nmol/L in healthy group (p < 0.001) |
| W. Gao et al. (2020) [75] | 120 cases of moderate to severe periodontitis after surgical treatment with 1000 IU/d VitD supplementation and 120 cases of 2000 IU/d VitD supplementation | 120 cases of periodontitis after surgical treatment without VitD supplementation | VitD supplementation of none, 1000 IU/d, and 2000 IU/d | Clinical examination | Groups with VitD supplementation had a significant decrease in attachment loss and probing depth compared to the control group |
| J. Han et al. (2019) [63] | Rat model for periodontitis with experimental group receiving VitD intraperitoneally vs. control group being treated with peanut oil | RANKL, TNF-α and interleukin serum levels | Examination of inflammatory status | VitD treatment alleviates inflammation by decreasing serum levels of RANKL, TNF-α, and interleukin-1 and increasing interleukin-10 levels | |
| J.L. Ebersole, J. Lambert, H. Bush, P. E. Huja, A. Basu (2018) [81] | Three NHANES cohort studies with 15,844 adults with periodontal examination, divided into healthy population and periodontitis population | Serum VitD level | Periodontal examination | Lower levels of VitD were seen in periodontitis population vs. healthy population | |
| G. Isola et al. (2020) [77] | 46 periodontitis cases | 43 healthy patients | Serum VitD level | Periodontal examination | Periodontitis group had a significantly lower mean concentration VitD levels (17.4 ± 5.2 ng/mL) vs. healthy group (29.9 ± 5.4 ng/mL) (p < 0.001) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ustianowski, Ł.; Ustianowska, K.; Gurazda, K.; Rusiński, M.; Ostrowski, P.; Pawlik, A. The Role of Vitamin C and Vitamin D in the Pathogenesis and Therapy of Periodontitis—Narrative Review. Int. J. Mol. Sci. 2023, 24, 6774. https://doi.org/10.3390/ijms24076774
Ustianowski Ł, Ustianowska K, Gurazda K, Rusiński M, Ostrowski P, Pawlik A. The Role of Vitamin C and Vitamin D in the Pathogenesis and Therapy of Periodontitis—Narrative Review. International Journal of Molecular Sciences. 2023; 24(7):6774. https://doi.org/10.3390/ijms24076774
Chicago/Turabian StyleUstianowski, Łukasz, Klaudia Ustianowska, Klaudia Gurazda, Marcin Rusiński, Piotr Ostrowski, and Andrzej Pawlik. 2023. "The Role of Vitamin C and Vitamin D in the Pathogenesis and Therapy of Periodontitis—Narrative Review" International Journal of Molecular Sciences 24, no. 7: 6774. https://doi.org/10.3390/ijms24076774
APA StyleUstianowski, Ł., Ustianowska, K., Gurazda, K., Rusiński, M., Ostrowski, P., & Pawlik, A. (2023). The Role of Vitamin C and Vitamin D in the Pathogenesis and Therapy of Periodontitis—Narrative Review. International Journal of Molecular Sciences, 24(7), 6774. https://doi.org/10.3390/ijms24076774






