Innate Immune Response of TmToll-3 Following Systemic Microbial Infection in Tenebrio molitor
Abstract
1. Introduction
2. Results
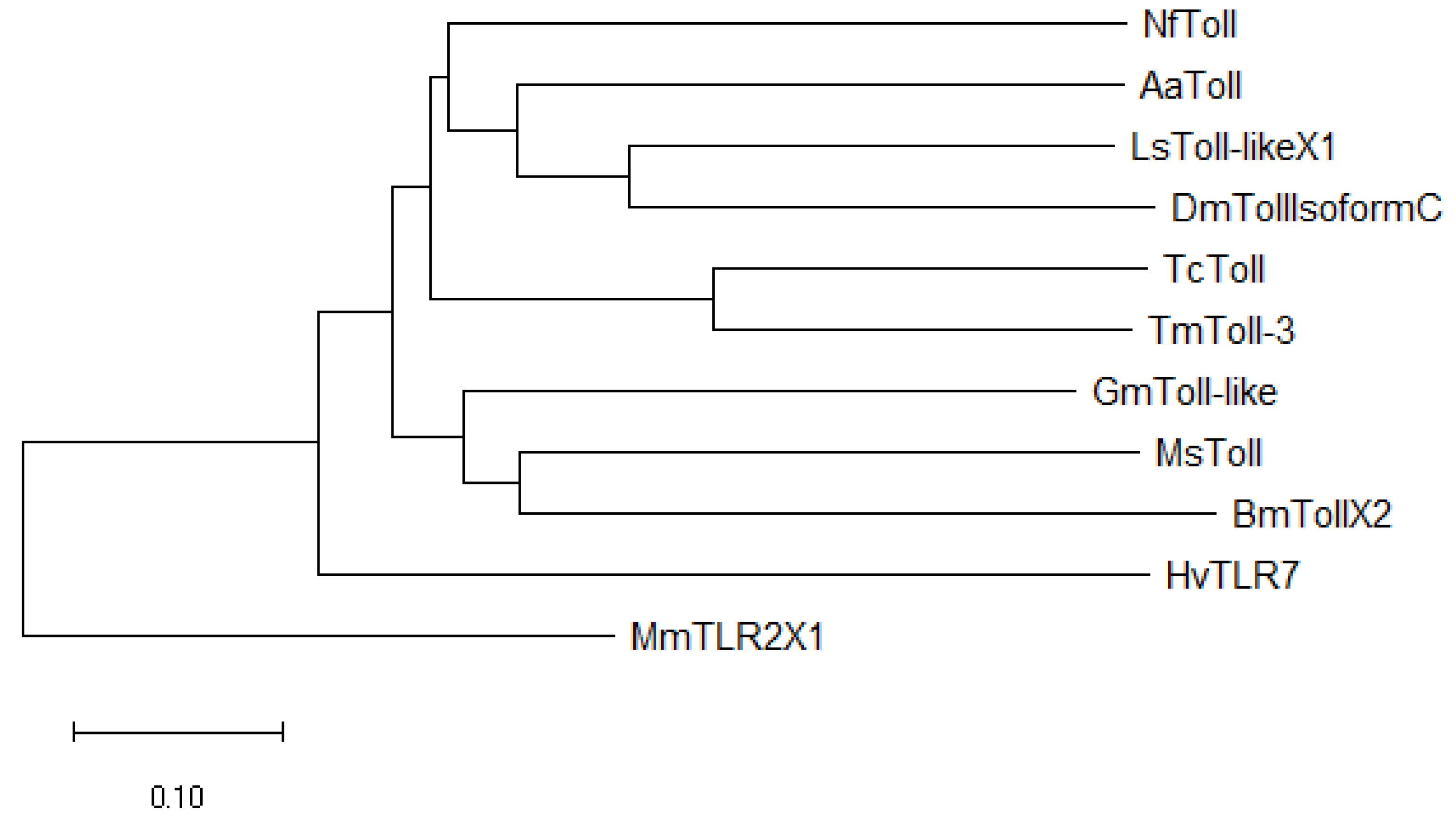
2.1. Sequence Analysis of TmToll-3
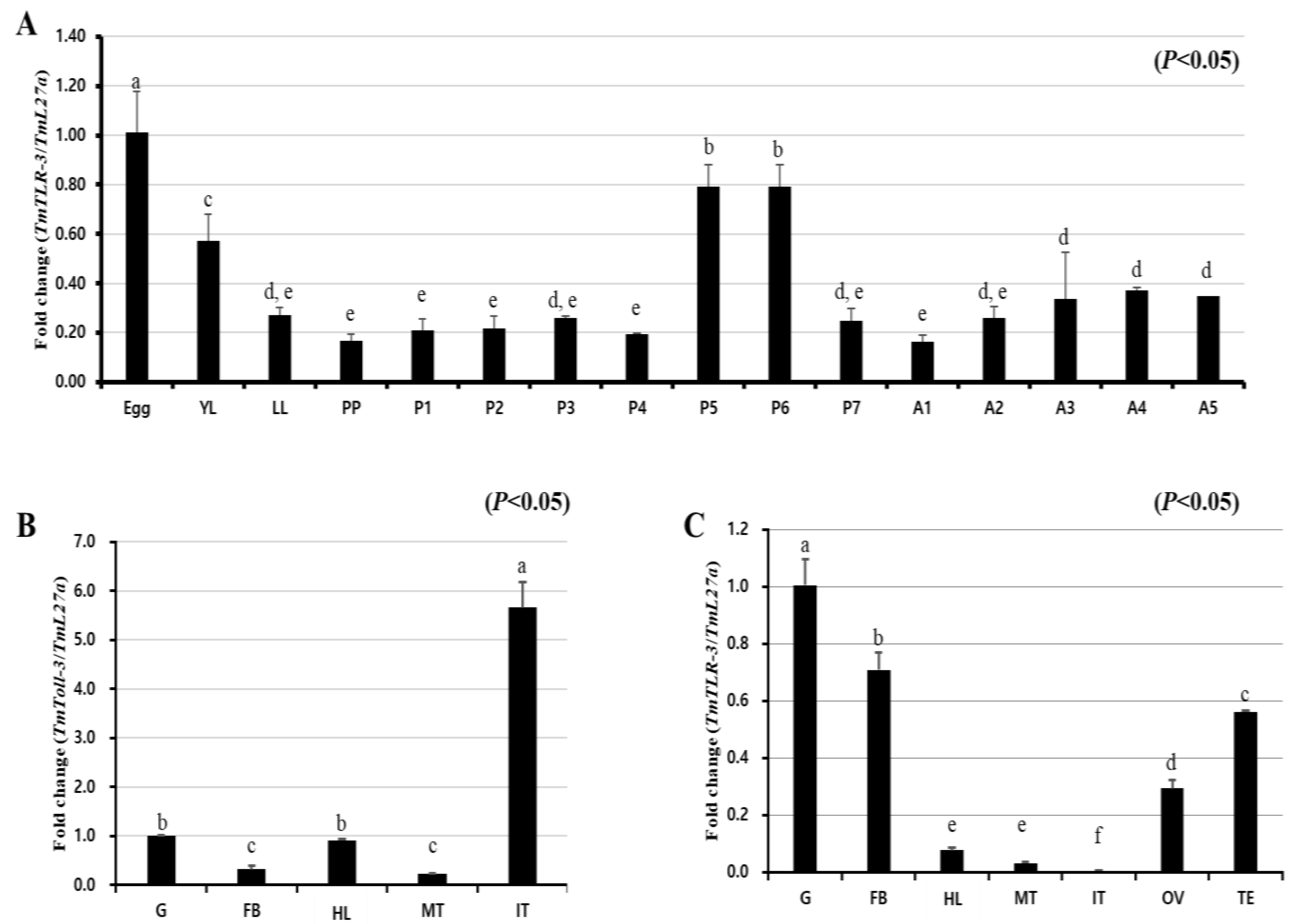
2.2. Expression Analysis of TmToll-3 Transcripts
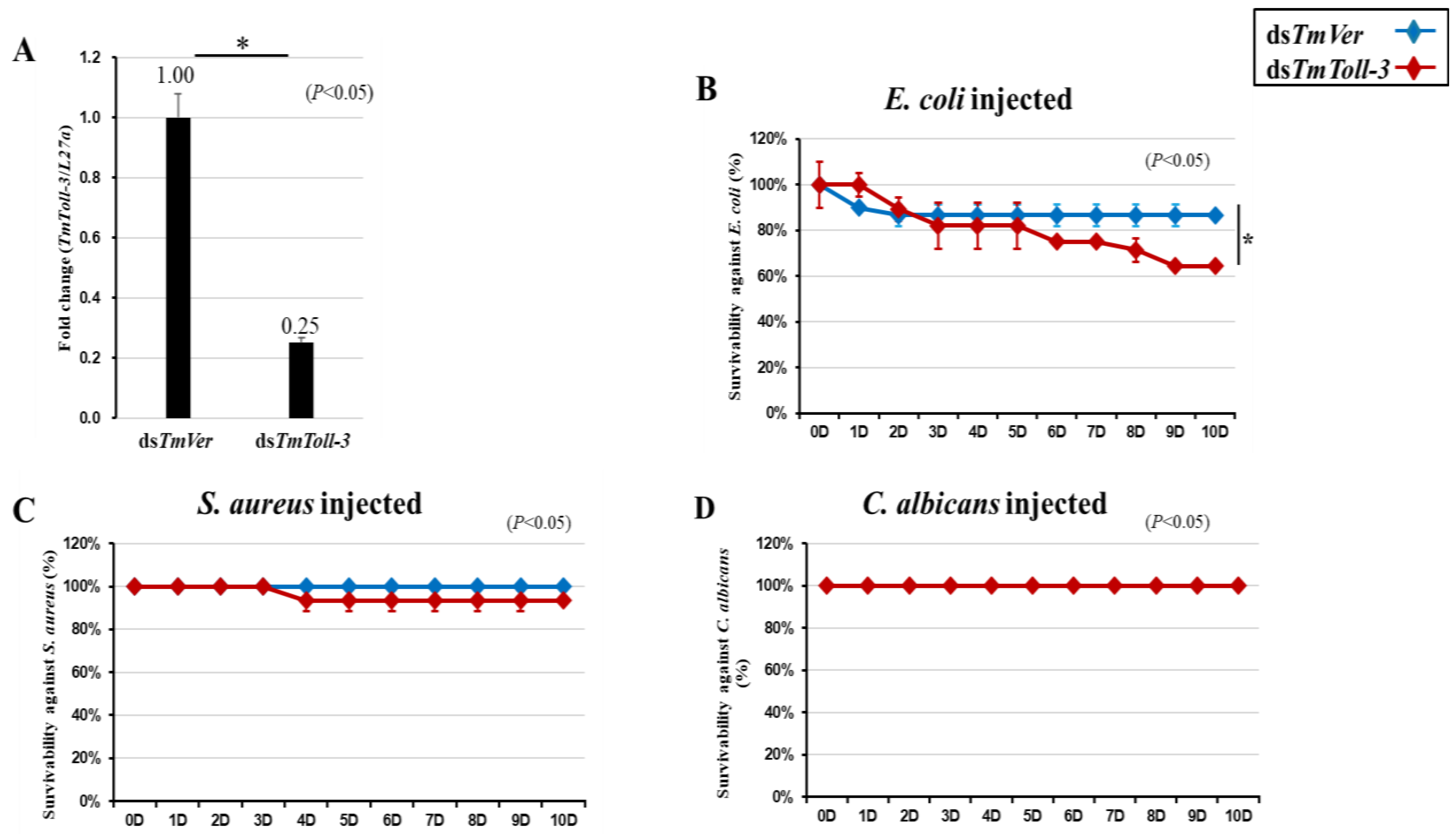
2.3. T. molitor Larval Mortality Assay
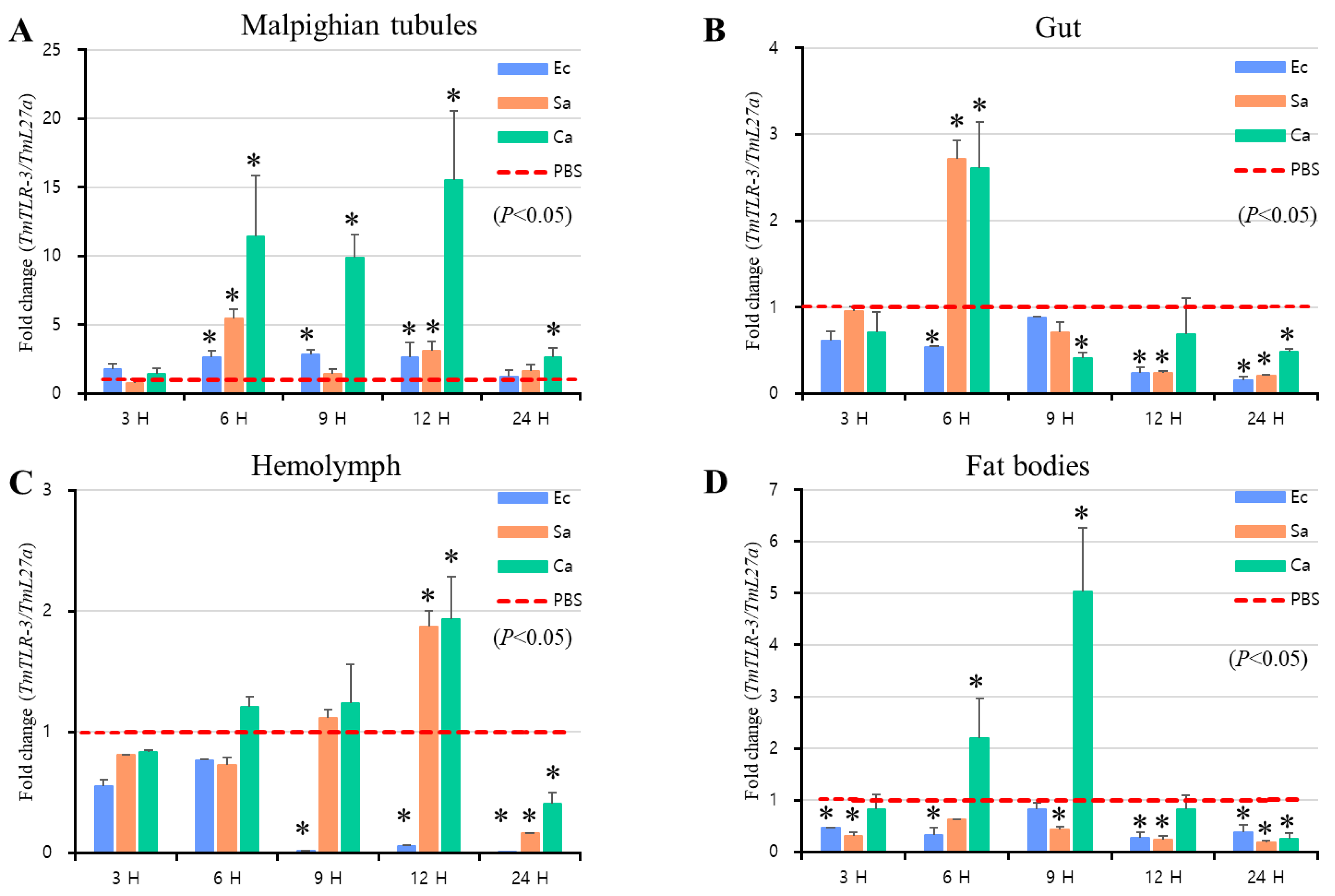
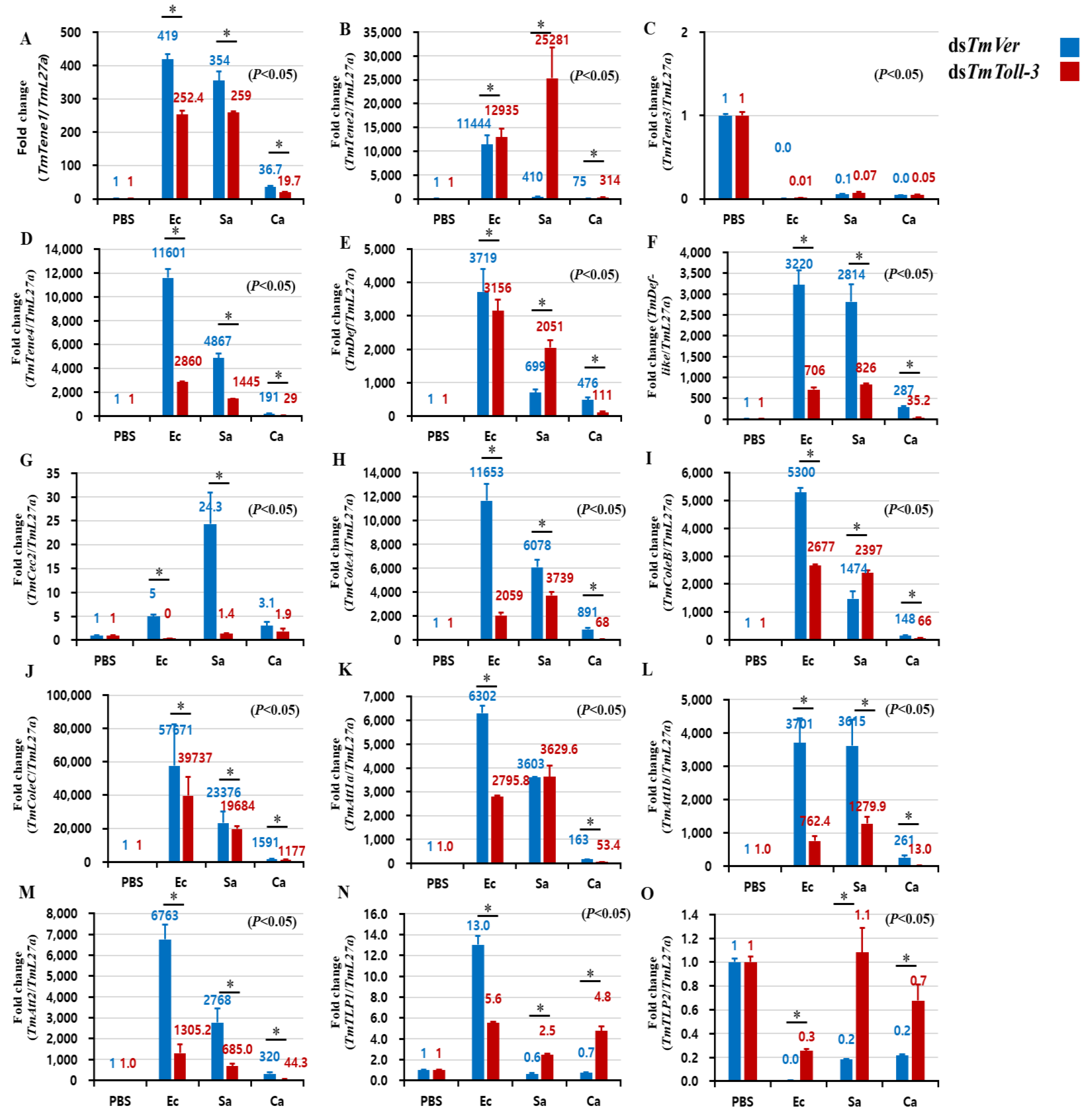
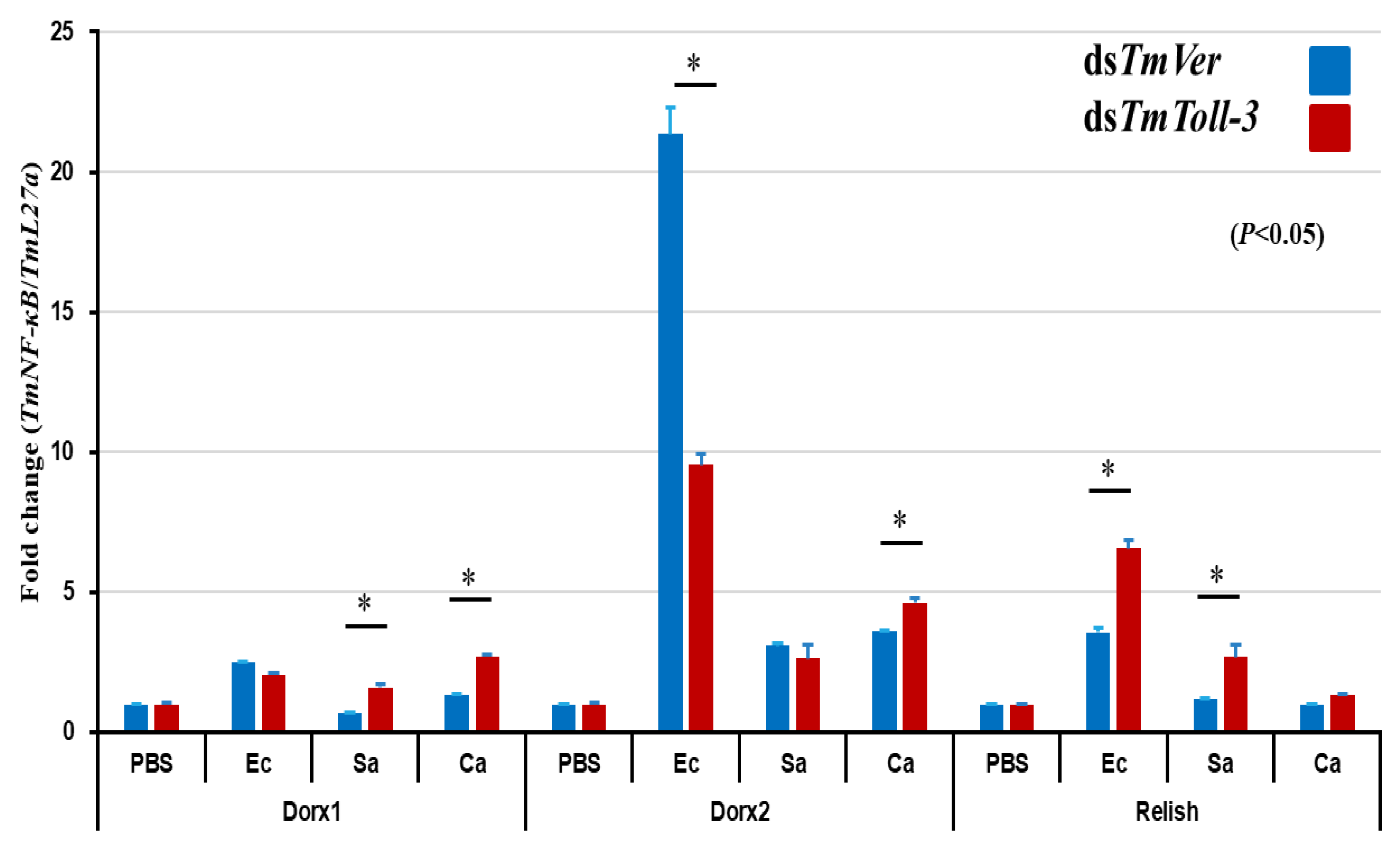
2.4. Effects of TmToll-3 RNAi on the Expression of AMP and Other Downstream Signaling Genes in Response to Microorganism Infection
3. Discussion
4. Materials and Methods
4.1. Insect Rearing and Microbial Infection
4.2. In Silico Analysis of TmToll-3
4.3. Expression and Induction Patterns of TmToll-3
4.4. RNA Interference Analysis
4.5. Effect of TmToll-3 Knockdown on the Response to Microorganisms
4.6. Effect of TmToll-3 RNAi on AMP Expression in Response to Microorganisms
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Uematsu, S.; Akira, S. Toll-like receptors and innate immunity. J. Mol. Med. 2006, 84, 712–725. [Google Scholar] [CrossRef] [PubMed]
- Belvin, M.P.; Anderson, K.V. A conserved signaling pathway: The Drosophila toll-dorsal pathway. Annu. Rev. Cell Dev. Biol. 1996, 12, 393–416. [Google Scholar] [CrossRef] [PubMed]
- Lemaitre, B.; Hoffmann, J. The host defense of Drosophila melanogaster. Annu. Rev. Immunol. 2007, 25, 697–743. [Google Scholar] [CrossRef]
- Bilak, H. Tauszig-Delamasure, S.; Imler, J.L. Toll and Toll-like receptors in Drosophila. Biochem. Society Trans. 2003, 31, 648–651. [Google Scholar] [CrossRef] [PubMed]
- Levin, T.C.; Malik, H.S. Rapidly evolving Toll-3/4 genes encode male-specific Toll-like receptors in Drosophila. Mol. Biol. Evol. 2017, 34, 2307–2323. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.V.; Bokla, L.; Nüsslein-Volhard, C. Establishment of dorsal-ventral polarity in the Drosophila embryo: The induction of polarity by the Toll gene product. Cell 1985, 42, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Tauszig, S.; Jouanguy, E.; Hoffmann, J.A.; Imler, J.L. Toll-related receptors and the control of antimicrobial peptide expression in Drosophila. Proc. Natl. Acad. Sci. USA 2000, 97, 10520–10525. [Google Scholar] [CrossRef]
- Yagi, Y.; Nishida, Y.; Ip, Y.T. Functional analysis of Toll-related genes in Drosophila. Dev. Growth Differ. 2010, 52, 771–783. [Google Scholar] [CrossRef]
- Lemaitre, B.; Nicolas, E.; Michaut, L.; Reichhart, J.M.; Hoffmann, J.A. The dorsoventral regulatory gene cassette spätzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell 1996, 86, 973–983. [Google Scholar] [CrossRef]
- Ooi, J.Y.; Yagi, Y.; Hu, X.; Ip, Y.T. The Drosophila Toll-9 activates a constitutive antimicrobial defense. EMBO Rep. 2002, 3, 82–87. [Google Scholar] [CrossRef]
- Bettencourt, R.; Tanji, T.; Yagi, Y.; Ip, Y.T. Toll and Toll-9 in Drosophila innate immune response. J. Endotoxin Res. 2004, 10, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, M.; Moy, R.H.; Xu, J.; Bambina, S.; Yasunaga, A.; Shelly, S.S.; Gold, B.; Cherry, S. Virus recognition by Toll-7 activates antiviral autophagy in Drosophila. Immunity 2012, 36, 658–667. [Google Scholar] [CrossRef]
- Eldon, E.; Kooyer, S.; D’Evelyn, D.; Duman, M.; Lawinger, P.; Botas, J.; Bellen, H. The Drosophila 18 wheeler is required for morphogenesis and has striking similarities to Toll. Development 1994, 120, 885–899. [Google Scholar] [CrossRef] [PubMed]
- Keith, F.J.; Gay, N.J. The Drosophila membrane receptor Toll can function to promote cellular adhesion. EMBO J. 1990, 9, 4299–4306. [Google Scholar] [CrossRef] [PubMed]
- Kolesnikov, T.; Beckendorf, S.K. 18 wheeler regulates apical constriction of salivary gland cells via the Rho-GTPase-signaling pathway. Dev. Biol. 2007, 307, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Umetsu, D. Cell mechanics and cell-cell recognition controls by Toll-like receptors in tissue morphogenesis and homeostasis. Fly 2002, 16, 233–247. [Google Scholar] [CrossRef]
- Ligoxygakis, P.; Bulet, P.; Reichhart, J.M. Critical evaluation of the role of the Toll-like receptor 18-Wheeler in the host defense of Drosophila. EMBO Rep. 2002, 3, 666–673. [Google Scholar] [CrossRef]
- Luo, C.; Shen, B.; Manley, J.; Zheng, L. Tehao functions in the Toll pathway in Drosophila melanogaster: Possible roles in development and innate immunity. Insect Mol. Biol. 2001, 10, 457–464. [Google Scholar] [CrossRef]
- McIlroy, G.; Foldi, I.; Aurikko, J.; Wentzell, J.S.; Lim, M.A.; Fenton, J.C.; Gay, N.J.; Hidalgo, A. Toll-6 and Toll-7 function as neurotrophin receptors in the Drosophila melanogaster CNS. Nat. Neurosci. 2013, 16, 1248–1256. [Google Scholar] [CrossRef]
- Ward, A.; Hong, W.; Favaloro, V.; Luo, L. Toll receptors instruct axon and dendrite targeting and participate in synaptic partner matching in a Drosophila olfactory circuit. Neuron 2015, 85, 1013–1028. [Google Scholar] [CrossRef]
- Ding, X.; Li, Z.; Lin, G.; Li, W.; Xue, L. Toll-7 promotes tumour growth and invasion in Drosophila. Cell Prolif. 2022, 55, e13188. [Google Scholar] [CrossRef] [PubMed]
- Narbonne-Reveau, K.; Charroux, B.; Royet, J. Lack of an antibacterial response defect in Drosophila Toll-9 mutant. PLoS ONE 2011, 6, e17470. [Google Scholar] [CrossRef] [PubMed]
- Shields, A.; Amcheslavsky, A.; Brown, E.; Lee, T.V.; Nie, Y.; Tanji, T.; Lp, Y.T.; Bergmann, A. Toll-9 interacts with Toll-1 to mediate a feedback loop during apoptosis-induced proliferation in Drosophila. Cell Rep. 2022, 2022, 110817. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.N.; Ren, F.F.; Zhou, C.B.; Xu, J.F.; Yi, H.Y.; Ye, M.Q.; Deng, X.J.; Cao, Y.; Yu, X.Q.; Yang, W.Y. An ML protein from the silkworm Bombyx mori may function as a key accessory protein for lipopolysaccharide signaling. Dev. Comp. Immunol. 2018, 88, 94–103. [Google Scholar] [CrossRef]
- Liu, Q.; Xu, D.; Jiang, S.; Huang, J.; Zhou, F.; Yang, Q.; Jiang, S.; Yang, L. Toll-receptor 9 gene in the black tiger shrimp (Penaeus monodon) induced the activation of the TLR–NF-κB signaling pathway. Gene 2018, 639, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Ali Mohammadie Kojour, M.; Han, Y.S.; Jo, Y.H. An overview of insect innate immunity. Entomol. Res. 2020, 50, 282–291. [Google Scholar] [CrossRef]
- Gay, N.J.; Keith, F.J. Drosophila Toll and IL-1 receptor. Nature 1991, 351, 355–356. [Google Scholar] [CrossRef]
- Hashimoto, C.; Hudson, K.L.; Anderson, K.V. The Toll gene of Drosophila, required for dorsal-ventral embryonic polarity, appears to encode a transmembrane protein. Cell 1998, 52, 269–279. [Google Scholar] [CrossRef]
- Lemaitre, B. The road to Toll. Nat. Rev. Immunol. 2004, 4, 521–527. [Google Scholar] [CrossRef]
- Ali Mohammadie Kojour, M.; Jang, H.A.; Lee, Y.S.; Jo, Y.H.; Han, Y.S. Immunological Roles of TmToll-2 in Response to Escherichia coli Systemic Infection in Tenebrio molitor. Int. J. Mol. Sci. 2022, 23, 14490. [Google Scholar] [CrossRef]
- Edosa, T.T.; Jo, Y.H.; Keshavarz, M.; Bae, Y.M.; Kim, D.H.; Lee, Y.S.; Han, Y.S. Tm Spz4 plays an important role in regulating the production of antimicrobial peptides in response to Escherichia coli and Candida albicans infections. Int. J. Mol. Sci. 2020, 21, 1878. [Google Scholar] [CrossRef]
- Edosa, T.T.; Jo, Y.H.; Keshavarz, M.; Bae, Y.M.; Kim, D.H.; Lee, Y.S.; Han, Y.S. Tm Spz6 is essential for regulating the immune response to Escherichia coli and Staphylococcus aureus infection in Tenebrio molitor. Insects 2020, 11, 105. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.A.; Patnaik, B.B.; Ali Mohammadie Kojour, M.; Kim, B.B.; Bae, Y.M.; Park, K.B.; Lee, Y.S.; Jo, Y.H.; Han, Y.S. Tm Spz-like plays a fundamental role in response to E. coli but not S. aureus or C. albican infection in Tenebrio molitor via regulation of antimicrobial peptide production. Int. J. Mol. Sci. 2021, 22, 10888. [Google Scholar] [CrossRef] [PubMed]
- Ali Mohammadie Kojour, M.; Edosa, T.T.; Jang, H.A.; Keshavarz, M.; Jo, Y.H.; Han, Y.S. Critical roles of spätzle5 in antimicrobial peptide production against Escherichia coli in Tenebrio molitor malpighian tubules. Front. Immunol. 2021, 12, 760475. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.M.; Jo, Y.H.; Patnaik, B.B.; Kim, B.B.; Park, K.B.; Edosa, T.T.; Keshavarz, M.; Kojour, M.A.M.; Lee, Y.S.; Han, Y.S. Tenebrio molitor spätzle 1b is required to confer antibacterial defense against gram-negative bacteria by regulation of antimicrobial peptides. Front. Physiology 2021, 18, 2084. [Google Scholar] [CrossRef]
- Yu, B.; Sang, Q.; Pan, G.; Li, C.; Zhou, Z. A Toll-Spätzle pathway in the immune response of Bombyx mori. Insects 2020, 11, 586. [Google Scholar] [CrossRef]
- Park, S.; Jo, Y.H.; Park, K.B.; Ko, H.J.; Kim, C.E.; Bae, Y.M.; Kim, B.; Jun, A.S.; Bang, I.S.; Lee, Y.S. TmToll-7 plays a crucial role in innate immune responses against Gram-negative bacteria by regulating 5 AMP genes in Tenebrio molitor. Front. Immunol. 2019, 10, 310. [Google Scholar] [CrossRef]
- Han, M.; Qin, S.; Song, X.; Li, Y.; Jin, P.; Chen, L.; Ma, F. Evolutionary rate patterns of genes involved in the Drosophila Toll and Imd signaling pathway. BMC Evol. Biol. 2013, 13, 245. [Google Scholar] [CrossRef]
- Yu, Y.; Park, J.W.; Kwon, H.M.; Hwang, H.O.; Jang, I.H.; Masuda, A.; Kurokawa, K.; Nakayama, H.; Lee, W.J.; Dohmae, N.; et al. Diversity of innate immune recognition mechanism for bacterial polymeric meso-diaminopimelic acid-type peptidoglycan in insects. J. Biol. Chem. 2010, 285, 32937–32945. [Google Scholar] [CrossRef]
- Lima, L.F.; Torres, A.Q.; Jardim, R.; Mesquita, R.D.; Schama, R. Evolution of Toll, Spatzle and MyD88 in insects: The problem of the Diptera bias. BMC Genom. 2021, 22, 562. [Google Scholar] [CrossRef]
- Keshavarz, M.; Jo, Y.H.; Edosa, T.T.; Bae, Y.M.; Han, Y.S. Tm PGRP-SA regulates antimicrobial response to bacteria and fungi n the fat body and gut of Tenebrio molitor. Int. J. Mol. Sci. 2020, 21, 2113. [Google Scholar] [CrossRef] [PubMed]
- Keshavarz, M.; Jo, Y.H.; Edosa, T.T.; Han, Y.S. Tenebrio molitor PGRP-LE plays a critical role in gut antimicrobial peptide production in response to Escherichia coli. Front. Physiol. 2020, 11, 320. [Google Scholar] [CrossRef] [PubMed]
- Johnston, P.R.; Makarova, O.; Rolff, J. Inducible defenses stay up late: Temporal patterns of immune gene expression in Tenebrio molitor. G3 Genes Genomes Genet. 2014, 4, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Kambris, Z.; Hoffmann, J.A.; Imler, J.L.; Capovilla, M. Tissue and stage-specific expression of the Tolls in Drosophila embryos. Gene Expr. Patterns 2002, 2, 311–317. [Google Scholar] [CrossRef]
- Noh, M.Y.; Muthukrishnan, S.; Kramer, K.J.; Arakane, Y. Cuticle formation and pigmentation in beetles. Curr. Opin. Insect Sci. 2016, 17, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.H.; Lee, J.H.; Patnaik, B.B.; Keshavarz, M.; Lee, Y.S.; Han, Y.S. Autophagy in Tenebrio molitor immunity: Conserved antimicrobial functions in insect defenses. Front. Immunol. 2021, 12, 667664. [Google Scholar] [CrossRef]
- Chamy, L.E.; Leclerc, V.; Caldelari, I.; Reichhart, J.M. Sensing of ‘danger signals’ and pathogen-associated molecular patterns defines binary signaling pathways ‘upstream’ of Toll. Nat. Immunol. 2008, 9, 1165–1170. [Google Scholar] [CrossRef]
- Zhang, R.; Li, X.; Zhang, J.; Li, Y.; Wang, Y.; Song, Y.; Ren, F.; Yi, H.; Deng, X.; Zhong, Y.; et al. Toll9 from Bombyx mori functions as a pattern recognition receptor that shares features with Toll-like receptor 4 from mammals. Proc. Natl. Acad. Sci. USA 2021, 118, e2103021118. [Google Scholar] [CrossRef]
- Ganz, T.; Selsted, M.E.; Szklarek, D.; Harwig, S.S.; Daher, K.; Bainton, D.F.; Lehrer, R.I. Defensins. Natural peptide antibiotics of human neutrophils. J. Clin. Investig. 1985, 76, 1427–1435. [Google Scholar] [CrossRef]
- Bulet, P.; Cociancich, S.; Dimarcq, J.L.; Lambert, J.; Richhart, J.M.; Hoffmann, D.; Hetru, C.; Hoffmann, J.A. Insect immunity. Isolation from a coleopteran insect of a novel inducible antibacterial peptide and of new members of the insect defensin family. J. Biol. Chem. 1991, 266, 24520–24525. [Google Scholar] [CrossRef]
- Jang, H.A.; Park, K.B.; Kim, B.B.; Ali Mohammadie Kojour, M.; Bae, Y.M.; Baliarsingh, S.; Lee, Y.S.; Han, Y.S.; Jo, Y.H. Bacterial but not fungal challenge up-regulates the transcription of Coleoptericin genes in Tenebrio molitor. Entomol. Res. 2020, 50, 440–449. [Google Scholar] [CrossRef]
- Jang, H.A.; Park, K.B.; Kim, B.B.; Ali Mohammadie Kojour, M.; Bae, Y.M.; Baliarsingh, S.; Lee, Y.S.; Han, Y.S.; Jo, Y.H. In Silico identification and expression analyses of Defensin genes in the mealworm beetle Tenebrio molitor. Entomol. Res. 2020, 50, 575–585. [Google Scholar] [CrossRef]
- Keshavarz, M.; Jo, Y.H.; Park, K.B.; Ko, H.J.; Edosa, T.T.; Lee, Y.S.; Han, Y.S. Tm DorX2 positively regulates antimicrobial peptides in Tenebrio molitor gut, fat body, and hemocytes in response to bacterial and fungal infection. Sci. Rep. 2019, 9, 16878. [Google Scholar] [CrossRef] [PubMed]
- Keshavarz, M.; Jo, Y.H.; Patnaik, B.B.; Park, K.B.; Ko, H.J.; Kim, C.E.; Edosa, T.T.; Lee, Y.S.; Han, Y.S. Tm Relish is required for regulating the antimicrobial responses to Escherichia coli and Staphylococcus aureus in Tenebrio molitor. Sci. Rep. 2020, 10, 4258. [Google Scholar] [CrossRef] [PubMed]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Lorenzen, M.D.; Brown, S.J.; Denell, R.E.; Beeman, R.W. Cloning and characterization of the Tribolium castaneum eye-color genes encoding tryptophan oxygenase and kynurenine 3-monooxygenase. Genetics 2022, 160, 225–234. [Google Scholar] [CrossRef]
| Name | Primer Sequences |
|---|---|
| TmToll-3_qPCR_Fw TmToll-3_qPCR_Rv | 5′-GTTGGAGAATGTTGTCGGTG-3′ 5′-CGAACGATGTCGTCAATCTG-3′ |
| TmToll-3_T7_Fw | 5′-TAATACGACTCACTATAGGGT GACACGTTCATCAACAACGG-3′ |
| TmToll-3_T7_Rv | 5′-TAATACGACTCACTATAGGGT CGTTTTGGTTAAAGGCGAAA-3′ |
| dsTmVermillion_Fw | 5′-TAATACGACTCACTATAGGGT TCGAGAAGTCAGAGCAGCAA-3′ |
| dsTmVermillion_Rv | 5′-TAATACGACTCACTATAGGGT ACCACCAGTTCCCAGTTGAG-3′ |
| TmTenecin-1_qPCR_Fw TmTenecin-1_qPCR_Rv | 5′-CAGCTGAAGAAATCGAACAAGG-3′ 5′-CAGACCCTCTTTCCGTTACAGT-3′ |
| TmTenecin-2_qPCR_Fw TmTenecin-2_qPCR_Rv | 5′-CAGCAAAACGGAGGATGGTC-3′ 5′-CGTTGAAATCGTGATCTTGTCC-3′ |
| TmTenecin-3_qPCR_Fw TmTenecin-3_qPCR_Rv | 5′-GATTTGCTTGATTCTGGTGGTC-3′ 5′-CTGATGGCCTCCTAAATGTCC-3′ |
| TmTenecin-4_qPCR_Fw TmTenecin-4_qPCR_Rv | 5′-GGACATTGAAGATCCAGGAAAG-3′ 5′-CGGTGTTCCTTATGTAGAGCTG-3′ |
| TmDefensin_qPCR_Fw TmDefensin_qPCR_Rv | 5′-AAATCGAACAAGGCCAACAC-3′ 5′-GCAAATGCAGACCCTCTTTC-3′ |
| TmDefensin-like_qPCR_Fw TmDefensin-like_qPCR_Rv | 5′-GGGATGCCTCATGAAGATGTAG-3′ 5′-CCAATGCAAACACATTCGTC-3′ |
| TmColeoptericin-A_qPCR_Fw TmColeoptericin-A_qPCR_Rv | 5′-GGACAGAATGGTGGATGGTC-3′ 5′-CTCCAACATTCCAGGTAGGC-3′ |
| TmColeoptericin-B_qPCR_Fw TmColeoptericin-B_qPCR_Rv | 5′-CAGCTGTTGCCCACAAAGTG-3′ 5′-CTCAACGTTGGTCCTGGTGT-3′ |
| TmColeoptericin-C_qPCR_Fw TmColeoptericin-C_qPCR_Rv | 5′-GGACGGTTCTGATCTTCTTGAT -3′ 5′-CAGCTGTTTGTTTGTTCTCGTC-3′ |
| TmAttacin-1a_qPCR_Fw TmAttacin-1a_qPCR_Rv | 5′-AAAGTGGTCCCCACCGATTC-3′ 5′-GCGCTGAATGTTTTCGGCTT-3′ |
| TmAttacin-1b_qPCR_Fw TmAttacin-1b_qPCR_Rv | 5′-GAGCTGTGAATGCAGGACAA-3′ 5′-CCCTCTGATGAAACCTCCAA-3′ |
| TmCecropin-2_qPCR_Fw TmCecropin-2_qPCR_Rv | 5′-TACTAGCAGCGCCAAAACCT-3′ 5′-CTGGAACATTAGGCGGAGAA-3′ |
| TmDorsal-1_qPCR_Fw TmDorsal-1_qPCR_Rv | 5′-AGCGTTGAGGTTTCGGTATG-3′ 5′-TCTTTGGTGACGCAAGACAC-3′ |
| TmDorsal-2_qPCR_Fw TmDorsal-2_qPCR_Rv | 5′-ACACCCCCGAAATCACAAAC-3′ 5′-TTTCAGAGCGCCAGGTTTTG-3′ |
| TmRelish_qPCR_Fw TmRelish_qPCR_Rv | 5′-AGCGTCAAGTTGGAGCAGAT-3′ 5′-GTCCGGACCTCAAGTGT-3′ |
| TmL27a_qPCR_Fw TmL27a_qPCR_Rv | 5′-TCATCCTGAAGGCAAAGCTCCAGT-3′ 5′-AGGTTGGTTAGGCAGGCACCTTTA-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali Mohammadie Kojour, M.; Jang, H.A.; Lee, Y.S.; Jo, Y.H.; Han, Y.S. Innate Immune Response of TmToll-3 Following Systemic Microbial Infection in Tenebrio molitor. Int. J. Mol. Sci. 2023, 24, 6751. https://doi.org/10.3390/ijms24076751
Ali Mohammadie Kojour M, Jang HA, Lee YS, Jo YH, Han YS. Innate Immune Response of TmToll-3 Following Systemic Microbial Infection in Tenebrio molitor. International Journal of Molecular Sciences. 2023; 24(7):6751. https://doi.org/10.3390/ijms24076751
Chicago/Turabian StyleAli Mohammadie Kojour, Maryam, Ho Am Jang, Yong Seok Lee, Yong Hun Jo, and Yeon Soo Han. 2023. "Innate Immune Response of TmToll-3 Following Systemic Microbial Infection in Tenebrio molitor" International Journal of Molecular Sciences 24, no. 7: 6751. https://doi.org/10.3390/ijms24076751
APA StyleAli Mohammadie Kojour, M., Jang, H. A., Lee, Y. S., Jo, Y. H., & Han, Y. S. (2023). Innate Immune Response of TmToll-3 Following Systemic Microbial Infection in Tenebrio molitor. International Journal of Molecular Sciences, 24(7), 6751. https://doi.org/10.3390/ijms24076751





