Insights into the Effects of Insecticides on Aphids (Hemiptera: Aphididae): Resistance Mechanisms and Molecular Basis
Abstract
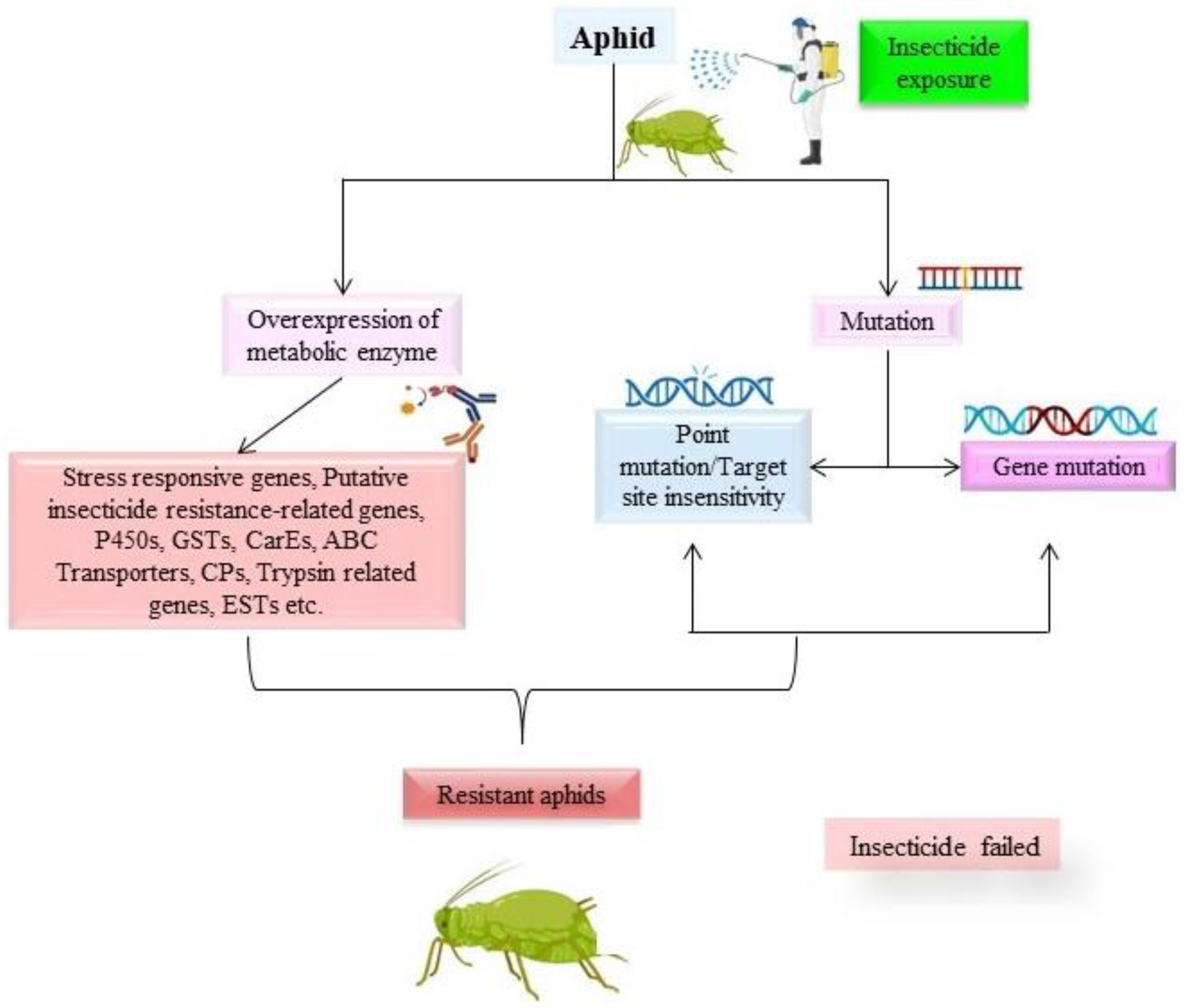
1. Introduction
2. Insecticide Resistance in Aphids to Different Insecticides
2.1. Organophosphate Resistance
Mechanism of Organiphosphate Resistance
2.2. Pyrethroid Resistance
Pyrethroid Resistance Allied to Na-Channel (Para) Gene
2.3. Carbamate Resistance
Mechanism of Carbamate Resistance
2.4. Neonicotinoid Resistance
Mechanism of Neonicotinoid Resistance
3. Gene Expression Studies
3.1. Stress-Responsive Genes
3.2. Putative Insecticide Resistance-Related Genes
3.3. Cytochrome P450 (P450)
3.4. Glutathione S-Transferase (GST)
- Delta
- Epsilon
- Omega
- Sigma
- Theta
- Zeta
3.5. Carboxylesterase CarEs
3.6. ABC Transporters
3.7. Cuticle Protein Genes
3.8. Trypsin-Related Genes
3.9. Other Insecticide Resistance-Related Genes and Insecticide Receptors
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, G.; Zhong, T. The Economic Insect Fauna of China; Science Press: Beijing, China, 1983. [Google Scholar]
- Blackman, R.L.; Eastop, V.F. Aphids on the World’s Crops: An Identification and Information Guide; Natural History Museum Publications; Wiley: Chichester, UK, 2000; ISBN 9780471851912. [Google Scholar]
- Tu, X.-B.; Zhao, H.-L.; Zhang, Z.-H. Transcriptome approach to understand the potential mechanisms of resistant and susceptible alfalfa (Medicago sativa L.) cultivars in response to aphid feeding. J. Integr. Agric. 2018, 17, 2518–2527. [Google Scholar] [CrossRef]
- Wang, M.; Yuan, G.; Chen, J.; Lei, Z.; Wu, Z. Research advances of occurrence pattern, damage characteristics of wheat aphid and resistance identification of wheat. J. Hennan Agric. Sci. 2006, 7, 58–60. [Google Scholar]
- Caillauda, M.; Edwardsb, O.; Fieldc, L.; GiblotDucrayd, D.; Graye, S.; Hawthornef, D.; Hunterg, W.; Janderh, G.; Morani, N.; Moyaj, A.; et al. Proposal to Sequence the Genome of the Pea Aphid (Acyrthosiphum pisum); International Aphid Genomics Consortium (IAGG): Coral Gables, FL, USA, 2004. [Google Scholar]
- Srigiriraju, L.; Semtner, P.J.; Bloomquist, J.R. Monitoring for imidacloprid resistance in the tobacco-adapted form of the green peach aphid, Myzus persicae (Sulzer) (Hemiptera: Aphididae), in the eastern United States. Pest Manag. Sci. 2010, 66, 676–685. [Google Scholar] [CrossRef]
- Mazzoni, E.; Cravedi, P. Analysis of insecticide-resistant Myzus persicae (Sulzer) populations collected in Italian peach orchards. Pest Manag. Sci. 2002, 58, 975–980. [Google Scholar] [CrossRef]
- Blackman, R.L.; Eastop, V.F. Aphids on the World’ s Herbaceous Plants and Shrubs; John Wiley & Sons: Hoboken, NJ, USA, 2006; ISBN 9780471489733. [Google Scholar]
- Schoonhoven, L.M.; Van Loon, J.J.A.; Dicke, M. Insect-Plant Biology; Oxford University Press: Oxford, UK, 2005; ISBN 019852594X. [Google Scholar]
- Liu, D.; Jiang, H.X.; Wang, Z.F.; Cao, Y.; Zhang, S.E.; Zhai, G.Y. The prevention and control of alfalfa aphid. Shandong J. Anim. Husb. Vet. 2012, 33, 94–96. [Google Scholar]
- Guo, K.; Yang, P.; Chen, J.; Lu, H.; Cui, F. Transcriptomic responses of three aphid species to chemical insecticide stress. Sci. China Life Sci. 2017, 60, 931–934. [Google Scholar] [CrossRef]
- Bass, C.; Puinean, A.M.; Zimmer, C.T.; Denholm, I.; Field, L.M.; Foster, S.P.; Gutbrod, O.; Nauen, R.; Slater, R.; Williamson, M.S. The evolution of insecticide resistance in the peach potato aphid, Myzus persicae. Insect Biochem. Mol. Biol. 2014, 51, 41–51. [Google Scholar] [CrossRef]
- Carletto, J.; Martin, T.; Vanlerberghe-Masutti, F.; Brévault, T. Insecticide resistance traits differ among and within host races in Aphis gossypii. Pest Manag. Sci. 2010, 66, 301–307. [Google Scholar] [CrossRef]
- Gong, P.; Li, X.; Wang, C.; Zhu, S.; Li, Q.; Zhang, Y.; Li, X.; Li, G.; Liu, E.; Gao, H.; et al. The sensitivity of field populations of Metopolophium dirhodum (Walker) (Hemiptera: Aphididae) to seven insecticides in Northern China. Agronomy 2021, 11, 1556. [Google Scholar] [CrossRef]
- Khan Mirza, F.; Yarahmadi, F.; Lotfi Jalal-Abadi, A.; Meraaten, A.A. Enzymes mediating resistance to chlorpyriphos in Aphis fabae (Homoptera: Aphididae). Ecotoxicol. Environ. Saf. 2020, 206, 111335. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, H.; Guo, K.; Yao, S.; Cui, F. Insecticide resistance status and detoxification enzymes of wheat aphids Sitobion avenae and Rhopalosiphum padi. Sci. China Life Sci. 2017, 60, 927–930. [Google Scholar] [CrossRef]
- Gong, P.; Li, X.; Gao, H.; Wang, C.; Li, M.; Zhang, Y.; Li, X.; Liu, E.; Zhu, X. Field evolved resistance to pyrethroids, neonicotinoids, organophosphates and macrolides in Rhopalosiphum padi (Linnaeus) and Sitobion avenae (Fabricius) from China. Chemosphere 2021, 269, 128747. [Google Scholar] [CrossRef]
- Nauen, R.; Elbert, A.; McCaffery, A.; Slater, R.; Sparks, T. IRAC: Insecticide resistance, and mode of action classification of insecticides. Mod. Crops Prot. Compd. 2011, 3, 935–955. [Google Scholar]
- Yu, S.J. The Toxicology and Biochemistry of Insecticides, 1st ed.; CRC Press: Boca Raton, FL, USA, 2011; ISBN 9780429136870. [Google Scholar]
- Foster, S.P.; Devine, G.; Devonshire, A.L. Insecticide Resistance. In Aphids as Crop Pests; CABI: Wallingford, UK, 2007; pp. 261–268. ISBN 9780851998190. [Google Scholar]
- Hanson, A.A.; Menger-Anderson, J.; Silverstein, C.; Potter, B.D.; Macrae, I.V.; Hodgson, E.W.; Koch, R.L. Evidence for soybean aphid (Hemiptera: Aphididae) resistance to pyrethroid insecticides in the Upper Midwestern United States. J. Econ. Entomol. 2017, 110, 2235–2246. [Google Scholar] [CrossRef]
- Xu, Y.; Xiurong, Y.; Changhui, Z.; Huizhen, W.; Xinzuo, C. Susceptibility of Helicoverpa Armigera, Aphis Gossypii, and T.Turkestani(Ugarovet Nikolski)to insecticides in Xinjiang. ACTA Agric. Boreali Occident. Sin. 2004, 13, 74–78. [Google Scholar]
- Li, F.; Han, Z.J.; Wu, Z.F.; Wang, Y.C. Insecticide resistance of Aphis gossypii Glover in cotton in China. Cott. Sci. 2001, 13, 121–124. [Google Scholar]
- Anstead, J.A.; Mallet, J.; Denholm, I. Temporal and spatial incidence of alleles conferring knockdown resistance to pyrethroids in the peach-potato aphid, Myzus persicae (Hemiptera: Aphididae), and their association with other insecticide resistance mechanisms. Bull. Entomol. Res. 2007, 97, 243–252. [Google Scholar] [CrossRef]
- Kennedy, J.S.; Stroyan, H.L.G. Biology of Aphids. Annu. Rev. Entomol. 1959, 4, 139–160. [Google Scholar] [CrossRef]
- Bwye, A.M.; Proudlove, W.; Berlandier, F.A.; Jones, R.A.C. Effects of applying insecticides to control aphid vectors and cucumber mosaic virus in narrow-leafed lupins Lupinus angustifolius). Aust. J. Exp. Agric. 1997, 37, 93–102. [Google Scholar] [CrossRef]
- Edwards, O.R.; Franzmann, B.; Thackray, D.; Micic, S. Insecticide resistance and implications for future aphid management in Australian grains and pastures: A review. Aust. J. Exp. Agric. 2008, 48, 1523–1530. [Google Scholar] [CrossRef]
- Whalon, M.E.; Mota-Sanchez, D.; Hollingworth, R.M. Global pesticide resistance in arthropods. In Global Pesticide Resistance in Arthropods; Whalon, M.E., Mota-Sanchez, D., Hollingworth, R.M., Eds.; CABI Publishing: Wallingford, UK, 2008; ISBN 9781845933531. [Google Scholar]
- APRD Arthropod Pesticide Resistance Database; Michigan State University: East Lansing, MI, USA, 2022.
- Ware, G.W. The Pesticide Book, 4th ed.; Thomson Publications: Ann Arbor, MI, USA, 1994; ISBN 978-0913702581. [Google Scholar]
- Rodríguez, M.A.; Bosch, D.; Sauphanor, B.; Avilla, J. Susceptibility to organophosphate insecticides and activity of detoxifying enzymes in Spanish populations of Cydia pomonella (Lepidoptera: Tortricidae). J. Econ. Entomol. 2010, 103, 482–491. [Google Scholar] [CrossRef]
- Rodríguez, M.A.; Marques, T.; Bosch, D.; Avilla, J. Assessment of insecticide resistance in eggs and neonate larvae of Cydia pomonella (Lepidoptera: Tortricidae). Pestic. Biochem. Physiol. 2011, 100, 151–159. [Google Scholar] [CrossRef]
- Reyes, M.; Franck, P.; Charmillot, P.-J.; Ioriatti, C.; Olivares, J.; Pasqualini, E.; Sauphanor, B. Diversity of insecticide resistance mechanisms and spectrum in European populations of the codling moth, Cydia pomonella. Pest Manag. Sci. 2007, 63, 890–902. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Contreras, E.; Reyes, M.; Barros, W.; Sauphanor, B. Evaluation of azinphos-methyl resistance and activity of detoxifying enzymes in codling moth (Lepidoptera: Tortricidae) from central Chile. J. Econ. Entomol. 2007, 100, 551–556. [Google Scholar] [CrossRef]
- Bush, M.R.; Abdel-All, Y.A.I.; Rock, G.C. Parathion resistance and esterase activity in Codling Moth (Lepidoptera: Tortricidae) from North Carolina. J. Econ. Entomol. 1993, 86, 660–666. [Google Scholar] [CrossRef]
- Reyes, M.; Collange, B.; Rault, M.; Casanelli, S.; Sauphanor, B. Combined detoxification mechanisms and target mutation fail to confer a high level of resistance to organophosphates in Cydia pomonella (L.) (Lepidoptera: Tortricidae). Pestic. Biochem. Physiol. 2011, 99, 25–32. [Google Scholar] [CrossRef]
- Gu, Z.Y.; Sun, S.S.; Wang, Y.H.; Wang, B.B.; Xie, Y.; Ma, L.; Wang, J.M.; Shen, W.D.; Li, B. Transcriptional characteristics of gene expression in the midgut of domestic silkworms (Bombyx mori) exposed to phoxim. Pestic. Biochem. Physiol. 2013, 105, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.Q. Gene expression analysis and enzyme assay reveal a potential role of the carboxylesterase gene CpCE-1 from Cydia pomonella in detoxification of insecticides. Pestic. Biochem. Physiol. 2016, 129, 56–62. [Google Scholar] [CrossRef]
- Beranek, A.P. Esterase variation and organophosphate resistance in populations of Aphis fabae and Myzus persicae. Entomol. Exp. Appl. 1974, 17, 129–142. [Google Scholar] [CrossRef]
- Kerns, D.L.; Gaylor, M.J. Insecticide resistance in field populations of the cotton aphid (Homoptera: Aphididae). J. Econ. Entomol. 1992, 85, 1–8. [Google Scholar] [CrossRef]
- Chen, M.; Han, Z.; Qiao, X.; Qu, M. Mutations in acetylcholinesterase genes of Rhopalosiphum padi resistant to organophosphate and carbamate insecticides. Genome 2007, 50, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Rufingier, C.; Pasteur, N.; Lagnel, J.; Martin, C.; Navajas, M. Mechanisms of insecticide resistance in the aphid Nasonovia ribisnigri (Mosley) (Homoptera: Aphididae) from France. Insect Biochem. Mol. Biol. 1999, 29, 385–391. [Google Scholar] [CrossRef]
- Herron, G.; Powis, K.; Rophail, J. Baseline studies and preliminary resistance survey of Australian populations of cotton aphid Aphis gossypii Glover (Hemiptera: Aphididae). Aust. J. Entomol. 2001, 39, 33–38. [Google Scholar] [CrossRef]
- Umina, P.A.; Edwards, O.; Carson, P.; Van Rooyen, A.; Anderson, A. High levels of resistance to carbamate and pyrethroid chemicals widespread in Australian Myzus persicae (Hemiptera: Aphididae) populations. J. Econ. Entomol. 2014, 107, 1626–1638. [Google Scholar] [CrossRef]
- Xi, J.; Pan, Y.; Bi, R.; Gao, X.; Chen, X.; Peng, T.; Zhang, M.; Zhang, H.; Hu, X.; Shang, Q. Elevated expression of esterase and cytochrome P450 are related with lambda-cyhalothrin resistance and lead to cross resistance in Aphis glycines Matsumura. Pestic. Biochem. Physiol. 2015, 118, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Nauen, R.; Denholm, I. Resistance of insect pests to neonicotinoid insecticides: Current status and future prospects. Arch. Insect Biochem. Physiol. 2005, 58, 200–215. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Jiang, L.; Wang, H.; Qiao, K.; Wang, D.; Wang, K. Toxicities and sublethal effects of seven neonicotinoid insecticides on survival, growth and reproduction of imidacloprid-resistant cotton aphid, Aphis gossypii. Pest Manag. Sci. 2011, 67, 1528–1533. [Google Scholar] [CrossRef]
- Hirata, K.; Kiyota, R.; Matsuura, A.; Toda, S.; Yamamoto, A.; Iwasa, T. Association between the R81T mutation in the nicotinic acetylcholine receptor β1 subunit of Aphis gossypii and the differential resistance to acetamiprid and imidacloprid. J. Pestic. Sci. 2015, 40, 25–31. [Google Scholar] [CrossRef]
- Devine, G.J.; Harling, Z.K.; Scarr, A.W.; Devonshire, A.L. Lethal and sublethal effects of imidacloprid on Nicotine-Tolerant Myzus nicotianae and Myzus persicae. Pestic. Sci. 1996, 48, 57–62. [Google Scholar] [CrossRef]
- Nauen, R.; Strobel, J.; Tietjen, K.; Otsu, Y.; Erdelen, C.; Elbert, A. Aphicidal activity of imidacloprid against a tobacco feeding strain of Myzus persicae (Homoptera: Aphididae) from Japan closely related to Myzus nicotianae and highly resistant to carbamates and organophosphates. Bull. Entomol. Res. 1996, 86, 165–171. [Google Scholar] [CrossRef]
- Soderlund, D.M. Molecular Mechanisms of Insecticide Resistance. In Molecular Mechanisms of Resistance to Agrochemicals; Sjut, V., Ed.; Springer: Berlin/Heidelberg, Germany, 1997; pp. 21–56. ISBN 978-3-662-03458-3. [Google Scholar]
- Oppenoorth, F.J. Biochemistry and genetics of insecticide resistance. Compr. Insect Physiol. Biochem. Pharmacol. Insect Control 1985, 12, 731–733. [Google Scholar]
- Soderlund, D.M.; Bloomquist, J.R. Molecular mechanisms of insecticide resistance. In Pesticide Resistance in Arthropods; Springer: Berlin/Heidelberg, Germany, 1990; pp. 58–96. [Google Scholar]
- Perry, A.S.; Yamamoto, I.; Ishaaya, P.D.I.; Perry, R.N. Insecticides in Agriculture and Environment: Retrospects and Prospects; Part of the Book Series: Applied Agriculture (APPLAGRIC); Springer: Berlin/Heidelberg, Germany, 1998. [Google Scholar]
- Hu, C.; Wei, Z.H.; Li, P.R.; Harwood, J.D.; Li, X.Y.; Yang, X.Q. Identification and functional characterization of a sigma glutathione S-transferase CpGSTs2 involved in λ-cyhalothrin resistance in the codling moth Cydia pomonella. J. Agric. Food Chem. 2020, 68, 12585–12594. [Google Scholar] [CrossRef]
- Li, F.; Han, Z. Mutations in acetylcholinesterase associated with insecticide resistance in the cotton aphid, Aphis gossypii Glover. Insect Biochem. Mol. Biol. 2004, 34, 397–405. [Google Scholar] [CrossRef]
- Sun, L.; Zhou, X.; Zhang, J.; Gao, X. Polymorphisms in a Carboxylesterase Gene Between Organophosphate-Resistant and -Susceptible Aphis gossypii (Homoptera: Aphididae). J. Econ. Entomol. 2005, 98, 1325–1332. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.-H.; Ai, G.-M.; Li, M.; Shi, X.-Y.; Diao, Q.-Y.; Gao, X.-W. Functional characterization of carboxylesterase gene mutations involved in Aphis gossypii resistance to organophosphate insecticides. Insect Mol. Biol. 2017, 26, 702–714. [Google Scholar] [CrossRef] [PubMed]
- Field, L.M.; Blackman, R.L. Insecticide resistance in the aphid Myzus persicae (Sulzer): Chromosome location and epigenetic effects on esterase gene expression in clonal lineages. Biol. J. Linn. Soc. 2003, 79, 107–113. [Google Scholar] [CrossRef]
- Javed, N.; Viner, R.; Williamson, M.S.; Field, L.M.; Devonshire, A.L.; Moores, G.D. Characterization of acetylcholinesterases, and their genes, from the hemipteran species Myzus persicae (Sulzer), Aphis gossypii (Glover), Bemisia tabaci (Gennadius) and Trialeurodes vaporariorum (Westwood). Insect Mol. Biol. 2003, 12, 613–620. [Google Scholar] [CrossRef]
- Nabeshima, T.; Kozaki, T.; Tomita, T.; Kono, Y. An amino acid substitution on the second acetylcholinesterase in the pirimicarb-resistant strains of the peach potato aphid, Myzus persicae. Biochem. Biophys. Res. Commun. 2003, 307, 15–22. [Google Scholar] [CrossRef]
- Andrews, M.C.; Callaghan, A.; Field, L.M.; Williamson, M.S.; Moores, G.D. Identification of mutations conferring insecticide-insensitive AChE in the cotton-melon aphid, Aphis gossypii Glover. Insect Mol. Biol. 2004, 13, 555–561. [Google Scholar] [CrossRef]
- Andrews, M.C.; Bass, C.G.; Williamson, M.S.; Callaghan, A.; Field, L.M.; Moores, G.D. A single amino acid substitution found in pirimicarb- insensitive acetylcholinesterase (AChE) of the peach-potato aphid, Myzus persicae (Sulzer). In Cholinergic Mechanisms: Function and Dysfunction; Taylor & Francis: London, UK, 2004; p. 38. [Google Scholar]
- MacKenzie, T.D.B.; Arju, I.; Poirier, R.; Singh, M. A Genetic survey of pyrethroid insecticide resistance in aphids in New Brunswick, Canada, with particular emphasis on aphids as vectors of potato virus Y. J. Econ. Entomol. 2018, 111, 1361–1368. [Google Scholar] [CrossRef] [PubMed]
- Eleftherianos, I.; Foster, S.P.; Williamson, M.S.; Denholm, I. Characterization of the M918T sodium channel gene mutation associated with strong resistance to pyrethroid insecticides in the peach-potato aphid, Myzus persicae (Sulzer). Bull. Entomol. Res. 2008, 98, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Foster, S.P.; Paul, V.L.; Slater, R.; Warren, A.; Denholm, I.; Field, L.M.; Williamson, M.S. A mutation (L1014F) in the voltage-gated sodium channel of the grain aphid, Sitobion avenae, is associated with resistance to pyrethroid insecticides. Pest Manag. Sci. 2014, 70, 1249–1253. [Google Scholar] [CrossRef]
- Puinean, A.M.; Foster, S.P.; Oliphant, L.; Denholm, I.; Field, L.M.; Millar, N.S.; Williamson, M.S.; Bass, C. Amplification of a cytochrome P450 gene is associated with resistance to neonicotinoid insecticides in the aphid Myzus persicae. PLoS Genet. 2010, 6, e1000999. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Qi, H.; Yang, D.; Yuan, H.; Rui, C. Cycloxaprid: A novel cis-nitromethylene neonicotinoid insecticide to control imidacloprid-resistant cotton aphid (Aphis gossypii). Pestic. Biochem. Physiol. 2016, 132, 96–101. [Google Scholar] [CrossRef]
- Xu, X.; Ding, Q.; Wang, X.; Wang, R.; Ullah, F.; Gao, X.; Song, D. V101I and R81T mutations in the nicotinic acetylcholine receptor β1 subunit are associated with neonicotinoid resistance in Myzus persicae. Pest Manag. Sci. 2022, 78, 1500–1507. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, F.; Chen, A.; Ma, K.; Liang, P.; Liu, Y.; Song, D.; Gao, X. Both point mutations and low expression levels of the nicotinic acetylcholine receptor β1 subunit are associated with imidacloprid resistance in an Aphis gossypii (Glover) population from a Bt cotton field in China. Pestic. Biochem. Physiol. 2017, 141, 1–8. [Google Scholar] [CrossRef]
- Agosin, M. Role of microsomal oxidations in insecticide degradation. In Comprehensive Insect Physiology, Biochemistry and Pharmacology; Ga, K., Li, G., Eds.; Pergamon Press: Oxford, UK, 1985; pp. 279–311. [Google Scholar]
- Scott, J.G. Cytochromes P450 and insecticide resistance. Insect Biochem. Mol. Biol. 1999, 29, 757–777. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, E. Microsomal mono-oxygenases. Compr. Insect Physiol. Biochem. Pharmacol. Pharmacol. 1985, 7, 223–321. [Google Scholar]
- Terriere, L.C. Induction of detoxication enzymes in insects. Annu. Rev. Entomol. 1984, 29, 71–88. [Google Scholar] [CrossRef]
- Chandrasena, D.; DiFonzo, C.; Byrne, A. An aphid-dip bioassay to evaluate susceptibility of soybean aphid (Hemiptera: Aphididae) to pyrethroid, organophosphate, and neonicotinoid insecticides. J. Econ. Entomol. 2011, 104, 1357–1363. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, W.J.; Yan, S.C.; Wang, C. Research on resistance of Aphis Glycines in Hei Longjiang Province. Adv. Mater. Res. 2012, 393–395, 926–929. [Google Scholar] [CrossRef]
- Ribeiro, L.P.; Akhtar, Y.; Vendramim, J.D.; Isman, M.B. Comparative bioactivity of selected seed extracts from Brazilian Annona species and an acetogenin-based commercial bioinsecticide against Trichoplusia ni and Myzus persicae. Crop Prot. 2014, 62, 100–106. [Google Scholar] [CrossRef]
- Koch, R.L.; Hodgson, E.W.; Knodel, J.J.; Varenhorst, A.J.; Potter, B.D. Management of insecticide-resistant soybean aphids in the Upper Midwest of the United States. J. Integr. Pest Manag. 2018, 9, 23. [Google Scholar] [CrossRef]
- Orantes, L.C.; Zhang, W.; Mian, M.A.R.; Michel, A.P. Maintaining genetic diversity and population panmixia through dispersal and not gene flow in a holocyclic heteroecious aphid species. Heredity 2012, 109, 127–134. [Google Scholar] [CrossRef]
- Schmidt, N.P.; O’Neal, M.E.; Anderson, P.F.; Lagos, D.; Voegtlin, D.; Bailey, W.; Caragea, P.; Cullen, E.; DiFonzo, C.; Elliott, K.; et al. Spatial distribution of Aphis glycines (Hemiptera: Aphididae): A summary of the suction trap network. J. Econ. Entomol. 2012, 105, 259–271. [Google Scholar] [CrossRef]
- Hodgson, E.W.; Burkness, E.C.; Hutchison, W.D.; Ragsdale, D.W. Enumerative and binomial sequential sampling plans for soybean aphid (Homoptera: Aphididae) in soybean. J. Econ. Entomol. 2004, 97, 2127–2136. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hodgson, E.W.; McCornack, B.P.; Koch, K.A.; Ragsdale, D.W.; Johnson, K.D.; O’Neal, M.E.; Cullen, E.M.; Kraiss, H.J.; DiFonzo, C.D.; Behnken, L.M. Field validation of speed scouting for soybean aphid. Crop Manag. 2007, 6, 1–8. [Google Scholar] [CrossRef]
- Ragsdale, D.W.; McCornack, B.P.; Venette, R.C.; Potter, B.D.; MacRae, I.V.; Hodgson, E.W.; O’Neal, M.E.; Johnson, K.D.; O’Neil, R.J.; DiFonzo, C.D.; et al. Economic threshold for soybean aphid (Hemiptera: Aphididae). J. Econ. Entomol. 2007, 100, 1258–1267. [Google Scholar] [CrossRef]
- Koch, R.L.; Potter, B.D.; Glogoza, P.A.; Hodgson, E.W.; Krupke, C.H.; Tooker, J.F.; DiFonzo, C.D.; Michel, A.P.; Tilmon, K.J.; Prochaska, T.J.; et al. Biology and economics of recommendations for insecticide-based management of soybean aphid. Plant Health Prog. 2016, 17, 265–269. [Google Scholar] [CrossRef]
- McCord, E.; Yu, S.J. The mechanisms of carbaryl resistance in the fall armyworm, Spodoptera frugiperda (J. E. Smith). Pestic. Biochem. Physiol. 1987, 27, 114–122. [Google Scholar] [CrossRef]
- Ku, T.-Y.; Bishop, J.L. Penetration, Excretion, and Metabolism of Carbaryl in Susceptible and Resistant German Cockroaches. J. Econ. Entomol. 1967, 60, 1328–1332. [Google Scholar] [CrossRef]
- Devonshire, A.L.; Moores, G.D. A carboxylesterase with broad substrate specificity causes organophosphorus, carbamate and pyrethroid resistance in peach-potato aphids (Myzus persicae). Pestic. Biochem. Physiol. 1982, 18, 235–246. [Google Scholar] [CrossRef]
- Srigiriraju, L.; Semtner, P.J.; Anderson, T.D.; Bloomquist, J.R. Monitoring for MACE resistance in the tobacco-adapted form of the green peach aphid, Myzus persicae (Sulzer) (Hemiptera: Aphididae) in the eastern United States. Crop Prot. 2010, 29, 197–202. [Google Scholar] [CrossRef]
- Zepeda-Paulo, F.A.; Simon, J.-C.; Ramírez, C.C.; Fuentes-Contreras, E.; Margaritopoulos, J.T.; Wilson, A.C.C.; Sorenson, C.E.; Briones, L.M.; Azevedo, R.; Ohashi, D.V.; et al. The invasion route for an insect pest species: The tobacco aphid in the New World. Mol. Ecol. 2010, 19, 4738–4752. [Google Scholar] [CrossRef] [PubMed]
- Devonshire, A.L.; Field, L.M.; Foster, S.P.; Moores, G.D.; Williamson, M.S.; Blackman, R.L. The evolution of insecticide resistance in the peach-potato aphid, Myzus persicae. Philos. Trans. R. Soc. B Biol. Sci. 1998, 353, 1677–1684. [Google Scholar] [CrossRef]
- Blackman, R.L.; Spence, J.M.; Field, L.M.; Devonshire, A.L. Chromosomal location of the amplified esterase genes conferring resistance to insecticides in Myzus persicae (Homoptera: Aphididae). Heredity 1995, 75, 297–302. [Google Scholar] [CrossRef]
- Field, L.M.; Devonshire, A.L. Structure and organization of amplicons containing the E4 esterase genes responsible for insecticide resistance in the aphid Myzus persicae (Sulzer). Biochem. J. 1997, 322 Pt 3, 867–871. [Google Scholar] [CrossRef]
- Blackman, R.L.; Spence, J.M.; Normark, B.B. High diversity of structurally heterozygous karyotypes and rDNA arrays in parthenogenetic aphids of the genus Trama (Aphididae: Lachninae). Heredity 2000, 84 Pt 2, 254–260. [Google Scholar] [CrossRef]
- Ffrench-Constant, R.H.; Clark, S.J.; Devonshire, A.L. Effect of decline of insecticide residues on selection for insecticide resistance in Myzus persicae (Sulzer) (Hemiptera: Aphididae). Bull. Entomol. Res. 1988, 78, 19–29. [Google Scholar] [CrossRef]
- Field, L.M.; Blackman, R.L.; Tyler-Smith, C.; Devonshire, A.L. Relationship between amount of esterase and gene copy number in insecticide-resistant Myzus persicae (Sulzer). Biochem. J. 1999, 339 Pt 3, 737–742. [Google Scholar] [CrossRef]
- Field, L.M. Methylation and expression of amplified esterase genes in the aphid Myzus persicae (Sulzer). Biochem. J. 2000, 349 Pt 3, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Blackman, R.L.; Spence, J.M.; Field, L.M.; Devonshire, A.L. Variation in the chromosomal distribution of amplified esterase (FE4) genes in Greek field populations of Myzus persicae (Sulzer). Heredity 1999, 82, 180–186. [Google Scholar] [CrossRef]
- Moores, G.D.; Devine, G.J.; Devonshire, A.L. Insecticide-Insensitive acetylcholinesterase can enhance esterase-based resistance in Myzus persicae and Myzus nicotianae. Pestic. Biochem. Physiol. 1994, 49, 114–120. [Google Scholar] [CrossRef]
- Benting, J.; Nauen, R. Biochemical evidence that an S431F mutation in acetylcholinesterase-1 of Aphis gossypii mediates resistance to pirimicarb and omethoate. Pest Manag. Sci. 2004, 60, 1051–1055. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.-L.; Ma, K.-S.; Hou, Y.-M.; Gao, X.-W. Monitoring insecticide resistance and diagnostics of resistance mechanisms in the green peach aphid, Myzus persicae (Sulzer) (Hemiptera: Aphididae) in China. Pestic. Biochem. Physiol. 2017, 143, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Mutero, A.; Pralavorio, M.; Bride, J.M.; Fournier, D. Resistance-associated point mutations in insecticide-insensitive acetylcholinesterase. Proc. Natl. Acad. Sci. USA 1994, 91, 5922–5926. [Google Scholar] [CrossRef]
- Lenormand, T.; Guillemaud, T.; Bourguet, D.; Raymond, M. Appearance and Sweep of a Gene Duplication: Adaptive Response and Potential for New Functions in the Mosquito Culex pipiens. Evolution 1998, 52, 1705–1712. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, P.; Nauen, R.; Schindler, M.; Elbert, A. Overview of the status and global strategy for neonicotinoids. J. Agric. Food Chem. 2011, 59, 2897–2908. [Google Scholar] [CrossRef]
- Ford, K.A.; Casida, J.E. Unique and common metabolites of thiamethoxam, clothianidin, and dinotefuran in mice. Chem. Res. Toxicol. 2006, 19, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Bass, C.; Field, L.M. Gene amplification and insecticide resistance. Pest Manag. Sci. 2011, 67, 886–890. [Google Scholar] [CrossRef] [PubMed]
- Foster, S.P.; Cox, D.; Oliphant, L.; Mitchinson, S.; Denholm, I. Correlated responses to neonicotinoid insecticides in clones of the peach-potato aphid, Myzus persicae (Hemiptera: Aphididae). Pest Manag. Sci. 2008, 64, 1111–1114. [Google Scholar] [CrossRef] [PubMed]
- Wei, N.; Zhong, Y.; Lin, L.; Xie, M.; Zhang, G.; Su, W.; Li, C.; Chen, H. Transcriptome analysis and identification of insecticide tolerance-related genes after exposure to insecticide in Sitobion avenae. Genes 2019, 10, 951. [Google Scholar] [CrossRef]
- Hrdlickova, R.; Toloue, M.; Tian, B. RNA-Seq methods for transcriptome analysis. Wiley Interdiscip. Rev. RNA 2017, 8, e1364. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Duan, X.; Lv, Y.; Zhang, X.; Nie, Z.; Xie, C.; Ni, Z.; Liang, R. Silencing of an aphid carboxylesterase gene by use of plant-mediated RNAi impairs Sitobion avenae tolerance of Phoxim insecticides. Transgenic Res. 2014, 23, 389–396. [Google Scholar] [CrossRef]
- Xiao, L.; Zhong, Y.P.; Wang, B.; Wu, T.L. Mapping an aphid resistance gene in soybean [Glycine max (L.) Merr.] P746. Genet. Mol. Res. 2014, 13, 9152–9160. [Google Scholar] [CrossRef]
- Enders, L.S.; Miller, N.J. Aphid Molecular Stress Biology. In Biology and Ecology of Aphids; Vilcinskas, A., Ed.; CRC Press: Boca Raton, FL, USA, 2016; pp. 135–151. ISBN 9780429161094. [Google Scholar]
- Pan, Y.; Peng, T.; Gao, X.; Zhang, L.; Yang, C.; Xi, J.; Xin, X.; Bi, R.; Shang, Q. Transcriptomic comparison of thiamethoxam-resistance adaptation in resistant and susceptible strains of Aphis gossypii Glover. Comp. Biochem. Physiol. Part D Genom. Proteom. 2015, 13, 10–15. [Google Scholar] [CrossRef]
- Cabrera-Brandt, M.; Silva, A.X.; Le Trionnaire, G.; Tagu, D.; Figueroa, C.C. Transcriptomic responses of the aphid Myzus persicae nicotianae Blackman (Hemiptera: Aphididae) to insecticides: Analyses in the single Chilean clone of the tobacco aphid. Chil. J. Agric. Res. 2014, 74, 191–199. [Google Scholar] [CrossRef]
- Silva, A.X.; Jander, G.; Samaniego, H.; Ramsey, J.S.; Figueroa, C.C. Insecticide resistance mechanisms in the green peach aphid Myzus persicae (Hemiptera: Aphididae) I: A transcriptomic survey. PLoS ONE 2012, 7, e36366. [Google Scholar] [CrossRef] [PubMed]
- Feyereisen, R. Insect P450 enzymes. Annu. Rev. Entomol. 1999, 44, 507–533. [Google Scholar] [CrossRef] [PubMed]
- Daimon, T.; Kozaki, T.; Niwa, R.; Kobayashi, I.; Furuta, K.; Namiki, T.; Uchino, K.; Banno, Y.; Katsuma, S.; Tamura, T.; et al. Precocious metamorphosis in the juvenile hormone-deficient mutant of the silkworm, Bombyx mori. PLoS Genet. 2012, 8, e1002486. [Google Scholar] [CrossRef]
- Niwa, R.; Matsuda, T.; Yoshiyama, T.; Namiki, T.; Mita, K.; Fujimoto, Y.; Kataoka, H. CYP306A1, a cytochrome P450 enzyme, is essential for ecdysteroid biosynthesis in the prothoracic glands of Bombyx and Drosophila. J. Biol. Chem. 2004, 279, 35942–35949. [Google Scholar] [CrossRef]
- Daborn, P.J.; Lumb, C.; Boey, A.; Wong, W.; Ffrench-Constant, R.H.; Batterham, P. Evaluating the insecticide resistance potential of eight Drosophila melanogaster cytochrome P450 genes by transgenic over-expression. Insect Biochem. Mol. Biol. 2007, 37, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Guo, X.; Zhang, Y.; Dong, Y.; Xing, D.; Yan, T.; Wang, G.; Zhang, H.; Zhao, T. Identification of genes involved in pyrethroid-, propoxur-, and dichlorvos- insecticides resistance in the mosquitoes, Culex pipiens complex (Diptera: Culicidae). Acta Trop. 2016, 157, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xu, H.; Pan, Y.; Gao, X.; Xi, J.; Zhang, J.; Shang, Q. Expression profile changes of cytochrome P450 genes between thiamethoxam susceptible and resistant strains of Aphis gossypii Glover. Pestic. Biochem. Physiol. 2018, 149, 1–7. [Google Scholar] [CrossRef]
- Ma, K.; Tang, Q.; Zhang, B.; Liang, P.; Wang, B.; Gao, X. Overexpression of multiple cytochrome P450 genes associated with sulfoxaflor resistance in Aphis gossypii Glover. Pestic. Biochem. Physiol. 2019, 157, 204–210. [Google Scholar] [CrossRef]
- Dermauw, W.; Van Leeuwen, T.; Feyereisen, R. Diversity and evolution of the P450 family in arthropods. Insect Biochem. Mol. Biol. 2020, 127, 103490. [Google Scholar] [CrossRef] [PubMed]
- Feyereisen, R. Insect CYP Genes and P450 Enzymes. In Insect Molecular Biology and Biochemistry; Gilbert, L.I., Ed.; Academic Press: San Diego, CA, USA, 2012; pp. 236–316. ISBN 978-0-12-384747-8. [Google Scholar]
- Sezutsu, H.; Le Goff, G.; Feyereisen, R. Origins of P450 diversity. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2013, 368, 20120428. [Google Scholar] [CrossRef]
- Feyereisen, R. Arthropod CYPomes illustrate the tempo and mode in P450 evolution. Biochim. Biophys. Acta 2011, 1814, 19–28. [Google Scholar] [CrossRef]
- Bass, C.; Zimmer, C.T.; Riveron, J.M.; Wilding, C.S.; Wondji, C.S.; Kaussmann, M.; Field, L.M.; Williamson, M.S.; Nauen, R. Gene amplification and microsatellite polymorphism underlie a recent insect host shift. Proc. Natl. Acad. Sci. USA 2013, 110, 19460–19465. [Google Scholar] [CrossRef]
- Singh, K.S.; Troczka, B.J.; Duarte, A.; Balabanidou, V.; Trissi, N.; Carabajal Paladino, L.Z.; Nguyen, P.; Zimmer, C.T.; Papapostolou, K.M.; Randall, E.; et al. The genetic architecture of a host shift: An adaptive walk protected an aphid and its endosymbiont from plant chemical defenses. Sci. Adv. 2020, 6, eaba1070. [Google Scholar] [CrossRef]
- Chen, C.; Shan, T.; Liu, Y.; Wang, C.; Shi, X.; Gao, X. Identification and functional analysis of a cytochrome P450 gene involved in imidacloprid resistance in Bradysia odoriphaga Yang et Zhang. Pestic. Biochem. Physiol. 2019, 153, 129–135. [Google Scholar] [CrossRef]
- Karunker, I.; Morou, E.; Nikou, D.; Nauen, R.; Sertchook, R.; Stevenson, B.J.; Paine, M.J.I.; Morin, S.; Vontas, J. Structural model and functional characterization of the Bemisia tabaci CYP6CM1vQ, a cytochrome P450 associated with high levels of imidacloprid resistance. Insect Biochem. Mol. Biol. 2009, 39, 697–706. [Google Scholar] [CrossRef]
- Dauterman, W.C. Insect metabolism: Extramicrosomal. Compr. Insect Physiol. Biochem. Pharmacol. 1985, 12, 713–730. [Google Scholar]
- Yu, S.J. Insect gutathione S-transferases. Zool. Stud. 1996, 35, 9–19. [Google Scholar]
- Wang, W.; Hu, C.; Li, X.-R.; Wang, X.-Q.; Yang, X.-Q. CpGSTd3 is a lambda-cyhalothrin metabolizing glutathione s-transferase from Cydia pomonella (L.). J. Agric. Food Chem. 2019, 67, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, X.; Zhang, Y. Characterization of a lambda-cyhalothrin metabolizing glutathione S-transferase CpGSTd1 from Cydia pomonella (L.). Appl. Microbiol. Biotechnol. 2014, 98, 8947–8962. [Google Scholar] [CrossRef]
- Ranson, H.; Hemingway, J. Mosquito Glutathione Transferases. In Gluthione Transferases and Gamma-Glutamyl Transpeptidases; Sies, H., Packer, L., Eds.; Methods in Enzymology; Academic Press: Cambridge, MA, USA, 2005; Volume 401, pp. 226–241. [Google Scholar]
- Li, X.; Zhang, X.; Zhang, J.; Zhang, X.; Starkey, S.R.; Zhu, K.Y. Identification and characterization of eleven glutathione S-transferase genes from the aquatic midge Chironomus tentans (Diptera: Chironomidae). Insect Biochem. Mol. Biol. 2009, 39, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Kostaropoulos, I.; Papadopoulos, A.I.; Metaxakis, A.; Boukouvala, E.; Papadopoulou-Mourkidou, E. The role of glutathione S-transferases in the detoxification of some organophosphorus insecticides in larvae and pupae of the yellow mealworm, Tenebrio molitor (Coleoptera: Tenebrionidae). Pest Manag. Sci. 2001, 57, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Wang, W.; Ju, D.; Chen, G.-M.; Tan, X.-L.; Mota-Sanchez, D.; Yang, X.-Q. Functional characterization of a novel λ-cyhalothrin metabolizing glutathione S-transferase, CpGSTe3, from the codling moth Cydia pomonella. Pest Manag. Sci. 2020, 76, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, B.; Su, S.; Zhang, C.; Chen, M. Identification and Functional characterization of two sigma glutathione s-transferase genes from bird cherry-oat aphid (Hemiptera: Aphididae). J. Econ. Entomol. 2019, 112, 416–424. [Google Scholar] [CrossRef]
- Li, P.-R.; Shi, Y.; Ju, D.; Liu, Y.-X.; Wang, W.; He, Y.-S.; Zhang, Y.-Y.; Yang, X.-Q. Metabolic functional redundancy of the CYP9A subfamily members leads to P450-mediated lambda-cyhalothrin resistance in Cydia pomonella. Pest Manag. Sci. 2022, 79, 1452–1466. [Google Scholar] [CrossRef] [PubMed]
- Mannervik, B.; Danielson, U.H. Glutathione transferases—Structure and catalytic activity. CRC Crit. Rev. Biochem. 1988, 23, 283–337. [Google Scholar] [CrossRef]
- Samuel, P. Transcriptional Profiling of Aphid Resistant and Susceptible Melon (Cucumis melo) following Cotton-Melon Aphid (Aphis gossypii) Feeding. Ph.D. Thesis, University of Arkansas, Little Rock, AR, USA, 2008. [Google Scholar]
- Ranson, H.; Claudianos, C.; Ortelli, F.; Abgrall, C.; Hemingway, J.; Sharakhova, M.V.; Unger, M.F.; Collins, F.H.; Feyereisen, R. Evolution of supergene families associated with insecticide resistance. Science 2002, 298, 179–181. [Google Scholar] [CrossRef]
- Jedlička, P.; Jedličková, V.; Lee, H.-J. Expression of stress-related genes in the parthenogenetic forms of the pea aphid, Acyrthosiphon pisum. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2015, 180, 32–37. [Google Scholar] [CrossRef]
- Gong, Y.-H.; Yu, X.-R.; Shang, Q.-L.; Shi, X.-Y.; Gao, X.-W. Oral delivery mediated RNA interference of a carboxylesterase gene results in reduced resistance to organophosphorus insecticides in the cotton aphid, Aphis gossypii Glover. PLoS ONE 2014, 9, e102823. [Google Scholar] [CrossRef]
- Li, F.; Ma, K.S.; Liang, P.Z.; Chen, X.W.; Liu, Y.; Gao, X.W. Transcriptional responses of detoxification genes to four plant allelochemicals in Aphis gossypii. J. Econ. Entomol. 2017, 110, 624–631. [Google Scholar] [CrossRef]
- Sun, H.; Pu, J.; Chen, F.; Wang, J.; Han, Z. Multiple ATP-binding cassette transporters are involved in insecticide resistance in the small brown planthopper, Laodelphax striatellus. Insect Mol. Biol. 2017, 26, 343–355. [Google Scholar] [CrossRef]
- Mastrantonio, V.; Ferrari, M.; Negri, A.; Sturmo, T.; Favia, G.; Porretta, D.; Epis, S.; Urbanelli, S. Insecticide Exposure Triggers a Modulated Expression of ABC Transporter Genes in Larvae of Anopheles gambiae s.s. Insects 2019, 10, 66. [Google Scholar] [CrossRef]
- Ju, D.; Dewer, Y.; Zhang, S.; Hu, C.; Li, P.; Yang, X. Genome-wide identification, characterization, and expression profiling of ATP-binding cassette (ABC) transporter genes potentially associated with abamectin detoxification in Cydia pomonella. Ecotoxicol. Environ. Saf. 2022, 230, 113152. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lv, Y.; Fang, F.; Hong, S.; Guo, Q.; Hu, S.; Zou, F.; Shi, L.; Lei, Z.; Ma, K.; et al. Identification of proteins associated with pyrethroid resistance by iTRAQ-based quantitative proteomic analysis in Culex pipiens pallens. Parasit. Vectors 2015, 8, 95. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Yang, H.; Wang, Z.; Long, G.; Jin, D. Comparative transcriptome analysis of Sogatella furcifera (Horváth) exposed to different insecticides. Sci. Rep. 2018, 8, 8773. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Huang, Z.; Rebeca, C.-L.; Zhu, X.; Guo, Y.; Lin, Q.; Hu, X.; Wang, R.; Liang, G.; Guan, X.; et al. De novo characterization of the pine aphid Cinara pinitabulaeformis Zhang et Zhang transcriptome and analysis of genes relevant to pesticides. PLoS ONE 2017, 12, e0178496. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Contreras, E.; Figueroa, C.C.; Silva, A.X.; Bacigalupe, L.D.; Briones, L.M.; Foster, S.P.; Unruh, T.R. Survey of resistance to four insecticides and their associated mechanisms in different genotypes of the green peach aphid (Hemiptera: Aphididae) from Chile. J. Econ. Entomol. 2013, 106, 400–407. [Google Scholar] [CrossRef] [PubMed]
| Species Name | Insecticide | Insecticide Group | Level of Resistance | References |
|---|---|---|---|---|
| Aphis gossypii, Myzus persicae, A. fabae and Rhopalosiphum padi | Methmidophos, monocrotophos, and phosphamidon | Organophosphates | 1- to 85-fold | [39,40,41] |
| M. persicae, A. gosypii, R. padi and Nasonovia ribisnigri | Pirmicarb and Propoxur | Carbamates | up to 4500-fold | [41,42,43,44] |
| M. persicae and A. glycines | Lambda-cyhalothrin, bifenthrin and alpha-cypermethrin | Pyrethroids | up to 1000-fold | [21,45,46] |
| M. persicae and A. gosypii | Imidacloprid, nitenpyram, thiacloprid, acetamiprid, Clothianidin, Thiamethoxam, and Dinotefuran | Neonicotinoids | 3- to 394-fold | [46,47,48,49,50] |
| Insecticide | Mutation Involved | Enzyme/Gene Involved | References |
|---|---|---|---|
| Organophosphate | A128V, H104R, T333P, A302S, F139L, F368(290)L, S329(228)P, V435(356)A, Thr210→Met210, Asn294→Lys294, Gly408→Asp408 and Ser441→Phe441 | Carboxylesterases, AChE genes (Ace1 and Ace2) | [41,56,57] |
| Carbamate | S431F, kdr (L1014F) mutation | AChE (Ace1 and Ace2), GST, carboxylesterases, E4, or FE4 | [59,60,61,62,63] |
| Pyrethroids | kdr (L1014 and M918 mutation), I935M, I936V, L925F, M957I, M957L, R980C, T929S and V931A | Na-gated channel, P450s | [64,65,66] |
| Neonicotinoids | R81T, arginine to threonine mutation, V101I and V62I, K264E | P450 gene (CYP6CY3), Carboxylesterase, MACE and nicotinic acetylcholine receptor (nAChR) β1 subunit | [12,67,68,69,70] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaleem Ullah, R.M.; Gao, F.; Sikandar, A.; Wu, H. Insights into the Effects of Insecticides on Aphids (Hemiptera: Aphididae): Resistance Mechanisms and Molecular Basis. Int. J. Mol. Sci. 2023, 24, 6750. https://doi.org/10.3390/ijms24076750
Kaleem Ullah RM, Gao F, Sikandar A, Wu H. Insights into the Effects of Insecticides on Aphids (Hemiptera: Aphididae): Resistance Mechanisms and Molecular Basis. International Journal of Molecular Sciences. 2023; 24(7):6750. https://doi.org/10.3390/ijms24076750
Chicago/Turabian StyleKaleem Ullah, Rana Muhammad, Fukun Gao, Aatika Sikandar, and Haiyan Wu. 2023. "Insights into the Effects of Insecticides on Aphids (Hemiptera: Aphididae): Resistance Mechanisms and Molecular Basis" International Journal of Molecular Sciences 24, no. 7: 6750. https://doi.org/10.3390/ijms24076750
APA StyleKaleem Ullah, R. M., Gao, F., Sikandar, A., & Wu, H. (2023). Insights into the Effects of Insecticides on Aphids (Hemiptera: Aphididae): Resistance Mechanisms and Molecular Basis. International Journal of Molecular Sciences, 24(7), 6750. https://doi.org/10.3390/ijms24076750








