Distinctive Kaposi Sarcoma-Associated Herpesvirus Serological Profile during Acute Plasmodium falciparum Malaria Episodes
Abstract
1. Introduction
2. Results
2.1. Characteristics of Study Participants
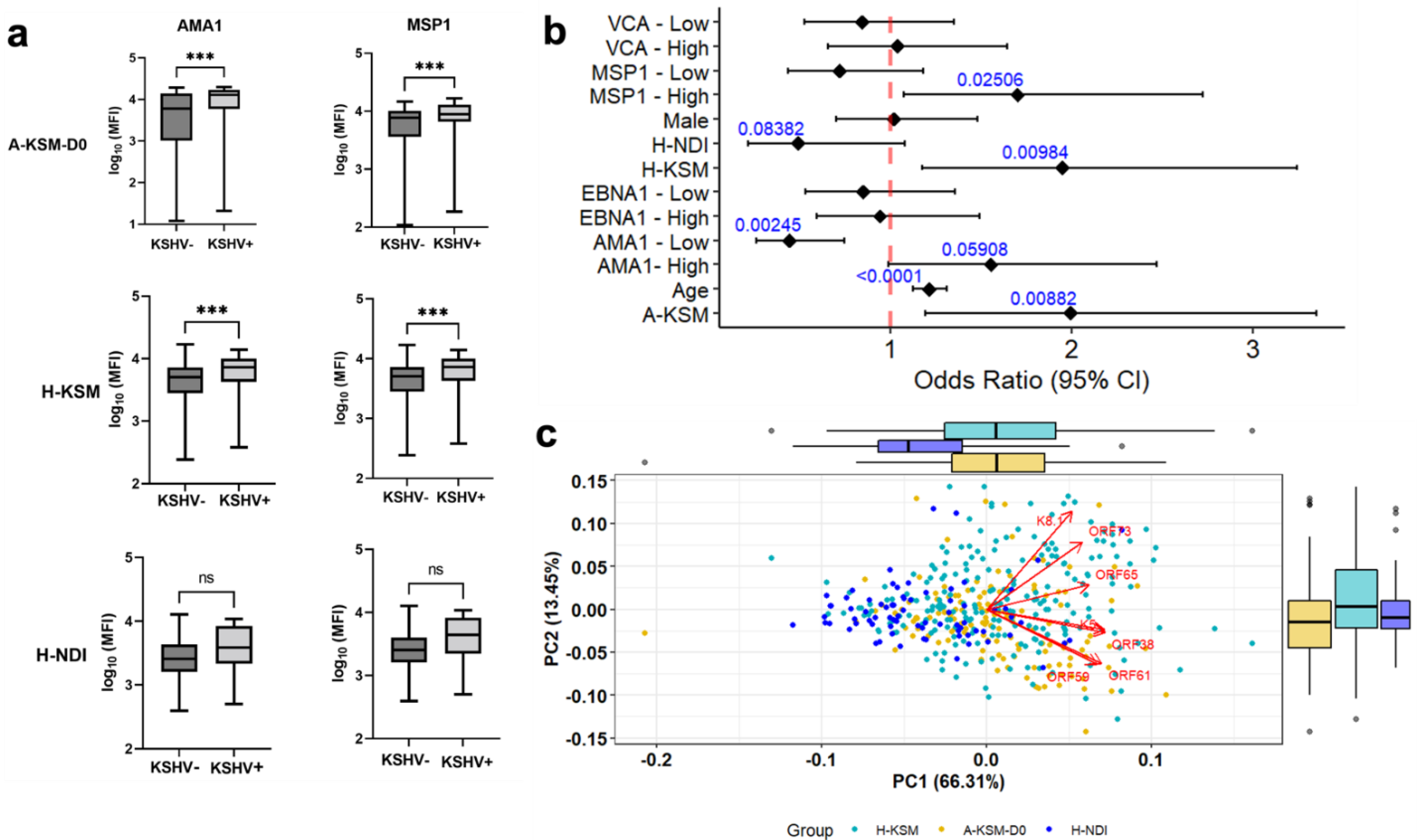
2.2. Acute P. falciparum Malaria Is Characterized by Distinct Serological Profiles in KSHV Seropositive Children
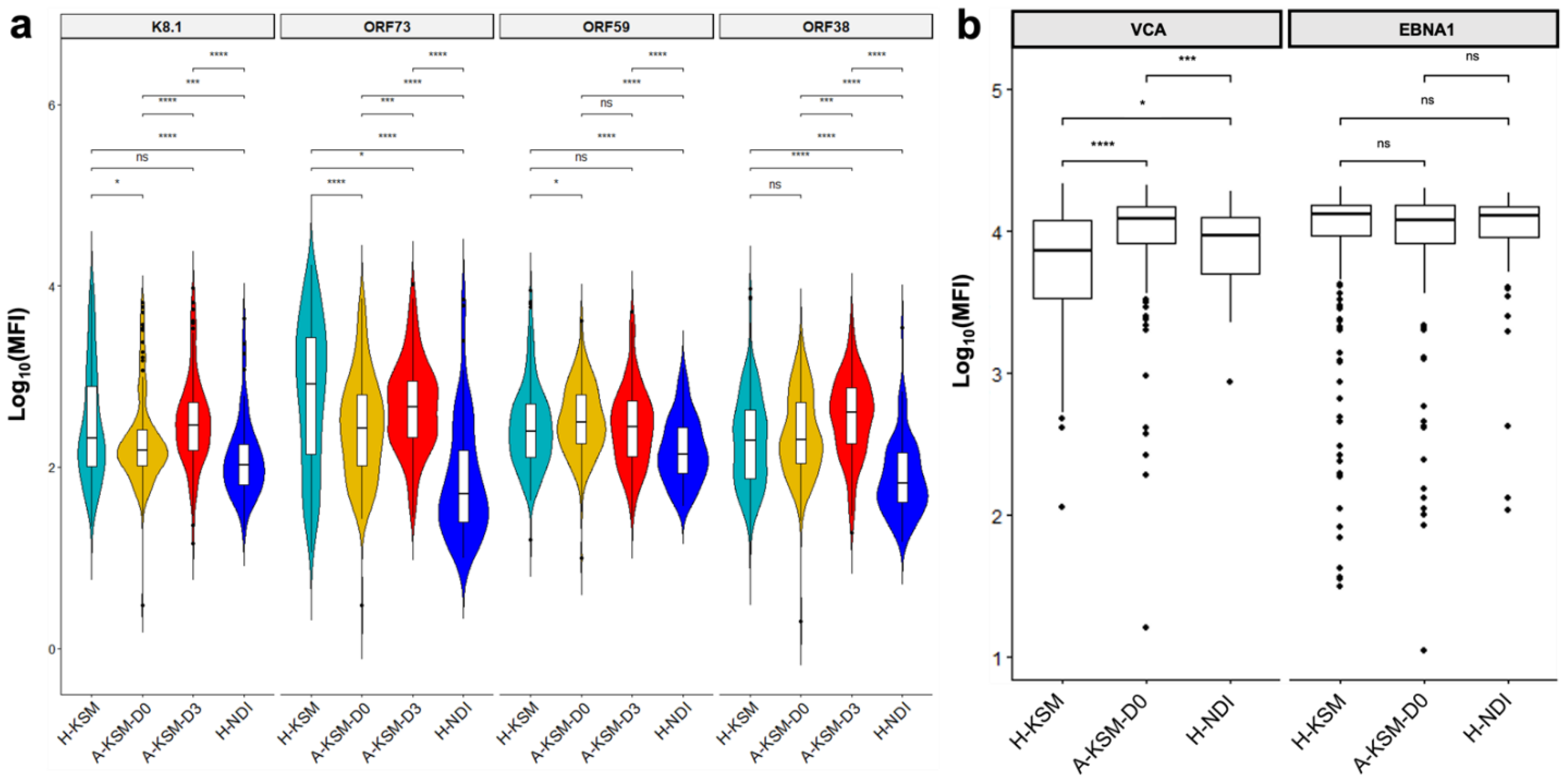
2.3. KSHV Lytic Antigens Are Increased in Patients with Acute Malaria
2.4. Pairwise Comparison of Antibody Responses Reveals Distinct IgG and IgM Antibodies to KSHV Antigens during Acute Malaria Episodes
3. Discussion
4. Materials and Methods
4.1. Study Area, Participants, and Ethical Approval
4.2. Antibody Serology Assay
4.3. Parasite Density and Viral Load Quantification
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goncalves, P.H.; Ziegelbauer, J.; Uldrick, T.S.; Yarchoan, R. Kaposi Sarcoma Herpesvirus-Associated Cancers and Related Diseases. Curr. Opin. HIV AIDS 2017, 12, 47–56. [Google Scholar] [CrossRef]
- Butler, L.M.; Were, W.A.; Balinandi, S.; Downing, R.; Dollard, S.; Neilands, T.B.; Gupta, S.; Rutherford, G.W.; Mermin, J. Human Herpesvirus 8 Infection in Children and Adults in a Population-Based Study in Rural Uganda. J. Infect. Dis. 2011, 203, 625–634. [Google Scholar] [CrossRef]
- Minhas, V.; Wood, C. Epidemiology and Transmission of Kaposi’s Sarcoma-Associated Herpesvirus. Viruses 2014, 6, 4178–4194. [Google Scholar] [CrossRef]
- Nalwoga, A.; Cose, S.; Wakeham, K.; Miley, W.; Ndibazza, J.; Drakeley, C.; Elliott, A.; Whitby, D.; Newton, R. Association between Malaria Exposure and Kaposi’s Sarcoma-Associated Herpes Virus Seropositivity in Uganda. Trop. Med. Int. Health 2015, 20, 665–672. [Google Scholar] [CrossRef]
- Nalwoga, A.; Cose, S.; Nash, S.; Miley, W.; Asiki, G.; Kusemererwa, S.; Yarchoan, R.; Labo, N.; Whitby, D.; Newton, R. Relationship Between Anemia, Malaria Coinfection, and Kaposi Sarcoma-Associated Herpesvirus Seropositivity in a Population-Based Study in Rural Uganda. J. Infect. Dis. 2018, 218, 1061–1065. [Google Scholar] [CrossRef]
- Sabourin, K.R.; Daud, I.; Ogolla, S.; Labo, N.; Miley, W.; Lamb, M.; Newton, R.; Whitby, D.; Rochford, R. Malaria Is Associated With Kaposi Sarcoma-Associated Herpesvirus Seroconversion in a Cohort of Western Kenyan Children. J. Infect. Dis. 2021, 224, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Sabourin, K.R.; Ogolla, S.; Daud, I.I.; Jackson, C.L.; Miley, W.; Labo, N.; Whitby, D.; Rochford, R. Malaria during Pregnancy and Transplacental Transfer of Kaposi Sarcoma-Associated Herpesvirus (KSHV) Antibodies: A Cohort Study of Kenyan Mother and Child Pairs. Infect. Agents Cancer 2020, 15, 71. [Google Scholar] [CrossRef] [PubMed]
- Oluoch, P.O.; Oduor, C.I.; Forconi, C.S.; Ong’echa, J.M.; Münz, C.; Dittmer, D.P.; Bailey, J.A.; Moormann, A.M. Kaposi Sarcoma-Associated Herpesvirus Infection and Endemic Burkitt Lymphoma. J. Infect. Dis. 2020, 222, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Cook-Mozaffari, P.; Newton, R.; Beral, V.; Burkitt, D.P. The Geographical Distribution of Kaposi’s Sarcoma and of Lymphomas in Africa before the AIDS Epidemic. Br. J. Cancer 1998, 78, 1521–1528. [Google Scholar] [CrossRef]
- Whitby, D.; Marshall, V.A.; Bagni, R.K.; Miley, W.J.; McCloud, T.G.; Hines-Boykin, R.; Goedert, J.J.; Conde, B.A.; Nagashima, K.; Mikovits, J.; et al. Reactivation of Kaposi’s Sarcoma-Associated Herpesvirus by Natural Products from Kaposi’s Sarcoma Endemic Regions. Int. J. Cancer 2007, 120, 321–328. [Google Scholar] [CrossRef]
- Ruocco, V.; Ruocco, E.; Schwartz, R.A.; Janniger, C.K. Kaposi Sarcoma and Quinine: A Potentially Overlooked Triggering Factor in Millions of Africans. J. Am. Acad. Dermatol. 2011, 64, 434–436. [Google Scholar] [CrossRef]
- Rochford, R.; Moormann, A.M. Burkitt’s Lymphoma. Curr. Top. Microbiol. Immunol. 2015, 390, 267–285. [Google Scholar]
- Quintana, M.D.P.; Smith-Togobo, C.; Moormann, A.; Hviid, L. Endemic Burkitt Lymphoma—An Aggressive Childhood Cancer Linked to Plasmodium Falciparum Exposure, but Not to Exposure to Other Malaria Parasites. APMIS 2020, 128, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Moormann, A.M.; Bailey, J.A. Malaria—How This Parasitic Infection Aids and Abets EBV-Associated Burkitt Lymphomagenesis. Curr. Opin. Virol. 2016, 20, 78–84. [Google Scholar] [CrossRef]
- Chêne, A.; Donati, D.; Guerreiro-Cacais, A.O.; Levitsky, V.; Chen, Q.; Falk, K.I.; Orem, J.; Kironde, F.; Wahlgren, M.; Bejarano, M.T. A Molecular Link between Malaria and Epstein–Barr Virus Reactivation. PLoS Pathog. 2007, 3, e80. [Google Scholar] [CrossRef]
- Conant, K.L.; Marinelli, A.; Kaleeba, J.A.R. Dangerous Liaisons: Molecular Basis for a Syndemic Relationship between Kaposi’s Sarcoma and P. Falciparum Malaria. Front. Microbiol. 2013, 4, 35. [Google Scholar] [PubMed]
- Wilmore, J.R.; Asito, A.S.; Wei, C.; Piriou, E.; Sumba, P.O.; Sanz, I.; Rochford, R. AID Expression in Peripheral Blood of Children Living in a Malaria Holoendemic Region Is Associated with Changes in B Cell Subsets and Epstein-Barr Virus. Int. J. Cancer 2015, 136, 1371–1380. [Google Scholar] [CrossRef]
- Robbiani, D.F.; Deroubaix, S.; Feldhahn, N.; Oliveira, T.Y.; Callen, E.; Wang, Q.; Jankovic, M.; Silva, I.T.; Rommel, P.C.; Bosque, D.; et al. Plasmodium Infection Promotes Genomic Instability and AID-Dependent B Cell Lymphoma. Cell 2015, 162, 727–737. [Google Scholar] [CrossRef]
- Thorley-Lawson, D.; Deitsch, K.W.; Duca, K.A.; Torgbor, C. The Link between Plasmodium Falciparum Malaria and Endemic Burkitt’s Lymphoma-New Insight into a 50-Year-Old Enigma. PLoS Pathog. 2016, 12, e1005331. [Google Scholar] [CrossRef]
- Nalwoga, A.; Webb, E.L.; Muserere, C.; Chihota, B.; Miley, W.; Labo, N.; Elliott, A.; Cose, S.; Whitby, D.; Newton, R. Variation in KSHV Prevalence between Geographically Proximate Locations in Uganda. Infect. Agents Cancer 2020, 15, 49. [Google Scholar] [CrossRef] [PubMed]
- Ogolla, S.; Daud, I.I.; Asito, A.S.; Sumba, O.P.; Ouma, C.; Vulule, J.; Middeldorp, J.M.; Dent, A.E.; Mehta, S.; Rochford, R. Reduced Transplacental Transfer of a Subset of Epstein-Barr Virus-Specific Antibodies to Neonates of Mothers Infected with Plasmodium Falciparum Malaria during Pregnancy. Clin. Vaccine Immunol. 2015, 22, 1197–1205. [Google Scholar] [CrossRef]
- Njie, R.; Bell, A.I.; Jia, H.; Croom-Carter, D.; Chaganti, S.; Hislop, A.D.; Whittle, H.; Rickinson, A.B. The Effects of Acute Malaria on Epstein-Barr Virus (EBV) Load and EBV-Specific T Cell Immunity in Gambian Children. J. Infect. Dis. 2009, 199, 31–38. [Google Scholar] [CrossRef]
- Whittle, H.C.; Brown, J.; Marsh, K.; Greenwood, B.M.; Seidelin, P.; Tighe, H.; Wedderburn, L. T-Cell Control of Epstein-Barr Virus-Infected B Cells Is Lost during P. falciparum Malaria. Nature 1984, 312, 449–450. [Google Scholar] [CrossRef]
- Chêne, A.; Nylén, S.; Donati, D.; Bejarano, M.T.; Kironde, F.; Wahlgren, M.; Falk, K.I. Effect of Acute Plasmodium Falciparum Malaria on Reactivation and Shedding of the Eight Human Herpes Viruses. PLoS ONE 2011, 6, e26266. [Google Scholar] [CrossRef]
- Strahan, R.C.; McDowell-Sargent, M.; Uppal, T.; Purushothaman, P.; Verma, S.C. KSHV Encoded ORF59 Modulates Histone Arginine Methylation of the Viral Genome to Promote Viral Reactivation. PLoS Pathog. 2017, 13, e1006482. [Google Scholar] [CrossRef]
- Wu, J.-J.; Avey, D.; Li, W.; Gillen, J.; Fu, B.; Miley, W.; Whitby, D.; Zhu, F. ORF33 and ORF38 of Kaposi’s Sarcoma-Associated Herpesvirus Interact and Are Required for Optimal Production of Infectious Progeny Viruses. J. Virol. 2016, 90, 1741–1756. [Google Scholar] [CrossRef] [PubMed]
- Nalwoga, A.; Roshan, R.; Moore, K.; Marshall, V.; Miley, W.; Labo, N.; Nakibuule, M.; Cose, S.; Rochford, R.; Newton, R.; et al. Kaposi’s Sarcoma-Associated Herpesvirus T Cell Responses in HIV Seronegative Individuals from Rural Uganda. Nat. Commun. 2021, 12, 7323. [Google Scholar] [CrossRef] [PubMed]
- Portugal, S.; Tipton, C.M.; Sohn, H.; Kone, Y.; Wang, J.; Li, S.; Skinner, J.; Virtaneva, K.; Sturdevant, D.E.; Porcella, S.F.; et al. Malaria-Associated Atypical Memory B Cells Exhibit Markedly Reduced B Cell Receptor Signaling and Effector Function. Elife 2015, 4, e07218. [Google Scholar] [CrossRef] [PubMed]
- Hopp, C.S.; Sekar, P.; Diouf, A.; Miura, K.; Boswell, K.; Skinner, J.; Tipton, C.M.; Peterson, M.E.; Chambers, M.J.; Andrews, S.; et al. Plasmodium Falciparum-Specific IgM B Cells Dominate in Children, Expand with Malaria, and Produce Functional IgM. J. Exp. Med. 2021, 218, e20200901. [Google Scholar] [CrossRef] [PubMed]
- Ikoma, M.; Gantt, S.; Casper, C.; Ogata, Y.; Zhang, Q.; Basom, R.; Dyen, M.R.; Rose, T.M.; Barcy, S. KSHV Oral Shedding and Plasma Viremia Result in Significant Changes in the Extracellular Tumorigenic miRNA Expression Profile in Individuals Infected with the Malaria Parasite. PLoS ONE 2018, 13, e0192659. [Google Scholar] [CrossRef]
- Nalwoga, A.; Nakibuule, M.; Marshall, V.; Miley, W.; Labo, N.; Cose, S.; Whitby, D.; Newton, R. Risk Factors for Kaposi’s Sarcoma-Associated Herpesvirus DNA in Blood and in Saliva in Rural Uganda. Clin. Infect. Dis. 2020, 71, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- McHugh, D.; Caduff, N.; Barros, M.H.M.; Rämer, P.C.; Raykova, A.; Murer, A.; Landtwing, V.; Quast, I.; Styles, C.T.; Spohn, M.; et al. Persistent KSHV Infection Increases EBV-Associated Tumor Formation In Vivo via Enhanced EBV Lytic Gene Expression. Cell Host Microbe 2017, 22, 61–73.e7. [Google Scholar] [CrossRef] [PubMed]
- Caduff, N.; McHugh, D.; Rieble, L.; Forconi, C.S.; Ong’echa, J.M.; Oluoch, P.O.; Raykova, A.; Murer, A.; Böni, M.; Zuppiger, L.; et al. KSHV Infection Drives Poorly Cytotoxic CD56-Negative Natural Killer Cell Differentiation in Vivo upon KSHV/EBV Dual Infection. Cell Rep. 2021, 35, 109056. [Google Scholar] [CrossRef]
- Faure, A.; Hayes, M.; Sugden, B. How Kaposi’s Sarcoma-Associated Herpesvirus Stably Transforms Peripheral B Cells towards Lymphomagenesis. Proc. Natl. Acad. Sci. USA 2019, 116, 16519–16528. [Google Scholar] [CrossRef]
- Bigi, R.; Landis, J.T.; An, H.; Caro-Vegas, C.; Raab-Traub, N.; Dittmer, D.P. Epstein-Barr Virus Enhances Genome Maintenance of Kaposi Sarcoma-Associated Herpesvirus. Proc. Natl. Acad. Sci. USA 2018, 115, E11379–E11387. [Google Scholar] [CrossRef]
- Labo, N.; Marshall, V.; Miley, W.; Davis, E.; McCann, B.; Stolka, K.B.; Ndom, P.; Hemingway-Foday, J.J.; Abassora, M.; Newton, R.; et al. Mutual Detection of Kaposi’s Sarcoma-Associated Herpesvirus and Epstein-Barr Virus in Blood and Saliva of Cameroonians with and without Kaposi’s Sarcoma. Int. J. Cancer 2019, 145, 2468–2477. [Google Scholar] [CrossRef] [PubMed]
- Sallah, N.; Miley, W.; Labo, N.; Carstensen, T.; Fatumo, S.; Gurdasani, D.; Pollard, M.O.; Dilthey, A.T.; Mentzer, A.J.; Marshall, V.; et al. Distinct Genetic Architectures and Environmental Factors Associate with Host Response to the γ2-Herpesvirus Infections. Nat. Commun. 2020, 11, 3849. [Google Scholar] [CrossRef]
- Motlhale, M.; Sitas, F.; Bradshaw, D.; Chen, W.C.; Singini, M.G.; de Villiers, C.B.; Lewis, C.M.; Muchengeti, M.; Waterboer, T.; Mathew, C.G.; et al. Epidemiology of Kaposi’s Sarcoma in Sub-Saharan Africa. Cancer Epidemiol. 2022, 78, 102167. [Google Scholar] [CrossRef]
- Topazian, H.; Moser, K.; Ngasala, B.; Oluoch, P.; Forconi, C.; Mhamilawa, L.; Aydemir, O.; Kharabora, O.; Deutsch-Feldman, M.; Read, A.; et al. Low Complexity of Infection Is Associated with Molecular Persistence of Plasmodium Falciparum in Kenya and Tanzania. Front. Epidemiol. 2022, 2. [Google Scholar] [CrossRef]
- Alegana, V.A.; Macharia, P.M.; Muchiri, S.; Mumo, E.; Oyugi, E.; Kamau, A.; Chacky, F.; Thawer, S.; Molteni, F.; Rutazanna, D.; et al. Plasmodium Falciparum Parasite Prevalence in East Africa: Updating Data for Malaria Stratification. PLoS Glob. Public Health 2021, 1, e0000014. [Google Scholar] [CrossRef]
- Labo, N.; Miley, W.; Marshall, V.; Gillette, W.; Esposito, D.; Bess, M.; Turano, A.; Uldrick, T.; Polizzotto, M.N.; Wyvill, K.M.; et al. Heterogeneity and Breadth of Host Antibody Response to KSHV Infection Demonstrated by Systematic Analysis of the KSHV Proteome. PLoS Pathog. 2014, 10, e1004046. [Google Scholar] [CrossRef] [PubMed]
- Cham, G.K.K.; Turner, L.; Lusingu, J.; Vestergaard, L.; Mmbando, B.P.; Kurtis, J.D.; Jensen, A.T.R.; Salanti, A.; Lavstsen, T.; Theander, T.G. Sequential, Ordered Acquisition of Antibodies to Plasmodium Falciparum Erythrocyte Membrane Protein 1 Domains. J. Immunol. 2009, 183, 3356–3363. [Google Scholar] [CrossRef] [PubMed]
- Parr, J.B.; Belson, C.; Patel, J.C.; Hoffman, I.F.; Kamthunzi, P.; Martinson, F.; Tegha, G.; Thengolose, I.; Drakeley, C.; Meshnick, S.R.; et al. Estimation of Plasmodium Falciparum Transmission Intensity in Lilongwe, Malawi, by Microscopy, Rapid Diagnostic Testing, and Nucleic Acid Detection. Am. J. Trop. Med. Hyg. 2016, 95, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Forconi, C.S.; Oduor, C.I.; Oluoch, P.O.; Ong’echa, J.M.; Münz, C.; Bailey, J.A.; Moormann, A.M. A New Hope for CD56negCD16pos NK Cells as Unconventional Cytotoxic Mediators: An Adaptation to Chronic Diseases. Front. Cell. Infect. Microbiol. 2020, 10, 162. [Google Scholar] [CrossRef]

| Malaria-Infected Children (A-KSM; n = 134) | Healthy Children-KSM (H-KSM; n = 221) | Healthy Children-Nandi (H-NDI; n = 77) | p-Value | |
|---|---|---|---|---|
| Female, n (%) | 63 (47%) | 109 (49%) | 38 (49%) | - |
| Age (years), Median (Range) | 4.8 (1.7–9.9) | 5.6 (1.3–15.8) | 5.3 (0.5–16.6) | - |
| KSHV seropositive (n, %) | 95 (71%) | 171 (77%) | 21 (28%) | <0.001 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oluoch, P.O.; Forconi, C.S.; Oduor, C.I.; Ritacco, D.A.; Akala, H.M.; Bailey, J.A.; Juliano, J.J.; Ong’echa, J.M.; Münz, C.; Moormann, A.M. Distinctive Kaposi Sarcoma-Associated Herpesvirus Serological Profile during Acute Plasmodium falciparum Malaria Episodes. Int. J. Mol. Sci. 2023, 24, 6711. https://doi.org/10.3390/ijms24076711
Oluoch PO, Forconi CS, Oduor CI, Ritacco DA, Akala HM, Bailey JA, Juliano JJ, Ong’echa JM, Münz C, Moormann AM. Distinctive Kaposi Sarcoma-Associated Herpesvirus Serological Profile during Acute Plasmodium falciparum Malaria Episodes. International Journal of Molecular Sciences. 2023; 24(7):6711. https://doi.org/10.3390/ijms24076711
Chicago/Turabian StyleOluoch, Peter O., Catherine S. Forconi, Cliff I. Oduor, Dominic A. Ritacco, Hoseah M. Akala, Jeffrey A. Bailey, Jonathan J. Juliano, John M. Ong’echa, Christian Münz, and Ann M. Moormann. 2023. "Distinctive Kaposi Sarcoma-Associated Herpesvirus Serological Profile during Acute Plasmodium falciparum Malaria Episodes" International Journal of Molecular Sciences 24, no. 7: 6711. https://doi.org/10.3390/ijms24076711
APA StyleOluoch, P. O., Forconi, C. S., Oduor, C. I., Ritacco, D. A., Akala, H. M., Bailey, J. A., Juliano, J. J., Ong’echa, J. M., Münz, C., & Moormann, A. M. (2023). Distinctive Kaposi Sarcoma-Associated Herpesvirus Serological Profile during Acute Plasmodium falciparum Malaria Episodes. International Journal of Molecular Sciences, 24(7), 6711. https://doi.org/10.3390/ijms24076711






