The Anticancer Drug Daunomycin Directly Affects Gene Expression and DNA Structure
Abstract
1. Introduction
2. Results
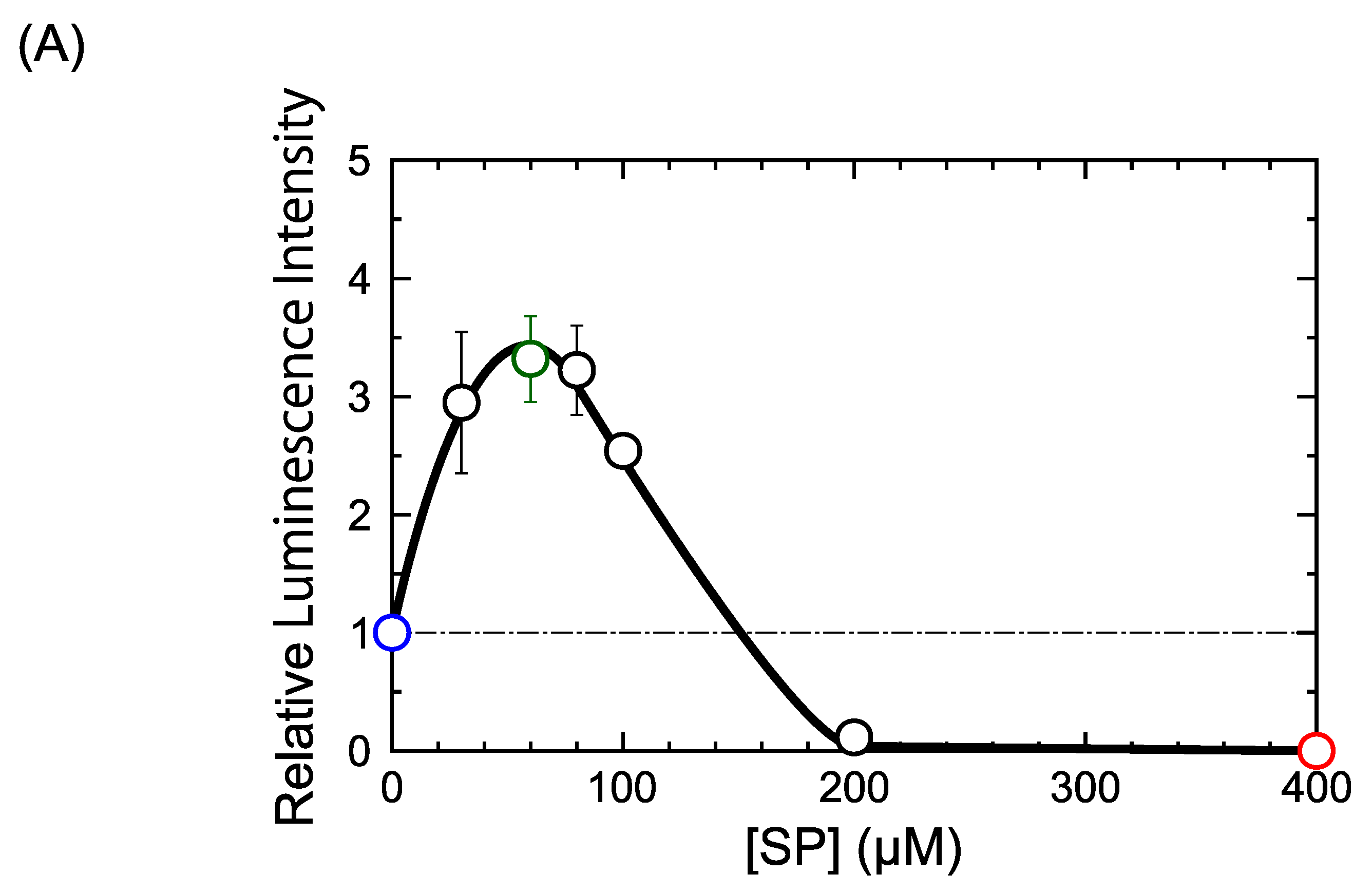
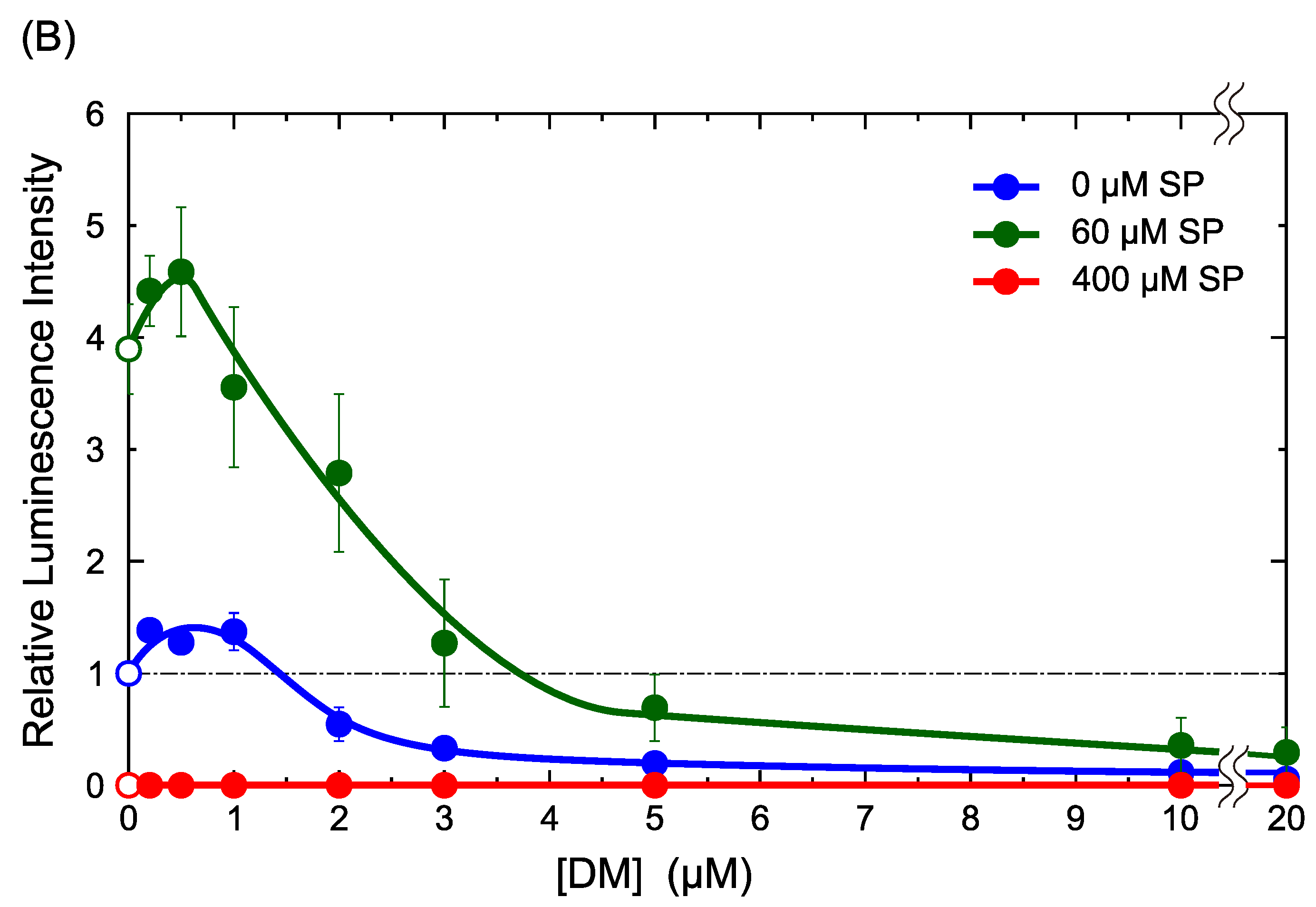
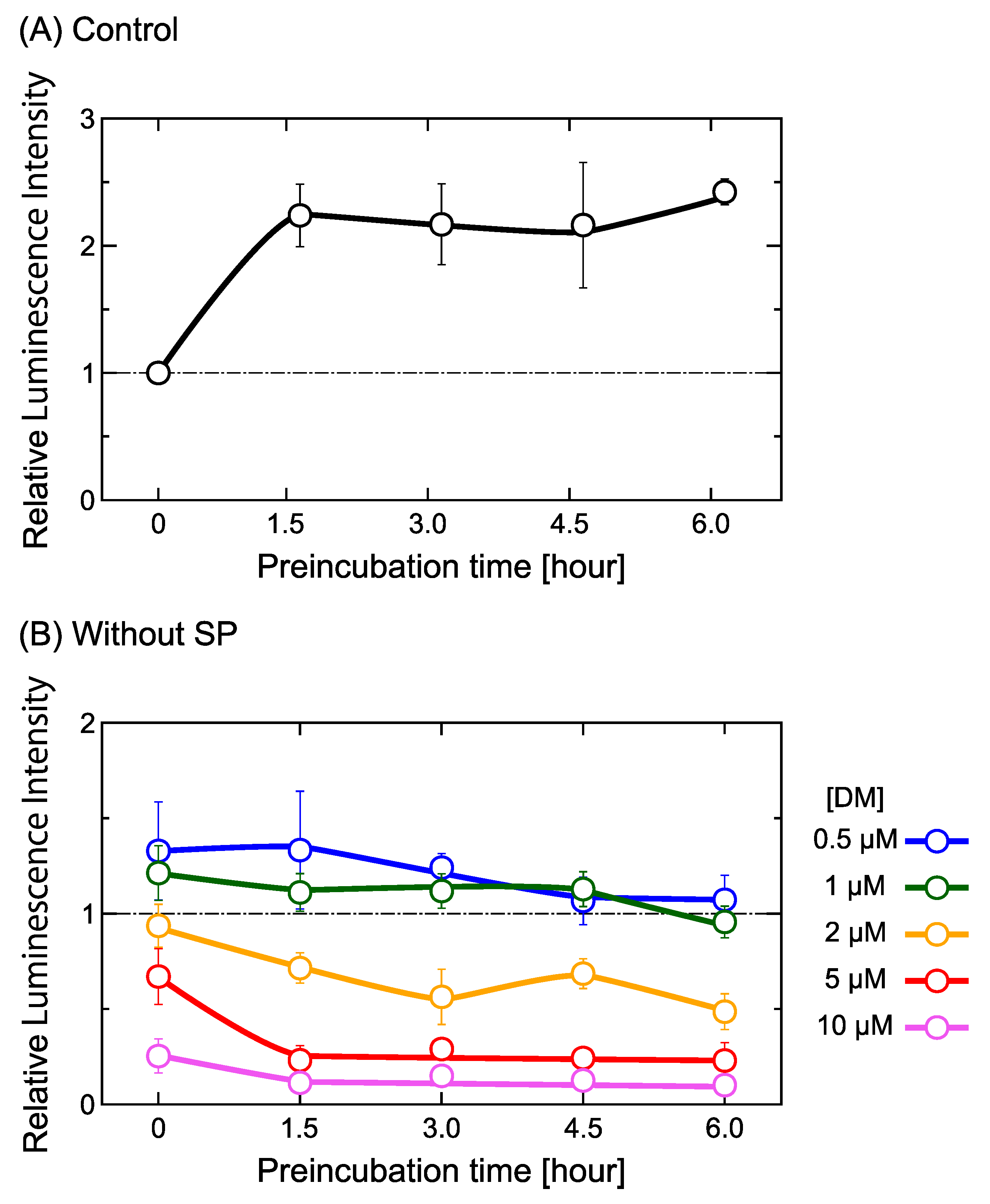
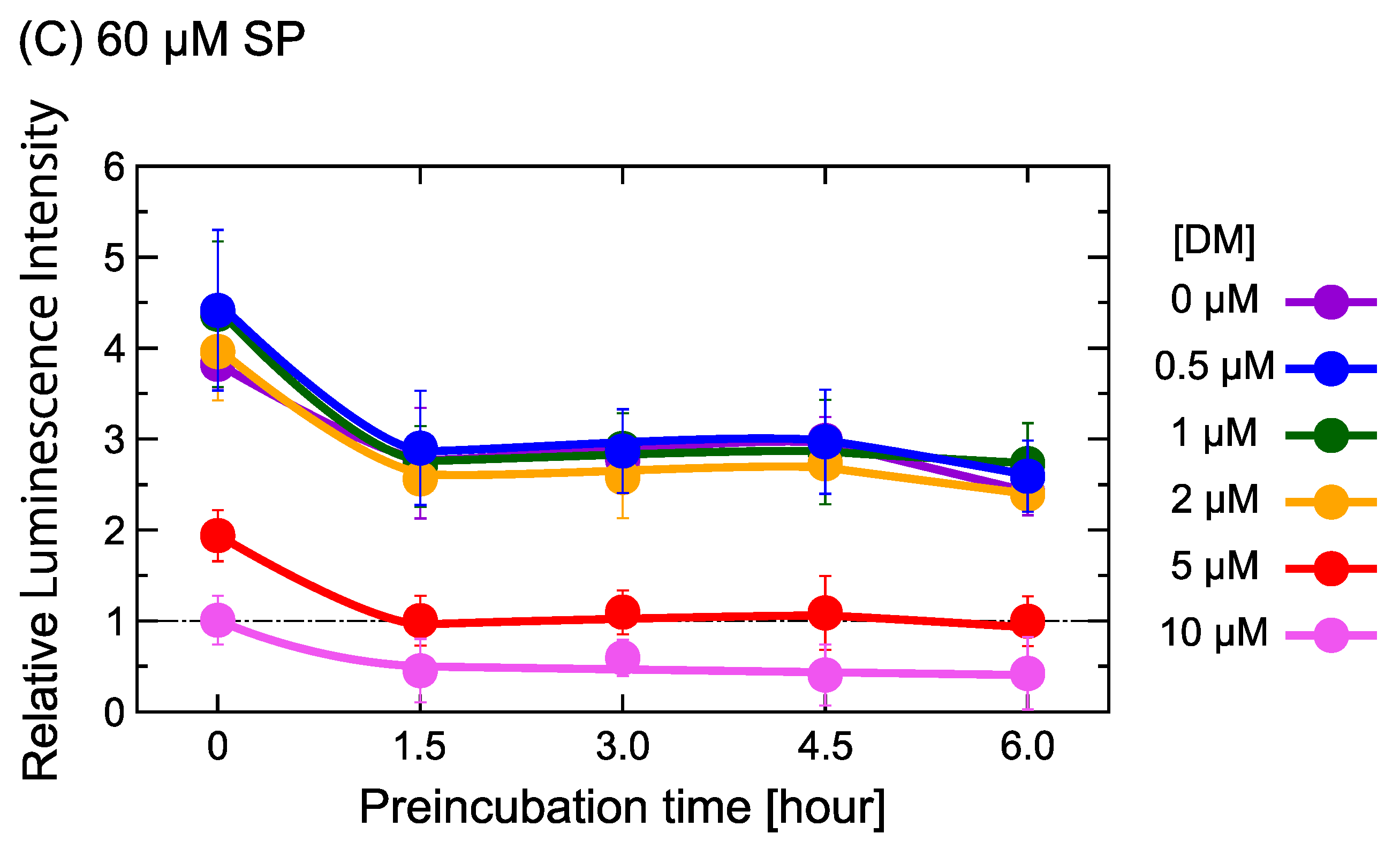
2.1. DM Affects Gene Expression
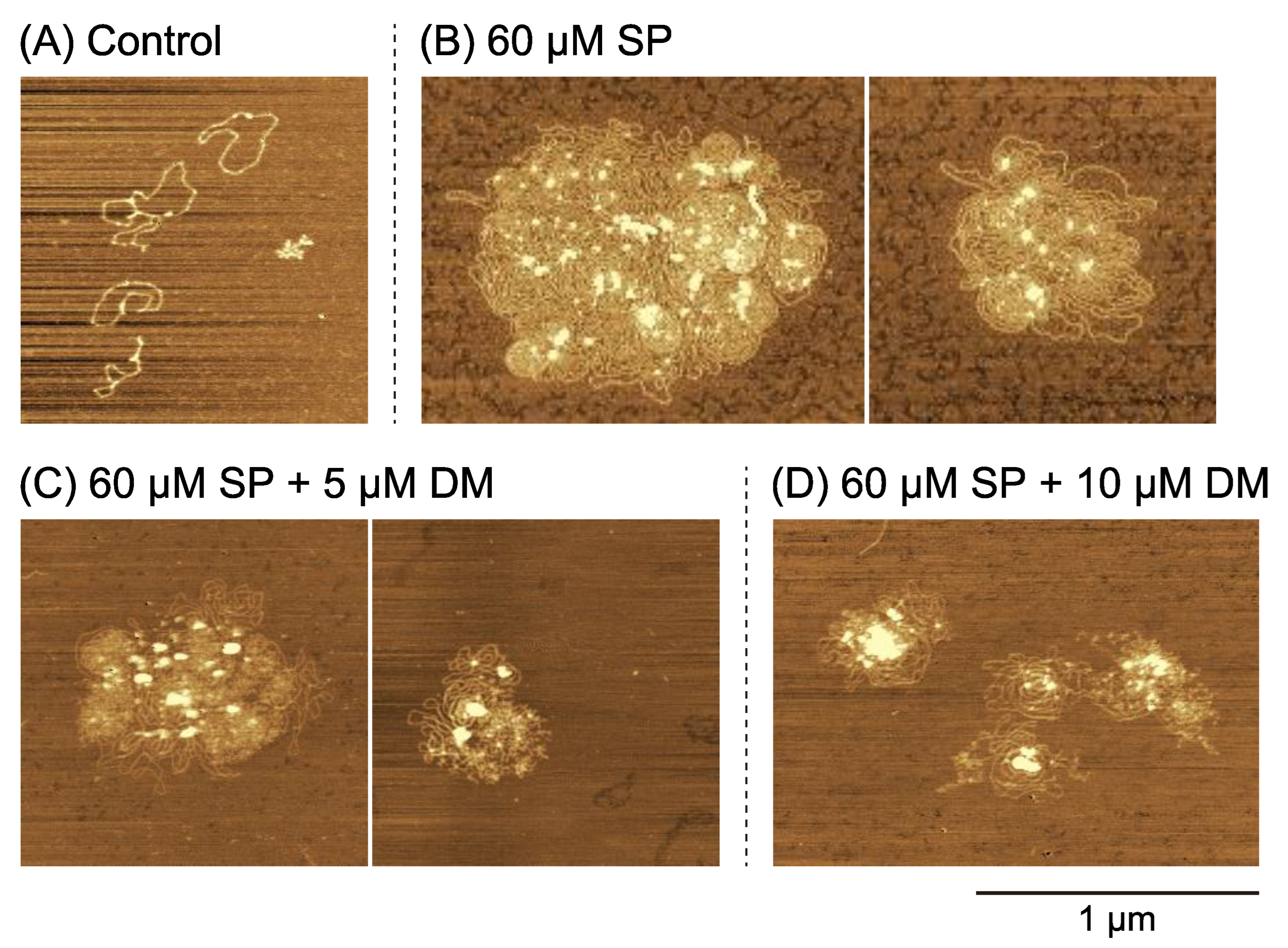
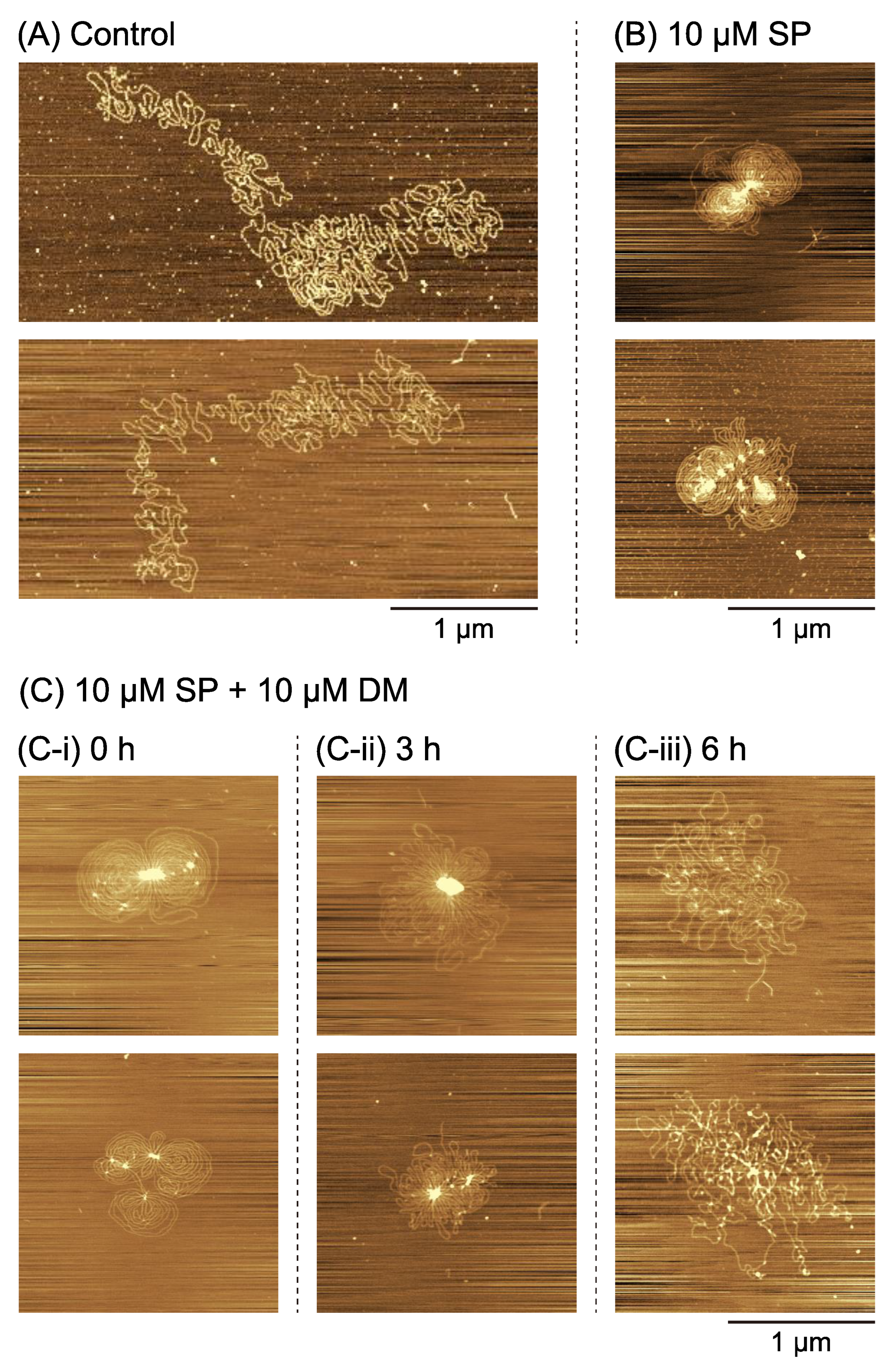
2.2. Higher-Order Conformational Changes of DNA Caused by DM
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Luciferase Assay for Gene Expression
4.2.2. Atomic Force Microscopy (AFM)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carbone, G.M.; McGuffie, E.; Napoli, S.; Flanagan, C.E.; Dembech, C.; Negri, U.; Arcamone, F.; Capobianco, M.L.; Catapano, C.V. DNA binding and antigene activity of a daunomycin-conjugated triplex-forming oligonucleotide targeting the P2 promoter of the human c-myc gene. Nucleic Acids Res. 2004, 32, 2396–2410. [Google Scholar] [CrossRef]
- Orbán, E.; Manea, M.; Marquadt, A.; Bánóczi, Z.; Csík, G.; Fellinger, E.; Bosze, S.; Hudecz, F. A new daunomycin–peptide conjugate: Synthesis, characterization and the effect on the protein expression profile of HL-60 cells in vitro. Bioconjug. Chem. 2011, 22, 2154–2165. [Google Scholar] [CrossRef]
- Pang, B.; Qiao, X.; Janssen, L.; Velds, A.; Groothuis, T.; Kerkhoven, R.; Nieuwland, M.; Ovaa, H.; Rottenberg, S.; Van Tellingen, O. Drug-induced histone eviction from open chromatin contributes to the chemotherapeutic effects of doxorubicin. Nat. Commun. 2013, 4, 1908. [Google Scholar] [CrossRef]
- Wójcik, K.; Zarębski, M.; Cossarizza, A.; Dobrucki, J.W. Daunomycin, an antitumor DNA intercalator, influences histone–DNA interactions. Cancer Biol. Ther. 2013, 14, 823–832. [Google Scholar] [CrossRef]
- Yang, F.; Teves, S.S.; Kemp, C.J.; Henikoff, S. Doxorubicin, DNA torsion, and chromatin dynamics. Biochim. Biophys. Acta 2014, 1845, 84–89. [Google Scholar] [CrossRef]
- Imai, H.; Dansako, H.; Ueda, Y.; Satoh, S.; Kato, N. Daunorubicin, a topoisomerase II poison, suppresses viral production of hepatitis B virus by inducing cGAS-dependent innate immune response. Biochem. Biophys. Res. Commun. 2018, 504, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Marinello, J.; Delcuratolo, M.; Capranico, G. Anthracyclines as topoisomerase II poisons: From early studies to new perspectives. Int. J. Mol. Sci. 2018, 19, 3480. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; van der Zanden, S.Y.; Wander, D.P.; Borràs, D.M.; Song, J.-Y.; Li, X.; van Duikeren, S.; van Gils, N.; Rutten, A.; van Herwaarden, T. Uncoupling DNA damage from chromatin damage to detoxify doxorubicin. Proc. Natl. Acad. Sci. USA 2020, 117, 15182–15192. [Google Scholar] [CrossRef] [PubMed]
- Visone, V.; Szabó, I.; Perugino, G.; Hudecz, F.; Bánóczi, Z.; Valenti, A. Topoisomerases inhibition and DNA binding mode of daunomycin–oligoarginine conjugate. J. Enzym. Inhib. Med. Chem. 2020, 35, 1363–1371. [Google Scholar] [CrossRef]
- van der Zanden, S.Y.; Qiao, X.; Neefjes, J. New insights into the activities and toxicities of the old anticancer drug doxorubicin. FEBS J. 2021, 288, 6095–6111. [Google Scholar] [CrossRef]
- Giráldez-Pérez, R.M.; Grueso, E.; Montero-Hidalgo, A.J.; Luque, R.M.; Carnerero, J.M.; Kuliszewska, E.; Prado-Gotor, R. Gold nanosystems covered with doxorubicin/DNA complexes: A therapeutic target for prostate and liver cancer. Int. J. Mol. Sci. 2022, 23, 15575. [Google Scholar] [CrossRef]
- WHO. World Health Organization Model List of Essential Medicines: 22nd List (2021); World Health Organization: Geneva, Switzerland, 2021.
- Wang, A.H.; Ughetto, G.; Quigley, G.J.; Rich, A. Interactions between an anthracycline antibiotic and DNA: Molecular structure of daunomycin complexed to d (CpGpTpApCpG) at 1.2-Å resolution. Biochemistry 1987, 26, 1152–1163. [Google Scholar] [CrossRef] [PubMed]
- Deweese, J.E.; Osheroff, N. The DNA cleavage reaction of topoisomerase II: Wolf in sheep’s clothing. Nucleic Acids Res. 2009, 37, 738–748. [Google Scholar] [CrossRef]
- Pommier, Y.; Leo, E.; Zhang, H.; Marchand, C. DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem. Biol. 2010, 17, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C. Contemporary challenges in the design of topoisomerase II inhibitors for cancer chemotherapy. Chem. Rev. 2012, 112, 3611–3640. [Google Scholar] [CrossRef]
- Kaczorowska, A.; Lamperska, W.; Frączkowska, K.; Masajada, J.; Drobczyński, S.; Sobas, M.; Wróbel, T.; Chybicka, K.; Tarkowski, R.; Kraszewski, S. Profound nanoscale structural and biomechanical changes in DNA helix upon treatment with anthracycline drugs. Int. J. Mol. Sci. 2020, 21, 4142. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, Y.; Yoshikawa, K.; Kanbe, T. Daunomycin unfolds compactly packed DNA. Biophys. Chem. 1996, 61, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, Y.; Kanbe, T.; Yoshikawa, K. Daunomycin triggers membrane blebbing and breakage of giant DNA encapsulated in a cell-sized liposome. Chem. Phys. Lett. 2002, 366, 305–310. [Google Scholar] [CrossRef]
- Shandilya, M.; Sharma, S.; Das, P.P.; Charak, S. Molecular-level understanding of the anticancer action mechanism of anthracyclines. In Advances in Precision Medicine Oncology; IntechOpen: London, UK, 2020. [Google Scholar]
- Tabor, C.W.; Tabor, H. Polyamines. Annu. Rev. Biochem. 1984, 53, 749–790. [Google Scholar] [CrossRef]
- Miller-Fleming, L.; Olin-Sandoval, V.; Campbell, K.; Ralser, M. Remaining mysteries of molecular biology: The role of polyamines in the cell. J. Mol. Biol. 2015, 427, 3389–3406. [Google Scholar] [CrossRef]
- Alcázar, R.; Tiburcio, A.F. Polyamines; Humana New York: New York, NY, USA, 2018. [Google Scholar]
- Yoshikawa, K.; Takahashi, M.; Vasilevskaya, V.; Khokhlov, A. Large discrete transition in a single DNA molecule appears continuous in the ensemble. Phys. Rev. Lett. 1996, 76, 3029–3031. [Google Scholar] [CrossRef]
- Takahashi, M.; Yoshikawa, K.; Vasilevskaya, V.; Khokhlov, A. Discrete coil− globule transition of single duplex DNAs induced by polyamines. J. Phys. Chem. B 1997, 101, 9396–9401. [Google Scholar] [CrossRef]
- Yoshikawa, K.; Yoshikawa, Y.; Kanbe, T. All-or-none folding transition in giant mammalian DNA. Chem. Phys. Lett. 2002, 354, 354–359. [Google Scholar] [CrossRef]
- Venancio-Marques, A.; Bergen, A.; Rossi-Gendron, C.; Rudiuk, S.; Baigl, D. Photosensitive polyamines for high-performance photocontrol of DNA higher-order structure. ACS Nano 2014, 8, 3654–3663. [Google Scholar] [CrossRef] [PubMed]
- Muramatsu, A.; Shimizu, Y.; Yoshikawa, Y.; Fukuda, W.; Umezawa, N.; Horai, Y.; Higuchi, T.; Fujiwara, S.; Imanaka, T.; Yoshikawa, K. Naturally occurring branched-chain polyamines induce a crosslinked meshwork structure in a giant DNA. J. Chem. Phys. 2016, 145, 235103. [Google Scholar] [CrossRef] [PubMed]
- Kanemura, A.; Yoshikawa, Y.; Fukuda, W.; Tsumoto, K.; Kenmotsu, T.; Yoshikawa, K. Opposite effect of polyamines on In vitro gene expression: Enhancement at low concentrations but inhibition at high concentrations. PLoS ONE 2018, 13, e0193595. [Google Scholar] [CrossRef]
- Nishio, T.; Yoshikawa, Y.; Shew, C.Y.; Umezawa, N.; Higuchi, T.; Yoshikawa, K. Specific effects of antitumor active norspermidine on the structure and function of DNA. Sci. Rep. 2019, 9, 14971. [Google Scholar] [CrossRef]
- Kitagawa, T.; Nishio, T.; Yoshikawa, Y.; Umezawa, N.; Higuchi, T.; Shew, C.-Y.; Kenmotsu, T.; Yoshikawa, K. Effects of structural isomers of spermine on the higher-order structure of DNA and gene expression. Int. J. Mol. Sci. 2021, 22, 2355. [Google Scholar] [CrossRef]
- Thomas, T.; Thomas, T.J. Polyamines in cell growth and cell death: Molecular mechanisms and therapeutic applications. Cell. Mol. Life Sci. 2001, 58, 244–258. [Google Scholar] [CrossRef]
- Desforges, B.; Curmi, P.A.; Bounedjah, O.; Nakib, S.; Hamon, L.; De Bandt, J.P.; Pastre, D. An intercellular polyamine transfer via gap junctions regulates proliferation and response to stress in epithelial cells. Mol. Biol. Cell 2013, 24, 1529–1543. [Google Scholar] [CrossRef]
- Handa, A.K.; Fatima, T.; Mattoo, A.K. Polyamines: Bio-molecules with diverse functions in plant and human health and disease. Front. Chem. 2018, 6, 2169–2174. [Google Scholar] [CrossRef]
- Zinchenko, A. DNA conformational behavior and compaction in biomimetic systems: Toward better understanding of DNA packaging in cell. Adv. Colloid Interface Sci. 2016, 232, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Livolant, F.; Levelut, A.; Doucet, J.; Benoit, J. The highly concentrated liquid-crystalline phase of DNA is columnar hexagonal. Nature 1989, 339, 724–726. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.J.; Wynveen, A.; Albrecht, T.; Kornyshev, A.A. Which way up? Recognition of homologous DNA segments in parallel and antiparallel alignments. J. Chem. Phys. 2015, 142, 01B618_1. [Google Scholar]
- Manning, G.S. The persistence length of DNA is reached from the persistence length of its null isomer through an internal electrostatic stretching force. Biophys. J. 2006, 91, 3607–3616. [Google Scholar] [CrossRef] [PubMed]
- Schiessel, H. Biophysics for Beginners: A Journey through the Cell Nucleus, 2nd ed.; Jenny Stanford Publishing: Singapore, 2021. [Google Scholar]
- Nishio, T.; Yoshikawa, Y.; Yoshikawa, K.; Sato, S.-i. Longer DNA exhibits greater potential for cell-free gene expression. Sci. Rep. 2021, 11, 11739. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishio, T.; Shimada, Y.; Yoshikawa, Y.; Kenmotsu, T.; Schiessel, H.; Yoshikawa, K. The Anticancer Drug Daunomycin Directly Affects Gene Expression and DNA Structure. Int. J. Mol. Sci. 2023, 24, 6631. https://doi.org/10.3390/ijms24076631
Nishio T, Shimada Y, Yoshikawa Y, Kenmotsu T, Schiessel H, Yoshikawa K. The Anticancer Drug Daunomycin Directly Affects Gene Expression and DNA Structure. International Journal of Molecular Sciences. 2023; 24(7):6631. https://doi.org/10.3390/ijms24076631
Chicago/Turabian StyleNishio, Takashi, Yohji Shimada, Yuko Yoshikawa, Takahiro Kenmotsu, Helmut Schiessel, and Kenichi Yoshikawa. 2023. "The Anticancer Drug Daunomycin Directly Affects Gene Expression and DNA Structure" International Journal of Molecular Sciences 24, no. 7: 6631. https://doi.org/10.3390/ijms24076631
APA StyleNishio, T., Shimada, Y., Yoshikawa, Y., Kenmotsu, T., Schiessel, H., & Yoshikawa, K. (2023). The Anticancer Drug Daunomycin Directly Affects Gene Expression and DNA Structure. International Journal of Molecular Sciences, 24(7), 6631. https://doi.org/10.3390/ijms24076631






