Exploring the Dose–Effect Relationship of Bifidobacterium longum in Relieving Loperamide Hydrochloride-Induced Constipation in Rats through Colon-Released Capsules
Abstract
1. Introduction
2. Results
2.1. Viable Number of Bifidobacterium longum in Capsules
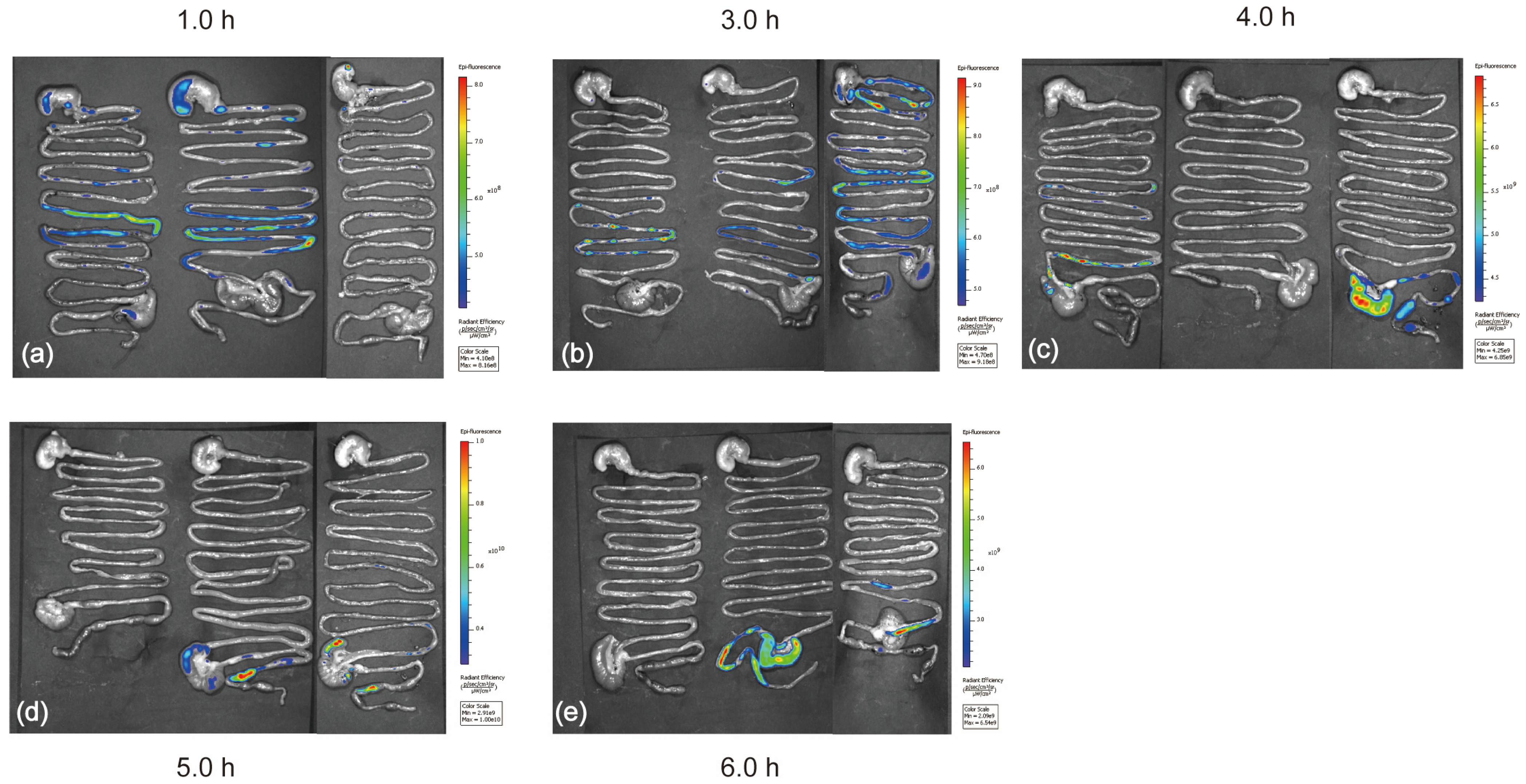
2.2. IVIS Imaging Shows That the Capsule Was Released at the Colon Site
2.3. B. longum Can Effectively Relieve the Apparent Symptoms of Constipation
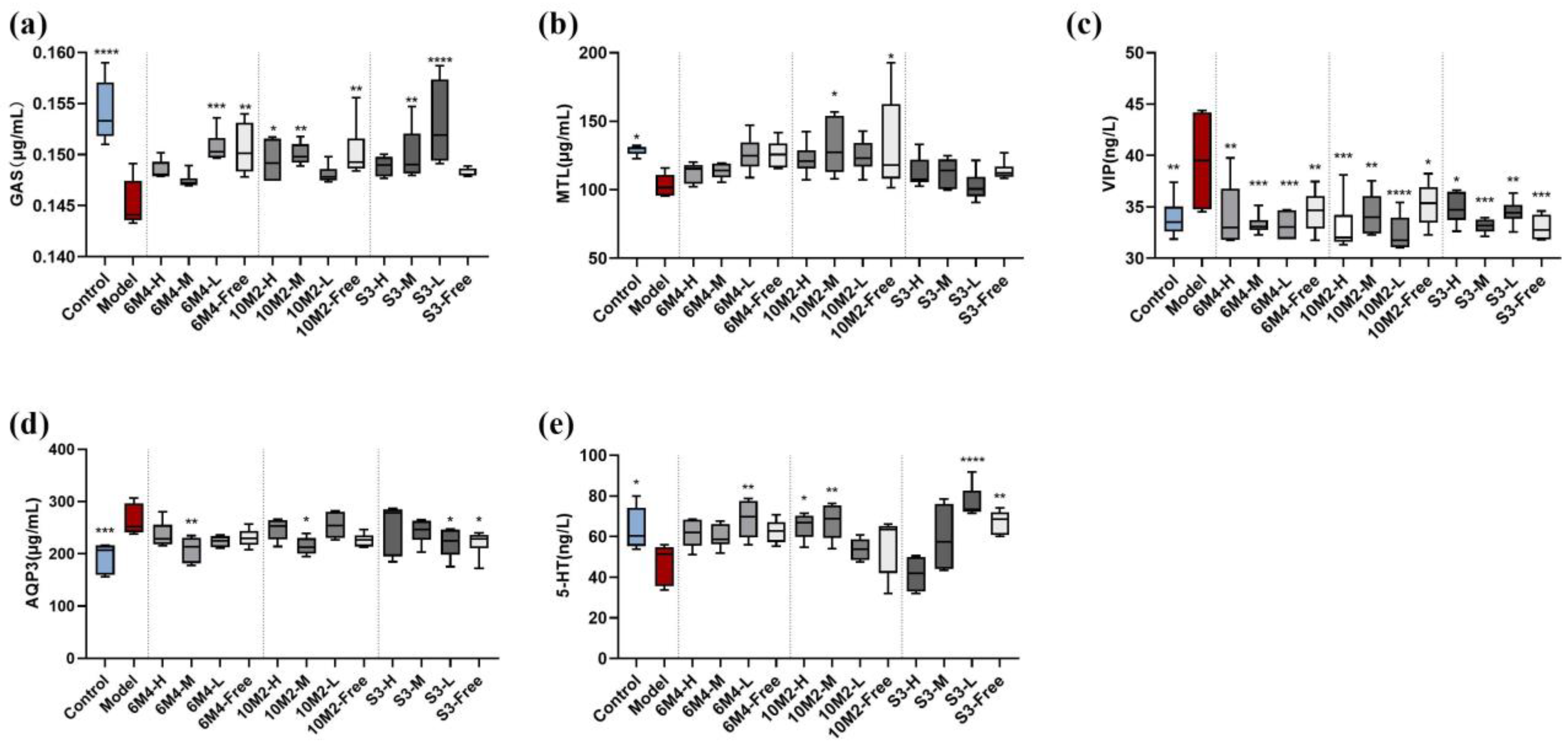
2.4. B. longum Exhibits a Dose–Effect Relationship in the Regulation of Gastrointestinal Active Peptides in Constipated Rats
2.5. B. longum Can Decrease the Content of Aquaporin-3 and Increase Serotonin Content in Colon Tissue to Promote Intestinal Peristalsis
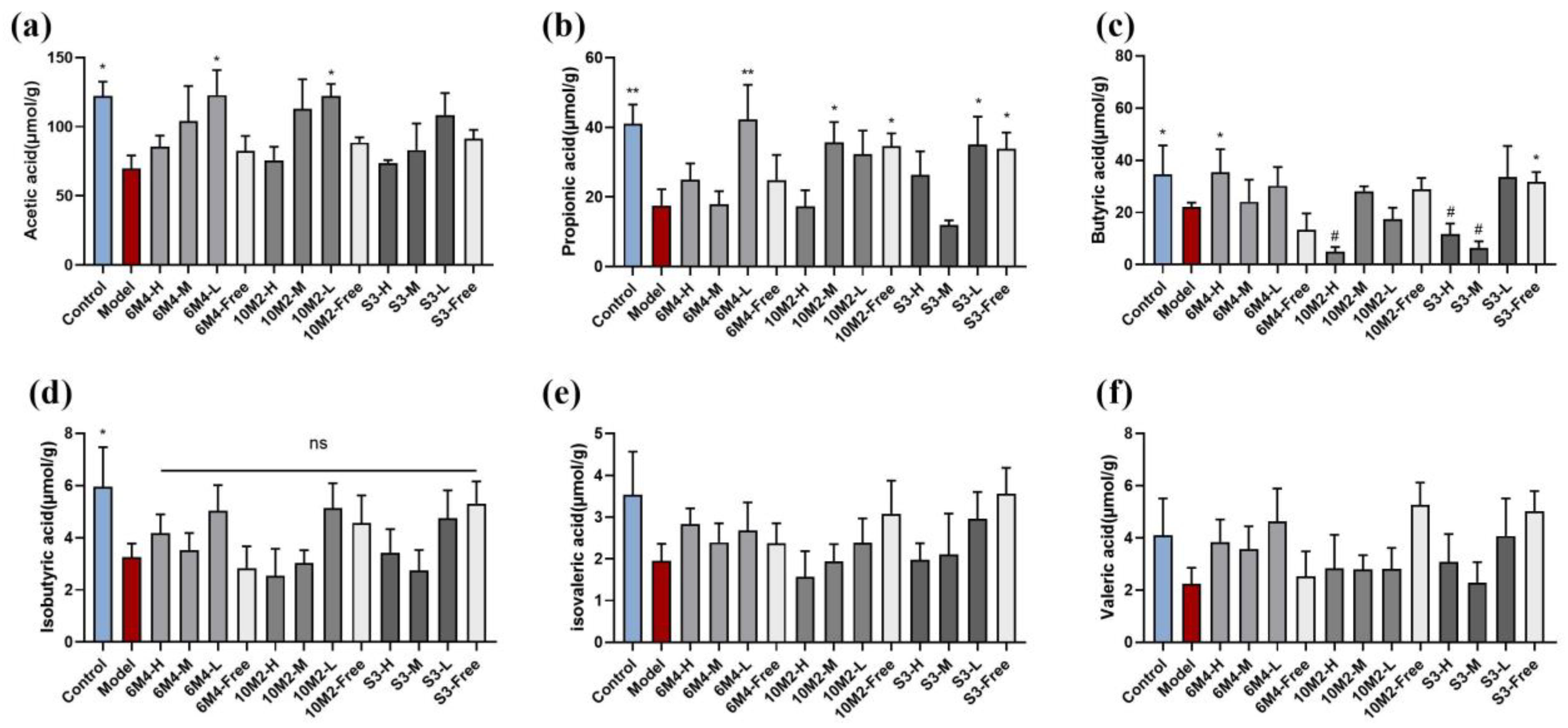
2.6. B. longum Can Increase the Content of SCFAs in Feces to Promote Intestinal Motility
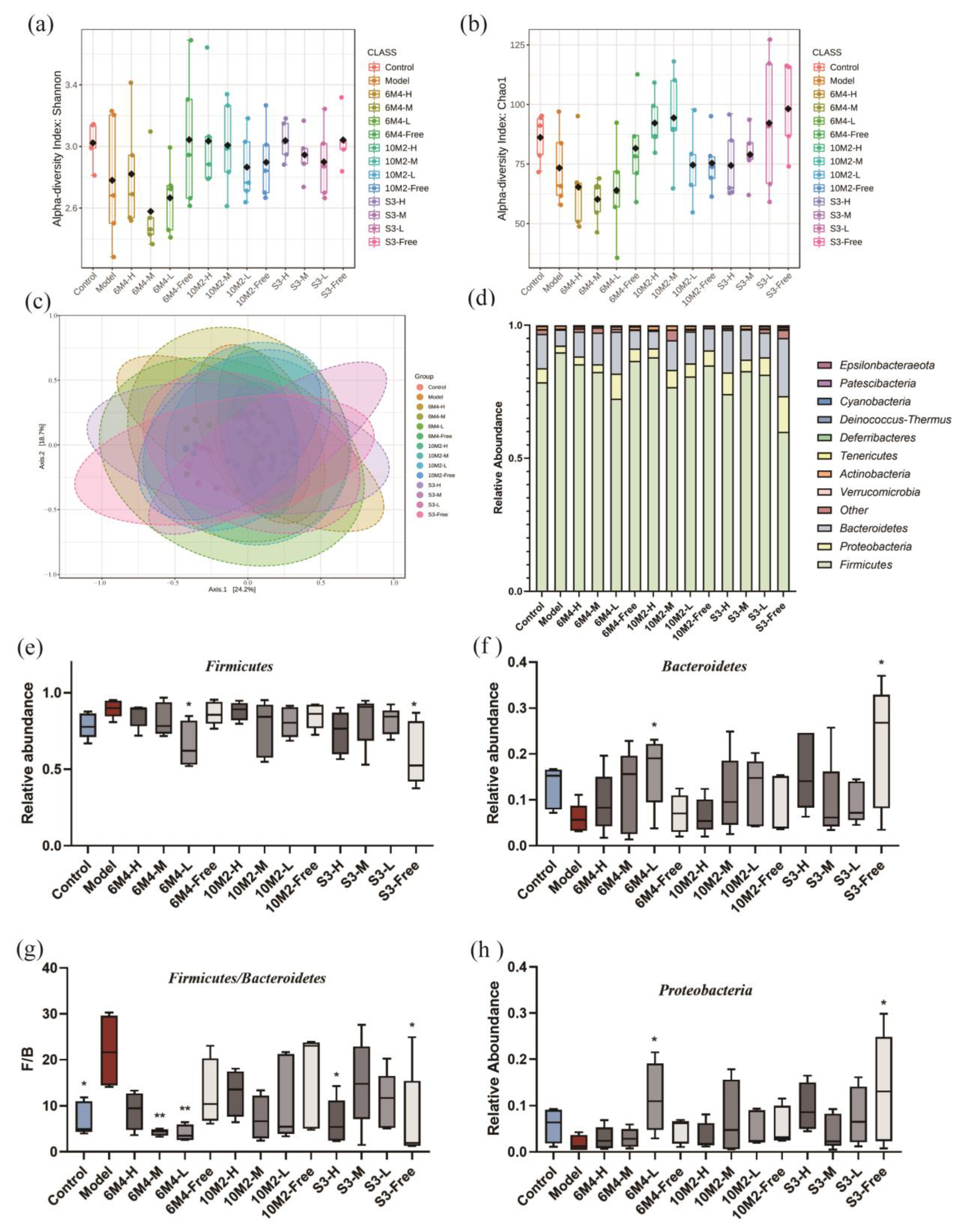
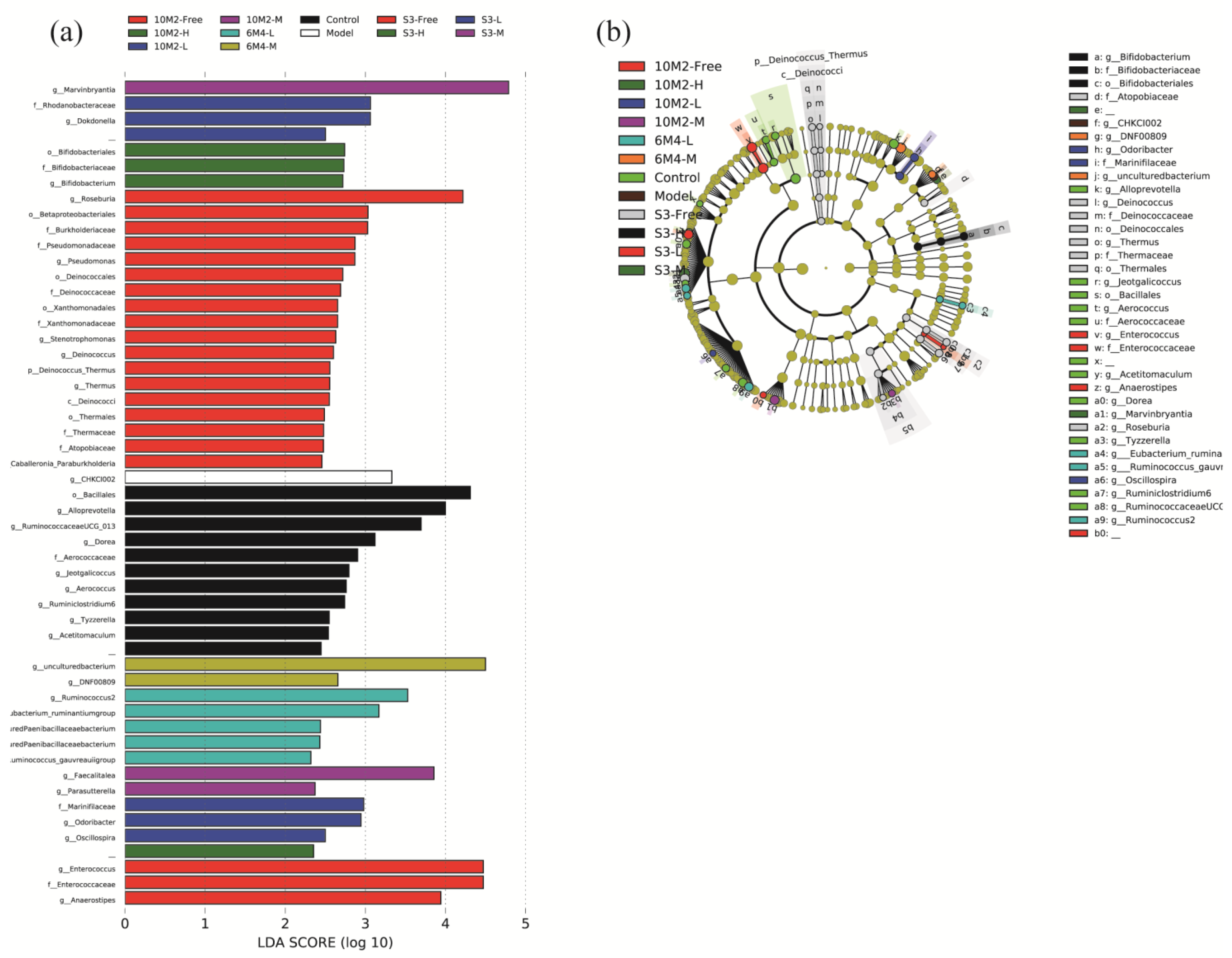
2.7. Gut Microbiota
3. Discussion
4. Materials and Methods
4.1. Strain Preparation
4.2. Encapsulation of Bifidobacterium longum Capsules
4.3. Colonic Release Validation of Capsules
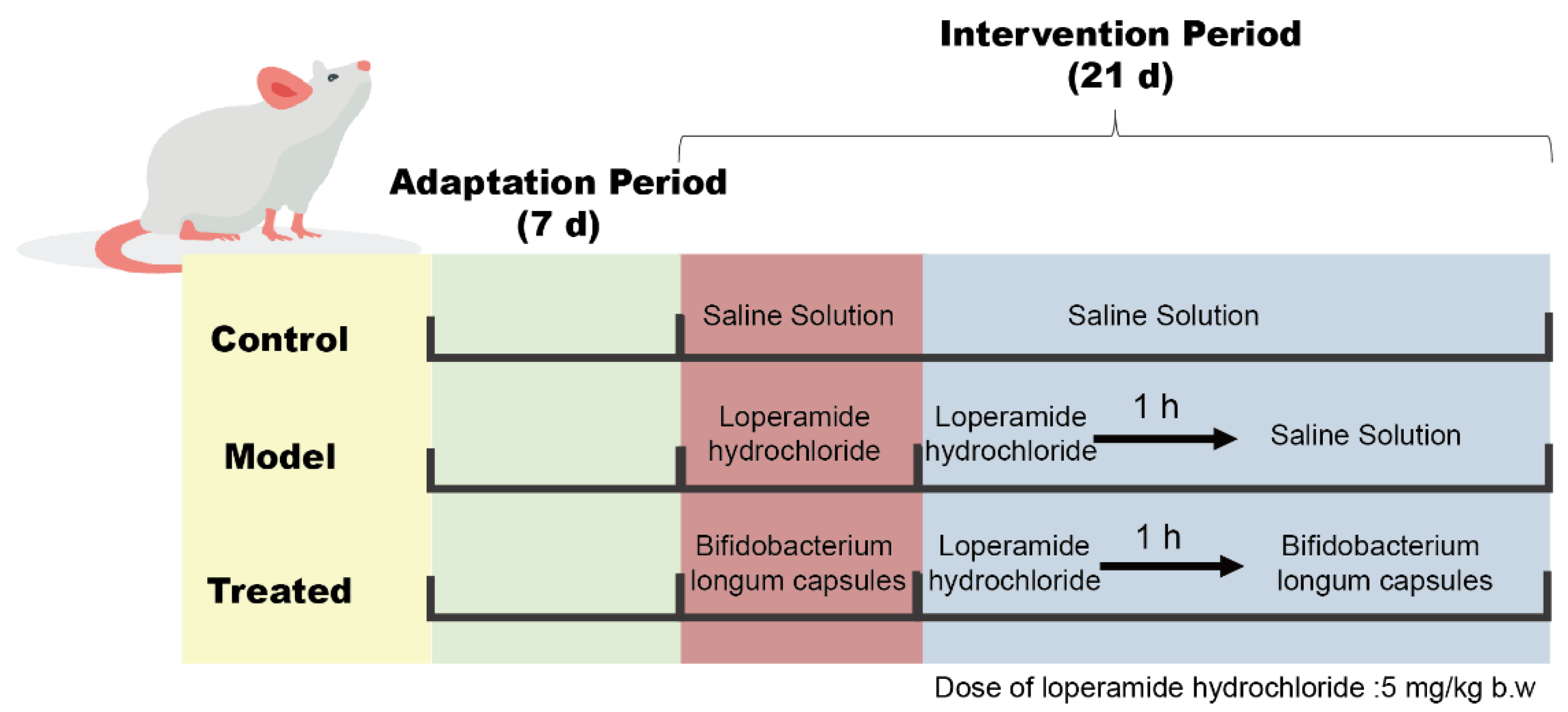
4.4. Animal Experimental Design
4.4.1. Water Content of Feces
4.4.2. Time Determination of First Black Stool
4.4.3. Determination of Gastrointestinal Mobility Rate
4.5. Determination of Gastrointestinal Active Peptide
4.6. Determination of Short-Chain Fatty Acid Content in Feces
4.7. 16S rDNA Sequencing and Bioinformatics Analysis
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vriesman, M.H.; Koppen, I.J.N.; Camilleri, M.; Di Lorenzo, C.; Benninga, M.A. Management of Functional Constipation in Children and Adults. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 21–39. [Google Scholar] [CrossRef] [PubMed]
- Krammer, H.-J.; von Seggern, H.; Schaumburg, J.; Neumer, F. Effect of Lactobacillus Casei Shirota on Colonic Transit Time in Patients with Chronic Constipation. Coloproctology 2011, 33, 109–113. [Google Scholar] [CrossRef]
- Bharucha, A.E.; Wald, A. Chronic Constipation. Mayo Clin. Proc. 2019, 94, 2340–2357. [Google Scholar] [CrossRef] [PubMed]
- Forootan, M.; Bagheri, N.; Darvishi, M. Chronic Constipation: A Review of Literature. Medicine 2018, 97, e10631. [Google Scholar] [CrossRef]
- Bharucha, A.E.; Pemberton, J.H.; Locke, G.R. American Gastroenterological Association Technical Review on Constipation. Gastroenterology 2013, 144, 218–238. [Google Scholar] [CrossRef]
- Suares, N.C.; Ford, A.C. Systematic Review: The Effects of Fibre in the Management of Chronic Idiopathic Constipation: Systematic Review: Effect of Fibre in Constipation. Aliment. Pharmacol. Ther. 2011, 33, 895–901. [Google Scholar] [CrossRef]
- Nelson, A.D.; Camilleri, M.; Chirapongsathorn, S.; Vijayvargiya, P.; Valentin, N.; Shin, A.; Erwin, P.J.; Wang, Z.; Murad, M.H. Comparison of Efficacy of Pharmacological Treatments for Chronic Idiopathic Constipation: A Systematic Review and Network Meta-Analysis. Gut 2017, 66, 1611–1622. [Google Scholar] [CrossRef]
- Rao, S.S.C.; Rattanakovit, K.; Patcharatrakul, T. Diagnosis and Management of Chronic Constipation in Adults. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 295–305. [Google Scholar] [CrossRef]
- Lee-Robichaud, H.; Thomas, K.; Morgan, J.; Nelson, R.L. Lactulose versus Polyethylene Glycol for Chronic Constipation. Cochrane Database Syst. Rev. 2010, 7, 1465–1858. [Google Scholar] [CrossRef]
- Rao, S.S.C.; Brenner, D.M. Efficacy and Safety of over-the-Counter Therapies for Chronic Constipation: An Updated Systematic Review. Am. J. Gastroenterol. 2021, 116, 1156–1181. [Google Scholar] [CrossRef]
- Chey, W.D.; Lembo, A.J.; Lavins, B.J.; Shiff, S.J.; Kurtz, C.B.; Currie, M.G.; MacDougall, J.E.; Jia, X.D.; Shao, J.Z.; Fitch, D.A.; et al. Linaclotide for Irritable Bowel Syndrome with Constipation: A 26-Week, Randomized, Double-Blind, Placebo-Controlled Trial to Evaluate Efficacy and Safety. Am. J. Gastroenterol. 2012, 107, 1702–1712. [Google Scholar] [CrossRef] [PubMed]
- Mueller-Lissner, S.; Kamm, M.A.; Wald, A.; Hinkel, U.; Koehler, U.; Richter, E.; Bubeck, J. Multicenter, 4-Week, Double-Blind, Randomized, Placebo-Controlled Trial of Sodium Picosulfate in Patients with Chronic Constipation. Am. J. Gastroenterol. 2010, 105, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Kubota, Y.; Iso, H.; Tamakoshi, A. Bowel Movement Frequency, Laxative Use, and Mortality from Coronary Heart Disease and Stroke among Japanese Men and Women: The Japan Collaborative Cohort (JACC) Study. J. Epidemiol. 2016, 26, 242–248. [Google Scholar] [CrossRef]
- Sumida, K.; Molnar, M.Z.; Potukuchi, P.K.; Thomas, F.; Lu, J.L.; Yamagata, K.; Kalantar-Zadeh, K.; Kovesdy, C.P. Constipation and Risk of Death and Cardiovascular Events. Atherosclerosis 2019, 281, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Li, S.; Wang, Q.; Guan, X.; Qian, L.; Li, J.; Zheng, Y.; Lin, B. Pediococcus Pentosaceus B49 from Human Colostrum Ameliorates Constipation in Mice. Food Funct. 2020, 11, 5607–5620. [Google Scholar] [CrossRef]
- Dimidi, E.; Christodoulides, S.; Scott, S.M.; Whelan, K. Mechanisms of Action of Probiotics and the Gastrointestinal Microbiota on Gut Motility and Constipation. Adv. Nutr. 2017, 8, 484–494. [Google Scholar] [CrossRef]
- Martoni, C.J.; Evans, M.; Chow, C.T.; Chan, L.S.; Leyer, G. Impact of a Probiotic Product on Bowel Habits and Microbial Profile in Participants with Functional Constipation: A Randomized Controlled Trial. J. Dig. Dis. 2019, 20, 435–446. [Google Scholar] [CrossRef]
- Ge, X.; Zhao, W.; Ding, C.; Tian, H.; Xu, L.; Wang, H.; Ni, L.; Jiang, J.; Gong, J.; Zhu, W.; et al. Potential Role of Fecal Microbiota from Patients with Slow Transit Constipation in the Regulation of Gastrointestinal Motility. Sci. Rep. 2017, 7, 441. [Google Scholar] [CrossRef]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous Bacteria from the Gut Microbiota Regulate Host Serotonin Biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef]
- van de Wouw, M.; Boehme, M.; Lyte, J.M.; Wiley, N.; Strain, C.; O’Sullivan, O.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Short-Chain Fatty Acids: Microbial Metabolites That Alleviate Stress-Induced Brain-Gut Axis Alterations: SCFAs Alleviate Stress-Induced Brain-Gut Axis Alterations. J. Physiol. 2018, 596, 4923–4944. [Google Scholar] [CrossRef]
- Christiansen, C.B.; Gabe, M.B.N.; Svendsen, B.; Dragsted, L.O.; Rosenkilde, M.M.; Holst, J.J. The Impact of Short-Chain Fatty Acids on GLP-1 and PYY Secretion from the Isolated Perfused Rat Colon. Am. J. Physiol.-Gastrointest. Liver Physiol. 2018, 315, G53–G65. [Google Scholar] [CrossRef] [PubMed]
- Rondeau, M.P.; Meltzer, K.; Michel, K.E.; McManus, C.M.; Washabau, R.J. Short Chain Fatty Acids Stimulate Feline Colonic Smooth Muscle Contraction. J. Feline Med. Surg. 2003, 5, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, J.; Zhang, Z.; Liang, X.; Liu, T.; Yi, H.; Gong, P.; Wang, L.; Yang, W.; Zhang, X.; et al. Konjac Glucomannan with Probiotics Acts as a Combination Laxative to Relieve Constipation in Mice by Increasing Short-Chain Fatty Acid Metabolism and 5-Hydroxytryptamine Hormone Release. Nutrition 2021, 84, 111112. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, H.; Kamikado, K.; Aoki, R.; Suganuma, N.; Nishijima, T.; Nakatani, A.; Kimura, I. Bifidobacterium Animalis Subsp. Lactis GCL2505 Modulates Host Energy Metabolism via the Short-Chain Fatty Acid Receptor GPR43. Sci. Rep. 2020, 10, 4158. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chai, M.; Wang, J.; Yu, Q.; Wang, G.; Zhang, H.; Zhao, J.; Chen, W. Bifidobacterium Longum Relieves Constipation by Regulating the Intestinal Barrier of Mice. Food Funct. 2022, 13, 5037–5049. [Google Scholar] [CrossRef] [PubMed]
- Chai, M.; Wang, L.; Li, X.; Zhao, J.; Zhang, H.; Wang, G.; Chen, W. Different Bifidobacterium Bifidum Strains Change the Intestinal Flora Composition of Mice via Different Mechanisms to Alleviate Loperamide-Induced Constipation. Food Funct. 2021, 12, 6058–6069. [Google Scholar] [CrossRef] [PubMed]
- Ouwehand, A.C. A Review of Dose-Responses of Probiotics in Human Studies. Benef. Microbes 2017, 8, 143–151. [Google Scholar] [CrossRef]
- Prudhviraj, G.; Vaidya, Y.; Singh, S.K.; Yadav, A.K.; Kaur, P.; Gulati, M.; Gowthamarajan, K. Effect of Co-Administration of Probiotics with Polysaccharide Based Colon Targeted Delivery Systems to Optimize Site Specific Drug Release. Eur. J. Pharm. Biopharm. 2015, 97, 164–172. [Google Scholar] [CrossRef]
- Ohkusa, T.; Koido, S.; Nishikawa, Y.; Sato, N. Gut Microbiota and Chronic Constipation: A Review and Update. Front. Med. 2019, 6, 19. [Google Scholar] [CrossRef]
- Whorwell, P.J.; Altringer, L.; Morel, J.; Bond, Y.; Charbonneau, D.; O’Mahony, L.; Kiely, B.; Shanahan, F.; Quigley, E.M.M. Efficacy of an Encapsulated Probiotic Bifidobacterium Infantis 35624 in Women with Irritable Bowel Syndrome. Am. J. Gastroenterol. 2006, 101, 1581–1590. [Google Scholar] [CrossRef]
- Larsen, C.N.; Nielsen, S.; Kaestel, P.; Brockmann, E.; Bennedsen, M.; Christensen, H.R.; Eskesen, D.C.; Jacobsen, B.L.; Michaelsen, K.F. Dose-Response Study of Probiotic Bacteria Bifidobacterium Animalis Subsp Lactis BB-12 and Lactobacillus Paracasei Subsp Paracasei CRL-341 in Healthy Young Adults. Eur. J. Clin. Nutr. 2006, 60, 1284–1293. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.-G.; Wen, P.; Fu, H.-Z.; Lin, G.-Y.; Liao, S.-T.; Zou, Y.-X. Protective Effect of Mulberry (Morus Atropurpurea ) Fruit against Diphenoxylate-Induced Constipation in Mice through the Modulation of Gut Microbiota. Food Funct. 2019, 10, 1513–1528. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-Q.; Li, D.-W.; Chen, Y.-Y.; Tao, H.-J.; Pu, Z.-J.; Zhang, J.; Tan, Y.-J.; Shi, X.-Q.; Yue, S.-J.; Zhou, G.-S.; et al. Elucidating Dosage-Effect Relationship of Different Efficacy of Rhubarb in Constipation Model Rats by Factor Analysis. J. Ethnopharmacol. 2019, 238, 111868. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Yi, R.; Qian, Y.; Park, K.-Y. Lactobacillus Plantarum YS-3 Prevents Activated Carbon-Induced Constipation in Mice. J. Med. Food 2018, 21, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Feighner, S.D.; Tan, C.P.; McKee, K.K.; Palyha, O.C.; Hreniuk, D.L.; Pong, S.-S.; Austin, C.P.; Figueroa, D.; MacNeil, D.; Cascieri, M.A.; et al. Receptor for Motilin Identified in the Human Gastrointestinal System. Science 1999, 284, 2184–2188. [Google Scholar] [CrossRef]
- Giancola, F.; Torresan, F.; Repossi, R.; Bianco, F.; Latorre, R.; Ioannou, A.; Guarino, M.; Volta, U.; Clavenzani, P.; Mazzoni, M.; et al. Downregulation of Neuronal Vasoactive Intestinal Polypeptide in Parkinson’s Disease and Chronic Constipation. Neurogastroenterol. Motil. 2017, 29, e12995. [Google Scholar] [CrossRef]
- Li, Y.; Long, S.; Liu, Q.; Ma, H.; Li, J.; Xiaoqing, W.; Yuan, J.; Li, M.; Hou, B. Gut Microbiota Is Involved in the Alleviation of Loperamide-induced Constipation by Honey Supplementation in Mice. Food Sci. Nutr. 2020, 8, 4388–4398. [Google Scholar] [CrossRef]
- Ikarashi, N.; Kon, R.; Sugiyama, K. Aquaporins in the Colon as a New Therapeutic Target in Diarrhea and Constipation. Int. J. Mol. Sci. 2016, 17, 1172. [Google Scholar] [CrossRef]
- Ikarashi, N.; Mochiduki, T.; Takasaki, A.; Ushiki, T.; Baba, K.; Ishii, M.; Kudo, T.; Ito, K.; Toda, T.; Ochiai, W.; et al. A Mechanism by Which the Osmotic Laxative Magnesium Sulphate Increases the Intestinal Aquaporin 3 Expression in HT-29 Cells. Life Sci. 2011, 88, 194–200. [Google Scholar] [CrossRef]
- Silberstein, C.; Kierbel, A.; Amodeo, G.; Zotta, E.; Bigi, F.; Berkowski, D.; Ibarra, C. Functional Characterization and Localization of AQP3 in the Human Colon. Braz. J. Med. Biol. Res. 1999, 32, 1303–1313. [Google Scholar] [CrossRef]
- Yin, J.; Liang, Y.; Wang, D.; Yan, Z.; Yin, H.; Wu, D.; Su, Q. Naringenin Induces Laxative Effects by Upregulating the Expression Levels of C-Kit and SCF, as Well as Those of Aquaporin 3 in Mice with Loperamide-Induced Constipation. Int. J. Mol. Med. 2018, 41, 649–658. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Z.; Tong, L.; Zhou, X.; Liang, X.; Yi, H.; Gong, P.; Liu, T.; Zhang, L.; Yang, L.; et al. Mechanisms Underlying the Promotion of 5-hydroxytryptamine Secretion in Enterochromaffin Cells of Constipation Mice by Bifidobacterium and Lactobacillus. Neurogastroenterol. Motil. 2021, 33, e14082. [Google Scholar] [CrossRef]
- Ren, X.; Liu, L.; Gamallat, Y.; Zhang, B.; Xin, Y. Enteromorpha and Polysaccharides from Enteromorpha Ameliorate Loperamide-Induced Constipation in Mice. Biomed. Pharmacother. 2017, 96, 1075–1081. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Liu, X.; An, Y.; Zhou, G.; Liu, Y.; Xu, M.; Dong, W.; Wang, S.; Yan, F.; Jiang, K.; et al. Dysbiosis Contributes to Chronic Constipation Development via Regulation of Serotonin Transporter in the Intestine. Sci. Rep. 2017, 7, 10322. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, S.; Tatewaki, M.; Yamada, T.; Fujimiya, M.; Mantyh, C.; Voss, M.; Eubanks, S.; Harris, M.; Pappas, T.N.; Takahashi, T. Short-Chain Fatty Acids Stimulate Colonic Transit via Intraluminal 5-HT Release in Rats. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2003, 284, R1269–R1276. [Google Scholar] [CrossRef]
- Liu, S.; Li, E.; Sun, Z.; Fu, D.; Duan, G.; Jiang, M.; Yu, Y.; Mei, L.; Yang, P.; Tang, Y.; et al. Altered Gut Microbiota and Short Chain Fatty Acids in Chinese Children with Autism Spectrum Disorder. Sci. Rep. 2019, 9, 287. [Google Scholar] [CrossRef]
- Zhuang, M.; Shang, W.; Ma, Q.; Strappe, P.; Zhou, Z. Abundance of Probiotics and Butyrate-Production Microbiome Manages Constipation via Short-Chain Fatty Acids Production and Hormones Secretion. Mol. Nutr. Food Res. 2019, 63, 1801187. [Google Scholar] [CrossRef]
- Yang, L.; Wang, Y.; Zhang, Y.; Li, W.; Jiang, S.; Qian, D.; Duan, J. Gut Microbiota: A New Avenue to Reveal Pathological Mechanisms of Constipation. Appl. Microbiol. Biotechnol. 2022, 106, 6899–6913. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, S.; Joseph, T.P.; Owusu, L.; Xiaomeng, R.; Meiqi, L.; Yi, X. A Polysaccharide Isolated from Dictyophora Indusiata Promotes Recovery from Antibiotic-Driven Intestinal Dysbiosis and Improves Gut Epithelial Barrier Function in a Mouse Model. Nutrients 2018, 10, 1003. [Google Scholar] [CrossRef] [PubMed]
- Sorbara, M.T.; Littmann, E.R.; Fontana, E.; Moody, T.U.; Kohout, C.E.; Gjonbalaj, M.; Eaton, V.; Seok, R.; Leiner, I.M.; Pamer, E.G. Functional and Genomic Variation between Human-Derived Isolates of Lachnospiraceae Reveals Inter- and Intra-Species Diversity. Cell Host Microbe 2020, 28, 134–146.e4. [Google Scholar] [CrossRef]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Ju, T.; Kong, J.Y.; Stothard, P.; Willing, B.P. Defining the Role of Parasutterella, a Previously Uncharacterized Member of the Core Gut Microbiota. ISME J. 2019, 13, 1520–1534. [Google Scholar] [CrossRef] [PubMed]
- Bourriaud, C.; Robins, R.J.; Martin, L.; Kozlowski, F.; Tenailleau, E.; Cherbut, C.; Michel, C. Lactate Is Mainly Fermented to Butyrate by Human Intestinal Microfloras but Inter-Individual Variation Is Evident. J. Appl. Microbiol. 2005, 99, 201–212. [Google Scholar] [CrossRef]
- Geva-Zatorsky, N.; Alvarez, D.; Hudak, J.E.; Reading, N.C.; Erturk-Hasdemir, D.; Dasgupta, S.; von Andrian, U.H.; Kasper, D.L. In Vivo Imaging and Tracking of Host–Microbiota Interactions via Metabolic Labeling of Gut Anaerobic Bacteria. Nat. Med. 2015, 21, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Xia, F.; Fan, W.; Jiang, S.; Ma, Y.; Lu, Y.; Qi, J.; Ahmad, E.; Dong, X.; Zhao, W.; Wu, W. Size-Dependent Translocation of Nanoemulsions via Oral Delivery. ACS Appl. Mater. Interfaces 2017, 9, 21660–21672. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]

| Group | Viable Count | Group | Viable Count | Group | Viable Count |
|---|---|---|---|---|---|
| 6M4-H | (9.0 ± 0.1) × 108 | 10M2-H | (7.8 ± 0.2) × 108 | S3-H | (4.3 ± 0.1) × 108 |
| 6M4-M | (9.8 ± 0.2) × 106 | 10M2-M | (1.8 ± 0.1) × 107 | S3-M | (4.1 ± 0.2) × 106 |
| 6M4-L | (2.1 ± 0.1) × 104 | 10M2-L | (2.3 ± 0.2) × 104 | S3-L | (9.7 ± 0.1) × 103 |
| 6M4-Free | (1.4 ± 0.3) × 109 | 10M2-Free | (1.9 ± 0.1) × 109 | S3-Free | (5.2 ± 0.3) × 108 |
| Group | Treatment | Count | Group | Treatment | Count |
|---|---|---|---|---|---|
| Control | Saline | 6 | Model | loperamide hydrochloride | 6 |
| 6M4-H | Capsule (108 CFU) + loperamide hydrochloride | 6 | 6M4-M | capsule (106 CFU) + loperamide hydrochloride | 6 |
| 6M4-L | Capsule (104 CFU) + loperamide hydrochloride | 6 | 6M4-Free | free (108 CFU/mL) + loperamide hydrochloride | 6 |
| 10M2-H | Capsule (108 CFU) + loperamide hydrochloride | 6 | 10M2-M | Capsule (106 CFU) + loperamide hydrochloride | 6 |
| 10M2-L | Capsule (104 CFU) + loperamide hydrochloride | 6 | 10M2-Free | Free (108 CFU/mL) + loperamide hydrochloride | 6 |
| S3-H | Capsule (108 CFU) + loperamide hydrochloride | 6 | S3-M | Capsule (106 CFU) + loperamide hydrochloride | 6 |
| S3-M | Capsule (104 CFU) + loperamide hydrochloride | 6 | S3-Free | Free (108 CFU/mL) + loperamide hydrochloride | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Mao, B.; Tang, X.; Zhang, Q.; Zhao, J.; Zhang, H.; Cui, S. Exploring the Dose–Effect Relationship of Bifidobacterium longum in Relieving Loperamide Hydrochloride-Induced Constipation in Rats through Colon-Released Capsules. Int. J. Mol. Sci. 2023, 24, 6585. https://doi.org/10.3390/ijms24076585
Zhou X, Mao B, Tang X, Zhang Q, Zhao J, Zhang H, Cui S. Exploring the Dose–Effect Relationship of Bifidobacterium longum in Relieving Loperamide Hydrochloride-Induced Constipation in Rats through Colon-Released Capsules. International Journal of Molecular Sciences. 2023; 24(7):6585. https://doi.org/10.3390/ijms24076585
Chicago/Turabian StyleZhou, Xin, Bingyong Mao, Xin Tang, Qiuxiang Zhang, Jianxin Zhao, Hao Zhang, and Shumao Cui. 2023. "Exploring the Dose–Effect Relationship of Bifidobacterium longum in Relieving Loperamide Hydrochloride-Induced Constipation in Rats through Colon-Released Capsules" International Journal of Molecular Sciences 24, no. 7: 6585. https://doi.org/10.3390/ijms24076585
APA StyleZhou, X., Mao, B., Tang, X., Zhang, Q., Zhao, J., Zhang, H., & Cui, S. (2023). Exploring the Dose–Effect Relationship of Bifidobacterium longum in Relieving Loperamide Hydrochloride-Induced Constipation in Rats through Colon-Released Capsules. International Journal of Molecular Sciences, 24(7), 6585. https://doi.org/10.3390/ijms24076585






