Abstract
Myocardial infarction (MI), heart failure, cardiomyopathy, myocarditis, and myocardial ischemia-reperfusion injury (I/R) are the most common heart diseases, yet there is currently no effective therapy due to their complex pathogenesis. Cardiomyocytes (CMs), fibroblasts (FBs), endothelial cells (ECs), and immune cells are the primary cell types involved in heart disorders, and, thus, targeting a specific cell type for the treatment of heart disease may be more effective. The same interleukin may have various effects on different kinds of cell types in heart disease, yet the exact role of interleukins and their pathophysiological pathways on primary cell types remain largely unexplored. This review will focus on the pathophysiological effects of various interleukins including the IL-1 family (IL-1, IL-18, IL-33, IL-37), IL-2, IL-4, the IL-6 family (IL-6 and IL-11), IL-8, IL-10, IL-17 on primary cell types in common heart disease, which may contribute to the more precise and effective treatment of heart disease.
1. Introduction
Heart disease is the leading cause of death worldwide [1]. Heart disease generally includes myocardial infarction (MI), heart failure, cardiomyopathy, myocarditis, and myocardial ischemia-reperfusion injury (I/R) [2]. There are no effective therapy methods currently because of their complex pathogenesis. Heart disease is characterized by the involvement of four major cell types: cardiomyocytes (CMs), fibroblasts (FBs), endothelial cells (ECs), and immune cells [3]. CMs, FBs, and ECs are resident cells in the heart; however, immune cells are infiltrated during inflammation, and they play a significant role in the pathophysiological process of heart disease. Thus, targeting a specific cell type for the treatment of heart disease may be more effective.
Inflammation has been identified as a potential initiator and promoter of heart disease. Different interleukin (IL) families have been linked to the development of heart disease, either positively or negatively. Proinflammatory factors such as IL-1β antagonists or antibodies have shown promising results in early clinical trials for heart disease [2]. Despite this, researchers are still exploring more effective cytokine treatment methods. However, the same interleukin may have various effects on different cell types of the heart. For instance, IL-11 has been found to mediate cytoprotective signals in cardiomyocytes [4], yet it has also been reported to have a profibrotic effect in cardiac fibrosis [5].
The IL-1 family is a major cytokine family associated with various cardiovascular diseases, initially comprising only two forms, IL-1α and IL-1β. To date, the IL-1 family has expanded to include 11 members (IL-1α, IL-1β, IL-1ra, IL-18, IL-33, IL-36Ra, IL-36α, IL-36β, IL-36γ, IL-37, and IL-38), with most of them being proinflammatory, while IL-1ra, IL-36Ra, IL-37, and IL-38 are anti-inflammatory [6].
IL-2 is an O-glycosylated four alpha-helix bundle cytokine that is primarily produced by activated T cells, dendritic cells, and B cells. It plays a pivotal role in the immune response to heart disease by regulating B-cell proliferation and immunoglobulin production, as well as maintaining T-cell homeostasis [7].
IL-4 is a glycosylated, type I cytokine with three intrachain disulfide bridges. It is mainly produced by T cells, natural killer T cells, mast cells, and eosinophils. In addition, it plays a central dual role in the development of inflammation in heart disease [8].
IL-6 is the founding member of the IL-6 cytokine family, which also includes IL-11, IL-27, IL-30, IL-31, leukemia inhibitory factor (LIF), oncostatin M (OSM), cardiotrophin-like cytokine (CLC), ciliary neurotrophic factor (CNTF), cardiotrophin-1 (CT-1), and neuropoietin. It is produced by numerous different cell types and is essential for regulating heart disease progression. IL-6-IL-6 R alpha complex promotes gp130 dimerization and the formation of a heterohexameric complex [9].
The CXCL8 gene encodes interleukin-8 (IL-8), a key mediator with both deleterious and beneficial properties. Multiple studies have reported elevated levels of IL-8 in various cardiac pathologies, including MI, suggesting that IL-8 could be a potential therapeutic target for heart disease [10].
The IL-10 family is composed of six members, namely IL-10, IL-19, IL-20, IL-22, IL-24, and IL-26. As the founding member, IL-10 has anti-inflammatory and immunosuppressive properties that serve to prevent excessive inflammation. It has been demonstrated to inhibit the antigen presentation capabilities of monocytes, macrophages, and dendritic cells, while simultaneously enhancing their tolerance-inducing, scavenger, and phagocytic functions. Additionally, IL-10 has been shown to suppress Th1-, Th2-, and Th17-mediated immune responses by inhibiting the proliferation of CD4+ T cells and their ability to produce proinflammatory cytokines. Furthermore, it has been observed to inhibit the secretion of proinflammatory mediators by neutrophils, eosinophils, and mast cells, as well as mast cell development.
IL-17 has been demonstrated to exert a wide range of biological activities on a variety of cell types, including CMs, ECs, neutrophils, monocytes, and macrophages. This cytokine has been shown to be involved in the inflammatory response in heart disease, suggesting a potential role in the pathogenesis of this condition [11].
Collectively, these findings suggest that IL plays a significant role in the pathogenesis of heart disease, and further research is needed to elucidate the precise mechanisms by which it modulates primary cell types in the heart. We have searched for papers using PubMed and Google Scholar with keywords: interleukins, IL-1, IL-18, IL-33, IL-37, IL-2, IL-4, IL-6, IL-11, IL-8, IL-10, IL-17, and heart disease. Then, we selected approximately 120 papers that involved the pathophysiological role of interleukins on primary cell types in heart disease as a reference. This review will primarily focus on the pathophysiological effects of various interleukins including the IL-1 family (IL-1, IL-18, IL-33, IL-37), IL-2, IL-4, the IL-6 family (IL-6 and IL-11), IL-8, IL-10, IL-17 on primary cells of heart disease-CMs, FBs, ECs, and immune cells. It would be more effective to focus on a certain cell type for the therapy of heart disease.
2. Cardiomyocytes (CMs)
CMs are the beating muscle cells that make up the atria and ventricles and are being targeted primarily in heart disease therapy. The specific effects of different interleukins on CMs in common heart disease are listed in Figure 1.

Figure 1.
The specific pathophysiological effects of different interleukins on CMs in common heart disease. The box in red represents the deleterious role, and the box in green represents the protective role.
2.1. IL-1
IL-1α and IL-1β are proinflammatory cytokines and their levels are correlated with the severity and pathogenesis of heart disease. Targeting the IL-1 signaling cascade including IL-1α and IL-1β may be a promising therapeutic target for patients with MI [12,13]. Moreover, in vivo MI mouse models have shown that inhibition of IL-1α reduces myocardial I/R damage, resulting in the retention of left ventricular function, reduced infarction area, and decreased activation of inflammatory bodies [14]. Thus, IL-1α blockers may represent an effective therapeutic approach to reduce I/R damage to the heart.
In a mouse model of MI, dead cardiomyocytes will release IL-1α [15,16]. In addition, the release of IL-1β in fulminant myocarditis leads to extensive inflammation, leading to the further death of cardiomyocytes, the gradual loss of active contracted tissue, and the development of cardiomyopathy and HF.
IL-1β is concentration-dependent and may elevate myocardial ring GMP through the myocardial L-arginine-NO pathway, leading to the restriction of systolic ejection and cardiac depression [13]. The regulation of the excitatory-contractile coupling of cardiomyocytes is reflected in changes in contractile force, and cytokine-specific effects appear to exist in the excitatory–contraction coupling, with TNF-α and IL-1β affecting inward calcium currents [17,18]. IL-1β has been shown to significantly prolong the duration of the action potential of guinea pig ventricular cells by changing the conductance of calcium channels [19]. IL-1β has been shown to rapidly inhibit the voltage-dependent Ca2+ current in adult rat ventricular muscle cells. Consistent with these data, IL-1β has been shown to inhibit systolic cardiomyocyte function by potentially involving the destruction of calcium processing or the inhibition of a β-adrenergic response [20,21]. In patients with HF, IL-1β has been demonstrated to decrease the beta-adrenergic responsiveness of L-type calcium channels, as well as decrease calcium homeostasis genes, including phospholamban and sarcoplasmic reticulum calcium ATPase [22,23]. Furthermore, IL-1β has been shown to have a proapoptotic effect on cardiomyocytes [24] and exert negative inspiratory effects on both isolated cardiomyocytes and intact hearts [13,25].
2.2. IL-2
Recent studies have demonstrated that high doses of IL-2 may induce AMI [26]. In isolated normal myocytes, IL-2 was found to decrease the amplitude of calcium transients induced by electrical stimulation, likely through blocking Ca2+ ATPase activity in the sarcoplasmic reticulum [27]. Furthermore, IL-2 concentrations produced by CD4+ T lymphocytes were abnormally elevated in patients with DCM, which may reflect deficiencies in T-cell function in these patients [28].
However, there are also studies demonstrating the protective role of IL-2 in MI. The injection of IL-2-activated NK cells has been shown to promote vascular remodeling through a4b7 integrin and killer cell lectin-like receptor (KLRG)-1 and promote cardiac repair after MI [29,30]. In addition, Cao et al. have reported that IL-2 could reduce infarct size by activating kappa-opioid receptors [31]. Moreover, IL-2 can be stimulated by the IL-2IgG2b fusion protein to improve left ventricular (LV) contraction function and remodeling in an MI rat model [30].
In conclusion, additional experimental studies are needed to fully elucidate the role of IL-2 and develop its therapeutic potential in heart disease.
2.3. IL-4
IL-4 is generally regarded as an anti-inflammatory cytokine. A recent study by Wan et al. showed that Vγ1+ γδT cells, as one of the main early producers of IL-4 after acute viral infection, protect the mouse heart from acute viral myocarditis. Moreover, the neutralization of IL-4 in mice led to exacerbations of acute myocarditis, confirming the IL-4-mediated Vγ1 protective mechanism [32]. This finding was further supported by another study on viral myocarditis, which showed elevated levels of IL-4 in mice with attenuated viral myocarditis and elevated levels of heart expression [33].
However, a contradictory finding was reported in the context of autoimmune myocarditis, where eosinophils are the predominant cell type in the heart expressing IL-4, and eosinophil-specific IL-4 deletion leads to improved cardiac function. In this regard, eosinophils have been shown to drive myocarditis progression to inflammatory dilated cardiomyopathy (DCMi), and this process is mediated by IL-4 [34].
2.4. IL-6
IL-6, as an upstream marker of inflammation, is independently associated with the risk of major adverse cardiovascular disease events, MI, HF, and cancer mortality stable coronary heart disease [35]. IL-6 and sIL-6R have been associated with AMI and cardiac injury; binding to trans-IL-6 receptors alters intracellular signaling, and blocking IL-6 receptor binding may be a causative factor in AMIs [36]. Hypothetically validated, the IL-6 receptor antagonist tocilizumab reduces inflammation and the release of TnT in non-ST-segment elevation MI (NSTEMI). Therefore, IL-6 is a potential therapeutic target for MI [37].
Cytokine-specific action appears to be present in the excitatory–contraction coupling and TNF-α IL-6 modulates Ca2+ ATPase activity of the sarcoplasmic reticulum in cardiomyocytes [38]. It was reported that the degradation of IL-6 mRNA inhibits the proinflammatory action in the stress-overloaded myocardium [39]. Moreover, the gene deletion of IL-6 improves cardiac function and weakens hypertrophy by eliminating the dependent effects of CaMKII on cardiomyocytes in the stress-overloaded myocardium [40]. In addition, in the model of LV remodeling after MI, a drug blockade of IL-6 through the administration of an anti-IL6R antibody weakens dilation and improves contraction function [41]. IL-6 can indirectly enhance the expression of iNOS, and excess nitric oxide may reduce myocardial contractility and may have toxic effects by triggering apoptosis [42].
IL-6 exerts negative inotropic action [43] and promotes a hypertrophy response in cardiomyocytes [44,45,46] through the gp130/STAT3 pathway, but can also enable protective action, mediated by mitochondrial function preservation [47]. Recombinant IL-6 induces a cytoprotective effect to prevent I/R damage and activates ERK1/2, JNK1/2, p38-MAPK, and PI3K without inducing STAT1/3 phosphorylation. These data suggest that the cardiomyocyte protective effect of IL-6 in I/R occurs through ERK1/2 and PI3K activation, but is not related to sIL-6R and JAK/STAT signaling [48].
2.5. IL-8
IL-8 is important in the development of MI. Serum IL-8 concentrations show a transient increase in the very early stages of AMI [49]. Specific monoclonal antibodies that neutralize IL-8 significantly reduce the degree of necrosis in rabbit myocardial I/R injury models [50]. High levels of IL-8 in STEMI patients with HF are associated with less improvement in left ventricular function in the first 6 weeks after PCI, suggesting that IL-8 may play a role in reperfusion-related injuries to the myocardium after ischemia [51].
2.6. IL-10
Cardioprotective effects of IL-10 on the cardiomyocytes of heart diseases were found in a variety of previous studies. The involvement of the Akt and Jak/Stat pathways in regulating TNF-induced cardiomyocyte apoptosis by IL-10 has been studied [52]. Subsequent research revealed that IL-10’s negative control of TNF-induced apoptosis was mediated by Akt via STAT3 activation [53]. In addition, there is a study revealing that IL-10-induced antiapoptotic signaling in cardiomyocytes includes upregulating TLR4 through MyD88 activation [54]. Moreover, Kishore, R et al. found that IL-10 attenuates pressure overload-induced hypertrophic remodeling and improves heart function via STAT3-dependent inhibition of NF-κB [55]. Exercise reduces HFD-induced cardiomyopathy by reducing obesity, inducing IL-10, and reducing TNF-α [56].
2.7. IL-11
IL-11 mediates cytoprotective signals in cardiomyocytes by activating phosphorylated STAT3 translocating into nuclei [57]. IL-11 attenuated cardiac remodeling after MI through the gp130/STAT3 axis [5]. In addition, it also reduced the I/R injury through STAT3 activation in the hearts [58]. This evidence demonstrates the therapeutic role of IL-11 in heart disease.
2.8. IL-17
As a proinflammatory cytokine, IL-17 participates in an array of heart diseases. IL-17 was reported to have contributed to the process of cardiac fibrosis, the activation of matrix metalloproteinases, and enhanced cardiac cell death. IL-17 induces mouse cardiomyocyte apoptosis via Stat3-iNOS activation, suggesting that IL-17 contributes to cardiac damage [59]. It has also been observed that IL-17A induces cardiomyocyte apoptosis through the p38 mitogen-activated protein kinase (MAPK)-p53-Bax signaling pathway and promotes both early- and late-phase post-MI ventricular remodeling [60]. Pan et al. found that IL-17 affects the calcium-handling process involved in HF. The treatment of neonatal cardiomyocytes with steady-state concentrations of IL-17 suppressed transient calcium and decreased SERCA2a and Cav1.2 expression, this effect is mediated via the NF-κB pathway [61].
2.9. IL-18
IL-18 is a proinflammatory cytokine produced during various heart diseases. IL-18 was discovered to be elevated in animal models of AMI, HF, pressure overload, and LPS-induced dysfunction. Furthermore, IL-18 has been shown to regulate cardiomyocyte hypertrophy, induce cardiac systolic dysfunction, and lead to extracellular matrix remodeling [62,63]. Several observations suggest that IL-18BP is a potential therapeutic tool for reducing myocardial dysfunction caused by ischemia [64,65,66].
The treatment of HL-1 cardiomyocytes with IL-18 resulted in hypertrophy and elevated levels of ANP, likely via the activation of signaling pathways involving PI3K, Akt, and the transcription factor GATA4 [67]. In vitro studies found that the IL-18 treatment of cardiomyocytes increased peak and diastolic calcium transients and decreased the shortening of isolated cardiomyocytes [68]. Moreover, an increase in serum IL-18 concentration may induce apoptosis in cardiomyocytes, leading to ongoing myocardial injury in acute MI [69].
However, there are some controversial studies; for example, the expression of IL-18RNA in the myocardium of patients with dilated cardiomyopathy is downregulated [70], and IL-18 has been shown to play a beneficial role in viral myocarditis caused by the cerebrocarditis virus. The systemic administration of IL-18 is beneficial in mice with myocarditis and may be mediated by reducing the expression of TNF-α in the heart [65].
Overall, the role of IL-18 in heart disease is primarily in amplifying myocardial dysfunction, and the level also was recognized as a marker of heart injury in patients.
2.10. IL-33
IL-33 belongs to the IL-1 family. IL-33 and its receptor ST2 (located on the membrane of CMs) were demonstrated to be cardioprotective. The highly localized signaling pathway mediated by ST2 regulates the heart’s response to pressure overload. It was suggested that IL-33 secretion by endothelial cells is crucial in converting myocardial pressure overload into a selective systemic inflammatory state [71]. In a study involving wild-type mice, treatment with recombinant IL-33 was found to reduce angiotensin II and phenylephrine-induced cardiomyocyte hypertrophy and fibrosis. Furthermore, IL-33 treatment improved survival after transverse aortic constriction (TAC), a surgical procedure used to induce cardiac hypertrophy and heart failure in animal models [72].
2.11. IL-37
IL-37, like IL-10, is an anti-inflammatory interleukin generated by a variety of cell types. IL-37 is the main cytokine in the regulation of immune response, mainly inhibits the expression, production, and effect of proinflammatory cytokines, and plays a role in autoimmune diseases and organ transplantation [73]. The expression level of IL-37 is known to be low under normal physiological conditions; however, the expression level of IL-37 is significantly upregulated in response to an inflammatory environment, such as in patients with acute myocardial infarction (AMI) [74].
IL-37 plays an active role in a variety of cardiovascular diseases [75]. The Zeng group reported that human recombinant IL-37 can inhibit neutrophil infiltration and reduce cardiomyocyte apoptosis through a tail vein injection into myocardial I/R mice, thereby alleviating myocardial I/R injury in mice [76]. The team also reported that the intraperitoneal injection of human recombinant IL-37 and the intravenous injection of IL-37 and troponin co-induced dendritic cells can alleviate adverse ventricular remodeling after MI and cardiomyocyte apoptosis in mice, also attenuating the degree of cardiac fibrosis [77]. Overall, the positive role of IL-37 on other heart diseases, i.e., HF, needs to be further elucidated.
Overall, the effects of interleukins on CMs in heart disease are complex and context-dependent, and more research is needed to fully understand their roles in these conditions.
3. Fibroblasts (FBs)
Fibroblasts are the most abundant cell type found in connective tissue and are responsible for secreting collagen proteins that provide a structural framework for many tissues. Additionally, fibroblasts play an important role in wound healing. In heart disease, however, fibroblasts are transdifferentiated into activated myofibroblasts, which express α-smooth muscle actin (ACTA2) and secrete extracellular matrix (ECM) proteins and are a defining feature of fibrosis. The specific effects of different interleukins on FBs in common heart disease are summarized in Figure 2.
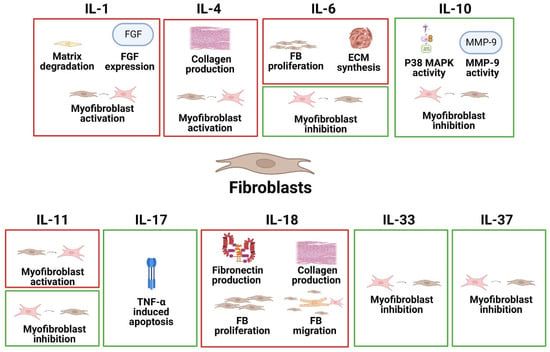
Figure 2.
The specific pathophysiological effects of different interleukins on FBs in common heart disease. The box in red represents the deleterious role, and the box in green represents the protective role.
3.1. IL-1
In fibroblasts, IL-1 can induce the expression of extracellular matrix proteins, including collagen, and promote fibroblast proliferation and differentiation into myofibroblasts. IL-1 promotes matrix degradation phenotypes in fibroblasts in an IL-1R1-dependent manner and helps disrupt key stromal–cardiomyocyte interactions needed for cell survival in the infarcted myocardium [78]. IL-1-driven matrix degradation may eventually activate fibroblast-mediated matrix protein synthesis, leading to increased fibrosis by increasing the expression of fibroblast growth factor [79].
3.2. IL-4
IL-4 stimulates the inflammatory response, activates collagen synthesis, promotes fibrosis progression, and inhibits the production of anti-inflammatory cytokines. Urine IL-4 is associated with myocardial fibrosis and remodeling in heart failure by the concentration of urine IL-4 in patients with HF and its relationship to markers of myocardial fibrosis and left ventricular volume [80].
IL-4 mediates the upregulation of proto-collagen genes through IL-4 receptor α and stimulates collagen production in mouse cardiac fibroblasts. This sheds light on the critical role of IL-4 in angiotensin-II-induced cardiac injury and provides a strong basis for IL-4 as an additional target for the treatment of cardiac fibrosis [81].
3.3. IL-6
IL-6 is another proinflammatory cytokine that has been implicated in the development of fibrosis. In an array of heart diseases, IL-6 promotes fibroblast proliferation and stimulates ECM synthesis [82,83,84].
Silencing IL-6 in EDC eliminated most of the benefits of cell transplantation and showed that IL-6 promotes cardiac fibroblasts and macrophages to reduce myocardial scarring while increasing the production of new cardiomyocytes and the recruitment of hematopoietic stem cells. IL-6 plays a key role in EDC-mediated cardiac repair and may provide a way to increase cell-mediated ischemic myocardial repair [85].
Both pro- and anti-inflammatory effects of IL-6 have been reported. In the short term, IL-6 has a protective effect and limits host damage, and it is precisely when this acute response remains chronically activated that IL-6 becomes pathogenic to the host; long-term elevated IL-6 levels lead to chronic inflammation and fibrotic disease. It is supposed that short-term IL-6 signaling protects and preserves heart tissue in response to acute injury; moreover, long-term IL-6 signaling or overproduction of the IL-6R protein plays a causal role in cardiovascular disease. Depending on the kinetics of the host response, IL-6 can be both protective and pathogenic [86].
3.4. IL-10
IL-10 is an anti-inflammatory cytokine that can have protective effects on the heart. IL-10 knockout mice presented worse left ventricular function and fibrosis following MI [87]. The IL-10 treatment demonstrated the downregulation of p38 mitogen-activated protein kinase activation, reduced expression of the cytokine mRNA-stabilizing protein Hur, a decreased metalloproteinase-9 (MMP-9) activity, and inhibited fibrosis after MI [88]. IL-10 gene expression inhibition is associated with the suppression of TLR4 and IL-1 receptor-associated kinase-1 (IRAK1) activation along with the upregulation of TLR2 and IRAK2, resulting in fibrosis.
3.5. IL-11
It is reported that IL-11 plays a profibrotic effect as a downstream effector following TGFβ1 exposure; the genetic deletion of IL-11 protects the heart from fibrosis [4]. In contrast to this, IL-11 attenuated cardiac fibrosis and remodeling after MI through the gp130/STAT3 axis [5]. Therefore, the exact role of IL-11 in heart fibrosis needs to be further elucidated.
3.6. IL-17
Th17 cells produce MMP through the IL-17-RANKL/OPG system in cardiac fibroblasts, or regulate cardiac fibrosis by stabilizing mRNAs from proinflammatory cytokines in various cardiomyocytes and immune cells [89]. IL-17 has been shown to exaggerate the extent of cardiac fibrosis, the activation of MMPs, and enhanced cardiac cell death.
3.7. IL-18
IL-18 and osteopontin (OPN) gene and protein expression were found elevated in pressure-overload mice; IL-18 has an effect on mice cardiac fibroblasts to induce OPN [90]. In addition to this, IL-18 has profibrotic effects on human cardiac fibroblasts by inducing fibronectin production [91]. Furthermore, IL-18 has profibrotic effects on rat cardiac fibroblasts by producing collagen type I and III, MMP-2, and then activating the JNK and PI3-kinase pathways. In addition to this, IL-18 leads to fibroblast migration and proliferation. These studies illustrate that IL-18 plays an important role in cardiac fibrosis-related disorders [92].
3.8. IL-33
As we mentioned before, recombinant IL-33 treatment reduced angiotensin II- and phenylephrine-induced fibrosis and improved survival after TAC in WT mice [72].
3.9. IL-37
The Zeng group reported that the intraperitoneal injection of human recombinant IL-37 and the intravenous injection of IL-37 and troponin co-induced dendritic cells can alleviate the adverse ventricular remodeling after MI and attenuated the degree of cardiac fibrosis in mice [77].
Overall, the effects of interleukins on fibroblasts in heart disease are complex and context-dependent, and more research is needed to fully understand their roles in the development of fibrosis and other pathological processes in the heart.
4. Endothelial Cells (ECs)
ECs are regarded as the major non-CM population in the heart, suggesting that their physiological and therapeutic importance may be greater than previously appreciated. ECs comprise approximately 95% of the blood vascular and 5% of the lymphatic system and are responsible for forming blood vessels and valves.
Furthermore, ECs play an important role in angiogenesis, which is the formation of new blood vessels from pre-existing ones. ECs also participate in the development of the heart and lymphatic system during embryonic development. The specific effects of different interleukins on ECs in common heart disease are summarized in Figure 3.

Figure 3.
The specific pathophysiological effects of different interleukins on ECs in common heart disease. The box in red represents the deleterious role, and the box in green represents the protective role.
4.1. IL-1
IL-1α has been shown to stimulate the expression of macrophage colony-stimulating factor (MCSF) in both ECs and SMCs at both the mRNA and protein levels during atherogenesis. This upregulation of MCSF may play a critical role in the formation of atherosclerotic lesions and the progression of MI. ECs and SMCs are known to contribute to the development of atherosclerosis and MI, and the increased expression of MCSF may promote the recruitment of monocytes and macrophages to the site of injury, leading to the formation of atherosclerotic plaques. These findings suggest that targeting the IL-1α-MCSF signaling pathway may hold therapeutic potential for the prevention and treatment of atherosclerosis and MI. Further studies are needed to fully elucidate the mechanisms underlying this pathway and to identify potential therapeutic targets [93].
4.2. IL-2
In a recent study, it was found that the injection of recombinant human interleukin-2 (rhIL-2) into mice led to a significant increase in the proliferation index of endothelial cells in the infarcted heart following MI. Specifically, the EC proliferation index was enhanced by 1.6-fold compared to control mice, indicating a potential role for rhIL-2 in promoting cardiac tissue repair following MI. These findings suggest that rhIL-2 may hold therapeutic potential for the treatment of MI and other cardiovascular diseases by promoting EC proliferation and angiogenesis. Further studies are needed to fully elucidate the underlying mechanisms and to optimize the dosing and delivery of rhIL-2 for clinical use [29].
4.3. IL-8
IL-8 may play a protective role in angiogenesis function; Haleagrahara et al. reported that in rats receiving insulin-like growth factor 1 (IGF-1) treatment, the enhanced angiogenetic impact of IL-8 is linked to the protection of ischemic myocardium and isoproterenol-induced cardiac damage [94]. In AMIs, IL-8 is associated with circulating progenitor cells, and in addition to the proangiogenesis function of IL-8 and VEGF, this mechanism may contribute to the production of new blood vessels and ECs, thereby improving myocardial function [95]. Therefore, the therapeutic potential in heart disease of IL-8 should be further developed in the future.
4.4. IL-10
IL-10 deficiency and inflammation have been shown to affect the function and content of exosomes derived from endothelial progenitor cells (EPCs), which in turn affects their therapeutic efficacy in myocardial repair. This effect is mediated by an upregulation of integrin-linked kinase (ILK) enrichment in exosomes, which activates the NF-κB pathway in recipient cells. Conversely, the knockdown of ILK in exosomes attenuates NF-κB activation and reduces the inflammatory response. These findings suggest that ILK may play a crucial role in modulating the therapeutic potential of EPC-derived exosomes in the context of myocardial repair [96]. Further research is needed to elucidate the underlying mechanisms and potential clinical applications of these findings.
4.5. IL-17
IL-17 has been shown to increase nitric oxide (NO) synthesis, leading to endothelial cell (EC) injury in an oxygen-dependent manner [97]. Additionally, IL-17 has been found to induce the expression of adhesion molecules, including CXCL1 (GRO-α) and CXCL8 in ECs [98]. These findings suggest that IL-17 may play a role in the development of vascular inflammation and injury, which are key components of many cardiovascular diseases. Further research is needed to fully elucidate the mechanisms underlying the effects of IL-17 on EC function and to explore potential therapeutic targets for the treatment of cardiovascular disease.
4.6. IL-18
In patients with MI who undergo coronary artery bypass grafting (CABG), elevated levels of IL-18 in the systemic circulation have been reported to activate lymphocytes, which may subsequently lead to EC cytotoxicity [99]. I/R injury triggers the activation of the NLRP3/IL-18 signaling pathway, which in turn induces the transcription of CXCL16 in cardiac vascular endothelial cells (VECs). Furthermore, it was revealed that the transcription of CXCL16 is dependent on the transcription factor FOXO3 and that IL-18-mediated STAT3 phosphorylation promotes nuclear translocation of FOXO3, which enhances CXCL16 promoter activity by binding to the FOXO3 binding site. These findings suggest that CXCL16 may serve as a potential therapeutic target for treating IL-18-mediated myocardial I/R injury by reducing cardiac inflammation and improving cardiac remodeling and dysfunction [100]. It was suggested that IL-18 may play a significant role in the pathogenesis of cardiovascular disease by promoting inflammation and vascular injury.
4.7. IL-33
IL-33 is a cytokine that can promote angiogenesis and vascular leakage. Experimental studies have shown that IL-33 stimulates ECs to produce nitric oxide (NO) through the ST2/TRAF6-Akt-eNOS signaling pathway, thereby promoting the process of angiogenesis and vascular leakage. This discovery reveals the mechanism of action of IL-33 in vascular diseases and provides new ideas for the development of treatments for related diseases. This study provides new evidence for revealing the mechanism of the IL-33 signaling pathway in angiogenesis and vascular leakage and provides new ideas and strategies for the treatment of related diseases [101].
Overall, the effects of interleukins on ECs in heart disease are mostly on EC proliferation and angiogenesis. More research is needed to fully understand other interleukins in the development of angiogenesis and other pathological processes in the heart.
5. Immune Cells
Immune cells are implicated in the pathophysiology of heart diseases and include several types of cells in the heart, such as T cells, B cells, macrophages, neutrophils, and NK cells. The specific effects of different interleukins on these immune cells in common heart disease are summarized in Figure 4.
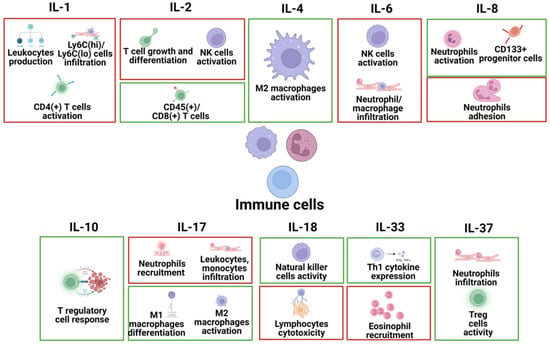
Figure 4.
The specific pathophysiological effects of different interleukins on immune cells in common heart disease. The box in red represents the deleterious role, and the box in green represents the protective role.
5.1. IL-1
IL-1 critically participates in the process of post-MI by activating leukocytes and fibroblasts, and IL-1 signals the absence of globally attenuated leukocyte recruitment, reducing the number of infiltrating Ly6C(hi) and Ly6C(lo) cells [78]. IL-1α was suggested to be a mediator of M2-like macrophage-induced fibroblast activation [102]. In acute MI, targeting IL-1β reduces leukocyte production and inflammation by suppressing bone marrow hematopoietic stem cell proliferation, demonstrating the positive influence of IL-1β on leukocyte production [103]. Furthermore, IL-1 receptor type 1 (IL-1R1) is critical for the induction of autoimmune myocarditis; it is required for dendritic cells producing TNF-α, IL-1, IL-6, and IL-12p70. In addition, the triggering of IL-1R1 induces CD4(+) T-cell activation and autoimmunity [104].
5.2. IL-2
It is confirmed that a high level of IL-2 can induce T-cell growth and differentiation and enhances NK cell activation and mediated cell death [7]. In DCM patients, elevated IL-2 levels expressed by CD4+ T lymphocytes were observed, then the cytokine stimulates macrophages to regulate the innate immune process [105]. In contrast, IL-2 plus IL-2 monoclonal antibody clone JES6-1 complexes (IL2/JES6-1) administration increased Tregs, suppressed leukocyte infiltration, including decreasing CD45(+) cells, macrophages, CD8(+) T cells, and effector memory CD8(+) in mice hearts before TAC-induced CHF, then attenuated the development of LV hypertrophy and dysfunction [106]. The injection of IL-2-activated NK cells promotes vascular remodeling and promotes cardiac repair after MI, as mentioned earlier [29].
5.3. IL-4
Recombinant protein IL-4 was used as a drug for treating MI and may activate M2 macrophage accumulation to enhance cardiac function, reduce infarction size, and repair damaged tissues [8]. Another study demonstrated that microRNA-155(−/−) mice developed attenuated viral myocarditis because of expressing increased levels of IL-4, affecting macrophage polarization to confer potential therapeutic targets for viral myocarditis [33].
5.4. IL-6
Systemically high levels of IL-6 produced by PBMC in HF patients affect NK cells through signal transduction pathways and may also affect other cells involved in heart disease, including cardiomyocytes, and possibly the function of other immune cells. In the model of LV remodeling after MI, a drug blockade of IL-6, through the administration of the anti-IL6R antibody, decreases neutrophil and macrophage infiltration in the infarct region, and the administration of anti-IL6R antibody after MI suppressed myocardial inflammation resulted in the amelioration of LV remodeling [41]. Another study has demonstrated that an IL-6 receptor antagonist can effectively attenuate the inflammatory response in patients with non-ST-elevation myocardial infarction (NSTEMI) [37]. In conclusion, all of these results show the harmful effect of IL-6 on the immune system in heart disease.
5.5. IL-8
IL-8 has been identified as a chemokine that plays a critical role in mediating neutrophil activation and recruitment, as well as promoting angiogenesis in healing infarcts [107]. IL-8 was seen to be associated with increasing circulating CD133+ progenitor cells in AMI patients, which then leads to new vessel generation and improved MI function [108]. However, recombinant canine IL-8 markedly increased the adhesion of neutrophils to CMs resulting in cytotoxicity in I/R canine myocardium [109].
5.6. IL-10
Anti-IL-10 therapy canceled the protective effect mediated by H310A1 infection by inhibiting T regulatory cell response, indicating that IL-10 is the main immunomodulatory factor in viral myocarditis [110].
5.7. IL-17
IL-17 plays an important role in the inflammation phase of heart disease. It has biological effects on neutrophil recruitment, leukocytes, and monocyte infiltration; however, there is also evidence of its activation of M2 macrophages for restoring cardiac damage.
IL-17A knockout mice significantly attenuated cardiomyocyte apoptosis and neutrophil infiltration in myocardial I/R injury, demonstrating the primary role of IL-17 in neutrophil recruitment [111]. In addition to this, IL-17 induces the accumulation of leukocytes in an inflammatory state, such as AMI, because of the adhesion molecules effect [112]. IL-17 is essential for the infiltration of Ly6C hi monocytes into inflammation sites of infarcted myocardial tissue [113]. This evidence suggests the deleterious property of IL-17 on the inflammation phase of heart disease.
On the other hand, it induces the production of GM-CSF, which could contribute to the differentiation of M1 macrophages. It also participates in the activation of M2 macrophages, which promote inflammatory healing, angiogenesis, and tissue remodeling [114]. Therefore, IL-17 plays a dual role in the inflammatory response of heart disease.
5.8. IL-18
The role of IL-18 in different types of heart disease is not consistent and is even controversial. For instance, IL-18 reduces the severity of viral myocarditis by inducing the cardiac expression of IFN-γ mRNA and increasing the activity of natural killer cells in the spleen [115]. In addition, in MI patients undergoing coronary artery bypass grafting (CABG), IL-18 in systemic circulation leads to the activation of lymphocyte cytotoxicity, then enhancing circulating lymphocyte activity [99].
5.9. IL-33
IL-33 knockout mice showed elevated Th1 cytokine expression levels and the infiltration of inflammatory cells in the myocardium of HF induced by mechanical stress [116]. In contrast to this finding, IL-33-treated mice displayed eosinophil recruitment and worsened systolic dysfunction at 7 days post-MI [117].
5.10. IL-37
As an innate immune anti-inflammatory interleukin, human recombinant IL-37 inhibits neutrophil infiltration through tail vein injection into myocardial I/R mice [76]. IL-37 has a protective effect on myocardial I/R injury by promoting Treg cell activation [118]. Overall, the therapeutic potential of IL-37 needs to be further developed and investigated.
Therefore, it is important to note that the effects of interleukins on immune cells in heart disease can vary depending on the specific type of heart disease and the stage of the disease. Further research is needed to fully understand the complex interactions between immune cells and interleukins in the pathophysiology of heart disease.
The various deleterious, protective, and dual roles of interleukin family members on primary cell types of heart disease are summarized in Figure 5.

Figure 5.
The overall effects of interleukin family members on primary cell types in heart disease. The heart comprises these major cell types: cardiomyocytes (CMs), fibroblasts (FBs), endothelial cells (ECs), and immune cells. An interleukin marked with a red circle represents the deleterious effect on a specific cell type, a green circle represents the protective effect on a specific cell type, and an orange circle represents a double-sided effect.
6. Conclusions
The inflammation process might be responsible for the initiation and progression of heart disease. Different IL families have been shown to be associated with the development of heart disease. The use of proinflammatory factors such as IL-1β antagonists or antibodies has made positive progress in heart disease early clinical trials and researchers continue to explore more effective cytokine treatment methods. However, the exact role of interleukins and their pathophysiological pathways on different cell types remain largely unknown. Additionally, several interleukins can act as both pro- and anti-inflammatory factors, and have either cardioprotective or deleterious properties, depending on the cell type.
In this review, the pathophysiological effects of interleukins including the IL-1 family (IL-1, IL-18, IL-33, and IL-37), IL-2, IL-4, the IL-6 family (IL-6 and IL-11), IL-8, IL-10, and IL-17 on primary cell types of heart disease is elucidated (Figure 1). It is concluded that determining the exact role of any particular cytokine in the pathogenesis and progression of different cell types in the heart is of significance. Targeting a specific cell type for the treatment of heart disease is likely to be more effective, and further research into IL therapy for specific cell types is needed to improve the quality of life and survival rate of patients.
Author Contributions
D.Y. and Y.L. reviewed the literature and drafted and wrote the manuscript; D.Z. and D.Y. revised the manuscript; D.Y. proposed the topic of this manuscript and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Natural Science Foundation for Young Scientists of Hubei Province, China to D.Y. (2021CFB041).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; de Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C.; et al. Heart Disease and Stroke Statistics—2017 Update: A Report From the American Heart Association. Circulation 2017, 135, e146–e603. [Google Scholar] [PubMed]
- Bartekova, M.; Radosinska, J.; Jelemensky, M.; Dhalla, N.S. Role of cytokines and inflammation in heart function during health and disease. Heart Fail. Rev. 2018, 23, 733–758. [Google Scholar] [PubMed]
- Litvinukova, M.; Talavera-Lopez, C.; Maatz, H.; Reichart, D.; Worth, C.L.; Lindberg, E.L.; Kanda, M.; Polanski, K.; Heinig, M.; Lee, M.; et al. Cells of the adult human heart. Nature 2020, 588, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Schafer, S.; Viswanathan, S.; Widjaja, A.A.; Lim, W.W.; Moreno-Moral, A.; DeLaughter, D.M.; Ng, B.; Patone, G.; Chow, K.; Khin, E.; et al. IL-11 is a crucial determinant of cardiovascular fibrosis. Nature 2017, 552, 110–115. [Google Scholar]
- Obana, M.; Maeda, M.; Takeda, K.; Hayama, A.; Mohri, T.; Yamashita, T.; Nakaoka, Y.; Komuro, I.; Takeda, K.; Matsumiya, G.; et al. Therapeutic activation of signal transducer and activator of transcription 3 by interleukin-11 ameliorates cardiac fibrosis after myocardial infarction. Circulation 2010, 121, 684–691. [Google Scholar] [CrossRef]
- Garlanda, C.; Dinarello, C.A.; Mantovani, A. The interleukin-1 family: Back to the future. Immunity 2013, 39, 1003–1018. [Google Scholar]
- Liao, W.; Lin, J.X.; Leonard, W.J. IL-2 family cytokines: New insights into the complex roles of IL-2 as a broad regulator of T helper cell differentiation. Curr. Opin. Immunol. 2011, 23, 598–604. [Google Scholar]
- Shintani, Y.; Ito, T.; Fields, L.; Shiraishi, M.; Ichihara, Y.; Sato, N.; Podaru, M.; Kainuma, S.; Tanaka, H.; Suzuki, K. IL-4 as a Repurposed Biological Drug for Myocardial Infarction through Augmentation of Reparative Cardiac Macrophages: Proof-of-Concept Data in Mice. Sci. Rep. 2017, 7, 6877. [Google Scholar] [CrossRef]
- Feng, Y.; Ye, D.; Wang, Z.; Pan, H.; Lu, X.; Wang, M.; Xu, Y.; Yu, J.; Zhang, J.; Zhao, M.; et al. The Role of Interleukin-6 Family Members in Cardiovascular Diseases. Front. Cardiovasc. Med. 2022, 9, 818890. [Google Scholar] [CrossRef]
- Apostolakis, S.; Vogiatzi, K.; Amanatidou, V.; Spandidos, D.A. Interleukin 8 and cardiovascular disease. Cardiovasc. Res. 2009, 84, 353–360. [Google Scholar] [CrossRef]
- Mora-Ruiz, M.D.; Blanco-Favela, F.; Chavez Rueda, A.K.; Legorreta-Haquet, M.V.; Chavez-Sanchez, L. Role of interleukin-17 in acute myocardial infarction. Mol. Immunol. 2019, 107, 71–78. [Google Scholar] [CrossRef]
- Saxena, A.; Russo, I.; Frangogiannis, N.G. Inflammation as a therapeutic target in myocardial infarction: Learning from past failures to meet future challenges. Transl. Res. 2016, 167, 152–166. [Google Scholar] [CrossRef] [PubMed]
- Evans, H.G.; Lewis, M.J.; Shah, A.M. Interleukin-1β modulates myocardial contraction via dexamethasone sensitive production of nitric oxide. Cardiovasc. Res. 1993, 27, 1486–1490. [Google Scholar] [CrossRef] [PubMed]
- Mauro, A.G.; Mezzaroma, E.; Torrado, J.; Kundur, P.; Joshi, P.; Stroud, K.; Quaini, F.; Lagrasta, C.A.; Abbate, A.; Toldo, S. Reduction of Myocardial Ischemia—Reperfusion Injury by Inhibiting Interleukin-1α. J. Cardiovasc. Pharmacol. 2017, 69, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Lugrin, J.; Parapanov, R.; Rosenblatt-Velin, N.; Rignault-Clerc, S.; Feihl, F.; Waeber, B.; Muller, O.; Vergely, C.; Zeller, M.; Tardivel, A.; et al. Cutting edge: IL-1α is a crucial danger signal triggering acute myocardial inflammation during myocardial infarction. J. Immunol. 2015, 194, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, G.; Colafrancesco, S.; Emmi, G.; Imazio, M.; Lopalco, G.; Maggio, M.C.; Sota, J.; Dinarello, C.A. Interleukin 1α: A comprehensive review on the role of IL-1α in the pathogenesis and treatment of autoimmune and inflammatory diseases. Autoimmun. Rev. 2021, 20, 102763. [Google Scholar] [CrossRef]
- Schreur, K.D.; Liu, S. Involvement of ceramide in inhibitory effect of IL-1β on L-type Ca2+ current in adult rat ventricular myocytes. Am. J. Physiol. 1997, 272, H2591–H2598. [Google Scholar]
- Liu, S.; Schreur, K.D. G protein-mediated suppression of L-type Ca2+ current by interleukin-1β in cultured rat ventricular myocytes. Am. J. Physiol. 1995, 268, C339–C349. [Google Scholar] [CrossRef]
- Li, Y.-H.; Rozanski, G.J. Effects of human recombinant interleukin-1 on electrical properties of guinea pig ventricular cells. Cardiovasc. Res. 1993, 27, 525–530. [Google Scholar] [CrossRef]
- Kumar, A.; Thota, V.; Dee, L.; Olson, J.; Uretz, E.; Parrillo, J.E. Tumor necrosis factor α and interleukin 1β are responsible for in vitro myocardial cell depression induced by human septic shock serum. J. Exp. Med. 1996, 183, 949–958. [Google Scholar] [CrossRef]
- Gulick, T.; Chung, M.K.; Pieper, S.J.; Lange, L.G.; Schreiner, G.F. Interleukin 1 and tumor necrosis factor inhibit cardiac myocyte β-adrenergic responsiveness. Proc. Natl. Acad. Sci. USA 1989, 86, 6753–6757. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.J.; Zhou, W.; Kennedy, R.H. Suppression of β-adrenergic responsiveness of L-type Ca2+ current by IL-1β in rat ventricular myocytes. Am. J. Physiol. 1999, 276, H141–H148. [Google Scholar] [CrossRef] [PubMed]
- Combes, A.; Frye, C.S.; Lemster, B.H.; Brooks, S.S.; Watkins, S.C.; Feldman, A.M.; McTiernan, C.F. Chronic exposure to interleukin 1β induces a delayed and reversible alteration in excitation-contraction coupling of cultured cardiomyocytes. Pflügers Arch. 2002, 445, 246–256. [Google Scholar] [CrossRef]
- Ing, D.J.; Zang, J.; Dzau, V.J.; Webster, K.A.; Bishopric, N.H. Modulation of Cytokine-Induced Cardiac Myocyte Apoptosis by Nitric Oxide, Bak, and Bcl-X. Circ. Res. 1999, 84, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Weisensee, D.; Bereiter-Hahn, J.; Schoeppe, W.; Löw-Friedrich, I. Effects of cytokines on the contractility of cultured cardiac myocytes. Int. J. Immunopharmacol. 1993, 15, 581–587. [Google Scholar] [CrossRef]
- Eisner, R.M.; Husain, A.; Clark, J.I. Case report and brief review: IL-2-induced myocarditis. Cancer Investig. 2004, 22, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.M.; Xia, Q.; Bruce, I.C.; Shen, Y.L.; Ye, Z.G.; Lin, G.H.; Chen, J.Z.; Li, G.R. Influence of interleukin-2 on Ca2+ handling in rat ventricular myocytes. J. Mol. Cell. Cardiol. 2003, 35, 1491–1503. [Google Scholar] [CrossRef]
- Marriott, J.; Goldman, J.H.; Keeling, P.J.; Baig, M.K.; Dalgleish, A.; McKenna, W. Abnormal cytokine profiles in patients with idiopathic dilated cardiomyopathy and their asymptomatic relatives. Heart 1996, 75, 287–290. [Google Scholar] [CrossRef]
- Bouchentouf, M.; Williams, P.; Forner, K.A.; Cuerquis, J.; Michaud, V.; Paradis, P.; Schiffrin, E.L.; Galipeau, J. Interleukin-2 enhances angiogenesis and preserves cardiac function following myocardial infarction. Cytokine 2011, 56, 732–738. [Google Scholar] [CrossRef]
- Koch, M.; Savvatis, K.; Scheeler, M.; Dhayat, S.; Bonaventura, K.; Pohl, T.; Riad, A.; Bulfone-Paus, S.; Schultheiss, H.P.; Tschope, C. Immunosuppression with an interleukin-2 fusion protein leads to improved LV function in experimental ischemic cardiomyopathy. Int. Immunopharmacol. 2010, 10, 207–212. [Google Scholar] [CrossRef]
- Cao, C.M.; Xia, Q.; Tu, J.; Chen, M.; Wu, S.; Wong, T.M. Cardioprotection of interleukin-2 is mediated via kappa-opioid receptors. J. Pharmacol. Exp. Ther. 2004, 309, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Wan, F.; Yan, K.; Xu, D.; Qian, Q.; Liu, H.; Li, M.; Xu, W. Vγ1(+)γδT, early cardiac infiltrated innate population dominantly producing IL-4, protect mice against CVB3 myocarditis by modulating IFNγ(+) T response. Mol. Immunol. 2017, 81, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, M.; Li, X.; Tang, Z.; Wang, X.; Zhong, M.; Suo, Q.; Zhang, Y.; Lv, K. Silencing MicroRNA-155 Attenuates Cardiac Injury and Dysfunction in Viral Myocarditis via Promotion of M2 Phenotype Polarization of Macrophages. Sci. Rep. 2016, 6, 22613. [Google Scholar] [CrossRef]
- Diny, N.L.; Baldeviano, G.C.; Talor, M.V.; Barin, J.G.; Ong, S.; Bedja, D.; Hays, A.G.; Gilotra, N.A.; Coppens, I.; Rose, N.R.; et al. Eosinophil-derived IL-4 drives progression of myocarditis to inflammatory dilated cardiomyopathy. J. Exp. Med. 2017, 214, 943–957. [Google Scholar] [CrossRef]
- Held, C.; White, H.D.; Stewart, R.A.H.; Budaj, A.; Cannon, C.P.; Hochman, J.S.; Koenig, W.; Siegbahn, A.; Steg, P.G.; Soffer, J.; et al. Inflammatory Biomarkers Interleukin-6 and C-Reactive Protein and Outcomes in Stable Coronary Heart Disease: Experiences from the Stability (Stabilization of Atherosclerotic Plaque by Initiation of Darapladib Therapy) Trial. J. Am. Heart Assoc. 2017, 214, 943–957. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.R.; Poterucha, J.T.; Mikuls, T.R.; Duryee, M.J.; Garvin, R.P.; Klassen, L.W.; Shurmur, S.W.; Thiele, G.M. IL-6 and its receptors in coronary artery disease and acute myocardial infarction. Cytokine 2013, 62, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Kleveland, O.; Kunszt, G.; Bratlie, M.; Ueland, T.; Broch, K.; Holte, E.; Michelsen, A.E.; Bendz, B.; Amundsen, B.H.; Espevik, T.; et al. Effect of a single dose of the interleukin-6 receptor antagonist tocilizumab on inflammation and troponin T release in patients with non-ST-elevation myocardial infarction: A double-blind, randomized, placebo-controlled phase 2 trial. Eur. Heart J. 2016, 37, 2406–2413. [Google Scholar] [CrossRef]
- Sugishita, K.; Kinugawa K-i Shimizu, T.; Harada, K.; Matsui, H.; Takahashi, T.; Serizawa, T.; Kohmoto, O. Cellular basis for the acute inhibitory effects of IL-6 and TNF-α on excitation-contraction coupling. J. Mol. Cell. Cardiol. 1999, 31, 1457–1467. [Google Scholar] [CrossRef] [PubMed]
- Omiya, S.; Omori, Y.; Taneike, M.; Murakawa, T.; Ito, J.; Tanada, Y.; Nishida, K.; Yamaguchi, O.; Satoh, T.; Shah, A.M. Cytokine mRNA degradation in cardiomyocytes restrains sterile inflammation in pressure-overloaded hearts. Circulation 2020, 141, 667–677. [Google Scholar] [CrossRef]
- Zhao, L.; Cheng, G.; Jin, R.; Afzal, M.R.; Samanta, A.; Xuan, Y.T.; Girgis, M.; Elias, H.K.; Zhu, Y.; Davani, A.; et al. Deletion of Interleukin-6 Attenuates Pressure Overload-Induced Left Ventricular Hypertrophy and Dysfunction. Circ. Res. 2016, 118, 1918–1929. [Google Scholar] [CrossRef]
- Kobara, M.; Noda, K.; Kitamura, M.; Okamoto, A.; Shiraishi, T.; Toba, H.; Matsubara, H.; Nakata, T. Antibody against interleukin-6 receptor attenuates left ventricular remodelling after myocardial infarction in mice. Cardiovasc. Res. 2010, 87, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Roig, E.; Orús, J.; Paré, C.; Azqueta, M.; Filella, X.; Perez-Villa, F.; Heras, M.; Sanz, G. Serum interleukin-6 in congestive heart failure secondary to idiopathic dilated cardiomyopathy. Am. J. Cardiol. 1998, 82, 688–690. [Google Scholar] [CrossRef] [PubMed]
- Kinugawa, K.-I.; Takahashi, T.; Kohmoto, O.; Yao, A.; Aoyagi, T.; Momomura S-i Hirata, Y.; Serizawa, T. Nitric oxide-mediated effects of interleukin-6 on [Ca2+] i and cell contraction in cultured chick ventricular myocytes. Circ. Res. 1994, 75, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Melendez, G.C.; McLarty, J.L.; Levick, S.P.; Du, Y.; Janicki, J.S.; Brower, G.L. Interleukin 6 mediates myocardial fibrosis, concentric hypertrophy, diastolic dysfunction in rats. Hypertension 2010, 56, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Sano, M.; Fukuda, K.; Kodama, H.; Pan, J.; Saito, M.; Matsuzaki, J.; Takahashi, T.; Makino, S.; Kato, T.; Ogawa, S. Interleukin-6 family of cytokines mediate angiotensin II-induced cardiac hypertrophy in rodent cardiomyocytes. J. Biol. Chem. 2000, 275, 29717–29723. [Google Scholar] [CrossRef]
- Kunisada, K.; Tone, E.; Fujio, Y.; Matsui, H.; Yamauchi-Takihara, K.; Kishimoto, T. Activation of gp130 transduces hypertrophic signals via STAT3 in cardiac myocytes. Circulation 1998, 98, 346–352. [Google Scholar] [CrossRef]
- Smart, N.; Mojet, M.H.; Latchman, D.S.; Marber, M.S.; Duchen, M.R.; Heads, R.J. IL-6 induces PI 3-kinase and nitric oxide-dependent protection and preserves mitochondrial function in cardiomyocytes. Cardiovasc. Res. 2006, 69, 164–177. [Google Scholar] [CrossRef]
- Fahmi, A.; Smart, N.; Punn, A.; Jabr, R.; Marber, M.; Heads, R. p42/p44-MAPK and PI3K are sufficient for IL-6 family cytokines/gp130 to signal to hypertrophy and survival in cardiomyocytes in the absence of JAK/STAT activation. Cell Signal. 2013, 25, 898–909. [Google Scholar] [CrossRef]
- Abe, Y.; Kawakami, M.; Kuroki, M.; Yamamoto, T.; Fujii, M.; Kobayashi, H.; Yaginuma, T.; Kashii, A.; Saito, M.; Matsushima, K. Transient rise in serum interleukin-8 concentration during acute myocardial infarction. Heart 1993, 70, 132–134. [Google Scholar] [CrossRef]
- Boyle, E.M., Jr.; Kovacich, J.C.; Hèbert, C.A.; Canty, T.G., Jr.; Chi, E.; Morgan, E.N.; Pohlman, T.H.; Verrier, E.D. Inhibition of interleukin-8 blocks myocardial ischemia-reperfusion injury. J. Thorac. Cardiovasc. Surg. 1998, 116, 114–121. [Google Scholar] [CrossRef]
- Husebye, T.; Eritsland, J.; Arnesen, H.; Bjornerheim, R.; Mangschau, A.; Seljeflot, I.; Andersen, G.O. Association of interleukin 8 and myocardial recovery in patients with ST-elevation myocardial infarction complicated by acute heart failure. PLoS ONE 2014, 9, e112359. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, S.; Sharma, A.K.; Singla, D.K.; Singal, P.K. p38 and ERK1/2 MAPKs mediate the interplay of TNFα and IL-10 in regulating oxidative stress and cardiac myocyte apoptosis. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H3524–H3531. [Google Scholar] [CrossRef]
- Dhingra, S.; Bagchi, A.K.; Ludke, A.L.; Sharma, A.K.; Singal, P.K. Akt regulates IL-10 mediated suppression of TNFα-induced cardiomyocyte apoptosis by upregulating Stat3 phosphorylation. PLoS ONE 2011, 6, e25009. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, A.K.; Sharma, A.; Dhingra, S.; Lehenbauer Ludke, A.R.; Al-Shudiefat, A.A.; Singal, P.K. Interleukin-10 activates Toll-like receptor 4 and requires MyD88 for cardiomyocyte survival. Cytokine 2013, 61, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.K.; Krishnamurthy, P.; Barefield, D.; Singh, N.; Gupta, R.; Lambers, E.; Thal, M.; Mackie, A.; Hoxha, E.; Ramirez, V.; et al. Interleukin-10 treatment attenuates pressure overload-induced hypertrophic remodeling and improves heart function via signal transducers and activators of transcription 3-dependent inhibition of nuclear factor-kappaB. Circulation 2012, 126, 418–429. [Google Scholar] [CrossRef]
- Kesherwani, V.; Chavali, V.; Hackfort, B.T.; Tyagi, S.C.; Mishra, P.K. Exercise ameliorates high fat diet induced cardiac dysfunction by increasing interleukin 10. Front. Physiol. 2015, 6, 124. [Google Scholar] [CrossRef]
- Kimura, R.; Maeda, M.; Arita, A.; Oshima, Y.; Obana, M.; Ito, T.; Yamamoto, Y.; Mohri, T.; Kishimoto, T.; Kawase, I.; et al. Identification of cardiac myocytes as the target of interleukin 11, a cardioprotective cytokine. Cytokine 2007, 38, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Obana, M.; Miyamoto, K.; Murasawa, S.; Iwakura, T.; Hayama, A.; Yamashita, T.; Shiragaki, M.; Kumagai, S.; Miyawaki, A.; Takewaki, K.; et al. Therapeutic administration of IL-11 exhibits the postconditioning effects against ischemia-reperfusion injury via STAT3 in the heart. Am. J. Physiol. Heart Circ. Physiol. 2012, 303, H569–H577. [Google Scholar] [CrossRef]
- Su, S.A.; Yang, D.; Zhu, W.; Cai, Z.; Zhang, N.; Zhao, L.; Wang, J.A.; Xiang, M. Interleukin-17A mediates cardiomyocyte apoptosis through Stat3-iNOS pathway. Biochim. Biophys. Acta 2016, 1863, 2784–2794. [Google Scholar] [CrossRef]
- Zhou, S.F.; Yuan, J.; Liao, M.Y.; Xia, N.; Tang, T.T.; Li, J.J.; Jiao, J.; Dong, W.Y.; Nie, S.F.; Zhu, Z.F.; et al. IL-17A promotes ventricular remodeling after myocardial infarction. J. Mol. Med. 2014, 92, 1105–1116. [Google Scholar] [CrossRef]
- Xue, G.L.; Li, D.S.; Wang, Z.Y.; Liu, Y.; Yang, J.M.; Li, C.Z.; Li, X.D.; Ma, J.D.; Zhang, M.M.; Lu, Y.J.; et al. Interleukin-17 upregulation participates in the pathogenesis of heart failure in mice via NF-kappaB-dependent suppression of SERCA2a and Cav1.2 expression. Acta Pharmacol. Sin. 2021, 42, 1780–1789. [Google Scholar] [CrossRef] [PubMed]
- Reidar Woldbaek, P. Increased cardiac IL-18 mRNA, pro-IL-18 and plasma IL-18 after myocardial infarction in the mouse: A potential role in cardiac dysfunction. Cardiovasc. Res. 2003, 59, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka-Tojo, M.; Tojo, T.; Inomata, T.; Machida, Y.; Osada, K.; Izumi, T. Circulating levels of interleukin 18 reflect etiologies of heart failure: Th1/Th2 cytokine imbalance exaggerates the pathophysiology of advanced heart failure. J. Card. Fail. 2002, 8, 21–27. [Google Scholar] [CrossRef]
- Gluck, B.; Schmidtke, M.; Merkle, I.; Stelzner, A.; Gemsa, D. Persistent expression of cytokines in the chronic stage of CVB3-induced myocarditis in NMRI mice. J. Mol. Cell. Cardiol. 2001, 33, 1615–1626. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, A.; Kanda, T.; Tanaka, T.; Yokoyama, T.; Kurimoto, M.; Tamura, J.I.; Kobayashi, I. Interleukin-18 reduces expression of cardiac tumor necrosis factor-α and atrial natriuretic peptide in a murine model of viral myocarditis. Life Sci. 2002, 70, 1225–1234. [Google Scholar] [CrossRef] [PubMed]
- Pomerantz, B.J.; Reznikov, L.L.; Harken, A.H.; Dinarello, C.A. Inhibition of caspase 1 reduces human myocardial ischemic dysfunction via inhibition of IL-18 and IL-1β. Proc. Natl. Acad. Sci. USA 2001, 98, 2871–2876. [Google Scholar] [CrossRef]
- Chandrasekar, B.; Mummidi, S.; Claycomb, W.C.; Mestril, R.; Nemer, M. Interleukin-18 is a pro-hypertrophic cytokine that acts through a phosphatidylinositol 3-kinase-phosphoinositide-dependent kinase-1-Akt-GATA4 signaling pathway in cardiomyocytes. J. Biol. Chem. 2005, 280, 4553–4567. [Google Scholar] [CrossRef]
- Woldbaek, P.R.; Sande, J.B.; Stromme, T.A.; Lunde, P.K.; Djurovic, S.; Lyberg, T.; Christensen, G.; Tonnessen, T. Daily administration of interleukin-18 causes myocardial dysfunction in healthy mice. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H708–H714. [Google Scholar] [CrossRef] [PubMed]
- Seta, Y.; Kanda, T.; Tanaka, T.; Arai, M.; Sekiguchi, K.; Yokoyama, T.; Kurimoto, M.; Tamura, J.I.; Kurabayashi, M. Interleukin 18 in acute myocardial infarction. Heart 2000, 84, 668–669. [Google Scholar] [CrossRef]
- Westphal, E.; Rohrbach, S.; Buerke, M.; Behr, H.; Darmer, D.; Silber, R.E.; Werdan, K.; Loppnow, H. Altered interleukin-1 receptor antagonist and interleukin-18 mRNA expression in myocardial tissues of patients with dilatated cardiomyopathy. Mol. Med. 2008, 14, 55–63. [Google Scholar] [CrossRef]
- Chen, W.Y.; Hong, J.; Gannon, J.; Kakkar, R.; Lee, R.T. Myocardial pressure overload induces systemic inflammation through endothelial cell IL-33. Proc. Natl. Acad. Sci. USA 2015, 112, 7249–7254. [Google Scholar] [CrossRef]
- Sanada, S.; Hakuno, D.; Higgins, L.J.; Schreiter, E.R.; McKenzie, A.N.; Lee, R.T. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J. Clin. Investig. 2007, 117, 1538–1549. [Google Scholar] [CrossRef]
- Nold, M.F.; Nold-Petry, C.A.; Zepp, J.A.; Palmer, B.E.; Bufler, P.; Dinarello, C.A. IL-37 is a fundamental inhibitor of innate immunity. Nat. Immunol. 2010, 11, 1014–1022. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Zeng, Q.; Huang, Y.; Shi, Y.; Lin, Y.; Lu, Z.; Meng, K.; Wu, B.; Yu, K.; Chai, M.; et al. Elevated plasma IL-37, IL-18, IL-18BP concentrations in patients with acute coronary syndrome. Mediat. Inflamm. 2014, 2014, 165742. [Google Scholar] [CrossRef]
- Zhuang, X.; Wu, B.; Li, J.; Shi, H.; Jin, B.; Luo, X. The emerging role of interleukin-37 in cardiovascular diseases. Immun. Inflamm. Dis. 2017, 5, 373–379. [Google Scholar] [CrossRef]
- Wu, B.; Meng, K.; Ji, Q.; Cheng, M.; Yu, K.; Zhao, X.; Tony, H.; Liu, Y.; Zhou, Y.; Chang, C.; et al. Interleukin-37 ameliorates myocardial ischaemia/reperfusion injury in mice. Clin. Exp. Immunol. 2014, 176, 438–451. [Google Scholar] [CrossRef]
- Zhu, R.; Sun, H.; Yu, K.; Zhong, Y.; Shi, H.; Wei, Y.; Su, X.; Xu, W.; Luo, Q.; Zhang, F.; et al. Interleukin-37 and Dendritic Cells Treated with Interleukin-37 Plus Troponin I Ameliorate Cardiac Remodeling After Myocardial Infarction. J. Am. Heart Assoc. 2014, 176, 438–451. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.; Chen, W.; Su, Y.; Rai, V.; Uche, O.U.; Li, N.; Frangogiannis, N.G. IL-1 induces proinflammatory leukocyte infiltration and regulates fibroblast phenotype in the infarcted myocardium. J. Immunol. 2013, 191, 4838–4848. [Google Scholar] [CrossRef] [PubMed]
- Bageghni, S.A.; Hemmings, K.E.; Yuldasheva, N.Y.; Maqbool, A.; Gamboa-Esteves, F.O.; Humphreys, N.E.; Jackson, M.S.; Denton, C.P.; Francis, S.; Porter, K.E.; et al. Fibroblast-specific deletion of interleukin-1 receptor-1 reduces adverse cardiac remodeling following myocardial infarction. JCI Insight 2013, 191, 4838–4848. [Google Scholar]
- Roselló-Lletí, E.; Rivera, M.; Bertomeu, V.; Cortés, R.; Jordán, A.; González-Molina, A. Interleukin-4 and Cardiac Fibrosis in Patients with Heart Failure. Rev. Española Cardiol. Engl. Ed. 2007, 60, 777–780. [Google Scholar] [CrossRef]
- Peng, H.; Sarwar, Z.; Yang, X.P.; Peterson, E.L.; Xu, J.; Janic, B.; Rhaleb, N.; Carretero, O.A.; Rhaleb, N.E. Profibrotic Role for Interleukin-4 in Cardiac Remodeling and Dysfunction. Hypertension 2015, 66, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.H.; Hung, C.S.; Liao, C.W.; Wei, L.H.; Chen, C.W.; Shun, C.T.; Wen, W.F.; Wan, C.H.; Wu, X.M.; Chang, Y.Y.; et al. IL-6 trans-signalling contributes to aldosterone-induced cardiac fibrosis. Cardiovasc. Res. 2018, 114, 690–702. [Google Scholar] [CrossRef]
- Mir, S.A.; Chatterjee, A.; Mitra, A.; Pathak, K.; Mahata, S.K.; Sarkar, S. Inhibition of signal transducer and activator of transcription 3 (STAT3) attenuates interleukin-6 (IL-6)-induced collagen synthesis and resultant hypertrophy in rat heart. J. Biol. Chem. 2012, 287, 2666–2677. [Google Scholar] [CrossRef] [PubMed]
- Leicht, M.; Briest, W.; Zimmer, H.-G. Regulation of norepinephrine-induced proliferation in cardiac fibroblasts by interleukin-6 and p42/p44 mitogen activated protein kinase. Mol. Cell. Biochem. 2003, 243, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Mayfield, A.E.; Kanda, P.; Nantsios, A.; Parent, S.; Mount, S.; Dixit, S.; Ye, B.; Seymour, R.; Stewart, D.J.; Davis, D.R. Interleukin-6 Mediates Post-Infarct Repair by Cardiac Explant-Derived Stem Cells. Theranostics 2017, 7, 4850–4861. [Google Scholar] [CrossRef]
- Fontes, J.A.; Rose, N.R.; Cihakova, D. The varying faces of IL-6: From cardiac protection to cardiac failure. Cytokine 2015, 74, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, P.; Lambers, E.; Verma, S.; Thorne, T.; Qin, G.; Losordo, D.W.; Kishore, R. Myocardial knockdown of mRNA-stabilizing protein HuR attenuates post-MI inflammatory response and left ventricular dysfunction in IL-10-null mice. FASEB J. 2010, 24, 2484–2494. [Google Scholar] [CrossRef]
- Krishnamurthy, P.; Rajasingh, J.; Lambers, E.; Qin, G.; Losordo, D.W.; Kishore, R. IL-10 inhibits inflammation and attenuates left ventricular remodeling after myocardial infarction via activation of STAT3 and suppression of HuR. Circ. Res. 2009, 104, e9–e18. [Google Scholar] [CrossRef]
- Sun, K.; Li, Y.Y.; Jin, J. A double-edged sword of immuno-microenvironment in cardiac homeostasis and injury repair. Signal. Transduct. Target. Ther. 2021, 6, 79. [Google Scholar] [CrossRef]
- Yu, Q.; Vazquez, R.; Khojeini, E.V.; Patel, C.; Venkataramani, R.; Larson, D.F. IL-18 induction of osteopontin mediates cardiac fibrosis and diastolic dysfunction in mice. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H76–H85. [Google Scholar] [CrossRef]
- Reddy, V.S.; Harskamp, R.E.; van Ginkel, M.W.; Calhoon, J.; Baisden, C.E.; Kim, I.S.; Valente, A.J.; Chandrasekar, B. Interleukin-18 stimulates fibronectin expression in primary human cardiac fibroblasts via PI3K-Akt-dependent NF-kappaB activation. J. Cell. Physiol. 2008, 215, 697–707. [Google Scholar] [CrossRef]
- Fix, C.; Bingham, K.; Carver, W. Effects of interleukin-18 on cardiac fibroblast function and gene expression. Cytokine 2011, 53, 19–28. [Google Scholar] [CrossRef]
- Clinton, S.K.; Underwood, R.; Hayes, L.; Sherman, M.L.; Kufe, D.W.; Libby, P. Macrophage colony-stimulating factor gene expression in vascular cells and in experimental and human atherosclerosis. Am. J. Pathol. 1992, 140, 301–316. [Google Scholar] [PubMed]
- Haleagrahara, N.; Chakravarthi, S.; Mathews, L. Insulin like growth factor-1 (IGF-1) causes overproduction of IL-8, an angiogenic cytokine and stimulates neovascularization in isoproterenol-induced myocardial infarction in rats. Int. J. Mol. Sci. 2011, 12, 8562–8574. [Google Scholar] [CrossRef] [PubMed]
- Wojakowski, W.; Ratajczak, M.Z.; Tendera, M. Interleukin-8: More on the mechanisms of progenitor cells mobilization in acute coronary syndromes. Eur. Heart J. 2006, 27, 1013–1015. [Google Scholar] [CrossRef]
- Yue, Y.; Wang, C.; Benedict, C.; Huang, G.; Truongcao, M.; Roy, R.; Cimini, M.; Garikipati, V.N.S.; Cheng, Z.; Koch, W.J.; et al. Interleukin-10 Deficiency Alters Endothelial Progenitor Cell-Derived Exosome Reparative Effect on Myocardial Repair via Integrin-Linked Kinase Enrichment. Circ. Res. 2020, 126, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Krstic, J.; Jaukovic, A.; Mojsilovic, S.; Ethordevic, I.O.; Trivanovic, D.; Ilic, V.; Santibanez, J.F.; Bugarski, D. In vitro effects of IL-17 on angiogenic properties of endothelial cells in relation to oxygen levels. Cell Biol. Int. 2013, 37, 1162–1170. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Zhang, S.; Zhuang, Y.; Zhang, H.; Bai, J.; Hou, Q. Interleukin-17 Stimulates STAT3-Mediated Endothelial Cell Activation for Neutrophil Recruitment. Cell Physiol. Biochem. 2015, 36, 2340–2356. [Google Scholar] [CrossRef]
- Sokolic, J.; Tokmadzic, V.S.; Knezevic, D.; Medved, I.; Vukelic Damjani, N.; Balen, S.; Rakic, M.; Lanca Bastiancic, A.; Laskarin, G. Endothelial dysfunction mediated by interleukin-18 in patients with ischemic heart disease undergoing coronary artery bypass grafting surgery. Med. Hypotheses 2017, 104, 20–24. [Google Scholar]
- Zhao, G.; Zhang, H.; Zhu, S.; Wang, S.; Zhu, K.; Zhao, Y.; Xu, L.; Zhang, P.; Xie, J.; Sun, A.; et al. Interleukin-18 accelerates cardiac inflammation and dysfunction during ischemia/reperfusion injury by transcriptional activation of CXCL16. Cell Signal. 2021, 87, 110141. [Google Scholar] [CrossRef]
- Choi, Y.S.; Choi, H.J.; Min, J.K.; Pyun, B.J.; Maeng, Y.S.; Park, H.; Kim, J.; Kim, Y.M.; Kwon, Y.G. Interleukin-33 induces angiogenesis and vascular permeability through ST2/TRAF6-mediated endothelial nitric oxide production. Blood 2009, 114, 3117–3126. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, M.; Shintani, Y.; Shintani, Y.; Ishida, H.; Saba, R.; Yamaguchi, A.; Adachi, H.; Yashiro, K.; Suzuki, K. Alternatively activated macrophages determine repair of the infarcted adult murine heart. J. Clin. Investig. 2016, 126, 2151–2166. [Google Scholar] [CrossRef] [PubMed]
- Sager, H.B.; Heidt, T.; Hulsmans, M.; Dutta, P.; Courties, G.; Sebas, M.; Wojtkiewicz, G.R.; Tricot, B.; Iwamoto, Y.; Sun, Y.; et al. Targeting Interleukin-1β Reduces Leukocyte Production After Acute Myocardial Infarction. Circulation 2015, 132, 1880–1890. [Google Scholar] [CrossRef]
- Eriksson, U.; Kurrer, M.O.; Sonderegger, I.; Iezzi, G.; Tafuri, A.; Hunziker, L.; Suzuki, S.; Bachmaier, K.; Bingisser, R.M.; Penninger, J.M.; et al. Activation of dendritic cells through the interleukin 1 receptor 1 is critical for the induction of autoimmune myocarditis. J. Exp. Med. 2003, 197, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Kuethe, F.; Braun, R.K.; Foerster, M.; Schlenker, Y.; Sigusch, H.H.; Kroegel, C.; Figulla, H.R. Immunopathogenesis of dilated cardiomyopathy. Evidence for the role of TH2-type CD4+T lymphocytes and association with myocardial HLA-DR expression. J. Clin. Immunol. 2006, 26, 33–39. [Google Scholar] [CrossRef]
- Wang, H.; Hou, L.; Kwak, D.; Fassett, J.; Xu, X.; Chen, A.; Chen, W.; Blazar, B.R.; Xu, Y.; Hall, J.L.; et al. Increasing Regulatory T Cells with Interleukin-2 and Interleukin-2 Antibody Complexes Attenuates Lung Inflammation and Heart Failure Progression. Hypertension 2016, 68, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G.; Entman, M.L. Chemokines in myocardial ischemia. Trends Cardiovasc. Med. 2005, 15, 163–169. [Google Scholar] [CrossRef]
- Schomig, K.; Busch, G.; Steppich, B.; Sepp, D.; Kaufmann, J.; Stein, A.; Schomig, A.; Ott, I. Interleukin-8 is associated with circulating CD133+ progenitor cells in acute myocardial infarction. Eur. Heart J. 2006, 27, 1032–1037. [Google Scholar] [CrossRef]
- Kukielka, G.L.; Smith, C.W.; LaRosa, G.J.; Manning, A.M.; Mendoza, L.H.; Daly, T.J.; Hughes, B.J.; Youker, K.A.; Hawkins, H.K.; Michael, L.H.; et al. Interleukin-8 gene induction in the myocardium after ischemia and reperfusion in vivo. J. Clin. Investig. 1995, 95, 89–103. [Google Scholar] [CrossRef]
- Huber, S.A.; Feldman, A.M.; Sartini, D. Coxsackievirus B3 induces T regulatory cells, which inhibit cardiomyopathy in tumor necrosis factor-α transgenic mice. Circ. Res. 2006, 99, 1109–1116. [Google Scholar] [CrossRef]
- Liao, Y.H.; Xia, N.; Zhou, S.F.; Tang, T.T.; Yan, X.X.; Lv, B.J.; Nie, S.F.; Wang, J.; Iwakura, Y.; Xiao, H.; et al. Interleukin-17A contributes to myocardial ischemia/reperfusion injury by regulating cardiomyocyte apoptosis and neutrophil infiltration. J. Am. Coll. Cardiol. 2012, 59, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Carreau, A.; Kieda, C.; Grillon, C. Nitric oxide modulates the expression of endothelial cell adhesion molecules involved in angiogenesis and leukocyte recruitment. Exp. Cell Res. 2011, 317, 29–41. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. The inflammatory response in myocardial injury, repair, and remodelling. Nat. Rev. Cardiol. 2014, 11, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Isailovic, N.; Daigo, K.; Mantovani, A.; Selmi, C. Interleukin-17 and innate immunity in infections and chronic inflammation. J. Autoimmun. 2015, 60, 1–11. [Google Scholar] [CrossRef]
- Kanda, T.; Tanaka, T.; Sekiguchi, K.; Seta, Y.; Kurimoto, M.; Wilson McManus, J.E.; Nagai, R.; Yang, D.; McManus, B.M.; Kobayashi, I. Effect of interleukin-18 on viral myocarditis: Enhancement of interferon- gamma and natural killer cell activity. J. Mol. Cell. Cardiol. 2000, 32, 2163–2171. [Google Scholar] [CrossRef]
- Veeraveedu, P.T.; Sanada, S.; Okuda, K.; Fu, H.Y.; Matsuzaki, T.; Araki, R.; Yamato, M.; Yasuda, K.; Sakata, Y.; Yoshimoto, T.; et al. Ablation of IL-33 gene exacerbate myocardial remodeling in mice with heart failure induced by mechanical stress. Biochem. Pharmacol. 2017, 138, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Ghali, R.; Habeichi, N.J.; Kaplan, A.; Tannous, C.; Abidi, E.; Bekdash, A.; Farhat, R.; Itani, H.; Jurjus, A.; Booz, G.W.; et al. IL-33 induces type-2-cytokine phenotype but exacerbates cardiac remodeling post-myocardial infarction with eosinophil recruitment, worsened systolic dysfunction, and ventricular wall rupture. Clin. Sci. 2020, 134, 1191–1218. [Google Scholar] [CrossRef]
- Dutta, D.; Barr, V.A.; Akpan, I.; Mittelstadt, P.R.; Singha, L.I.; Samelson, L.E.; Ashwell, J.D. Recruitment of calcineurin to the TCR positively regulates T cell activation. Nat. Immunol. 2017, 18, 196–204. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).