Kappa-Carrageenan/Chitosan/Gelatin Scaffolds Provide a Biomimetic Microenvironment for Dentin-Pulp Regeneration
Abstract
1. Introduction
2. Results
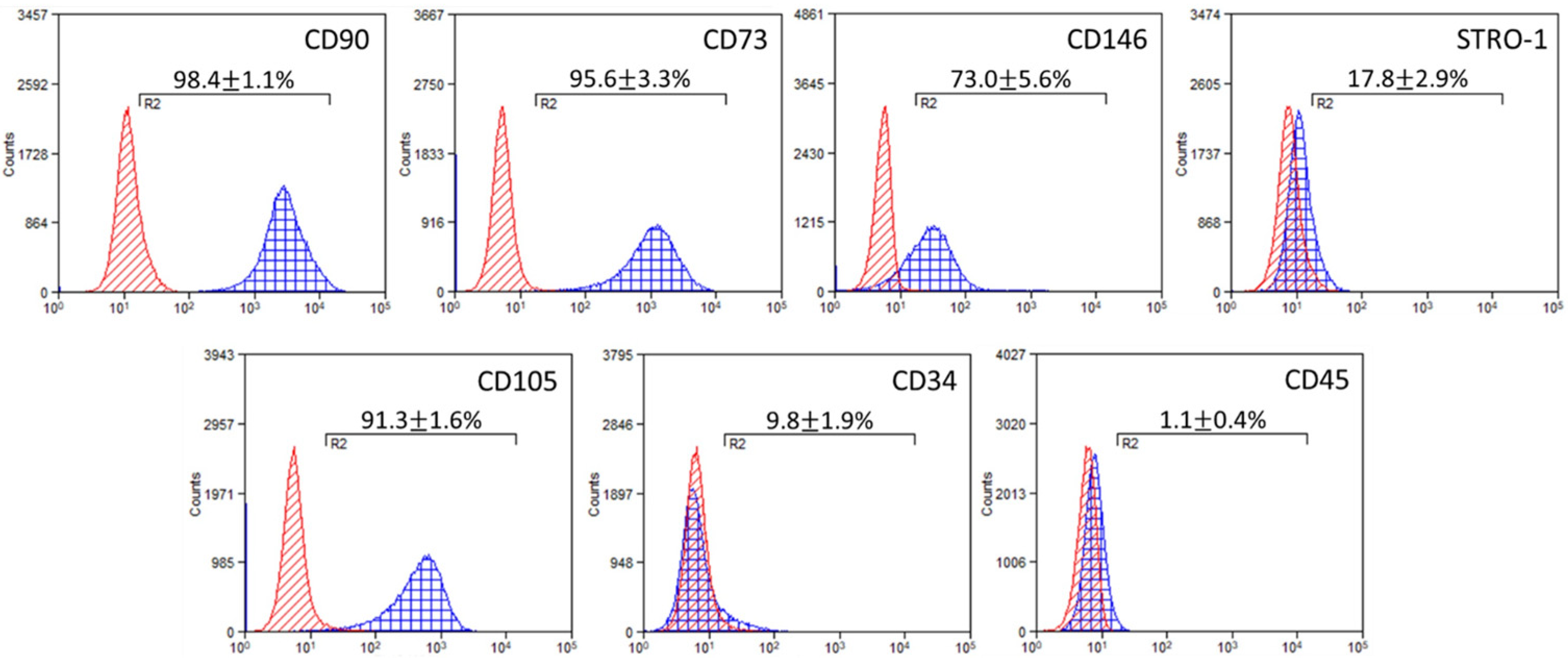
2.1. Immunophenotypic Characterization of DPSC Cultures
2.2. DPSCs Contribution to the Mechanical Integrity of the Scaffolds
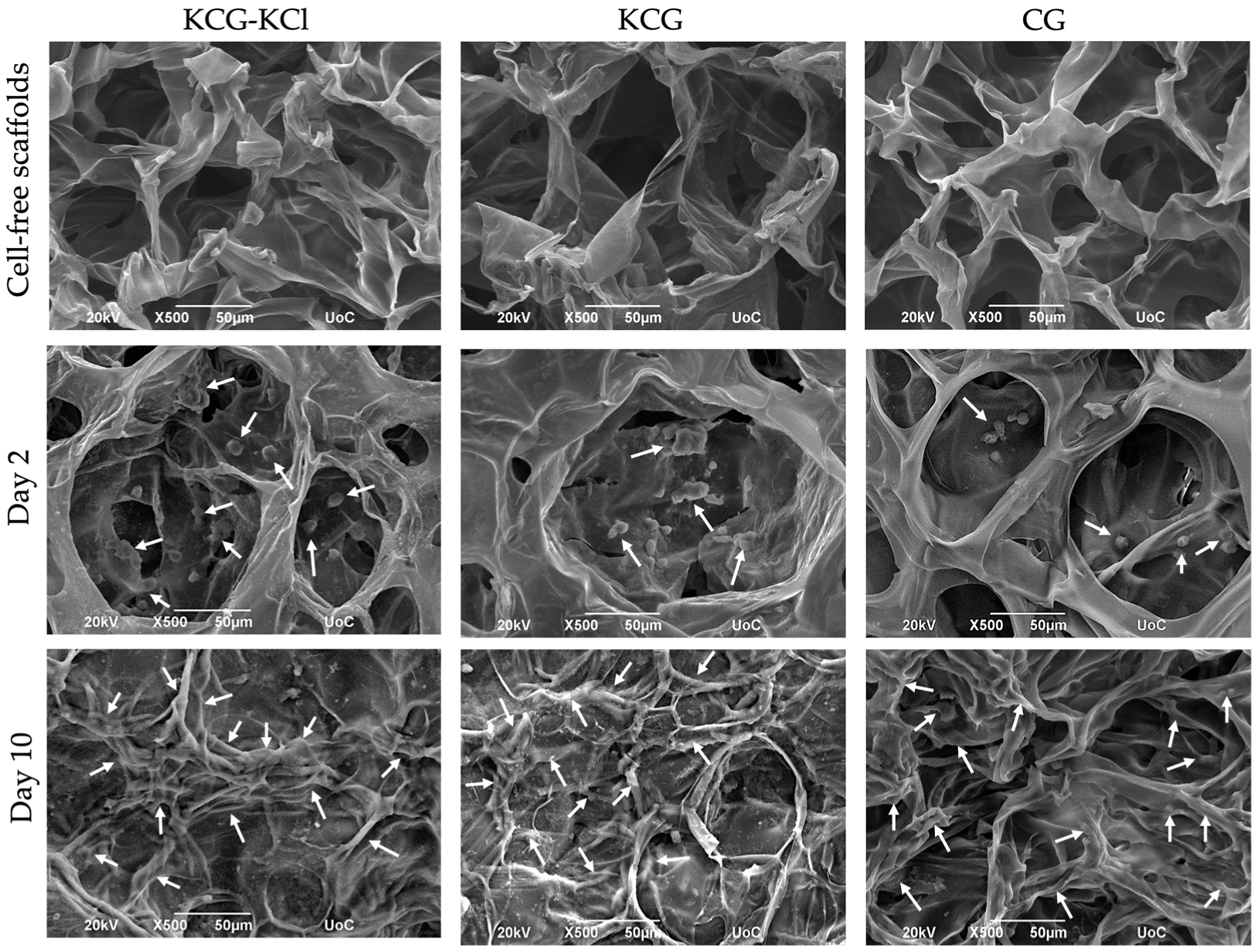
2.3. SEM Analysis of DPSCs Morphology and Adhesion
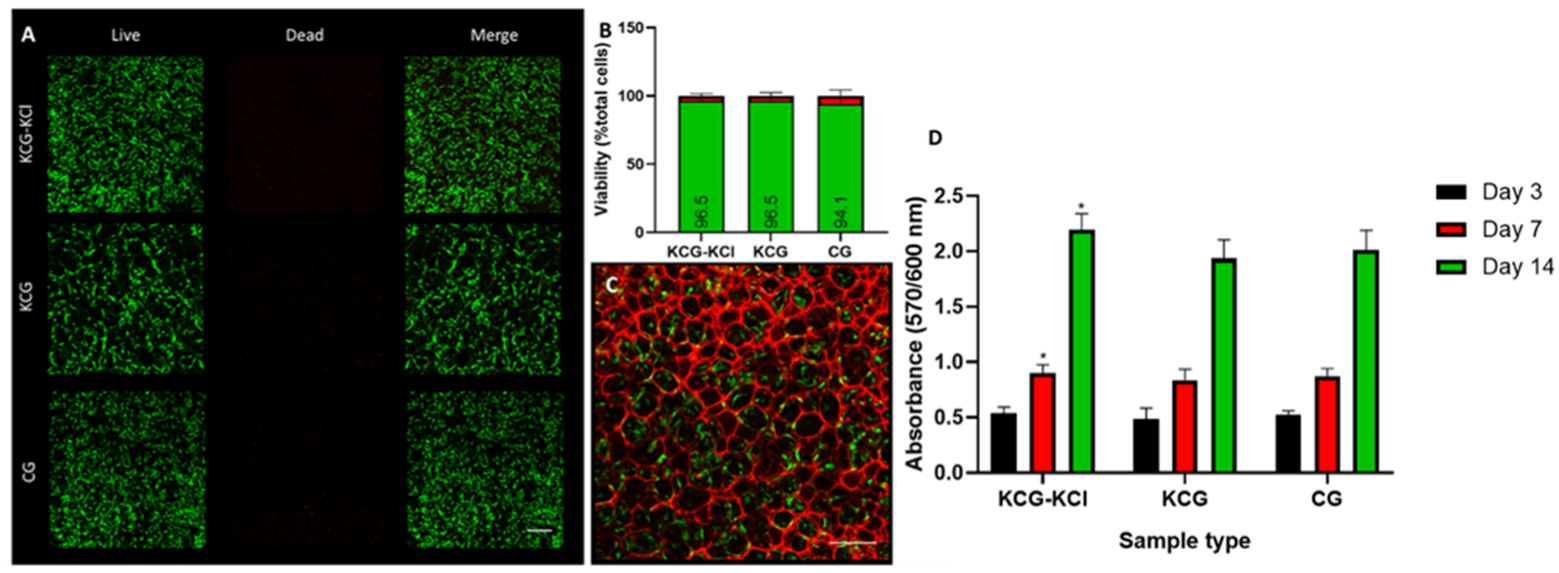
2.4. DPSCs’ Growth and Proliferation within the Various Scaffold Types
2.5. Odontogenic Differentiation of DPSCs in the Presence of the Scaffolds
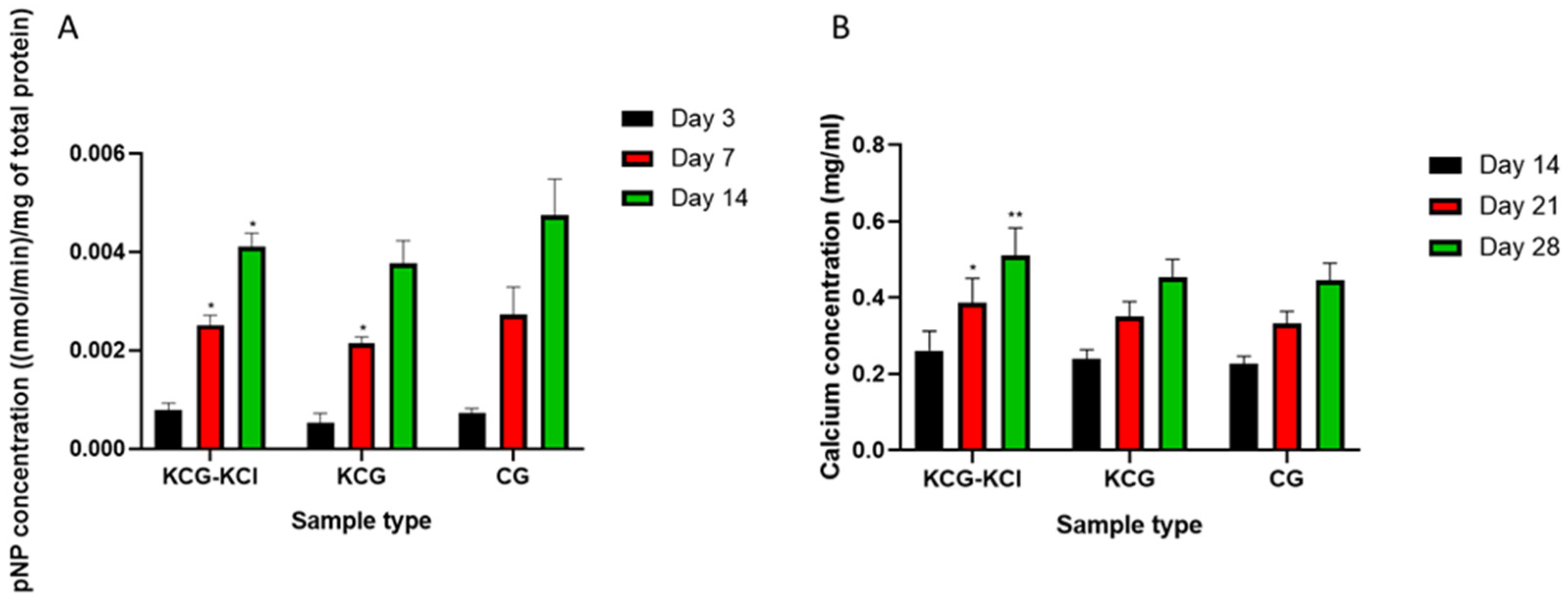
2.5.1. Determination of the ALP Activity of DPSCs
2.5.2. Evaluation of the Secreted Calcium in Supernatants
2.5.3. Real-Time PCR Odontogenic Markers
3. Discussion
4. Materials and Methods
4.1. Materials
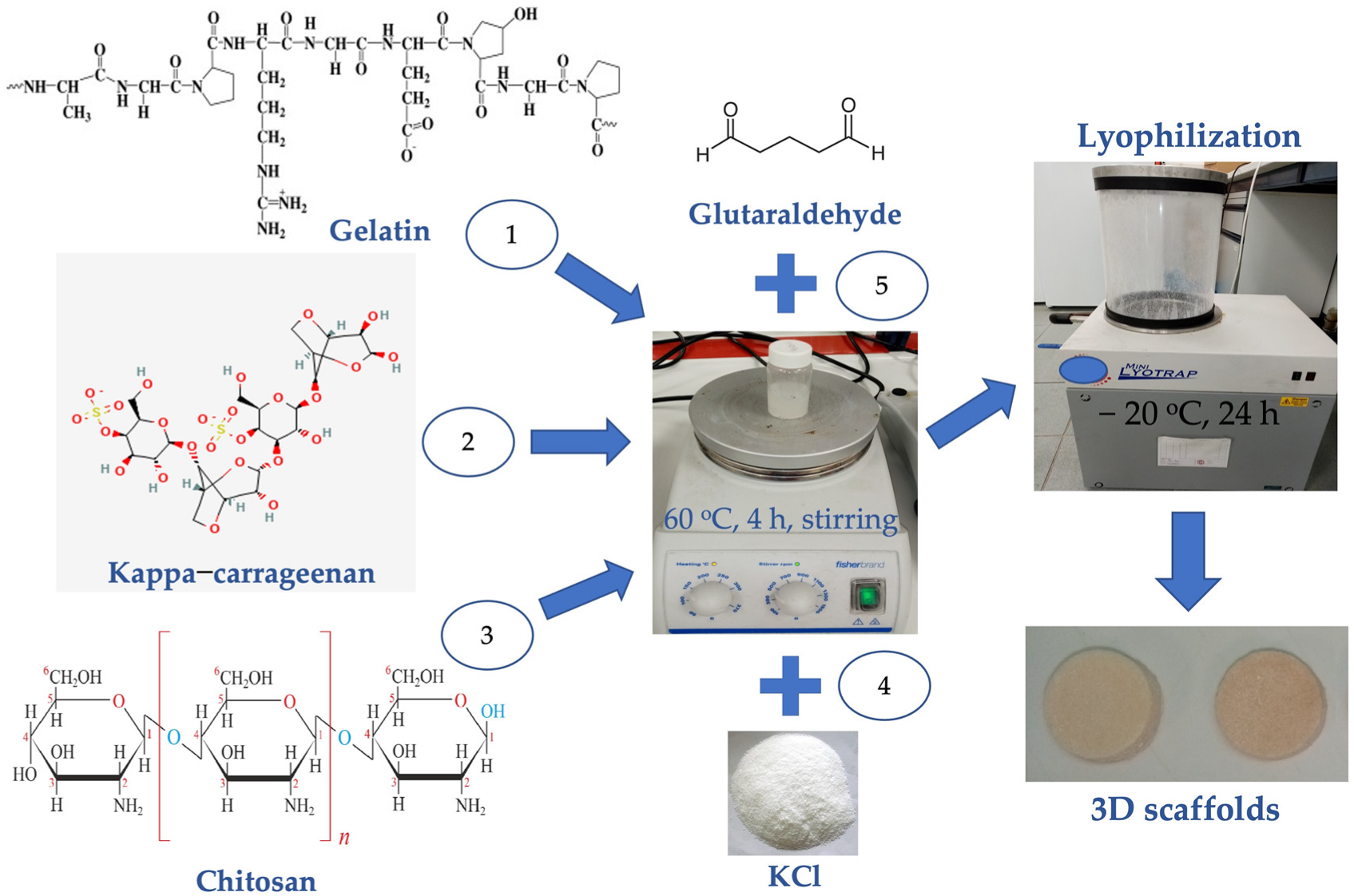
4.2. Scaffolds Preparation
4.3. Establishment and Culture of DPSCs
4.4. Immunophenotypic Characterization of DPSCs
4.5. Cell Viability Assessment
4.5.1. Cell Viability Assessment with the PrestoBlueTM Metabolic Assay
4.5.2. Cell Viability Assessment by Live/Dead Staining
4.6. Mechanical Properties of the DPSCs Seeded Scaffolds
4.7. Scanning Electron Microscopy (SEM)
4.8. Odontogenic Differentiation
4.8.1. Alkaline Phosphatase Activity
4.8.2. Calcium Secretion Levels Determination
4.8.3. Gene Expression of DPSCs
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yelick, P.C.; Sharpe, P.T. Tooth Bioengineering and Regenerative Dentistry. J. Dent. Res. 2019, 98, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Machla, F.; Angelopoulos, I.; Epple, M.; Chatzinikolaidou, M.; Bakopoulou, A. Biomolecule-Mediated Therapeutics of the Dentin–Pulp Complex: A Systematic Review. Biomolecules 2022, 12, 285. [Google Scholar] [CrossRef] [PubMed]
- Giraud, T.; Jeanneau, C.; Rombouts, C.; Bakhtiar, H.; Laurent, P.; About, I. Pulp capping materials modulate the balance between inflammation and regeneration. Dent. Mater. 2019, 35, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Egusa, H.; Sonoyama, W.; Nishimura, M.; Atsuta, I.; Akiyama, K. Stem cells in dentistry—Part I: Stem cell sources. J. Prosthodont. Res. 2012, 56, 151–165. [Google Scholar] [CrossRef]
- Feng, R.; Lengner, C. Application of Stem Cell Technology in Dental Regenerative Medicine. Adv. Wound Care 2013, 2, 296–305. [Google Scholar] [CrossRef]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) In Vitro and In Vivo. Proc. Natl. Acad. Sci. USA 2001, 97, 13625–13630. [Google Scholar] [CrossRef]
- La Noce, M.; Stellavato, A.; Vassallo, V.; Cammarota, M.; Laino, L.; Desiderio, V.; Del Vecchio, V.; Nicoletti, G.F.; Tirino, V.; Papaccio, G.; et al. Hyaluronan-Based Gel Promotes Human Dental Pulp Stem Cells Bone Differentiation by Activating YAP/TAZ Pathway. Cells 2021, 10, 2899. [Google Scholar] [CrossRef]
- Casagrande, L.; Cordeiro, M.M.; Nor, S.A.; Nor, J.E. Dental pulp stem cells in regenerative dentistry. Odontology 2011, 99, 1–7. [Google Scholar] [CrossRef]
- Demarco, F.F.; Conde, M.C.M.; Cavalcanti, B.N.; Casagrande, L.; Sakai, V.T.; Nör, J.E. Dental pulp tissue engineering. Braz. Dent. J. 2011, 22, 3–13. [Google Scholar] [CrossRef]
- Lymperi, S.; Ligoudistianou, C.; Taraslia, V.; Kontakiotis, E.; Anastasiadou, E. Dental stem cells and their applications in dental tissue engineering. Open Dent. J. 2013, 7, 76. [Google Scholar] [CrossRef]
- Bordini, E.; Cassiano, F.; Bronze-Uhle, E.; Alamo, L.; Hebling, J.; Costa, C.; Soares, D. Chitosan in association with osteogenic factors as a cell-homing platform for dentin regeneration: Analysis in a pulp-in-a-chip model. Dent. Mater. 2022, 38, 655–669. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, A.; Sittinger, M.; Risbud, M.V. Chitosan: A versatile biopolymer for orthopaedic tissue-engineering. Biomaterials 2005, 26, 5983–5990. [Google Scholar] [CrossRef]
- Hsu, S.-h.; Chang, Y.-B.; Tsai, C.-L.; Fu, K.-Y.; Wang, S.-H.; Tseng, H.-J. Characterization and biocompatibility of chitosan nanocomposites. Colloids Surf. B Biointerfaces 2011, 85, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Aider, M. Chitosan application for active bio-based films production and potential in the food industry: Review. LWT—Food Sci. Technol. 2010, 43, 837–842. [Google Scholar] [CrossRef]
- Georgopoulou, A.; Papadogiannis, F.; Batsali, A.; Marakis, J.; Alpantaki, K.; Eliopoulos, A.G.; Pontikoglou, C.; Chatzinikolaidou, M. Chitosan/gelatin scaffolds support bone regeneration. J. Mater. Sci. Mater. Med. 2018, 29, 59. [Google Scholar] [CrossRef]
- Aguilar, A.; Zein, N.; Harmouch, E.; Hafdi, B.; Bornert, F.; Offner, D.; Clauss, F.; Fioretti, F.; Huck, O.; Benkirane-Jessel, N.; et al. Application of Chitosan in Bone and Dental Engineering. Molecules 2019, 24, 3009. [Google Scholar] [CrossRef] [PubMed]
- Kiernan, C.H.; Wolvius, E.B.; Brama, P.A.J.; Farrell, E. The Immune Response to Allogeneic Differentiated Mesenchymal Stem Cells in the Context of Bone Tissue Engineering. Tissue Eng. Part B Rev. 2017, 24, 75–83. [Google Scholar] [CrossRef]
- Santoro, M.; Tatara, A.; Mikos, A. Gelatin carriers for drug and cell delivery in tissue engineering. J. Control. Release 2014, 190, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Smith, L.A.; Hu, J.; Ma, P.X. Biomimetic nanofibrous gelatin/apatite composite scaffolds for bone tissue engineering. Biomaterials 2009, 30, 2252–2258. [Google Scholar] [CrossRef]
- Jiang, G.; Li, S.; Yu, K.; He, B.; Hong, J.; Xu, T.; Meng, J.; Ye, C.; Chen, Y.; Shi, Z.; et al. A 3D-printed PRP-GelMA hydrogel promotes osteochondral regeneration through M2 macrophage polarization in a rabbit model. Acta Biomater. 2021, 128, 150–162. [Google Scholar] [CrossRef]
- Peter, M.; Ganesh, N.; Selvamurugan, N.; Nair, S.V.; Furuike, T.; Tamura, H.; Jayakumar, R. Preparation and characterization of chitosan–gelatin/nanohydroxyapatite composite scaffolds for tissue engineering applications. Carbohydr. Polym. 2010, 80, 687–694. [Google Scholar] [CrossRef]
- Bakopoulou, A.; Georgopoulou, A.; Grivas, I.; Bekiari, C.; Prymak, O.; Loza, Κ.; Epple, M.; Papadopoulos, G.C.; Koidis, P.; Chatzinikolaidou, Μ. Dental pulp stem cells in chitosan/gelatin scaffolds for enhanced orofacial bone regeneration. Dent. Mater. 2019, 35, 310–327. [Google Scholar] [CrossRef] [PubMed]
- Vagropoulou, G.; Trentsiou, M.; Anthie, G.; Papachristou, E.; Prymak, O.; Kritis, A.; Epple, M.; Chatzinikolaidou, M.; Bakopoulou, A.; Koidis, P. Hybrid chitosan/gelatin/nanohydroxyapatite scaffolds promote odontogenic differentiation of dental pulp stem cells and in vitro biomineralization. Dent. Mater. 2020, 37, e23–e36. [Google Scholar] [CrossRef] [PubMed]
- Pereda, M.; Ponce, A.G.; Marcovich, N.E.; Ruseckaite, R.A.; Martucci, J.F. Chitosan-gelatin composites and bi-layer films with potential antimicrobial activity. Food Hydrocoll. 2011, 25, 1372–1381. [Google Scholar] [CrossRef]
- Roshanfar, F.; Hesaraki, S.; Dolatshahi-Pirouz, A. Electrospun Silk Fibroin/kappa-Carrageenan Hybrid Nanofibers with Enhanced Osteogenic Properties for Bone Regeneration Applications. Biology 2022, 11, 751. [Google Scholar] [CrossRef]
- Rode, M.; Angulski, A.; Gomes, F.; Silva, M.; Jeremias, T.; Carvalho, R.; Vieira, D.; De Oliveira, L.F.; Maia, L.; Trentin, A.; et al. Carrageenan hydrogel as a scaffold for skin-derived multipotent stromal cells delivery. J. Biomater. Appl. 2018, 33, 422–434. [Google Scholar] [CrossRef]
- Gashti, M.P.; Stir, M.; Hulliger, J. Synthesis of bone-like micro-porous calcium phosphate/iota-carrageenan composites by gel diffusion. Colloids Surf. B Biointerfaces 2013, 110, 426–433. [Google Scholar] [CrossRef]
- Campo, V.L.; Kawano, D.F.; Silva, D.B.d.; Carvalho, I. Carrageenans: Biological properties, chemical modifications and structural analysis—A review. Carbohydr. Polym. 2009, 77, 167–180. [Google Scholar] [CrossRef]
- Nukavarapu, S.; Dorcemus, D. Osteochondral Tissue Engineering: Current Strategies and Challenges. Biotechnol. Adv. 2012, 31, 706–721. [Google Scholar] [CrossRef]
- Hermansson, A.M.; Eriksson, E.; Jordansson, E. Effects of potassium, sodium and calcium on the microstructure and rheological behaviour of kappa-carrageenan gels. Carbohydr. Polym. 1991, 16, 297–320. [Google Scholar] [CrossRef]
- Tavakoli, S.; Kharaziha, M.; Kermanpur, A.; Mokhtari, H. Sprayable and injectable visible-light Kappa-carrageenan hydrogel for in-situ soft tissue engineering. Int. J. Biol. Macromol. 2019, 138, 590–601. [Google Scholar] [CrossRef] [PubMed]
- Yegappan, R.; Selvaprithiviraj, V.; Amirthalingam, S.; Jayakumar, R. Carrageenan based hydrogels for drug delivery, tissue engineering and wound healing. Carbohydr. Polym. 2018, 198, 385–400. [Google Scholar] [CrossRef]
- Loukelis, K.; Papadogianni, D.; Chatzinikolaidou, M. Kappa-carrageenan/chitosan/gelatin scaffolds enriched with potassium chloride for bone tissue engineering. Int. J. Biol. Macromol. 2022, 209, 1720–1730. [Google Scholar] [CrossRef] [PubMed]
- Peres, M.A.; Macpherson, L.M.D.; Weyant, R.J.; Daly, B.; Venturelli, R.; Mathur, M.R.; Listl, S.; Celeste, R.K.; Guarnizo-Herreño, C.C.; Kearns, C.; et al. Oral diseases: A global public health challenge. Lancet 2019, 394, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Dzobo, K.; Thomford, N.E.; Senthebane, D.; Shipanga, H.; Rowe, A.; Dandara, C.; Pillay, M.; Motaung, S. Advances in Regenerative Medicine and Tissue Engineering: Innovation and Transformation of Medicine. Stem Cells Int. 2018, 2018, 2495848. [Google Scholar] [CrossRef]
- Kim, B.-S.; Baez, C.E.; Atala, A. Biomaterials for tissue engineering. World J. Urol. 2000, 18, 2–9. [Google Scholar] [CrossRef]
- Araujo, J.V.; Davidenko, N.; Danner, M.; Cameron, R.E.; Best, S. Novel Porous scaffolds of pH Responsive Chitosan/Carrageenan-based Polyelectrolyte Complexes for Tissue Engineering. J. Biomed. Mater. Res. Part A 2014, 102, 4415–4426. [Google Scholar] [CrossRef]
- Tziveleka, L.A.; Sapalidis, A.; Kikionis, S.; Aggelidou, E.; Demiri, E.; Kritis, A.; Ioannou, E.; Roussis, V. Hybrid Sponge-Like Scaffolds Based on Ulvan and Gelatin: Design, Characterization and Evaluation of Their Potential Use in Bone Tissue Engineering. Materials 2020, 13, 1763. [Google Scholar] [CrossRef]
- Lin, S.-L.; Lee, S.-Y.; Lin, Y.-C.; Huang, Y.-H.; Yang, J.-C.; Huang, H.-M. Evaluation of mechanical and histological properties of cryopreserved human premolars under short-term preservation: A preliminary study. J. Dent. Sci. 2014, 9, 244–248. [Google Scholar] [CrossRef]
- Yamada, S.; Wirtz, D.; Kuo, S.C. Mechanics of Living Cells Measured by Laser Tracking Microrheology. Biophys. J. 2000, 78, 1736–1747. [Google Scholar] [CrossRef]
- Bausch, A.R.; Ziemann, F.; Boulbitch, A.A.; Jacobson, K.; Sackmann, E. Local Measurements of Viscoelastic Parameters of Adherent Cell Surfaces by Magnetic Bead Microrheometry. Biophys. J. 1998, 75, 2038–2049. [Google Scholar] [CrossRef] [PubMed]
- Guz, N.; Dokukin, M.; Kalaparthi, V.; Sokolov, I. If Cell Mechanics Can Be Described by Elastic Modulus: Study of Different Models and Probes Used in Indentation Experiments. Biophys. J. 2014, 107, 564–575. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, M.; Shin, J.H.; Kim, J. Characterization of cellular elastic modulus using structure based double layer model. Med. Biol. Eng. Comput. 2011, 49, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Prakoso, A.T.; Basri, H.; Adanta, D.; Yani, I.; Ammarullah, M.I.; Akbar, I.; Ghazali, F.A.; Syahrom, A.; Kamarul, T. The Effect of Tortuosity on Permeability of Porous Scaffold. Biomedicines 2023, 11, 427. [Google Scholar] [CrossRef] [PubMed]
- Samourides, A.; Browning, L.; Hearnden, V.; Chen, B. The effect of porous structure on the cell proliferation, tissue ingrowth and angiogenic properties of poly(glycerol sebacate urethane) scaffolds. Mater. Sci. Eng. C 2020, 108, 110384. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Feng, G.; Zhang, J.; Xing, J.; Huang, D.; Lian, M.; Zhang, W.; Wu, W.; Hu, Y.; Lu, X.; et al. Basic Fibroblast Growth Factor Promotes Human Dental Pulp Stem Cells cultured in 3D Porous Chitosan Scaffolds to Neural Differentiation. Int. J. Neurosci. 2020, 131, 625–633. [Google Scholar] [CrossRef]
- González Ocampo, J.I.; Machado de Paula, M.M.; Bassous, N.J.; Lobo, A.O.; Ossa Orozco, C.P.; Webster, T.J. Osteoblast responses to injectable bone substitutes of kappa-carrageenan and nano hydroxyapatite. Acta Biomater. 2019, 83, 425–434. [Google Scholar] [CrossRef]
- Abuarqoub, D.; Awidi, A.; Abuharfeil, N. Comparison of osteo/odontogenic differentiation of human adult dental pulp stem cells and stem cells from apical papilla in the presence of platelet lysate. Arch. Oral Biol. 2015, 60, 1545–1553. [Google Scholar] [CrossRef]
- Yegappan, R.; Selvaprithiviraj, V.; Amirthalingam, S.; Mohandas, A.; Hwang, N.; Rangasamy, J. Injectable angiogenic and osteogenic carrageenan nanocomposite hydrogel for bone tissue engineering. Int. J. Biol. Macromol. 2018, 122, 320–328. [Google Scholar] [CrossRef]
- Yamakoshi, Y. Dentinogenesis and Dentin Sialophosphoprotein (DSPP). J. Oral Biosci. 2009, 51, 134–142. [Google Scholar] [CrossRef]
- Qin, C.; Brunn, J.C.; Cadena, E.; Ridall, A.; Tsujigiwa, H.; Nagatsuka, H.; Nagai, N.; Butler, W.T. The Expression of Dentin Sialophosphoprotein Gene in Bone. J. Dent. Res. 2002, 81, 392–394. [Google Scholar] [CrossRef] [PubMed]
- Lei, S.; Liu, X.-M.; Liu, Y.; Bi, J.; Zhu, S.; Chen, X. Lipopolysaccharide Downregulates the Osteo-/Odontogenic Differentiation of Stem Cells From Apical Papilla by Inducing Autophagy. J. Endod. 2020, 46, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Machla, F.; Sokolova, V.; Platania, V.; Prymak, O.; Kostka, K.; Kruse, B.; Agrymakis, M.; Pasadaki, S.; Kritis, A.; Alpantaki, K.; et al. Tissue engineering at the dentin-pulp interface using human treated dentin scaffolds conditioned with DMP1 or BMP2 plasmid DNA-carrying calcium phosphate nanoparticles. Acta Biomater. 2023, 159, 156–172. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, S.; McDonald, F. Runx2 and dental development. Eur. J. Oral Sci. 2006, 114, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Widbiller, M.; Lindner, S.R.; Buchalla, W.; Eidt, A.; Hiller, K.A.; Schmalz, G.; Galler, K.M. Three-dimensional culture of dental pulp stem cells in direct contact to tricalcium silicate cements. Clin. Oral Investig. 2016, 20, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Papadogiannhs, F.; Batsali, A.; Klontzas, M.; Karabela, M.; Anthie, G.; Mantalaris, A.; Zafeiropoulos, N.; Chatzinikolaidou, M.; Pontikoglou, C. Osteogenic differentiation of bone marrow mesenchymal stem cells on chitosan/gelatin scaffolds: Gene expression profile and mechanical analysis. Biomed. Mater. 2020, 15, 064101. [Google Scholar] [CrossRef]
- Bakopoulou, A.; Leyhausen, G.; Volk, J.; Tsiftsoglou, A.; Garefis, P.; Koidis, P.; Geurtsen, W. Assessment of the Impact of Two Different Isolation Methods on the Osteo/Odontogenic Differentiation Potential of Human Dental Stem Cells Derived from Deciduous Teeth. Calcif. Tissue Int. 2011, 88, 130–141. [Google Scholar] [CrossRef]
- Ding, Y.; Xu, G.; Wang, G. On the determination of elastic moduli of cells by AFM based indentation. Sci. Rep. 2017, 2017, 45575. [Google Scholar] [CrossRef]
- Hadjicharalambous, C.; Kozlova, D.; Sokolova, V.; Epple, M.; Chatzinikolaidou, M. Calcium phosphate nanoparticles carrying BMP-7 plasmid DNA induce an osteogenic response in MC3T3-E1 pre-osteoblasts. J. Biomed. Mater. Res. Part A 2015, 103, 3834–3842. [Google Scholar] [CrossRef]
- Wang, X.; Wenk, E.; Zhang, X.; Meinel, L.; Vunjak-Novakovic, G.; Kaplan, D.L. Growth factor gradients via microsphere delivery in biopolymer scaffolds for osteochondral tissue engineering. J. Control. Release 2009, 134, 81–90. [Google Scholar] [CrossRef]


| Type | Scaffold Acronym | Composition |
|---|---|---|
| (i) | KCG-KCl | 1% w/v kappa-carrageenan, 1.67% w/v chitosan, 1.67% w/v gelatin, 0.25 M KCl |
| (ii) | KCG | 1% w/v kappa-carrageenan, 1.67% w/v chitosan, 1.67% w/v gelatin |
| (iii) | CG | 1.67% w/v chitosan, 1.67% w/v gelatin |
| Gene Symbol | Forward (5′–3′) | Reverse (5′–3′) | Amplicon Size (bp) |
|---|---|---|---|
| DSPP | GCTGGCCTGGATAATTCCGA | CTCCTGGCCCTTGCTGTTAT | 135 |
| ALP | CCGTGGCAACTCTATCTTTGG | CAGGCCCATTGCCATACAG | 89 |
| RUNX2 | CCACCGAGACCAACAGAGTC | TCACTGTGCTGAAGAGGCTG | 118 |
| B2M | TGTCTTTCAGCAAGGACTGGT | ACATGTCTCGATCCCACTTAAC | 138 |
| SDHA | GCATGCCAGGGAAGACTACA | GCCAACGTCCACATAGGACA | 127 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loukelis, K.; Machla, F.; Bakopoulou, A.; Chatzinikolaidou, M. Kappa-Carrageenan/Chitosan/Gelatin Scaffolds Provide a Biomimetic Microenvironment for Dentin-Pulp Regeneration. Int. J. Mol. Sci. 2023, 24, 6465. https://doi.org/10.3390/ijms24076465
Loukelis K, Machla F, Bakopoulou A, Chatzinikolaidou M. Kappa-Carrageenan/Chitosan/Gelatin Scaffolds Provide a Biomimetic Microenvironment for Dentin-Pulp Regeneration. International Journal of Molecular Sciences. 2023; 24(7):6465. https://doi.org/10.3390/ijms24076465
Chicago/Turabian StyleLoukelis, Konstantinos, Foteini Machla, Athina Bakopoulou, and Maria Chatzinikolaidou. 2023. "Kappa-Carrageenan/Chitosan/Gelatin Scaffolds Provide a Biomimetic Microenvironment for Dentin-Pulp Regeneration" International Journal of Molecular Sciences 24, no. 7: 6465. https://doi.org/10.3390/ijms24076465
APA StyleLoukelis, K., Machla, F., Bakopoulou, A., & Chatzinikolaidou, M. (2023). Kappa-Carrageenan/Chitosan/Gelatin Scaffolds Provide a Biomimetic Microenvironment for Dentin-Pulp Regeneration. International Journal of Molecular Sciences, 24(7), 6465. https://doi.org/10.3390/ijms24076465








