Evaluation of Mitochondrial Function in Blood Samples Shows Distinct Patterns in Subjects with Thyroid Carcinoma from Those with Hyperplasia
Abstract
1. Introduction
2. Results
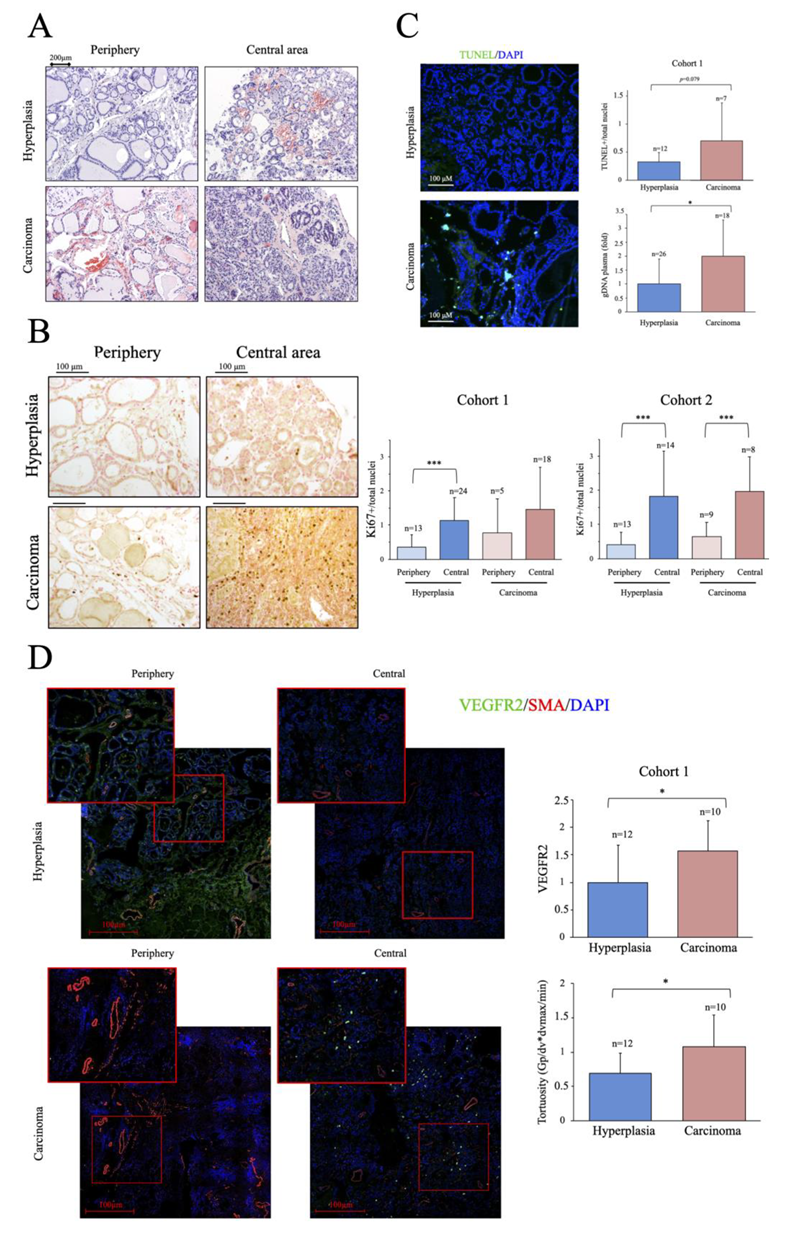
2.1. Characterization of the Cohort: Basic Metabolic Risk Factors and Histological Analysis
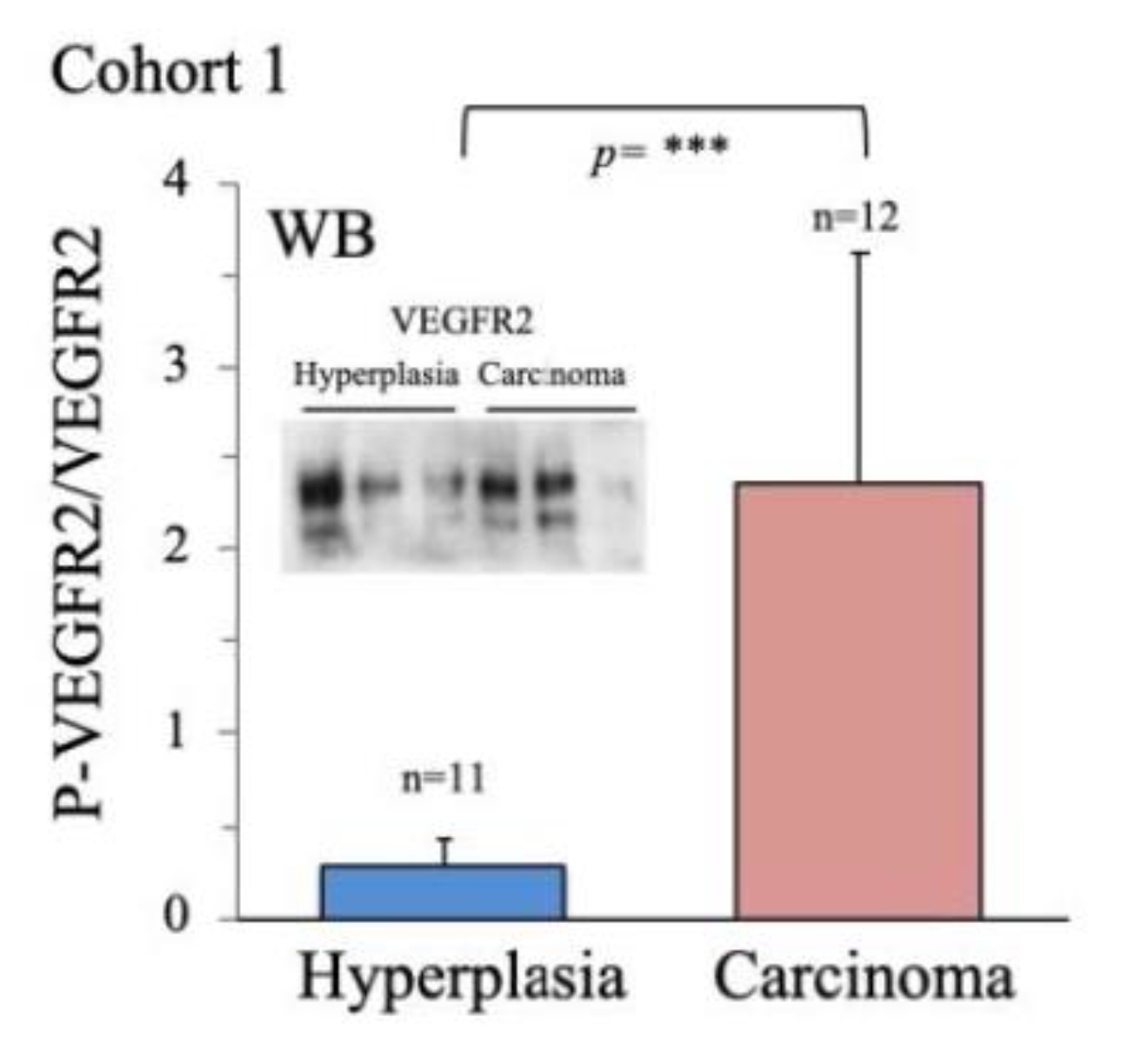
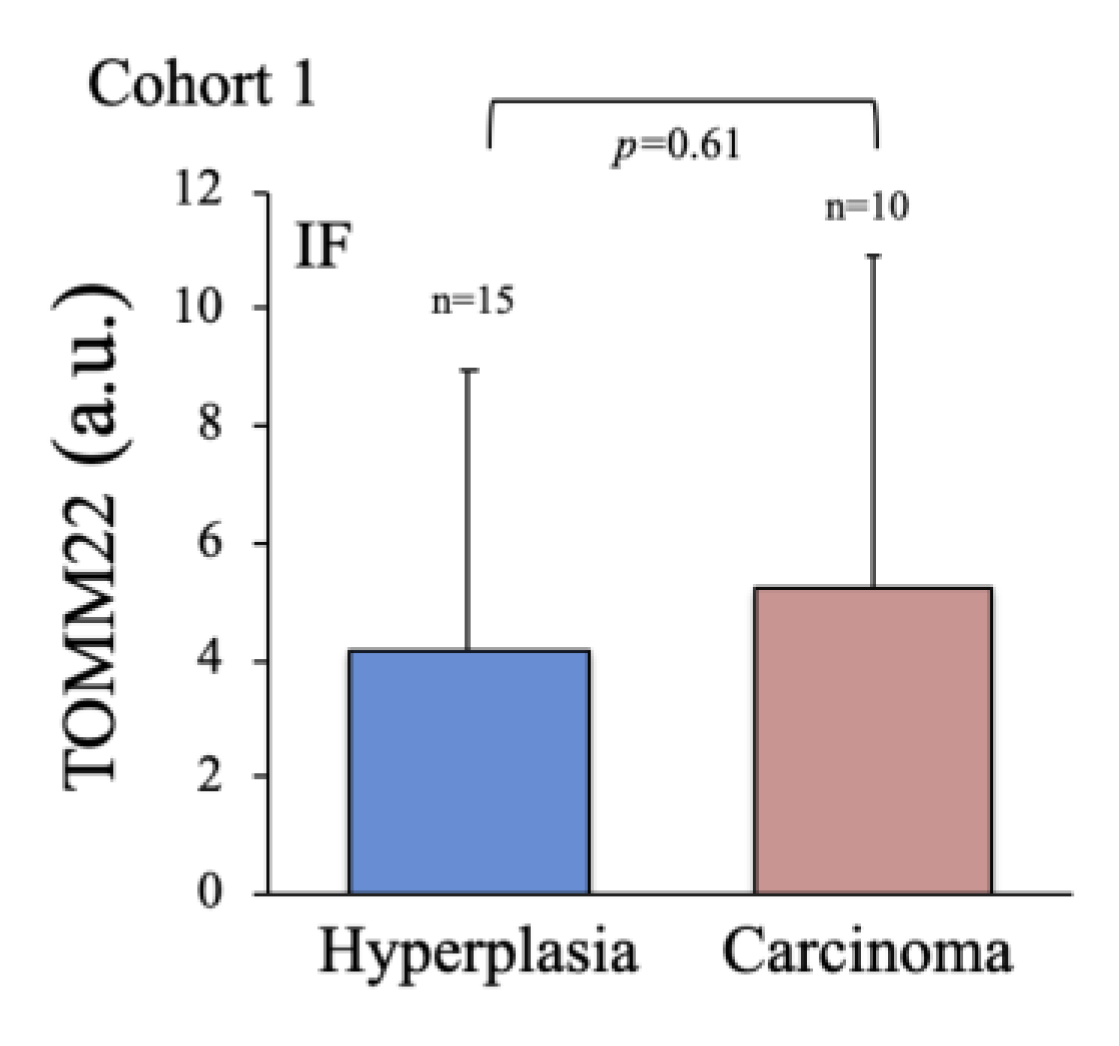
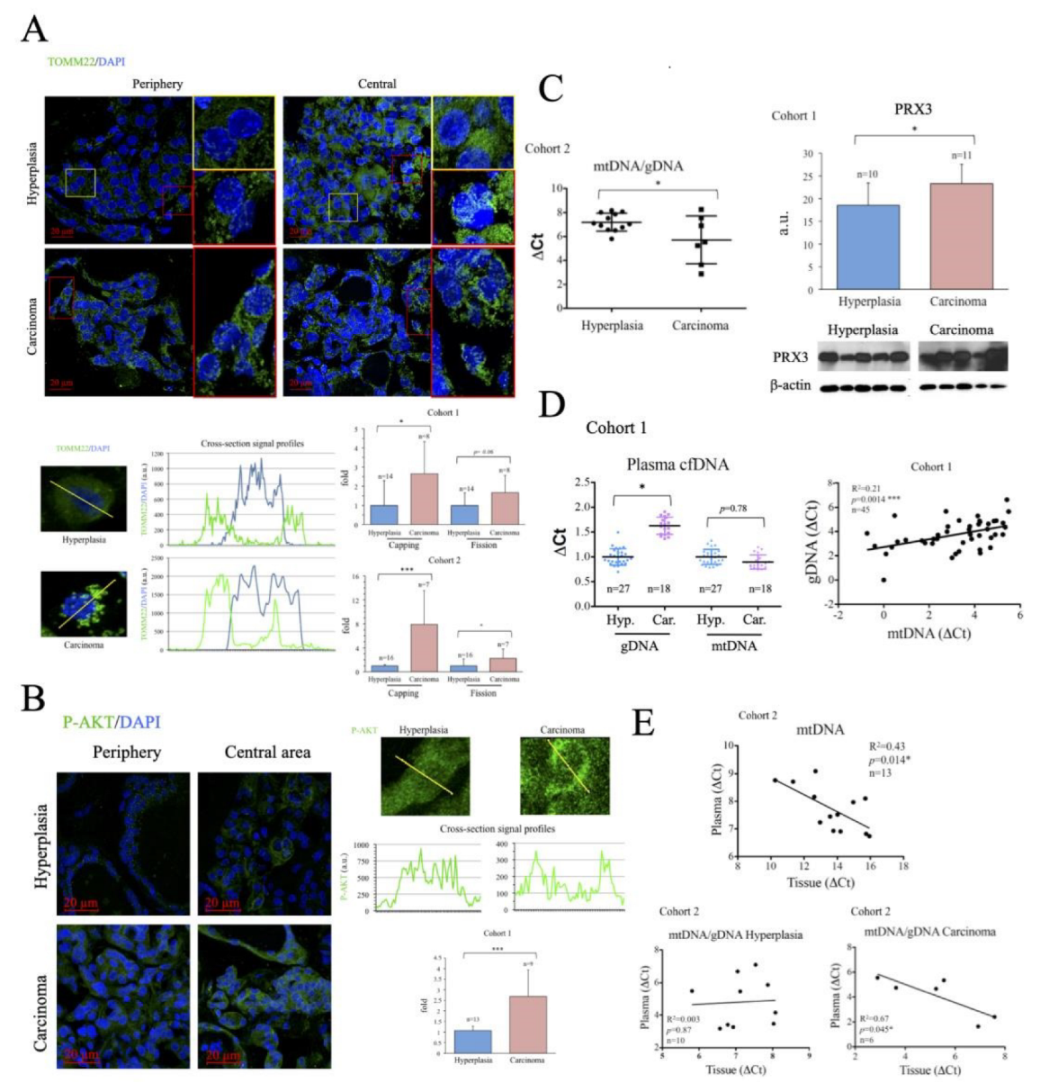
2.2. Characterization of Thyroid Tissue Metabolic Status
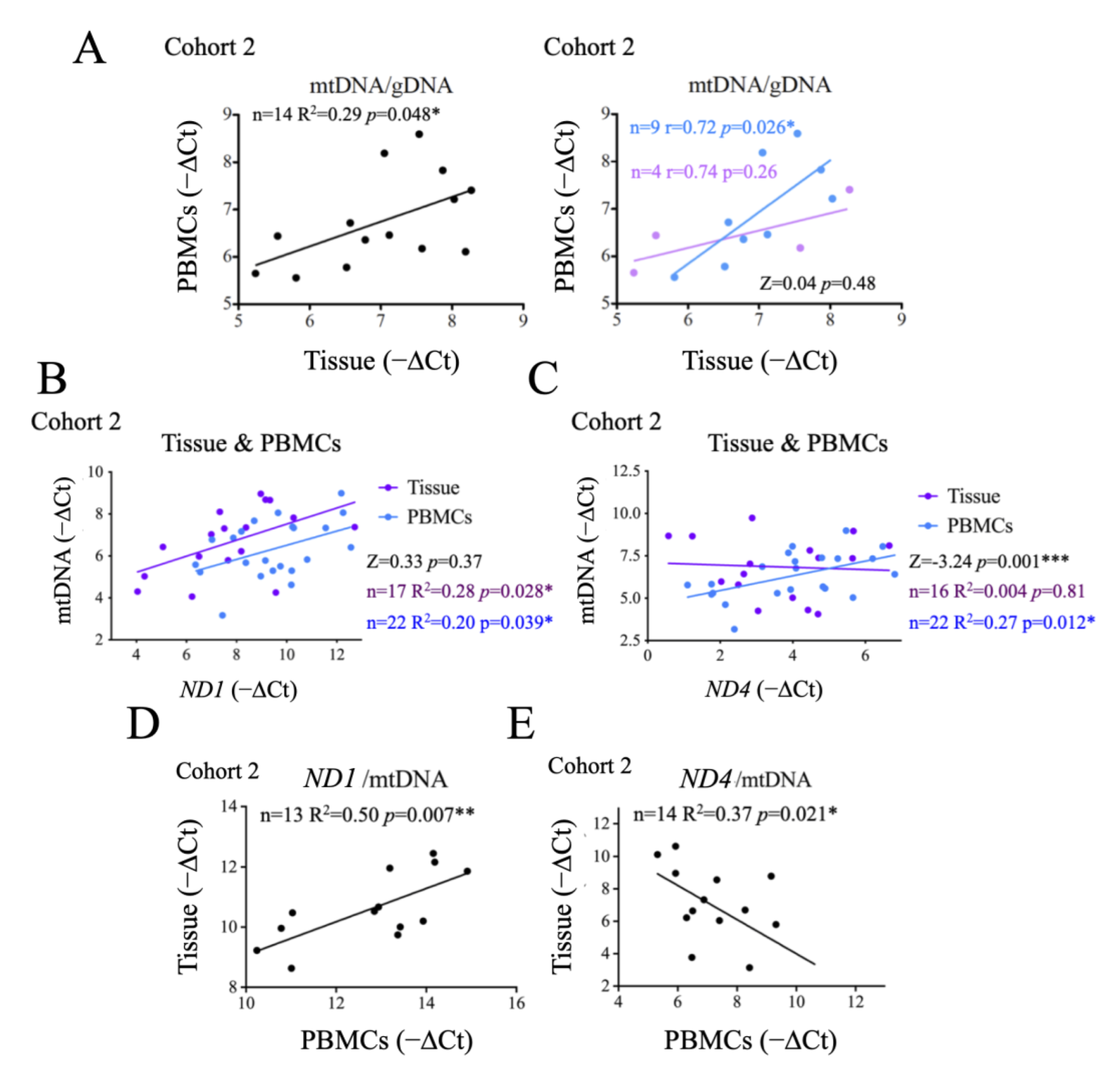
2.3. Evaluation of Thyroid DNA in Plasma Samples
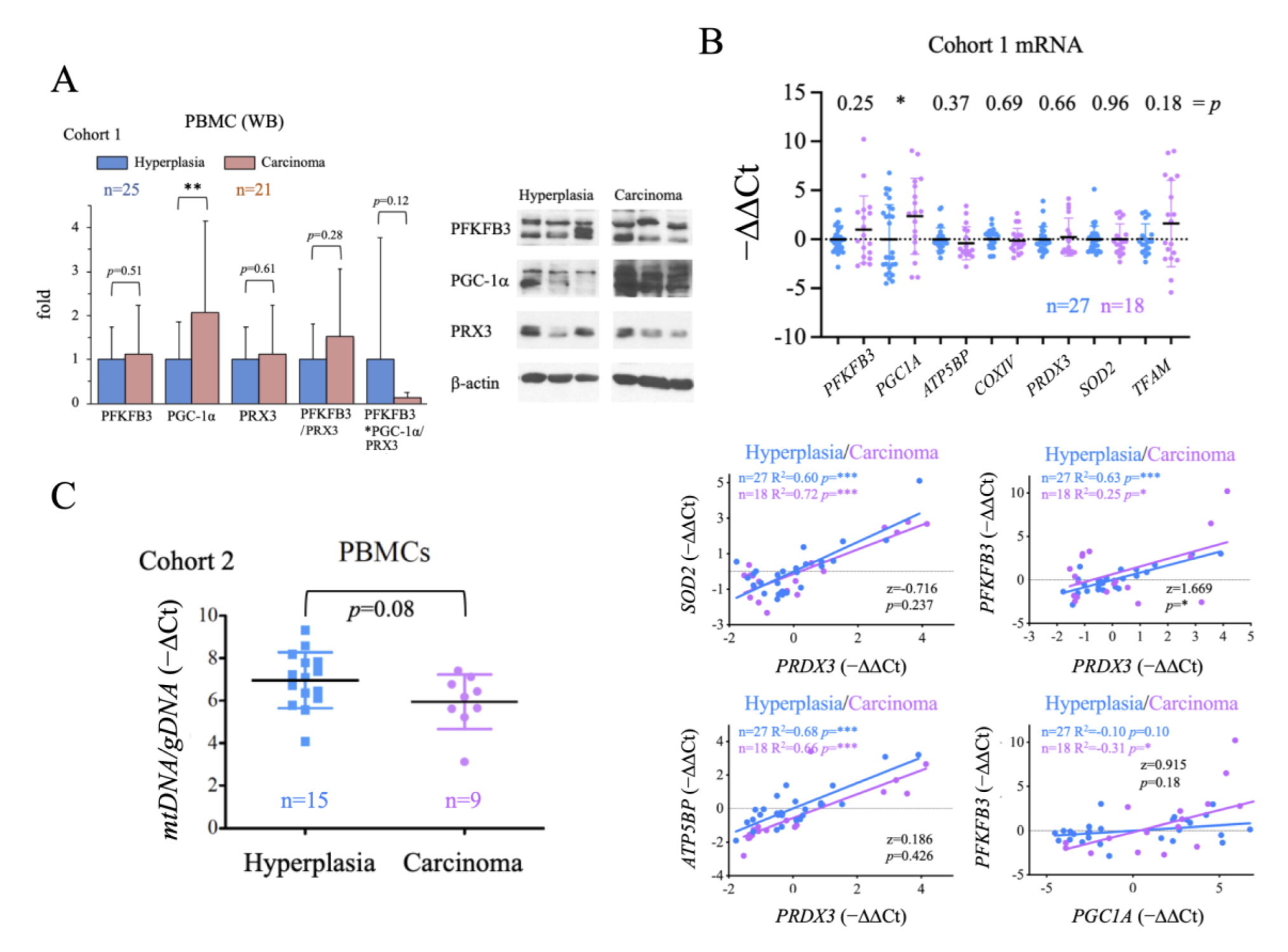
2.4. Evaluation of the Systemic Metabolic Status of Patient-Derived PBMCs
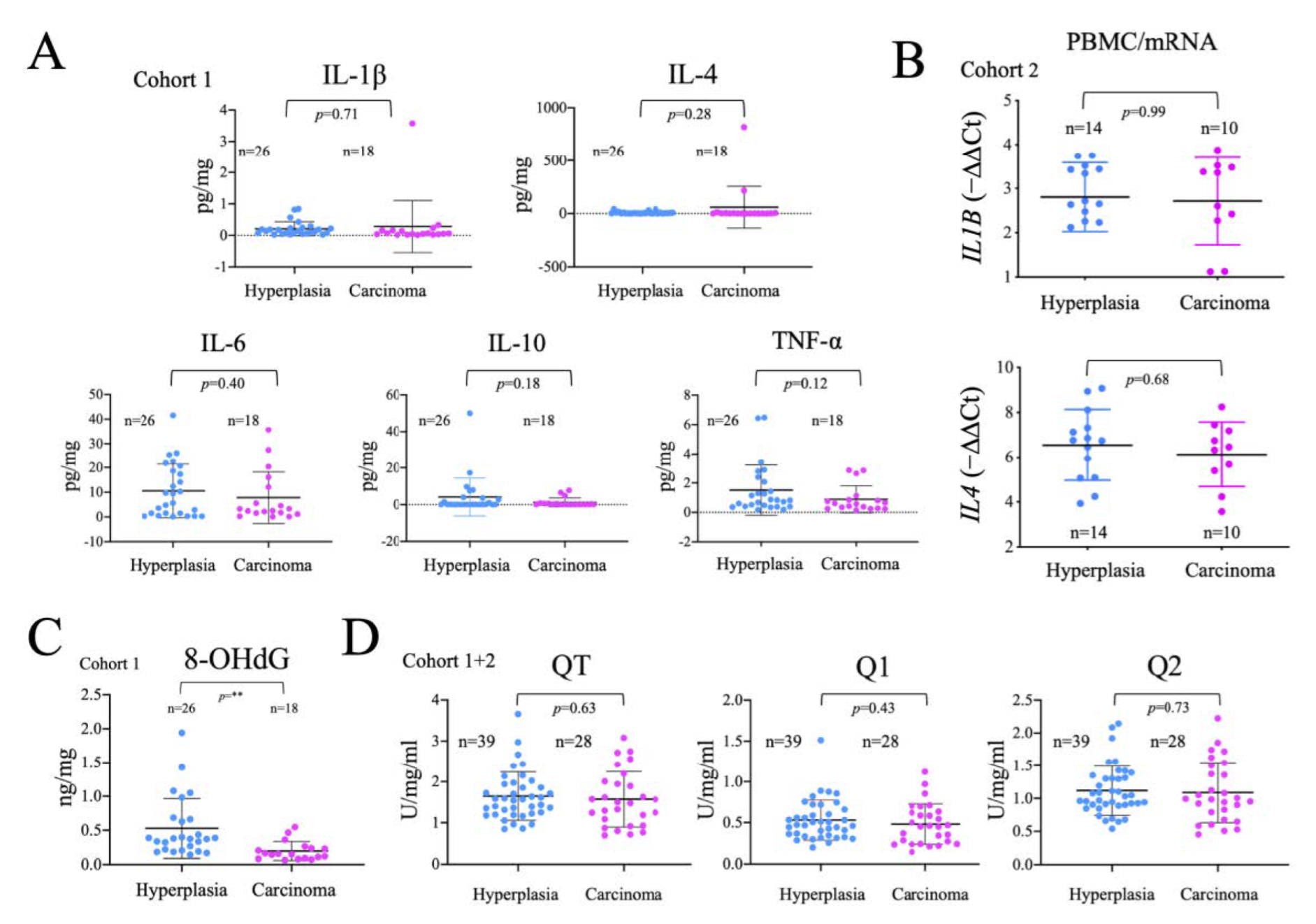
2.5. Analysis of the Systemic Inflammatory Status
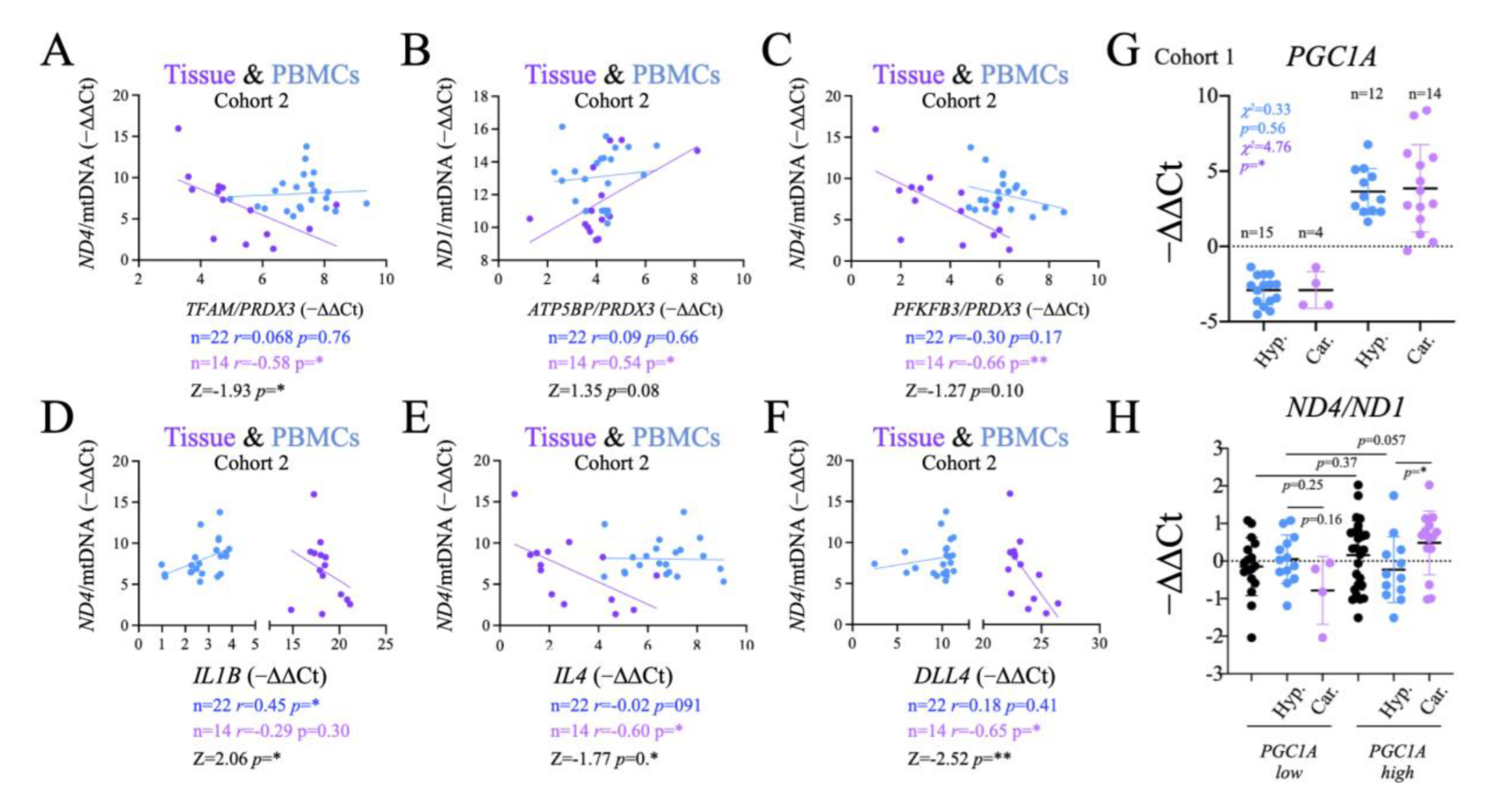
2.6. Comparison of Systemic and Tumor Metabolism
2.7. Origin and Significance of cfDNA Detectable in Crude Plasma Samples
2.8. Functional Significance of the Observed Changes in ND1 and ND4
3. Discussion
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Leal-Esteban, L.C.; Fajas, L. Cell cycle regulators in cancer cell metabolism. Biochim. Et Biophys. Acta (BBA)-Mol. Basis Dis. 2020, 1866, 165715. [Google Scholar] [CrossRef]
- Salazar-Roa, M.; Malumbres, M. Fueling the Cell Division Cycle. Trends Cell Biol. 2017, 27, 69–81. [Google Scholar] [CrossRef]
- Thompson, C.B.; Bielska, A.A. Growth factors stimulate anabolic metabolism by directing nutrient uptake. J. Biol. Chem. 2019, 294, 17883–17888. [Google Scholar] [CrossRef]
- Theurey, P.; Rieusset, J. Mitochondria-Associated Membranes Response to Nutrient Availability and Role in Metabolic Diseases. Trends Endocrinol Metab. 2017, 28, 32–45. [Google Scholar] [CrossRef]
- Dalla Pozza, E.; Dando, I.; Pacchian, A.R.; Liboi, E.; Scupoli, M.T.; Donadelli, M. Regulation of succinate dehydrogenase and role of succinate in cancer. Semin. Cell Dev. Biol. 2020, 98, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Le, A. Glutamine Metabolism in Cancer. Adv. Exp. Med. Biol. 2018, 1063, 13–32. [Google Scholar]
- Kiesel, V.A.; Sheeley, M.P.; Coleman, M.F.; Cotul, E.K.; Donkin, S.S.; Hursting, S.D.; Wendt, M.K.; Teegarden, D. Pyruvate carboxylase and cancer progression. Cancer Metab. 2021, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Karakhanova, S.; Hartwig, W.; D’Haese, J.G.; Philippov, P.P.; Werner, J.; Bazhin, A.V. Mitochondria and Mitochondrial ROS in Cancer: Novel Targets for Anticancer Therapy. J. Cell Physiol. 2016, 231, 2570–2581. [Google Scholar] [CrossRef]
- Battaglia, A.M.; Chirillo, R.; Aversa, I.; Sacco, A.; Costanzo, F.; Biamonte, F. Ferroptosis and Cancer: Mitochondria Meet the “Iron Maiden” Cell Death. Cells 2020, 9, E1505. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, S.; Hushmandi, K.; Zabolian, A.; Saleki, H.; Torabi, S.; Ranjbar, A.; SeyedSaleh, S.; Sharifzadeh, S.; Khan, H.; Ashrafizadeh, M.; et al. Elucidating Role of Reactive Oxygen Species (ROS) in Cisplatin Chemotherapy: A Focus on Molecular Pathways and Possible Therapeutic Strategies. Molecules 2021, 26, 2382. [Google Scholar] [CrossRef]
- Sharma, P.; Sampath, H. Mitochondrial DNA Integrity: Role in Health and Disease. Cells 2019, 8, E100. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Ju, Y.S.; Kim, Y.; Li, J.; Wang, Y.; Yoon, C.J.; Yang, Y.; Martincorena, I.; Creighton, C.J.; Weinstein, J.N.; et al. Comprehensive molecular characterization of mitochondrial genomes in human cancers. Nat. Genet. 2020, 52, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Qiao, L.; Wang, Y.; Zeng, J.; Chen, D.; Guo, H.; Zhang, Y. Mitochondrial DNA D-loop lesions with the enhancement of DNA repair contribute to gastrointestinal cancer progression. Oncol. Rep. 2018, 40, 3694–3704. [Google Scholar] [CrossRef] [PubMed]
- Nencioni, A.; Caffa, I.; Cortellino, S.; Longo, V.D. Fasting and cancer: Molecular mechanisms and clinical application. Nat. Rev. Cancer 2018, 18, 707–719. [Google Scholar] [CrossRef] [PubMed]
- Zong, W.X.; Rabinowitz, J.D.; White, E. Mitochondria and Cancer. Mol. Cell. 2016, 61, 667–676. [Google Scholar] [CrossRef]
- Poulet, G.; Massias, J.; Taly, V. Liquid Biopsy: General Concepts. Acta. Cytol. 2019, 63, 449–455. [Google Scholar] [CrossRef]
- Weerts, M.J.A.; Timmermans, E.C.; van de Stolpe, A.; Vossen, R.H.; Anvar, S.Y.; Foekens, J.A.; Sleijfer, S.; Martens, J.W.M. Tumor-Specific Mitochondrial DNA Variants Are Rarely Detected in Cell-Free DNA. Neoplasia 2018, 20, 687–696. [Google Scholar] [CrossRef]
- Creed, J.; Klotz, L.; Harbottle, A.; Maggrah, A.; Reguly, B.; George, A.; Gnanapragasm, V. A single mitochondrial DNA deletion accurately detects significant prostate cancer in men in the PSA “grey zone”. World J. Urol. 2018, 36, 341–348. [Google Scholar] [CrossRef]
- Cabanillas, M.E.; McFadden, D.G.; Durante, C. Thyroid cancer. Lancet 2016, 388, 2783–2795. [Google Scholar] [CrossRef]
- Kitahara, C.M.; Sosa, J.A. The changing incidence of thyroid cancer. Nat. Rev. Endocrinol. 2016, 12, 646–653. [Google Scholar] [CrossRef]
- Kim, W.G.; Cheng, S.Y. Mechanisms Linking Obesity and Thyroid Cancer Development and Progression in Mouse Models. Horm Cancer 2018, 9, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, P.; Abd Elmageed, Z.Y.; Friedlander, P.; Aslam, R.; Kandil, E. The utility of molecular markers in pre-operative assessment of thyroid nodules. Future Oncol. 2015, 11, 2343–2350. [Google Scholar] [CrossRef] [PubMed]
- Sciacchitano, S.; Lavra, L.; Ulivieri, A.; Magi, F.; De Francesco, G.P.; Bellotti, C.; Salehi, L.B.; Trovato, M.; Drago, C.; Bartolazzi, A. Comparative analysis of diagnostic performance, feasibility and cost of different test-methods for thyroid nodules with indeterminate cytology. Oncotarget 2017, 8, 49421–49442. [Google Scholar] [CrossRef]
- Titov, S.E.; Ivanov, M.K.; Demenkov, P.S.; Katanyan, G.A.; Kozorezova, E.S.; Malek, A.V.; Veryaskina, Y.A.; Zhimulev, I.F. Combined quantitation of HMGA2 mRNA, microRNAs, and mitochondrial-DNA content enables the identification and typing of thyroid tumors in fine-needle aspiration smears. BMC Cancer 2019, 19, 1010. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Cui, N.H.; Zhang, S.; Wang, X.B.; Ming, L. Leukocyte Mitochondrial DNA Copy Number and Risk of Thyroid Cancer: A Two-Stage Case-Control Study. Front. Endocrinol 2019, 10, 421. [Google Scholar] [CrossRef]
- Lyu, L.; Wang, Q.; Song, S.; Li, L.; Zhou, H.; Li, M.; Jiang, Z.; Zhou, C.; Chen, G.; Lyu, J.; et al. Oncocytic tumors are marked by enhanced mitochondrial content and mtDNA mutations of complex I in Chinese patients. Mitochondrion 2019, 45, 1–6. [Google Scholar] [CrossRef]
- Máximo, V.; Soares, P.; Lima, J.; Cameselle-Teijeiro, J.; Sobrinho-Simões, M. Mitochondrial DNA somatic mutations (point mutations and large deletions) and mitochondrial DNA variants in human thyroid pathology: A study with emphasis on Hürthle cell tumors. Am. J. Pathol. 2002, 160, 1857–1865. [Google Scholar] [CrossRef]
- Su, X.; Wang, W.; Ruan, G.; Liang, M.; Zheng, J.; Chen, Y.; Wu, H.; Fahey, T.J.; Guan, M.; Teng, L. A Comprehensive Characterization of Mitochondrial Genome in Papillary Thyroid Cancer. Int. J. Mol. Sci. 2016, 17, 1594. [Google Scholar] [CrossRef]
- Jiang, Z.; Bahr, T.; Zhou, C.; Jin, T.; Chen, H.; Song, S.; Ikeno, Y.; Tian, H.; Bai, Y. Diagnostic value of circulating cell-free mtDNA in patients with suspected thyroid cancer: ND4/ND1 ratio as a new potential plasma marker. Mitochondrion 2020, 55, 145–153. [Google Scholar] [CrossRef]
- Dabravolski, S.A.; Nikiforov, N.G.; Zhuravlev, A.D.; Orekhov, N.A.; Mikhaleva, L.M.; Orekhov, A.N. The Role of Altered Mitochondrial Metabolism in Thyroid Cancer Development and Mitochondria-Targeted Thyroid Cancer Treatment. Int. J. Mol. Sci. 2022, 23, 460. [Google Scholar] [CrossRef]
- Park, J.H.; Choi, M.; Kim, J.H.; Kim, J.; Han, K.; Kim, B.; Kim, D.H.; Park, Y.G. Metabolic Syndrome and the Risk of Thyroid Cancer: A Nationwide Population-Based Cohort Study. Thyroid 2020, 30, 1496–1504. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.N.; Kim, J.H.; Kim, M.K. Association of Metabolic Health and Central Obesity with the Risk of Thyroid Cancer: Data from the Korean Genome and Epidemiology Study. Cancer Epidemiol. Biomarkers Prev. 2022, 31, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.S.; Maygarden, S.; Dodd, L.G. Pathologic Diagnosis of Thyroid Cancer. Mancino, A., Kim, L., Eds.; In Management of Differentiated Thyroid Cancer; Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Prieto, I.; Alarcón, C.R.; García-Gómez, R.; Berdún, R.; Urgel, T.; Portero, M.; Pamplona, R.; Martínez-Ruiz, A.; Ruiz-Sanz, J.I.; Ruiz-Larrea, M.B.; et al. Metabolic adaptations in spontaneously immortalized PGC-1α knock-out mouse embryonic fibroblasts increase their oncogenic potential. Redox Biol. 2020, 29, 101396. [Google Scholar] [CrossRef]
- Sánchez-Ramos, C.; Prieto, I.; Tierrez, A.; Laso, J.; Valdecantos, M.P.; Bartrons, R.; Roselló-Catafau, J.; Monsalve, M. PGC-1α Downregulation in Steatotic Liver Enhances Ischemia-Reperfusion Injury and Impairs Ischemic Preconditioning. Antioxid Redox Signal. 2017, 27, 1332–1346. [Google Scholar] [CrossRef]
- Yan, Y.-Y.; Guo, Q.-R.; Wang, F.-H.; Adhikari, R.; Zhu, Z.-Y.; Zhang, H.-Y.; Zhou, W.-M.; Yu, H.; Li, J.-Q.; Zhang, J.-Y. Cell-Free DNA: Hope and Potential Application in Cancer. Front Cell Dev. Biol. 2021, 9, 639233. [Google Scholar] [CrossRef] [PubMed]
- Merker, J.D.; Oxnard, G.R.; Compton, C.; Diehn, M.; Hurley, P.; Lazar, A.J.; Lindeman, N.; Lockwood, C.M.; Rai, A.J.; Schilsky, R.L.; et al. Circulating Tumor DNA Analysis in Patients with Cancer: American Society of Clinical Oncology and College of American Pathologists Joint Review. J. Clin. Oncol. 2018, 36, 1631–1641. [Google Scholar] [CrossRef]
- Siemann, D.W. The unique characteristics of tumor vasculature and preclinical evidence for its selective disruption by Tumor-Vascular Disrupting Agents. Cancer Treat. Rev. 2011, 37, 63–74. [Google Scholar] [CrossRef]
- García-Quintans, N.; Sánchez-Ramos, C.; Prieto, I.; Tierrez, A.; Arza, E.; Alfranca, A.; Redondo, J.M.; Monsalve, M. Oxidative stress induces loss of pericyte coverage and vascular instability in PGC-1α-deficient mice. Angiogenesis 2016, 19, 217–228. [Google Scholar] [CrossRef]
- Shah, A.A.; Kamal, M.A.; Akhtar, S. Tumor Angiogenesis and VEGFR-2: Mechanism, Pathways and Current Biological Therapeutic Interventions. Curr. Drug Metab. 2021, 22, 50–59. [Google Scholar]
- García-Quintans, N.; Prieto, I.; Sánchez-Ramos, C.; Luque, A.; Arza, E.; Olmos, Y.; Monsalve, M. Regulation of endothelial dynamics by PGC-1α relies on ROS control of VEGF-A signaling. Free. Radic. Biol. Med. 2016, 93, 41–51. [Google Scholar] [CrossRef]
- Raimondi, V.; Ciccarese, F.; Ciminale, V. Oncogenic pathways and the electron transport chain: A dangeROS liaison. Br. J.Cancer 2020, 122, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Harris, I.S.; DeNicola, G.M. The Complex Interplay between Antioxidants and ROS in Cancer. Trends Cell Biol. 2020, 30, 440–451. [Google Scholar] [CrossRef]
- Ghosh, P.; Vidal, C.; Dey, S.; Zhang, L. Mitochondria Targeting as an Effective Strategy for Cancer Therapy. Int. J. Mol. Sci. 2020, 21, E3363. [Google Scholar] [CrossRef] [PubMed]
- Savagner, F.; Franc, B.; Guyetant, S.; Rodien, P.; Reynier, P.; Malthiery, Y. Defective Mitochondrial ATP Synthesis in Oxyphilic Thyroid Tumors. J. Clin. Endocrinol. Metab. 2001, 86, 4920–4925. [Google Scholar] [CrossRef] [PubMed]
- Callegari, S.; Cruz-Zaragoza, L.D.; Rehling, P. From TOM to the TIM23 complex-handing over of a precursor. Biol. Chem. 2020, 401, 709–721. [Google Scholar] [CrossRef]
- Mohd Khair, S.Z.N.; Abd Radzak, S.M.; Mohamed Yusoff, A.A. The Uprising of Mitochondrial DNA Biomarker in Cancer. Dis. Markers 2021, 2021, 7675269. [Google Scholar] [CrossRef]
- Kraft, L.M.; Lackner, L.L. Mitochondrial anchors: Positioning mitochondria and more. Biochem. Biophys Res. Commun. 2018, 500, 2–8. [Google Scholar] [CrossRef]
- Maycotte, P.; Marín-Hernández, A.; Goyri-Aguirre, M.; Anaya-Ruiz, M.; Reyes-Leyva, J.; Cortés-Hernández, P. Mitochondrial dynamics and cancer. Tumour. Biol. 2017, 39, 1010428317698391. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Q.; Guan, H.; Shi, B.; Ji, M.; Hou, P. ZNF677 Suppresses Akt Phosphorylation and Tumorigenesis in Thyroid Cancer. Cancer Res. 2018, 78, 5216–5228. [Google Scholar] [CrossRef]
- Manning, B.D.; Cantley, L.C. AKT/PKB Signaling: Navigating Downstream. Cell 2007, 129, 1261–1274. [Google Scholar] [CrossRef]
- Coelho, R.G.; Fortunato, R.S.; Carvalho, D.P. Metabolic Reprogramming in Thyroid Carcinoma. Front. Oncol. 2018, 8, 82. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Lindsay, J.G. The Peroxiredoxin Family: An Unfolding Story. Subcell Biochem. 2017, 83, 127–147. [Google Scholar]
- Wonsey, D.R.; Zeller, K.I.; Dang, C.V. The c-Myc target gene PRDX3 is required for mitochondrial homeostasis and neoplastic transformation. Proc. Natl. Acad. Sci. USA 2002, 99, 6649–6654. [Google Scholar] [CrossRef]
- Fontana, G.A.; Gahlon, H.L. Mechanisms of replication and repair in mitochondrial DNA deletion formation. Nucleic. Acids Res. 2020, 48, 11244–11258. [Google Scholar] [CrossRef] [PubMed]
- Rostami, A.; Lambie, M.; Yu, C.W.; Stambolic, V.; Waldron, J.N.; Bratman, S.V. Senescence, Necrosis, and Apoptosis Govern Circulating Cell-free DNA Release Kinetics. Cell Rep. 2020, 31, 107830. [Google Scholar] [CrossRef] [PubMed]
- Martins, I.; Ribeiro, I.P.; Jorge, J.; Gonçalves, A.C.; Sarmento-Ribeiro, A.B.; Melo, J.B.; Carreira, I.M. Liquid Biopsies: Applications for Cancer Diagnosis and Monitoring. Genes 2021, 12, 349. [Google Scholar] [CrossRef] [PubMed]
- Di, W.; Lv, J.; Jiang, S.; Lu, C.; Yang, Z.; Ma, Z.; Hu, W.; Yang, Y.; Xu, B. PGC-1: The Energetic Regulator in Cardiac Metabolism. Curr. Issues Mol. Biol. 2018, 28, 29–46. [Google Scholar] [CrossRef]
- Valle, I.; Álvarez-Barrientos, A.; Arza, E.; Lamas, S.; Monsalve, M. PGC-1α regulates the mitochondrial antioxidant defense system in vascular endothelial cells. Cardiovasc. Res. 2005, 66, 562–573. [Google Scholar] [CrossRef]
- Cantelmo, A.R.; Conradi, L.-C.; Brajic, A.; Goveia, J.; Kalucka, J.; Pircher, A.; Chaturvedi, P.; Hol, J.; Thienpont, B.; Teuwen, L.-A.; et al. Inhibition of the Glycolytic Activator PFKFB3 in Endothelium Induces Tumor Vessel Normalization, Impairs Metastasis, and Improves Chemotherapy. Cancer Cell. 2016, 30, 968–985. [Google Scholar] [CrossRef]
- Neagu, M.; Constantin, C.; Popescu, I.D.; Zipeto, D.; Tzanakakis, G.; Nikitovic, D.; Fenga, C.; Stratakis, C.A.; Spandidos, D.A.; Tsatsakis, A.M. Inflammation and Metabolism in Cancer Cell—Mitochondria Key Player. Front. Oncol. 2019, 9, 348. [Google Scholar] [CrossRef]
- Ziegler, A.; Koch, A.; Krockenberger, K.; Großhennig, A. Personalized medicine using DNA biomarkers: A review. Hum Genet. 2012, 131, 1627–1638. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Jia, Q.; Tan, J.; Meng, Z. Serum biomarkers for thyroid cancer. Biomark Med. 2020, 14, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Pessoa, L.S.; Heringer, M.; Ferrer, V.P. ctDNA as a cancer biomarker: A broad overview. Crit. Rev. Oncol. Hematol. 2020, 155, 103109. [Google Scholar] [CrossRef] [PubMed]
- Kustanovich, A.; Schwartz, R.; Peretz, T.; Grinshpun, A. Life and death of circulating cell-free DNA. Cancer Biol. Ther. 2019, 20, 1057–1067. [Google Scholar] [CrossRef]
- Reznik, E.; Miller, M.L.; Şenbabaoğlu, Y.; Riaz, N.; Sarungbam, J.; Tickoo, S.K.; Al-Ahmadie, H.A.; Lee, W.; Seshan, V.E.; Hakimi, A.A.; et al. Mitochondrial DNA copy number variation across human cancers. eLife 2016, 5, e10769. [Google Scholar] [CrossRef]
- Chan, D.C. Mitochondrial Dynamics and Its Involvement in Disease. Annu. Rev. Pathol. 2020, 15, 235–259. [Google Scholar] [CrossRef]
- Srinivasan, S.; Guha, M.; Kashina, A.; Avadhani, N.G. Mitochondrial dysfunction and mitochondrial dynamics-The cancer connection. Biochim. Biophys Acta Bioenerg 2017, 1858, 602–614. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- Ismail, T.; Kim, Y.; Lee, H.; Lee, D.S.; Lee, H.S. Interplay Between Mitochondrial Peroxiredoxins and ROS in Cancer Development and Progression. Int. J. Mol. Sci. 2019, 20, E4407. [Google Scholar] [CrossRef]
- Fabregat-Andrés, Ó.; Tierrez, A.; Mata, M.; Estornell-Erill, J.; Ridocci-Soriano, F.; Monsalve, M. Induction of PGC-1α expression can be detected in blood samples of patients with ST-segment elevation acute myocardial infarction. PLoS One. 2011, 6, e26913. [Google Scholar] [CrossRef]
- Kang, Y.E.; Kim, H.J.; Shong, M. Regulation of Systemic Glucose Homeostasis by T Helper Type 2 Cytokines. Diabetes Metab. J. 2019, 43, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-C.; Xu, X.; Hajra, A.; Apple, S.; Kharawala, A.; Duarte, G.; Liaqat, W.; Fu, Y.; Li, W.; Chen, Y.; et al. Current Advancement in Diagnosing Atrial Fibrillation by Utilizing Wearable Devices and Artificial Intelligence: A Review Study. Diagnostics 2022, 12, 689. [Google Scholar] [CrossRef] [PubMed]
- Prieto, I.; Zambrano, A.; Laso, J.; Aranda, A.; Samper, E.; Monsalve, M. Early induction of senescence and immortalization in PGC-1α-deficient mouse embryonic fibroblasts. Free Radic. Biol. Med. 2019, 138, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Borniquel, S.; Valle, I.; Cadenas, S.; Lamas, S.; Monsalve, M. Nitric oxide regulates mitochondrial oxidative stress protection via the transcriptional coactivator PGC-1alpha. FASEB J. 2006, 20, 1889–1891. [Google Scholar] [CrossRef]
- Monsalve, M.; Wu, Z.; Adelmant, G.; Puigserver, P.; Fan, M.; Spiegelman, B.M. Direct coupling of transcription and mRNA processing through the thermogenic coactivator PGC-1. Mol. Cell. 2000, 6, 307–316. [Google Scholar] [CrossRef]
- Olmos, Y.; Sanchez-Gomez, F.J.; Wild, B.; Garcia-Quintans, N.; Cabezudo, S.; Lamas, S.; Monsalve, M. SirT1 regulation of antioxidant genes is dependent on the formation of a FoxO3a/PGC-1α complex. Antioxid. Redox Signal. 2013, 19, 1507–1521. [Google Scholar] [CrossRef]

| Cohort 1 n = 45 | p | Cohort 2 n = 28 | p | |||
|---|---|---|---|---|---|---|
| Hyperplasias n = 24 | Carcinomas n = 21 | ns | Hyperplasias n = 16 | Carcinomas n = 12 | ns | |
| Male | 3 | 5 | ns | 3 | 2 | ns |
| Female | 21 | 16 | ns | 13 | 10 | ns |
| Age | 62.43+/−12.87 | 52.76+/−16 | 0.032 | 53.06+/−12.80 | 53.83+/−13.5 | ns |
| BMI | 25.72+/−4.49 | 27.27+/−4.40 | ns | 29.67+/−7.37 | 28.76+/−5.10 | ns |
| Fasting glucose (mg/dl) | 98.33+/−14.86 | 93.85+/−10.80 | ns | 104.13+/−45.43 | 107.17+/−24.96 | ns |
| Cholesterol (mg/dl) | 209.69+/−25.98 | 206.00+/−43.17 | ns | 184.64+/−41.09 | 191.83+/−37.56 | ns |
| Triglycerides (mg/dl) | 122.13+/−84.18 | 116.83+/−67.43 | ns | 98.77+/−53.40 | 138.83+/−65.88 | ns |
| Smokers | 3 | 7 | ns | 2 | 3 | ns |
| Blood pressure (Max. mm Hg) | 137.33+/−18.41 | 135.50+/−15.58 | ns | 141.87+/−23.16 | 127.18+/−28.20 | ns |
| Blood pressure (Min. mm Hg) | 78.14+/−10.41 | 79.70+/−10.53 | ns | 81.31+/−15.70 | 79.18+/−11.81 | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernal-Tirapo, J.; Bayo Jiménez, M.T.; Yuste-García, P.; Cordova, I.; Peñas, A.; García-Borda, F.-J.; Quintela, C.; Prieto, I.; Sánchez-Ramos, C.; Ferrero-Herrero, E.; et al. Evaluation of Mitochondrial Function in Blood Samples Shows Distinct Patterns in Subjects with Thyroid Carcinoma from Those with Hyperplasia. Int. J. Mol. Sci. 2023, 24, 6453. https://doi.org/10.3390/ijms24076453
Bernal-Tirapo J, Bayo Jiménez MT, Yuste-García P, Cordova I, Peñas A, García-Borda F-J, Quintela C, Prieto I, Sánchez-Ramos C, Ferrero-Herrero E, et al. Evaluation of Mitochondrial Function in Blood Samples Shows Distinct Patterns in Subjects with Thyroid Carcinoma from Those with Hyperplasia. International Journal of Molecular Sciences. 2023; 24(7):6453. https://doi.org/10.3390/ijms24076453
Chicago/Turabian StyleBernal-Tirapo, Julia, María Teresa Bayo Jiménez, Pedro Yuste-García, Isabel Cordova, Ana Peñas, Francisco-Javier García-Borda, Cesar Quintela, Ignacio Prieto, Cristina Sánchez-Ramos, Eduardo Ferrero-Herrero, and et al. 2023. "Evaluation of Mitochondrial Function in Blood Samples Shows Distinct Patterns in Subjects with Thyroid Carcinoma from Those with Hyperplasia" International Journal of Molecular Sciences 24, no. 7: 6453. https://doi.org/10.3390/ijms24076453
APA StyleBernal-Tirapo, J., Bayo Jiménez, M. T., Yuste-García, P., Cordova, I., Peñas, A., García-Borda, F.-J., Quintela, C., Prieto, I., Sánchez-Ramos, C., Ferrero-Herrero, E., & Monsalve, M. (2023). Evaluation of Mitochondrial Function in Blood Samples Shows Distinct Patterns in Subjects with Thyroid Carcinoma from Those with Hyperplasia. International Journal of Molecular Sciences, 24(7), 6453. https://doi.org/10.3390/ijms24076453





