Mir-302a/TWF1 Axis Impairs the Myogenic Differentiation of Progenitor Cells through F-Actin-Mediated YAP1 Activation
Abstract
1. Introduction
2. Results
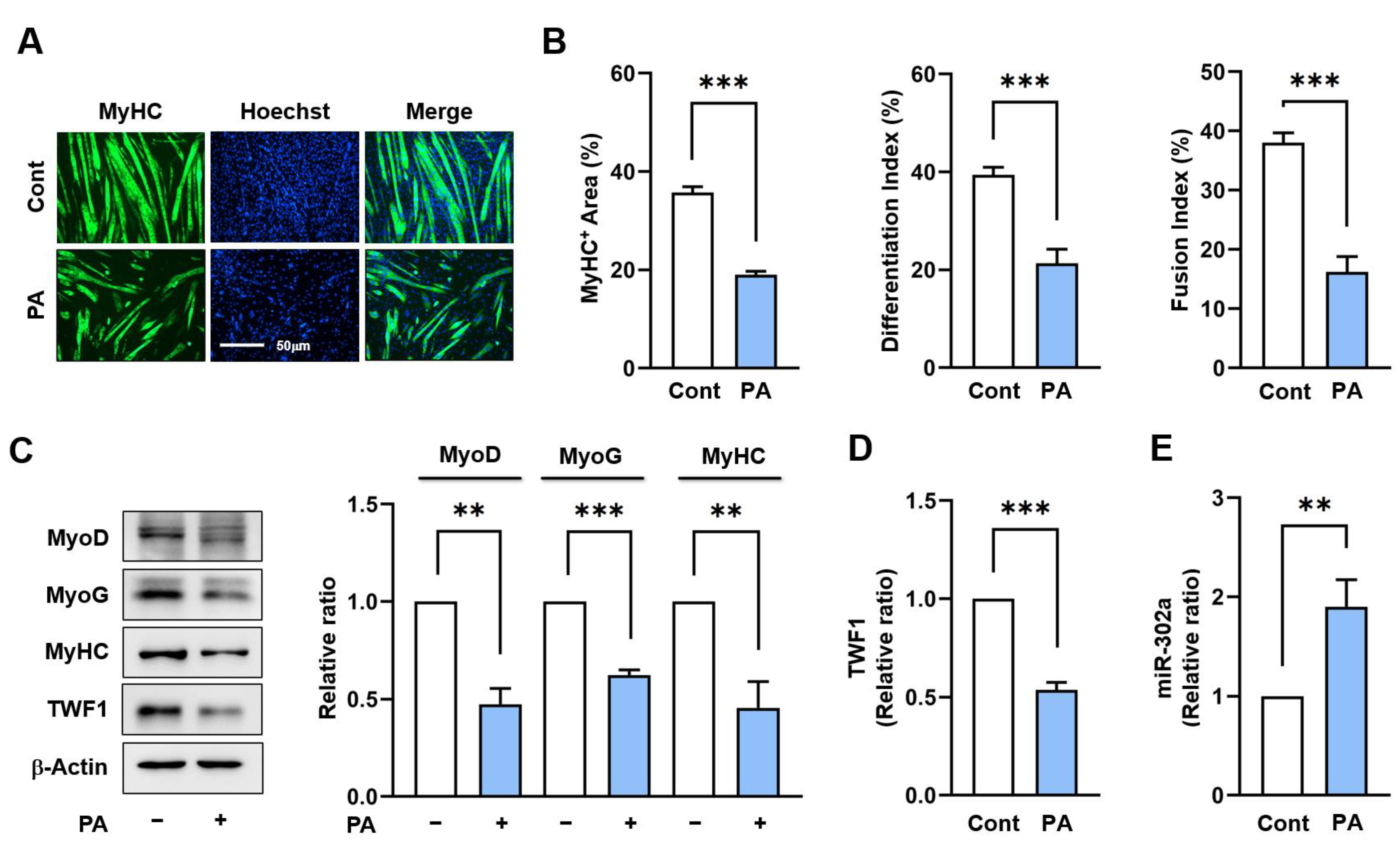
2.1. PA Suppresses TWF1 but Increases miR-302a Expression in Myoblasts
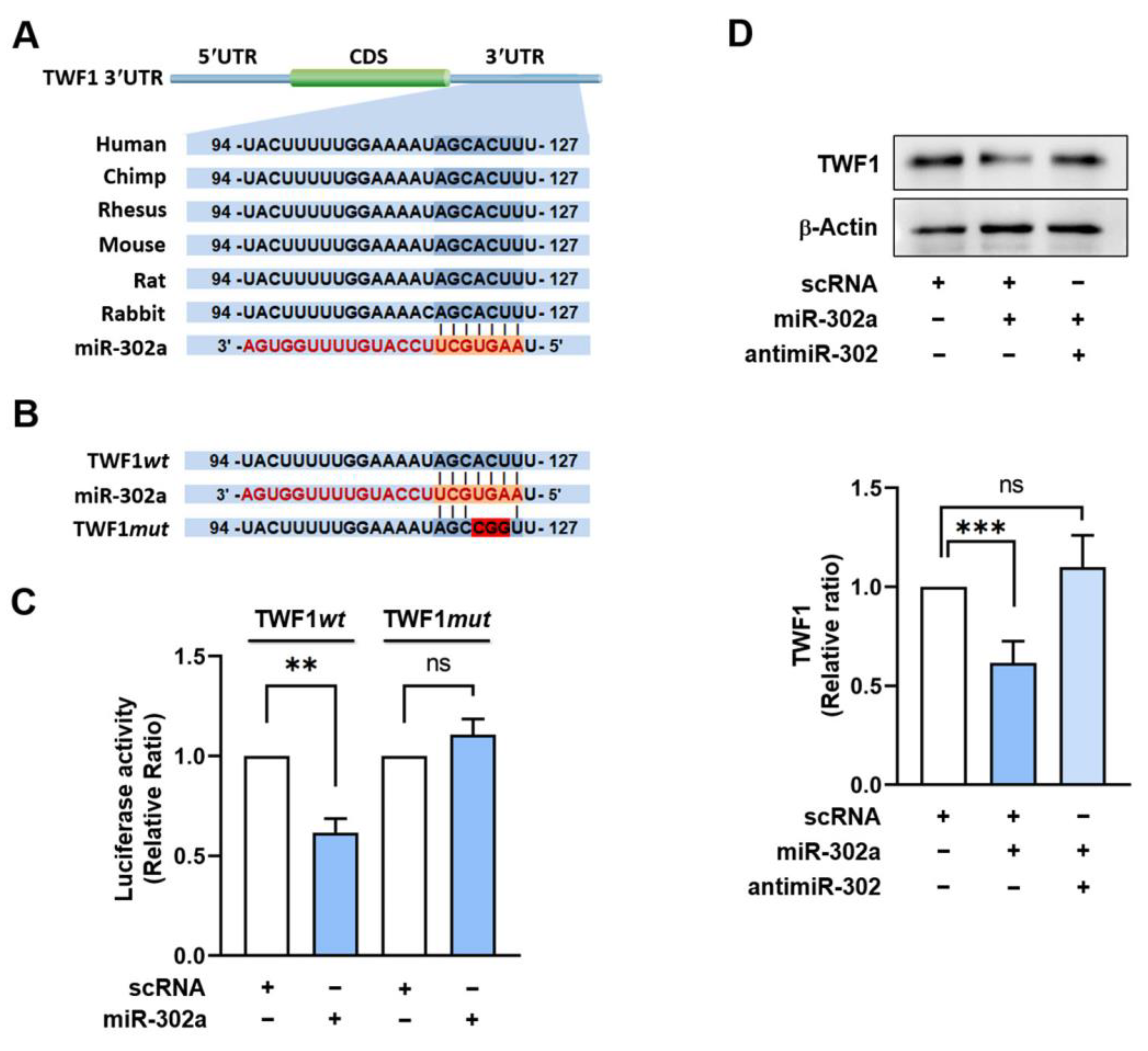
2.2. TWF1 Is Directly Targeted by miR-302a
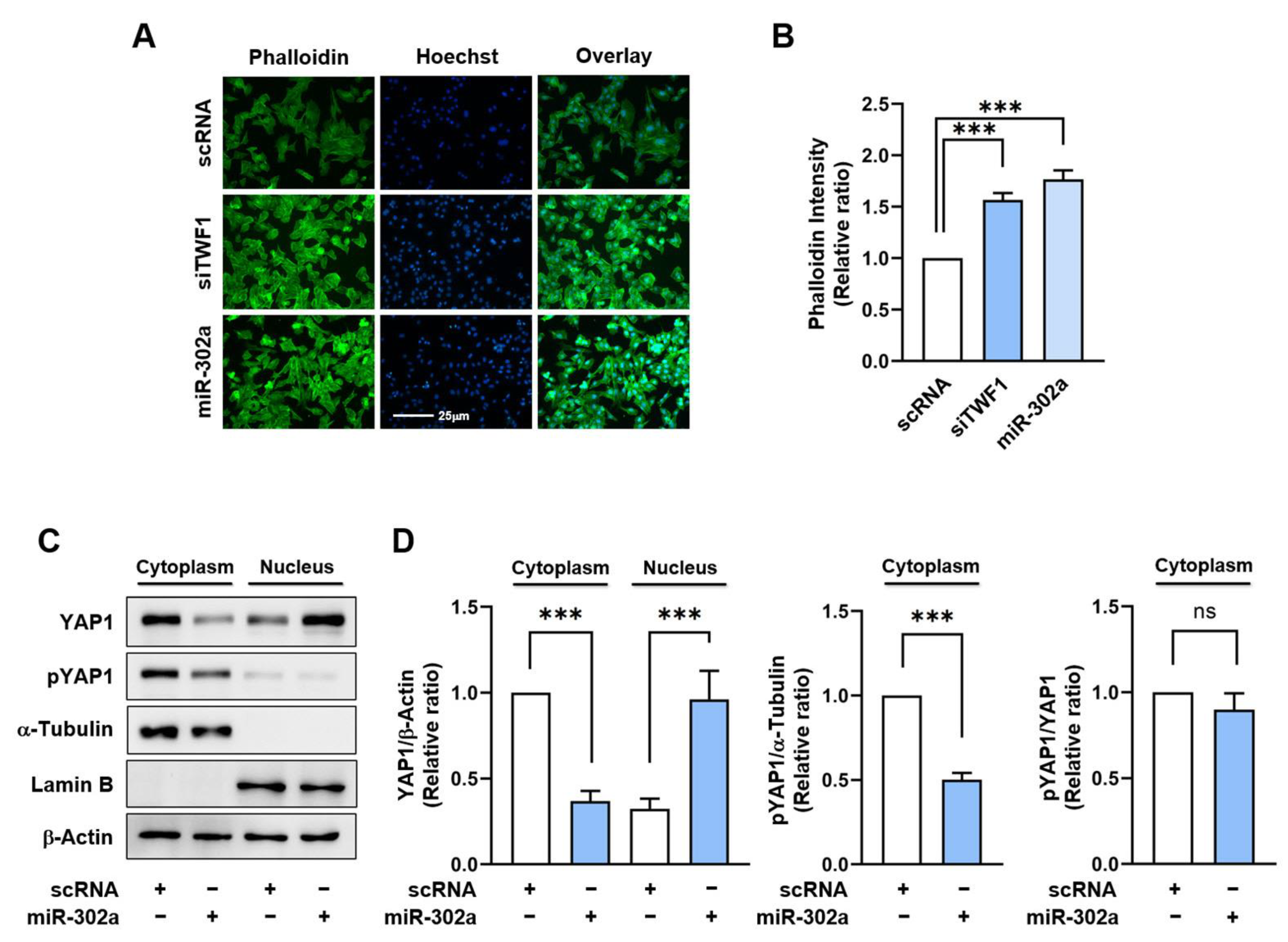
2.3. MiR-302a Increases F-Actin Accumulation and the Nuclear Translocation of YAP1
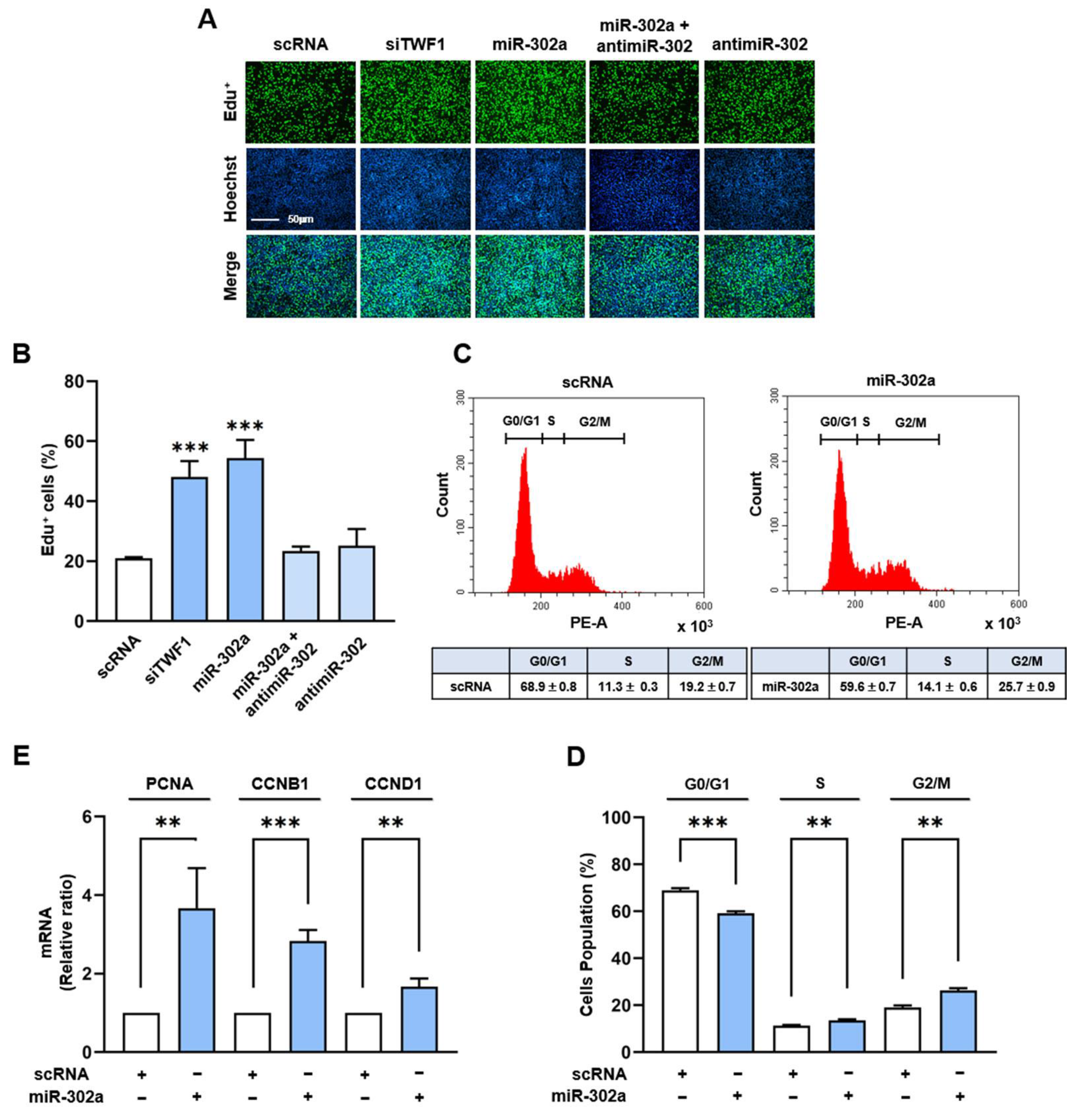
2.4. MiR-302a Increases Myoblast Proliferation
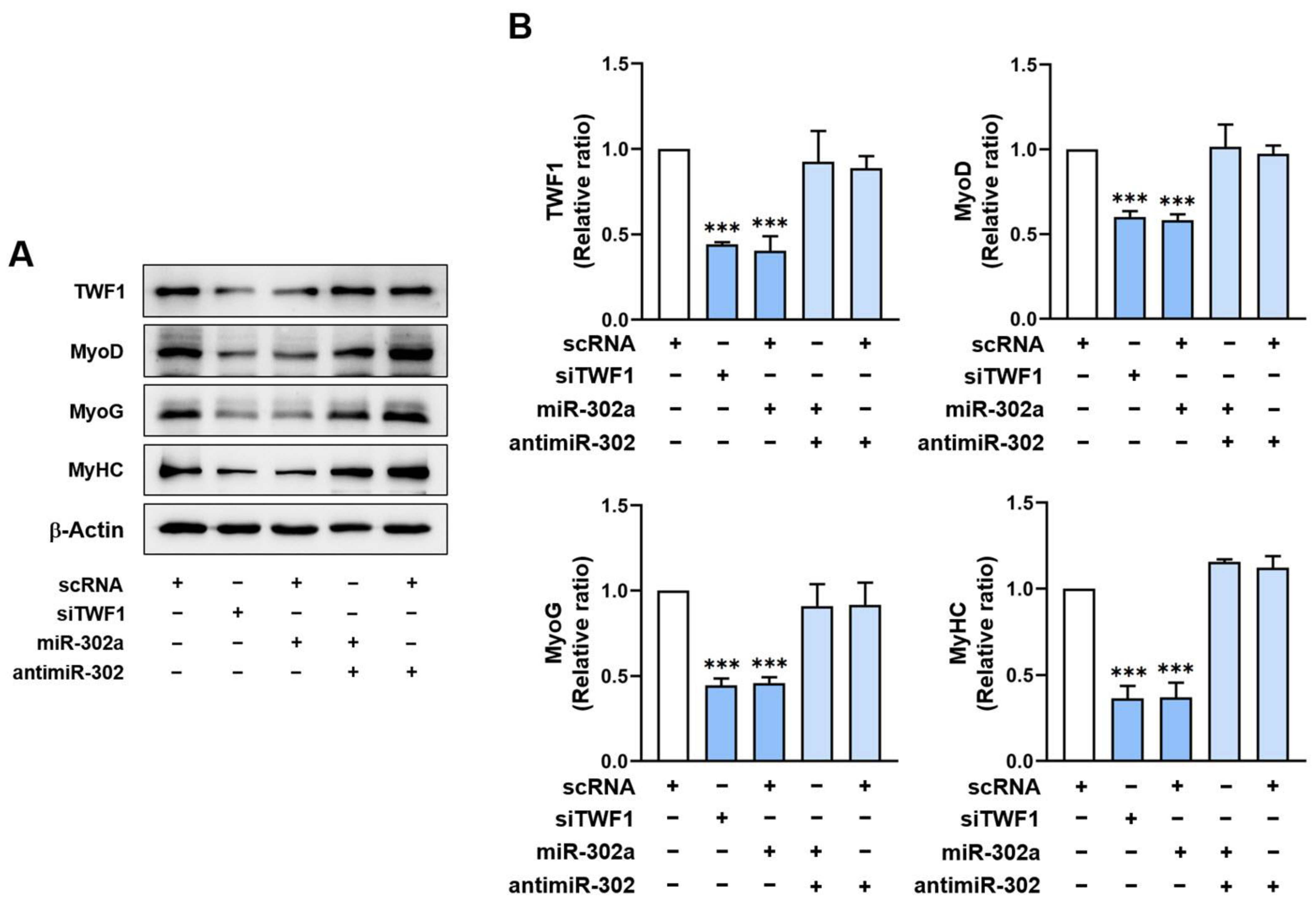
2.5. MiR-302a Suppresses Myogenic Factor Expressions
2.6. MiR-302a Impairs Differentiation and Myotube Formation of Myoblasts
3. Discussion
4. Materials and Methods
4.1. Cell Culture and PA Treatment
4.2. Transfection of Oligonucleotides
4.3. Dual-Luciferase Reporter Assay
4.4. RT-qPCR
4.5. Cell Fractionation for Cytoplasm and Nucleus
4.6. Immunoblotting
4.7. Immunofluorescence Analysis
4.8. Ethynyl Deoxyuridine (EdU) Assay
4.9. Flow Cytometry Assay
4.10. Database and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mukund, K.; Subramaniam, S. Skeletal muscle: A review of molecular structure and function, in health and disease. Wiley Interdiscip. Rev. Syst. Biol. Med. 2020, 12, e1462. [Google Scholar] [CrossRef]
- Yusuf, F.; Brand-Saberi, B. Myogenesis and muscle regeneration. Histochem. Cell Biol. 2012, 138, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Chal, J.; Pourquie, O. Making muscle: Skeletal myogenesis in vivo and in vitro. Development 2017, 144, 2104–2122. [Google Scholar] [CrossRef]
- Sartori, R.; Romanello, V.; Sandri, M. Mechanisms of muscle atrophy and hypertrophy: Implications in health and disease. Nat. Commun. 2021, 12, 330. [Google Scholar] [CrossRef] [PubMed]
- Akhmedov, D.; Berdeaux, R. The effects of obesity on skeletal muscle regeneration. Front. Physiol. 2013, 4, 371. [Google Scholar] [CrossRef] [PubMed]
- Teng, S.; Huang, P. The effect of type 2 diabetes mellitus and obesity on muscle progenitor cell function. Stem Cell Res. Ther. 2019, 10, 103. [Google Scholar] [CrossRef] [PubMed]
- Abramovici, H.; Gee, S.H. Morphological changes and spatial regulation of diacylglycerol kinase-zeta, syntrophins, and Rac1 during myoblast fusion. Cell Motil. Cytoskelet. 2007, 64, 549–567. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, S.; Shin, H.; Uhm, C.S. Intercellular interaction observed by atomic force microscopy. Ultramicroscopy 2008, 108, 1148–1151. [Google Scholar] [CrossRef]
- Guerin, C.M.; Kramer, S.G. Cytoskeletal remodeling during myotube assembly and guidance: Coordinating the actin and microtubule networks. Commun. Integr. Biol. 2009, 2, 452–457. [Google Scholar] [CrossRef]
- Heng, Y.W.; Koh, C.G. Actin cytoskeleton dynamics and the cell division cycle. Int. J. Biochem. Cell Biol. 2010, 42, 1622–1633. [Google Scholar] [CrossRef]
- Watt, K.I.; Goodman, C.A.; Hornberger, T.A.; Gregorevic, P. The Hippo Signaling Pathway in the Regulation of Skeletal Muscle Mass and Function. Exerc. Sport Sci. Rev. 2018, 46, 92–96. [Google Scholar] [CrossRef]
- Fischer, M.; Rikeit, P.; Knaus, P.; Coirault, C. YAP-Mediated Mechanotransduction in Skeletal Muscle. Front. Physiol. 2016, 7, 41. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Nakamura, F. Actin-Associated Proteins and Small Molecules Targeting the Actin Cytoskeleton. Int. J. Mol. Sci. 2022, 23, 2118. [Google Scholar] [CrossRef] [PubMed]
- Balaban, C.; Sztacho, M.; Blazikova, M.; Hozak, P. The F-Actin-Binding MPRIP Forms Phase-Separated Condensates and Associates with PI(4,5)P2 and Active RNA Polymerase II in the Cell Nucleus. Cells 2021, 10, 848. [Google Scholar] [CrossRef] [PubMed]
- Balaban, C.; Sztacho, M.; Antiga, L.; Miladinović, A.; Harata, M.; Hozák, P. PIP2-Effector Protein MPRIP Regulates RNA Polymerase II Condensation and Transcription. Biomolecules 2023, 13, 426. [Google Scholar] [CrossRef]
- Nguyen, N.U.; Liang, V.R.; Wang, H.V. Actin-associated protein palladin is required for migration behavior and differentiation potential of C2C12 myoblast cells. Biochem. Biophys. Res. Commun. 2014, 452, 728–733. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Hou, L.; Zhang, Y.; Jiang, F.; Zhu, Y.; Li, Q.X.; Hu, C.Y.; Wang, C. PFN2a Suppresses C2C12 Myogenic Development by Inhibiting Proliferation and Promoting Apoptosis via the p53 Pathway. Cells 2019, 8, 959. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.T.; Min, K.H.; Kim, D.; Park, S.Y.; Lee, W. CFL2 is an essential mediator for myogenic differentiation in C2C12 myoblasts. Biochem. Biophys. Res. Commun. 2020, 533, 710–716. [Google Scholar] [CrossRef]
- Dupont, S.; Morsut, L.; Aragona, M.; Enzo, E.; Giulitti, S.; Cordenonsi, M.; Zanconato, F.; Le Digabel, J.; Forcato, M.; Bicciato, S.; et al. Role of YAP/TAZ in mechanotransduction. Nature 2011, 474, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Aragona, M.; Panciera, T.; Manfrin, A.; Giulitti, S.; Michielin, F.; Elvassore, N.; Dupont, S.; Piccolo, S. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell 2013, 154, 1047–1059. [Google Scholar] [CrossRef]
- Nguyen, M.T.; Won, Y.H.; Kwon, T.W.; Lee, W. Twinfilin-1 is an essential regulator of myogenic differentiation through the modulation of YAP in C2C12 myoblasts. Biochem. Biophys. Res. Commun. 2022, 599, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Krol, J.; Loedige, I.; Filipowicz, W. The widespread regulation of microRNA biogenesis, function and decay. Nat. Rev. Genet. 2010, 11, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.T.; Lee, W. MiR-141-3p regulates myogenic differentiation in C2C12 myoblasts via CFL2-YAP-mediated mechanotransduction. BMB Rep. 2022, 55, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.T.; Min, K.H.; Lee, W. MiR-96-5p Induced by Palmitic Acid Suppresses the Myogenic Differentiation of C2C12 Myoblasts by Targeting FHL1. Int. J. Mol. Sci. 2020, 21, 9445. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, Y.; Song, C.; Chen, Y.; Wang, Y.; Lai, M.; Zhang, C.; Fang, X. MiR-424-5p targets HSP90AA1 to facilitate proliferation and restrain differentiation in skeletal muscle development. Anim. Biotechnol. 2022, 1–13. [Google Scholar] [CrossRef]
- Dowling, L.; Duseja, A.; Vilaca, T.; Walsh, J.S.; Goljanek-Whysall, K. MicroRNAs in obesity, sarcopenia, and commonalities for sarcopenic obesity: A systematic review. J. Cachexia Sarcopenia Muscle 2022, 13, 68–85. [Google Scholar] [CrossRef]
- Silveira, A.; Gomes, J.; Roque, F.; Fernandes, T.; de Oliveira, E.M. MicroRNAs in Obesity-Associated Disorders: The Role of Exercise Training. Obes. Facts 2022, 15, 105–117. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, M.; Lian, D.; Li, Y.; Li, Y.; Wang, J.; Deng, S.; Yu, K.; Lian, Z. Non-Coding RNA Regulates the Myogenesis of Skeletal Muscle Satellite Cells, Injury Repair and Diseases. Cells 2019, 8, 988. [Google Scholar] [CrossRef]
- Mok, G.F.; Lozano-Velasco, E.; Munsterberg, A. microRNAs in skeletal muscle development. Semin. Cell Dev. Biol. 2017, 72, 67–76. [Google Scholar] [CrossRef]
- Sannicandro, A.J.; Soriano-Arroquia, A.; Goljanek-Whysall, K. Micro(RNA)-managing muscle wasting. J. Appl. Physiol. 2019, 127, 619–632. [Google Scholar] [CrossRef]
- Ji, C.; Guo, X. The clinical potential of circulating microRNAs in obesity. Nat. Rev. Endocrinol. 2019, 15, 731–743. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Dosal, A.; Rodil-Garcia, P.; Salazar-Olivo, L.A. Circulating microRNAs in human obesity: A systematic review. Biomarkers 2019, 24, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Gan, L.; Si, J.; Zhang, J.; Liu, Z.; Zhao, J.; Gou, Z.; Zhang, H. Role of miR-302/367 cluster in human physiology and pathophysiology. Acta Biochim. Biophys. Sin. 2020, 52, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.; Jiang, J.; Li, Y.; Fu, L.; Meng, F.; Li, J. MiR-302a-3p aggravates myocardial ischemia-reperfusion injury by suppressing mitophagy via targeting FOXO3. Exp. Mol. Pathol. 2020, 117, 104522. [Google Scholar] [CrossRef]
- Yang, W.M.; Min, K.H.; Lee, W. MicroRNA expression analysis in the liver of high fat diet-induced obese mice. Data Brief 2016, 9, 1155–1159. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.M.; Min, K.H.; Lee, W. Data for differentially expressed microRNAs in saturated fatty acid palmitate-treated HepG2 cells. Data Brief 2016, 9, 996–999. [Google Scholar] [CrossRef] [PubMed]
- Hohjoh, H.; Fukushima, T. Marked change in microRNA expression during neuronal differentiation of human teratocarcinoma NTera2D1 and mouse embryonal carcinoma P19 cells. Biochem. Biophys. Res. Commun. 2007, 362, 360–367. [Google Scholar] [CrossRef]
- Zhao, B.; Huang, B.; Li, W.; Jin, Y. MicroRNA expression profiling during neural differentiation of mouse embryonic carcinoma P19 cells. Methods Mol. Biol. 2013, 936, 105–116. [Google Scholar] [CrossRef]
- Nguyen, M.T.; Lee, W. MiR-320-3p Regulates the Proliferation and Differentiation of Myogenic Progenitor Cells by Modulating Actin Remodeling. Int. J. Mol. Sci. 2022, 23, 801. [Google Scholar] [CrossRef] [PubMed]
- Dupont, S. Role of YAP/TAZ in cell-matrix adhesion-mediated signalling and mechanotransduction. Exp. Cell Res. 2016, 343, 42–53. [Google Scholar] [CrossRef]
- Heng, B.C.; Zhang, X.H.; Aubel, D.; Bai, Y.Y.; Li, X.C.; Wei, Y.; Fussenegger, M.; Deng, X.L. An overview of signaling pathways regulating YAP/TAZ activity. Cell Mol. Life Sci. 2020, 78, 497–512. [Google Scholar] [CrossRef] [PubMed]
- Sansores-Garcia, L.; Bossuyt, W.; Wada, K.; Yonemura, S.; Tao, C.; Sasaki, H.; Halder, G. Modulating F-actin organization induces organ growth by affecting the Hippo pathway. EMBO J. 2011, 30, 2325–2335. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Gu, W.; Zhang, Y.; Jiang, B.; Qiao, X.; Wen, Y. Activated Yes-Associated Protein Accelerates Cell Cycle, Inhibits Apoptosis, and Delays Senescence in Human Periodontal Ligament Stem Cells. Int. J. Med. Sci. 2018, 15, 1241–1250. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Cho, Y.S.; Wang, X.; Park, O.; Ma, X.; Kim, H.; Gan, W.; Jho, E.H.; Cha, B.; Jeung, Y.J.; et al. Hippo signaling is intrinsically regulated during cell cycle progression by APC/C(Cdh1). Proc. Natl. Acad. Sci. USA 2019, 116, 9423–9432. [Google Scholar] [CrossRef] [PubMed]
- Abreu, P.; Leal-Cardoso, J.H.; Ceccatto, V.M.; Hirabara, S.M. Regulation of muscle plasticity and trophism by fatty acids: A short review. Rev. Assoc. Med. Bras. (1992) 2017, 63, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Poukkula, M.; Kremneva, E.; Serlachius, M.; Lappalainen, P. Actin-depolymerizing factor homology domain: A conserved fold performing diverse roles in cytoskeletal dynamics. Cytoskeleton 2011, 68, 471–490. [Google Scholar] [CrossRef]
- Palmgren, S.; Vartiainen, M.; Lappalainen, P. Twinfilin, a molecular mailman for actin monomers. J. Cell Sci. 2002, 115, 881–886. [Google Scholar] [CrossRef]
- Johnston, A.B.; Collins, A.; Goode, B.L. High-speed depolymerization at actin filament ends jointly catalysed by Twinfilin and Srv2/CAP. Nat. Cell Biol. 2015, 17, 1504–1511. [Google Scholar] [CrossRef]
- Moseley, J.B.; Okada, K.; Balcer, H.I.; Kovar, D.R.; Pollard, T.D.; Goode, B.L. Twinfilin is an actin-filament-severing protein and promotes rapid turnover of actin structures in vivo. J. Cell Sci. 2006, 119, 1547–1557. [Google Scholar] [CrossRef]
- Wang, Y.J.; Yu, C.; Wu, W.W.; Ju, Y.Y.; Liu, Y.; Xu, C.; Long, J.D.; Zan, G.Y.; Wei, X.Y.; Zhang, L.S.; et al. Alteration of twinfilin1 expression underlies opioid withdrawal-induced remodeling of actin cytoskeleton at synapses and formation of aversive memory. Mol. Psychiatry 2021, 26, 6218–6236. [Google Scholar] [CrossRef]
- Hakala, M.; Wioland, H.; Tolonen, M.; Kotila, T.; Jegou, A.; Romet-Lemonne, G.; Lappalainen, P. Twinfilin uncaps filament barbed ends to promote turnover of lamellipodial actin networks. Nat. Cell Biol. 2021, 23, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Mendez, M.G.; Janmey, P.A. Transcription factor regulation by mechanical stress. Int. J. Biochem. Cell Biol. 2012, 44, 728–732. [Google Scholar] [CrossRef] [PubMed]
- Pan, D. The hippo signaling pathway in development and cancer. Dev. Cell 2010, 19, 491–505. [Google Scholar] [CrossRef] [PubMed]
- Pike, L.S.; Smift, A.L.; Croteau, N.J.; Ferrick, D.A.; Wu, M. Inhibition of fatty acid oxidation by etomoxir impairs NADPH production and increases reactive oxygen species resulting in ATP depletion and cell death in human glioblastoma cells. Bba-Bioenergetics 2011, 1807, 726–734. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, M.T.; Lee, W. Mir-302a/TWF1 Axis Impairs the Myogenic Differentiation of Progenitor Cells through F-Actin-Mediated YAP1 Activation. Int. J. Mol. Sci. 2023, 24, 6341. https://doi.org/10.3390/ijms24076341
Nguyen MT, Lee W. Mir-302a/TWF1 Axis Impairs the Myogenic Differentiation of Progenitor Cells through F-Actin-Mediated YAP1 Activation. International Journal of Molecular Sciences. 2023; 24(7):6341. https://doi.org/10.3390/ijms24076341
Chicago/Turabian StyleNguyen, Mai Thi, and Wan Lee. 2023. "Mir-302a/TWF1 Axis Impairs the Myogenic Differentiation of Progenitor Cells through F-Actin-Mediated YAP1 Activation" International Journal of Molecular Sciences 24, no. 7: 6341. https://doi.org/10.3390/ijms24076341
APA StyleNguyen, M. T., & Lee, W. (2023). Mir-302a/TWF1 Axis Impairs the Myogenic Differentiation of Progenitor Cells through F-Actin-Mediated YAP1 Activation. International Journal of Molecular Sciences, 24(7), 6341. https://doi.org/10.3390/ijms24076341






