Learning in the Single-Cell Organism Physarum polycephalum: Effect of Propofol
Abstract
1. Introduction
2. Results
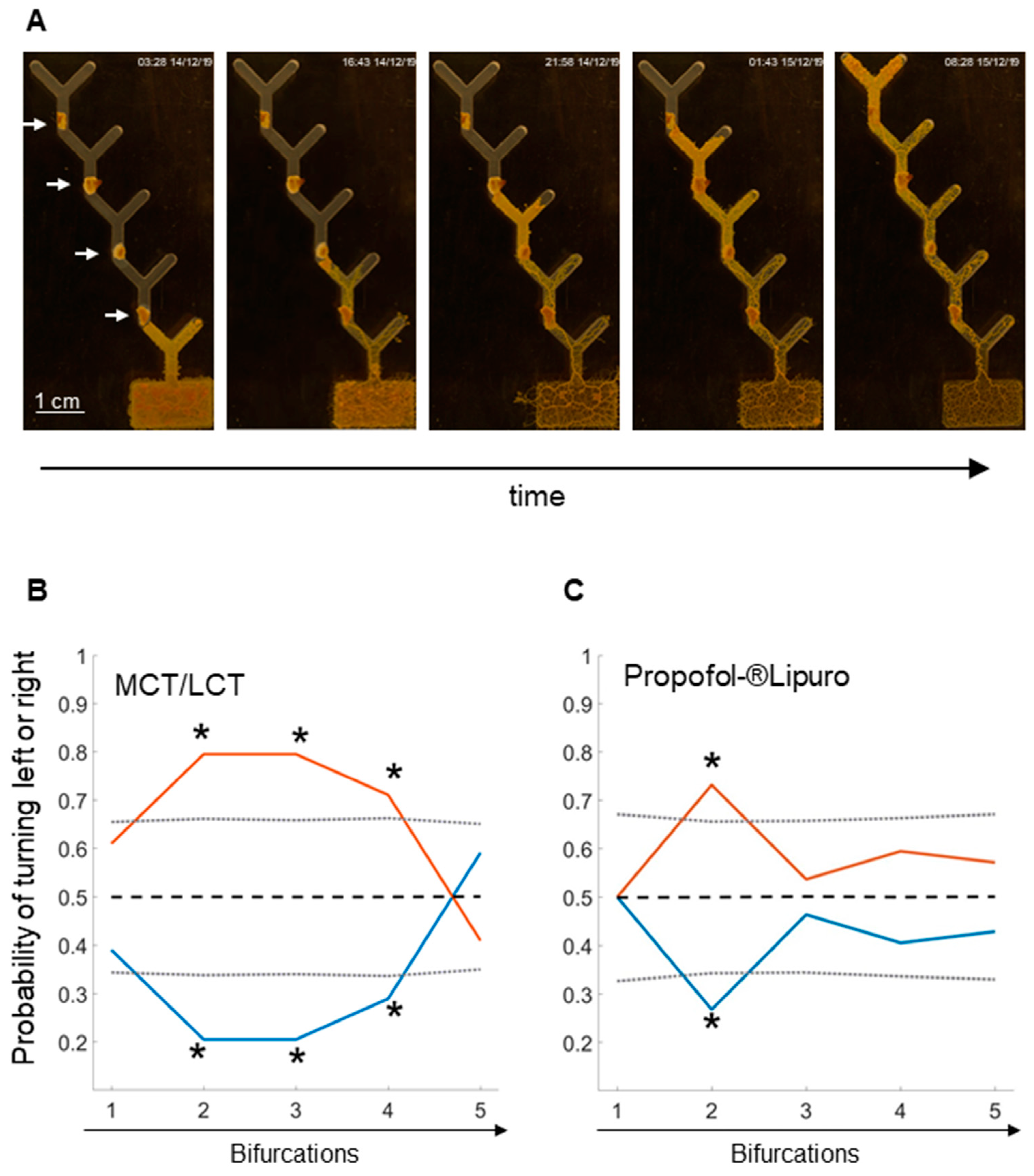
2.1. Learning in the 5-Level Y-Maze
2.2. Propofol Repels Physarum in the 1-Level Y-Maze
2.3. GABA Receptor Transcripts in Physarum
3. Discussion
4. Materials and Methods
4.1. Culturing of Physarum polycephalum
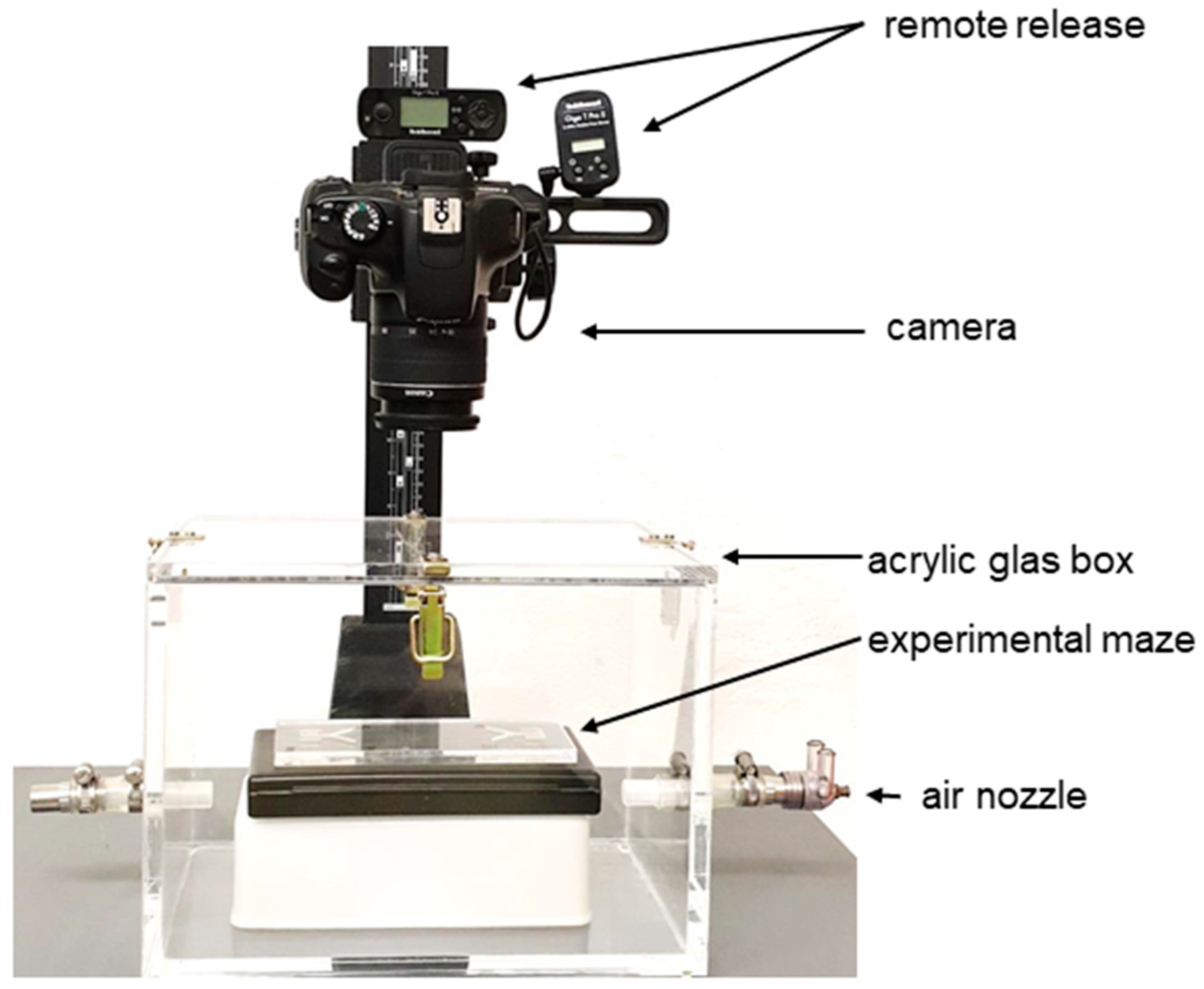
4.2. Experimental Procedure
4.3. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ben Jacob, E.; Becker, I.; Shapira, Y.; Levine, H. Bacterial linguistic communication and social intelligence. Trends Microbiol. 2004, 12, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Hellingwerf, K.J. Bacterial observations: A rudimentary form of intelligence? Trends Microbiol. 2005, 13, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Armus, H.L.; Montgomery, A.R.; Gurney, R.L. Discrimination learning and extinction in paramecia (P. caudatum). Psychol. Rep. 2006, 98, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Kunita, I.; Yamaguchi, T.; Tero, A.; Akiyama, M.; Kuroda, S.; Nakagaki, T. A ciliate memorizes the geometry of a swimming arena. J. R. Soc. Interface R. Soc. 2016, 13, 20160155. [Google Scholar] [CrossRef]
- Nakagaki, T.; Yamada, H.; Toth, A. Maze-solving by an amoeboid organism. Nature 2000, 407, 470. [Google Scholar] [CrossRef]
- Tero, A.; Takagi, S.; Saigusa, T.; Ito, K.; Bebber, D.P.; Fricker, M.D.; Yumiki, K.; Kobayashi, R.; Nakagaki, T. Rules for Biologically Inspired Adaptive Network Design. Science 2010, 327, 439–442. [Google Scholar] [CrossRef]
- Zhu, L.; Aono, M.; Kim, S.J.; Hara, M. Amoeba-based computing for traveling salesman problem: Long-term correlations between spatially separated individual cells of Physarum polycephalum. Biosystems 2013, 112, 1–10. [Google Scholar] [CrossRef]
- Saigusa, T.; Tero, A.; Nakagaki, T.; Kuramoto, Y. Amoebae anticipate periodic events. Phys. Rev. Lett. 2008, 100, 018101. [Google Scholar] [CrossRef]
- Shirakawa, T.; Gunji, Y.-P.; Miyake, Y. An associative learning experiment using the plasmodium of Physarum polycephalum. Nano Commun. Netw. 2011, 2, 99–105. [Google Scholar] [CrossRef]
- Shirakawa, T.; Sato, H. Construction of a Molecular Learning Network. J. Adv. Comput. Intell. 2013, 17, 913–918. [Google Scholar] [CrossRef]
- Vogel, D.; Dussutour, A. Direct transfer of learned behaviour via cell fusion in non-neural organisms. Proc. R. Soc. B Biol. Sci. 2016, 283, 20162382. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Cho, Y.J.; Ahn, E.J.; Choi, G.J.; Kang, H. Pharmacological strategies to prevent postoperative delirium: A systematic review and network meta-analysis. Anesth. Pain Med. 2021, 16, 28–48. [Google Scholar] [CrossRef]
- Walsh, C.T. Propofol: Milk of Amnesia. Cell 2018, 175, 10–13. [Google Scholar] [CrossRef]
- Schaap, P.; Barrantes, I.; Minx, P.; Sasaki, N.; Anderson, R.W.; Bénard, M.; Biggar, K.K.; Buchler, N.E.; Bundschuh, R.; Chen, X.; et al. The Physarum polycephalum Genome Reveals Extensive Use of Prokaryotic Two-Component and Metazoan-Type Tyrosine Kinase Signaling. Genome Biol. Evol. 2015, 8, 109–125. [Google Scholar] [CrossRef] [PubMed]
- Boly, M.; Seth, A.K.; Wilke, M.; Ingmundson, P.; Baars, B.; Laureys, S.; Edelman, D.B.; Tsuchiya, N. Consciousness in humans and non-human animals: Recent advances and future directions. Front. Psychol. 2013, 4, 625. [Google Scholar] [CrossRef] [PubMed]
- Friston, K. Prediction, perception and agency. Int. J. Psychophysiol. Off. J. Int. Organ. Psychophysiol. 2012, 83, 248–252. [Google Scholar] [CrossRef]
- Antkowiak, B. How do general anaesthetics work? Naturwissenschaften 2001, 88, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Kelz, M.B.; Mashour, G.A. The Biology of General Anesthesia from Paramecium to Primate. Curr. Biol. 2019, 29, R1199–R1210. [Google Scholar] [CrossRef]
- Oestergren, G. Colchicine mitosis, chromosome contraction, narcosis and protein chain folding. Hereditas 1944, 30, 429–467. [Google Scholar] [CrossRef]
- Ramesh, S.A.; Tyerman, S.D.; Gilliham, M.; Xu, B. γ-Aminobutyric acid (GABA) signalling in plants. Cell. Mol. Life Sci. 2017, 74, 1577–1603. [Google Scholar] [CrossRef]
- Wu, Y.; Janetopoulos, C. Systematic analysis of γ-aminobutyric acid (GABA) metabolism and function in the social amoeba Dictyostelium discoideum. J. Biol. Chem. 2013, 288, 15280–15290. [Google Scholar] [CrossRef] [PubMed]
- Craddock, T.J.; Tuszynski, J.A.; Hameroff, S. Cytoskeletal signaling: Is memory encoded in microtubule lattices by CaMKII phosphorylation? PLoS Comput. Biol. 2012, 8, e1002421. [Google Scholar] [CrossRef] [PubMed]
- Salles-Passador, I.; Moisand, A.; Planques, V.; Wright, M. Physarum plasmodia do contain cytoplasmic microtubules! J. Cell Sci. 1991, 100 Pt 3, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Ueda, M.; Ogihara, S. Microtubules are required in amoeba chemotaxis for preferential stabilization of appropriate pseudopods. J. Cell Sci. 1994, 107 Pt 8, 2071–2079. [Google Scholar] [CrossRef]
- Allison, A.C.; Nunn, J.F. Effects of general anaesthetics on microtubules: A possible mechanism of anaesthesia. Lancet 1968, 2, 1326–1329. [Google Scholar] [CrossRef]
- Craddock, T.J.A.; Kurian, P.; Preto, J.; Sahu, K.; Hameroff, S.R.; Klobukowski, M.; Tuszynski, J.A. Anesthetic Alterations of Collective Terahertz Oscillations in Tubulin Correlate with Clinical Potency: Implications for Anesthetic Action and Post-Operative Cognitive Dysfunction. Sci. Rep. 2017, 7, 9877. [Google Scholar] [CrossRef]
- Lee, J.; Oettmeier, C.; Döbereiner, H.-G. A novel growth mode of Physarum polycephalum during starvation. J. Phys. D Appl. Phys. 2018, 51, 244002. [Google Scholar] [CrossRef]
- Adamatzky, A. Routing Physarum with repellents. Eur. Phys. J. E 2010, 31, 403–410. [Google Scholar] [CrossRef]
- Boussard, A.; Delescluse, J.; Pérez-Escudero, A.; Dussutour, A. Memory inception and preservation in slime moulds: The quest for a common mechanism. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2019, 374, 20180368. [Google Scholar] [CrossRef]
- Alim, K.; Andrew, N.; Pringle, A.; Brenner, M.P. Mechanism of signal propagation in Physarum polycephalum. Proc. Natl. Acad. Sci. USA 2017, 114, 5136–5141. [Google Scholar] [CrossRef]
- Kramar, M.; Alim, K. Encoding memory in tube diameter hierarchy of living flow network. Proc. Natl. Acad. Sci. USA 2021, 118, e2007815118. [Google Scholar] [CrossRef] [PubMed]
- Kippenberger, S.; Kleemann, J.; Kaufmann, R.; Meissner, M. Acting without Central Agent-Considerations for a Self-Model at the Cellular Level. Front. Hum. Neurosci. 2017, 11, 191. [Google Scholar] [CrossRef] [PubMed]
- Gershman, S.J.; Balbi, P.E.M.; Gallistel, C.R.; Gunawardena, J. Reconsidering the evidence for learning in single cells. eLife 2021, 10, e61907. [Google Scholar] [CrossRef]
- McConnell, J.V. Comparative Physiology: Learning in Invertebrates. Annu. Rev. Physiol. 1966, 28, 107–136. [Google Scholar] [CrossRef] [PubMed]
- Dee, J.; Foxon, J.L.; Hill, W.; Roberts, E.M.; Walker, M.H. Contact with a solid substratum induces cysts in axenic cultures of Physarum polycephalum amoebae: Mannitol-induced detergent-resistant cells are not true cysts. Microbiology 1997, 143, 1059–1069. [Google Scholar] [CrossRef]


| Query | UniProt | Name | Score | E-Value |
|---|---|---|---|---|
| PhyPoly_transcript_03383 | sp|O88871 | GABR2_RAT Gamma-aminobutyric acid type B receptor subunit 2 | 109 | 1 × 10−22 |
| PhyPoly_transcript_03383 | sp|Q80T41 | GABR2_MOUSE Gamma-aminobutyric acid type B receptor subunit 2 | 108 | 3 × 10−22 |
| PhyPoly_transcript_03383 | sp|O75899 | GABR2_HUMAN Gamma-aminobutyric acid type B receptor subunit 2 | 108 | 3 × 10−22 |
| PhyPoly_transcript_04011 | sp|Q9Z0U4 | GABR1_RAT Gamma-aminobutyric acid type B receptor subunit 1 | 77 | 8 × 10−13 |
| PhyPoly_transcript_04011 | sp|Q9WV18 | GABR1_MOUSE Gamma-aminobutyric acid type B receptor subunit 1 | 77 | 8 × 10−13 |
| PhyPoly_transcript_04011 | sp|Q9UBS5 | GABR1_HUMAN Gamma-aminobutyric acid type B receptor subunit 1 | 77 | 8 × 10−13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kippenberger, S.; Pipa, G.; Steinhorst, K.; Zöller, N.; Kleemann, J.; Özistanbullu, D.; Kaufmann, R.; Scheller, B. Learning in the Single-Cell Organism Physarum polycephalum: Effect of Propofol. Int. J. Mol. Sci. 2023, 24, 6287. https://doi.org/10.3390/ijms24076287
Kippenberger S, Pipa G, Steinhorst K, Zöller N, Kleemann J, Özistanbullu D, Kaufmann R, Scheller B. Learning in the Single-Cell Organism Physarum polycephalum: Effect of Propofol. International Journal of Molecular Sciences. 2023; 24(7):6287. https://doi.org/10.3390/ijms24076287
Chicago/Turabian StyleKippenberger, Stefan, Gordon Pipa, Katja Steinhorst, Nadja Zöller, Johannes Kleemann, Deniz Özistanbullu, Roland Kaufmann, and Bertram Scheller. 2023. "Learning in the Single-Cell Organism Physarum polycephalum: Effect of Propofol" International Journal of Molecular Sciences 24, no. 7: 6287. https://doi.org/10.3390/ijms24076287
APA StyleKippenberger, S., Pipa, G., Steinhorst, K., Zöller, N., Kleemann, J., Özistanbullu, D., Kaufmann, R., & Scheller, B. (2023). Learning in the Single-Cell Organism Physarum polycephalum: Effect of Propofol. International Journal of Molecular Sciences, 24(7), 6287. https://doi.org/10.3390/ijms24076287





