Activation of TRPV1-Expressing Renal Sensory Nerves of Rats with N-Oleoyldopamine Attenuates High-Fat-Diet-Induced Impairment of Renal Function
Abstract
1. Introduction
2. Results
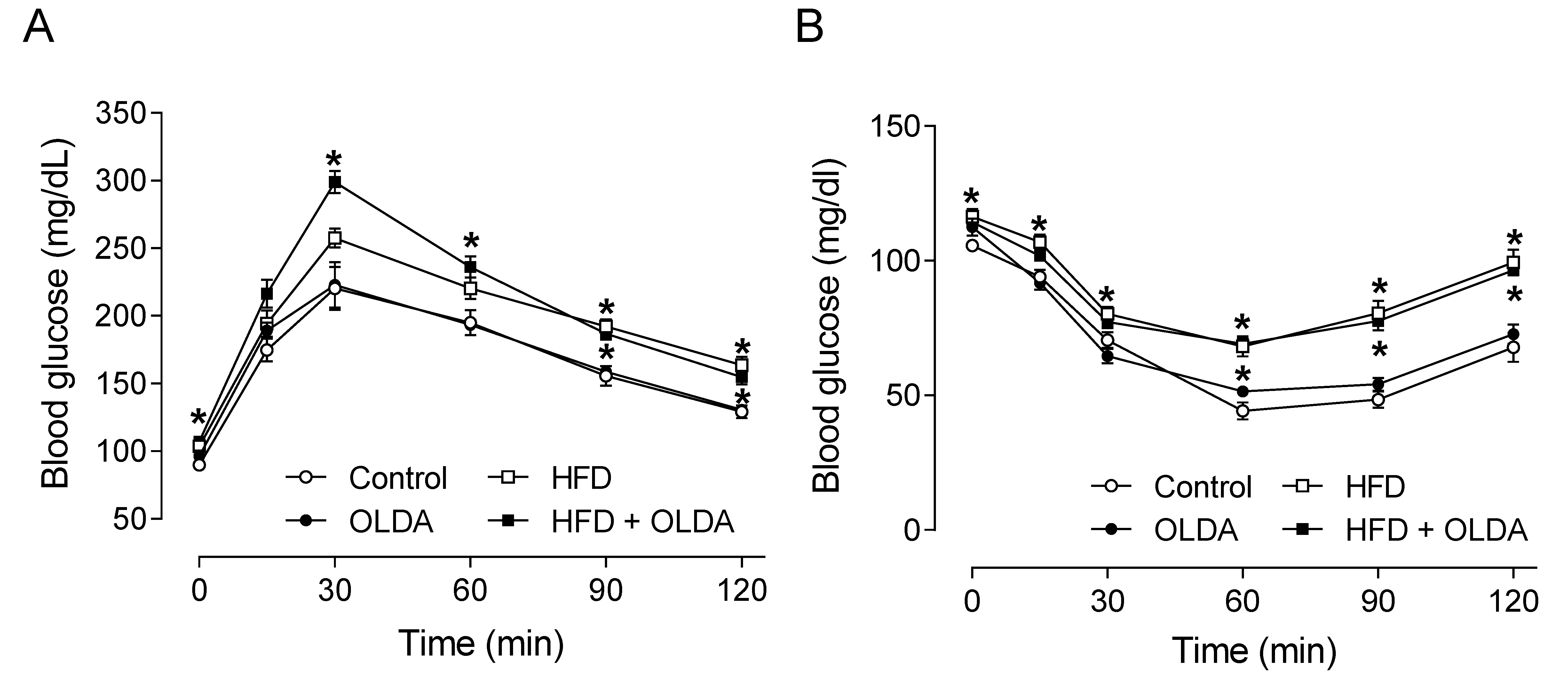
2.1. OLDA Does Not Affect Glucose Tolerance and Insulin Sensitivity in HFD-Fed Mice
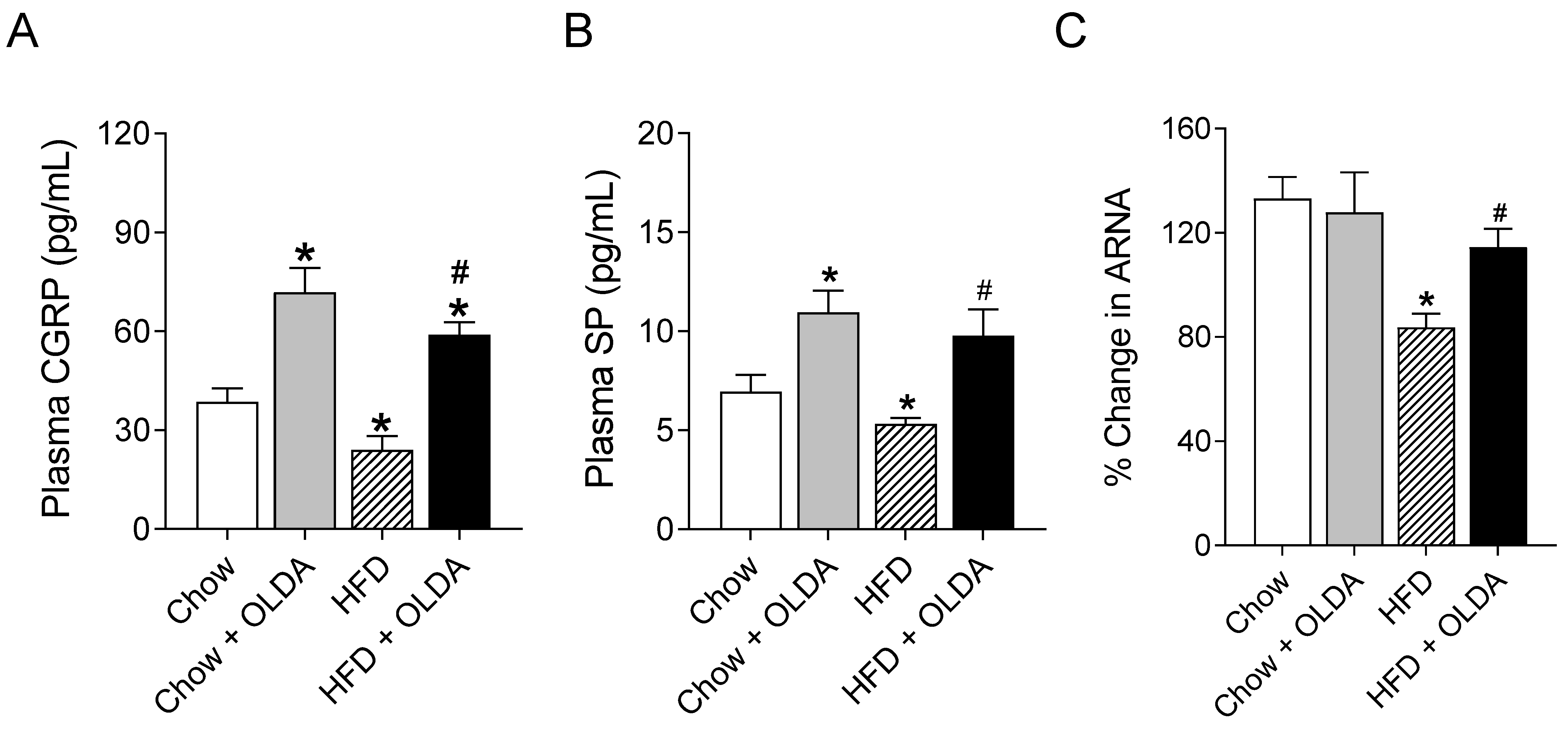
2.2. OLDA Attenuates HFD-Induced Impairment in Afferent Renal Nerve Activity
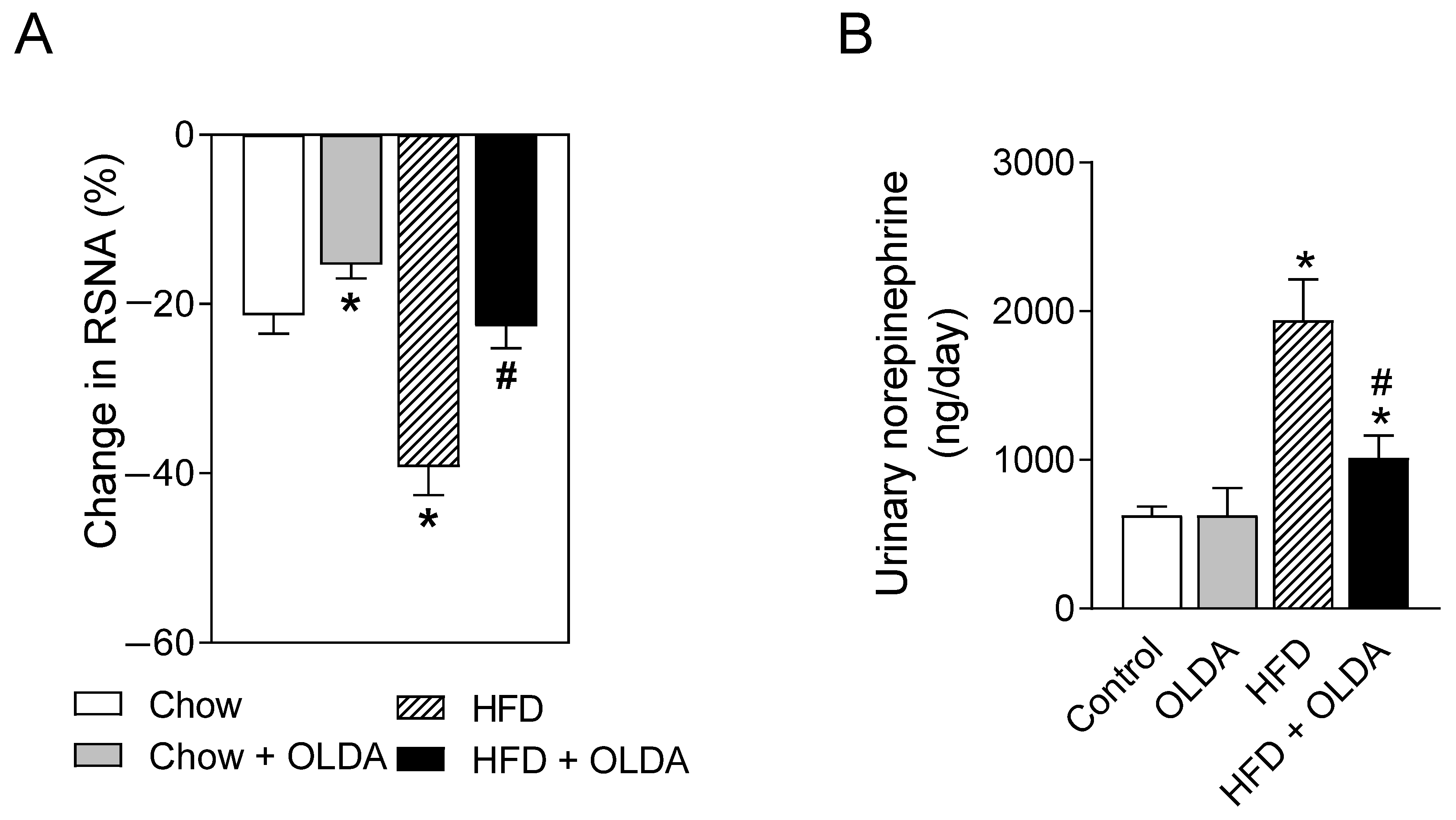
2.3. OLDA Blunts HFD-Induced Enhancement of Renal Sympathetic Nerve Activity
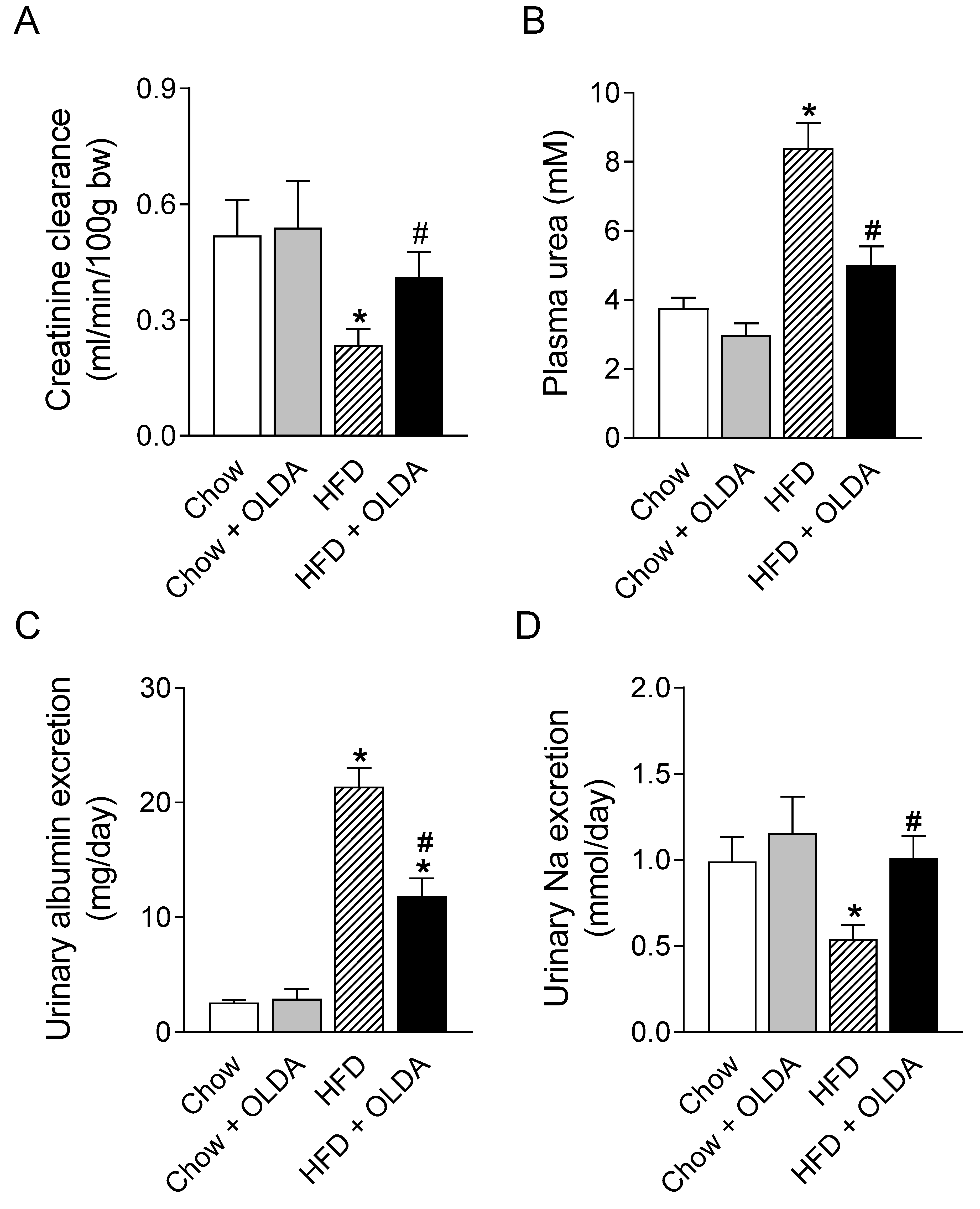
2.4. OLDA Attenuates HFD-Induced Renal Dysfunction
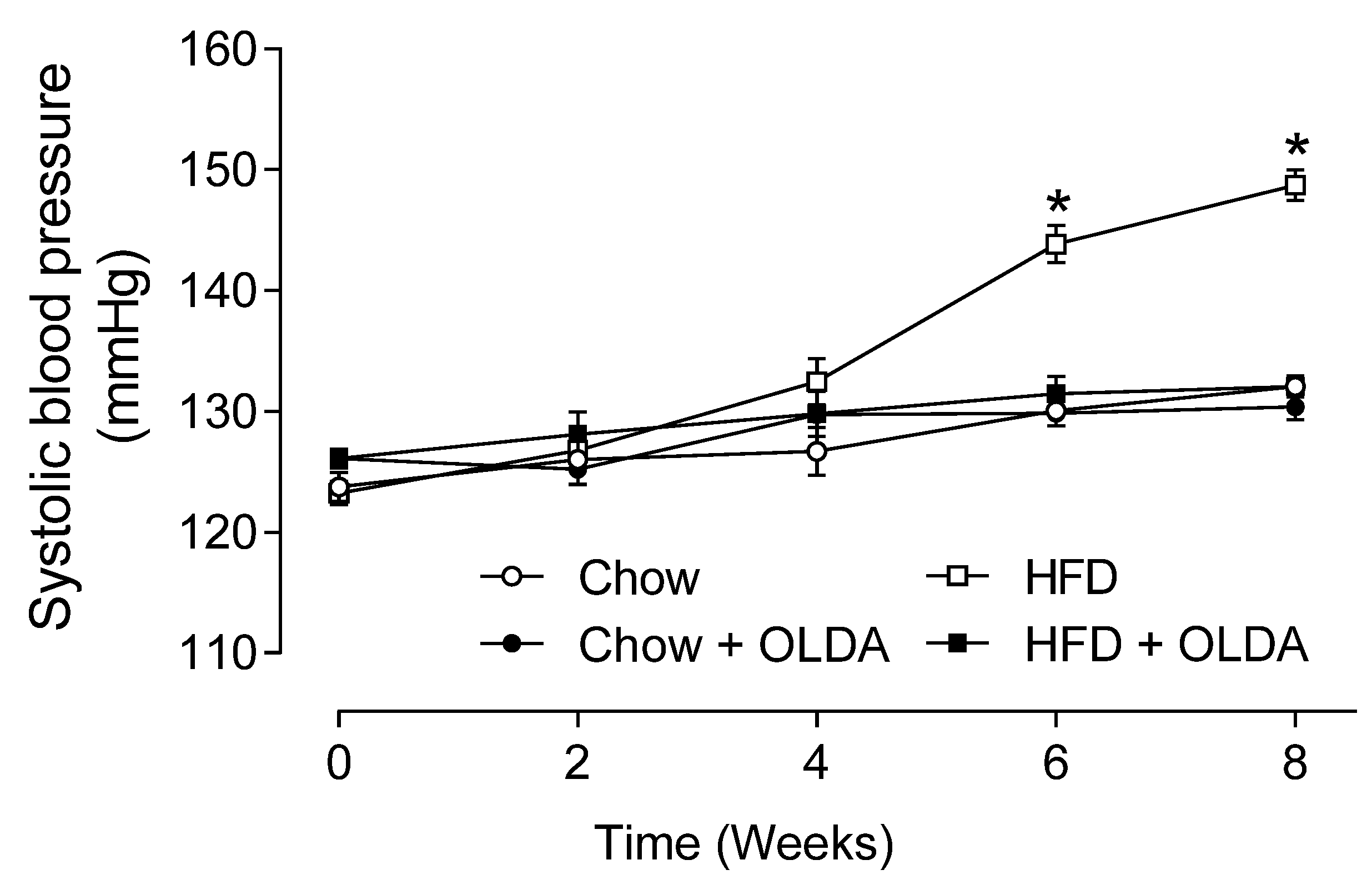
2.5. OLDA Prevents HFD-Induced Development of Hypertension
3. Discussion
4. Materials and Methods
4.1. Drugs
4.2. Animals
4.3. Surgery of Intrathecal Catheter Implantation and Intrathecal Injection
4.4. Measurement of Systolic Blood Pressure
4.5. Glucose Tolerance Test and Insulin Tolerance Test
4.6. Plasma and Urine Assays
4.7. Recording of the Activity of Afferent and Efferent Renal Nerve
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hall, J.E.; Mouton, A.J.; da Silva, A.A.; Omoto, A.C.M.; Wang, Z.; Li, X.; do Carmo, J.M. Obesity, kidney dysfunction, and inflammation: Interactions in hypertension. Cardiovasc. Res. 2021, 117, 1859–1876. [Google Scholar] [CrossRef] [PubMed]
- Rahmouni, K. Obesity-associated hypertension: Recent progress in deciphering the pathogenesis. Hypertension 2014, 64, 215–221. [Google Scholar] [CrossRef]
- Lohmeier, T.E.; Iliescu, R.; Liu, B.; Henegar, J.R.; Maric-Bilkan, C.; Irwin, E.D. Systemic and renal-specific sympathoinhibition in obesity hypertension. Hypertension 2012, 59, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, D.H.; Galligan, J.J. P2Y2 receptors mediate ATP-induced resensitization of TRPV1 expressed by kidney projecting sensory neurons. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R1634–R1641. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Luo, Z.; Ma, S.; Wong, W.T.; Ma, L.; Zhong, J.; He, H.; Zhao, Z.; Cao, T.; Yan, Z.; et al. Activation of TRPV1 by dietary capsaicin improves endothelium-dependent vasorelaxation and prevents hypertension. Cell Metab. 2010, 12, 130–141. [Google Scholar] [CrossRef]
- Zhong, B.; Ma, S.; Wang, D.H. TRPV1 protects renal ischemia-reperfusion injury in diet-induced obese mice by enhancing CGRP release and increasing renal blood flow. PeerJ 2019, 7, e6505. [Google Scholar] [CrossRef]
- Zhong, B.; Ma, S.; Wang, D.H. Knockout of TRPV1 Exacerbates Ischemia-reperfusion-induced Renal Inflammation and Injury in Obese Mice. In Vivo 2020, 34, 2259–2268. [Google Scholar] [CrossRef]
- Zhong, B.; Ma, S.; Wang, D.H. Ablation of TRPV1 Elevates Nocturnal Blood Pressure in Western Diet-fed Mice. Curr. Hypertens. Rev. 2019, 15, 144–153. [Google Scholar] [CrossRef]
- Yu, S.Q.; Ma, S.; Wang, D.H. Selective ablation of TRPV1 by intrathecal injection of resiniferatoxin in rats increases renal sympathoexcitatory responses and salt sensitivity. Hypertens. Res. 2018, 41, 679–690. [Google Scholar] [CrossRef]
- Wang, D.H.; Wu, W.; Lookingland, K.J. Degeneration of capsaicin-sensitive sensory nerves leads to increased salt sensitivity through enhancement of sympathoexcitatory response. Hypertension 2001, 37, 440–443. [Google Scholar] [CrossRef]
- Xie, C.; Wang, D.H. Effects of a high-salt diet on TRPV-1-dependent renal nerve activity in Dahl salt-sensitive rats. Am. J. Nephrol. 2010, 32, 194–200. [Google Scholar] [CrossRef]
- Yu, S.Q.; Ma, S.; Wang, D.H. TRPV1 Activation Prevents Renal Ischemia-Reperfusion Injury-Induced Increase in Salt Sensitivity by Suppressing Renal Sympathetic Nerve Activity. Curr. Hypertens. Rev. 2020, 16, 148–155. [Google Scholar] [CrossRef]
- Saino-Saito, S.; Sasaki, H.; Volpe, B.T.; Kobayashi, K.; Berlin, R.; Baker, H. Differentiation of the dopaminergic phenotype in the olfactory system of neonatal and adult mice. J. Comp. Neurol. 2004, 479, 389–398. [Google Scholar] [CrossRef]
- Yu, S.Q.; Wang, D.H. Enhanced salt sensitivity following shRNA silencing of neuronal TRPV1 in rat spinal cord. Acta Pharmacol. Sin. 2011, 32, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.Q.; Wang, D.H. Intrathecal injection of TRPV1 shRNA leads to increases in blood pressure in rats. Acta Physiol. 2011, 203, 139–147. [Google Scholar] [CrossRef]
- Zhu, Y.; Xie, C.; Wang, D.H. TRPV1-mediated diuresis and natriuresis induced by hypertonic saline perfusion of the renal pelvis. Am. J. Nephrol. 2007, 27, 530–537. [Google Scholar] [CrossRef]
- Xie, C.; Wang, D.H. Ablation of transient receptor potential vanilloid 1 abolishes endothelin-induced increases in afferent renal nerve activity: Mechanisms and functional significance. Hypertension 2009, 54, 1298–1305. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Sachs, J.R.; Wang, D.H. Interdependent regulation of afferent renal nerve activity and renal function: Role of transient receptor potential vanilloid type 1, neurokinin 1, and calcitonin gene-related peptide receptors. J. Pharmacol. Exp. Ther. 2008, 325, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Wang, D.H. Inhibition of renin release by arachidonic acid metabolites, 12(s)-HPETE and 12-HETE: Role of TRPV1 channels. Endocrinology 2011, 152, 3811–3819. [Google Scholar] [CrossRef]
- Shimeda, Y.; Hirotani, Y.; Akimoto, Y.; Shindou, K.; Ijiri, Y.; Nishihori, T.; Tanaka, K. Protective effects of capsaicin against cisplatin-induced nephrotoxicity in rats. Biol. Pharm. Bull. 2005, 28, 1635–1638. [Google Scholar] [CrossRef]
- Mizutani, A.; Okajima, K.; Murakami, K.; Mizutani, S.; Kudo, K.; Uchino, T.; Kadoi, Y.; Noguchi, T. Activation of sensory neurons reduces ischemia/reperfusion-induced acute renal injury in rats. Anesthesiology 2009, 110, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Jones, W.K.; Fan, G.C.; Liao, S.; Zhang, J.M.; Wang, Y.; Weintraub, N.L.; Kranias, E.G.; Schultz, J.E.; Lorenz, J.; Ren, X. Peripheral nociception associated with surgical incision elicits remote nonischemic cardioprotection via neurogenic activation of protein kinase C signaling. Circulation 2009, 120, S1–S9. [Google Scholar] [CrossRef] [PubMed]
- Waters, J.; Schaefer, A.; Sakmann, B. Backpropagating action potentials in neurones: Measurement, mechanisms and potential functions. Prog. Biophys. Mol. Biol. 2005, 87, 145–170. [Google Scholar] [CrossRef] [PubMed]
- Chu, Z.L.; Carroll, C.; Chen, R.; Alfonso, J.; Gutierrez, V.; He, H.; Lucman, A.; Xing, C.; Sebring, K.; Zhou, J.; et al. N-oleoyldopamine enhances glucose homeostasis through the activation of GPR119. Mol. Endocrinol. 2010, 24, 161–170. [Google Scholar] [CrossRef]
- Ma, L.; Zhong, J.; Zhao, Z.; Luo, Z.; Ma, S.; Sun, J.; He, H.; Zhu, T.; Liu, D.; Zhu, Z.; et al. Activation of TRPV1 reduces vascular lipid accumulation and attenuates atherosclerosis. Cardiovasc. Res. 2011, 92, 504–513. [Google Scholar] [CrossRef]
- Wang, P.; Yan, Z.; Zhong, J.; Chen, J.; Ni, Y.; Li, L.; Ma, L.; Zhao, Z.; Liu, D.; Zhu, Z. Transient receptor potential vanilloid 1 activation enhances gut glucagon-like peptide-1 secretion and improves glucose homeostasis. Diabetes 2012, 61, 2155–2165. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, S.-Q.; Ma, S.; Wang, D.H. Activation of TRPV1-Expressing Renal Sensory Nerves of Rats with N-Oleoyldopamine Attenuates High-Fat-Diet-Induced Impairment of Renal Function. Int. J. Mol. Sci. 2023, 24, 6207. https://doi.org/10.3390/ijms24076207
Yu S-Q, Ma S, Wang DH. Activation of TRPV1-Expressing Renal Sensory Nerves of Rats with N-Oleoyldopamine Attenuates High-Fat-Diet-Induced Impairment of Renal Function. International Journal of Molecular Sciences. 2023; 24(7):6207. https://doi.org/10.3390/ijms24076207
Chicago/Turabian StyleYu, Shuang-Quan, Shuangtao Ma, and Donna H. Wang. 2023. "Activation of TRPV1-Expressing Renal Sensory Nerves of Rats with N-Oleoyldopamine Attenuates High-Fat-Diet-Induced Impairment of Renal Function" International Journal of Molecular Sciences 24, no. 7: 6207. https://doi.org/10.3390/ijms24076207
APA StyleYu, S.-Q., Ma, S., & Wang, D. H. (2023). Activation of TRPV1-Expressing Renal Sensory Nerves of Rats with N-Oleoyldopamine Attenuates High-Fat-Diet-Induced Impairment of Renal Function. International Journal of Molecular Sciences, 24(7), 6207. https://doi.org/10.3390/ijms24076207



