Targeting Nociceptive Neurons and Transient Receptor Potential Channels for the Treatment of Migraine
Abstract
1. Introduction
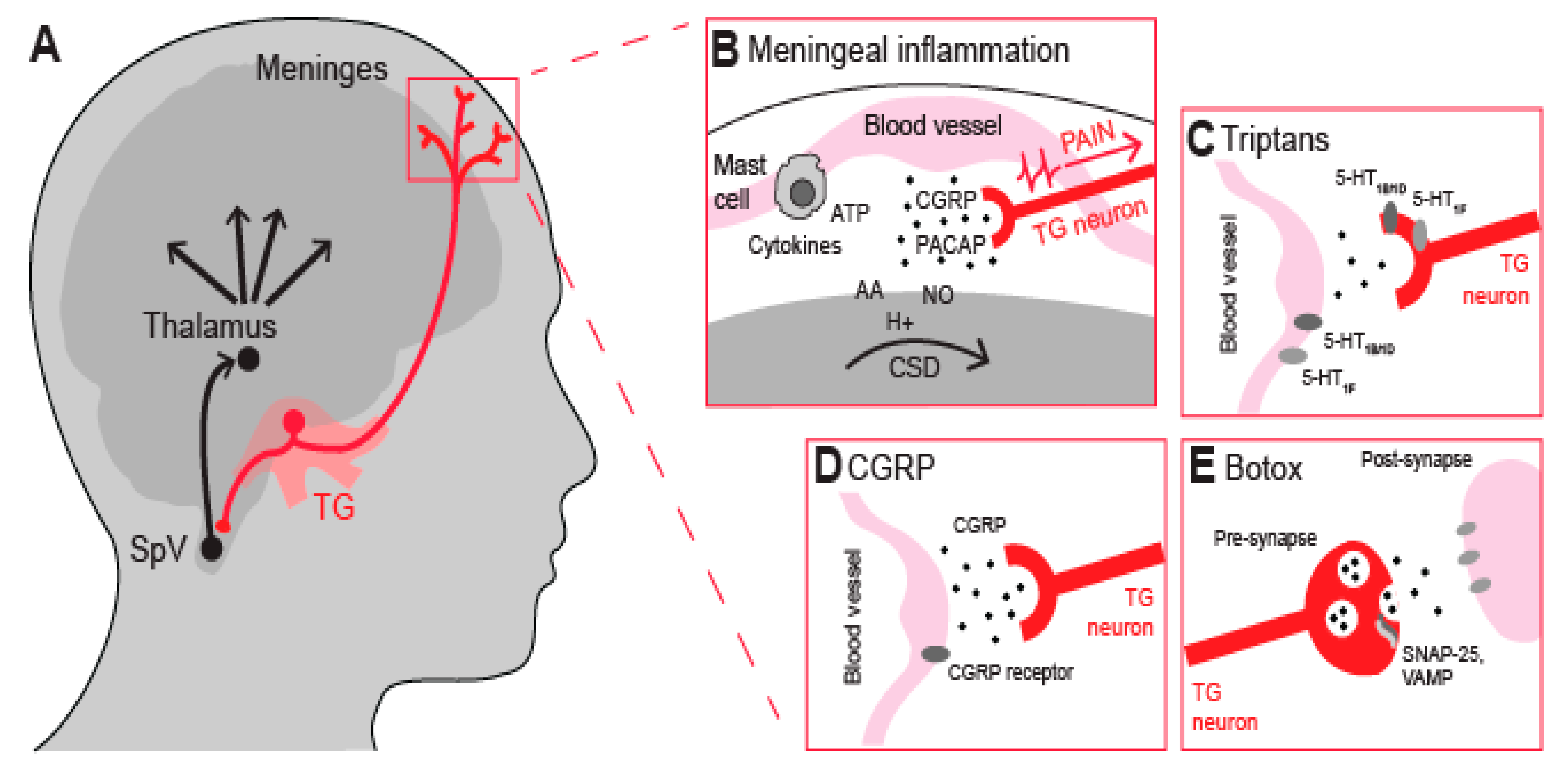
2. Nociceptive Neurons and Meningeal Inflammation
3. Current Anti-Migraine Drugs
4. TRP Channels as Targets for Anti-Migraine Drugs
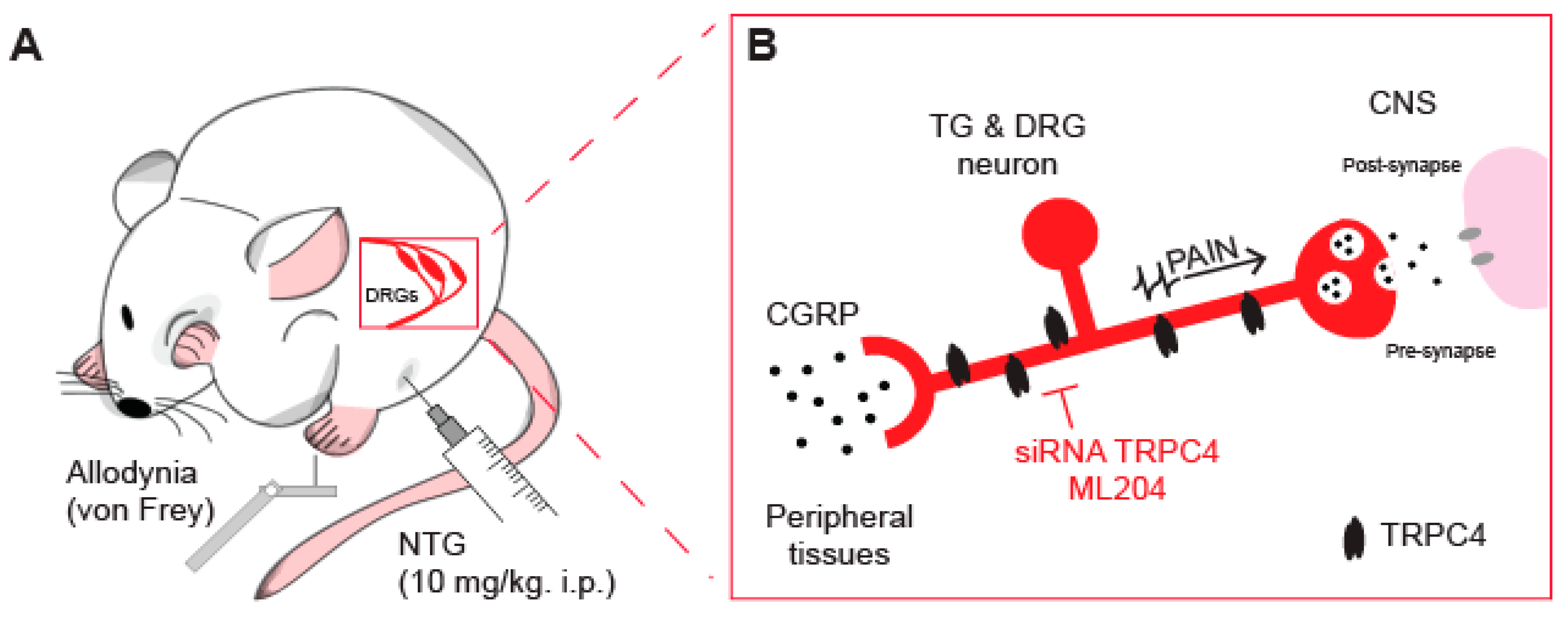
5. TRPC4 Channel as an Emerging Target for Anti-Migraine Drugs
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Burch, R.C.; Buse, D.C.; Lipton, R.B. Migraine: Epidemiology, Burden, and Comorbidity. Neurol. Clin. 2019, 37, 631–649. [Google Scholar] [CrossRef]
- Charles, A. The pathophysiology of migraine: Implications for clinical management. Lancet Neurol. 2018, 17, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Dodick, D.W. Migraine. Lancet 2018, 391, 1315–1330. [Google Scholar] [CrossRef] [PubMed]
- Mason, B.N.; Russo, A.F. Vascular Contributions to Migraine: Time to Revisit? Front. Cell. Neurosci. 2018, 12, 233. [Google Scholar] [CrossRef] [PubMed]
- Burstein, R.; Noseda, R.; Borsook, D. Migraine: Multiple Processes, Complex Pathophysiology. J. Neurosci. 2015, 35, 6619–6629. [Google Scholar] [CrossRef]
- Noseda, R.; Burstein, R. Migraine pathophysiology: Anatomy of the trigeminovascular pathway and associated neurological symptoms, CSD, sensitization and modulation of pain. Pain 2013, 154 (Suppl. S1), S44–S53. [Google Scholar] [CrossRef] [PubMed]
- Goadsby, P.; Holland, P.; Martins-Oliveira, M.; Hoffmann, J.; Schankin, C.; Akerman, S. Pathophysiology of Migraine: A Disorder of Sensory Processing. Physiol. Rev. 2017, 97, 553–622. [Google Scholar] [CrossRef]
- Dodick, D.W. A Phase-by-Phase Review of Migraine Pathophysiology. Headache 2018, 58, 4–16. [Google Scholar] [CrossRef]
- Zhang, X.; Levy, D.; Noseda, R.; Kainz, V.; Jakubowski, M.; Burstein, R. Activation of Meningeal Nociceptors by Cortical Spreading Depression: Implications for Migraine with Aura. J. Neurosci. 2010, 30, 8807–8814. [Google Scholar] [CrossRef]
- Dux, M.; Rosta, J.; Messlinger, K. TRP Channels in the Focus of Trigeminal Nociceptor Sensitization Contributing to Primary Headaches. Int. J. Mol. Sci. 2020, 21, 342. [Google Scholar] [CrossRef] [PubMed]
- Olesen, J.; Burstein, R.; Ashina, M.; Tfelt-Hansen, P. Origin of pain in migraine: Evidence for peripheral sensitisation. Lancet Neurol. 2009, 8, 679–690. [Google Scholar] [CrossRef]
- Levy, D. Migraine pain and nociceptor activation—Where do we stand? Headache 2010, 50, 909–916. [Google Scholar] [CrossRef]
- Berta, T.; Qadri, Y.; Tan, P.-H.; Ji, R.-R. Targeting dorsal root ganglia and primary sensory neurons for the treatment of chronic pain. Expert Opin. Ther. Targets 2017, 21, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Benemei, S.; Dussor, G. TRP Channels and Migraine: Recent Developments and New Therapeutic Opportunities. Pharmaceuticals 2019, 12, 54. [Google Scholar] [CrossRef] [PubMed]
- Dussor, G.; Yan, J.; Xie, J.Y.; Ossipov, M.H.; Dodick, D.W.; Porreca, F. Targeting TRP Channels For Novel Migraine Therapeutics. ACS Chem. Neurosci. 2014, 5, 1085–1096. [Google Scholar] [CrossRef]
- Artero-Morales, M.; González-Rodríguez, S.; Ferrer-Montiel, A. TRP Channels as Potential Targets for Sex-Related Differences in Migraine Pain. Front. Mol. Biosci. 2018, 5, 73. [Google Scholar] [CrossRef] [PubMed]
- Basbaum, A.I.; Bautista, D.M.; Scherrer, G.; Julius, D. Cellular and Molecular Mechanisms of Pain. Cell 2009, 139, 267–284. [Google Scholar] [CrossRef] [PubMed]
- Woolf, C.J.; Ma, Q. Nociceptors--noxious stimulus detectors. Neuron 2007, 55, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Edvinsson, L.; Goadsby, P.J. Neuropeptides in Migraine and Cluster Headache. Cephalalgia 1994, 14, 320–327. [Google Scholar] [CrossRef]
- Iyengar, S.; Ossipov, M.H.; Johnson, K.W. The role of calcitonin gene–related peptide in peripheral and central pain mechanisms including migraine. Pain 2017, 158, 543–559. [Google Scholar] [CrossRef]
- Markovics, A.; Kormos, V.; Gaszner, B.; Lashgarara, A.; Szoke, E.; Sandor, K.; Szabadfi, K.; Tuka, B.; Tajti, J.; Szolcsanyi, J.; et al. Pituitary adenylate cyclase-activating polypeptide plays a key role in nitroglycerol-induced trigeminovascular activation in mice. Neurobiol. Dis. 2012, 45, 633–644. [Google Scholar] [CrossRef]
- Aggarwal, M.; Puri, V.; Puri, S. Serotonin and CGRP in migraine. Ann. Neurosci. 2012, 19, 88–94. [Google Scholar] [CrossRef]
- Zhang, X.; Burstein, R.; Levy, D. Local action of the proinflammatory cytokines IL-1β and IL-6 on intracranial meningeal nociceptors. Cephalalgia 2011, 32, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Edvinsson, L.; Haanes, K.A.; Warfvinge, K. Does inflammation have a role in migraine? Nat. Rev. Neurol. 2019, 15, 483–490. [Google Scholar] [CrossRef]
- Lukacs, M.; Haanes, K.A.; Majláth, Z.; Tajti, J.; Vécsei, L.; Warfvinge, K.; Edvinsson, L. Dural administration of inflammatory soup or Complete Freund’s Adjuvant induces activation and inflammatory response in the rat trigeminal ganglion. J. Headache Pain 2015, 16, 564. [Google Scholar] [CrossRef] [PubMed]
- Conti, P.; D’ovidio, C.; Conti, C.; Gallenga, C.E.; Lauritano, D.; Caraffa, A.; Kritas, S.K.; Ronconi, G. Progression in migraine: Role of mast cells and pro-inflammatory and anti-inflammatory cytokines. Eur. J. Pharmacol. 2018, 844, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Pietrobon, D.; Moskowitz, M.A. Pathophysiology of migraine. Annu. Rev. Physiol. 2013, 75, 365–391. [Google Scholar] [CrossRef] [PubMed]
- Gürsoy-Ozdemir, Y.; Qiu, J.; Matsuoka, N.; Bolay, H.; Bermpohl, D.; Jin, H.; Wang, X.; Rosenberg, G.A.; Lo, E.H.; Moskowitz, M.A. Cortical spreading depression activates and upregulates MMP-9. J. Clin. Investig. 2004, 113, 1447–1455. [Google Scholar] [CrossRef] [PubMed]
- Karatas, H.; Erdener, S.E.; Gursoy-Ozdemir, Y.; Lule, S.; Eren-Koçak, E.; Sen, Z.D.; Dalkara, T. Spreading Depression Triggers Headache by Activating Neuronal Panx1 Channels. Science 2013, 339, 1092–1095. [Google Scholar] [CrossRef]
- Chen, S.-P.; Qin, T.; Seidel, J.L.; Zheng, Y.; Eikermann, M.; Ferrari, M.D.; Maagdenberg, A.M.J.M.V.D.; Moskowitz, M.A.; Ayata, C.; Eikermann-Haerter, K. Inhibition of the P2X7–PANX1 complex suppresses spreading depolarization and neuroinflammation. Brain 2017, 140, 1643–1656. [Google Scholar] [CrossRef]
- Hassler, S.N.; Ahmad, F.B.; Burgos-Vega, C.C.; Boitano, S.; Vagner, J.; Price, T.J.; Dussor, G. Protease activated receptor 2 (PAR2) activation causes migraine-like pain behaviors in mice. Cephalalgia 2018, 39, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, R.; Wang, Z.; Saavedra, C.; Dinardo, A.; Corr, M.; Powell, S.B.; Yaksh, T.L. Role of Toll-like receptor 4 signaling in mast cell-mediated migraine pain pathway. Mol. Pain 2019, 15, 1744806919867842. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, J.; Goadsby, P.J. Emerging Targets in Migraine. CNS Drugs 2013, 28, 11–17. [Google Scholar] [CrossRef]
- Belvis, R.; Mas, N.; Aceituno, A. Migraine attack treatment: A tailor-made suit, not one size fits all. Recent Pat. CNS Drug Discov. 2014, 9, 26–40. [Google Scholar] [CrossRef]
- Tso, A.R.; Goadsby, P.J. Anti-CGRP Monoclonal Antibodies: The Next Era of Migraine Prevention? Curr. Treat. Options Neurol. 2017, 19, 27. [Google Scholar] [CrossRef] [PubMed]
- Diener, H.-C.; Charles, A.; Goadsby, P.J.; Holle, D. New therapeutic approaches for the prevention and treatment of migraine. Lancet Neurol. 2015, 14, 1010–1022. [Google Scholar] [CrossRef]
- Lipton, R.B.; Silberstein, S.D. Episodic and Chronic Migraine Headache: Breaking Down Barriers to Optimal Treatment and Prevention. Headache 2015, 55, 103–122. [Google Scholar] [CrossRef]
- Pardutz, A.; Schoenen, J. NSAIDs in the Acute Treatment of Migraine: A Review of Clinical and Experimental Data. Pharmaceuticals 2010, 3, 1966–1987. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.J.Y.; De Felice, M. Migraine Treatment: Current Acute Medications and Their Potential Mechanisms of Action. Neurotherapeutics 2017, 15, 274–290. [Google Scholar] [CrossRef]
- Cannon, C.P.; Cannon, P.J. COX-2 Inhibitors and Cardiovascular Risk. Science 2012, 336, 1386–1387. [Google Scholar] [CrossRef]
- Negro, A.; Koverech, A.; Martelletti, P. Serotonin receptor agonists in the acute treatment of migraine: A review on their therapeutic potential. J. Pain Res. 2018, 11, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Diener, H. The Risks or Lack Thereof of Migraine Treatments in Vascular Disease. Headache 2020, 60, 649–653. [Google Scholar] [CrossRef] [PubMed]
- Victor, T.; Hu, X.; Campbell, J.; Buse, D.; Lipton, R. Migraine prevalence by age and sex in the United States: A life-span study. Cephalalgia 2010, 30, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Meng, I.D.; Dodick, D.; Ossipov, M.H.; Porreca, F. Pathophysiology of medication overuse headache: Insights and hypotheses from preclinical studies. Cephalalgia 2011, 31, 851–860. [Google Scholar] [CrossRef]
- Kuca, B.; Silberstein, S.D.; Wietecha, L.; Berg, P.H.; Dozier, G.; Lipton, R.B.; on behalf of the COL MIG-301 Study Group. Lasmiditan is an effective acute treatment for migraine: A phase 3 randomized study. Neurology 2018, 91, e2222–e2232. [Google Scholar] [CrossRef] [PubMed]
- Wattiez, A.-S.; Sowers, L.P.; Russo, A.F. Calcitonin gene-related peptide (CGRP): Role in migraine pathophysiology and therapeutic targeting. Expert Opin. Ther. Targets 2020, 24, 91–100. [Google Scholar] [CrossRef]
- de Vries, T.; Villalón, C.M.; MaassenVanDenBrink, A. Pharmacological treatment of migraine: CGRP and 5-HT beyond the triptans. Pharmacol. Ther. 2020, 211, 107528. [Google Scholar] [CrossRef]
- Saely, S.; Croteau, D.; Jawidzik, L.; Brinker, A.; Kortepeter, C.; Saely, B.S. Hypertension: A new safety risk for patients treated with erenumab. Headache 2021, 61, 202–208. [Google Scholar] [CrossRef] [PubMed]
- MaassenVanDenBrink, A.; Meijer, J.; Villalón, C.M.; Ferrari, M.D. Wiping Out CGRP: Potential Cardiovascular Risks. Trends Pharmacol. Sci. 2016, 37, 779–788. [Google Scholar] [CrossRef]
- Raciti, L.; Raciti, G.; Militi, D.; Casella, C.; Calabrò, R.S. Chronic Migraine: A Narrative Review on the Use of Botulinum Toxin with Clinical Indications and Future Directions. J. Integr. Neurosci. 2022, 21, 141. [Google Scholar] [CrossRef]
- Rossetto, O.; Pirazzini, M.; Montecucco, C. Botulinum neurotoxins: Genetic, structural and mechanistic insights. Nat. Rev. Microbiol. 2014, 12, 535–549. [Google Scholar] [CrossRef] [PubMed]
- Burstein, R.; Blumenfeld, A.M.; Silberstein, S.D.; Adams, A.M.; Brin, M.F. Mechanism of Action of OnabotulinumtoxinA in Chronic Migraine: A Narrative Review. Headache 2020, 60, 1259–1272. [Google Scholar] [CrossRef] [PubMed]
- Cernuda-Morollón, E.; Ramón, C.; Martínez-Camblor, P.; Serrano-Pertierra, E.; Larrosa, D.; Pascual, J. OnabotulinumtoxinA decreases interictal CGRP plasma levels in patients with chronic migraine. Pain 2015, 156, 820–824. [Google Scholar] [CrossRef] [PubMed]
- Go, E.J.; Ji, J.; Kim, Y.H.; Berta, T.; Park, C.-K. Transient Receptor Potential Channels and Botulinum Neurotoxins in Chronic Pain. Front. Mol. Neurosci. 2021, 14, 772719. [Google Scholar] [CrossRef] [PubMed]
- Gazerani, P.; Pedersen, N.S.; Staahl, C.; Drewes, A.; Arendt-Nielsen, L. Subcutaneous Botulinum toxin type A reduces capsaicin-induced trigeminal pain and vasomotor reactions in human skin. Pain 2009, 141, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Mayordomo, R.; Ruiz, M.; Pascual, J.; De La Sacristana, M.G.; Vidriales, I.; Sobrado, M.; Cernuda-Morollon, E.; Gago-Veiga, A.B.; Garcia-Azorin, D.; Telleria, J.J.; et al. CALCA and TRPV1 genes polymorphisms are related to a good outcome in female chronic migraine patients treated with OnabotulinumtoxinA. J. Headache Pain 2019, 20, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Montell, C. Physiology, phylogeny, and functions of the TRP superfamily of cation channels. Sci. STKE 2001, 2001, re1. [Google Scholar] [CrossRef]
- Clapham, D.E.; Runnels, L.W.; Strübing, C. The trp ion channel family. Nat. Rev. Neurosci. 2001, 2, 387–396. [Google Scholar] [CrossRef]
- Koivisto, A.-P.; Belvisi, M.G.; Gaudet, R.; Szallasi, A. Advances in TRP channel drug discovery: From target validation to clinical studies. Nat. Rev. Drug Discov. 2021, 21, 41–59. [Google Scholar] [CrossRef]
- Nilius, B.; Voets, T.; Peters, J. TRP Channels in Disease. Sci. STKE 2005, 2005, 8. [Google Scholar] [CrossRef]
- Patapoutian, A.; Tate, S.; Woolf, C.J. Transient receptor potential channels: Targeting pain at the source. Nat. Rev. Drug Discov. 2009, 8, 55–68. [Google Scholar] [CrossRef]
- Kunkler, P.E.; Ballard, C.J.; Oxford, G.S.; Hurley, J.H. TRPA1 receptors mediate environmental irritant-induced meningeal vasodilatation. Pain 2011, 152, 38–44. [Google Scholar] [CrossRef]
- Nassini, R.; Materazzi, S.; Vriens, J.; Prenen, J.; Benemei, S.; De Siena, G.; la Marca, G.; Andrè, E.; Preti, D.; Avonto, C.; et al. The ‘headache tree’ via umbellulone and TRPA1 activates the trigeminovascular system. Brain 2011, 135, 376–390. [Google Scholar] [CrossRef]
- Prince, P.B.; Rapoport, A.M.; Sheftell, F.D.; Tepper, S.J.; Bigal, M.E. The Effect of Weather on Headache. Headache 2004, 44, 596–602. [Google Scholar] [CrossRef]
- Gavva, N.R.; Sandrock, R.; Arnold, G.E.; Davis, M.; Lamas, E.; Lindvay, C.; Li, C.-M.; Smith, B.; Backonja, M.; Gabriel, K.; et al. Reduced TRPM8 expression underpins reduced migraine risk and attenuated cold pain sensation in humans. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Meents, J.E.; Hoffmann, J.; Chaplan, S.R.; Neeb, L.; Schuh-Hofer, S.; Wickenden, A.; Reuter, U. Two TRPV1 receptor antagonists are effective in two different experimental models of migraine. J. Headache Pain 2015, 16, 57. [Google Scholar] [CrossRef]
- Zhang, X.; Strassman, A.M.; Novack, V.; Brin, M.F.; Burstein, R. Extracranial injections of botulinum neurotoxin type A inhibit intracranial meningeal nociceptors’ responses to stimulation of TRPV1 and TRPA1 channels: Are we getting closer to solving this puzzle? Cephalalgia 2016, 36, 875–886. [Google Scholar] [CrossRef]
- Hoffmann, J.; Supronsinchai, W.; Andreou, A.P.; Summ, O.; Akerman, S.; Goadsby, P.J. Olvanil acts on transient receptor potential vanilloid channel 1 and cannabinoid receptors to modulate neuronal transmission in the trigeminovascular system. Pain 2012, 153, 2226–2232. [Google Scholar] [CrossRef]
- Nicoletti, P.; Trevisani, M.; Manconi, M.; Gatti, R.; De Siena, G.; Zagli, G.; Benemei, S.; Capone, J.G.; Geppetti, P.; Pini, L.A. Ethanol Causes Neurogenic Vasodilation by TRPV1 Activation and CGRP Release in the Trigeminovascular System of The Guinea Pig. Cephalalgia 2007, 28, 9–17. [Google Scholar] [CrossRef]
- Zakharov, A.; Vitale, C.; Kilinc, E.; Koroleva, K.; Fayuk, D.; Shelukhina, I.; Naumenko, N.; Khazipov, R.; Skorinkin, A.; Giniatullin, R. Hunting for origins of migraine pain: Cluster analysis of spontaneous and capsaicin-induced firing in meningeal trigeminal nerve fibers. Front. Cell. Neurosci. 2015, 9, 287. [Google Scholar] [CrossRef]
- Evans, M.S.; Cheng, X.; Jeffry, J.A.; Disney, K.E.; Premkumar, L.S. Sumatriptan Inhibits TRPV1 Channels in Trigeminal Neurons. Headache 2012, 52, 773–784. [Google Scholar] [CrossRef]
- Demartini, C.; Tassorelli, C.; Zanaboni, A.M.; Tonsi, G.; Francesconi, O.; Nativi, C.; Greco, R. The role of the transient receptor potential ankyrin type-1 (TRPA1) channel in migraine pain: Evaluation in an animal model. J. Headache Pain 2017, 18, 94. [Google Scholar] [CrossRef] [PubMed]
- Marone, I.M.; De Logu, F.; Nassini, R.; De Carvalho Goncalves, M.; Benemei, S.; Ferreira, J.; Puma, S.L.; Bunnett, N.W.; Geppetti, P.; Materazzi, S. TRPA1/NOX in the soma of trigeminal ganglion neurons mediates migraine-related pain of glyceryl trinitrate in mice. Brain 2018, 141, 2312–2328. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-J.; Yeo, J.-H.; Yoon, S.-Y.; Roh, D.-H. Different Involvement of ASIC and TRPA1 in Facial and Hindpaw Allodynia in Nitroglycerin-Induced Peripheral Hypersensitivities in Mice. Life 2022, 12, 1294. [Google Scholar] [CrossRef] [PubMed]
- Edelmayer, R.M.; Le, L.N.; Yan, J.; Wei, X.; Nassini, R.; Materazzi, S.; Preti, D.; Appendino, G.; Geppetti, P.; Dodick, D.W.; et al. Activation of TRPA1 on dural afferents: A potential mechanism of headache pain. Pain 2012, 153, 1949–1958. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, Y.; Xu, Y.; Ma, D.; Wang, M. The Transient Receptor Potential Ankyrin Type 1 Plays a Critical Role in Cortical Spreading Depression. Neuroscience 2018, 382, 23–34. [Google Scholar] [CrossRef]
- Materazzi, S.; Benemei, S.; Fusi, C.; Gualdani, R.; De Siena, G.; Vastani, N.; Andersson, D.A.; Trevisan, G.; Moncelli, M.R.; Wei, X.; et al. Parthenolide inhibits nociception and neurogenic vasodilatation in the trigeminovascular system by targeting the TRPA1 channel. Pain 2013, 154, 2750–2758. [Google Scholar] [CrossRef]
- Benemei, S.; De Logu, F.; Puma, S.L.; Marone, I.M.; Coppi, E.; Ugolini, F.; Liedtke, W.; Pollastro, F.; Appendino, G.; Geppetti, P.; et al. The anti-migraine component of butterbur extracts, isopetasin, desensitizes peptidergic nociceptors by acting on TRPA1 cation channel. Br. J. Pharmacol. 2017, 174, 2897–2911. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Vega, C.C.; Ahn, D.D.-U.; Bischoff, C.; Wang, W.; Horne, D.; Wang, J.; Gavva, N.; Dussor, G. Meningeal transient receptor potential channel M8 activation causes cutaneous facial and hindpaw allodynia in a preclinical rodent model of headache. Cephalalgia 2015, 36, 185–193. [Google Scholar] [CrossRef]
- Ren, L.; Dhaka, A.; Cao, Y.-Q. Function and Postnatal Changes of Dural Afferent Fibers Expressing TRPM8 Channels. Mol. Pain 2015, 11, 37. [Google Scholar] [CrossRef]
- Kayama, Y.; Shibata, M.; Takizawa, T.; Ibata, K.; Shimizu, T.; Ebine, T.; Toriumi, H.; Yuzaki, M.; Suzuki, N. Functional interactions between transient receptor potential M8 and transient receptor potential V1 in the trigeminal system: Relevance to migraine pathophysiology. Cephalalgia 2017, 38, 833–845. [Google Scholar] [CrossRef]
- Haghighi, A.B.; Motazedian, S.; Rezaii, R.; Mohammadi, F.; Salarian, L.; Pourmokhtari, M.; Khodaei, S.; Vossoughi, M.; Miri, R. Cutaneous application of menthol 10% solution as an abortive treatment of migraine without aura: A randomised, double-blind, placebo-controlled, crossed-over study. Int. J. Clin. Pract. 2010, 64, 451–456. [Google Scholar] [CrossRef]
- Chen, Y.; Kanju, P.; Fang, Q.; Lee, S.H.; Parekh, P.K.; Lee, W.; Moore, C.; Brenner, D.; Gereau, R.W.; Wang, F.; et al. TRPV4 is necessary for trigeminal irritant pain and functions as a cellular formalin receptor. Pain 2014, 155, 2662–2672. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Edelmayer, R.M.; Yan, J.; Dussor, G. Activation of TRPV4 on dural afferents produces headache-related behavior in a preclinical rat model. Cephalalgia 2011, 31, 1595–1600. [Google Scholar] [CrossRef] [PubMed]
- Sadler, K.E.; Moehring, F.; Shiers, S.I.; Laskowski, L.J.; Mikesell, A.R.; Plautz, Z.R.; Brezinski, A.N.; Mecca, C.M.; Dussor, G.; Price, T.J.; et al. Transient receptor potential canonical 5 mediates inflammatory mechanical and spontaneous pain in mice. Sci. Transl. Med. 2021, 13, eabd7702. [Google Scholar] [CrossRef] [PubMed]
- Cohen, C.F.; Prudente, A.S.; Berta, T.; Lee, S.H. Transient Receptor Potential Channel 4 Small-Molecule Inhibition Alleviates Migraine-Like Behavior in Mice. Front. Mol. Neurosci. 2021, 14, 260. [Google Scholar] [CrossRef]
- Caterina, M.J.; Rosen, T.A.; Tominaga, M.; Brake, A.J.; Julius, D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature 1999, 398, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef]
- Gladkikh, I.N.; Sintsova, O.V.; Leychenko, E.V.; Kozlov, S.A. TRPV1 Ion Channel: Structural Features, Activity Modulators, and Therapeutic Potential. Biochemistry (Moscow) 2021, 86, S50–S70. [Google Scholar] [CrossRef] [PubMed]
- Takayama, Y.; Derouiche, S.; Maruyama, K.; Tominaga, M. Emerging Perspectives on Pain Management by Modulation of TRP Channels and ANO1. Int. J. Mol. Sci. 2019, 20, 3411. [Google Scholar] [CrossRef]
- Hung, C.-Y.; Tan, C.-H. TRP Channels in Nociception and Pathological Pain. In Advances in Pain Research: Mechanisms and Modulation of Chronic Pain; Advances in Experimental Medicine and Biology; Springer: Singapore, 2018; Volume 1099, pp. 13–27. [Google Scholar] [CrossRef]
- Iannone, L.F.; De Logu, F.; Geppetti, P.; De Cesaris, F. The role of TRP ion channels in migraine and headache. Neurosci. Lett. 2021, 768, 136380. [Google Scholar] [CrossRef]
- Huang, D.; Li, S.; Dhaka, A.; Story, G.M.; Cao, Y.-Q. Expression of the Transient Receptor Potential Channels TRPV1, TRPA1 and TRPM8 in Mouse Trigeminal Primary Afferent Neurons Innervating the Dura. Mol. Pain 2012, 8, 1744–8069. [Google Scholar] [CrossRef] [PubMed]
- Price, T.J.; Flores, C.M. Critical Evaluation of the Colocalization Between Calcitonin Gene-Related Peptide, Substance P, Transient Receptor Potential Vanilloid Subfamily Type 1 Immunoreactivities, and Isolectin B4 Binding in Primary Afferent Neurons of the Rat and Mouse. J. Pain 2007, 8, 263–272. [Google Scholar] [CrossRef]
- Shimizu, T.; Toriumi, H.; Sato, H.; Shibata, M.; Nagata, E.; Gotoh, K.; Suzuki, N. Distribution and origin of TRPV1 receptor-containing nerve fibers in the dura mater of rat. Brain Res. 2007, 1173, 84–91. [Google Scholar] [CrossRef]
- Carreño, O.; Corominas, R.; Fernández-Morales, J.; Camiña, M.; Sobrido, M.; Fernández-Fernández, J.M.; Pozo-Rosich, P.; Cormand, B.; Macaya, A. SNP variants within the vanilloid TRPV1 and TRPV3 receptor genes are associated with migraine in the Spanish population. Am. J. Med Genet. Part B Neuropsychiatr. Genet. 2011, 159B, 94–103. [Google Scholar] [CrossRef]
- Meents, J.E.; Neeb, L.; Reuter, U. TRPV1 in migraine pathophysiology. Trends Mol. Med. 2010, 16, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Diamond, S.; Freitag, F.; Phillips, S.B.; Bernstein, J.E.; Saper, J.R. Intranasal Civamide for the Acute Treatment of Migraine Headache. Cephalalgia 2000, 20, 597–602. [Google Scholar] [CrossRef]
- Szallasi, A.; Sheta, M. Targeting TRPV1 for pain relief: Limits, losers and laurels. Expert Opin. Investig. Drugs 2012, 21, 1351–1369. [Google Scholar] [CrossRef]
- Roh, J.; Go, E.J.; Park, J.-W.; Kim, Y.H.; Park, C.-K. Resolvins: Potent Pain Inhibiting Lipid Mediators via Transient Receptor Potential Regulation. Front. Cell Dev. Biol. 2020, 8, 584206. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Tonello, R.; Im, S.-T.; Jeon, H.; Park, J.; Ford, Z.; Davidson, S.; Kim, Y.H.; Park, C.-K.; Berta, T. Resolvin D3 controls mouse and human TRPV1-positive neurons and preclinical progression of psoriasis. Theranostics 2020, 10, 12111–12126. [Google Scholar] [CrossRef]
- Story, G.M.; Peier, A.M.; Reeve, A.J.; Eid, S.R.; Mosbacher, J.; Hricik, T.R.; Earley, T.J.; Hergarden, A.C.; Andersson, D.A.; Hwang, S.W.; et al. ANKTM1, a TRP-like Channel Expressed in Nociceptive Neurons, Is Activated by Cold Temperatures. Cell 2003, 112, 819–829. [Google Scholar] [CrossRef]
- Jordt, S.-E.; Bautista, D.M.; Chuang, H.-H.; McKemy, D.D.; Zygmunt, P.M.; Högestätt, E.D.; Meng, I.D.; Julius, D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 2004, 427, 260–265. [Google Scholar] [CrossRef] [PubMed]
- del Camino, D.; Murphy, S.; Heiry, M.; Barrett, L.B.; Earley, T.J.; Cook, C.A.; Petrus, M.J.; Zhao, M.; D’Amours, M.; Deering, N.; et al. TRPA1 contributes to cold hypersensitivity. J. Neurosci. 2010, 30, 15165–15174. [Google Scholar] [CrossRef] [PubMed]
- Bandell, M.; Story, G.M.; Hwang, S.W.; Viswanath, V.; Eid, S.R.; Petrus, M.J.; Earley, T.J.; Patapoutian, A. Noxious Cold Ion Channel TRPA1 Is Activated by Pungent Compounds and Bradykinin. Neuron 2004, 41, 849–857. [Google Scholar] [CrossRef]
- Trevisani, M.; Siemens, J.; Materazzi, S.; Bautista, D.M.; Nassini, R.; Campi, B.; Imamachi, N.; Andrè, E.; Patacchini, R.; Cottrell, G.S.; et al. 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc. Natl. Acad. Sci. USA 2007, 104, 13519–13524. [Google Scholar] [CrossRef]
- Bautista, D.M.; Jordt, S.-E.; Nikai, T.; Tsuruda, P.R.; Read, A.J.; Poblete, J.; Yamoah, E.N.; Basbaum, A.I.; Julius, D. TRPA1 Mediates the Inflammatory Actions of Environmental Irritants and Proalgesic Agents. Cell 2006, 124, 1269–1282. [Google Scholar] [CrossRef]
- Zhong, J.; Minassi, A.; Prenen, J.; Taglialatela-Scafati, O.; Appendino, G.; Nilius, B. Umbellulone modulates TRP channels. Pflügers Arch.-Eur. J. Physiol. 2011, 462, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Wang, S.; Tominaga, M.; Yamamoto, S.; Fukuoka, T.; Higashi, T.; Kobayashi, K.; Obata, K.; Yamanaka, H.; Noguchi, K. Sensitization of TRPA1 by PAR2 contributes to the sensation of inflammatory pain. J. Clin. Investig. 2007, 117, 1979–1987. [Google Scholar] [CrossRef]
- Kopruszinski, C.M.; Thornton, P.; Arnold, J.; Newton, P.; Lowne, D.; Navratilova, E.; Swiokla, J.; Dodick, D.W.; Dobson, C.; Gurrell, I.; et al. Characterization and preclinical evaluation of a protease activated receptor 2 (PAR2) monoclonal antibody as a preventive therapy for migraine. Cephalalgia 2020, 40, 1535–1550. [Google Scholar] [CrossRef]
- McKemy, D.D.; Neuhausser, W.M.; Julius, D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 2002, 416, 52–58. [Google Scholar] [CrossRef]
- Peier, A.M.; Moqrich, A.; Hergarden, A.C.; Reeve, A.J.; Andersson, D.A.; Story, G.M.; Earley, T.J.; Dragoni, I.; McIntyre, P.; Bevan, S.; et al. A TRP Channel that Senses Cold Stimuli and Menthol. Cell 2002, 108, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Zakharian, E.; Cao, C.; Rohacs, T. Gating of Transient Receptor Potential Melastatin 8 (TRPM8) Channels Activated by Cold and Chemical Agonists in Planar Lipid Bilayers. J. Neurosci. 2010, 30, 12526–12534. [Google Scholar] [CrossRef]
- Lippoldt, E.K.; Elmes, R.R.; McCoy, D.D.; Knowlton, W.M.; McKemy, D.D. Artemin, a Glial Cell Line-Derived Neurotrophic Factor Family Member, Induces TRPM8-Dependent Cold Pain. J. Neurosci. 2013, 33, 12543–12552. [Google Scholar] [CrossRef]
- Asuthkar, S.; Demirkhanyan, L.; Sun, X.; Elustondo, P.A.; Krishnan, V.; Baskaran, P.; Velpula, K.K.; Thyagarajan, B.; Pavlov, E.V.; Zakharian, E. The TRPM8 protein is a testosterone receptor: II. Functional evidence for an ionotropic effect of testosterone on TRPM8. J. Biol. Chem. 2015, 290, 2670–2688. [Google Scholar] [CrossRef]
- Sousa-Valente, J.; Andreou, A.; Urban, L.; Nagy, I. Transient receptor potential ion channels in primary sensory neurons as targets for novel analgesics. Br. J. Pharmacol. 2014, 171, 2508–2527. [Google Scholar] [CrossRef] [PubMed]
- Chasman, D.I.; Schürks, M.; Anttila, V.; De Vries, B.; Schminke, U.; Launer, L.J.; Terwindt, G.M.; van den Maagdenberg, A.M.J.M.; Fendrich, K.; Völzke, H.; et al. Genome-wide association study reveals three susceptibility loci for common migraine in the general population. Nat. Genet. 2011, 43, 695–698. [Google Scholar] [CrossRef]
- Freilinger, T.; International Headache Genetics Consortium; Anttila, V.; De Vries, B.; Malik, R.; Kallela, M.; Terwindt, G.M.; Pozo-Rosich, P.; Winsvold, B.S.; Nyholt, D.; et al. Genome-wide association analysis identifies susceptibility loci for migraine without aura. Nat. Genet. 2012, 44, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Sintas, C.; Fernández-Morales, J.; Vila-Pueyo, M.; Narberhaus, B.; Arenas, C.; Pozo-Rosich, P.; Macaya, A.; Cormand, B.; Arenas, C. Replication study of previous migraine genome-wide association study findings in a Spanish sample of migraine with aura. Cephalalgia 2014, 35, 776–782. [Google Scholar] [CrossRef]
- Ling, Y.-H.; Chen, S.-P.; Fann, C.S.-J.; Wang, S.-J.; Wang, Y.-F. TRPM8 genetic variant is associated with chronic migraine and allodynia. J. Headache Pain 2019, 20, 1–8. [Google Scholar] [CrossRef]
- Burstein, R.; Yarnitsky, D.; Goor-Aryeh, I.; Ransil, B.J.; Bajwa, Z.H. An association between migraine and cutaneous allodynia. Ann. Neurol. 2000, 47, 614–624. [Google Scholar] [CrossRef]
- Takashima, Y.; Daniels, R.L.; Knowlton, W.; Teng, J.; Liman, E.R.; McKemy, D.D. Diversity in the Neural Circuitry of Cold Sensing Revealed by Genetic Axonal Labeling of Transient Receptor Potential Melastatin 8 Neurons. J. Neurosci. 2007, 27, 14147–14157. [Google Scholar] [CrossRef]
- Dhaka, A.; Earley, T.J.; Watson, J.; Patapoutian, A. Visualizing Cold Spots: TRPM8-Expressing Sensory Neurons and Their Projections. J. Neurosci. 2008, 28, 566–575. [Google Scholar] [CrossRef]
- Kobayashi, K.; Fukuoka, T.; Obata, K.; Yamanaka, H.; Dai, Y.; Tokunaga, A.; Noguchi, K. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with adelta/c-fibers and colocalization with trk receptors. J. Comp. Neurol. 2005, 493, 596–606. [Google Scholar] [CrossRef]
- Dussor, G.; Cao, Y.Q. TRPM8 and Migraine. Headache 2016, 56, 1406–1417. [Google Scholar] [CrossRef]
- Alarcón-Alarcón, D.; Cabañero, D.; de Andrés-López, J.; Nikolaeva-Koleva, M.; Giorgi, S.; Fernández-Ballester, G.; Fernández-Carvajal, A.; Ferrer-Montiel, A. TRPM8 contributes to sex dimorphism by promoting recovery of normal sensitivity in a mouse model of chronic migraine. Nat. Commun. 2022, 13, 1–17. [Google Scholar] [CrossRef]
- Wei, C.; Kim, B.; McKemy, D.D. Transient receptor potential melastatin 8 is required for nitroglycerin- and calcitonin gene-related peptide–induced migraine-like pain behaviors in mice. Pain 2022, 163, 2380–2389. [Google Scholar] [CrossRef]
- Liedtke, W.; Choe, Y.; Martí-Renom, M.A.; Bell, A.M.; Denis, C.S.; AndrejŠali; Hudspeth, A.J.; Friedman, J.M.; Heller, S. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell 2000, 103, 525–535. [Google Scholar] [CrossRef]
- Vriens, J.; Watanabe, H.; Janssens, A.; Droogmans, G.; Voets, T.; Nilius, B. Cell swelling, heat, and chemical agonists use distinct pathways for the activation of the cation channel TRPV4. Proc. Natl. Acad. Sci. USA 2004, 101, 396–401. [Google Scholar] [CrossRef]
- Grant, A.D.; Cottrell, G.S.; Amadesi, S.; Trevisani, M.; Nicoletti, P.; Materazzi, S.; Altier, C.; Cenac, N.; Zamponi, G.W.; Bautista-Cruz, F.; et al. Protease-activated receptor 2 sensitizes the transient receptor potential vanilloid 4 ion channel to cause mechanical hyperalgesia in mice. J. Physiol. 2007, 578, 715–733. [Google Scholar] [CrossRef]
- Abramowitz, J.; Birnbaumer, L. Physiology and pathophysiology of canonical transient receptor potential channels. FASEB J. 2008, 23, 297–328. [Google Scholar] [CrossRef]
- Löf, C.; Viitanen, T.; Sukumaran, P.; Törnquist, K. TRPC2: Of Mice But Not Men. Transient Recept. Potential Channels 2010, 704, 125–134. [Google Scholar] [CrossRef]
- Chen, X.; Sooch, G.; Demaree, I.S.; White, F.A.; Obukhov, A.G. Transient Receptor Potential Canonical (TRPC) Channels: Then and Now. Cells 2020, 9, 1983. [Google Scholar] [CrossRef]
- Okada, T.; Inoue, R.; Yamazaki, K.; Maeda, A.; Kurosaki, T.; Yamakuni, T.; Tanaka, I.; Shimizu, S.; Ikenaka, K.; Imoto, K.; et al. Molecular and functional characterization of a novel mouse transient receptor potential protein homologue TRP7. Ca(2+)-permeable cation channel that is constitutively activated and enhanced by stimulation of G protein-coupled receptor. J. Biol. Chem. 1999, 274, 27359–27370. [Google Scholar] [CrossRef]
- Hofmann, T.; Obukhov, A.G.; Schaefer, M.; Harteneck, C.; Gudermann, T.; Schultz, G. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature 1999, 397, 259–263. [Google Scholar] [CrossRef]
- Storch, U.; Forst, A.-L.; Pardatscher, F.; Erdogmus, S.; Philipp, M.; Gregoritza, M.; Schnitzler, M.M.Y.; Gudermann, T. Dynamic NHERF interaction with TRPC4/5 proteins is required for channel gating by diacylglycerol. Proc. Natl. Acad. Sci. USA 2017, 114, E37–E46. [Google Scholar] [CrossRef]
- Chen, X.; Li, W.; Riley, A.M.; Soliman, M.; Chakraborty, S.; Stamatkin, C.W.; Obukhov, A.G. Molecular Determinants of the Sensitivity to Gq/11-Phospholipase C-dependent Gating, Gd3+ Potentiation, and Ca2+ Permeability in the Transient Receptor Potential Canonical Type 5 (TRPC5) Channel. J. Biol. Chem. 2017, 292, 898–911. [Google Scholar] [CrossRef]
- Bollimuntha, S.; Selvaraj, S.; Singh, B.B. Emerging Roles of Canonical TRP Channels in Neuronal Function. Transient Recept. Potential Channels 2010, 704, 573–593. [Google Scholar] [CrossRef]
- Riccio, A.; Li, Y.; Tsvetkov, E.; Gapon, S.; Yao, G.L.; Smith, K.S.; Engin, E.; Rudolph, U.; Bolshakov, V.Y.; Clapham, D.E. Decreased Anxiety-Like Behavior and Gαq/11-Dependent Responses in the Amygdala of Mice Lacking TRPC4 Channels. J. Neurosci. 2014, 34, 3653–3667. [Google Scholar] [CrossRef]
- Just, S.; Chenard, B.L.; Ceci, A.; Strassmaier, T.; Chong, J.A.; Blair, N.T.; Gallaschun, R.J.; Del Camino, D.; Cantin, S.; D’amours, M.; et al. Treatment with HC-070, a potent inhibitor of TRPC4 and TRPC5, leads to anxiolytic and antidepressant effects in mice. PLoS ONE 2018, 13, e0191225. [Google Scholar] [CrossRef]
- Riccio, A.; Li, Y.; Moon, J.; Kim, K.S.; Smith, K.S.; Rudolph, U.; Gapon, S.; Yao, G.L.; Tsvetkov, E.; Rodig, S.J.; et al. Essential role for TRPC5 in amygdala function and fear-related behavior. Cell 2009, 137, 761–772. [Google Scholar] [CrossRef]
- Lee, S.H.; Tonello, R.; Choi, Y.; Jung, S.J.; Berta, T. Sensory Neuron–Expressed TRPC4 Is a Target for the Relief of Psoriasiform Itch and Skin Inflammation in Mice. J. Investig. Dermatol. 2020, 140, 2221–2229.e6. [Google Scholar] [CrossRef]
- Wu, D.; Huang, W.; Richardson, P.M.; Priestley, J.V.; Liu, M. TRPC4 in Rat Dorsal Root Ganglion Neurons Is Increased after Nerve Injury and Is Necessary for Neurite Outgrowth. J. Biol. Chem. 2008, 283, 416–426. [Google Scholar] [CrossRef]
- Westlund, K.; Zhang, L.; Ma, F.; Nesemeier, R.; Ruiz, J.; Ostertag, E.; Crawford, J.; Babinski, K.; Marcinkiewicz, M. A rat knockout model implicates TRPC4 in visceral pain sensation. Neuroscience 2014, 262, 165–175. [Google Scholar] [CrossRef]
- Lee, S.H.; Cho, P.S.; Tonello, R.; Lee, H.; Jang, J.H.; Park, G.Y.; Hwang, S.W.; Park, C.-K.; Jung, S.J.; Berta, T. Peripheral serotonin receptor 2B and transient receptor potential channel 4 mediate pruritus to serotonergic antidepressants in mice. J. Allergy Clin. Immunol. 2018, 142, 1349–1352.e16. [Google Scholar] [CrossRef]
- Alom, F.; Miyakawa, M.; Matsuyama, H.; Nagano, H.; Tanahashi, Y.; Unno, T. Possible antagonistic effects of the TRPC4 channel blocker ML204 on M2 and M3 muscarinic receptors in mouse ileal and detrusor smooth muscles and atrial myocardium. J. Vet. Med. Sci. 2018, 80, 1407–1415. [Google Scholar] [CrossRef]
- Miller, M.; Shi, J.; Zhu, Y.; Kustov, M.; Tian, J.-B.; Stevens, A.; Wu, M.; Xu, J.; Long, S.; Yang, P.; et al. Identification of ML204, a Novel Potent Antagonist That Selectively Modulates Native TRPC4/C5 Ion Channels. J. Biol. Chem. 2011, 286, 33436–33446. [Google Scholar] [CrossRef]
- Pradhan, A.A.; Smith, M.L.; McGuire, B.; Tarash, I.; Evans, C.J.; Charles, A. Characterization of a novel model of chronic migraine. Pain 2014, 155, 269–274. [Google Scholar] [CrossRef]
- Mathew, N.T.; Kailasam, J.; Seifert, T. Clinical recognition of allodynia in migraine. Neurology 2004, 63, 848–852. [Google Scholar] [CrossRef]
- Avona, A.; Burgos-Vega, C.; Burton, M.D.; Akopian, A.N.; Price, T.J.; Dussor, G. Dural Calcitonin Gene-Related Peptide Produces Female-Specific Responses in Rodent Migraine Models. J. Neurosci. 2019, 39, 4323–4331. [Google Scholar] [CrossRef]
- Stewart, W.F.; Wood, C.; Reed, M.L.; Roy, J.; Lipton, R.B. Cumulative Lifetime Migraine Incidence in Women and Men. Cephalalgia 2008, 28, 1170–1178. [Google Scholar] [CrossRef]
- May, A.; Goadsby, P.J. Substance P receptor antagonists in the therapy of migraine. Expert Opin. Investig. Drugs 2001, 10, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Eftekhari, S.; Edvinsson, L. Calcitonin gene-related peptide (CGRP) and its receptor components in human and rat spinal trigeminal nucleus and spinal cord at C1-level. BMC Neurosci. 2011, 12, 112. [Google Scholar] [CrossRef] [PubMed]
- Maleki, N.; Androulakis, X.M. Is There Any MRI Pattern That Discriminates Female From Male Migraine Patients? Front. Neurol. 2019, 10, 961. [Google Scholar] [CrossRef]
| Model Tested | Approach | Effect | Reference | |
|---|---|---|---|---|
| TRPV1 | Capsaicin injection in the carotid artery–rats | TRPV1 antagonists: JNJ-38893777, JNJ-17203212 | ↓ CGRP | [66] |
| Dural application of TRPV1 agonist capsaicin–rats | Botulinum toxin A Injections | ↓ response of C-type meningeal nociceptive neurons | [67] | |
| Electrode stimulation of the dura above the middle meningeal artery–rats | TRPV1 agonist: olvanil | ↓ activity of the trigeminocervical complex | [68] | |
| EtOH-treated TG tissue slices–guinea pigs | TRPV1 antagonist: capsazepine | ↓CGRP & SP | [69] | |
| Meningeal application of TRPV1 agonist capsaicin-rats | TRPV1 antagonist: capsazepine | ↓ activity TG fibers | [70] | |
| Capsaicin-treated TG neurons and trigeminal nucleus caudalis–rats | Sumatriptan | ↓ TRPV1 current ↓ sEPSC frequency | [71] | |
| TRPA1 | Systemic NTG administration and orofacial formalin test–rats | TRPA1 antagonist–ADM_12 | ↓ nocifensive behavior | [72] |
| Systemic Glyceryl trinitrate administration-mice | TRPA1 antagonist–HC-030031, TRPA1-conditional KO mice | ↓ mechanical allodynia and activity of TG neurons | [73] | |
| Systemic NTG administration–mice | TRPA1 antagonist–HC-030031 | ↓ mechanical allodynia | [74] | |
| Dural application of TRPA1 agonists: mustard oil or umbellulone-rats | TRPA1 antagonist–HC-030031 | ↓ mechanical allodynia, ↑ rearing in Open Field Test | [75] | |
| Mouse brain slice cortical spreading depression (CSD) model treated with TRPA1 agonist–Umbellulone | TRPA1 antagonists: HC-030031, A967079, anti-TRPA1 antibody | ↓ CSD | [76] | |
| Dural application of TRPA1 agonist-allyl isothiocyanate | TRPA1 partial agonist–parthenolide | ↓ mechanical allodynia | [77] | |
| Facial or bath (culture) application of TRPA1 agonist-allyl isothiocyanate | TRPA1 agonist–isopetasin | ↓ nocifensive behavior and activity of TG neurons | [78] | |
| TRPM8 | Dural application of icilin–rats | TRPM8 antagonist–AMG-1161 | ↓ mechanical allodynia | [79] |
| Dural application of inflammatory mediators–mouse | TRPM8 agonist–menthol | ↓ nocifensive behavior | [80] | |
| Dural application of inflammatory soup-mice | TRPM8 agonist–icilin | ↑ heat threshold temperature | [81] | |
| Cutaneous application of menthol–human | TRPM8 agonist–menthol | ↓ migraine | [82] | |
| TRPV4 | Formalin injections to whiskerpad-rats | TRPV4-KO mice and TRPV4 antagonist–HC067047 | ↓ nocifensive behavior | [83] |
| Dural application of hypotonic solution TRPV4 agonist–4α-PDD–rats | TRPV4 antagonist -RN1734 | ↓ nocifensive behavior | [84] | |
| TRPC4/5 | Dural injections of lysophosphatidylcholine-mice | TRPC5 antagonist–AC1903 | ↓ mechanical allodynia | [85] |
| Systemic NTG administration-mice | TRPC4 antagonist-ML204 | ↓ mechanical allodynia; ↓ CGRP plasma levels | [86] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cohen, C.F.; Roh, J.; Lee, S.H.; Park, C.-K.; Berta, T. Targeting Nociceptive Neurons and Transient Receptor Potential Channels for the Treatment of Migraine. Int. J. Mol. Sci. 2023, 24, 7897. https://doi.org/10.3390/ijms24097897
Cohen CF, Roh J, Lee SH, Park C-K, Berta T. Targeting Nociceptive Neurons and Transient Receptor Potential Channels for the Treatment of Migraine. International Journal of Molecular Sciences. 2023; 24(9):7897. https://doi.org/10.3390/ijms24097897
Chicago/Turabian StyleCohen, Cinder Faith, Jueun Roh, Sang Hoon Lee, Chul-Kyu Park, and Temugin Berta. 2023. "Targeting Nociceptive Neurons and Transient Receptor Potential Channels for the Treatment of Migraine" International Journal of Molecular Sciences 24, no. 9: 7897. https://doi.org/10.3390/ijms24097897
APA StyleCohen, C. F., Roh, J., Lee, S. H., Park, C.-K., & Berta, T. (2023). Targeting Nociceptive Neurons and Transient Receptor Potential Channels for the Treatment of Migraine. International Journal of Molecular Sciences, 24(9), 7897. https://doi.org/10.3390/ijms24097897







