Fatty Acid 2-Hydroxylase and 2-Hydroxylated Sphingolipids: Metabolism and Function in Health and Diseases
Abstract
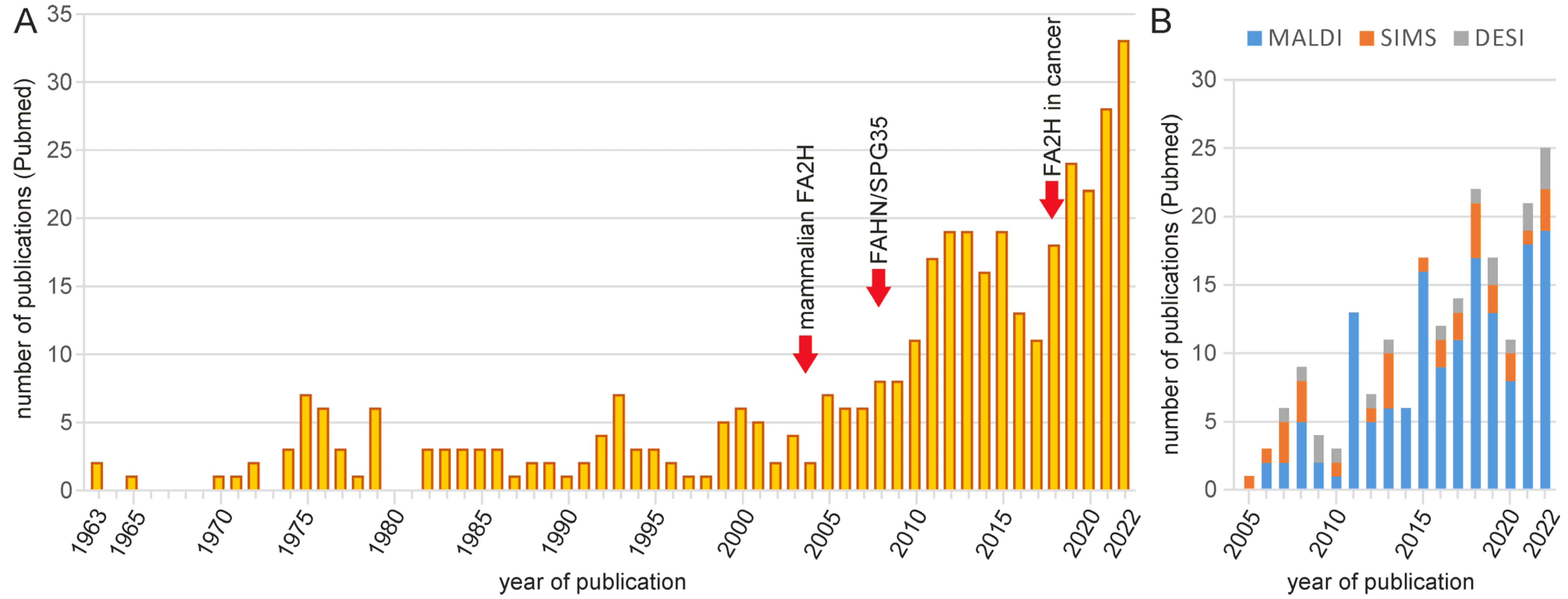
1. Introduction
2. Analytical Methods for 2hFA-SL and Other Sphingolipids
3. Biosynthesis of hFA and hFA-SL
3.1. Fatty Acid 2-Hydroxylase (FA2H)
3.2. Alternative Pathways of 2hFA Synthesis
3.3. Degradation of 2hFA-SL

4. 2hFA-SL in Membrane Trafficking and Microdomains
5. FA2H Expression and Function in Different Tissues
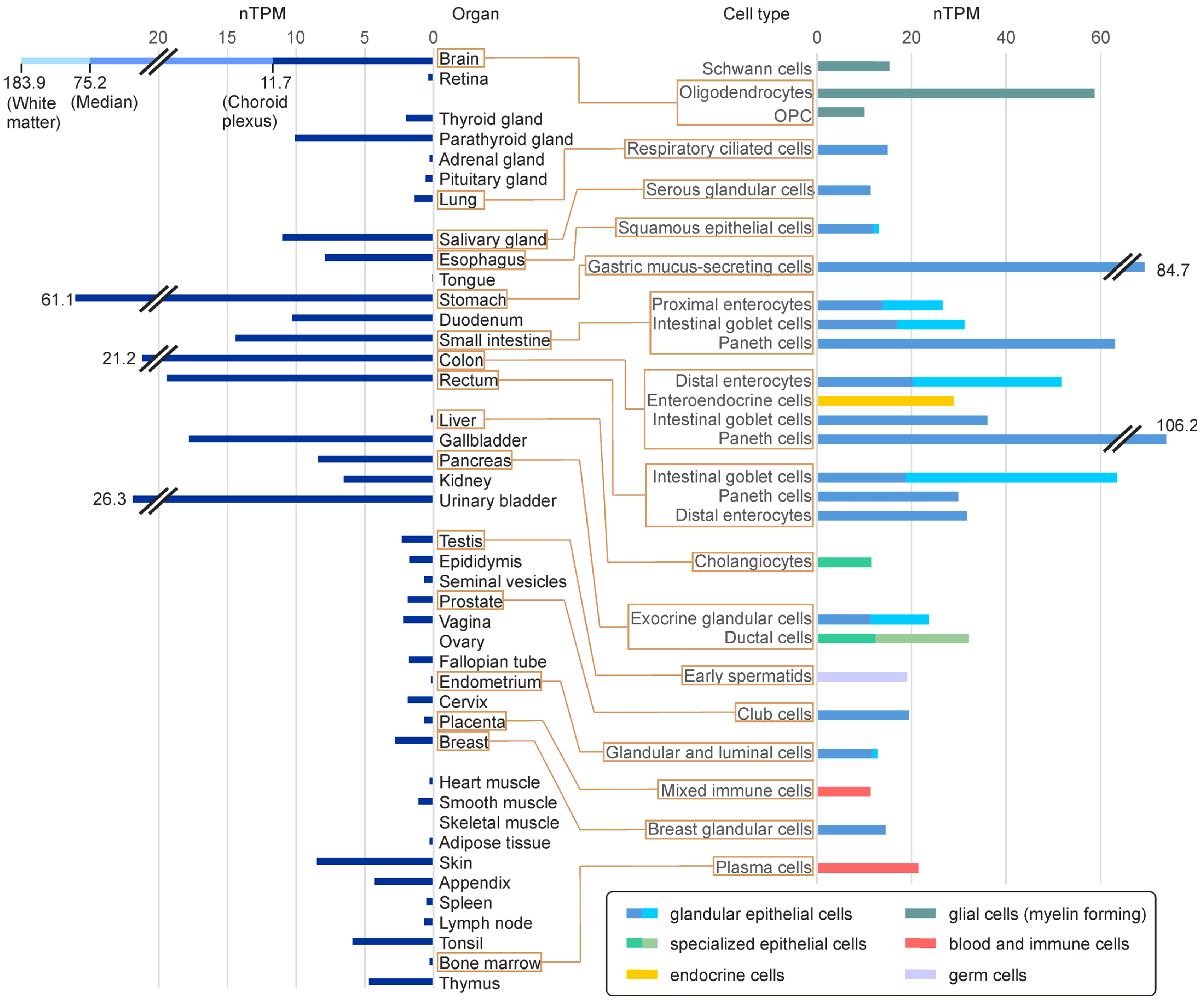
5.1. FA2H Expression Levels in Mammalian Tissues and Different Cell Types
5.2. FA2H in the Nervous System
5.3. 2hFA-SL and FA2H in the Skin
6. FA2H, 2hFA-SL and 2hFA in Human Diseases
6.1. Fatty Acid Hydroxylase-Associated Neurodegeneration (FAHN)
6.2. Altered 2hFA-SL Levels or FA2H Expression in Other Diseases
6.3. FA2H and 2hFA-SL in Cancer
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hannun, Y.A.; Obeid, L.M. Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 175–191. [Google Scholar] [CrossRef]
- Kawana, M.; Miyamoto, M.; Ohno, Y.; Kihara, A. Comparative profiling and comprehensive quantification of stratum corneum ceramides in humans and mice by LC/MS/MS. J. Lipid Res. 2020, 61, 884–895. [Google Scholar] [CrossRef]
- Kishimoto, Y.; Radin, N.S. Occurrence of 2-hydroxy fatty acids in animal tissues. J. Lipid Res. 1963, 4, 139–143. [Google Scholar] [CrossRef]
- Hama, H. Fatty acid 2-Hydroxylation in mammalian sphingolipid biology. Biochim. Biophys. Acta 2010, 1801, 405–414. [Google Scholar] [CrossRef]
- Tatsumi, K.; Kishimoto, Y.; Hignite, C. Stereochemical aspects of synthetic and naturally occurring 2-hydroxy fatty acids. Their absolute configurations and assays of optical purity. Arch. Biochem. Biophys. 1974, 165, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Jenske, R.; Vetter, W. Enantioselective analysis of 2- and 3-hydroxy fatty acids in food samples. J. Agric. Food Chem. 2008, 56, 11578–11583. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, M.; Hiraishi, A. Taxonomic significance of 2-hydroxy fatty acid profiles of the species in the genus Sphingomonas and related taxa. IFO Res. Commun. 2001, 20, 72–82. [Google Scholar]
- Kawahara, K.; Seydel, U.; Matsuura, M.; Danbara, H.; Rietschel, E.T.; Zähringer, U. Chemical structure of glycosphingolipids isolated from Sphingomonas paucimobilis. FEBS Lett. 1991, 292, 107–110. [Google Scholar] [CrossRef]
- Wollenweber, H.W.; Rietschel, E.T. Analysis of lipopolysaccharide (lipid A) fatty acids. J. Microbiol. Methods 1990, 11, 195–211. [Google Scholar] [CrossRef]
- Matsunaga, I.; Yokotani, N.; Gotoh, O.; Kusunose, E.; Yamada, M.; Ichihara, K. Molecular cloning and expression of fatty acid alpha-hydroxylase from Sphingomonas paucimobilis. J. Biol. Chem. 1997, 272, 23592–23596. [Google Scholar] [CrossRef] [PubMed]
- Fujishiro, T.; Shoji, O.; Nagano, S.; Sugimoto, H.; Shiro, Y.; Watanabe, Y. Crystal structure of H2O2-dependent cytochrome P450SPalpha with its bound fatty acid substrate: Insight into the regioselective hydroxylation of fatty acids at the alpha position. J. Biol. Chem. 2011, 286, 29941–29950. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, I.; Sumimoto, T.; Ueda, A.; Kusunose, E.; Ichihara, K. Fatty acid-specific, regiospecific, and stereospecific hydroxylation by cytochrome P450 (CYP152B1) from Sphingomonas paucimobilis: Substrate structure required for alpha-hydroxylation. Lipids 2000, 35, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Ring, M.W.; Schwär, G.; Bode, H.B. Biosynthesis of 2-hydroxy and iso-even fatty acids is connected to sphingolipid formation in myxobacteria. Chembiochem 2009, 10, 2003–2010. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Matyash, V.; Liebisch, G.; Kurzchalia, T.V.; Shevchenko, A.; Schwudke, D. Lipid extraction by methyl-tert-butyl ether for high-throughput lipidomics. J. Lipid Res. 2008, 49, 1137–1146. [Google Scholar] [CrossRef]
- Löfgren, L.; Forsberg, G.B.; Ståhlman, M. The BUME method: A new rapid and simple chloroform-free method for total lipid extraction of animal tissue. Sci. Rep. 2016, 6, 27688. [Google Scholar] [CrossRef]
- Breil, C.; Abert Vian, M.; Zemb, T.; Kunz, W.; Chemat, F. “Bligh and Dyer” and Folch Methods for Solid-Liquid-Liquid Extraction of Lipids from Microorganisms. Comprehension of Solvatation Mechanisms and towards Substitution with Alternative Solvents. Int. J. Mol. Sci. 2017, 18, 708. [Google Scholar] [CrossRef]
- Pati, S.; Nie, B.; Arnold, R.D.; Cummings, B.S. Extraction, chromatographic and mass spectrometric methods for lipid analysis. Biomed. Chromatogr. 2016, 30, 695–709. [Google Scholar] [CrossRef]
- Saini, R.K.; Prasad, P.; Shang, X.; Keum, Y.S. Advances in Lipid Extraction Methods-A Review. Int. J. Mol. Sci. 2021, 22, 13643. [Google Scholar] [CrossRef]
- Köfeler, H.C.; Ahrends, R.; Baker, E.S.; Ekroos, K.; Han, X.; Hoffmann, N.; Holčapek, M.; Wenk, M.R.; Liebisch, G. Recommendations for good practice in MS-based lipidomics. J. Lipid Res. 2021, 62, 100138. [Google Scholar] [CrossRef]
- Li, A.; Hines, K.M.; Xu, L. Lipidomics by HILIC-Ion Mobility-Mass Spectrometry. Methods Mol. Biol. 2020, 2084, 119–132. [Google Scholar] [CrossRef]
- Barrientos, R.C.; Zhang, Q. Recent advances in the mass spectrometric analysis of glycosphingolipidome—A review. Anal. Chim. Acta 2020, 1132, 134–155. [Google Scholar] [CrossRef]
- Tsugawa, H.; Ikeda, K.; Tanaka, W.; Senoo, Y.; Arita, M.; Arita, M. Comprehensive identification of sphingolipid species by in silico retention time and tandem mass spectral library. J. Cheminform. 2017, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wu, G.; Zhang, W.; Dong, M.; Xia, Y. Resolving Modifications on Sphingoid Base and N-Acyl Chain of Sphingomyelin Lipids in Complex Lipid Extracts. Anal. Chem. 2020, 92, 14775–14782. [Google Scholar] [CrossRef]
- von Gerichten, J.; Schlosser, K.; Lamprecht, D.; Morace, I.; Eckhardt, M.; Wachten, D.; Jennemann, R.; Gröne, H.J.; Mack, M.; Sandhoff, R. Diastereomer-specific quantification of bioactive hexosylceramides from bacteria and mammals. J. Lipid Res. 2017, 58, 1247–1258. [Google Scholar] [CrossRef] [PubMed]
- Züllig, T.; Köfeler, H.C. High resolution mass spectrometry in lipidomics. Mass Spectrom. Rev. 2021, 40, 162–176. [Google Scholar] [CrossRef]
- Luberto, C.; Haley, J.D.; Del Poeta, M. Imaging with mass spectrometry, the next frontier in sphingolipid research? A discussion on where we stand and the possibilities ahead. Chem. Phys. Lipids 2019, 219, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.X.; Whitehead, S.N. Imaging mass spectrometry allows for neuroanatomic-specific detection of gangliosides in the healthy and diseased brain. Analyst 2020, 145, 2473–2481. [Google Scholar] [CrossRef] [PubMed]
- Unsihuay, D.; Mesa Sanchez, D.; Laskin, J. Quantitative Mass Spectrometry Imaging of Biological Systems. Annu. Rev. Phys. Chem. 2021, 72, 307–329. [Google Scholar] [CrossRef]
- Marsching, C.; Eckhardt, M.; Gröne, H.J.; Sandhoff, R.; Hopf, C. Imaging of complex sulfatides SM3 and SB1a in mouse kidney using MALDI-TOF/TOF mass spectrometry. Anal. Bioanal. Chem. 2011, 401, 53–64. [Google Scholar] [CrossRef]
- Wang, H.Y.; Jackson, S.N.; Post, J.; Woods, A.S. A Minimalist Approach to MALDI Imaging of Glycerophospholipids and Sphingolipids in Rat Brain Sections. Int. J. Mass Spectrom. 2008, 278, 143–149. [Google Scholar] [CrossRef]
- Maganti, R.J.; Hronowski, X.L.; Dunstan, R.W.; Wipke, B.T.; Zhang, X.; Jandreski, L.; Hamann, S.; Juhasz, P. Defining Changes in the Spatial Distribution and Composition of Brain Lipids in the Shiverer and Cuprizone Mouse Models of Myelin Disease. J. Histochem. Cytochem. 2019, 67, 203–219. [Google Scholar] [CrossRef] [PubMed]
- Yuki, D.; Sugiura, Y.; Zaima, N.; Akatsu, H.; Hashizume, Y.; Yamamoto, T.; Fujiwara, M.; Sugiyama, K.; Setou, M. Hydroxylated and non-hydroxylated sulfatide are distinctly distributed in the human cerebral cortex. Neuroscience 2011, 193, 44–53. [Google Scholar] [CrossRef]
- Sjövall, P.; Lausmaa, J.; Johansson, B. Mass spectrometric imaging of lipids in brain tissue. Anal. Chem. 2004, 76, 4271–4278. [Google Scholar] [CrossRef] [PubMed]
- Pernber, Z.; Richter, K.; Mansson, J.E.; Nygren, H. Sulfatide with different fatty acids has unique distributions in cerebellum as imaged by time-of-flight secondary ion mass spectrometry (TOF-SIMS). Biochim. Biophys. Acta 2007, 1771, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Kadar, H.; Pham, H.; Touboul, D.; Brunelle, A.; Baud, O. Impact of inhaled nitric oxide on the sulfatide profile of neonatal rat brain studied by TOF-SIMS imaging. Int. J. Mol. Sci. 2014, 15, 5233–5245. [Google Scholar] [CrossRef] [PubMed]
- Girod, M.; Shi, Y.; Cheng, J.X.; Cooks, R.G. Mapping lipid alterations in traumatically injured rat spinal cord by desorption electrospray ionization imaging mass spectrometry. Anal. Chem. 2011, 83, 207–215. [Google Scholar] [CrossRef]
- Passarelli, M.K.; Pirkl, A.; Moellers, R.; Grinfeld, D.; Kollmer, F.; Havelund, R.; Newman, C.F.; Marshall, P.S.; Arlinghaus, H.; Alexander, M.R.; et al. The 3D OrbiSIMS-label-free metabolic imaging with subcellular lateral resolution and high mass-resolving power. Nat. Methods 2017, 14, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Hirahara, Y.; Wakabayashi, T.; Mori, T.; Koike, T.; Yao, I.; Tsuda, M.; Honke, K.; Gotoh, H.; Ono, K.; Yamada, H. Sulfatide species with various fatty acid chains in oligodendrocytes at different developmental stages determined by imaging mass spectrometry. J. Neurochem. 2017, 140, 435–450. [Google Scholar] [CrossRef]
- Nakashima, K.; Hirahara, Y.; Koike, T.; Tanaka, S.; Gamo, K.; Oe, S.; Hayashi, S.; Seki-Omura, R.; Nakano, Y.; Ohe, C.; et al. Sulfatide with ceramide composed of phytosphingosine (t18:0) and 2-hydroxy FAs in renal intercalated cells. J. Lipid Res. 2022, 63, 100210. [Google Scholar] [CrossRef] [PubMed]
- Alderson, N.L.; Rembiesa, B.M.; Walla, M.D.; Bielawska, A.; Bielawski, J.; Hama, H. The human FA2H gene encodes a fatty acid 2-hydroxylase. J. Biol. Chem. 2004, 279, 48562–48568. [Google Scholar] [CrossRef] [PubMed]
- Eckhardt, M.; Yaghootfam, A.; Fewou, S.N.; Zöller, I.; Gieselmann, V. A mammalian fatty acid hydroxylase responsible for the formation of alpha-hydroxylated galactosylceramide in myelin. Biochem. J. 2005, 388, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Haak, D.; Gable, K.; Beeler, T.; Dunn, T. Hydroxylation of Saccharomyces cerevisiae ceramides requires Sur2p and Scs7p. J. Biol. Chem. 1997, 272, 29704–29710. [Google Scholar] [CrossRef]
- Dunn, T.M.; Haak, D.; Monaghan, E.; Beeler, T.J. Synthesis of monohydroxylated inositolphosphorylceramide (IPC-C) in Saccharomyces cerevisiae requires Scs7p, a protein with both a cytochrome b5-like domain and a hydroxylase/desaturase domain. Yeast 1998, 14, 311–321. [Google Scholar] [CrossRef]
- Nagano, M.; Ihara-Ohori, Y.; Imai, H.; Inada, N.; Fujimoto, M.; Tsutsumi, N.; Uchimiya, H.; Kawai-Yamada, M. Functional association of cell death suppressor, Arabidopsis Bax inhibitor-1, with fatty acid 2-hydroxylation through cytochrome b5; Plant J. 2009, 58, 122–134. [Google Scholar] [CrossRef]
- Cid, N.G.; Puca, G.; Nudel, C.B.; Nusblat, A.D. Genome analysis of sphingolipid metabolism-related genes in Tetrahymena thermophila and identification of a fatty acid 2-hydroxylase involved in the sexual stage of conjugation. Mol. Microbiol. 2020, 114, 775–788. [Google Scholar] [CrossRef]
- Zhu, G.; Koszelak-Rosenblum, M.; Connelly, S.M.; Dumont, M.E.; Malkowski, M.G. The Crystal Structure of an Integral Membrane Fatty Acid α-Hydroxylase. J. Biol. Chem. 2015, 290, 29820–29833. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, X.; Zhou, D.; Okunade, A.L.; Su, X. Stereospecificity of fatty acid 2-hydroxylase and differential functions of 2-hydroxy fatty acid enantiomers. J. Lipid Res. 2012, 53, 1327–1335. [Google Scholar] [CrossRef]
- Ukawa, T.; Banno, F.; Ishikawa, T.; Kasahara, K.; Nishina, Y.; Inoue, R.; Tsujii, K.; Yamaguchi, M.; Takahashi, T.; Fukao, Y.; et al. Sphingolipids with 2-hydroxy fatty acids aid in plasma membrane nanodomain organization and oxidative burst. Plant Physiol. 2022, 189, 839–857. [Google Scholar] [CrossRef]
- Nagano, M.; Uchimiya, H.; Kawai-Yamada, M. Plant sphingolipid fatty acid 2-hydroxylases have unique characters unlike their animal and fungus counterparts. Plant Signal. Behav. 2012, 7, 1388–1392. [Google Scholar] [CrossRef] [PubMed]
- Nagano, M.; Takahara, K.; Fujimoto, M.; Tsutsumi, N.; Uchimiya, H.; Kawai-Yamada, M. Arabidopsis sphingolipid fatty acid 2-hydroxylases (AtFAH1 and AtFAH2) are functionally differentiated in fatty acid 2-hydroxylation and stress responses. Plant Physiol. 2012, 159, 1138–1148. [Google Scholar] [CrossRef]
- Alderson, N.L.; Walla, M.D.; Hama, H. A novel method for the measurement of in vitro fatty acid 2-hydroxylase activity by gas chromatography-mass spectrometry. J. Lipid Res. 2005, 46, 1569–1575. [Google Scholar] [CrossRef]
- Hardt, R.; Winter, D.; Gieselmann, V.; Eckhardt, M. Identification of progesterone receptor membrane component-1 as an interaction partner and possible regulator of fatty acid 2-hydroxylase. Biochem. J. 2018, 475, 853–871. [Google Scholar] [CrossRef]
- Piel, R.B., III; Shiferaw, M.T.; Vashisht, A.A.; Marcero, J.R.; Praissman, J.L.; Phillips, J.D.; Wohlschlegel, J.A.; Medlock, A.E. A Novel Role for Progesterone Receptor Membrane Component 1 (PGRMC1): A Partner and Regulator of Ferrochelatase. Biochemistry 2016, 55, 5204–5217. [Google Scholar] [CrossRef]
- Hughes, A.L.; Powell, D.W.; Bard, M.; Eckstein, J.; Barbuch, R.; Link, A.J.; Espenshade, P.J. Dap1/PGRMC1 binds and regulates cytochrome P450 enzymes. Cell Metab. 2007, 5, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Zöller, I.; Meixner, M.; Hartmann, D.; Büssow, H.; Meyer, R.; Gieselmann, V.; Eckhardt, M. Absence of 2-hydroxylated sphingolipids is compatible with normal neural development but causes late-onset axon and myelin sheath degeneration. J. Neurosci. 2008, 28, 9741–9754. [Google Scholar] [CrossRef] [PubMed]
- Potter, K.A.; Kern, M.J.; Fullbright, G.; Bielawski, J.; Scherer, S.S.; Yum, S.W.; Li, J.J.; Cheng, H.; Han, X.; Venkata, J.K.; et al. Central nervous system dysfunction in a mouse model of FA2H deficiency. Glia 2011, 59, 1009–1021. [Google Scholar] [CrossRef]
- Maier, H.; Meixner, M.; Hartmann, D.; Sandhoff, R.; Wang-Eckhardt, L.; Zöller, I.; Gieselmann, V.; Eckhardt, M. Normal fur development and sebum production depends on fatty acid 2-hydroxylase expression in sebaceous glands. J. Biol. Chem. 2011, 286, 25922–25934. [Google Scholar] [CrossRef]
- Dan, P.; Edvardson, S.; Bielawski, J.; Hama, H.; Saada, A. 2-Hydroxylated sphingomyelin profiles in cells from patients with mutated fatty acid 2-hydroxylase. Lipids Health Dis. 2011, 10, 84. [Google Scholar] [CrossRef]
- Kitamura, T.; Seki, N.; Kihara, A. Phytosphingosine degradation pathway includes fatty acid α-oxidation reactions in the endoplasmic reticulum. Proc. Natl. Acad. Sci. USA 2017, 114, E2616–E2623. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, J.; Kido, M.; Tadano-Aritomi, K.; Ishizuka, I.; Tominaga, K.; Toida, K.; Takeda, E.; Suzuki, K.; Kuroda, Y. Mutation in saposin D domain of sphingolipid activator protein gene causes urinary system defects and cerebellar Purkinje cell degeneration with accumulation of hydroxy fatty acid-containing ceramide in mouse. Hum. Mol. Genet. 2004, 13, 2709–2723. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, Y.; Kihara, A.; Chiba, H.; Tojo, H.; Igarashi, Y. 2-Hydroxy-ceramide synthesis by ceramide synthase family: Enzymatic basis for the preference of FA chain length. J. Lipid Res. 2008, 49, 2356–2364. [Google Scholar] [CrossRef]
- Foulon, V.; Sniekers, M.; Huysmans, E.; Asselberghs, S.; Mahieu, V.; Mannaerts, G.P.; Van Veldhoven, P.P.; Casteels, M. Breakdown of 2-hydroxylated straight chain fatty acids via peroxisomal 2-hydroxyphytanoyl-CoA lyase: A revised pathway for the alpha-oxidation of straight chain fatty acids. J. Biol. Chem. 2005, 280, 9802–9812. [Google Scholar] [CrossRef] [PubMed]
- Hebbar, S.; Schuhmann, K.; Shevchenko, A.; Knust, E. Hydroxylated sphingolipid biosynthesis regulates photoreceptor apical domain morphogenesis. J. Cell Biol. 2020, 219, e201911100. [Google Scholar] [CrossRef]
- Li, Y.; Wang, C.; Huang, Y.; Fu, R.; Zheng, H.; Zhu, Y.; Shi, X.; Padakanti, P.K.; Tu, Z.; Su, X.; et al. Elegans Fatty Acid Two-Hydroxylase Regulates Intestinal Homeostasis by Affecting Heptadecenoic Acid Production. Cell. Physiol. Biochem. 2018, 49, 947–960. [Google Scholar] [CrossRef]
- Sampaio, J.L.; Gerl, M.J.; Klose, C.; Ejsing, C.S.; Beug, H.; Simons, K.; Shevchenko, A. Membrane lipidome of an epithelial cell line. Proc. Natl. Acad. Sci. USA 2011, 108, 1903–1907. [Google Scholar] [CrossRef]
- Dingjan, T.; Futerman, A.H. The role of the ‘sphingoid motif’ in shaping the molecular interactions of sphingolipids in biomembranes. Biochim. Biophys. Acta Biomembr. 2021, 1863, 183701. [Google Scholar] [CrossRef]
- Szulc, Z.M.; Bai, A.; Bielawski, J.; Mayroo, N.; Miller, D.E.; Gracz, H.; Hannun, Y.A.; Bielawska, A. Synthesis, NMR characterization and divergent biological actions of 2′-hydroxy-ceramide/dihydroceramide stereoisomers in MCF7 cells. Bioorg. Med. Chem. 2010, 18, 7565–7579. [Google Scholar] [CrossRef]
- Pascher, I.; Sundell, S. Molecular arrangements in sphingolipids. The crystal structure of cerebroside. Chem. Phys. Lipids 1977, 20, 175–191. [Google Scholar] [CrossRef]
- Yahi, N.; Aulas, A.; Fantini, J. How cholesterol constrains glycolipid conformation for optimal recognition of Alzheimer’s beta amyloid peptide (Abeta1-40). PLoS ONE 2010, 5, e9079. [Google Scholar] [CrossRef] [PubMed]
- Cacas, J.L.; Buré, C.; Grosjean, K.; Gerbeau-Pissot, P.; Lherminier, J.; Rombouts, Y.; Maes, E.; Bossard, C.; Gronnier, J.; Furt, F.; et al. Revisiting Plant Plasma Membrane Lipids in Tobacco: A Focus on Sphingolipids. Plant Physiol. 2016, 170, 367–384. [Google Scholar] [CrossRef] [PubMed]
- Mamode Cassim, A.; Navon, Y.; Gao, Y.; Decossas, M.; Fouillen, L.; Grélard, A.; Nagano, M.; Lambert, O.; Bahammou, D.; Van Delft, P.; et al. Biophysical analysis of the plant-specific GIPC sphingolipids reveals multiple modes of membrane regulation. J. Biol. Chem. 2021, 296, 100602. [Google Scholar] [CrossRef]
- Nagano, M.; Ishikawa, T.; Fujiwara, M.; Fukao, Y.; Kawano, Y.; Kawai-Yamada, M.; Shimamoto, K. Plasma Membrane Microdomains Are Essential for Rac1-RbohB/H-Mediated Immunity in Rice. Plant Cell 2016, 28, 1966–1983. [Google Scholar] [CrossRef]
- Windschiegl, B.; Steinem, C. Influence of alpha-hydroxylation of glycolipids on domain formation in lipid monolayers. Langmuir 2006, 22, 7454–7457. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Zhou, D.; Pryse, K.M.; Okunade, A.L.; Su, X. Fatty acid 2-hydroxylase mediates diffusional mobility of Raft-associated lipids, GLUT4 level, and lipogenesis in 3T3-L1 adipocytes. J. Biol. Chem. 2010, 285, 25438–25447. [Google Scholar] [CrossRef] [PubMed]
- Jaikishan, S.; Slotte, J.P. Stabilization of sphingomyelin interactions by interfacial hydroxyls—A study of phytosphingomyelin properties. Biochim. Biophys. Acta 2013, 1828, 391–397. [Google Scholar] [CrossRef]
- The Human Protein Atlas. Available online: https://www.proteinatlas.org/ENSG00000103089-FA2H/tissue+cell+type (accessed on 14 February 2023).
- Karlsson, M.; Zhang, C.; Méar, L.; Zhong, W.; Digre, A.; Katona, B.; Sjöstedt, E.; Butler, L.; Odeberg, J.; Dusart, P.; et al. A single-cell type transcriptomics map of human tissues. Sci. Adv. 2021, 7, eabh2169. [Google Scholar] [CrossRef]
- Eckhardt, M. The role and metabolism of sulfatide in the nervous system. Mol. Neurobiol. 2008, 37, 93–103. [Google Scholar] [CrossRef]
- Ki, P.F.; Kishimoto, Y. The lipid composition of urodele myelin which lacks hydroxycerebroside and hydroxysulfatide. J. Neurochem. 1984, 42, 994–1000. [Google Scholar] [CrossRef]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Proteomics. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Zöller, I. Untersuchungen zur Biosynthese und Funktion von alpha-hydroxylierten Sphingolipiden Anhand von Mausmodellen. Ph.D. Thesis, University of Bonn, Bonn, Germany, 2007. [Google Scholar]
- Baba, H.; Ishibashi, T. The Role of Sulfatides in Axon-Glia Interactions. Adv. Exp. Med. Biol. 2019, 1190, 165–179. [Google Scholar] [CrossRef]
- Boggs, J.M.; Gao, W.; Hirahara, Y. Myelin glycosphingolipids, galactosylceramide and sulfatide, participate in carbohydrate-carbohydrate interactions between apposed membranes and may form glycosynapses between oligodendrocyte and/or myelin membranes. Biochim. Biophys. Acta 2008, 1780, 445–455. [Google Scholar] [CrossRef]
- Grassi, S.; Prioni, S.; Cabitta, L.; Aureli, M.; Sonnino, S.; Prinetti, A. The Role of 3-O-Sulfogalactosylceramide, Sulfatide, in the Lateral Organization of Myelin Membrane. Neurochem. Res. 2016, 41, 130–143. [Google Scholar] [CrossRef]
- Suzuki, M.; Ohno, Y.; Kihara, A. Whole picture of human stratum corneum ceramides, including the chain-length diversity of long-chain bases. J. Lipid Res. 2022, 63, 100235. [Google Scholar] [CrossRef]
- Uchida, Y.; Hama, H.; Alderson, N.L.; Douangpanya, S.; Wang, Y.; Crumrine, D.A.; Elias, P.M.; Holleran, W.M. Fatty acid 2-hydroxylase, encoded by FA2H, accounts for differentiation-associated increase in 2-OH ceramides during keratinocyte differentiation. J. Biol. Chem. 2007, 282, 13211–13219. [Google Scholar] [CrossRef]
- Mutagenetix. Phenotypic Mutation ‘Sparse’. Available online: https://mutagenetix.utsouthwestern.edu/phenotypic/phenotypic_rec.cfm?pk=2356 (accessed on 14 February 2023).
- Nicolaides, N.; Fu, H.C.; Ansari, M.N. Diester waxes in surface lipids of animal skin. Lipids 1970, 5, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Ebel, P.; Imgrund, S.; Vom Dorp, K.; Hofmann, K.; Maier, H.; Drake, H.; Degen, J.; Dörmann, P.; Eckhardt, M.; Franz, T.; et al. Ceramide synthase 4 deficiency in mice causes lipid alterations in sebum and results in alopecia. Biochem. J. 2014, 461, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.L.; Xu, R.; Yi, J.K.; Li, F.; Chen, J.; Jones, E.C.; Slutsky, J.B.; Huang, L.; Rigas, B.; Cao, J.; et al. Alkaline Ceramidase 1 Protects Mice from Premature Hair Loss by Maintaining the Homeostasis of Hair Follicle Stem Cells. Stem Cell Rep. 2017, 9, 1488–1500. [Google Scholar] [CrossRef] [PubMed]
- Jacinto, J.G.P.; Häfliger, I.M.; Veiga, I.M.B.; Letko, A.; Gentile, A.; Drögemüller, C. A frameshift insertion in FA2H causes a recessively inherited form of ichthyosis congenita in Chianina cattle. Mol. Genet. Genom. 2021, 296, 1313–1322. [Google Scholar] [CrossRef] [PubMed]
- Edvardson, S.; Hama, H.; Shaag, A.; Gomori, J.M.; Berger, I.; Soffer, D.; Korman, S.H.; Taustein, I.; Saada, A.; Elpeleg, O. Mutations in the fatty acid 2-hydroxylase gene are associated with leukodystrophy with spastic paraparesis and dystonia. Am. J. Hum. Genet. 2008, 83, 643–648. [Google Scholar] [CrossRef]
- Dick, K.J.; Eckhardt, M.; Paisán-Ruiz, C.; Alshehhi, A.A.; Proukakis, C.; Sibtain, N.A.; Maier, H.; Sharifi, R.; Patton, M.A.; Bashir, W.; et al. Mutation of FA2H underlies a complicated form of hereditary spastic paraplegia (SPG35). Hum. Mutat. 2010, 31, E1251–E1260. [Google Scholar] [CrossRef]
- Tesson, C.; Koht, J.; Stevanin, G. Delving into the complexity of hereditary spastic paraplegias: How unexpected phenotypes and inheritance modes are revolutionizing their nosology. Hum. Genet. 2015, 134, 511–538. [Google Scholar] [CrossRef] [PubMed]
- Dong, E.L.; Wang, C.; Wu, S.; Lu, Y.Q.; Lin, X.H.; Su, H.Z.; Zhao, M.; He, J.; Ma, L.X.; Wang, N.; et al. Clinical spectrum and genetic landscape for hereditary spastic paraplegias in China. Mol. Neurodegener. 2018, 13, 36. [Google Scholar] [CrossRef]
- Gregory, A.; Venkateswaran, S.; Hayflick, S.J. Fatty Acid Hydroxylase-Associated Neurodegeneration. In GeneReviews® [Internet]; Adam, M.P., Everman, D.B., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 2011; pp. 1993–2023. [Google Scholar]
- Kruer, M.C.; Paisán-Ruiz, C.; Boddaert, N.; Yoon, M.Y.; Hama, H.; Gregory, A.; Malandrini, A.; Woltjer, R.L.; Munnich, A.; Gobin, S.; et al. Defective FA2H leads to a novel form of neurodegeneration with brain iron accumulation (NBIA). Ann. Neurol. 2010, 68, 611–618. [Google Scholar] [CrossRef]
- Pierson, T.M.; Simeonov, D.R.; Sincan, M.; Adams, D.A.; Markello, T.; Golas, G.; Fuentes-Fajardo, K.; Hansen, N.F.; Cherukuri, P.F.; Cruz, P.; et al. NISC Comparative Sequencing Program. Exome sequencing and SNP analysis detect novel compound heterozygosity in fatty acid hydroxylase-associated neurodegeneration. Eur. J. Hum. Genet. 2012, 20, 476–479. [Google Scholar] [CrossRef] [PubMed]
- Pensato, V.; Castellotti, B.; Gellera, C.; Pareyson, D.; Ciano, C.; Nanetti, L.; Salsano, E.; Piscosquito, G.; Sarto, E.; Eoli, M.; et al. Overlapping phenotypes in complex spastic paraplegias SPG11, SPG15, SPG35 and SPG48. Brain 2014, 137, 1907–1920. [Google Scholar] [CrossRef] [PubMed]
- Donkervoort, S.; Dastgir, J.; Hu, Y.; Zein, W.M.; Marks, H.; Blackstone, C.; Bönnemann, C.G. Phenotypic variability of a likely FA2H founder mutation in a family with complicated hereditary spastic paraplegia. Clin. Genet. 2014, 85, 393–395. [Google Scholar] [CrossRef]
- Zaki, M.S.; Selim, L.; Mansour, L.; Mahmoud, I.G.; Fenstermaker, A.G.; Gabriel, S.B.; Gleeson, J.G. Mutations in FA2H in three Arab families with a clinical spectrum of neurodegeneration and hereditary spastic paraparesis. Clin. Genet. 2015, 88, 95–97. [Google Scholar] [CrossRef]
- Kara, E.; Tucci, A.; Manzoni, C.; Lynch, D.S.; Elpidorou, M.; Bettencourt, C.; Chelban, V.; Manole, A.; Hamed, S.A.; Haridy, N.A.; et al. Genetic and phenotypic characterization of complex hereditary spastic paraplegia. Brain 2016, 139, 1904–1918. [Google Scholar] [CrossRef]
- Soehn, A.S.; Rattay, T.W.; Beck-Wödl, S.; Schäferhoff, K.; Monk, D.; Döbler-Neumann, M.; Hörtnagel, K.; Schlüter, A.; Ruiz, M.; Pujol, A.; et al. Uniparental disomy of chromosome 16 unmasks recessive mutations of FA2H/SPG35 in 4 families. Neurology 2016, 87, 186–191. [Google Scholar] [CrossRef]
- Bektaş, G.; Yeşil, G.; Yıldız, E.P.; Aydınlı, N.; Çalışkan, M.; Özmen, M. Hereditary spastic paraplegia type 35 caused by a novel FA2H mutation. Turk. J. Pediatr. 2017, 59, 329–334. [Google Scholar] [CrossRef]
- Travaglini, L.; Aiello, C.; Stregapede, F.; D’Amico, A.; Alesi, V.; Ciolfi, A.; Bruselles, A.; Catteruccia, M.; Pizzi, S.; Zanni, G.; et al. The impact of next-generation sequencing on the diagnosis of pediatric-onset hereditary spastic paraplegias: New genotype-phenotype correlations for rare HSP-related genes. Neurogenetics 2018, 19, 111–121. [Google Scholar] [CrossRef]
- Benger, M.; Mankad, K.; Proukakis, C.; Mazarakis, N.D.; Kinali, M. The Interaction of Genetic Mutations in PARK2 and FA2H Causes a Novel Phenotype in a Case of Childhood-Onset Movement Disorder. Front. Neurol. 2019, 10, 555. [Google Scholar] [CrossRef]
- Landouré, G.; Dembélé, K.; Cissé, L.; Samassékou, O.; Diarra, S.; Bocoum, A.; Dembélé, M.E.; Fischbeck, K.H.; Guinto, C.O. Hereditary spastic paraplegia type 35 in a family from Mali. Am. J. Med. Genet. A 2019, 179, 1122–1125. [Google Scholar] [CrossRef]
- Rattay, T.W.; Lindig, T.; Baets, J.; Smets, K.; Deconinck, T.; Söhn, A.S.; Hörtnagel, K.; Eckstein, K.N.; Wiethoff, S.; Reichbauer, J.; et al. FAHN/SPG35: A narrow phenotypic spectrum across disease classifications. Brain 2019, 142, 1561–1572. [Google Scholar] [CrossRef] [PubMed]
- Shakya, S.; Kumari, R.; Suroliya, V.; Tyagi, N.; Joshi, A.; Garg, A.; Singh, I.; Kalikavil, P.D.; Cherian, A.; Mukerji, M.; et al. Whole exome and targeted gene sequencing to detect pathogenic recessive variants in early onset cerebellar ataxia. Clin. Genet. 2019, 96, 566–574. [Google Scholar] [CrossRef]
- Tsang, M.H.Y.; Kwong, A.K.Y.; Chan, K.L.S.; Fung, J.L.F.; Yu, M.H.C.; Mak, C.C.Y.; Yeung, K.S.; Rodenburg, R.J.T.; Smeitink, J.A.M.; Chan, R.; et al. Delineation of molecular findings by whole-exome sequencing for suspected cases of paediatric-onset mitochondrial diseases in the Southern Chinese population. Hum. Genomics 2020, 14, 28. [Google Scholar] [CrossRef] [PubMed]
- Hardt, R.; Jordans, S.; Winter, D.; Gieselmann, V.; Wang-Eckhardt, L.; Eckhardt, M. Decreased turnover of the CNS myelin protein Opalin in a mouse model of hereditary spastic paraplegia 35. Hum. Mol. Genet. 2021, 29, 3616–3630. [Google Scholar] [CrossRef] [PubMed]
- Meyer, E.; Kurian, M.A.; Hayflick, S.J. Neurodegeneration with Brain Iron Accumulation: Genetic Diversity and Pathophysiological Mechanisms. Annu. Rev. Genom. Hum. Genet. 2015, 16, 257–279. [Google Scholar] [CrossRef]
- Drecourt, A.; Babdor, J.; Dussiot, M.; Petit, F.; Goudin, N.; Garfa-Traoré, M.; Habarou, F.; Bole-Feysot, C.; Nitschké, P.; Ottolenghi, C.; et al. Impaired Transferrin Receptor Palmitoylation and Recycling in Neurodegeneration with Brain Iron Accumulation. Am. J. Hum. Genet. 2018, 102, 266–277. [Google Scholar] [CrossRef]
- Wang, Z.B.; Liu, J.Y.; Xu, X.J.; Mao, X.Y.; Zhang, W.; Zhou, H.H.; Liu, Z.Q. Neurodegeneration with brain iron accumulation: Insights into the mitochondria dysregulation. Biomed. Pharmacother. 2019, 118, 109068. [Google Scholar] [CrossRef] [PubMed]
- Mandik, F.; Kanana, Y.; Rody, J.; Misera, S.; Wilken, B.; Laabs von Holt, B.H.; Klein, C.; Vos, M. A new model for fatty acid hydroxylase-associated neurodegeneration reveals mitochondrial and autophagy abnormalities. Front. Cell Dev. Biol. 2022, 10, 1000553. [Google Scholar] [CrossRef] [PubMed]
- Axpe, I.R.; Blanco Martín, E.; Garcia Ribes, A.; Bermejo-Ramirez, R.; Arroyo Andújar, D. Sensory-motor neuropathy in a case with SPG35: Expanding the phenotype. J. Neurol. Sci. 2017, 380, 98–100. [Google Scholar] [CrossRef] [PubMed]
- Jordans, S.; Hardt, R.; Becker, I.; Winter, D.; Wang-Eckhardt, L.; Eckhardt, M. Age-Dependent Increase in Schmidt-Lanterman Incisures and a Cadm4-Associated Membrane Skeletal Complex in Fatty Acid 2-hydroxylase Deficient Mice: A Mouse Model of Spastic Paraplegia SPG35. Mol. Neurobiol. 2022, 59, 3969–3979. [Google Scholar] [CrossRef]
- van Kruining, D.; Luo, Q.; van Echten-Deckert, G.; Mielke, M.M.; Bowman, A.; Ellis, S.; Oliveira, T.G.; Martinez-Martinez, P. Sphingolipids as prognostic biomarkers of neurodegeneration, neuroinflammation, and psychiatric diseases and their emerging role in lipidomic investigation methods. Adv. Drug Deliv. Rev. 2020, 159, 232–244. [Google Scholar] [CrossRef]
- Han, X.; Holtzman, D.M.; McKeel, D.W.; Kelley, J.; Morris, J.C. Substantial sulfatide deficiency and ceramide elevation in very early Alzheimer’s disease: Potential role in disease pathogenesis. J. Neurochem. 2002, 82, 809–818. [Google Scholar] [CrossRef]
- Qiu, S.; Palavicini, J.P.; Wang, J.; Gonzalez, N.S.; He, S.; Dustin, E.; Zou, C.; Ding, L.; Bhattacharjee, A.; Van Skike, C.E.; et al. Adult-onset CNS myelin sulfatide deficiency is sufficient to cause Alzheimer’s disease-like neuroinflammation and cognitive impairment. Mol. Neurodegener. 2021, 16, 64. [Google Scholar] [CrossRef]
- Kanetake, T.; Sassa, T.; Nojiri, K.; Sawai, M.; Hattori, S.; Miyakawa, T.; Kitamura, T.; Kihara, A. Neural symptoms in a gene knockout mouse model of Sjögren-Larsson syndrome are associated with a decrease in 2-hydroxygalactosylceramide. FASEB J. 2019, 33, 928–941. [Google Scholar] [CrossRef]
- Fewou, S.N.; Büssow, H.; Schaeren-Wiemers, N.; Vanier, M.T.; Macklin, W.B.; Gieselmann, V.; Eckhardt, M. Reversal of non-hydroxy:alpha-hydroxy galactosylceramide ratio and unstable myelin in transgenic mice overexpressing UDP-galactose:ceramide galactosyltransferase. J. Neurochem. 2005, 94, 469–481. [Google Scholar] [CrossRef]
- Ji, Y.; Luo, Z.; Gao, H.; Dos Reis, F.C.G.; Bandyopadhyay, G.; Jin, Z.; Manda, K.A.; Isaac, R.; Yang, M.; Fu, W.; et al. Hepatocyte-derived exosomes from early onset obese mice promote insulin sensitivity through miR-3075. Nat. Metab. 2021, 3, 1163–1174. [Google Scholar] [CrossRef] [PubMed]
- Ogretmen, B. Sphingolipid metabolism in cancer signalling and therapy. Nat. Rev. Cancer 2018, 18, 33–50. [Google Scholar] [CrossRef]
- Companioni, O.; Mir, C.; Garcia-Mayea, Y.; Leonart, M.E. Targeting Sphingolipids for Cancer Therapy. Front. Oncol. 2021, 11, 745092. [Google Scholar] [CrossRef] [PubMed]
- Hakomori, S. Tumor malignancy defined by aberrant glycosylation and sphingo(glyco)lipid metabolism. Cancer Res. 1996, 56, 5309–5318. [Google Scholar]
- Nilsson, O.; Brezicka, F.T.; Holmgren, J.; Sörenson, S.; Svennerholm, L.; Yngvason, F.; Lindholm, L. Detection of a ganglioside antigen associated with small cell lung carcinomas using monoclonal antibodies directed against fucosyl-GM1. Cancer Res. 1986, 46, 1403–1407. [Google Scholar]
- Ladisch, S.; Sweeley, C.C.; Becker, H.; Gage, D. Aberrant fatty acyl alpha-hydroxylation in human neuroblastoma tumor gangliosides. J. Biol. Chem. 1989, 264, 12097–12105. [Google Scholar] [CrossRef] [PubMed]
- Iwamori, M.; Iwamori, Y.; Kubushiro, K.; Ishiwata, I.; Kiguchi, K. Characteristic expression of Lewis-antigenic glycolipids in human ovarian carcinoma-derived cells with anticancer drug-resistance. J. Biochem. 2007, 141, 309–317. [Google Scholar] [CrossRef]
- Kiguchi, K.; Iwamori, Y.; Suzuki, N.; Kobayashi, Y.; Ishizuka, B.; Ishiwata, I.; Kita, T.; Kikuchi, Y.; Iwamori, M. Characteristic expression of globotriaosyl ceramide in human ovarian carcinoma-derived cells with anticancer drug resistance. Cancer Sci. 2006, 97, 1321–1326. [Google Scholar] [CrossRef]
- Silsirivanit, A.; Phoomak, C.; Teeravirote, K.; Wattanavises, S.; Seubwai, W.; Saengboonmee, C.; Zhan, Z.; Inokuchiz, J.I.; Suzuki, A.; Wongkham, S. Overexpression of HexCer and LacCer containing 2-hydroxylated fatty acids in cholangiocarcinoma and the association of the increase of LacCer (d18:1-h23:0) with shorter survival of the patients. Glycoconj. J. 2019, 36, 103–111. [Google Scholar] [CrossRef]
- Lemay, A.M.; Courtemanche, O.; Couttas, T.A.; Jamsari, G.; Gagné, A.; Bossé, Y.; Joubert, P.; Don, A.S.; Marsolais, D. High FA2H and UGT8 transcript levels predict hydroxylated hexosylceramide accumulation in lung adenocarcinoma. J. Lipid Res. 2019, 60, 1776–1786. [Google Scholar] [CrossRef]
- Alderson, N.L.; Hama, H. Fatty acid 2-hydroxylase regulates cAMP-induced cell cycle exit in D6P2T schwannoma cells. J. Lipid Res. 2009, 50, 1203–1208. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Beeghly-Fadiel, A.; Cai, Q.; Cai, H.; Guo, X.; Shi, L.; Wu, J.; Ye, F.; Qiu, Q.; Zheng, Y.; et al. Gene expression in triple-negative breast cancer in relation to survival. Breast Cancer Res. Treat. 2018, 171, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Yang, X.; Sun, L.; Sun, S.; Huang, X.; Zhou, D.; Li, T.; Zhang, W.; Abumrad, N.A.; Zhu, X.; et al. Fatty acid 2-hydroxylation inhibits tumor growth and increases sensitivity to cisplatin in gastric cancer. EBioMedicine 2019, 41, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lin, J.; Yan, K.; Wang, J. Identification of a Robust Five-Gene Risk Model in Prostate Cancer: A Robust Likelihood-Based Survival Analysis. Int. J. Genom. 2020, 2020, 1097602. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Yu, W.; Shen, L.; Huang, T. MLKL is a potential prognostic marker in gastric cancer. Oncol. Lett. 2019, 18, 3830–3836. [Google Scholar] [CrossRef]
- Sun, L.; Yang, X.; Huang, X.; Yao, Y.; Wei, X.; Yang, S.; Zhou, D.; Zhang, W.; Long, Z.; Xu, X.; et al. 2-Hydroxylation of Fatty Acids Represses Colorectal Tumorigenesis and Metastasis via the YAP Transcriptional Axis. Cancer Res. 2021, 81, 289–302. [Google Scholar] [CrossRef]
- Hong, B.; Li, J.; Huang, C.; Huang, T.; Zhang, M.; Huang, L. miR-300/FA2H affects gastric cancer cell proliferation and apoptosis. Open Med. 2020, 15, 882–889. [Google Scholar] [CrossRef]
- Li, H.; Lian, B.; Liu, Y.; Chai, D.; Li, J. MicroRNA-1297 downregulation inhibits breast cancer cell epithelial-mesenchymal transition and proliferation in a FA2H-dependent manner. Oncol. Lett. 2020, 20, 277. [Google Scholar] [CrossRef]
- Herrero, A.B.; Astudillo, A.M.; Balboa, M.A.; Cuevas, C.; Balsinde, J.; Moreno, S. Levels of SCS7/FA2H-mediated fatty acid 2-hydroxylation determine the sensitivity of cells to antitumor PM02734. Cancer Res. 2008, 68, 9779–9787. [Google Scholar] [CrossRef]
- Dai, X.; Zhang, S.; Cheng, H.; Cai, D.; Chen, X.; Huang, Z. FA2H Exhibits Tumor Suppressive Roles on Breast Cancers via Cancer Stemness Control. Front. Oncol. 2019, 9, 1089. [Google Scholar] [CrossRef]
- Takeda, S.; Ikeda, E.; Su, S.; Harada, M.; Okazaki, H.; Yoshioka, Y.; Nishimura, H.; Ishii, H.; Kakizoe, K.; Taniguchi, A.; et al. Δ9-THC modulation of fatty acid 2-hydroxylase (FA2H) gene expression: Possible involvement of induced levels of PPARα in MDA-MB-231 breast cancer cells. Toxicology 2014, 326, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Hirao-Suzuki, M.; Takeda, S.; Watanabe, K.; Takiguchi, M.; Aramaki, H. Δ9-Tetrahydrocannabinol upregulates fatty acid 2-hydroxylase (FA2H) via PPARα induction: A possible evidence for the cancellation of PPARβ/δ-mediated inhibition of PPARα in MDA-MB-231 cells. Arch. Biochem. Biophys. 2019, 662, 219–225. [Google Scholar] [CrossRef]
- Sakai, G.; Hirao-Suzuki, M.; Koga, T.; Kobayashi, T.; Kamishikiryo, J.; Tanaka, M.; Fujii, K.; Takiguchi, M.; Sugihara, N.; Toda, A.; et al. Perfluorooctanoic acid (PFOA) as a stimulator of estrogen receptor-negative breast cancer MDA-MB-231 cell aggressiveness: Evidence for involvement of fatty acid 2-hydroxylase (FA2H) in the stimulated cell migration. J. Toxicol. Sci. 2022, 47, 159–168. [Google Scholar] [CrossRef]
- Zhou, X.; Huang, F.; Ma, G.; Wei, W.; Wu, N.; Liu, Z. Dysregulated ceramides metabolism by fatty acid 2-hydroxylase exposes a metabolic vulnerability to target cancer metastasis. Signal Transduct Target Ther. 2022, 7, 370. [Google Scholar] [CrossRef]
- Gong, B.; Wang, X.; Li, B.; Li, Y.; Lu, R.; Zhang, K.; Li, B.; Ma, Y.; Li, Y. miR-205-5p inhibits thymic epithelial cell proliferation via FA2H-TFAP2A feedback regulation in age-associated thymus involution. Mol. Immunol. 2020, 122, 173–185. [Google Scholar] [CrossRef]
- Llado, V.; Gutierrez, A.; Martínez, J.; Casas, J.; Terés, S.; Higuera, M.; Galmés, A.; Saus, C.; Besalduch, J.; Busquets, X.; et al. Minerval induces apoptosis in Jurkat and other cancer cells. J. Cell. Mol. Med. 2010, 14, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Khmelinskaia, A.; Ibarguren, M.; de Almeida, R.F.; López, D.J.; Paixão, V.A.; Ahyayauch, H.; Goñi, F.M.; Escribá, P.V. Changes in membrane organization upon spontaneous insertion of 2-hydroxylated unsaturated fatty acids in the lipid bilayer. Langmuir 2014, 30, 2117–2128. [Google Scholar] [CrossRef] [PubMed]
- Ibarguren, M.; López, D.J.; Escribá, P.V. The effect of natural and synthetic fatty acids on membrane structure, microdomain organizatioN.; cellular functions and human health. Biochim. Biophys. Acta 2014, 1838, 1518–1528. [Google Scholar] [CrossRef]
- Massalha, W.; Markovits, M.; Pichinuk, E.; Feinstein-Rotkopf, Y.; Tarshish, M.; Mishra, K.; Llado, V.; Weil, M.; Escriba, P.V.; Kakhlon, O. Minerval (2-hydroxyoleic acid) causes cancer cell selective toxicity by uncoupling oxidative phosphorylation and compromising bioenergetic compensation capacity. Biosci. Rep. 2019, 39, BSR20181661. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.L.; Barceló-Coblijn, G.; de Almeida, R.F.; Noguera-Salvà, M.A.; Terés, S.; Higuera, M.; Liebisch, G.; Schmitz, G.; Busquets, X.; Escribá, P.V. The role of membrane fatty acid remodeling in the antitumor mechanism of action of 2-hydroxyoleic acid. Biochim. Biophys. Acta 2013, 1828, 1405–1413. [Google Scholar] [CrossRef]
- Torgersen, M.L.; Klokk, T.I.; Kavaliauskiene, S.; Klose, C.; Simons, K.; Skotland, T.; Sandvig, K. The anti-tumor drug 2-hydroxyoleic acid (Minerval) stimulates signaling and retrograde transport. Oncotarget 2016, 7, 86871–86888. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eckhardt, M. Fatty Acid 2-Hydroxylase and 2-Hydroxylated Sphingolipids: Metabolism and Function in Health and Diseases. Int. J. Mol. Sci. 2023, 24, 4908. https://doi.org/10.3390/ijms24054908
Eckhardt M. Fatty Acid 2-Hydroxylase and 2-Hydroxylated Sphingolipids: Metabolism and Function in Health and Diseases. International Journal of Molecular Sciences. 2023; 24(5):4908. https://doi.org/10.3390/ijms24054908
Chicago/Turabian StyleEckhardt, Matthias. 2023. "Fatty Acid 2-Hydroxylase and 2-Hydroxylated Sphingolipids: Metabolism and Function in Health and Diseases" International Journal of Molecular Sciences 24, no. 5: 4908. https://doi.org/10.3390/ijms24054908
APA StyleEckhardt, M. (2023). Fatty Acid 2-Hydroxylase and 2-Hydroxylated Sphingolipids: Metabolism and Function in Health and Diseases. International Journal of Molecular Sciences, 24(5), 4908. https://doi.org/10.3390/ijms24054908





