Genome-Wide Meta-Analysis of QTLs Associated with Root Traits and Implications for Maize Breeding
Abstract
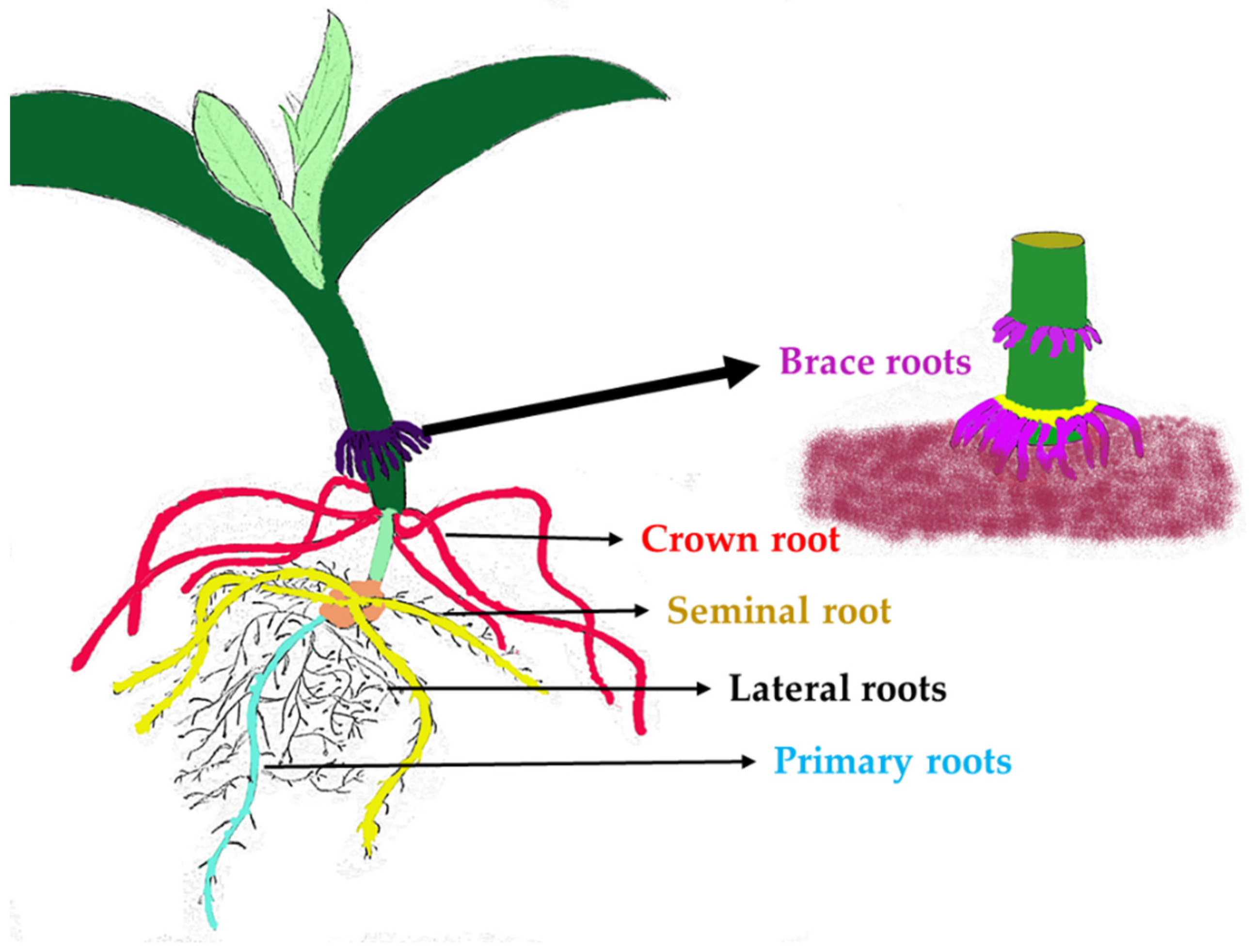
1. Introduction
2. Results
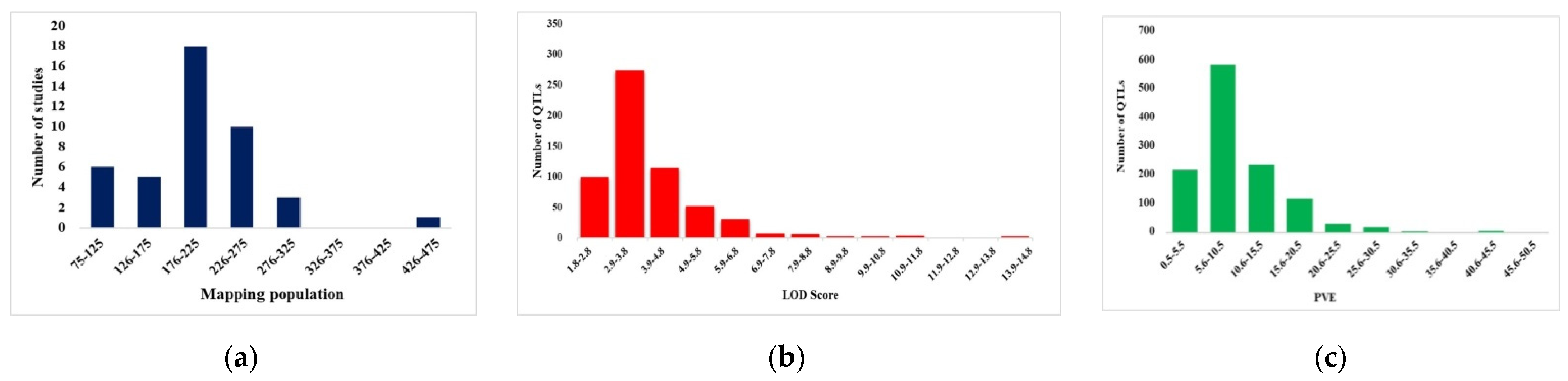
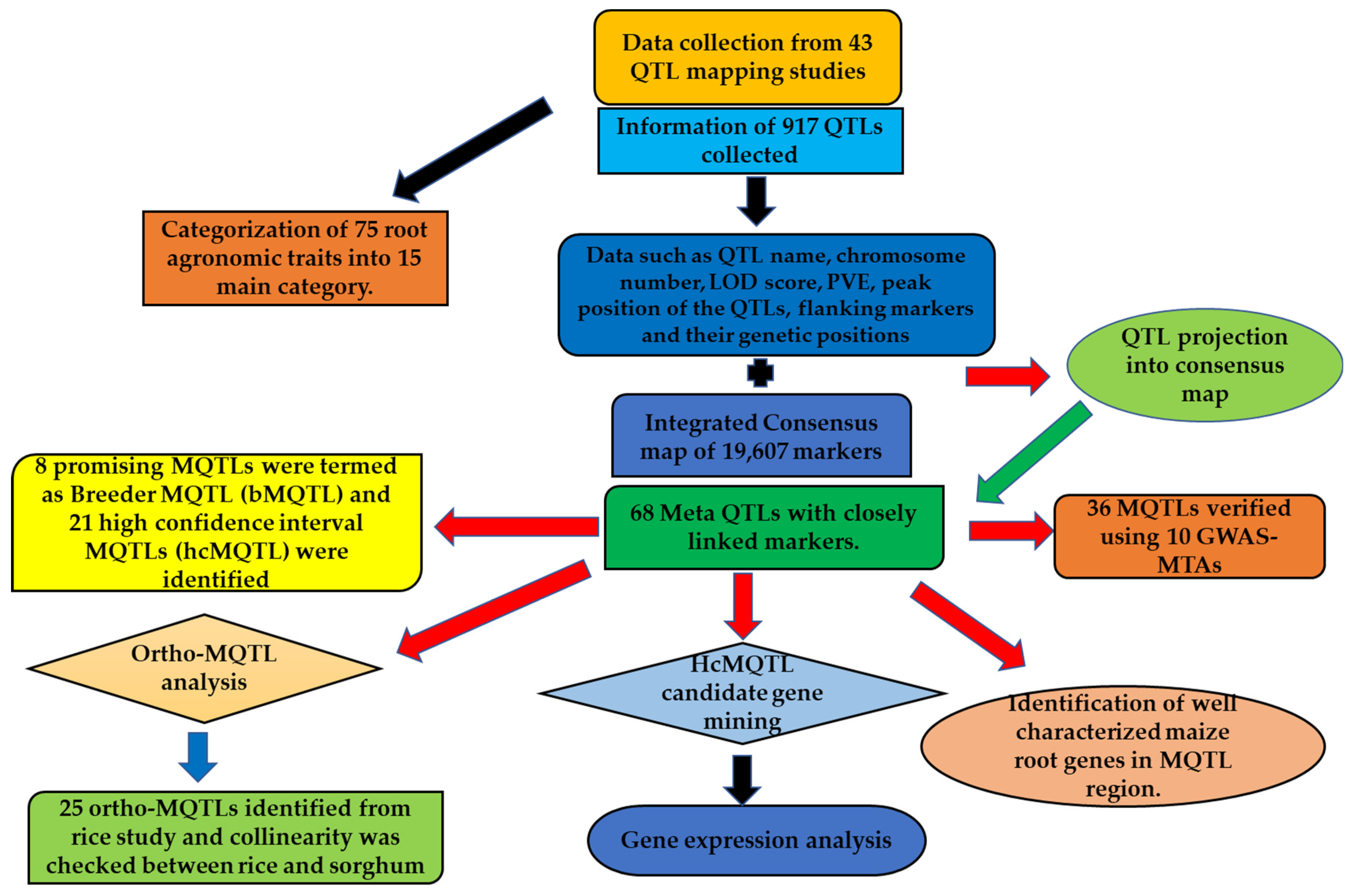
2.1. Characterization of QTLs Associated with Root Traits
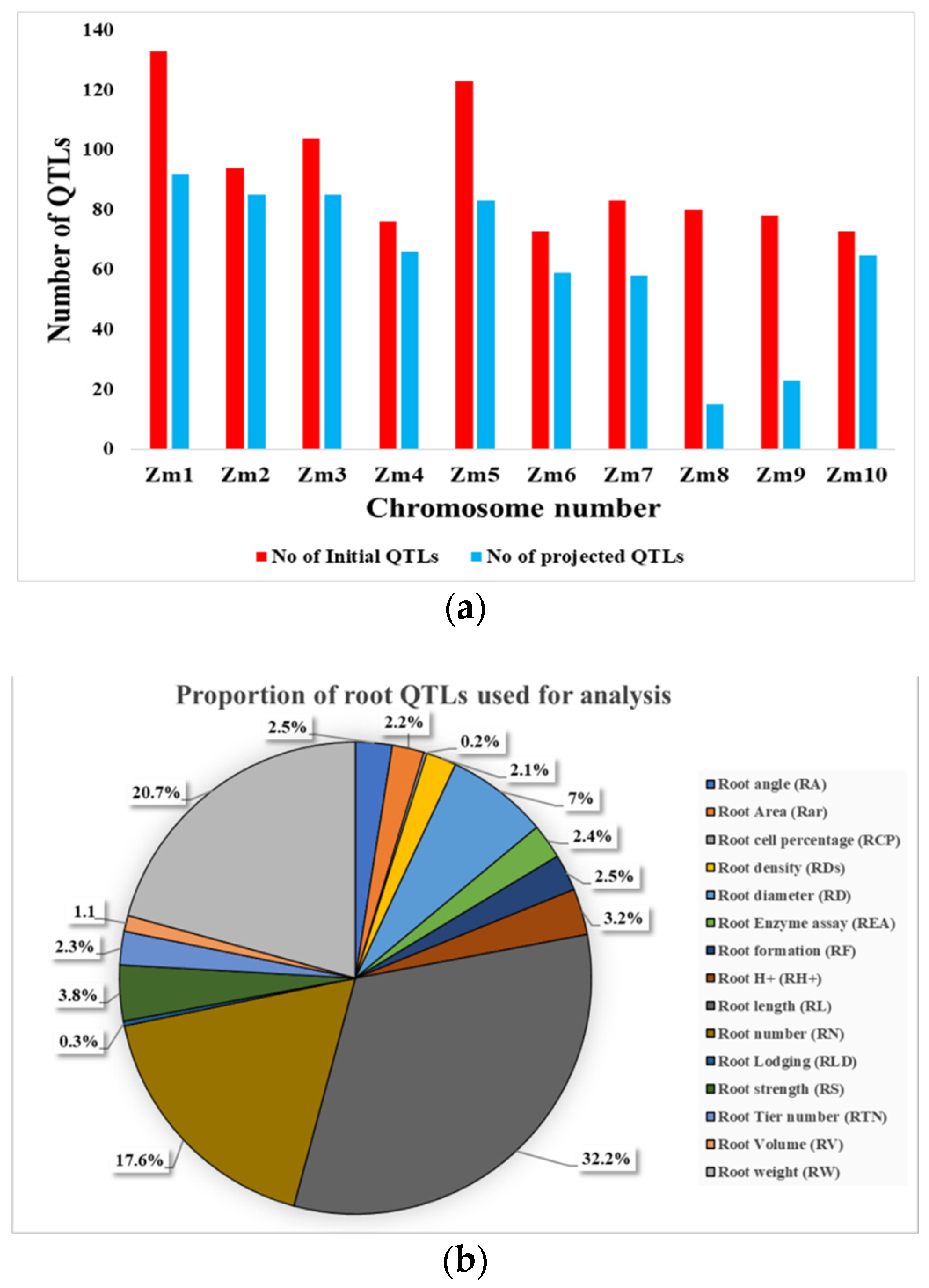
2.2. Consensus Map, Projected QTLs and MQTLs for Root Traits
2.3. GWAS Validated MQTLs
2.4. Candidate Genes Available from hcMQTL Regions and Their Expression Analysis
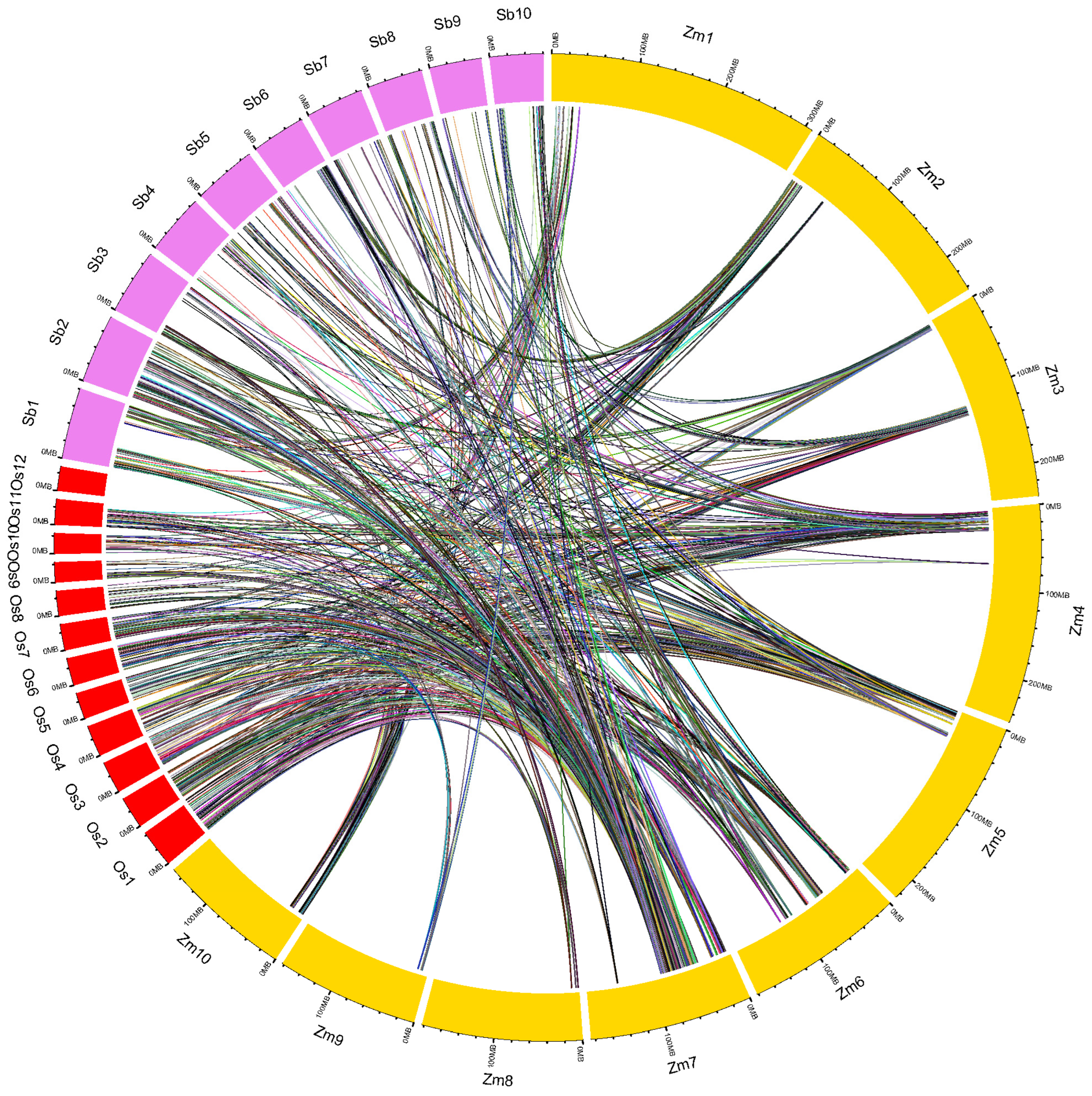
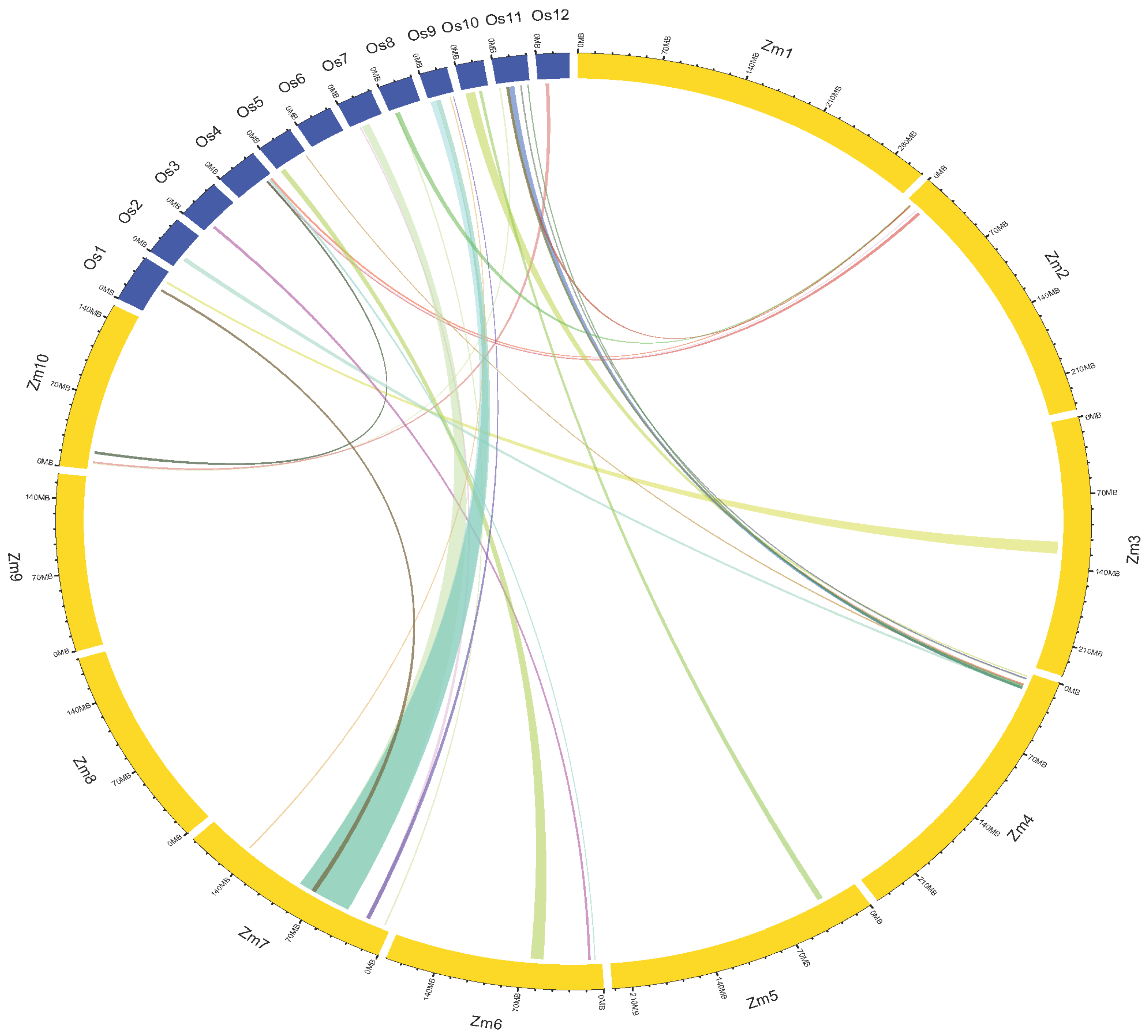
2.5. Conserved Genomic Regions and Ortho-MQTLs among the Cereals
2.6. Adjacent MQTLs with Known Characterized Maize Root Genes and Orthologs of Rice Genes in MQTL Regions
3. Discussion
4. Materials and Methods
4.1. Bibliographic Search for QTL Data Collection
4.2. Consensus Map and QTL Projection
4.3. Determining the Physical Positions of MQTLs and Validation with GWAS
4.4. Establishment of MQTLs for Gene Mining and Selection of Some Promising MQTLs for Breeding
4.5. Expression Analysis of Gene Models
4.6. Unravelling Conserved Genomic Regions Associated with Root Traits among the Cereals
4.7. MQTL Characterization Using Cloned Genes and Homology of MQTLs with Rice
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kong, D.; Wang, J.; Wu, H.; Valverde-Barrantes, O.J.; Wang, R.; Zeng, H.; Kardol, P.; Zhang, H.; Feng, Y. Nonlinearity of root trait relationships and the root economics spectrum. Nat. Commun. 2019, 10, 2203. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Sharif, R.; Xu, X.; Chen, X. Mechanisms of waterlogging tolerance in plants: Research progress and prospects. Front. Plant Sci. 2021, 11, 627331. [Google Scholar] [CrossRef]
- Rogers, E.D.; Benfey, P.N. Regulation of plant root system architecture: Implications for crop advancement. Curr. Opin. Biotechnol. 2015, 32, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Yan, Y.; Gu, S.; Wang, Y.; Xu, C.; Sheng, D.; Li, Y.; Wang, P.; Huang, S. Lodging resistance in maize: A function of root–shoot interactions. Eur. J. Agron. 2022, 132, 126393. [Google Scholar] [CrossRef]
- Khan, M.; Gemenet, D.C.; Villordon, A. Root system architecture and abiotic stress tolerance: Current knowledge in root and tuber crops. Front. Plant Sci. 2016, 7, 1584. [Google Scholar] [CrossRef]
- Hake, S.; Ross-Ibarra, J. The natural history of model organisms: Genetic, evolutionary and plant breeding insights from the domestication of maize. eLife 2015, 4, e05861. [Google Scholar] [CrossRef]
- Erenstein, O.; Jaleta, M.; Sonder, K.; Mottaleb, K.; Prasanna, B. Global maize production, consumption and trade: Trends and R&D implications. Food Secur. 2022, 14, 1295–1319. [Google Scholar]
- Yu, P.; He, X.; Baer, M.; Beirinckx, S.; Tian, T.; Moya, Y.A.; Zhang, X.; Deichmann, M.; Frey, F.P.; Bresgen, V. Plant flavones enrich rhizosphere Oxalobacteraceae to improve maize performance under nitrogen deprivation. Nat. Plants 2021, 7, 481–499. [Google Scholar] [CrossRef]
- Timsina, J.; Jat, M.L.; Majumdar, K. Rice-maize systems of South Asia: Current status, future prospects and research priorities for nutrient management. Plant Soil 2010, 335, 65–82. [Google Scholar] [CrossRef]
- Hochholdinger, F.; Yu, P.; Marcon, C. Genetic control of root system development in maize. Trends Plant Sci. 2018, 23, 79–88. [Google Scholar] [CrossRef]
- Yu, P.; Baldauf, J.A.; Lithio, A.; Marcon, C.; Nettleton, D.; Li, C.; Hochholdinger, F. Root type-specific reprogramming of maize pericycle transcriptomes by local high nitrate results in disparate lateral root branching patterns. Plant Physiol. 2016, 170, 1783–1798. [Google Scholar] [CrossRef]
- Bennetzen, J.L.; Hake, S. Handbook of Maize; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Orman-Ligeza, B.; Parizot, B.; Gantet, P.P.; Beeckman, T.; Bennett, M.J.; Draye, X. Post-embryonic root organogenesis in cereals: Branching out from model plants. Trends Plant Sci. 2013, 18, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Satbhai, S.B.; Ristova, D.; Busch, W. Underground tuning: Quantitative regulation of root growth. J. Exp. Bot. 2015, 66, 1099–1112. [Google Scholar] [CrossRef] [PubMed]
- Mi, G.; Chen, F.; Yuan, L.; Zhang, F. Ideotype root system architecture for maize to achieve high yield and resource use efficiency in intensive cropping systems. Adv. Agron. 2016, 139, 73–97. [Google Scholar]
- Bray, A.L.; Topp, C.N. The quantitative genetic control of root architecture in maize. PlantCell Physiol. 2018, 59, 1919–1930. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Liu, C.; Mei, X.; Jiang, D.; Wang, X.; Dong, E.; Zhang, J.; Cai, Y. QTL identification in backcross population for brace-root-related traits in maize. Euphytica 2020, 216, 32. [Google Scholar] [CrossRef]
- Cai, H.; Chen, F.; Mi, G.; Zhang, F.; Maurer, H.P.; Liu, W.; Reif, J.C.; Yuan, L. Mapping QTLs for root system architecture of maize (Zea mays L.) in the field at different developmental stages. Theor. Appl. Genet. 2012, 125, 1313–1324. [Google Scholar] [CrossRef]
- Li, P.; Fan, Y.; Yin, S.; Wang, Y.; Wang, H.; Xu, Y.; Yang, Z.; Xu, C. Multi-environment QTL mapping of crown root traits in a maize RIL population. Crop J. 2020, 8, 645–654. [Google Scholar] [CrossRef]
- Saini, D.K.; Chopra, Y.; Singh, J.; Sandhu, K.S.; Kumar, A.; Bazzer, S.; Srivastava, P. Comprehensive evaluation of mapping complex traits in wheat using genome-wide association studies. Mol. Breed. 2022, 42, 1. [Google Scholar] [CrossRef]
- Zhang, X.; Guan, Z.; Wang, L.; Fu, J.; Zhang, Y.; Li, Z.; Ma, L.; Liu, P.; Zhang, Y.; Liu, M. Combined GWAS and QTL analysis for dissecting the genetic architecture of kernel test weight in maize. Mol. Genet. Genom. 2020, 295, 409–420. [Google Scholar] [CrossRef]
- Goffinet, B.; Gerber, S. Quantitative trait loci: A meta-analysis. Genetics 2000, 155, 463–473. [Google Scholar] [CrossRef]
- Du, B.; Wu, J.; Islam, M.S.; Sun, C.; Lu, B.; Wei, P.; Liu, D.; Chen, C. Genome-wide meta-analysis of QTL for morphological related traits of flag leaf in bread wheat. PLoS ONE 2022, 17, e0276602. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Singh, V.P.; Saini, D.K.; Sharma, H.; Saripalli, G.; Kumar, S.; Balyan, H.S.; Gupta, P.K. Meta-QTLs, ortho-MQTLs, and candidate genes for thermotolerance in wheat (Triticum aestivum L.). Mol. Breed. 2021, 41, 69. [Google Scholar] [CrossRef]
- Pal, N.; Saini, D.K.; Kumar, S. Meta-QTLs, ortho-MQTLs and candidate genes for the traits contributing to salinity stress tolerance in common wheat (Triticum aestivum L.). Physiol. Mol. Biol. Plants 2021, 27, 2767–2786. [Google Scholar] [CrossRef] [PubMed]
- Saini, D.K.; Chopra, Y.; Pal, N.; Chahal, A.; Srivastava, P.; Gupta, P.K. Meta-QTLs, ortho-MQTLs and candidate genes for nitrogen use efficiency and root system architecture in bread wheat (Triticum aestivum L.). Physiol. Mol. Biol. Plants 2021, 27, 2245–2267. [Google Scholar] [CrossRef] [PubMed]
- Saini, D.K.; Srivastava, P.; Pal, N.; Gupta, P. Meta-QTLs, ortho-meta-QTLs and candidate genes for grain yield and associated traits in wheat (Triticum aestivum L.). Theor. Appl. Genet. 2022, 135, 1049–1081. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, N.; Pruthi, G.; Prakash Raigar, O.; Singh, M.P.; Phagna, K.; Kumar, A.; Sethi, M.; Singh, J.; Ade, P.A.; Saini, D.K. Meta-QTL analysis in rice and cross-genome talk of the genomic regions controlling nitrogen use efficiency in cereal crops revealing phylogenetic relationship. Front. Genet. 2021, 12, 807210. [Google Scholar] [CrossRef]
- Tanin, M.J.; Saini, D.K.; Sandhu, K.S.; Pal, N.; Gudi, S.; Chaudhary, J.; Sharma, A. Consensus genomic regions associated with multiple abiotic stress tolerance in wheat and implications for wheat breeding. Sci. Rep. 2022, 12, 13680. [Google Scholar] [CrossRef]
- Gudi, S.; Saini, D.K.; Singh, G.; Halladakeri, P.; Kumar, P.; Shamshad, M.; Tanin, M.J.; Singh, S.; Sharma, A. Unravelling consensus genomic regions associated with quality traits in wheat using meta-analysis of quantitative trait loci. Planta 2022, 255, 115. [Google Scholar] [CrossRef]
- Sheoran, S.; Gupta, M.; Kumari, S.; Kumar, S.; Rakshit, S. Meta-QTL analysis and candidate genes identification for various abiotic stresses in maize (Zea mays L.) and their implications in breeding programs. Mol. Breed. 2022, 42, 26. [Google Scholar] [CrossRef]
- Gupta, M.; Choudhary, M.; Singh, A.; Sheoran, S.; Singla, D.; Rakshit, S. Meta-QTL analysis for mining of candidate genes and constitutive gene network development for fungal disease resistance in maize (Zea mays L.). Crop J. 2022; in press. [Google Scholar]
- Guo, J.; Chen, L.; Li, Y.; Shi, Y.; Song, Y.; Zhang, D.; Li, Y.; Wang, T.; Yang, D.; Li, C. Meta-QTL analysis and identification of candidate genes related to root traits in maize. Euphytica 2018, 214, 223. [Google Scholar] [CrossRef]
- Kaur, S.; Rakshit, S.; Choudhary, M.; Das, A.K.; Kumar, R.R. Meta-analysis of QTLs associated with popping traits in maize (Zea mays L.). PLoS ONE 2021, 16, e0256389. [Google Scholar] [CrossRef] [PubMed]
- Khahani, B.; Tavakol, E.; Shariati, V.; Rossini, L. Meta-QTL and ortho-MQTL analyses identified genomic regions controlling rice yield, yield-related traits and root architecture under water deficit conditions. Sci. Rep. 2021, 11, 6942. [Google Scholar] [CrossRef] [PubMed]
- Daryani, P.; Darzi Ramandi, H.; Dezhsetan, S.; Mirdar Mansuri, R.; Hosseini Salekdeh, G.; Shobbar, Z.-S. Pinpointing genomic regions associated with root system architecture in rice through an integrative meta-analysis approach. Theor. Appl. Genet. 2022, 135, 81–106. [Google Scholar] [CrossRef] [PubMed]
- Soriano, J.M.; Alvaro, F. Discovering consensus genomic regions in wheat for root-related traits by QTL meta-analysis. Sci. Rep. 2019, 9, 10537. [Google Scholar] [CrossRef]
- Wei, Z.; Li, J. Receptor-like protein kinases: Key regulators controlling root hair development in Arabidopsis thaliana. J. Integr. Plant Biol. 2018, 60, 841–850. [Google Scholar] [CrossRef]
- Han, G.; Li, Y.; Qiao, Z.; Wang, C.; Zhao, Y.; Guo, J.; Chen, M.; Wang, B. Advances in the regulation of epidermal cell development by C2H2 zinc finger proteins in plants. Front. Plant Sci. 2021, 12, 754512. [Google Scholar] [CrossRef]
- Rosado, D.; Ackermann, A.; Spassibojko, O.; Rossi, M.; Pedmale, U.V. WRKY transcription factors and ethylene signaling modify root growth during the shade-avoidance response. Plant Physiol. 2022, 188, 1294–1311. [Google Scholar] [CrossRef]
- Kumar, K.; Yadava, P.; Gupta, M.; Choudhary, M.; Jha, A.K.; Wani, S.H.; Dar, Z.A.; Kumar, B.; Rakshit, S. Narrowing down molecular targets for improving phosphorus-use efficiency in maize (Zea mays L.). Mol. Biol. Rep. 2022, 49, 12091–12107. [Google Scholar] [CrossRef]
- Quraishi, U.M.; Abrouk, M.; Murat, F.; Pont, C.; Foucrier, S.; Desmaizieres, G.; Confolent, C.; Riviere, N.; Charmet, G.; Paux, E. Cross-genome map based dissection of a nitrogen use efficiency ortho-metaQTL in bread wheat unravels concerted cereal genome evolution. Plant J. 2011, 65, 745–756. [Google Scholar] [CrossRef]
- Li, W.; Zhao, X.; Zhang, X. Genome-wide analysis and expression patterns of the YUCCA genes in maize. J. Genet. Genom. 2015, 42, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Uribe, X.; Torres, M.A.; Capellades, M.; Puigdomenech, P.; Rigau, J. Maize α-tubulin genes are expressed according to specific patterns of cell differentiation. Plant Mol. Biol. 1998, 37, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Choudhary, M.; Halder, T.; Prakash, N.R.; Singh, V.; Sheoran, S.; Longmei, N.; Rakshit, S.; Siddique, K.H. Salinity stress tolerance and omics approaches: Revisiting the progress and achievements in major cereal crops. Heredity 2022, 128, 497–518. [Google Scholar] [CrossRef]
- Taramino, G.; Sauer, M.; Stauffer Jr, J.L.; Multani, D.; Niu, X.; Sakai, H.; Hochholdinger, F. The maize (Zea mays L.) RTCS gene encodes a LOB domain protein that is a key regulator of embryonic seminal and post-embryonic shoot-borne root initiation. Plant J. 2007, 50, 649–659. [Google Scholar] [CrossRef]
- Suzuki, M.; Sato, Y.; Wu, S.; Kang, B.-H.; McCarty, D.R. Conserved functions of the MATE transporter BIG EMBRYO1 in regulation of lateral organ size and initiation rate. Plant Cell 2015, 27, 2288–2300. [Google Scholar] [CrossRef]
- Cordoba, E.; Porta, H.; Arroyo, A.; San Román, C.; Medina, L.; Rodríguez-Concepción, M.; León, P. Functional characterization of the three genes encoding 1-deoxy-D-xylulose 5-phosphate synthase in maize. J. Exp. Bot. 2011, 62, 2023–2038. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wang, F.; Liu, B.; Xu, C.; He, Q.; Cheng, W.; Zhao, X.; Ding, Z.; Zhang, W.; Zhang, K. ZmSKS13, a cupredoxin domain-containing protein, is required for maize kernel development via modulation of redox homeostasis. New Phytol. 2021, 229, 2163–2178. [Google Scholar] [CrossRef] [PubMed]
- MaizeGDB. Newly Characterized Genes. Available online: https://www.maizegdb.org/new_genes?window=alltime (accessed on 14 June 2022).
- Cao, Y.; Zhang, M.; Liang, X.; Li, F.; Shi, Y.; Yang, X.; Jiang, C. Natural variation of an EF-hand Ca2+-binding-protein coding gene confers saline-alkaline tolerance in maize. Nat. Commun. 2020, 11, 186. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Xing, J.; Wan, J.; Wang, X.; Zhang, J.; Wang, X.; Li, Z.; Zhang, M. Copalyl diphosphate synthase mutation improved salt tolerance in maize (Zea mays. L) via enhancing vacuolar Na+ sequestration and maintaining ROS homeostasis. Front. Plant Sci. 2020, 11, 457. [Google Scholar] [CrossRef]
- Cao, X.; Yang, H.; Shang, C.; Ma, S.; Liu, L.; Cheng, J. The roles of auxin biosynthesis YUCCA gene family in plants. Int. J. Mol. Sci. 2019, 20, 6343. [Google Scholar] [CrossRef]
- Matsuyama, T.; Yasumura, N.; Funakoshi, M.; Yamada, Y.; Hashimoto, T. Maize genes specifically expressed in the outermost cells of root cap. Plant Cell Physiol. 1999, 40, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.-C.; Weir, N.R.; Hill, K.; Zhang, W.; Kim, H.J.; Shiu, S.-H.; Schaller, G.E.; Kieber, J.J. Characterization of genes involved in cytokinin signaling and metabolism from rice. Plant Physiol. 2012, 158, 1666–1684. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.S.; Kim, Y.S.; Baek, K.H.; Jung, H.; Ha, S.-H.; Do Choi, Y.; Kim, M.; Reuzeau, C.; Kim, J.-K. Root-specific expression of OsNAC10 improves drought tolerance and grain yield in rice under field drought conditions. Plant Physiol. 2010, 153, 185–197. [Google Scholar] [CrossRef]
- Lee, H.Y.; Chen, Z.; Zhang, C.; Yoon, G.M. Editing of the OsACS locus alters phosphate deficiency-induced adaptive responses in rice seedlings. J. Exp. Bot. 2019, 70, 1927–1940. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Xu, X.; Tang, Z.; Zhang, W.; Huang, X.-Y.; Zhao, F.-J. OsWRKY28 regulates phosphate and arsenate accumulation, root system architecture and fertility in rice. Front. Plant Sci. 2018, 9, 1330. [Google Scholar] [CrossRef]
- Suetsugu, N.; Takemiya, A.; Kong, S.-G.; Higa, T.; Komatsu, A.; Shimazaki, K.-I.; Kohchi, T.; Wada, M. RPT2/NCH1 subfamily of NPH3-like proteins is essential for the chloroplast accumulation response in land plants. Proc. Natl. Acad. Sci. USA 2016, 113, 10424–10429. [Google Scholar] [CrossRef]
- Zhang, X.-X.; Tang, Y.-J.; Ma, Q.-B.; Yang, C.-Y.; Mu, Y.-H.; Suo, H.-C.; Luo, L.-H.; Nian, H. OsDREB2A, a rice transcription factor, significantly affects salt tolerance in transgenic soybean. PLoS ONE 2013, 8, e83011. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, Y.K.; Kremling, K.A.; Ding, Y.; Bennett, J.S.; Bae, J.S.; Kim, D.K.; Ackerman, H.H.; Kolomiets, M.V.; Schmelz, E.A. Ethylene signaling regulates natural variation in the abundance of antifungal acetylated diferuloylsucroses and Fusarium graminearum resistance in maize seedling roots. New Phytol. 2019, 221, 2096–2111. [Google Scholar] [CrossRef]
- Wu, W.; Cheng, S. Root genetic research, an opportunity and challenge to rice improvement. Field Crops Res. 2014, 165, 111–124. [Google Scholar] [CrossRef]
- Banuelos, M.A.; Garciadeblas, B.; Cubero, B.; Rodrıguez-Navarro, A. Inventory and functional characterization of the HAK potassium transporters of rice. Plant Physiol. 2002, 130, 784–795. [Google Scholar] [CrossRef]
- Ranathunge, K.; El-Kereamy, A.; Gidda, S.; Bi, Y.-M.; Rothstein, S.J. AMT1;1 transgenic rice plants with enhanced NH4+ permeability show superior growth and higher yield under optimal and suboptimal NH4+ conditions. J. Exp. Bot. 2014, 65, 965–979. [Google Scholar] [CrossRef]
- Wan, B.; Lin, Y.; Mou, T. Expression of rice Ca2+-dependent protein kinases (CDPKs) genes under different environmental stresses. FEBS Lett. 2007, 581, 1179–1189. [Google Scholar] [CrossRef]
- Paez-Garcia, A.; Motes, C.M.; Scheible, W.-R.; Chen, R.; Blancaflor, E.B.; Monteros, M.J. Root traits and phenotyping strategies for plant improvement. Plants 2015, 4, 334–355. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Cui, Z.; Li, C.; Luo, J.; Guan, Y.; Liu, L.; Zhang, Z.; Zhang, L.; He, Y.; Ruan, Y. Identification of maize brace-root quantitative trait loci in a recombinant inbred line population. Euphytica 2018, 214, 168. [Google Scholar] [CrossRef]
- Ghaffari, R.; Cannon, E.K.S.; Kanizay, L.B.; Lawrence, C.J.; Dawe, R.K. Maize chromosomal knobs are located in gene-dense areas and suppress local recombination. Chromosoma 2012, 122, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Amo, A.; Wei, D.; Chai, Y.; Zheng, J.; Qiao, P.; Cui, C.; Lu, S.; Chen, L.; Hu, Y.-G. Large-scale integration of meta-QTL and genome-wide association study discovers the genomic regions and candidate genes for yield and yield-related traits in bread wheat. Theor. Appl. Genet. 2021, 134, 3083–3109. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ren, Z.; Li, L.; Du, Y.; Zhou, Y.; Zhang, M.; Li, Z.; Yi, F.; Duan, L. Meta-QTL analysis explores the key genes, especially hormone related genes, involved in the regulation of grain water content and grain dehydration rate in maize. BMC Plant Biol. 2022, 22, 346. [Google Scholar] [CrossRef]
- Bush, W.S.; Moore, J.H. Chapter 11: Genome-wide association studies. PLoS Comput. Biol. 2012, 8, e1002822. [Google Scholar] [CrossRef]
- Kaur, B.; Sandhu, K.S.; Kamal, R.; Kaur, K.; Singh, J.; Röder, M.S.; Muqaddasi, Q.H. Omics for the improvement of abiotic, biotic, and agronomic traits in major cereal crops: Applications, challenges, and prospects. Plants 2021, 10, 1989. [Google Scholar] [CrossRef]
- Devgan, M.; Gill, G.K.; Praba, U.P.; Singh, G.; Garg, T.; Karnatam, K.S.; Kaur, A.; Vikal, Y. Biochemical and molecular characterization of sub-tropical maize germplasm for tocopherols. J. Food Compos. Anal. 2022, 114, 104842. [Google Scholar] [CrossRef]
- Gudi, S.; Kumar, P.; Singh, S.; Tanin, M.J.; Sharma, A. Strategies for accelerating genetic gains in crop plants: Special focus on speed breeding. Physiol. Mol. Biol. Plants 2022, 28, 1921–1938. [Google Scholar] [CrossRef]
- Karnatam, K.S.; Jaganathan, D.; Dilip, K.R.; Boopathi, N.M.; Muthurajan, R. Shortlisting putative candidate genes underlying qDTY1. 1, a major effect drought tolerant QTL in rice (Oryza sativa L.). Electron. J. Plant Breed. 2020, 11, 916–924. [Google Scholar]
- Roppolo, D.; Boeckmann, B.; Pfister, A.; Boutet, E.; Rubio, M.C.; Dénervaud-Tendon, V.; Vermeer, J.E.; Gheyselinck, J.; Xenarios, I.; Geldner, N. Functional and evolutionary analysis of the CASPARIAN STRIP MEMBRANE DOMAIN PROTEIN family. Plant Physiol. 2014, 165, 1709–1722. [Google Scholar] [CrossRef] [PubMed]
- Abreu, I.; Saéz, Á.; Castro-Rodríguez, R.; Escudero, V.; Rodríguez-Haas, B.; Senovilla, M.; Larue, C.; Grolimund, D.; Tejada-Jiménez, M.; Imperial, J. Medicago truncatula Zinc-Iron Permease6 provides zinc to rhizobia-infected nodule cells. Plant Cell Environ. 2017, 40, 2706–2719. [Google Scholar] [CrossRef]
- Singh, P.; Kumari, A.; Gupta, K.J. Alternative oxidase plays a role in minimizing ROS and RNS produced under salinity stress in Arabidopsis thaliana. Physiol. Plant. 2022, 174, e13649. [Google Scholar]
- Han, X.; Zhao, F. OsYUCCA2 deficiency in rice growth and development. Ciência Rural 2021, 52, e20210327. [Google Scholar] [CrossRef]
- Hsieh, P.-H.; Kan, C.-C.; Wu, H.-Y.; Yang, H.-C.; Hsieh, M.-H. Early molecular events associated with nitrogen deficiency in rice seedling roots. Sci. Rep. 2018, 8, 12207. [Google Scholar] [CrossRef]
- Che, J.; Yamaji, N.; Shen, R.F.; Ma, J.F. An Al-inducible expansin gene, Os EXPA 10 is involved in root cell elongation of rice. Plant J. 2016, 88, 132–142. [Google Scholar] [CrossRef]
- Liu, Q.; Luo, L.; Wang, X.; Shen, Z.; Zheng, L. Comprehensive analysis of rice laccase gene (OsLAC) family and ectopic expression of OsLAC10 enhances tolerance to copper stress in Arabidopsis. Int. J. Mol. Sci. 2017, 18, 209. [Google Scholar] [CrossRef]
- Darvasi, A.; Soller, M. A simple method to calculate resolving power and confidence interval of QTL map location. Behav. Genet. 1997, 27, 125–132. [Google Scholar] [CrossRef]
- Guo, B.; Sleper, D.; Lu, P.; Shannon, J.; Nguyen, H.; Arelli, P. QTLs associated with resistance to soybean cyst nematode in soybean: Meta-analysis of QTL locations. Crop Sci. 2006, 46, 595–602. [Google Scholar] [CrossRef]
- Endelman, J.B.; Plomion, C. LPmerge: An R package for merging genetic maps by linear programming. Bioinformatics 2014, 30, 1623–1624. [Google Scholar] [CrossRef]
- Kumar, R.; Saini, D.K.; Kumar, M.; Priyanka, V.; Akhatar, J.; Kaushik, D.; Sharma, A.; Dhanda, P.S.; Kaushik, P. Revealing the Genetic Architecture of Yield-Related and Quality Traits in Indian Mustard [Brassica juncea(L.) Czern. and Coss.] Using Meta-QTL Analysis. Agronomy 2022, 12, 2442. [Google Scholar] [CrossRef]
- Sosnowski, O.; Charcosset, A.; Joets, J. BioMercator V3: An upgrade of genetic map compilation and quantitative trait loci meta-analysis algorithms. Bioinformatics 2012, 28, 2082–2083. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.-M.; Shao, X.-Y.; Pei, Y.-H.; Guo, X.-M.; Li, J.; Song, X.-Y.; Zhao, M.-A. Genetic diversity and genome-wide association study of major ear quantitative traits using high-density SNPs in maize. Front. Plant Sci. 2018, 9, 966. [Google Scholar] [CrossRef]
- Hu, G.; Li, Z.; Lu, Y.; Li, C.; Gong, S.; Yan, S.; Li, G.; Wang, M.; Ren, H.; Guan, H. Genome-wide association study identified multiple genetic loci on chilling resistance during germination in maize. Sci. Rep. 2017, 7, 10840. [Google Scholar] [CrossRef]
- Stelpflug, S.C.; Sekhon, R.S.; Vaillancourt, B.; Hirsch, C.N.; Buell, C.R.; de Leon, N.; Kaeppler, S.M. An expanded maize gene expression atlas based on RNA sequencing and its use to explore root development. Plant Genome 2016, 9, plantgenome2015.04.0025. [Google Scholar] [CrossRef]
- Walley, J.W.; Sartor, R.C.; Shen, Z.; Schmitz, R.J.; Wu, K.J.; Urich, M.A.; Nery, J.R.; Smith, L.G.; Schnable, J.C.; Ecker, J.R. Integration of omic networks in a developmental atlas of maize. Science 2016, 353, 814–818. [Google Scholar] [CrossRef]
- Yu, Y.; Ouyang, Y.; Yao, W. shinyCircos: An R/Shiny application for interactive creation of Circos plot. Bioinformatics 2018, 34, 1229–1231. [Google Scholar] [CrossRef] [PubMed]
- Burton, A.L.; Johnson, J.M.; Foerster, J.M.; Hirsch, C.N.; Buell, C.; Hanlon, M.T.; Kaeppler, S.M.; Brown, K.M.; Lynch, J.P. QTL mapping and phenotypic variation for root architectural traits in maize (Zea mays L.). Theor. Appl. Genet. 2014, 127, 2293–2311. [Google Scholar] [CrossRef]
- Burton, A.L.; Johnson, J.; Foerster, J.; Hanlon, M.T.; Kaeppler, S.M.; Lynch, J.P.; Brown, K.M. QTL mapping and phenotypic variation of root anatomical traits in maize (Zea mays L.). Theor. Appl. Genet. 2015, 128, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xu, L. Comparative mapping of QTLs for H+ secretion of root in maize (Zea mays L.) and cross phosphorus levels on two growth stages. Front. Agric. China 2011, 5, 284–290. [Google Scholar] [CrossRef]
- Chen, J.; Xu, L. The candidate QTLs affecting phosphorus absorption efficiency and root weight in maize (Zea mays L.). Front. Agric. China 2011, 5, 456–462. [Google Scholar] [CrossRef]
- Chen, J.; Xu, L.; Cai, Y.; Xu, J. QTL mapping of phosphorus efficiency and relative biologic characteristics in maize (Zea mays L.) at two sites. Plant Soil 2008, 313, 251–266. [Google Scholar] [CrossRef]
- Gu, D.; Mei, X.; Yu, T.; Sun, N.; Xu, D.; Liu, C.; Cai, Y. QTL identification for brace-root traits of maize in different generations and environments. Crop Sci. 2017, 57, 13–21. [Google Scholar] [CrossRef]
- Guingo, E.; Hébert, Y.; Charcosset, A. Genetic analysis of root traits in maize. Agronomie 1998, 18, 225–235. [Google Scholar] [CrossRef]
- Hu, S.; Lübberstedt, T.; Zhao, G.; Lee, M. QTL mapping of low-temperature germination ability in the maize IBM Syn4 RIL population. PLoS ONE 2016, 11, e0152795. [Google Scholar] [CrossRef]
- Hund, A.; Fracheboud, Y.; Soldati, A.; Frascaroli, E.; Salvi, S.; Stamp, P. QTL controlling root and shoot traits of maize seedlings under cold stress. Theor. Appl. Genet. 2004, 109, 618–629. [Google Scholar] [CrossRef]
- Jiang, X.; Tian, B.; Zhang, W.; Wang, G.; Wang, J. QTL mapping of coleorhiza length in maize (Zea mays L.) under two germination environmental conditions. Plant Breed. 2011, 130, 625–632. [Google Scholar] [CrossRef]
- Ju, C.; Zhang, W.; Liu, Y.; Gao, Y.; Wang, X.; Yan, J.; Yang, X.; Li, J. Genetic analysis of seedling root traits reveals the association of root trait with other agronomic traits in maize. BMC Plant Biol. 2018, 18, 171. [Google Scholar] [CrossRef]
- Landi, P.; Sanguineti, M.; Darrah, L.; Giuliani, M.; Salvi, S.; Conti, S.; Tuberosa, R. Detection of QTLs forvertical root pulling resistance in maize and overlap with QTLs for root traits in hydroponics. Maydica 2002, 47, 233–243. [Google Scholar]
- Li, P.; Chen, F.; Cai, H.; Liu, J.; Pan, Q.; Liu, Z.; Gu, R.; Mi, G.; Zhang, F.; Yuan, L. A genetic relationship between nitrogen use efficiency and seedling root traits in maize as revealed by QTL analysis. J. Exp. Bot. 2015, 66, 3175–3188. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhuang, Z.; Cai, H.; Cheng, S.; Soomro, A.A.; Liu, Z.; Gu, R.; Mi, G.; Yuan, L.; Chen, F. Use of genotype-environment interactions to elucidate the pattern of maize root plasticity to nitrogen deficiency. J. Integr. Plant Biol. 2016, 58, 242–253. [Google Scholar] [CrossRef]
- Liu, J.; Li, J.; Chen, F.; Zhang, F.; Ren, T.; Zhuang, Z.; Mi, G. Soil, Mapping QTLs for root traits under different nitrate levels at the seedling stage in maize (Zea mays L.). Plant Cell Physiol. 2008, 305, 253–265. [Google Scholar]
- Liu, Z.; Gao, K.; Shan, S.; Gu, R.; Wang, Z.; Craft, E.J.; Mi, G.; Yuan, L.; Chen, F. Comparative analysis of root traits and the associated QTLs for maize seedlings grown in paper roll, hydroponics and vermiculite culture system. Front. Plant Sci. 2017, 8, 436. [Google Scholar] [CrossRef] [PubMed]
- Ku, L.; Sun, Z.; Wang, C.; Zhang, J.; Zhao, R.; Liu, H.; Tai, G.; Chen, Y. QTL mapping and epistasis analysis of brace root traits in maize. Mol. Breed. 2012, 30, 697–708. [Google Scholar] [CrossRef]
- Mano, Y.; Muraki, M.; Fujimori, M.; Takamizo, T.; Kindiger, B. Identification of QTL controlling adventitious root formation during flooding conditions in teosinte (Zea mays ssp. huehuetenangensis) seedlings. Euphytica 2005, 142, 33–42. [Google Scholar] [CrossRef]
- Mano, Y.; Omori, F.; Muraki, M.; Takamizo, T. QTL mapping of adventitious root formation under flooding conditions in tropical maize (Zea mays L.) seedlings. Breed. Sci. 2005, 55, 343–347. [Google Scholar] [CrossRef]
- Mano, Y.; Omori, F.; Takamizo, T.; Kindiger, B.; Bird, R.M.; Loaisiga, C.; Takahashi, H. QTL mapping of root aerenchyma formation in seedlings of a maize× rare teosinte “Zea nicaraguensis” cross. Plant Soil 2007, 295, 103–113. [Google Scholar] [CrossRef]
- Mano, Y.; Omori, F.; Kindiger, B.; Takahashi, H. A linkage map of maize× teosinte Zealuxurians and identification of QTLs controlling root aerenchyma formation. Mol. Breed. 2008, 21, 327–337. [Google Scholar] [CrossRef]
- Mano, Y.; Omori, F. Verification of QTL controlling root aerenchyma formation in a maize× teosinte “Zea nicaraguensis” advanced backcross population. Breed. Sci. 2008, 58, 217–223. [Google Scholar] [CrossRef]
- Mano, Y.; Omori, F.; Loaisiga, C.H.; Bird, R.M. QTL mapping of above-ground adventitious roots during flooding in maize x teosinte” Zea nicaraguensis” backcross population. Plant Root 2009, 3, 3–9. [Google Scholar] [CrossRef]
- Mano, Y.; Omori, F. High-density linkage map around the root aerenchyma locus Qaer1. 06 in the backcross populations of maize Mi29× teosinte “Zea nicaraguensis”. Breed. Sci. 2009, 59, 427–433. [Google Scholar] [CrossRef]
- Omori, F.; Mano, Y. QTL mapping of root angle in F2 populations from maize ‘B73’× teosinte ‘Zealuxurians’. Plant Root 2007, 1, 57–65. [Google Scholar] [CrossRef]
- Osman, K.A.; Tang, B.; Wang, Y.; Chen, J.; Yu, F.; Li, L.; Han, X.; Zhang, Z.; Yan, J.; Zheng, Y. Dynamic QTL analysis and candidate gene mapping for waterlogging tolerance at maize seedling stage. PLoS ONE 2013, 8, e79305. [Google Scholar] [CrossRef]
- Pestsova, E.; Lichtblau, D.; Wever, C.; Presterl, T.; Bolduan, T.; Ouzunova, M.; Westhoff, P. QTL mapping of seedling root traits associated with nitrogen and water use efficiency in maize. Euphytica 2016, 209, 585–602. [Google Scholar] [CrossRef]
- Qiu, F.; Zheng, Y.; Zhang, Z.; Xu, S. Mapping of QTL associated with waterlogging tolerance during the seedling stage in maize. Ann. Bot. 2007, 99, 1067–1081. [Google Scholar] [CrossRef]
- Qiu, H.; Liu, C.; Yu, T.; Mei, X.; Wang, G.; Wang, J.; Cai, Y. Identification of QTL for acid phosphatase activity in root and rhizosphere soil of maize under low phosphorus stress. Euphytica 2014, 197, 133–143. [Google Scholar] [CrossRef]
- Ruta, N.; Liedgens, M.; Fracheboud, Y.; Stamp, P.; Hund, A. QTLs for the elongation of axile and lateral roots of maize in response to low water potential. Theor. Appl. Genet. 2010, 120, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Salvi, S.; Giuliani, S.; Ricciolini, C.; Carraro, N.; Maccaferri, M.; Presterl, T.; Ouzunova, M.; Tuberosa, R. Two major quantitative trait loci controlling the number of seminal roots in maize co-map with the root developmental genes rtcs and rum1. J. Exp. Bot. 2016, 67, 1149–1159. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Wang, B.; Hauck, A.L.; Dong, X.; Li, J.; Lai, J. Genetic dissection of maize seedling root system architecture traits using an ultra-high density bin-map and a recombinant inbred line population. J. Integr. Plant Biol. 2016, 58, 266–279. [Google Scholar] [CrossRef] [PubMed]
- Trachsel, S.; Messmer, R.; Stamp, P.; Hund, A. Mapping of QTLs for lateral and axile root growth of tropical maize. Theor. Appl. Genet. 2009, 119, 1413–1424. [Google Scholar] [CrossRef] [PubMed]
- Tuberosa, R.; Sanguineti, M.C.; Landi, P.; Michela Giuliani, M.; Salvi, S.; Conti, S. Identification of QTLs for root characteristics in maize grown in hydroponics and analysis of their overlap with QTLs for grain yield in the field at two water regimes. Plant Mol. Biol. 2002, 48, 697–712. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Liang, K.; Han, X.; Du, D.; Pan, Z.; Qiu, F. Major natural genetic variation contributes to waterlogging tolerance in maize seedlings. Mol. Breed. 2019, 39, 97. [Google Scholar] [CrossRef]
- Zaidi, P.H.; Rashid, Z.; Vinayan, M.T.; Almeida, G.D.; Phagna, R.K.; Babu, R. QTL mapping of agronomic waterlogging tolerance using recombinant inbred lines derived from tropical maize (Zea mays L) germplasm. PLoS ONE 2015, 10, e0124350. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tang, B.; Yu, F.; Li, L.; Wang, M.; Xue, Y.; Zhang, Z.; Yan, J.; Yue, B.; Zheng, Y. Identification of major QTL for waterlogging tolerance using genome-wide association and linkage mapping of maize seedlings. Plant Mol. Biol. Report. 2013, 31, 594–606. [Google Scholar] [CrossRef]
- Zhu, J.; Kaeppler, S.M.; Lynch, J.P. Mapping of QTLs for lateral root branching and length in maize (Zea mays L.) under differential phosphorus supply. Theor. Appl. Genet. 2005, 111, 688–695. [Google Scholar] [CrossRef]
- Zhu, J.; Kaeppler, S.M.; Lynch, J.P. Mapping of QTL controlling root hair length in maize (Zea mays L.) under phosphorus deficiency. Plant Soil 2005, 270, 299–310. [Google Scholar] [CrossRef]
- Pace, J.; Gardner, C.; Romay, C.; Ganapathysubramanian, B.; Lübberstedt, T. Genome-wide association analysis of seedling root development in maize (Zea mays L.). BMC Genom. 2015, 16, 47. [Google Scholar] [CrossRef]
- Sun, X.; Ren, W.; Wang, P.; Chen, F.; Yuan, L.; Pan, Q.; Mi, G. Evaluation of maize root growth and genome-wide association studies of root traits in response to low nitrogen supply at seedling emergence. Crop J. 2021, 9, 794–804. [Google Scholar] [CrossRef]
- Moussa, A.A.; Mandozai, A.; Jin, Y.; Qu, J.; Zhang, Q.; Zhao, H.; Anwari, G.; Khalifa, M.A.S.; Lamboro, A.; Noman, M. Genome-wide association screening and verification of potential genes associated with root architectural traits in maize (Zea mays L.) at multiple seedling stages. BMC Genom. 2021, 22, 558. [Google Scholar] [CrossRef]
- Wu, B.; Ren, W.; Zhao, L.; Li, Q.; Sun, J.; Chen, F.; Pan, Q. Genome-Wide Association Study of Root System Architecture in Maize. Genes 2022, 13, 181. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Tang, X.; Yang, X.; Fan, Y.; Xu, Y.; Li, P.; Xu, C.; Yang, Z. Exploiting natural variation in crown root traits via genome-wide association studies in maize. BMC Plant Biol. 2021, 21, 346. [Google Scholar] [CrossRef]
- Sun, D.; Chen, S.; Cui, Z.; Lin, J.; Liu, M.; Jin, Y.; Zhang, A.; Gao, Y.; Cao, H.; Ruan, Y. Genome-wide association study reveals the genetic basis of brace root angle and diameter in maize. Front. Genet. 2022, 13, 963852. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, H.; Wang, M.; Li, G.; Chen, Z.; Leiser, W.L.; Weiß, T.M.; Lu, X.; Wang, M.; Chen, S. Genetic Dissection of Phosphorus Use Efficiency in a Maize Association Population under Two P Levels in the Field. Int. J. Mol. Sci. 2021, 22, 9311. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Qing, C.; Frei, U.; Shen, Y.; Lübberstedt, T. Association mapping for root system architecture traits under two nitrogen conditions in germplasm enhancement of maize doubled haploid lines. Crop J. 2020, 8, 213–226. [Google Scholar] [CrossRef]
- Guo, J.; Li, C.; Zhang, X.; Li, Y.; Zhang, D.; Shi, Y.; Song, Y.; Li, Y.; Yang, D.; Wang, T. Transcriptome and GWAS analyses reveal candidate gene for seminal root length of maize seedlings under drought stress. Plant Sci. 2020, 292, 110380. [Google Scholar] [CrossRef]
- Liang, T.; Qing, C.; Liu, P.; Zou, C.; Yuan, G.; Pan, G.; Shen, Y.; Ma, L. Joint GWAS and WGCNA uncover the genetic control of calcium accumulation under salt treatment in maize seedlings. Physiol. Plant. 2022, 174, e13606. [Google Scholar] [CrossRef]
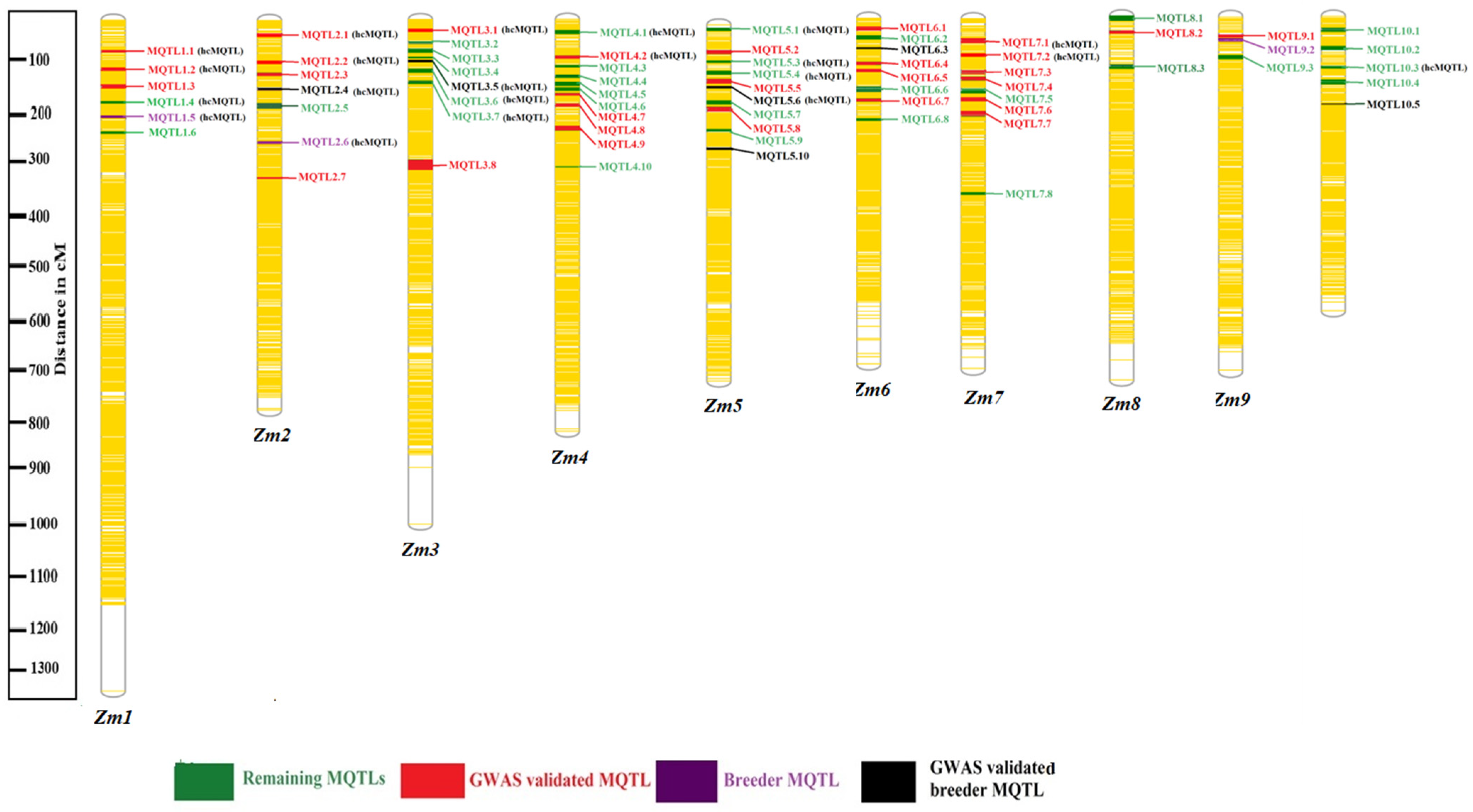
| S. No | MQTL Name | Chromosome | CI (95%) | Genetic Position (cM) | Flanking Maker (From-to) | Number of QTLs Involved | Root Trait Categories |
|---|---|---|---|---|---|---|---|
| 1 | MQTL1.1 | 1 | 2.35 | 60.39–62.74 | ofp1-gpm330 | 42 | RA, RD, REA, RL, RN, RS, RVT, and RW |
| 2 | MQTL1.2 | 1 | 2.55 | 95.86–98.41 | mrpa4-lls1 | 15 | RD, REA, RL, RN, RS, and RW |
| 3 | MQTL1.3 | 1 | 4.18 | 128.74–132.92 | sdg122-cox10b | 5 | RD, RL, RN, and RS |
| 4 | MQTL1.4 | 1 | 2.45 | 159.71–162.16 | TIDP3048-ereb35 | 13 | RA, RDT, RL, RN, RTN, and RW |
| 5 | MQTL1.5 | 1 | 0.95 | 187.73–188.68 | pco109407-pco107465a | 12 | RAR, RL, RN, RVT, and RW |
| 6 | MQTL1.6 | 1 | 2.75 | 218.80–221.55 | pmk1-pco141386 | 5 | RN, RLD, and RW |
| 7 | MQTL2.1 | 2 | 2.89 | 27.99–30.88 | isu144a-kcbp1 | 16 | RD, RH+, RL, RN, and RW |
| 8 | MQTL2.2 | 2 | 3.58 | 80.68–84.26 | ereb47-AY109603 | 14 | RA, RD, REA, RH+, RL, RN, and RTN |
| 9 | MQTL2.3 | 2 | 4.24 | 103.66–107.9 | gpm914a-TIDP3748 | 7 | RDT, RD, RL, and RTN |
| 10 | MQTL2.4 | 2 | 0.9 | 134.98–135.88 | Bin2-448-mex1 | 17 | RA, RDT, RL, RN, RS, and RW |
| 11 | MQTL2.5 | 2 | 9.53 | 162.31–171.84 | mybr20-IDP650 | 9 | RDT, RL, and RW |
| 12 | MQTL2.6 | 2 | 1.79 | 237.83–239.62 | TIDP8875-les1 | 13 | RAR, RL, RN, and RW |
| 13 | MQTL2.7 | 2 | 1.19 | 307.99–309.18 | abi17-TIDP3679 | 9 | RD, RF, RL, RN, and RW |
| 14 | MQTL3.1 | 3 | 2.54 | 19.79–22.33 | agrr209a-Bin3_33 | 21 | RAR, RD, RL, RN, RS, RTN, RVT, and RW |
| 15 | MQTL3.2 | 3 | 3.89 | 42.97–46.86 | mmp38-bcd734b | 5 | RDT, RL, RN, and RW |
| 16 | MQTL3.3 | 3 | 2.95 | 59.20–62.15 | asg30c-cdo345b | 5 | RAR, RH+, and RL |
| 17 | MQTL3.4 | 3 | 1.72 | 73.56–75.28 | bnlg1325-mHbrMC376-Mo17 | 7 | RD, REA, RL, RN, and RW |
| 18 | MQTL3.5 | 3 | 1.61 | 79.90–81.51 | umc1886-cr1 | 19 | RH+ RL, RN, and RW |
| 19 | MQTL3.6 | 3 | 5.21 | 97.57–102.78 | mHbrBA221_Mo17-plpb1 | 11 | RD, REA, RH+, RL, RN, and RW |
| 20 | MQTL3.7 | 3 | 2.43 | 122.41–124.84 | SC298C-lim66 | 15 | RA, RDT, RL, RN, and RW |
| 21 | MQTL3.8 | 3 | 18.56 | 273.95–292.51 | bhlh58-TIDP3495 | 2 | RLD, andRW |
| 22 | MQTL4.1 | 4 | 4.88 | 22.82–27.7 | IDP8455-UMC156A | 16 | RDT, RL, RN, RS, and RW |
| 23 | MQTL4.2 | 4 | 3.2 | 72.47–75.67 | IDP483-bnlg1318 | 12 | RD, RL, RN, RS, and RW |
| 24 | MQTL4.3 | 4 | 2.71 | 90.76–93.47 | uaz68b(zp19)-Bin4_384 | 5 | RL, RN, and RW |
| 25 | MQTL4.4 | 4 | 4.27 | 108.91–113.18 | PZE_108065982-GRMZM2G157241 | 6 | RDT, RD, REA, and RL |
| 26 | MQTL4.5 | 4 | 4.84 | 124.66–129.5 | phd28-zhd1 | 2 | RL, and RW |
| 27 | MQTL4.6 | 4 | 3.72 | 134.19–137.91 | alf18-bnlg292 | 6 | RL, and RN |
| 28 | MQTL4.7 | 4 | 2.18 | 146.18–148.36 | gpm147-IDP6896 | 4 | RL, RN, and RW |
| 29 | MQTL4.8 | 4 | 2.66 | 167.26–169.92 | PZE_105145239-gpm782 | 3 | RDT, RL, and RW |
| 30 | MQTL4.9 | 4 | 6.04 | 210.71–216.75 | TIDP2704-nbcs12 | 6 | RAR, RD, RL, RN, and RW |
| 31 | MQTL4.10 | 4 | 0.51 | 287.60–288.11 | gpm420a-umc42a | 6 | RA, RAR, RD, RL, and RW |
| 32 | MQTL5.1 | 5 | 3.15 | 7.38–10.53 | CDO87A-AY110625 | 13 | RAR, RD, RH+, RL, RVT, and RW |
| 33 | MQTL5.2 | 5 | 5.47 | 50.43–55.90 | umc1901-chi3 | 5 | RL |
| 34 | MQTL5.3 | 5 | 3.08 | 71.28–74.36 | umc2388-bnl7.21c | 15 | RDT, RH+, RL, RN, and RW |
| 35 | MQTL5.4 | 5 | 5.68 | 90.85–96.53 | cdpk18-rab28 | 13 | RD, REA, RH+, RL, and RW |
| 36 | MQTL5.5 | 5 | 2.7 | 112.13–114.83 | gpm921b-PGAMCGG330 | 5 | RAR, RN, and RTN |
| 37 | MQTL5.6 | 5 | 1.5 | 120.06–121.56 | orc5-SYN37265 | 14 | RDT, RD, RL, RN, and RW |
| 38 | MQTL5.7 | 5 | 5.02 | 148.31–153.33 | agrx43-IDP8129 | 3 | RL, and RN |
| 39 | MQTL5.8 | 5 | 2.71 | 163.09–165.80 | umc107b-bnlg1208 | 8 | RA, RAR, RL, and RN |
| 40 | MQTL5.9 | 5 | 2.12 | 205.46–207.58 | ca2p10-smh6 | 2 | RDT, and RVT |
| 41 | MQTL5.10 | 5 | 1.97 | 240.76–242.73 | IDP5960-bnlg1700 | 5 | RD, RL, and RN |
| 42 | MQTL6.1 | 6 | 4.8 | 17.05–21.85 | AX_86316369-umc1572 | 12 | RDT, RD, RL, RN, RS, and RW |
| 43 | MQTL6.2 | 6 | 4.66 | 35.11–39.77 | umc2698-si486014c02 | 9 | RAR, RH+, RL, RN, and RW |
| 44 | MQTL6.3 | 6 | 0.97 | 57.64–58.61 | lht1-gpm304a | 12 | RD, RH+, RL, RN, RS, and RW |
| 45 | MQTL6.4 | 6 | 3.47 | 86.335–89.805 | ago1a-magi84474 | 13 | RA, RCP, RD, RL, RN, RTN, and RW |
| 46 | MQTL6.5 | 6 | 3.85 | 101.36–105.21 | csu680e-abi29 | 4 | RL, and RW |
| 47 | MQTL6.6 | 6 | 8.01 | 134.25–142.26 | IDP350-mHbrBG152-B73 | 4 | RDT, RL, and RW |
| 48 | MQTL6.7 | 6 | 5.36 | 157.84–163.2 | SYN1223-c3h26 | 2 | RL |
| 49 | MQTL6.8 | 6 | 1.18 | 197.14–198.32 | IDP8221-pco062823a | 3 | RA, and RL |
| 50 | MQTL7.1 | 7 | 4.22 | 40.74–44.96 | phi057-umc1112 | 20 | RD, RL, RN, RS, RVT, and RW |
| 51 | MQTL7.2 | 7 | 4.07 | 68.59–72.66 | IDP1692-PGAMCGG275 | 14 | RD, REA, RL, RN, RS, and RW |
| 52 | MQTL7.3 | 7 | 3.87 | 102.66–106.53 | csu486b-npi400a | 8 | RA, RL, RN, RTN, and RW |
| 53 | MQTL7.4 | 7 | 3.84 | 114.17–118.01 | umc1888-umc2190 | 3 | RA, RL, and RN |
| 54 | MQTL7.5 | 7 | 3.92 | 140.84–144.76 | v5-pzb01228 | 4 | RD, RL, and RN |
| 55 | MQTL7.6 | 7 | 5.43 | 155.27–160.70 | gpm273b-sid1 | 4 | RA, RD, RL, and RN |
| 56 | MQTL7.7 | 7 | 7.76 | 180.84–188.6 | arftf37-sdg110a | 2 | RW |
| 57 | MQTL7.8 | 7 | 2.02 | 340.05–342.07 | pebp24-gpm369c | 3 | RL |
| 58 | MQTL8.1 | 8 | 10.64 | 0–10.36 | ms23-umc1786 | 3 | RL, and RN |
| 59 | MQTL8.2 | 8 | 6.79 | 27.56–34.35 | psei4-IDP1987 | 6 | RF, RH+, RL, and RN |
| 60 | MQTL8.3 | 8 | 8.49 | 95.06–103.55 | gpm932d-IDP5056 | 6 | RF, RL, and RW |
| 61 | MQTL9.1 | 9 | 2.13 | 36.17–38.30 | pip1f-mads74 | 11 | RD, RL, RN, and RW |
| 62 | MQTL9.2 | 9 | 1.31 | 44.93–46.24 | opr1-lim343 | 7 | RL, and RW |
| 63 | MQTL9.3 | 9 | 5.73 | 76.43–82.16 | IDP1969-agrr147 | 5 | RL, RN, and RW |
| 64 | MQTL10.1 | 10 | 3.24 | 25.84–29.08 | myb69-ksu1e | 7 | RA, RLT, and RW |
| 65 | MQTL10.2 | 10 | 5.79 | 59.40–65.19 | TIDP3129-phm3631 | 8 | RA, RD, RL, RN, and RS |
| 66 | MQTL10.3 | 10 | 2.16 | 99.34–101.5 | umc1053-dmag4 | 28 | RAR, RD, REA, RL, RN, RS, RTN, and RW |
| 67 | MQTL10.4 | 10 | 5.39 | 126.06–131.45 | SYN22956-umc2749 | 9 | RD, RL, RN, RLD, and RS |
| 68 | MQTL10.5 | 10 | 0.62 | 171.74–172.36 | IDP4076-de18 | 13 | RF, RH+, RL, RTN, and RW |
| Known Genes Found within the MQTL Regions | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| S. No | Gene Name | MQTL Name | Gene Stable ID | Chromosome | GENE Start (bp) | Gene End (bp) | Role | Gene Product | Reference |
| 1 | PHT1 | MQTL 5.1 | Zm00001eb222510 | 5 | 33,267,448 | 33,269,832 | Phosphate utilization activity | Phosphate permease | [38] |
| 2 | YUC3 | MQTL 4.7 | Zm00001eb168680 | 4 | 15,140,763 | 15,143,517 | Auxin biosynthesis in roots | Flavin monooxygenase | [39] |
| 3 | YUC6 | MQTL 8.2 | Zm00001eb332870 | 8 | 3,246,199 | 3,249,205 | Auxin biosynthesis in roots | Flavin monooxygenase | [39] |
| 4 | DXS2 | MQTL 7.5 | Zm00001eb302370 | 7 | 14,390,493 | 14,393,714 | Highly expressed in the primary root of young seedlings and complete function not known yet | Transketolase, N-terminal | [40] |
| 5 | TUA4 | MQTL 5.7 | Zm00001eb215710 | 5 | 9,999,369 | 10,002,552 | Structural maintenance and cell division in root cells | alpha Tubulin | [41] |
| 6 | RTP1 | MQTL 4.6 | Zm00001eb168290 | 4 | 13,009,615 | 13,011,188 | Highly expressed in roots of young seedlings; involved in suberin pathway | O-methyltransferase domain | [42] |
| Known Genes Found near the MQTL Regions (±1 Mb MQTL Regions) | |||||||||
| 1 | RTCS1 | MQTL1.2 | Zm00001eb003920 | 1 | 10,913,046 | 10,913,187 | Promotes primary root growth in plant | Rootless concerning crown and seminal roots1 | [43] |
| 2 | NSA1 | MQTL2.4 | Zm00001eb071820 | 2 | 12,627,413 | 12,631,767 | Involved in Na+ homeostasis; mutants of the gene promote root Na+ efflux | Na+ content under saline-alkaline conditions1 | [44] |
| 3 | EIN2 | MQTL3.1 | Zm00001eb119690 | 3 | 2,916,990 | 2,926,153 | Produces secondary metabolites in roots under stress | Ethylene insensitive 2 | [45] |
| 4 | NHX1 | MQTL4.2 | Zm00001eb165870 | 4 | 4,724,901 | 4,733,653 | Involved in Na+ homeostasis; mutants promote root Na+ efflux | Na+/H+ antiporter 1 | [46] |
| 5 | CPPS4 | MQTL4.3 | Zm00001eb167120 | 4 | 7,053,803 | 7,059,057 | Upregulates under root exposure to abiotic stress | Copalyl diphosphate synthase4 | [47] |
| 6 | BIGE1 | MQTL5.1 | Zm00001eb211050 | 5 | 1,856,121 | 1,860,385 | Mutant causes accelerated root initiation as well as enlargement of the embryo scutellum | big embryo1 | [48] |
| 7 | RCP1 | MQTL6.5 | Zm00001eb270090 | 6 | 78,255,812 | 78,258,499 | Highly expressed in outer most root cap cells and involved in root development | Root cap protein1 | [49] |
| 8 | SKUS13 | MQTL7.1 | Zm00001eb298980 | 7 | 2,650,096 | 2,655,635 | Involved in directional root growth | Skewed root growth similar 13 | [50] |
| 9 | YUC5 | MQTL7.5 | Zm00001eb306140 | 7 | 39,946,780 | 39,950,489 | Transcribed in the root apex (0–5mm) | Yucca5: flavin monooxygenase-like enzyme | [51] |
| 10 | AW330564 | MQTL10.1 | Zm00001eb405820 | 10 | 2,736,099 | 2,736,268 | Root phototrophism | Similar to M. truncatula Root phototropism protein | [52] |
| Known Rice Genes | Rice Gene Stable ID | Rice Chromosome | Encoded Proteins | Function Description | Corresponding Maize Gene | Maize MQTL | Reference |
|---|---|---|---|---|---|---|---|
| OsACS7 | Os07g0280200 | 7 | ACC synthase | Involved in phosphate uptake in plants | Zm00001eb308860 | MQTL7.7 | [53] |
| OsAMT1 | Os04g0509600 | 4 | Ammonium transporter | Ammonium ion uptake in roots | Zm00001eb077350 | MQTL2.6 | [54] |
| OsCDPK10 | Os03g0788500 | 3 | Calcium-dependent protein kinase | Signalling of various root related biological process | Zm00001eb213280 | MQTL5.4 | [55] |
| OsCIPK14 | Os12g0113500 | 12 | Serine/threonine protein kinase | Signalling of various root related biological process | Zm00001eb406220 | MQTL10.2 | [56] |
| OsCIPK15 | Os11g0113700 | 11 | Serine/threonine protein kinase | Signalling of various root related biological process | Zm00001eb406220 | MQTL10.3 | [56] |
| OsCIPK23 | Os07g0150700 | 7 | Serine/threonine protein kinase | Signalling of various root related biological process | Zm00001eb300010 | MQTL7.2 | [56] |
| OsCKX1 | Os01g0187600 | 1 | Cytokinin Oxidase | Cytokinin metabolism | Zm00001eb121500 | MQTL3.6 | [57] |
| OsEXPA10 | Os04g0583500 | 4 | Expansin | Root cell elongation | Zm00001eb072890 | MQTL2.5 | [58] |
| OsHAK10 | Os06g0625900 | 6 | Potassium transporter | K+ transport from soil to plants | Zm00001eb275630 | MQTL6.7 | [59] |
| OsLAC17 | Os10g0346300 | 10 | Laccase | Catalyses the synthesis of lignin under stress | Zm00001eb165090 | MQTL4.1 | [60] |
| OsMGT | Os06g0650800 | 6 | Magnesium transporters | Magnesiumion uptake and transport from root to other organs | Zm00001eb274990 | MQTL6.7 | [61] |
| OsNAC7 | Os06g0131700 | 6 | NAC TF | Modulates root growth | Zm00001eb269810 | MQTL6.5 | [62] |
| OsWRKY68 | Os04g0605100 | 4 | WRKY TF | Involved in phosphate uptake in plants | Zm00001eb071590 | MQTL2.4 | [63] |
| OsYUCCA10 | Os01g0274100 | 1 | Flavin monooxygenase-like enzyme | Auxin biosynthesis | Zm00001eb332870 | MQTL8.2 | [64] |
| OsYUCCA12 | Os02g0272200 | 2 | Flavin monooxygenase-like enzyme | Auxin biosynthesis | Zm00001eb168680 | MQTL4.7 | [64] |
| OsYUCCA13 | Os11g0207700 | 11 | Flavin monooxygenase-like enzyme | Auxin biosynthesis | Zm00001eb168680 | MQTL4.7 | [64] |
| OsYUCCA14 | Os11g0207900 | 11 | Flavin monooxygenase-like enzyme | Auxin biosynthesis | Zm00001eb168680 | MQTL4.7 | [64] |
| OsYUCCA9 | Os01g0273800 | 1 | Flavin monooxygenase-like enzyme | Auxin biosynthesis | Zm00001eb332870 | MQTL8.2 | [64] |
| OsDREB1E | Os04g0572400 | 4 | DREB1 TF | Upregulated in roots during stress | Zm00001eb073550 | MQTL2.5 | [65] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karnatam, K.S.; Chhabra, G.; Saini, D.K.; Singh, R.; Kaur, G.; Praba, U.P.; Kumar, P.; Goyal, S.; Sharma, P.; Ranjan, R.; et al. Genome-Wide Meta-Analysis of QTLs Associated with Root Traits and Implications for Maize Breeding. Int. J. Mol. Sci. 2023, 24, 6135. https://doi.org/10.3390/ijms24076135
Karnatam KS, Chhabra G, Saini DK, Singh R, Kaur G, Praba UP, Kumar P, Goyal S, Sharma P, Ranjan R, et al. Genome-Wide Meta-Analysis of QTLs Associated with Root Traits and Implications for Maize Breeding. International Journal of Molecular Sciences. 2023; 24(7):6135. https://doi.org/10.3390/ijms24076135
Chicago/Turabian StyleKarnatam, Krishna Sai, Gautam Chhabra, Dinesh Kumar Saini, Rajveer Singh, Gurwinder Kaur, Umesh Preethi Praba, Pankaj Kumar, Simran Goyal, Priti Sharma, Rumesh Ranjan, and et al. 2023. "Genome-Wide Meta-Analysis of QTLs Associated with Root Traits and Implications for Maize Breeding" International Journal of Molecular Sciences 24, no. 7: 6135. https://doi.org/10.3390/ijms24076135
APA StyleKarnatam, K. S., Chhabra, G., Saini, D. K., Singh, R., Kaur, G., Praba, U. P., Kumar, P., Goyal, S., Sharma, P., Ranjan, R., Sandhu, S. K., Kumar, R., & Vikal, Y. (2023). Genome-Wide Meta-Analysis of QTLs Associated with Root Traits and Implications for Maize Breeding. International Journal of Molecular Sciences, 24(7), 6135. https://doi.org/10.3390/ijms24076135







