Abstract
Despite laparoscopy being a standardized option to diagnose pelvic endometriotic implants, non-invasive biomarkers are necessary to avoid the discomfort of invasive procedures. Recent evidence suggests a potential role of microRNAs (miRNAs) as feasible biomarkers for the early diagnosis of endometriosis. Following the recommendations in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement, we systematically searched PubMed, EMBASE, Scopus, Cochrane Library, and Science Direct in January 2023. We provided no restriction on the country and year of publication, and considered English published articles. We selected studies including patients with endometriosis and describing miRNA regulation in the context of endometriosis. Overall, 45 studies fulfilled the inclusion criteria, and 2045 patients with endometriosis and 1587 controls were screened. Patients were analyzed concerning miRNAs expression and sources, stage of disease, and symptoms, and compared to controls. Among DEMs, the ones with the widest delta between endometriosis patients and controls—Relative Expression ≥ 4 Log2(ratio)—were miR-145, miR-191, miR-195, miR-21-5p, miR-106b-5p, miR-195-5p, miR-451a, miR-200c, miR-20a-5p, and miR-15a-5p. Although the epigenetic regulation is partially unclear, miRNAs are valid biomarkers to diagnose endometriotic lesions in symptomatic and non-symptomatic women. MiRNAs modulation should be clarified, especially during therapies or relapse, to plan targeted management protocols.
1. Introduction
Endometriosis diagnosis in childbearing-age women is often delayed due to the lack of pathognomonic signs and symptoms [1,2,3]. Nowadays, transvaginal ultrasound (TVS) is the most cost-effective method to detect endometriotic lesions, but the gold-standard methodology for diagnosis is laparoscopy [4]. Laparoscopy is also considered the gold-standard treatment for endometriosis [2], while the best treatment option should consider the age of the patient, the symptoms, the desire to conceive, and previous surgeries [4]. In older women who underwent previous surgeries, medical treatment and In Vitro Fertilization may be discussed [4]. That emphasizes the necessity of non-invasive biomarkers to avoid the discomfort of laparoscopic procedures and simplify the diagnosis. Recent studies suggest the possibility of using microRNAs (miRNAs)—as reliable markers from different compartments (serum, plasma, endometrial biopsies, etc.)—in the early diagnosis of endometriosis [5,6,7]. Minimally invasive methods are necessary to assess the influence of bio-behavioral disruptors on the prognosis, treatment response, and recurrence.
The miRNAs are a class of small RNA molecules, composed of 15–22 nucleotides each, post-transcriptionally regulating genes [8]. MiRNAs hybridize into complementary mRNAs, which are involved in different cellular features, such as implantation, embryo developmental processes, tumor suppression, apoptosis, proliferation, angiogenesis, and metastasization [9,10,11]. Despite endometriosis showing a benign histological connotation, it develops and disseminates as a neoplastic process [12,13]. Moreover, there are pieces of evidence that endometriotic implants could transform into cancerous lesions [14]. In that context, endometriosis pathogenesis contains genetic, angiogenic, metabolic, and immunological alterations [12]. Endometriotic implants could undergo malignant transformation via altered molecular pathways, showing characteristics of atypia, invasivity, and diffusion, especially in E-cadherin-negative endometriotic cells [12]. Moreover, in 30% of cases, an endometriosis diagnosis may be linked to ovarian cancer detection [12]. Indeed, there is evidence that miRNAs are also involved in the pathogenesis of ovarian cancer, even in presentations linked to endometriosis [15,16,17]. One of the advantages of miRNAs is that they are accessible to sample. They could be found in multiple cellular compartments—in the context of different human systems—and be up- or downregulated [18,19,20]. It is estimated that miRNAs could be feasible biomarkers in the early diagnosis and management of endometriosis progression [20]. For example, endometriotic lesion development may depend on lower-expression cell adhesion and cytoskeleton molecules and decreased proteolysis [21,22,23]. In those contexts, the proliferation, migration, and stemness of endometrial stromal cells (ESCs) are increased in endometriosis through miRNAs’ epigenetic transcription [24]. Most miRNAs can be found in the serum of endometriosis-affected patients, and they could be extracted through liquid biopsy [15,21]. Those data may pave the way for new strategies for early diagnosis of endometriotic implants. The present systematic review aimed to evaluate the distribution and regulation of the differently expressed miRNAs (DEMs) in the context of endometriosis.
2. Materials and Methods
The methods for this study were specified a priori based on the recommendations in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [25]. The present work is categorized on PROSPERO as: ID400389.
2.1. Search Method
We performed a systematic search for records about the expression of different miRNAs in endometriosis-affected patients in PubMed, EMBASE, Scopus, Cochrane Library, and Science Direct in January 2023. We made no restriction on the country or year of publication, and considered only studies published entirely in English. We adopted the following string of keywords in each database to identify studies that fit to the topic of our review: “(Cell-Derived Microparticles OR MicroRNAs) AND Endometriosis”.
2.2. Study Selection
The study selection was made independently by I.I. and P.F. In the case of a discrepancy, C.R. decided on inclusion or exclusion. Inclusion criteria were (1) studies including patients with endometriosis; (2) studies describing differently expressed miRNAs (DEMs) and their regulation in the context of endometriosis signs and symptoms; and (3) peer-reviewed articles, published originally. We excluded non-original studies, pre-clinical trials, animal trials, abstract-only publications, and articles in a language other than English. If possible, we tried to contact the authors of studies that were published as conference abstracts via e-mail and asked them to provide their data. We assessed all included studies concerning potential conflicts of interest.
2.3. Extraction and Quantification of miRNAs
Liquid biopsy is a minimally invasive procedure to extract microvesicles from serum [12]. Extraction, amplification, and quantization of miRNAs are based on different procedures.
2.3.1. Extracellular Vesicles Classification
Extracellular vesicles (EVs) are a class of various submicron vesicles that can be released by cells in different conditions [26,27,28,29,30,31,32,33,34]. EVs are classified into exosomes, microvesicles, and apoptotic bodies. Exosomes measure from 30 to 100 nm, and they are formed into endosomes. Microvesicles measure from 100 to 1000 nm, and they derive from the plasma membrane, whereas apoptotic bodies measure 0.1–5 μm [35]. In particular, exosomes derived from multivesicular bodies (MVBs), and the “endosomal sorting complex required for transport” (ESCRT) protein complex may regulate their release [36]. Secondarily, MVBs can fuse with the plasma membrane, releasing exosomes. Immunoelectron microscopy revealed tetraspanins CD9, CD63, and CD81 as key components of exosomes, which could be used as biomarkers [37,38,39,40,41,42,43,44], whereas apoptotic bodies are positive for caspases 3 and 7 [45]. Otherwise, EVs have recently been identified according to their dimensions as small, if less than 100 nm, and medium and/or large, when 100–200 nm [46].
2.3.2. Extracellular Vesicles Analysis
EVs are usually recognized through immunoblotting, detecting the presence of tetraspanins in samples [46,47]. Moreover, the transmission electron microscope (TEM) and scanning electron microscope (SEM) assess EVs’ dimensions [48,49,50,51,52], whereas EVs features like elasticity are tested by an atomic force microscope (AFM) [53,54,55]. Flow cytometry (FC) is the most feasible method to analyze EVs’ content [56,57,58,59]. An immunophenotypic assessment may be performed through polychromatic FC [44,60,61,62], whereas FC with fluorescence images guarantees a sensitive method for EVs analysis [63,64].
Other authors described miRNA isolation in endometrial stromal cells from biopsies of ectopic endometrial lesions or eutopic endometria, which were placed into two halves in buffered formaline for storage and molecular examination. The RNA quality was first evaluated according to the integrity of the strains in the samples, whereas further analysis was performed based on the RNA minimum degradation in each sample [65].
Among the DEMs isolated, only those with AUC (Area Under the Curve) > 0.6 and significant allele and genotype distribution frequencies (p < 0.05) were considered in the present study.
3. Results
3.1. Studies’ Characteristics
We mention the studies selected and all reasons for exclusion in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart (Figure 1). After the database search, 124 articles matched the search criteria. After removing records without full text, duplicates, and wrong study designs (e.g., reviews), 64 were eligible. Overall, 45 matched the inclusion criteria and were included in the systematic review. The countries where the studies were conducted, the year range, the studies’ design, and the number of participants are summarized in Table 1. Overall, the publication years ranged from 2013 to 2022. In total, 2045 patients with endometriosis and 1587 controls were analyzed.
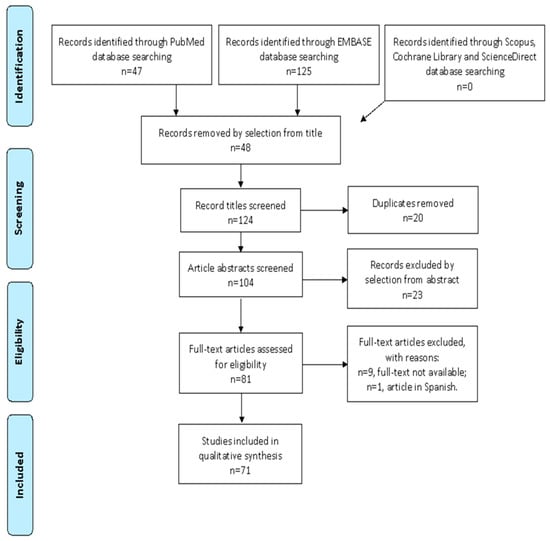
Figure 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) Flow-chart.

Table 1.
Characteristics of included studies.
3.2. Outcomes
A total of 2045 patients were included in the review. Regarding miRNA sources, miRNAs in endometrial stromal cells (ESCs) were extracted from the biopsies of ectopic endometrial lesions and/or eutopic endometria. Otherwise, miRNAs were extracted from serum, plasma, follicular fluid, and cumulus cells. Those data are summarized in Table 2 and Table 3.

Table 2.
Upregulated microRNA expression profiles in patients with endometriosis.

Table 3.
Downregulated microRNA expression profiles in patients with endometriosis.
3.2.1. Early Diagnosis
In total, 58 miRNAs were upregulated in endometriosis-affected patients, whereas, 67 miRNAs were downregulated. Those data are summarized in Table 2 and Table 3. Except for 8, the other 37 studies revealed the stage of disease. In total, 18 records involved patients with ASRM (American Society for Reproductive Medicine) stages III–IV of disease; 16 records involved patients with ASRM stages I–IV of disease; whereas only 3 studies included patients with low or intermediate stages of disease (I–III). In particular, Wang et al. enrolled 30 patients with stages I–II of disease and revealed upregulation of miR-20a-5p through liquid biopsy [71]. In parallel, liquid biopsy showed downregulation of miR-30c-5p, miR127-3p, miR-99b-5p, and miR-15b-5p in the same cohort [71]. Liu et al. isolated miRNAs from ESCs, both in eutopic and ectopic endometria, demonstrating that miR-449b-3p was downregulated in the early stages of endometriosis-affected women [74]. Petracco et al. enrolled patients with stages II–III of endometriosis [80]. MiRNA was isolated both from eutopic and ectopic endometrial samples, and miR-135a/b was downregulated [80]. In the last two studies, the difference in the relative expression of miRNAs between patients and controls was <2 Log2(ratio) [74,80].
3.2.2. Early Diagnosis
Pokrovenko et al. enrolled 64 endometriotic infertile patients with dysmenorrhea in stages I–IV of disease, and miRNA extraction revealed downregulation of miR-let-7 [94], although the relative expression in the Log2(ratio) between the patients and controls corresponded to 0.6 [94]. Regarding dysmenorrhea, in the Bendifallah et al. study, 100% of the patients with stages I–IV of endometriosis were dysmenorrheic [99]. In particular, liquid biopsy demonstrated that hsa-miR-29b-1-5p, hsa-miR-4748, hsa-miR-515-5p, hsa-miR-548j-5p, and hsa-miR-6502-5p were upregulated, whereas hsa-miR-3137 and hsa-miR-3168 were downregulated, with no specification of the relative expression pattern [99]. Both endometrial biopsies and plasma showed that miR-124-3p was downregulated in the Dabi et al. analysis, even though the authors did not declare the stage of disease of the patients enrolled [100]. Regarding infertility, Xu et al. enrolled 14 infertile endometriotic patients, with no specification of the stage of disease, in whose endometrial biopsies miR-1304-3p, miR-544b, miR-3684, miR-494-5p, miR-4683, and miR-6747-3p were upregulated, whereas miR-3935, miR-4427, miR-652-5p and miR-205-5p were downregulated [73]. In the da Silva et al. study, 100% of the patients with stages I–IV of disease were infertile, and miRNA extraction from cumulus cells revealed downregulation of miR-532-3p [84]. Only Li et al. analyzed the follicular fluid of infertile patients with stages III–IV of disease, revealing downregulation of miR-451 [78]. Those results are summarized in Table 4.

Table 4.
MicroRNA modulation in endometriosis.
3.2.3. MiRNAs Relative Expression in Patients and Controls
Among DEMs, the ones with the widest delta between endometriosis patients and controls—Relative Expression ≥ 4 Log2(ratio)—were miR-145, miR-191, miR-195, miR-21-5p, miR-106b-5p, miR-195-5p, miR-451a, miR-200c, miR-20a-5p, and miR-15a-5p [21,68,69,72,83,88,96]. In parallel, DEMs with an intermediate delta between patients and controls—Relative Expression ≥ 2 > 4 Log2(ratio)—were miR-146a rs2910164, miR-149 rs2292832, miR-16, miR-29c-3p, miR-451, miR-10b, miR-199a-3p, miR-205-5p/ZEB1, miR-519b-3p/PRRG4, and miR-423 rs6505162 [20,68,69,78,83,88,95,98,104]. For example, Borisov et al. highlighted the widest difference in the relative expression of upregulated miR-191 between endometriotic patients and controls [83]. It was isolated in ESCs from the biopsies of eutopic and ectopic endometria [83]. Afterwards, in the Braza-Boïls et al. study, we found that miR-21-5p was the second most upregulated miRNA in endometriotic patients compared to controls [68]. miRNA was extracted from eutopic endometria in that case also [68]. Secondarily, upregulated miR-145 and miR-451a show a difference ≥ 4 Log2(ratio) in relative expression between patients and controls (6.5 and 5.2, respectively), and they are extracted from plasma and serum, respectively [21,72]. Those results are summarized in Table 4.
4. Discussion
From a functional perspective, miRNAs are involved in intercellular crosstalk, both in eutopic endometrial tissue and endometriotic implants [70]. Scientific literature highlighted the potential role of DEMs as biomarkers for endometriosis-affected women. The expression and modulation of miRNAs are wide and heterogeneous, and we considered in our study only DEMs with the highest AUC (>0.6) and significant allele and genotype distribution frequencies (p < 0.05). Hypothetically, miRNAs may indirectly represent the cellular microenvironment that leads to the formation of endometriotic implants. Therefore, their research could help intercept endometriosis before macroscopic lesions are identifiable on an ultrasound. Although it is extremely difficult to determine the most sensitive and specific miRNAs in endometriosis pathogenesis, we have underlined miRNAs expression in symptomatic patients. For example, specific miRNAs are overexpressed in dysmenorrheic or infertile women. The presence of the symptom can help us in a twofold way. It can help us identify patients for further investigation by liquid biopsy. It can also give us information about how patients evolve to this symptomatology, helping us to understand the molecular mechanisms underlying the development of the symptomatology. This consideration is also interesting from the perspective of infertility symptoms without organic pelvic distorting lesions. In these cases, infertility is likely related to the uterine microenvironment corrupted by a chronic inflammatory state. The study of miRNAs in these patients can identify conditions invisible to the eye. Farsimadan et al. isolated miRNAs in infertile endometriotic patients without any declared symptoms, and they highlighted the upregulation of miR-146a rs2910164 and miR-149 rs2292832, which showed an intermediate delta of relative expression between patients and controls, e.g., ≥2 > 4 Log2(ratio) [20]. Further, Li et al. isolated miR-451 as upregulated miRNA in infertile endometriotic patients, but they did not declare whether those patients were suffering from other symptoms, such as dysmenorrhea [78]. However, their analysis revealed an intermediate difference in miR-451 relative expression between patients and controls [78]. On the other hand, there is little evidence about miRNA expression in endometriotic patients suffering from dysmenorrhea, but without infertility-related problems. Bendifallah et al. showed upregulation of hsa-miR-29b-1-5p, hsa-miR-4748, hsa-miR-515-5p, hsa-miR-548j-5p and hsa-miR-6502-5p, whereas Dabi et al. revealed upregulated miR-124-3p, even though neither of the studies specified the incidence of infertility in their cohorts [99,100]. Moreover, neither of the studies disclosed the difference in the relative expression of miRNAs between patients and controls [99,100]. Farsimadan et al. isolated miRNAs in infertile endometriotic patients without any declared symptoms, and they highlighted the upregulation of miR-146a rs2910164 and miR-149 rs2292832, which showed an intermediate delta of relative expression between patients and controls, e.g., ≥2 > 4 Log2(ratio) [20]. Further, Li et al. isolated miR-451 as an upregulated miRNA in infertile endometriotic patients, but they did not declare whether those patients were suffering from other symptoms, such as dysmenorrhea [78]. However, their analysis revealed an intermediate difference in miR-451 relative expression between patients and controls [78]. In our opinion, given the inaccurate definition of the signs and symptoms in different studies—mainly due to the heterogeneity of endometriosis presentation—it would be appropriate to focus on DEMs with the widest range in relative expression between patients and controls to avoid high false-positive rates during miRNA isolation. The real clinical use of miRNAs should lie in implementing the diagnostic capabilities of early forms. With this in mind, the different ways in which miRNA assays can be obtained should be emphasized. The site of expression probably influences miRNA modulation. Liquid and incisional endometrial biopsy may be valid options for miRNA extraction, even if eutopic endometrial tissue seems to have more defined expression profiles [68,83]. Surely, liquid biopsy through patients’ serum or plasma would be more feasible and cost-effective as a screening method in women suffering from dysmenorrhea, infertility, and dyschezia. An endometrial tissue biopsy may be a valid option in diagnosing endometriotic implants in patients with a suspected transvaginal ultrasound. Moreover, serum and plasma miRNAs are supposed to have a distant effect involving systemic organs. That suggests a potential role of liquid biopsy in detecting endometriotic implants of unknown location [20,106]. Although isolation strategies from saliva also revealed positive biomarkers for endometriosis detection, liquid biopsy could find a place in the application of screening programs on the fertile population, even in the complete absence of symptoms [106,107]. Unfortunately, this perspective needs more investigation of miRNA fluctuations in the early stages. For those reasons, one of the best candidates for early diagnosis of endometriosis may be miR-145 [21]. In their study, Bashti et al. showed an important advantage of miR-145—neither of the patients enrolled suffered from infertility or dysmenorrhea [21]. In addition, miR-145 expression is mostly upregulated in stages I–II of disease, suggesting its crucial role in tracking recent endometriotic lesions [21]. Finally, a hypothetical function of miRNAs could be related to the follow-up of patients on medical therapy. Any fluctuations could directly represent the response of endometriosis tissue to medical therapy, optimizing the chronification of treatment. Our review investigated all present literature on the topic. This represents its strength and weakness, related to the enormous heterogeneity of the data in the literature. The hope is that it will represent the basis for further investigation of the most interesting miRNAs useful for the clinical management of patients with endometriosis, with targeted studies specifically designed to investigate the various aspects.
5. Conclusions
Most recent evidence shows that intercellular crosstalk has a critical role in endometriosis pathogenesis, although there is heterogeneity of the data. In that context, specific molecular signatures could mark the homeostasis of endometrial tissue. Focusing on endometrial tissue, a pattern of miRNAs may be useful for the early diagnosis and management of endometriosis-affected women, mainly through liquid biopsy. Although there is a lack of data regarding DEMs in response to different therapeutic regimens, that analysis could be performed in women, administered with laparoscopy, oral contraceptives, Non-Steroidal Anti-Inflammatory Drugs (NSAIDs), or a GnRH antagonist during the FU period. To date, although the mechanism of epigenetic regulation remains unclear, the assessment of miRNAs’ expression could be a promising and cost-effective tool to detect the presence of endometriotic implants in symptomatic and non-symptomatic patients. Further studies are needed to clarify miRNAs’ modulation during treatment or the recurrence of disease in order to predict disease development and plan targeted management options.
Author Contributions
Conceptualization, C.R. and P.D.F.; methodology, I.I.; software, P.F.; validation, C.R., P.F.G., and P.D.F.; formal analysis, I.I.; investigation, I.I.; resources, I.I.; data curation, P.F.; writing—original draft preparation, I.I. and P.F.; writing—review and editing, I.I.; visualization, C.R.; supervision, L.C.; project administration, P.D.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created. Please see the References section for research data supporting reported results.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Burney, R.O.; Giudice, L.C. Pathogenesis and pathophysiology of endometriosis. Fertil. Steril. 2012, 98, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Jadoul, P.; Kitajima, M.; Donnez, O.; Squifflet, J.; Donnez, J. Surgical treatment of ovarian endometriomas: State of the art? Fertil. Steril. 2012, 98, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Giudice, L.C.; Kao, L.C. Endometriosis. Lancet 2004, 364, 1789–1799. [Google Scholar] [CrossRef]
- Brosens, I.; Brosens, J. Is laparoscopy the gold standard for the diagnosis of endometriosis? Eur. J. Obstet. Gynecol. Reprod. Biol. 2000, 88, 117–119. [Google Scholar] [CrossRef] [PubMed]
- Zachariah, R.; Schmid, S.; Radpour, R.; Buerki, N.; Fan, A.X.-C.; Hahn, S.; Holzgreve, W.; Zhong, X.Y. Circulating cell-free DNA as a potential biomarker for minimal and mild endometriosis. Reprod. Biomed. Online 2009, 18, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.-Z.; Yang, Y.; Lang, J.; Sun, P.; Leng, J. Plasma miR-17-5p, miR-20a and miR-22 are down-regulated in women with endometriosis. Hum. Reprod. 2013, 28, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Rekker, K.; Saare, M.; Roost, A.M.; Kaart, T.; Sõritsa, D.; Karro, H.; Sõritsa, A.; Simón, C.; Salumets, A.; Peters, M. Circulating miR-200–family micro-RNAs have altered plasma levels in patients with endometriosis and vary with blood collection time. Fertil. Steril. 2015, 104, 938–946.e2. [Google Scholar] [CrossRef]
- Zhang, B.; Pan, X.; Cobb, G.; Anderson, T. microRNAs as oncogenes and tumor suppressors. Dev. Biol. 2007, 302, 1–12. [Google Scholar] [CrossRef]
- Stepicheva, N.A.; Song, J.L. Function and regulation of microRNA-31 in development and disease. Mol. Reprod. Dev. 2016, 83, 654–674. [Google Scholar] [CrossRef]
- Acunzo, M.; Romano, G.; Wernicke, D.; Croce, C.M. MicroRNA and cancer—A brief overview. Adv. Biol. Regul. 2015, 57, 1–9. [Google Scholar] [CrossRef]
- Lynam-Lennon, N.; Maher, S.; Reynolds, J.V. The roles of microRNA in cancer and apoptosis. Biol. Rev. 2009, 84, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Varma, R.; Rollason, T.; Gupta, J.K.; Maher, E.R. Endometriosis and the neoplastic process. Reproduction 2004, 127, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.-W. Endometriosis and ovarian cancer: Potential benefits and harms of screening and risk-reducing surgery. Fertil. Steril. 2015, 104, 813–830. [Google Scholar] [CrossRef]
- Matalliotakis, M.; Matalliotaki, C.; Goulielmos, G.N.; Patelarou, E.; Tzardi, M.; Spandidos, D.; Arici, A.; Matalliotakis, I. Association between ovarian cancer and advanced endometriosis. Oncol. Lett. 2018, 15, 7689–7692. [Google Scholar] [CrossRef] [PubMed]
- Lucidi, A.; Buca, D.; Ronsini, C.; Tinari, S.; Bologna, G.; Buca, D.; Leombroni, M.; Liberati, M.; D’Antonio, F.; Scambia, G.; et al. Role of Extracellular Vesicles in Epithelial Ovarian Cancer: A Systematic Review. Int. J. Mol. Sci. 2020, 21, 8762. [Google Scholar] [CrossRef] [PubMed]
- Schmid, G.; Notaro, S.; Reimer, D.; Abdel-Azim, S.; Duggan-Peer, M.; Holly, J.; Fiegl, H.; Rössler, J.; Wiedemair, A.; Concin, N.; et al. Expression and promotor hypermethylation of miR-34a in the various histological subtypes of ovarian cancer. BMC Cancer 2016, 16, 102. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.D.; Gercel-Taylor, C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol. Oncol. 2008, 110, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ba, Y.; Ma, L.; Cai, X.; Yin, Y.; Wang, K.; Guo, J.; Zhang, Y.; Chen, J.; Guo, X.; et al. Characterization of microRNAs in serum: A novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008, 18, 997–1006. [Google Scholar] [CrossRef]
- Yang, H.; Kong, W.; He, L.; Zhao, J.-J.; O’Donnell, J.D.; Wang, J.; Wenham, R.M.; Coppola, D.; Kruk, P.A.; Nicosia, S.V.; et al. MicroRNA expression profiling in human ovarian cancer: miR-214 induces cell survival and cisplatin resistance by targeting PTEN. Cancer Res. 2008, 68, 425–433. [Google Scholar] [CrossRef]
- Farsimadan, M.; Haje, M.I.; Mawlood, C.K.; Arabipour, I.; Emamvirdizadeh, A.; Takamoli, S.; Masumi, M.; Vaziri, H. MicroRNA variants in endometriosis and its severity. Br. J. Biomed. Sci. 2021, 78, 206–210. [Google Scholar] [CrossRef]
- Noruzinia, M.; Bashti, O.; Garshasbi, M.; Abtahi, M. miR-31 and miR-145 as Potential Non-Invasive Regulatory Biomarkers in Patients with Endometriosis. Cell J. 2018, 20, 84–89. [Google Scholar] [CrossRef]
- Hidalgo, G.D.S.; Meola, J.; e Silva, J.C.R.; de Paz, C.C.P.; Ferriani, R.A. TAGLN expression is deregulated in endometriosis and may be involved in cell invasion, migration, and differentiation. Fertil. Steril. 2011, 96, 700–703. [Google Scholar] [CrossRef] [PubMed]
- Hull, M.L.; Escareno, C.R.; Godsland, J.M.; Doig, J.R.; Johnson, C.M.; Phillips, S.C.; Smith, S.K.; Tavaré, S.; Print, C.G.; Charnock-Jones, D.S. Endometrial-peritoneal interactions during endometriotic lesion establishment. Am. J. Pathol. 2008, 173, 700–715. [Google Scholar] [CrossRef] [PubMed]
- Eggers, J.C.; Martino, V.; Reinbold, R.; Schäfer, S.D.; Kiesel, L.; Starzinski-Powitz, A.; Schüring, A.N.; Kemper, B.; Greve, B.; Götte, M. microRNA miR-200b affects proliferation, invasiveness and stemness of endometriotic cells by targeting ZEB1, ZEB2 and KLF4. Reprod. Biomed. Online 2016, 32, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Franzago, M.; Lanuti, P.; Fraticelli, F.; Marchioni, M.; Buca, D.; Di Nicola, M.; Liberati, M.; Miscia, S.; Stuppia, L.; Vitacolonna, E. Biological insight into the extracellular vesicles in women with and without gestational diabetes. J. Endocrinol. Investig. 2021, 44, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Buca, D.; Bologna, G.; D’Amico, A.; Cugini, S.; Musca, F.; Febbo, M.; D’Arcangelo, D.; Buca, D.; Simeone, P.; Liberati, M.; et al. Extracellular Vesicles in Feto–Maternal Crosstalk and Pregnancy Disorders. Int. J. Mol. Sci. 2020, 21, 2120. [Google Scholar] [CrossRef]
- Goossens, N.; Nakagawa, S.; Sun, X.; Hoshida, Y. Cancer biomarker discovery and validation. Transl. Cancer Res. 2015, 4, 256–269. [Google Scholar] [CrossRef]
- Di Tomo, P.; Lanuti, P.; Di Pietro, N.; Baldassarre, M.P.A.; Marchisio, M.; Pandolfi, A.; Consoli, A.; Formoso, G. Liraglutide mitigates TNF-α induced pro-atherogenic changes and microvesicle release in HUVEC from diabetic women. Diabetes/Metab. Res. Rev. 2017, 33, e2925. [Google Scholar] [CrossRef]
- Pieragostino, D.; Lanuti, P.; Cicalini, I.; Cufaro, M.C.; Ciccocioppo, F.; Ronci, M.; Simeone, P.; Onofrj, M.; van der Pol, E.; Fontana, A.; et al. Proteomics characterization of extracellular vesicles sorted by flow cytometry reveals a disease-specific molecular cross-talk from cerebrospinal fluid and tears in multiple sclerosis. J. Proteom. 2019, 204, 103403. [Google Scholar] [CrossRef]
- Grande, R.; Dovizio, M.; Marcone, S.; Szklanna, P.B.; Bruno, A.; Ebhardt, H.A.; Cassidy, H.; Ní Áinle, F.; Caprodossi, A.; Lanuti, P.; et al. Platelet-Derived Microparticles from Obese Individuals: Characterization of Number, Size, Proteomics, and Crosstalk With Cancer and Endothelial Cells. Front. Pharmacol. 2019, 10, 7. [Google Scholar] [CrossRef] [PubMed]
- Pieragostino, D.; Cicalini, I.; Lanuti, P.; Ercolino, E.; Di Ioia, M.; Zucchelli, M.; Zappacosta, R.; Miscia, S.; Marchisio, M.; Sacchetta, P.; et al. Enhanced release of acid sphingomyelinase-enriched exosomes generates a lipidomics signature in CSF of Multiple Sclerosis patients. Sci. Rep. 2018, 8, 3071. [Google Scholar] [CrossRef] [PubMed]
- Codagnone, M.; Recchiuti, A.; Lanuti, P.; Pierdomenico, A.M.; Cianci, E.; Patruno, S.; Mari, V.C.; Simiele, F.; Di Tomo, P.; Pandolfi, A.; et al. Lipoxin A4 stimulates endothelial miR-126–5p expression and its transfer via microvesicles. FASEB J. 2017, 31, 1856–1866. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Lu, S.; Pu, D.; Zhang, H.; Yang, L.; Zeng, P.; Su, F.; Chen, Z.; Guo, M.; Gu, Y.; et al. Detection of fetal trisomy and single gene disease by massively parallel sequencing of extracellular vesicle DNA in maternal plasma: A proof-of-concept validation. BMC Med. Genom. 2019, 12, 151. [Google Scholar] [CrossRef] [PubMed]
- Cufaro, M.C.; Pieragostino, D.; Lanuti, P.; Rossi, C.; Cicalini, I.; Federici, L.; De Laurenzi, V.; Del Boccio, P. Extracellular Vesicles and Their Potential Use in Monitoring Cancer Progression and Therapy: The Contribution of Proteomics. J. Oncol. 2019, 2019, 1639854. [Google Scholar] [CrossRef] [PubMed]
- Andreu, Z.; Yáñez-Mó, M. Tetraspanins in Extracellular Vesicle Formation and Function. Front. Immunol. 2014, 5, 442. [Google Scholar] [CrossRef] [PubMed]
- Krause, M.; Rak-Raszewska, A.; Naillat, F.; Saarela, U.; Schmidt, C.; Ronkainen, V.-P.; Bart, G.; Ylä-Herttuala, S.; Vainio, S.J. Exosomes as secondary inductive signals involved in kidney organogenesis. J. Extracell. Vesicles 2018, 7, 1422675. [Google Scholar] [CrossRef]
- Helwa, I.; Cai, J.; Drewry, M.D.; Zimmerman, A.; Dinkins, M.B.; Khaled, M.L.; Seremwe, M.; Dismuke, W.M.; Bieberich, E.; Stamer, W.D.; et al. A Comparative Study of Serum Exosome Isolation Using Differential Ultracentrifugation and Three Commercial Reagents. PLoS ONE 2017, 12, e0170628. [Google Scholar] [CrossRef]
- Chairoungdua, A.; Smith, D.L.; Pochard, P.; Hull, M.; Caplan, M.J. Exosome release of β-catenin: A novel mechanism that antagonizes Wnt signaling. J. Cell Biol. 2010, 190, 1079–1091. [Google Scholar] [CrossRef]
- Buschow, S.I.; Nolte-‘t Hoen, E.N.M.N.; Van Niel, G.; Pols, M.S.; ten Broeke, T.T.; Lauwen, M.; Ossendorp, F.; Melief, C.J.M.; Raposo, G.; Wubbolts, R.; et al. MHC II in dendritic cells is targeted to lysosomes or t cell-induced exosomes via distinct multivesicular body pathways. Traffic 2009, 10, 1528–1542. [Google Scholar] [CrossRef]
- Escola, J.-M.; Kleijmeer, M.J.; Stoorvogel, W.; Griffith, J.M.; Yoshie, O.; Geuze, H.J. Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by humsan B-lymphocytes. J. Biol. Chem. 1998, 273, 20121–20127. [Google Scholar] [CrossRef] [PubMed]
- Nazarenko, I.; Rana, S.; Baumann, A.; McAlear, J.; Hellwig, A.; Trendelenburg, M.; Lochnit, G.; Preissner, K.T.; Zöller, M. Cell surface tetraspanin Tspan8 contributes to molecular pathways of exosome-induced endothelial cell activation. Cancer Res. 2010, 70, 1668–1678. [Google Scholar] [CrossRef] [PubMed]
- Villarroya-Beltri, C.; Baixauli, F.; Gutiérrez-Vázquez, C.; Sánchez-Madrid, F.; Mittelbrunn, M. Sorting it out: Regulation of exosome loading. Semin. Cancer Biol. 2014, 28, 3–13. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Poon, I.K.H.; Parkes, M.A.F.; Jiang, L.; Atkin-Smith, G.K.; Tixeira, R.; Gregory, C.D.; Ozkocak, D.C.; Rutter, S.F.; Caruso, S.; Santavanond, J.P.; et al. Moving beyond size and phosphatidylserine exposure: Evidence for a diversity of apoptotic cell-derived extracellular vesicles in vitro. J. Extracell. Vesicles 2019, 8, 1608786. [Google Scholar] [CrossRef]
- Ciardiello, C.; Leone, A.; Lanuti, P.; Roca, M.S.; Moccia, T.; Minciacchi, V.R.; Minopoli, M.; Gigantino, V.; De Cecio, R.; Rippa, M.; et al. Large oncosomes overexpressing integrin alpha-V promote prostate cancer adhesion and invasion via AKT activation. J. Exp. Clin. Cancer Res. 2019, 38, 317. [Google Scholar] [CrossRef]
- Hartjes, T.A.; Mytnyk, S.; Jenster, G.W.; van Steijn, V.; van Royen, M.E. Extracellular Vesicle Quantification and Characterization: Common Methods and Emerging Approaches. Bioengineering 2019, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.H.; Shin, H.W.; Jung, A.R.; Kwon, O.S.; Choi, Y.-J.; Park, J.; Lee, J.Y. Author Correction: Prostate-specific extracellular vesicles as a novel biomarker in human prostate cancer. Sci. Rep. 2019, 9, 6051. [Google Scholar] [CrossRef]
- Linares, R.; Tan, S.; Gounou, C.; Brisson, A.R. Imaging and Quantification of Extracellular Vesicles by Transmission Electron Microscopy. Methods Mol. Biol. 2017, 1545, 43–54. [Google Scholar] [CrossRef]
- Sokolova, V.; Ludwig, A.-K.; Hornung, S.; Rotan, O.; Horn, P.A.; Epple, M.; Giebel, B. Characterisation of exosomes derived from human cells by nanoparticle tracking analysis and scanning electron microscopy. Colloids Surf. B Biointerfaces 2011, 87, 146–150. [Google Scholar] [CrossRef]
- Casado, S.; Lobo, M.D.V.T.; Paíno, C.L. Dynamics of plasma membrane surface related to the release of extracellular vesicles by mesenchymal stem cells in culture. Sci. Rep. 2017, 7, 6767. [Google Scholar] [CrossRef] [PubMed]
- Nanou, A.; Crespo, M.; Flohr, P.; De Bono, J.S.; Terstappen, L.W.M.M. Scanning Electron Microscopy of Circulating Tumor Cells and Tumor-Derived Extracellular Vesicles. Cancers 2018, 10, 416. [Google Scholar] [CrossRef] [PubMed]
- Biggs, C.N.; Siddiqui, K.M.; Al-Zahrani, A.A.; Pardhan, S.; Brett, S.I.; Guo, Q.Q.; Yang, J.; Wolf, P.; Power, N.E.; Durfee, P.N.; et al. Prostate extracellular vesicles in patient plasma as a liquid biopsy platform for prostate cancer using nanoscale flow cytometry. Oncotarget 2016, 7, 8839–8849. [Google Scholar] [CrossRef] [PubMed]
- Hardij, J.; Cecchet, F.; Berquand, A.; Gheldof, D.; Chatelain, C.; Mullier, F.; Chatelain, B.; Dogné, J.-M. Characterisation of tissue factor-bearing extracellular vesicles with AFM: Comparison of air-tapping-mode AFM and liquid Peak Force AFM. J. Extracell. Vesicles 2013, 2, 21045. [Google Scholar] [CrossRef] [PubMed]
- Vorselen, D.; Marchetti, M.; López-Iglesias, C.; Peters, P.J.; Roos, W.H.; Wuite, G.J.L. Multilamellar nanovesicles show distinct mechanical properties depending on their degree of lamellarity. Nanoscale 2018, 10, 5318–5324. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, C.; Di Vizio, D.; Sahoo, S.; Théry, C.; Witwer, K.W.; Wauben, M.; Hill, A.F. Techniques used for the isolation and characterization of extracellular vesicles: Results of a worldwide survey. J. Extracell. Vesicles 2016, 5, 32945. [Google Scholar] [CrossRef] [PubMed]
- Poncelet, P.; Robert, S.; Bailly, N.; Garnache-Ottou, F.; Bouriche, T.; Devalet, B.; Segatchian, J.H.; Saas, P.; Mullier, F. Tips and tricks for flow cytometry-based analysis and counting of microparticles. Transfus. Apher. Sci. 2015, 53, 110–126. [Google Scholar] [CrossRef]
- Chandler, W.L. Measurement of microvesicle levels in human blood using flow cytometry. Cytom. Part B Clin. Cytom. 2016, 90, 326–336. [Google Scholar] [CrossRef]
- Deshmukh, A.; Kim, G.; Burke, M.; Anyanwu, E.; Jeevanandam, V.; Uriel, N.; Tung, R.; Ozcan, C. Atrial Arrhythmias and Electroanatomical Remodeling in Patients With Left Ventricular Assist Devices. J. Am. Heart Assoc. 2017, 6, e005340. [Google Scholar] [CrossRef]
- Cossarizza, A.; Chang, H.-D.; Radbruch, A.; Acs, A.; Adam, D.; Adam-Klages, S.; Agace, W.W.; Aghaeepour, N.; Akdis, M.; Allez, M.; et al. Guidelines for the use of flow cytometry and cell sorting in immunological studies (second edition). Eur. J. Immunol. 2019, 49, 1457–1973. [Google Scholar] [CrossRef]
- van der Pol, E.; Coumans, F.; Varga, Z.; Krumrey, M.; Nieuwland, R. Innovation in detection of microparticles and exosomes. J. Thromb. Haemost. 2013, 11, 36–45. [Google Scholar] [CrossRef]
- Stoner, S.A.; Duggan, E.; Condello, D.; Guerrero, A.; Turk, J.R.; Narayanan, P.K.; Nolan, J.P. High sensitivity flow cytometry of membrane vesicles. Cytom. Part A 2016, 89, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Erdbrügger, U.; Rudy, C.K.; Etter, M.E.; Dryden, K.A.; Yeager, M.; Klibanov, A.L.; Lannigan, J. Imaging flow cytometry elucidates limitations of microparticle analysis by conventional flow cytometry. Cytom. Part A 2014, 85, 756–770. [Google Scholar] [CrossRef] [PubMed]
- Mastoridis, S.; Bertolino, G.M.; Whitehouse, G.; Dazzi, F.; Sanchez-Fueyo, A.; Martinez-Llordella, M. Multiparametric Analysis of Circulating Exosomes and Other Small Extracellular Vesicles by Advanced Imaging Flow Cytometry. Front. Immunol. 2018, 9, 1583. [Google Scholar] [CrossRef] [PubMed]
- Laudanski, P.; Charkiewicz, R.; Kuzmicki, M.; Szamatowicz, J.; Charkiewicz, A.; Niklinski, J. MicroRNAs expression profiling of eutopic proliferative endometrium in women with ovarian endometriosis. Reprod. Biol. Endocrinol. 2013, 11, 78. [Google Scholar] [CrossRef]
- Wang, W.-T.; Zhao, Y.-N.; Han, B.-W.; Hong, S.-J.; Chen, Y.-Q. Circulating microRNAs identified in a genome-wide serum microRNA expression analysis as noninvasive biomarkers for endometriosis. J. Clin. Endocrinol. Metab. 2013, 98, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Suryawanshi, S.; Vlad, A.M.; Lin, H.-M.; Mantia-Smaldone, G.; Laskey, R.; Lee, M.; Lin, Y.; Donnellan, N.; Klein-Patel, M.; Lee, T.; et al. Plasma microRNAs as novel biomarkers for endometriosis and endometriosis-associated ovarian cancer. Clin. Cancer Res. 2013, 19, 1213–1224. [Google Scholar] [CrossRef]
- Braza-Boïls, A.; Salloum-Asfar, S.; Marí-Alexandre, J.; Arroyo, A.B.; González-Conejero, R.; Barceló-Molina, M.; García-Oms, J.; Vicente, V.; Estellés, A.; Gilabert-Estellés, J.; et al. Peritoneal fluid modifies the microRNA expression profile in endometrial and endometriotic cells from women with endometriosis. Hum. Reprod. 2015, 30, 2292–2302. [Google Scholar] [CrossRef]
- Cho, S.; Mutlu, L.; Grechukhina, O.; Taylor, H.S. Circulating microRNAs as potential biomarkers for endometriosis. Fertil. Steril. 2015, 103, 1252–1260.e1. [Google Scholar] [CrossRef]
- Cosar, E.; Mamillapalli, R.; Ersoy, G.S.; Cho, S.; Seifer, B.; Taylor, H.S. Serum microRNAs as diagnostic markers of endometriosis: A comprehensive array-based analysis. Fertil. Steril. 2016, 106, 402–409. [Google Scholar] [CrossRef]
- Wang, L.; Huang, W.; Ren, C.; Zhao, M.; Jiang, X.; Fang, X.; Xia, X. Analysis of Serum microRNA Profile by Solexa Sequencing in Women With Endometriosis. Reprod. Sci. 2016, 23, 1359–1370. [Google Scholar] [CrossRef] [PubMed]
- Nothnick, W.B.; Al-Hendy, A.; Lue, J.R. Circulating Micro-RNAs as Diagnostic Biomarkers for Endometriosis: Privation and Promise. J. Minim. Invasive Gynecol. 2015, 22, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Li, Z.; Liu, J.; Yu, S.; Wei, Z. MicroRNA expression profiling in endometriosis-associated infertility and its relationship with endometrial receptivity evaluated by ultrasound. J. X-Ray Sci. Technol. 2017, 25, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, J.; Zhu, X.; Tang, L.; Luo, X.; Shi, Y. Role of miR449b3p in endometriosis via effects on endometrial stromal cell proliferation and angiogenesis. Mol. Med. Rep. 2018, 18, 3359–3365. [Google Scholar] [CrossRef] [PubMed]
- Maged, A.M.; Deeb, W.S.; El Amir, A.; Zaki, S.S.; El Sawah, H.; Al Mohamady, M.; Metwally, A.A.; Katta, M.A. Diagnostic accuracy of serum miR-122 and miR-199a in women with endometriosis. Int. J. Gynecol. Obstet. 2018, 141, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Pateisky, P.; Pils, D.; Szabo, L.; Kuessel, L.; Husslein, H.; Schmitz, A.; Wenzl, R.; Yotova, I. hsa-miRNA-154-5p expression in plasma of endometriosis patients is a potential diagnostic marker for the disease. Reprod. Biomed. Online 2018, 37, 449–466. [Google Scholar] [CrossRef]
- Wang, F.; Wang, H.; Jin, D.; Zhang, Y. Serum miR-17, IL-4, and IL-6 levels for diagnosis of endometriosis. Medicine 2018, 97, e10853. [Google Scholar] [CrossRef]
- Li, X.; Zhang, W.; Fu, J.; Xu, Y.; Gu, R.; Qu, R.; Li, L.; Sun, Y.; Sun, X. MicroRNA-451 is downregulated in the follicular fluid of women with endometriosis and influences mouse and human embryonic potential. Reprod. Biol. Endocrinol. 2019, 17, 96. [Google Scholar] [CrossRef]
- Nabiel, Y.; Elshahawy, H.; Mosbah, A. Intrauterine Bacterial Colonization and Endometrial MicroRNA-17-5p Levels in Association to Endometriosis: A Study in an Egyptian Population. Immunol. Investig. 2020, 49, 611–621. [Google Scholar] [CrossRef]
- Petracco, R.; De Oliveira Dias, A.C.; Taylor, H.; Petracco, Á.; Badalotti, M.; Michelon, J.D.R.; Marinowic, D.R.; Hentschke, M.; De Azevedo, P.N.; Zanirati, G.; et al. Evaluation of miR135a/b expression in endometriosis lesions. Biomed. Rep. 2019, 11, 181–187. [Google Scholar] [CrossRef]
- Vanhie, A.O.D.; Peterse, D.; Beckers, A.; Cuéllar, A.; Fassbender, A.; Meuleman, C.; Mestdagh, P.; D’Hooghe, T. Plasma miRNAs as biomarkers for endometriosis. Hum. Reprod. 2019, 34, 1650–1660. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, S.; Tang, L.; Wang, X.; Zhang, T.; Xia, X.; Fang, X. Downregulated circular RNA hsa_circ_0067301 regulates epithelial-mesenchymal transition in endometriosis via the miR-141/Notch signaling pathway. Biochem. Biophys. Res. Commun. 2019, 514, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Borisov, E.; Knyazeva, M.; Novak, V.; Zabegina, L.; Prisyazhnaya, T.; Karizkiy, A.; Berlev, I.; Malek, A. Analysis of Reciprocally Dysregulated miRNAs in Eutopic Endometrium Is a Promising Approach for Low Invasive Diagnostics of Adenomyosis. Diagnostics 2020, 10, 782. [Google Scholar] [CrossRef]
- da Silva, L.F.I.; Da Broi, M.G.; da Luz, C.M.; da Silva, L.E.C.M.; Ferriani, R.A.; Meola, J.; Navarro, P.A. miR-532-3p: A possible altered miRNA in cumulus cells of infertile women with advanced endometriosis. Reprod. Biomed. Online 2021, 42, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.-L.; Zhang, Z.; Fan, W.-S.; Li, L.-A.; Ye, M.-X.; Zhang, Q.; Zhang, N.-N.; Li, Z.; Meng, Y.-G. Identification of MicroRNAs as Potential Biomarkers in Ovarian Endometriosis. Reprod. Sci. 2020, 27, 1715–1723. [Google Scholar] [CrossRef]
- Mai, H.; Xu, H.; Lin, H.; Wei, Y.; Yin, Y.; Huang, Y.; Huang, S.; Liao, Y. LINC01541 Functions as a ceRNA to Modulate the Wnt/β-Catenin Pathway by Decoying miR-506-5p in Endometriosis. Reprod. Sci. 2021, 28, 665–674. [Google Scholar] [CrossRef]
- Moustafa, S.; Burn, M.; Mamillapalli, R.; Nematian, S.; Flores, V.; Taylor, H.S. Accurate diagnosis of endometriosis using serum microRNAs. Am. J. Obstet. Gynecol. 2020, 223, 557.e1–557.e11. [Google Scholar] [CrossRef]
- Papari, E.; Noruzinia, M.; Kashani, L.; Foster, W.G. Identification of candidate microRNA markers of endometriosis with the use of next-generation sequencing and quantitative real-time polymerase chain reaction. Fertil. Steril. 2020, 113, 1232–1241. [Google Scholar] [CrossRef]
- Razi, M.H.; Eftekhar, M.; Ghasemi, N.; Sheikhha, M.H.; Firoozabadi, A.D. Expression levels of circulatory mir-185-5p, vascular endothelial growth factor, and platelet-derived growth factor target genes in endometriosis. Int. J. Reprod. Biomed. 2020, 18, 347–358. [Google Scholar] [CrossRef]
- Wu, J.; Huang, H.; Huang, W.; Wang, L.; Xia, X.; Fang, X. Analysis of exosomal lncRNA, miRNA and mRNA expression profiles and ceRNA network construction in endometriosis. Epigenomics 2020, 12, 1193–1213. [Google Scholar] [CrossRef]
- Zhang, L.; Li, H.; Yuan, M.; Li, D.; Sun, C.; Wang, G. Serum Exosomal MicroRNAs as Potential Circulating Biomarkers for Endometriosis. Dis. Markers 2020, 2020, 2456340. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Chen, S.; Wang, D.; Yang, Q. LINC01116 promotes proliferation and migration of endometrial stromal cells by targeting FOXP1 via sponging miR-9-5p in endometriosis. J. Cell. Mol. Med. 2020, 25, 2000–2012. [Google Scholar] [CrossRef] [PubMed]
- Misir, S.; Hepokur, C.; Oksasoglu, B.; Yildiz, C.; Yanik, A.; Aliyazicioglu, Y. Circulating serum miR-200c and miR-34a-5p as diagnostic biomarkers for endometriosis. J. Gynecol. Obstet. Hum. Reprod. 2021, 50, 102092. [Google Scholar] [CrossRef] [PubMed]
- Pokrovenko, D.A.; Vozniuk, V.; Medvediev, M.V. MicroRNA let-7: A promising non-invasive biomarker for diagnosing and treating external genital endometriosis. J. Turk. Soc. Obstet. Gynecol. 2021, 18, 291–297. [Google Scholar] [CrossRef]
- Wang, D.; Cui, L.; Yang, Q.; Wang, J. Circular RNA circZFPM2 promotes epithelial-mesenchymal transition in endometriosis by regulating miR-205-5p/ZEB1 signalling pathway. Cell. Signal. 2021, 87, 110145. [Google Scholar] [CrossRef]
- Wu, J.; Fang, X.; Huang, H.; Huang, W.; Wang, L.; Xia, X. Construction and topological analysis of an endometriosis-related exosomal circRNA-miRNA-mRNA regulatory network. Aging 2021, 13, 12607–12630. [Google Scholar] [CrossRef] [PubMed]
- Zafari, N.; Bahramy, A.; Zolbin, M.M.; Allahyari, S.E.; Farazi, E.; Hassannejad, Z.; Yekaninejad, M.S. microRNAs as novel diagnostic biomarkers in endometriosis patients: A systematic review and meta-analysis. Expert Rev. Mol. Diagn. 2022, 22, 479–495. [Google Scholar] [CrossRef]
- Bao, Q.; Zheng, Q.; Wang, S.; Tang, W.; Zhang, B. LncRNA HOTAIR regulates cell invasion and migration in endometriosis through miR-519b-3p/PRRG4 pathway. Front. Oncol. 2022, 12, 953055. [Google Scholar] [CrossRef]
- Bendifallah, S.; Dabi, Y.; Suisse, S.; Delbos, L.; Poilblanc, M.; Descamps, P.; Golfier, F.; Jornea, L.; Bouteiller, D.; Touboul, C.; et al. Endometriosis Associated-miRNome Analysis of Blood Samples: A Prospective Study. Diagnostics 2022, 12, 1150. [Google Scholar] [CrossRef]
- Dabi, Y.; Suisse, S.; Jornea, L.; Bouteiller, D.; Touboul, C.; Puchar, A.; Daraï, E.; Bendifallah, S. Clues for Improving the Pathophysiology Knowledge for Endometriosis Using Serum Micro-RNA Expression. Diagnostics 2022, 12, 175. [Google Scholar] [CrossRef]
- He, S.; Jing, L.; Ma, D.; Liu, Z.; Lv, N. MicroRNA-148a targets ADAMTS5 to inhibit proliferation of endometriosis cells. Pak. J. Pharm. Sci. 2022, 35, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhu, L.; Li, H.; Ye, J.; Lin, N.; Chen, M.; Pan, D.; Chen, Z. Endometriosis derived exosomal miR-301a-3p mediates macrophage polarization via regulating PTEN-PI3K axis. Biomed. Pharmacother. 2022, 147, 112680. [Google Scholar] [CrossRef] [PubMed]
- Iurova, I.M.; Eldarov, E.C.; Bobrov, B.M.; Khabas, K.G.; Pavlovich, P.S. Expression of exosomal microRNA in high-grade ovarian cancer and ovarian endometriotic cysts. Obstet. Gynecol. 2022, 3, 68–79. [Google Scholar] [CrossRef]
- Jaafar, S.O.; Jaffar, J.O.; Ibrahim, S.A.; Jarjees, K.K. MicroRNA Variants miR-27a rs895819 and miR-423 rs6505162, but not miR-124-1 rs531564, are Linked to Endometriosis and its Severity. Br. J. Biomed. Sci. 2022, 79, 10207. [Google Scholar] [CrossRef]
- Nai, M.; Zhang, Y.; Li, L.; Jin, Y.; Li, Y.; Wang, L.; Ren, C. Effects of miR-363 on the Biological Activities of Eutopic Endometrial Stromal Cells in Endometriosis. BioMed. Res. Int. 2022, 2022, 7663379. [Google Scholar] [CrossRef] [PubMed]
- Barnabas, G.D.; Bahar-Shany, K.; Sapoznik, S.; Helpman, L.; Kadan, Y.; Beiner, M.; Weitzner, O.; Arbib, N.; Korach, J.; Perri, T.; et al. Microvesicle Proteomic Profiling of Uterine Liquid Biopsy for Ovarian Cancer Early Detection. Mol. Cell. Proteom. 2019, 18, 865–875. [Google Scholar] [CrossRef]
- Dabi, Y.; Suisse, S.; Puchar, A.; Delbos, L.; Poilblanc, M.; Descamps, P.; Haury, J.; Golfier, F.; Jornea, L.; Bouteiller, D.; et al. Endometriosis-associated infertility diagnosis based on saliva microRNA signatures. Reprod. Biomed. Online 2023, 46, 138–149. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).