The Interaction Effect of the Design Parameters on the Water Absorption of the Hemp-Reinforced Biocarbon-Filled Bio-Epoxy Composites
Abstract
1. Introduction
2. Results and Discussion
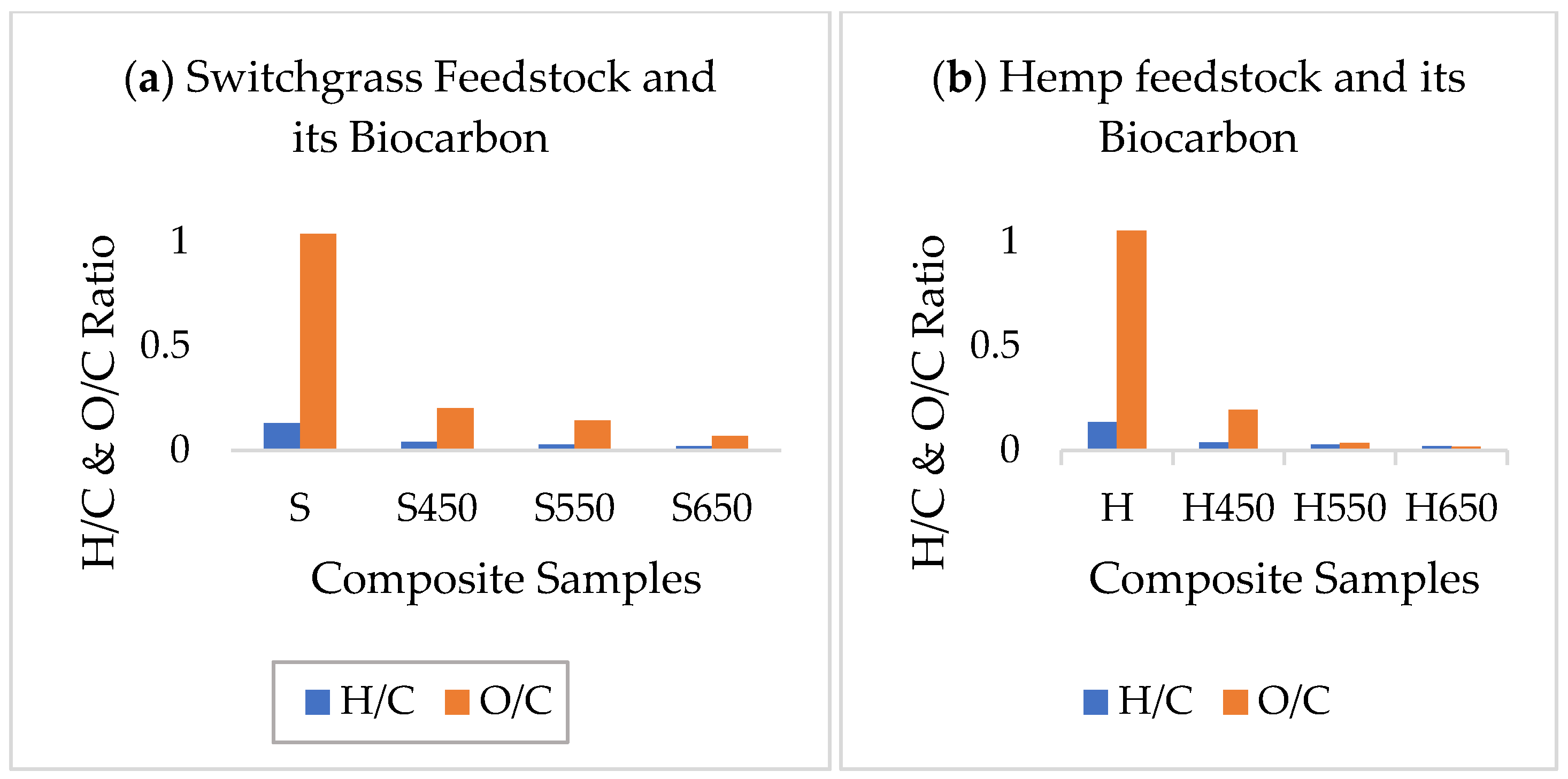
2.1. Biocarbon Characterization
2.1.1. Physiochemical Analyses of Biocarbon
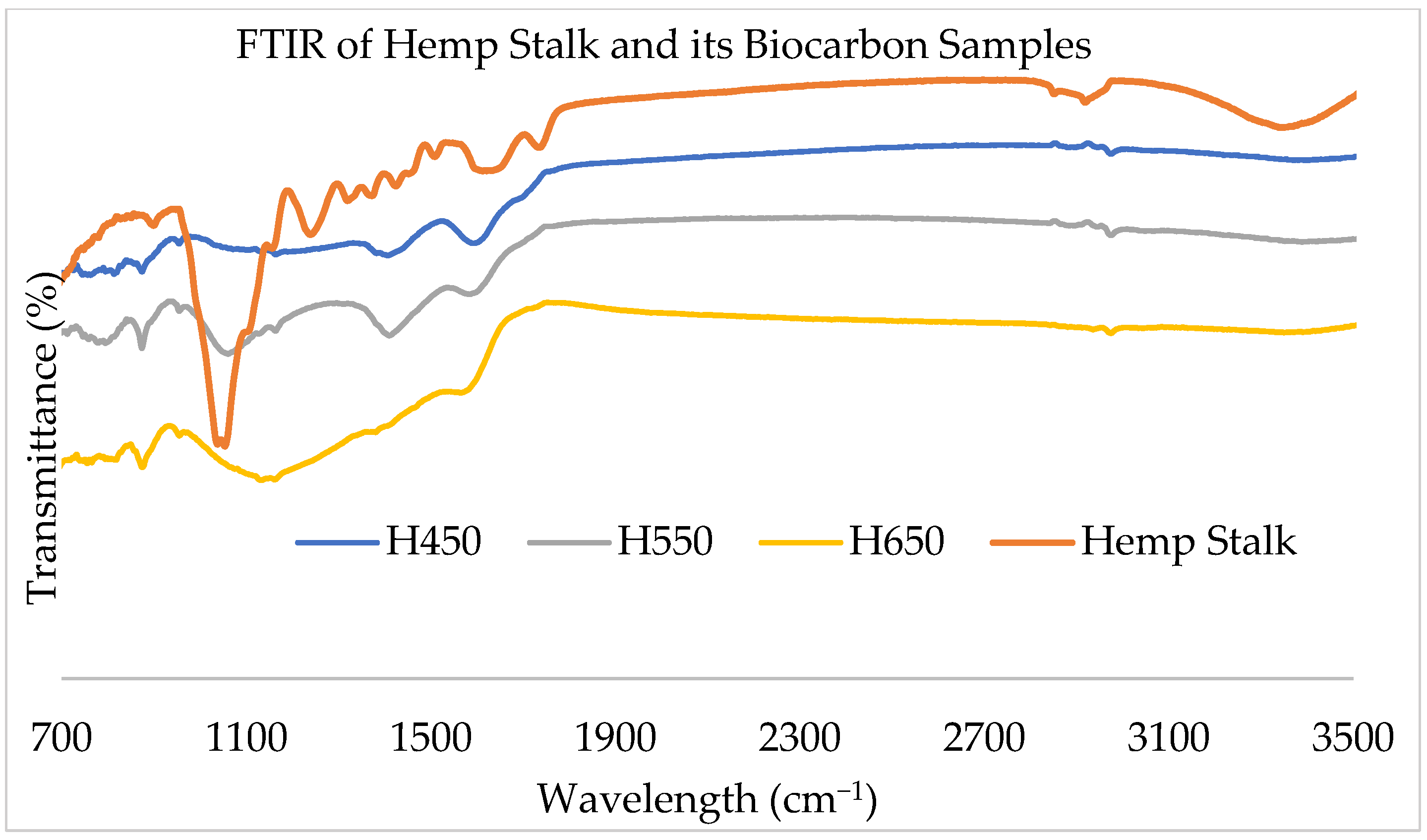
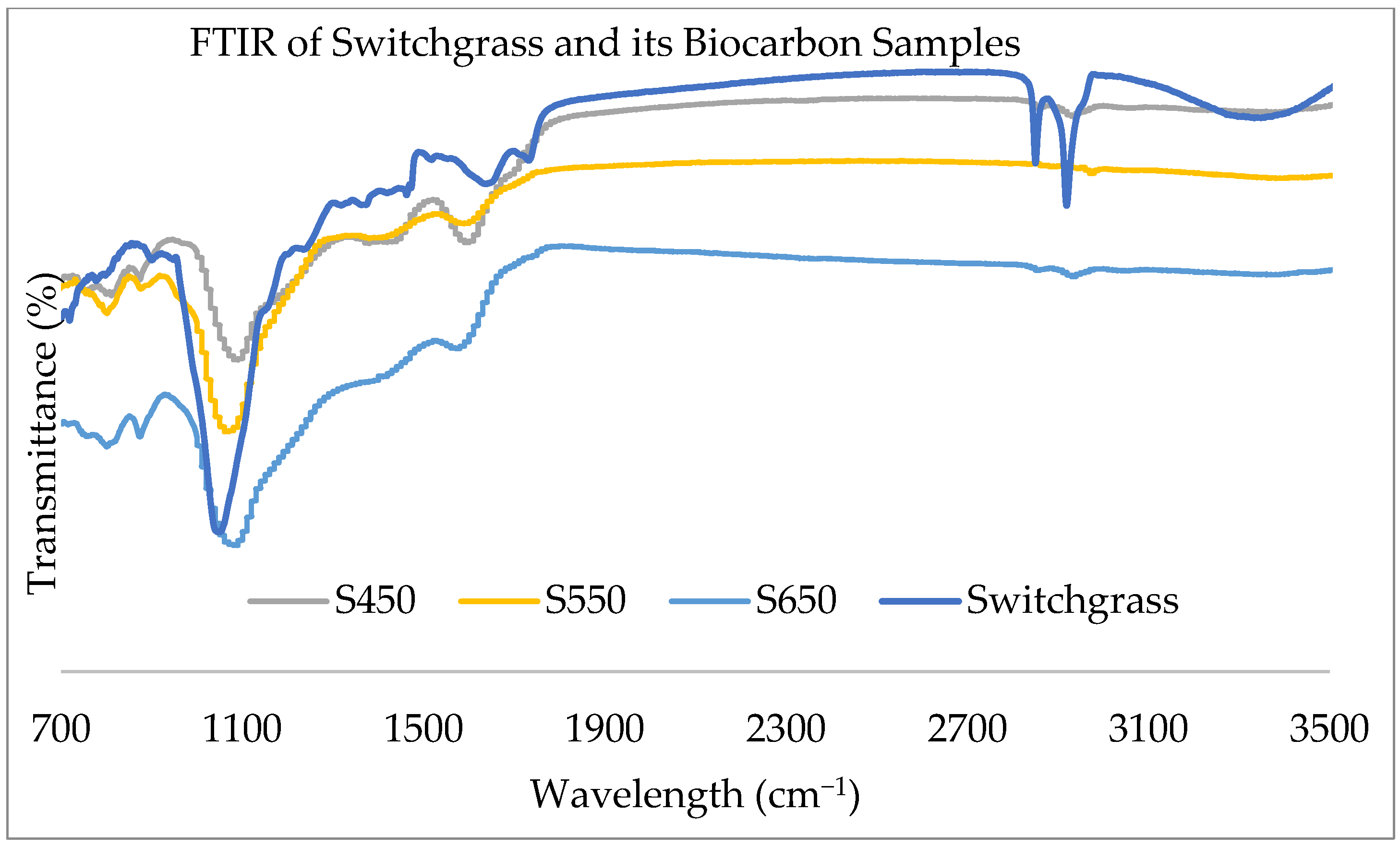
2.1.2. FTIR Analysis
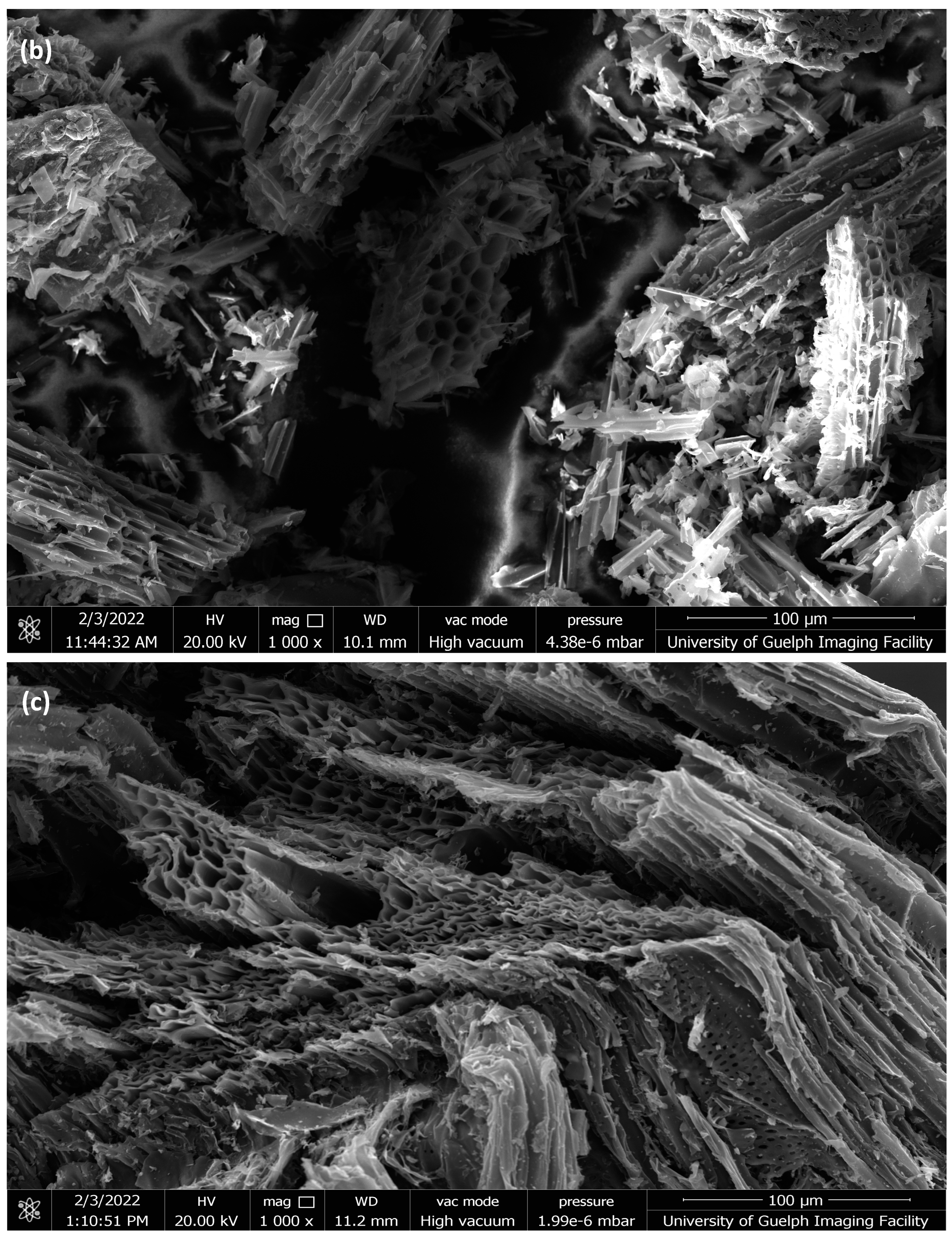
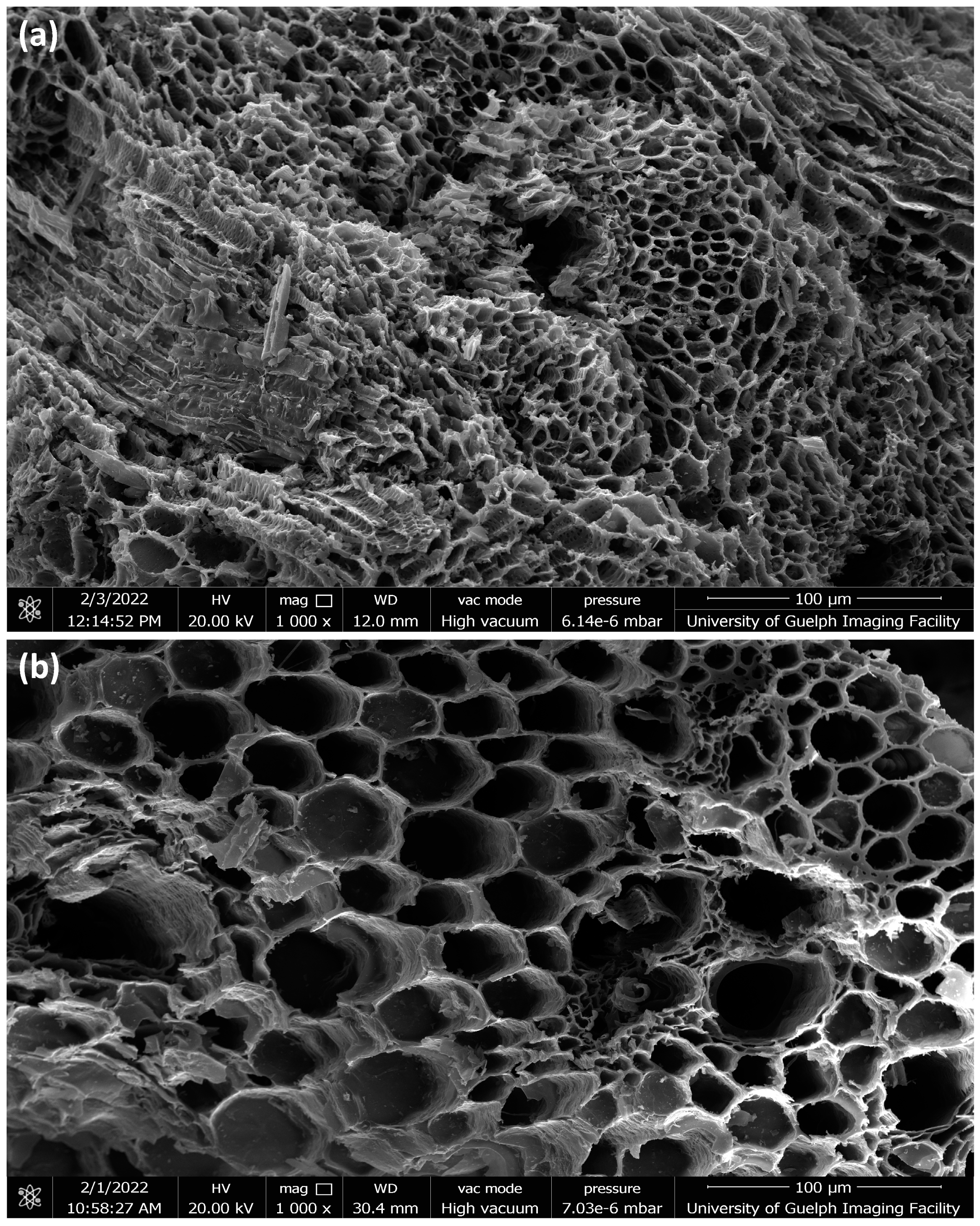
2.1.3. SEM Analysis
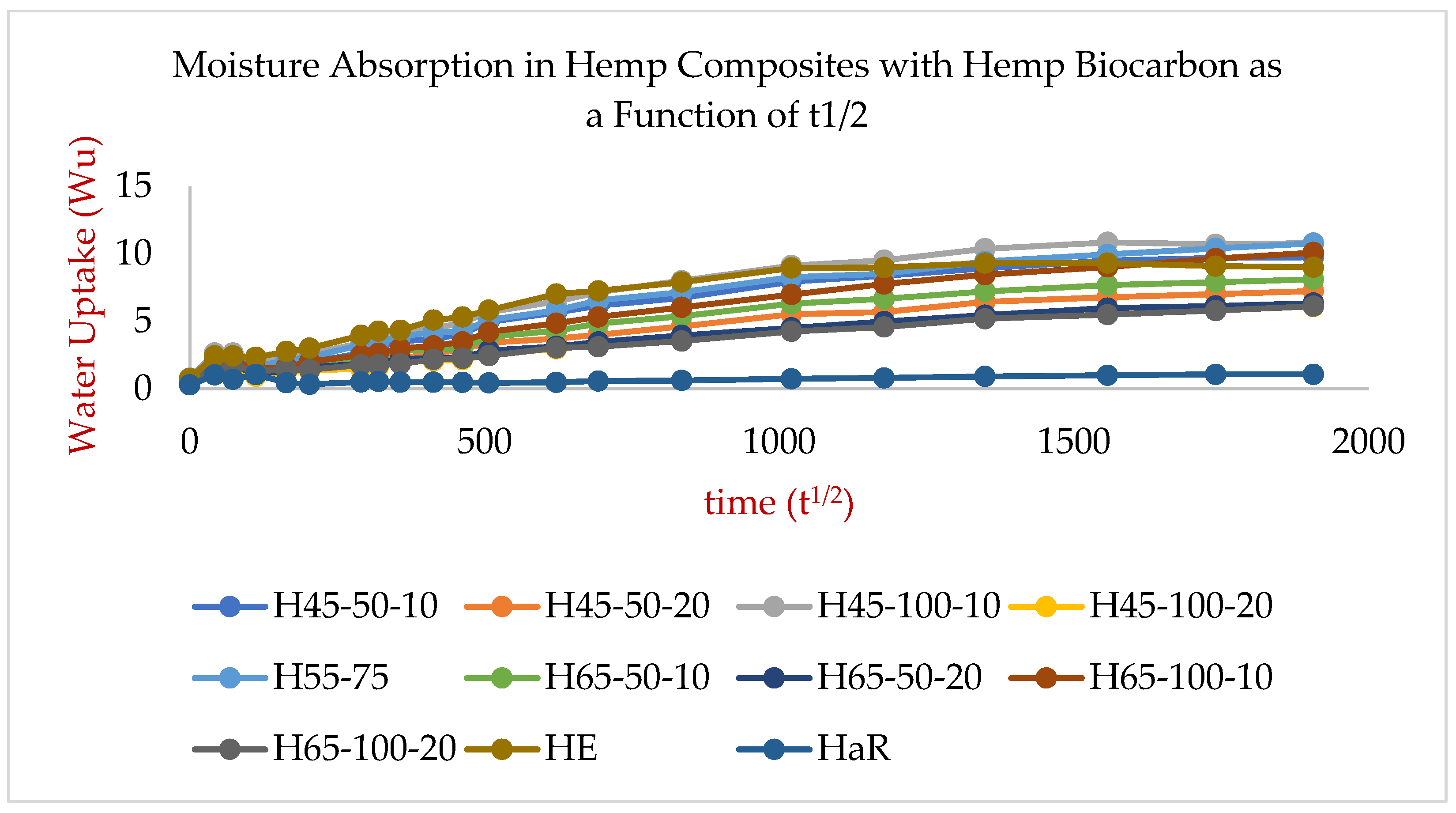
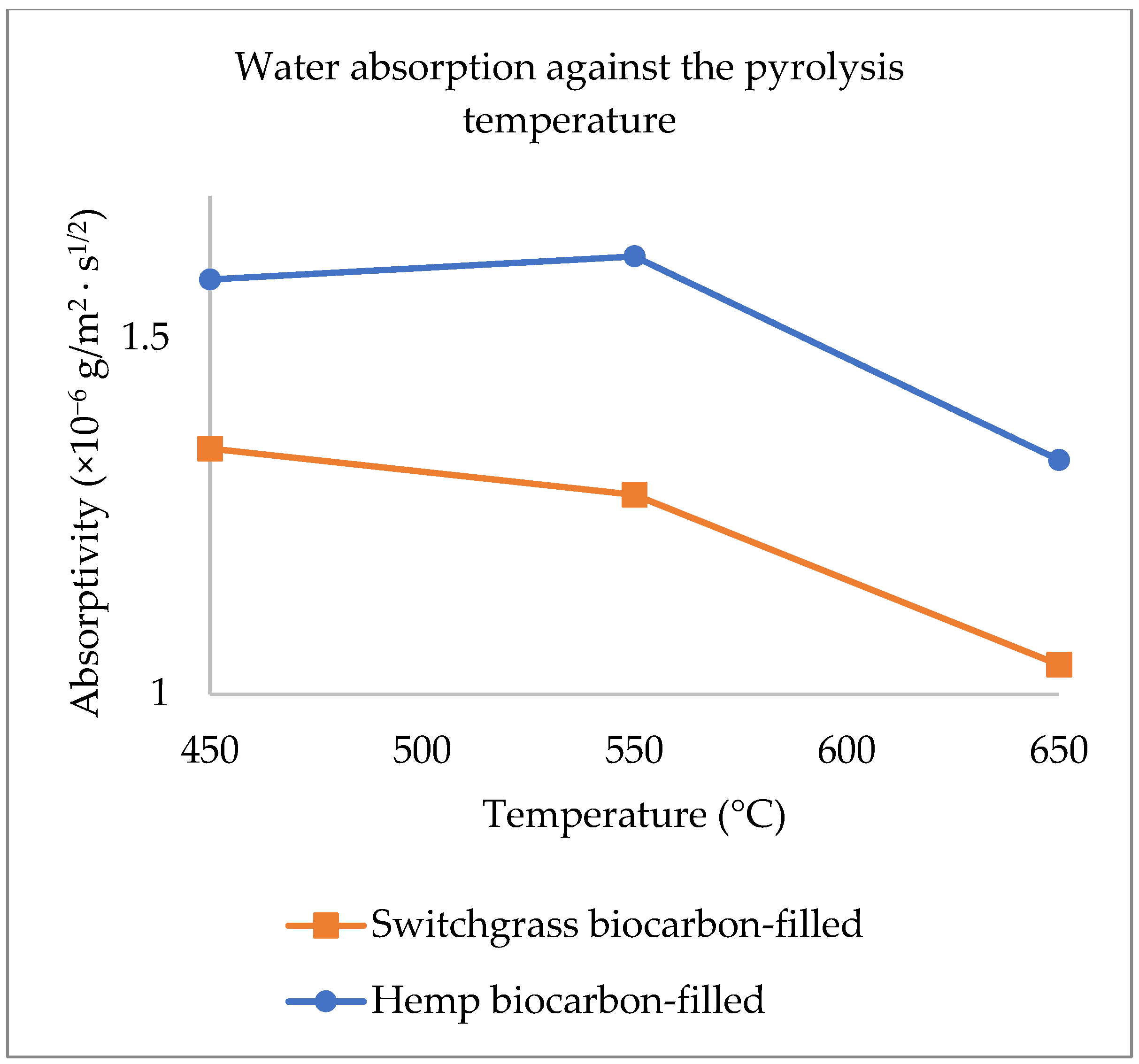
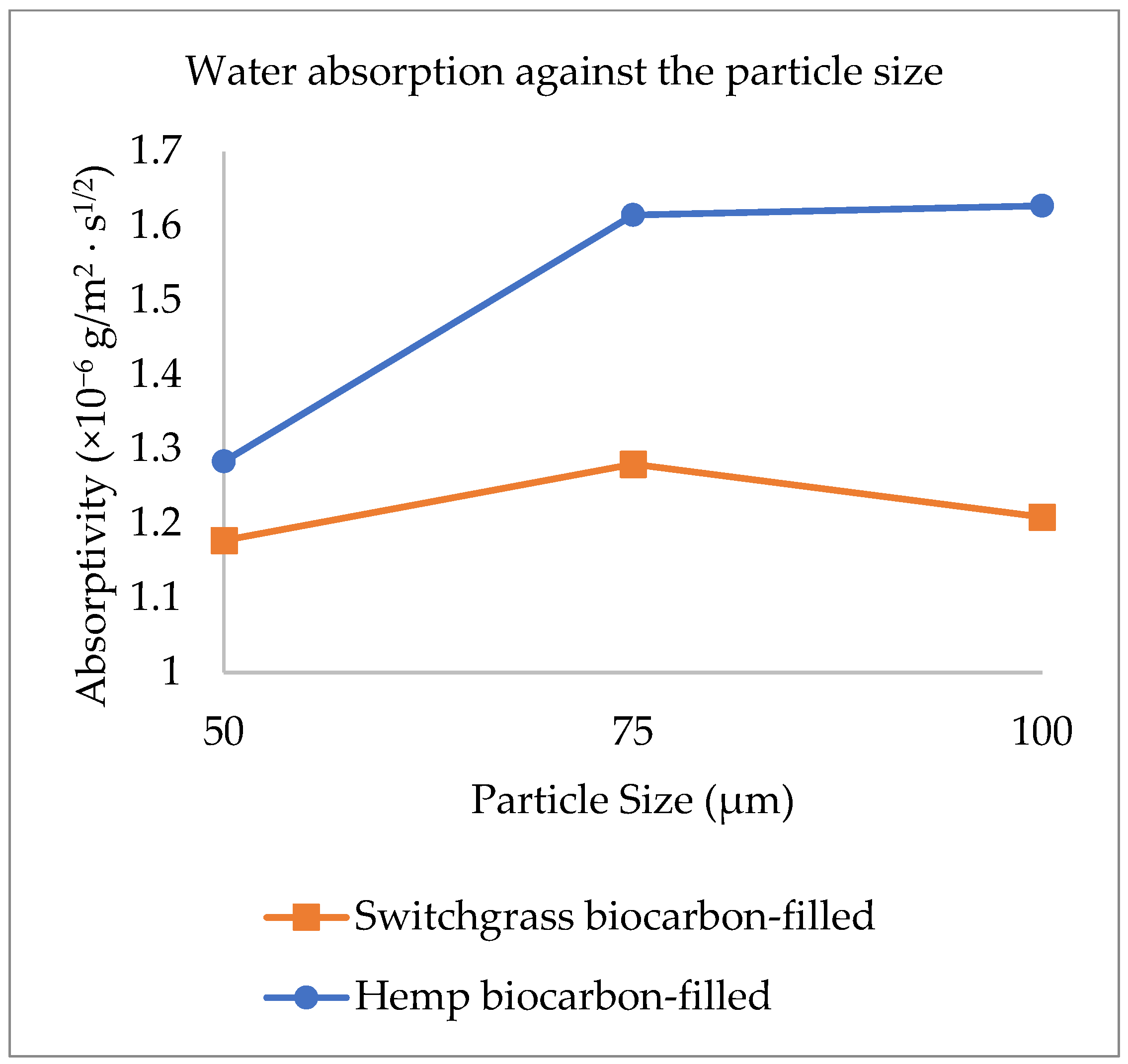
2.2. Explanations on the Composites’ Water Absorptivity and Diffusivity
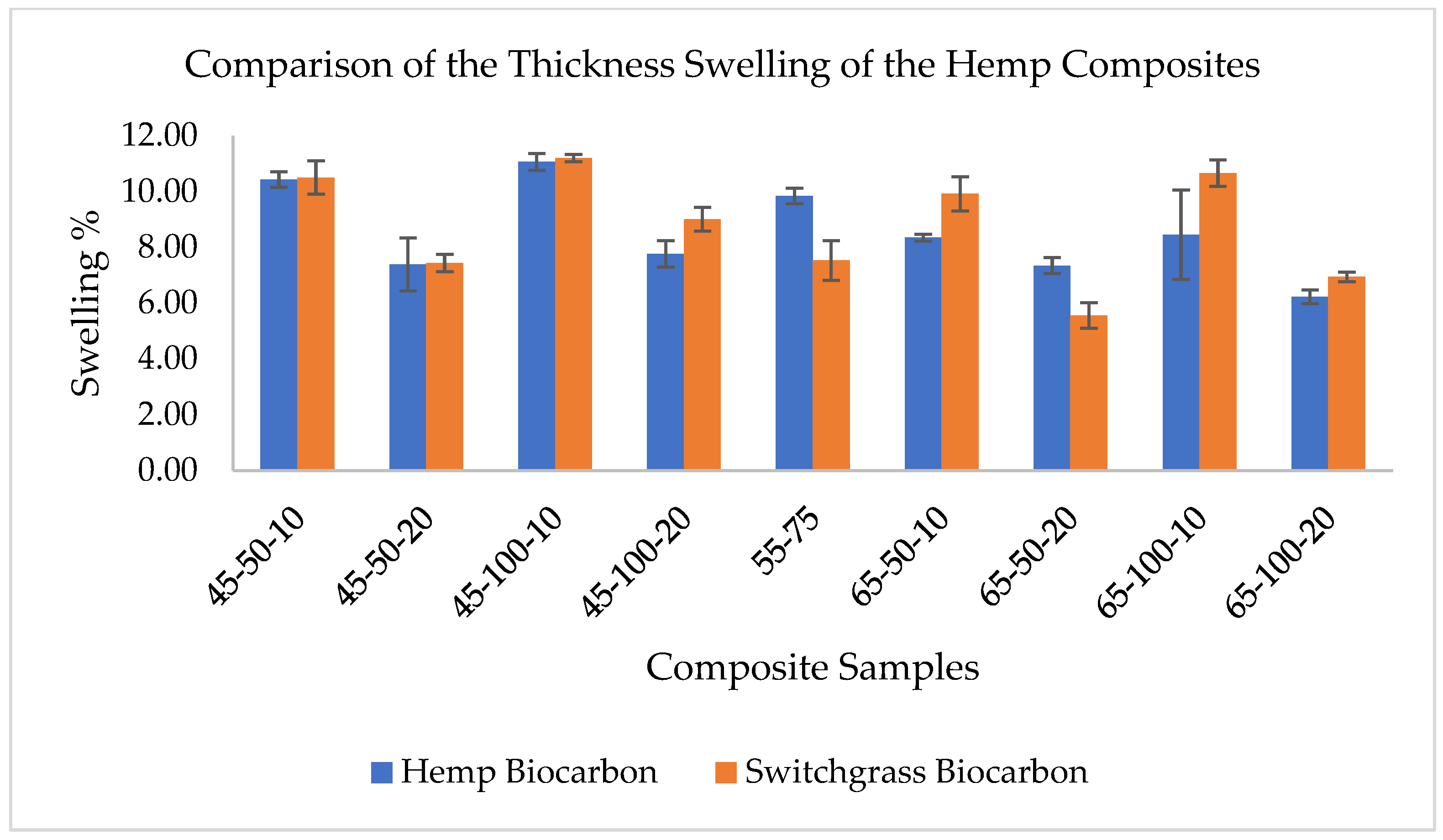
2.3. Water Absorption Effect on the Thickness Swelling of the Composites
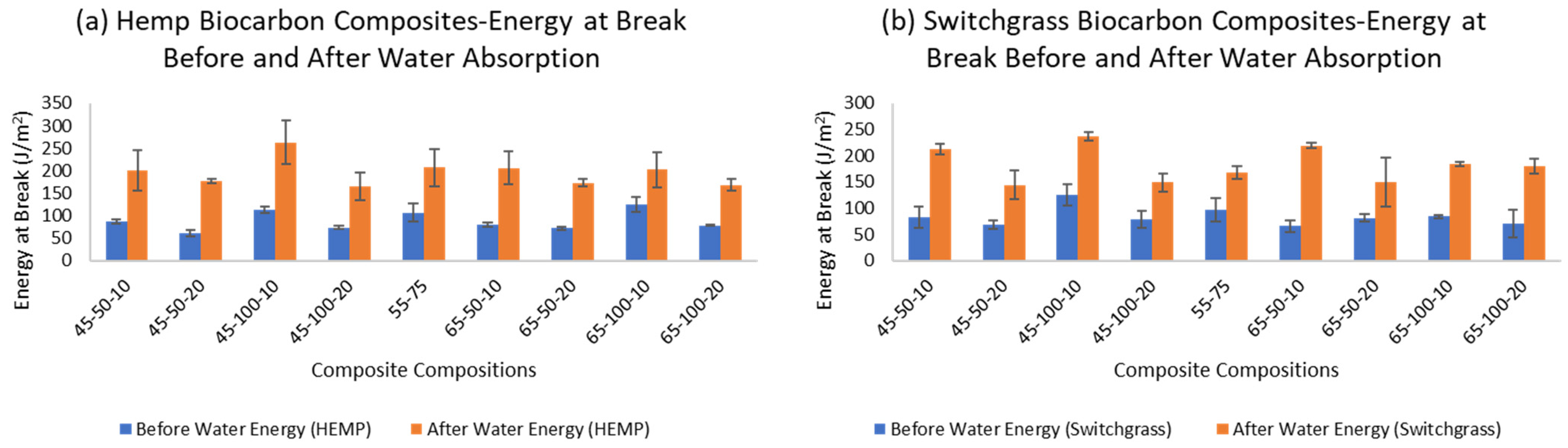
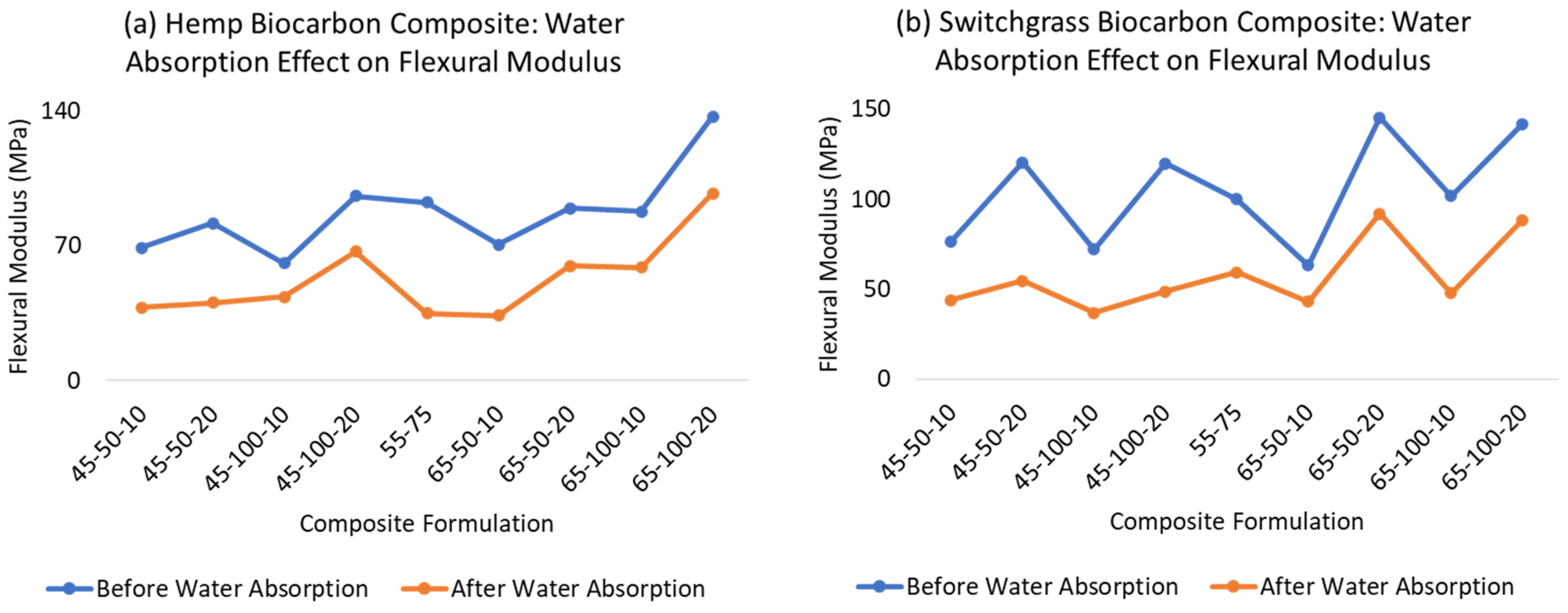
2.4. Water Absorption Effect on the Mechanical Properties of the Composite Materials
2.5. Biocarbon-Filled Composites’ Water Absorptivity Design of Experiments:
2.5.1. Biocarbon-Filled Composites Design of Experiments Based on Water Absorptivity
2.5.2. Statistical Analysis for the Biocarbon-Filled Hemp-Reinforced Biopolymer Composites
2.5.3. Model Adequacy Check
2.5.4. Response Surfaces and Contour Plots of the Biocarbon-Filled Hemp Reinforced Biopolymer Composites
2.5.5. Optimization of the Hemp Composites for the Water Absorptivity
3. Materials and Methods
3.1. Biocarbon and Biocomposite Samples Preparation
3.2. Physiochemical Analyses
3.3. Water Absorption Behaviour
3.4. Tensile Properties
3.5. Flexural Test
3.6. Composites’ Swelling Behaviour
3.7. Fourier Transform IR
3.8. Scanning Electron Microscopy
3.9. Experimental Design and Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tuzi, N.; Fraser, S.; Pietka, C. National Waste Characterization Report: The Composition of Canadian Residual Municipal Solid Waste. 2020. Available online: https://publications.gc.ca/site/archivee-archived.html?url=https://publications.gc.ca/collections/collection_2020/eccc/en14/En14-405-2020-eng.pdf (accessed on 7 March 2023).
- Khoshnava, S.; Rostami, R.; Zin, R.M.; Štreimikienė, D.; Mardani, A.; Ismail, M. The Role of Green Building Materials in Reducing Environmental and Human Health Impacts. Int. J. Environ. Res. Public Health 2020, 17, 2589. [Google Scholar] [CrossRef]
- Patel, P.; Patel, A. Use of sustainable green materials in construction of green buildings for sustainable development. IOP Conf. Ser. Earth Environ. Sci. 2021, 785, 012009. [Google Scholar] [CrossRef]
- Franzoni, E. Materials Selection for Green Buildings: Which Tools for Engineers and Architects? Procedia Eng. 2011, 21, 883–890. [Google Scholar] [CrossRef]
- Ncube, L.; Ude, A.; Ogunmuyiwa, E.; Zulkifli, R.; Beas, I. Environmental Impact of Food Packaging Materials: A Review of Contemporary Development from Conventional Plastics to Polylactic Acid Based Materials. Materials 2020, 13, 4994. [Google Scholar] [CrossRef]
- Yadav, M.; Agarwal, M. Biobased building materials for sustainable future: An overview. Mater. Today Proc. 2021, 43, 2895–2902. [Google Scholar] [CrossRef]
- Dabade, B.; Reddy, G.; Rajesham, S.; Kiran, C. Effect of Fiber Length and Fiber Weight Ratio on Tensile Properties of Sun hemp and Palmyra Fiber Reinforced Polyester Composites. J. Reinf. Plast. Compos. 2006, 25, 1733–1738. [Google Scholar] [CrossRef]
- Liu, M.; Thygesen, A.; Summerscales, J.; Meyer, A. Targeted pre-treatment of hemp bast fibres for optimal performance in biocomposite materials: A review. Ind. Crop. Prod. 2017, 108, 660–683. [Google Scholar] [CrossRef]
- Eberle, C.; Shortnacy, L. Sunn hemp planting date effect on growth, biomass accumulation, and nutritive value in southeastern Wyoming. Crop. Sci. 2021, 61, 4447–4457. [Google Scholar] [CrossRef]
- Ranalli, P. Advances in Hemp Research; CRC Press: Boca Raton, FL, USA, 1999. [Google Scholar]
- Jawaid, M.; Abdul Khalil, H.P.S.; Noorunnisa Khanam, P.; Abu Bakar, A. Hybrid Composites Made from Oil Palm Empty Fruit Bunches/Jute Fibres: Water Absorption, Thickness Swelling and Density Behaviours. J. Polym. Environ. 2011, 19, 106–109. [Google Scholar] [CrossRef]
- Yang, H.-S.; Kim, H.-J.; Park, H.-J.; Lee, B.-J.; Hwang, T.-S. Water absorption behavior and mechanical properties of lignocellulosic filler–polyolefin bio-composites. Compos. Struct. 2006, 72, 429–437. [Google Scholar] [CrossRef]
- Panthapulakkal, S.; Sain, M. Studies on the Water Absorption Properties of Short Hemp—Glass Fiber Hybrid Polypropylene Composites. J. Compos. Mater. 2007, 41, 1871–1883. [Google Scholar] [CrossRef]
- Shahzad, A. Effects of water absorption on mechanical properties of hemp fiber composites. Polym. Compos. 2012, 33, 120–128. [Google Scholar] [CrossRef]
- Dahal, R.; Acharya, B.; Dutta, A. Mechanical; Thermal, and Acoustic Properties of Hemp and Biocomposite Materials: A Review. J. Compos. Sci. 2022, 6, 373. [Google Scholar] [CrossRef]
- Dahal, R.; Acharya, B.; Dony, A. Dutta, Biocarbon Filled Hemp-Epoxy Composite: Its Physical, Mechanical, and Thermal Properties. Appl. Compos. Mater. 2022, 29, 2185–2202. [Google Scholar] [CrossRef]
- Brechtl, J.; Li, Y.; Li, K.; Kearney, L.; Nawaz, K.; Flores-Betancourt, A.; Thompson, M.; Rios, O.; Momen, A. Structural, Thermal, and Mechanical Characterization of a Thermally Conductive Polymer Composite for Heat Exchanger Applications. Polymers 2021, 13, 1970. [Google Scholar] [CrossRef]
- Chandramohan, D.; Kumar, A.P. Experimental data on the properties of natural fiber particle reinforced polymer composite material. Data Brief. 2017, 13, 460–468. [Google Scholar] [CrossRef]
- Abdellatef, Y.; Khan, M.; Khan, A.; Alam, M.; Kavgic, M. Mechanical, Thermal, and Moisture Buffering Properties of Novel Insulating Hemp-Lime Composite Building Materials. Materials 2020, 13, 5000. [Google Scholar] [CrossRef]
- Minugu, O.; Gujjala, R.; Shakuntala, O.; Manoj, P.; Chowdary, M. Effect of biomass derived biochar materials on mechanical properties of biochar epoxy composites. Proc. Inst. Mech. Eng. C J. Mech. Eng. Sci. 2021, 235, 5626–5638. [Google Scholar] [CrossRef]
- Dahal, R.; Acharya, B.; Saha, G.; Bissessur, R.; Dutta, A.; Farooque, A. Biochar as a filler in glassfiber reinforced composites: Experimental study of thermal and mechanical properties. Compos. B Eng. 2019, 175, 107169. [Google Scholar] [CrossRef]
- Suarez-Riera, D.; Restuccia, L.; Ferro, G. The use of Biochar to reduce the carbon footprint of cement-based materials. Procedia Struct. Integr. 2020, 26, 199–210. [Google Scholar] [CrossRef]
- Khitab, A.; Ahmad, S.; Khan, R.; Arshad, M.; Anwar, W.; Tariq, J.; Khan, A.; Khan, R.; Jalil, A.; Tariq, Z. Production of Biochar and Its Potential Application in Cementitious Composites. Crystals 2021, 11, 527. [Google Scholar] [CrossRef]
- Demirbas, A. Effects of temperature and particle size on bio-char yield from pyrolysis of agricultural residues. J. Anal. Appl. Pyrolysis 2004, 72, 243–248. [Google Scholar] [CrossRef]
- Muzyka, R.; Misztal, E.; Hrabak, J.; Banks, S.; Sajdak, M. Various biomass pyrolysis conditions influence the porosity and pore size distribution of biochar. Energy 2023, 263, 126128. [Google Scholar] [CrossRef]
- Yoo, S.; Kelley, S.; Tilotta, D.; Park, S. Structural Characterization of Loblolly Pine Derived Biochar by X-ray Diffraction and Electron Energy Loss Spectroscopy. ACS Sustain. Chem. Eng. 2018, 6, 2621–2629. [Google Scholar] [CrossRef]
- Bikbulatova, S.; Tahmasebi, A.; Zhang, Z.; Rish, S.; Yu, J. Understanding water retention behavior and mechanism in bio-char. Fuel Process. Technol. 2018, 169, 101–111. [Google Scholar] [CrossRef]
- Yousefi, E.; Shiri, M.; Rezaei, M.; Rezaei, S.; Band, S.; Mosavi, A. A novel long-term water absorption and thickness swelling deep learning forecast method for corn husk fiber-polypropylene composite. Case Stud. Constr. Mater. 2022, 17, e01268. [Google Scholar] [CrossRef]
- Sachin, K.O.; Maruthi, B.H.; Puttaswamaiah, S.; Satish, B. Effect of fillers on water and chemical absorption behavior of natural fiber reinforced epoxy composites. Int. J. Biosens. Bioelectron. 2021, 7, 69–72. [Google Scholar] [CrossRef]
- Gupta, S.; Kua, H.; Cynthia, S.T. Use of biochar-coated polypropylene fibers for carbon sequestration and physical improvement of mortar. Cem. Concr. Compos. 2017, 83, 171–187. [Google Scholar] [CrossRef]
- Qin, Y.; Pang, X.; Tan, K.; Bao, T. Evaluation of pervious concrete performance with pulverized biochar as cement replacement. Cem. Concr. Compos. 2021, 119, 104022. [Google Scholar] [CrossRef]
- Prabhu, P.; Jayabalakrishnan, D.; Balaji, V.; Bhaskar, K.; Maridurai, T.; Prakash, V. Mechanical, tribology, dielectric, thermal conductivity, and water absorption behaviour of Caryota urens woven fibre-reinforced coconut husk biochar toughened wood-plastic composite. Biomass Convers. Biorefin. 2022, 1–8. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, D.; Xu, H.; Lu, W.; Ren, X.; Cai, H.; Lei, H.; Huo, E.; Zhao, Y.; Qian, M.; et al. Biochar filled high-density polyethylene composites with excellent properties: Towards maximizing the utilization of agricultural wastes. Ind. Crop. Prod. 2020, 146, 112185. [Google Scholar] [CrossRef]
- Boateng, A.; Daugaard, D.; Goldberg, N.; Hicks, K. Bench-Scale Fluidized-Bed Pyrolysis of Switchgrass for Bio-Oil Production. Ind. Eng. Chem. Res. 2007, 46, 1891–1897. [Google Scholar] [CrossRef]
- Wallace, C.; Afzal, M.; Saha, G. Effect of feedstock and microwave pyrolysis temperature on physio-chemical and nano-scale mechanical properties of biochar. Bioresour. Bioprocess 2019, 6, 33. [Google Scholar] [CrossRef]
- Crombie, K.; Mašek, O.; Sohi, S.; Brownsort, P.; Cross, A. The effect of pyrolysis conditions on biochar stability as determined by three methods. GCB Bioenergy 2013, 5, 122–131. [Google Scholar] [CrossRef]
- Oleszczuk, P.; Ćwikła-Bundyra, W.; Bogusz, A.; Skwarek, E.; Ok, Y. Characterization of nanoparticles of biochars from different biomass. J. Anal. Appl. Pyrolysis 2016, 121, 165–172. [Google Scholar] [CrossRef]
- Xu, C.-Y.; Li, Q.-R.; Geng, Z.-C.; Hu, F.-N.; Zhao, S.-W. Surface properties and suspension stability of low-temperature pyrolyzed biochar nanoparticles: Effects of solution chemistry and feedstock sources. Chemosphere 2020, 259, 127510. [Google Scholar] [CrossRef]
- Keiluweit, M.; Nico, P.; Johnson, M.; Kleber, M. Dynamic Molecular Structure of Plant Biomass-Derived Black Carbon (Biochar). Environ. Sci. Technol. 2010, 44, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Sills, D.; Gossett, J. Using FTIR to predict saccharification from enzymatic hydrolysis of alkali-pretreated biomasses. Biotechnol. Bioeng. 2012, 109, 353–362. [Google Scholar] [CrossRef]
- Dai, D.; Fan, M. Characteristic and Performance of Elementary Hemp Fibre. Mater. Sci. Appl. 2010, 01, 336–342. [Google Scholar] [CrossRef]
- Kubo, S.; Kadla, J. Hydrogen Bonding in Lignin: A Fourier Transform Infrared Model Compound Study. Biomacromolecules 2005, 6, 2815–2821. [Google Scholar] [CrossRef]
- Asakawa, Y.; Ludwiczuk, A.; Nagashima, F. Phytochemical and biological studies of bryophytes. Phytochemistry 2013, 91, 52–80. [Google Scholar] [CrossRef]
- Cao, Z.; Wang, Z.; Shang, Z.; Zhao, J. Classification and identification of Rhodobryum roseum Limpr. and its adulterants based on fourier-transform infrared spectroscopy (FTIR) and chemometrics. PLoS ONE 2017, 12, e0172359. [Google Scholar] [CrossRef] [PubMed]
- Janu, R.; Mrlik, V.; Ribitsch, D.; Hofman, J.; Sedláček, P.; Bielská, L.; Soja, G. Biochar surface functional groups as affected by biomass feedstock, biochar composition and pyrolysis temperature. Carbon Resour. Convers. 2021, 4, 36–46. [Google Scholar] [CrossRef]
- Ramirez, F.; Luque, P.; Heredia, A.; Bukovac, M. Fourier transform IR study of enzymatically isolated tomato fruit cuticular membrane. Biopolymers 1992, 32, 1425–1429. [Google Scholar] [CrossRef]
- Zhuang, J.; Li, M.; Pu, Y.; Ragauskas, A.; Yoo, C. Observation of Potential Contaminants in Processed Biomass Using Fourier Transform Infrared Spectroscopy. Appl. Sci. 2020, 10, 4345. [Google Scholar] [CrossRef]
- Ouajai, S.; Shanks, R. Composition, structure and thermal degradation of hemp cellulose after chemical treatments. Polym. Degrad. Stab. 2005, 89, 327–335. [Google Scholar] [CrossRef]
- Himmelsbach, D.; Khalili, S.; Akin, D. The use of FT-IR microspectroscopic mapping to study the effects of enzymatic retting of flax (Linum usitatissimum L) stems. J. Sci. Food Agric. 2002, 82, 685–696. [Google Scholar] [CrossRef]
- Lin, D.; Pan, B.; Zhu, L.; Xing, B. Characterization and Phenanthrene Sorption of Tea Leaf Powders. J. Agric. Food Chem. 2007, 55, 5718–5724. [Google Scholar] [CrossRef]
- Nair, R.; Mondal, M.; Weichgrebe, D. Biochar from co-pyrolysis of urban organic wastes—Investigation of carbon sink potential using ATR-FTIR and TGA. Biomass Convers. Biorefin. 2022, 12, 4729–4743. [Google Scholar] [CrossRef]
- Abdel-Fattah, T.; Mahmoud, M.; Ahmed, S.; Huff, M.; Lee, J.; Kumar, S. Biochar from woody biomass for removing metal contaminants and carbon sequestration. J. Ind. Eng. Chem. 2015, 22, 103–109. [Google Scholar] [CrossRef]
- Schwarz, B. Capillary water absorption of building materials. Gesund. Ing. 1972, 93, 206–211. [Google Scholar]
- Krus, M. Moisture Transport and Storage Coefficients of Porous Mineral Building Materials: Theoretical Principles and New Test Methods; University of Stuttgart: Stuttgart, Germany, 1995. [Google Scholar]
- Marcovich, N.; Reboredo, M.; Aranguren, M. Moisture diffusion in polyester–woodflour composites. Polymer 1999, 40, 7313–7320. [Google Scholar] [CrossRef]
- Khazaei, J. Water absorption characteristics of three wood varieties. ALSE 2008, XLI, 134–145. [Google Scholar]
- Kumar, A.; Flynn, P. Uptake of fluids by boreal wood chips: Implications for bioenergy. Fuel Process. Technol. 2006, 87, 605–608. [Google Scholar] [CrossRef]
- Setyawan, P.; Martadinata, W.; Sugiman, S.; Salman, S.; Catur, A.; Zainuri, A. Water absorption and tensile properties of the hybrid of fly ash-CaCO3 /epoxy composites. IOP Conf. Ser. Mater. Sci. Eng. 2019, 694, 012037. [Google Scholar] [CrossRef]
- Tuna, S.; Keyf, F.; Gumus, H.; Uzun, C. The evaluation of water sorption/solubility on various acrylic resins. Eur. J. Dent. 2008, 2, 191–197. [Google Scholar] [CrossRef]
- Jasmi, N.; Kasim, J.; Ansar, M.; Maidin, I. The Role of Oil Palm (Elaeis guineensis) Frond as Filler in Polypropylene Matrix with Relation of Filler Loading and Particle Size Effects, Proceedings of the Regional Conference on Science, Technology and Social Sciences (RCSTSS 2014); Springer: Singapore, 2016; pp. 393–404. [Google Scholar] [CrossRef]
- Awad, S.; Jawaid, M.; Fouad, H.; Saba, N.; Dhakal, H.; Alothman, O.; Khalaf, E. A comparative assessment of chemical, mechanical, and thermal characteristics of treated oil palm/pineapple fiber/bio phenolic composites. Polym. Compos. 2022, 43, 2115–2128. [Google Scholar] [CrossRef]
- ASTM D3173; Standard Test Method for Moisture in the Analysis Sample of Coal and Coke. ASTM International (ASTM): Englewood, CO, USA, 2011.
- ASTM D3175-20; Standard Test Method for Volatile Matter in the Analysis Sample of Coal and Coke. ASTM International (ASTM): Englewood, CO, USA, 2020.
- ASTM E1755-01; Standard Test Method for Ash in Biomass. ASTM International (ASTM): Englewood, CO, USA, 2010.
- ASTM D570-98; Standard Test Method For Water Absorption Of Plastics. ASTM International (ASTM): Englewood, CO, USA, 2018.
- Sanjeevi, S.; Shanmugam, V.; Kumar, S.; Ganesan, V.; Sas, G.; Johnson, D.; Shanmugam, M.; Ayyanar, A.; Naresh, K.; Neisiany, R.; et al. Effects of water absorption on the mechanical properties of hybrid natural fibre/phenol formaldehyde composites. Sci. Rep. 2021, 11, 13385. [Google Scholar] [CrossRef]
- ASTM D790-17; Standard Test Methods for Flexural Properties of Unreinforced and Reinforced Plastics and Electrical Insulating Materials. ASTM International (ASTM): Englewood, CO, USA, 2017.



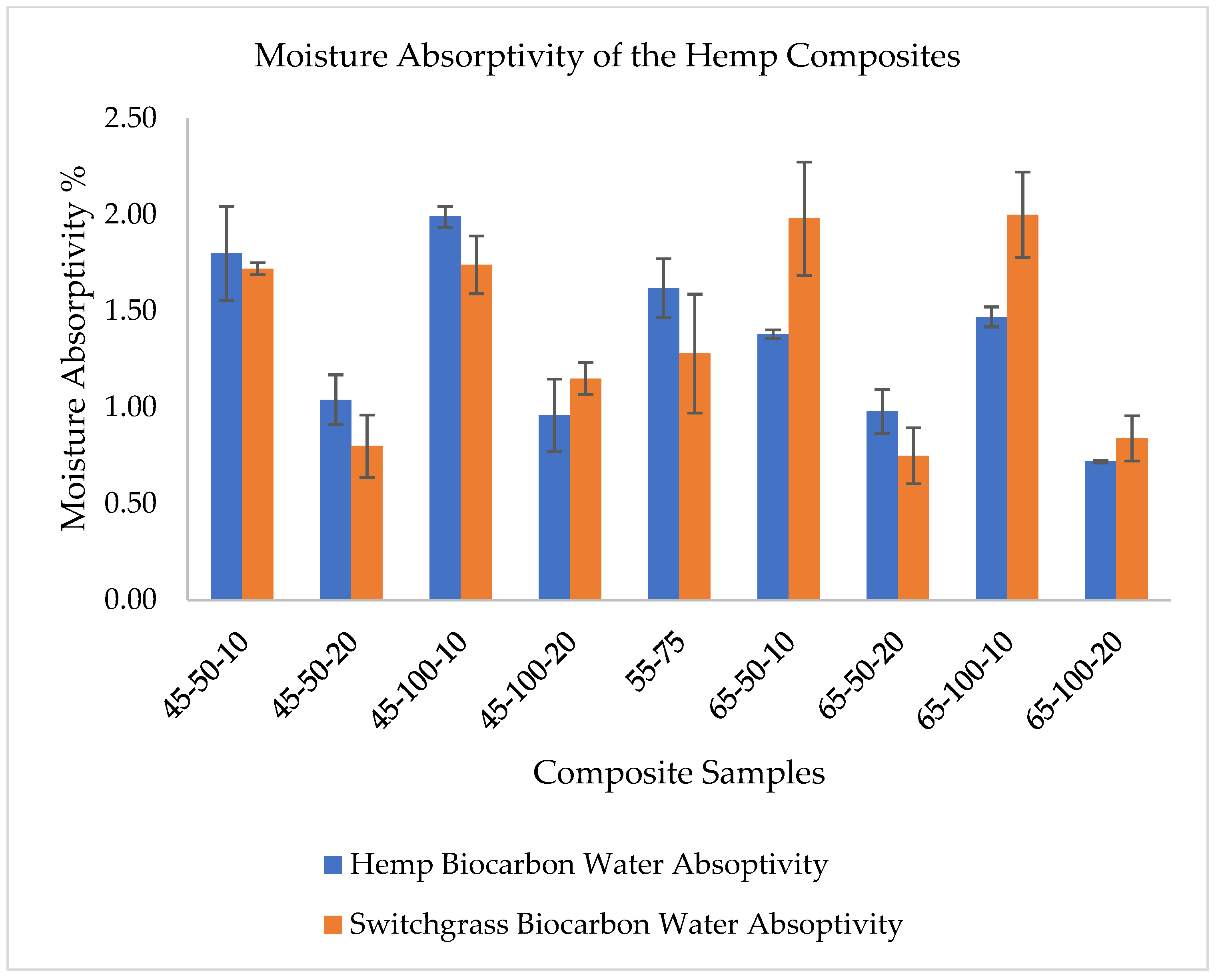
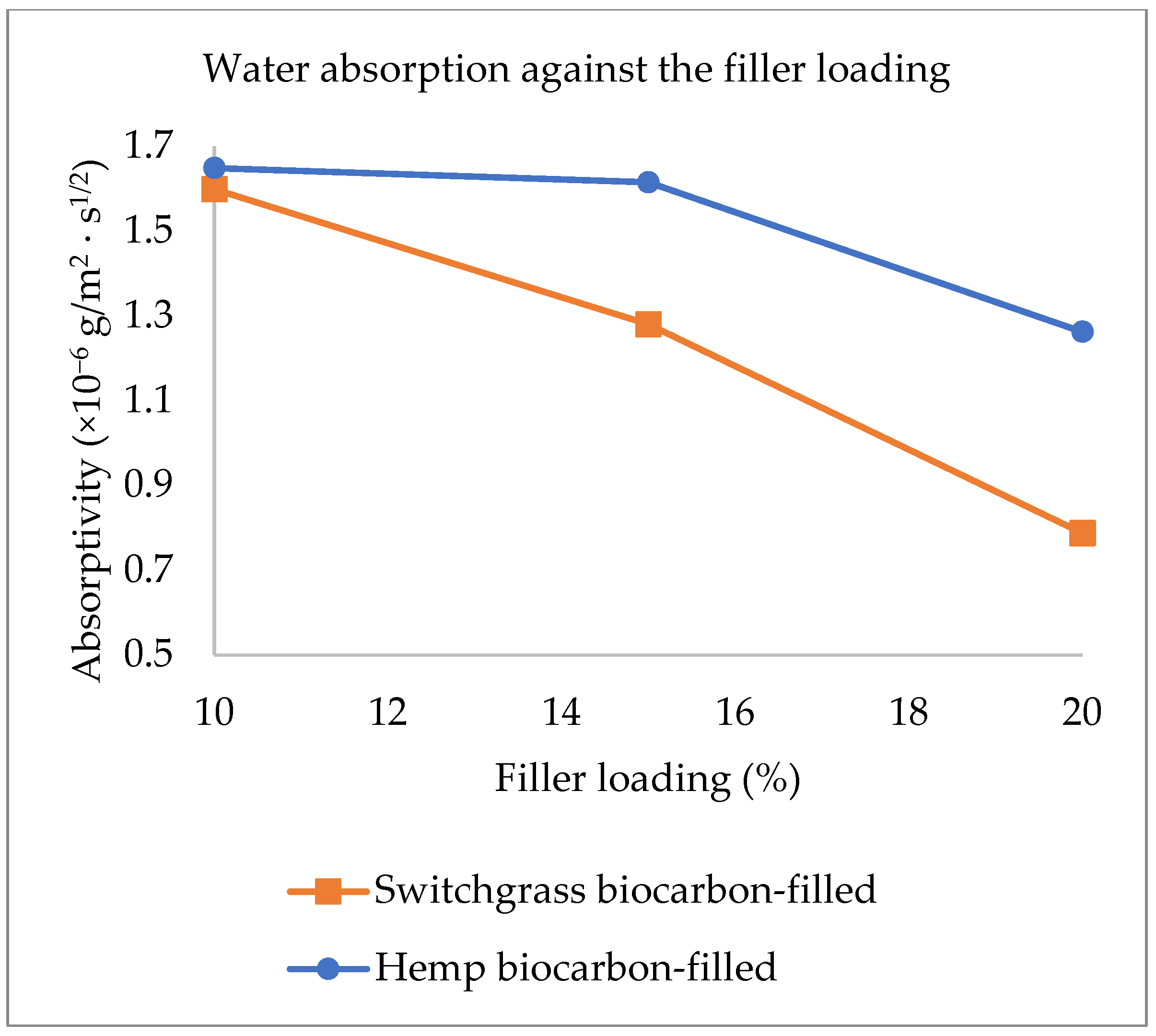
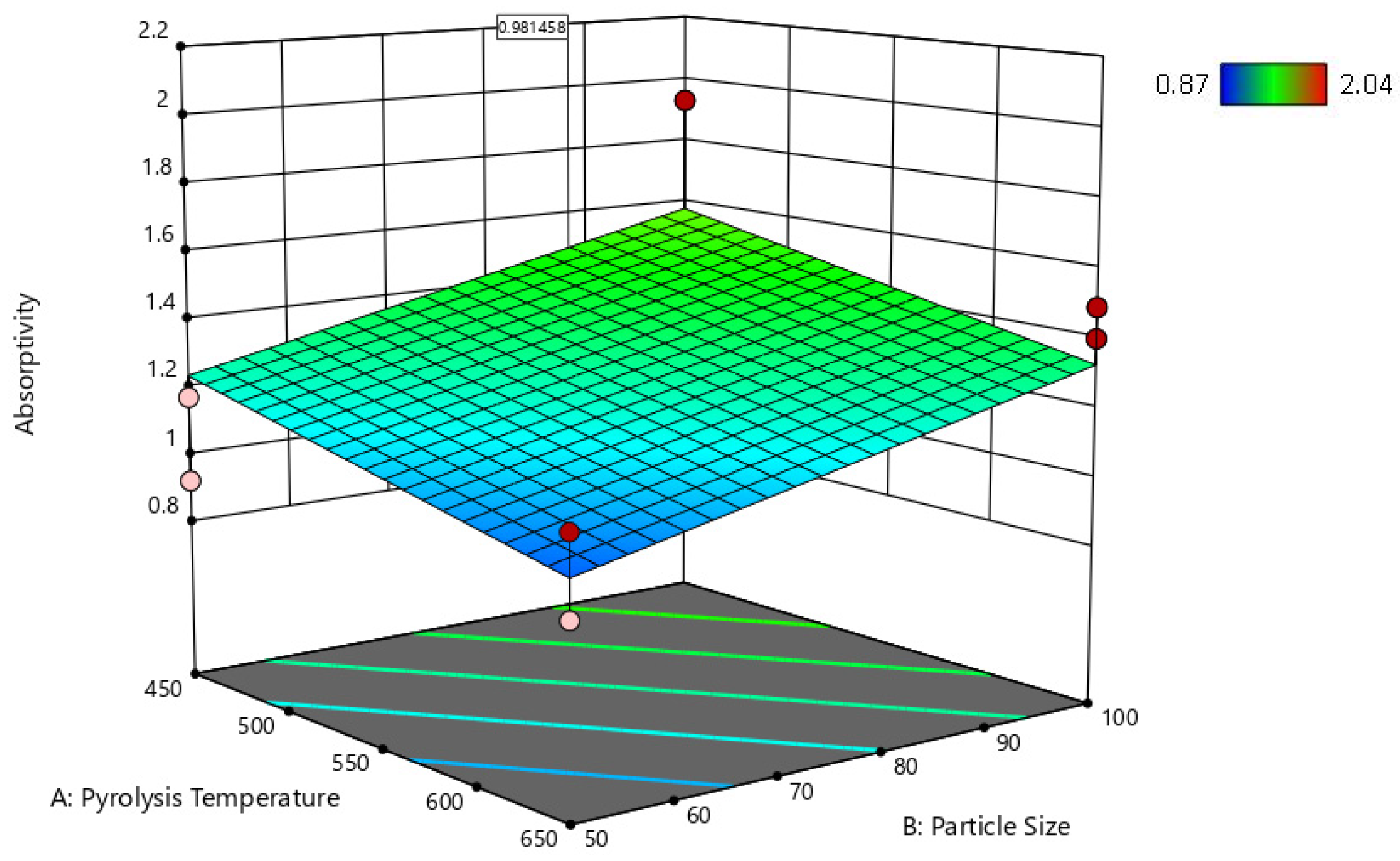
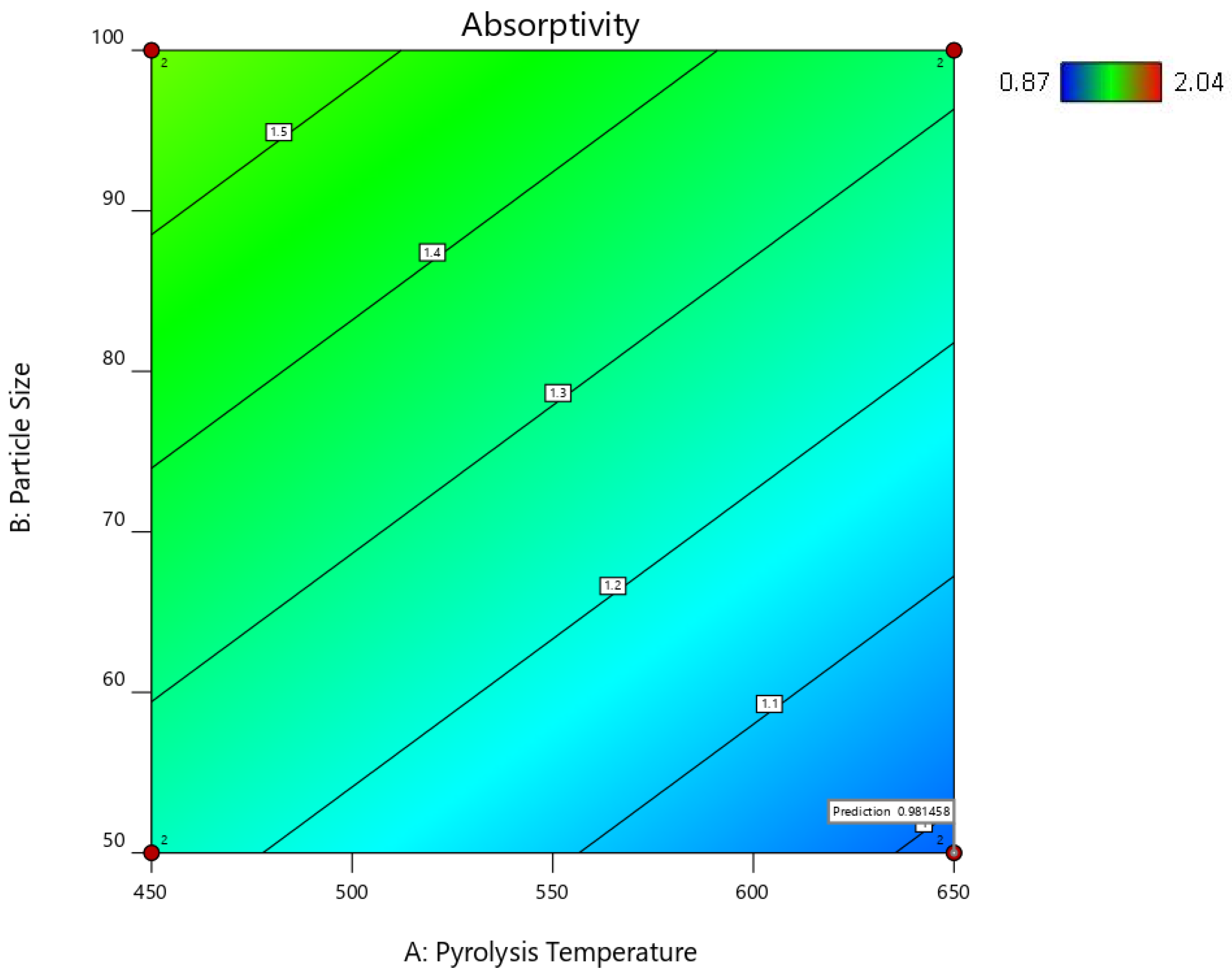
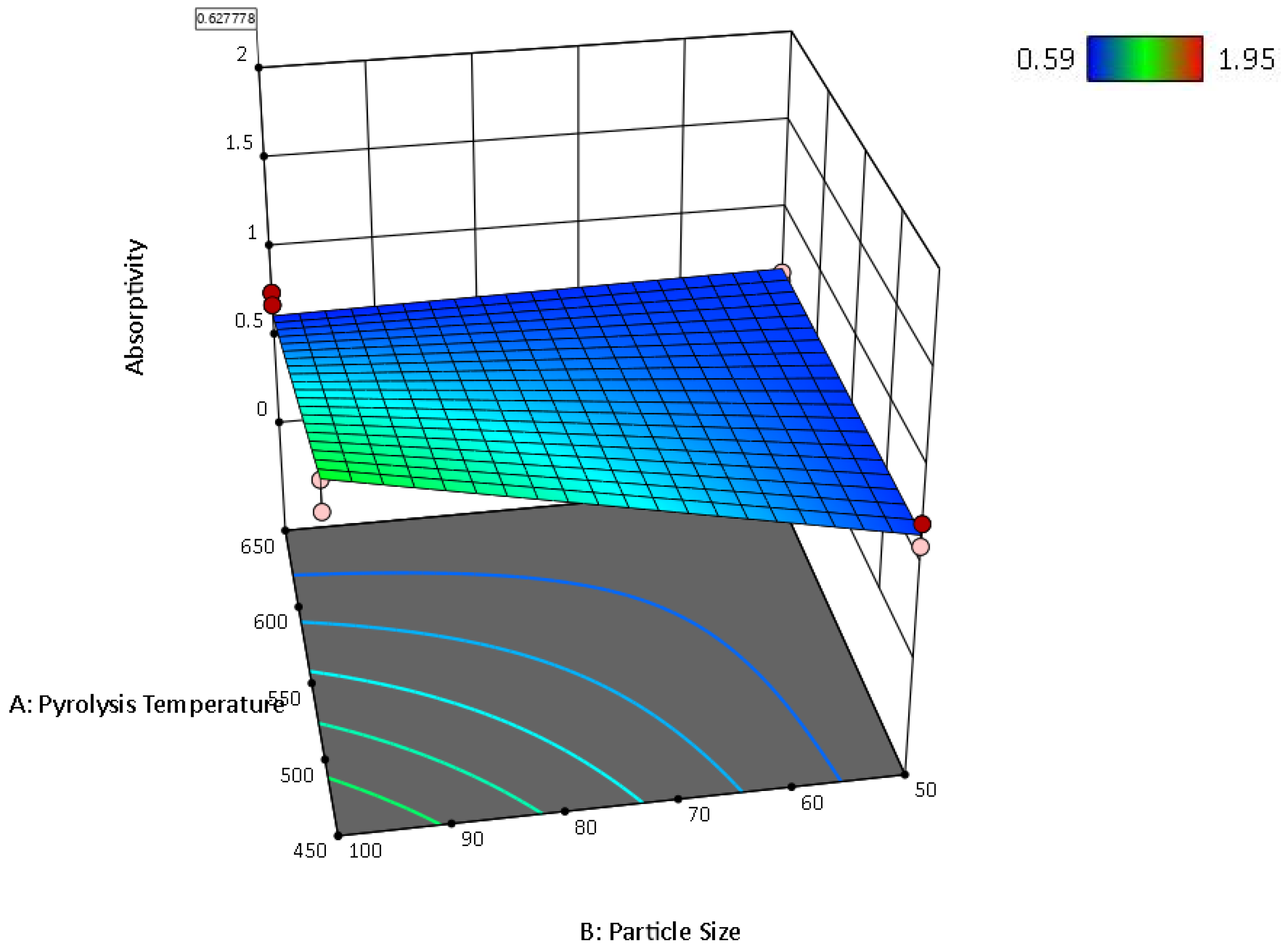
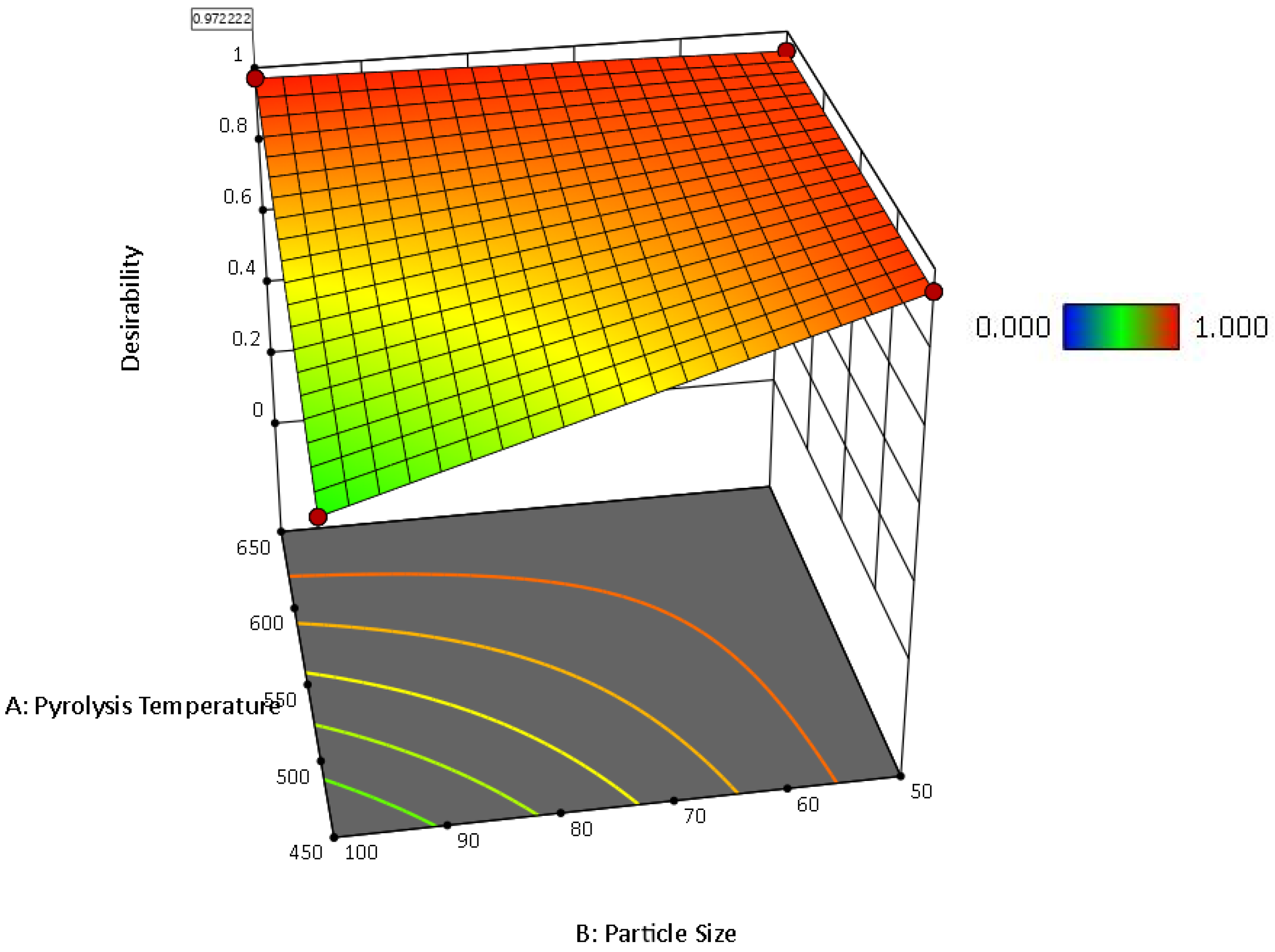
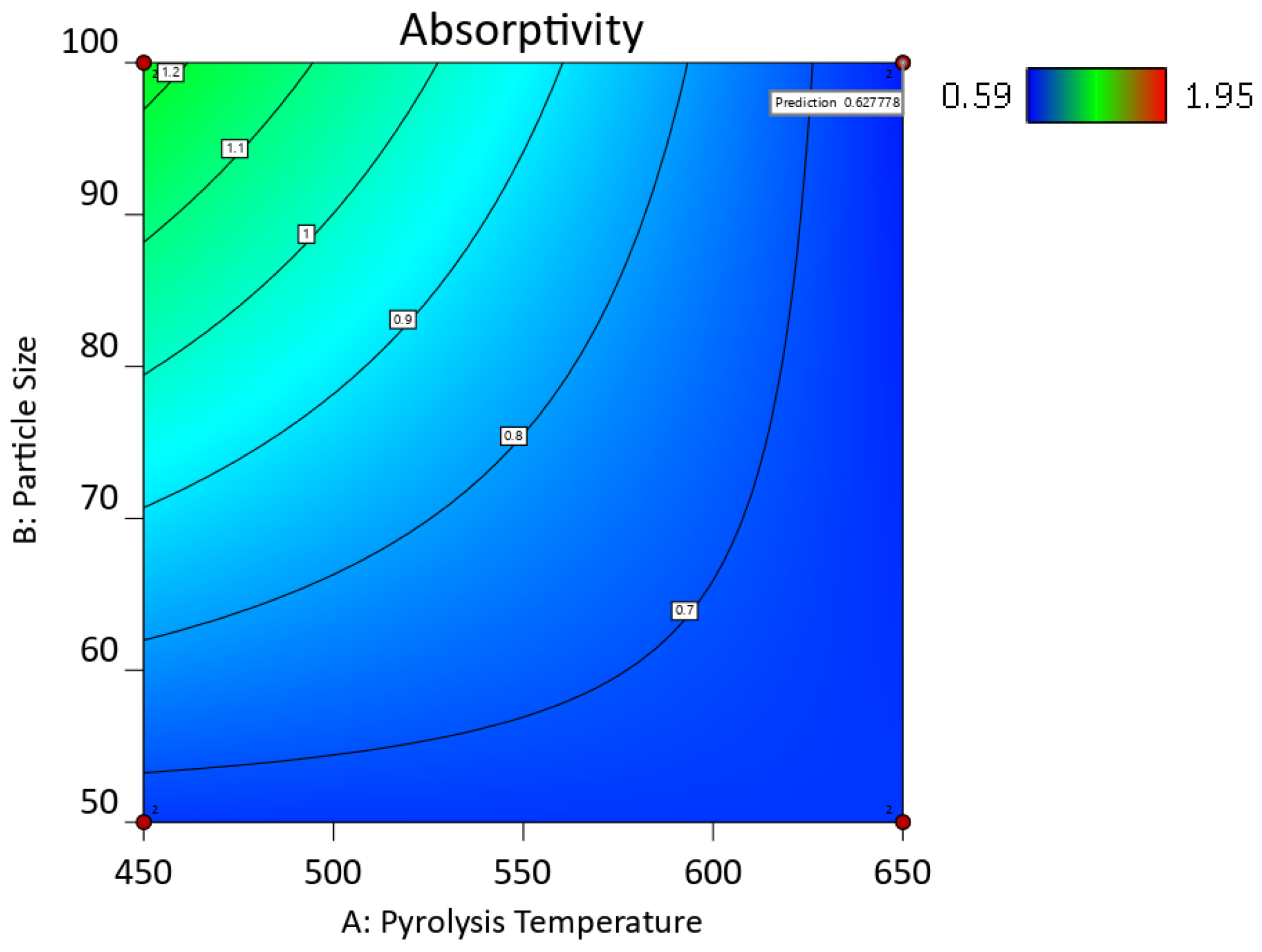
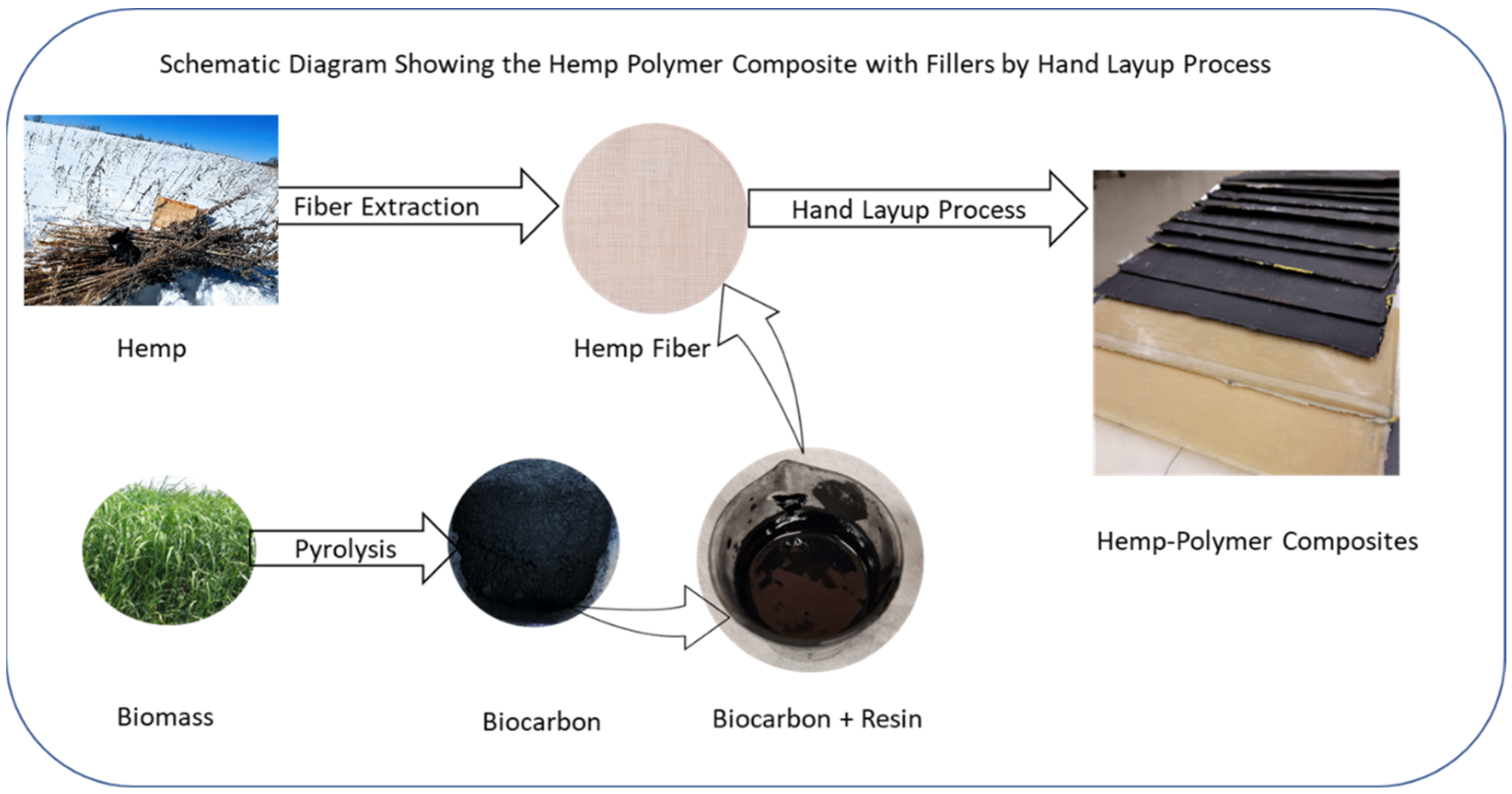
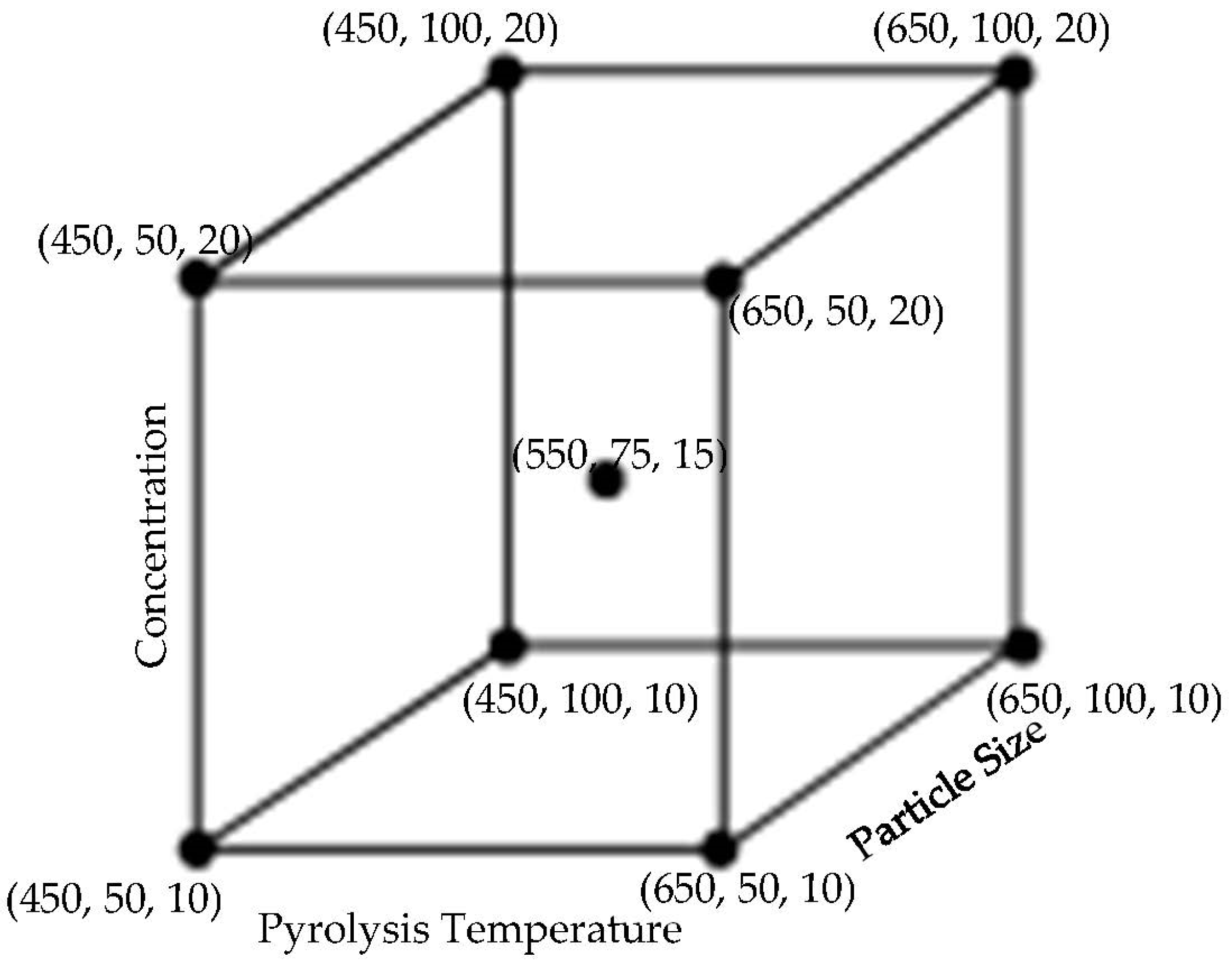
| Factor 1 | Factor 2 | Factor 3 | Switchgrass Biocarbon | Hemp Biocarbon |
|---|---|---|---|---|
| A: Pyrolysis Temperature | B: Particle Size | C: Filler Loading | Water Absorptivity | Water Absorptivity |
| 650 | 100 | 10 | 0.97 | 1.52 |
| 650 | 100 | 20 | 0.76 | 1.49 |
| 650 | 50 | 20 | 0.59 | 0.87 |
| 650 | 50 | 10 | 1.95 | 1.36 |
| 650 | 50 | 20 | 0.64 | 1.1 |
| 450 | 100 | 20 | 1.07 | 1.22 |
| 550 | 75 | 15 | 1.59 | 1.77 |
| 450 | 100 | 20 | 1.23 | 1.93 |
| 650 | 100 | 10 | 1.2 | 1.49 |
| 450 | 50 | 10 | 1.69 | 1.75 |
| 450 | 100 | 10 | 1.83 | 1.93 |
| 650 | 50 | 10 | 1.53 | 1.4 |
| 450 | 100 | 10 | 1.92 | 2.04 |
| 450 | 50 | 20 | 0.72 | 1.17 |
| 450 | 50 | 20 | 0.6 | 1.7 |
| 450 | 50 | 10 | 1.7 | 1.7 |
| 550 | 75 | 15 | 0.97 | 1.46 |
| 650 | 100 | 20 | 0.69 | 1.4 |
| Hemp Biocarbon-Filled Composites | Switchgrass Biocarbon-Filled Composites | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Source | SS | df | MS | F-Value | p-Value | SS | df | MS | F-Value | p-Value |
| Model | 1.530 | 7 | 0.219 | 5.310 | 0.012 | 3.730 | 7 | 0.533 | 14.350 | 0.000 |
| A-Pyrolysis Temperature | 0.258 | 1 | 0.258 | 6.260 | 0.034 | 0.369 | 1 | 0.369 | 9.930 | 0.012 |
| B-Particle Size | 0.473 | 1 | 0.473 | 11.480 | 0.008 | 0.004 | 1 | 0.004 | 0.105 | 0.753 |
| C-Filler Loading | 0.597 | 1 | 0.597 | 14.500 | 0.004 | 2.630 | 1 | 2.630 | 70.800 | 0.000 |
| AB | 0.011 | 1 | 0.011 | 0.255 | 0.626 | 0.369 | 1 | 0.369 | 9.930 | 0.012 |
| AC | 0.101 | 1 | 0.101 | 2.450 | 0.152 | 0.019 | 1 | 0.019 | 0.509 | 0.494 |
| BC | 0.092 | 1 | 0.092 | 2.220 | 0.170 | 0.289 | 1 | 0.289 | 7.770 | 0.021 |
| ABC | 0.001 | 1 | 0.001 | 0.026 | 0.876 | 0.052 | 1 | 0.052 | 1.390 | 0.268 |
| Curvature | 0.045 | 1 | 0.045 | 1.100 | 0.322 | 0.013 | 1 | 0.013 | 0.361 | 0.563 |
| Pure Error | 0.370 | 9 | 0.041 | 0.335 | 9 | 0.037 | ||||
| Total | 1.950 | 17 | 4.080 | 17 | ||||||
| Samples | Std. Dev. | Mean | C.V. % | R2 | Adjusted R2 | Predicted R2 |
|---|---|---|---|---|---|---|
| Hemp biocarbon-filled composites | 0.2029 | 1.47 | 13.77 | 0.8052 | 0.6537 | 0.2207 |
| Switchgrass biocarbon-filled composites | 0.1928 | 1.2 | 16.03 | 0.9178 | 0.8538 | 0.671 |
| Hemp Biocarbon-Filled Composites | Switchgrass Biocarbon-Filled Composites | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Source | SS | df | MS | F-Value | p-Value | SS | df | MS | F-Value | p-Value |
| Model | 1.330 | 3 | 0.442 | 10.000 | 0.001 | 3.660 | 4 | 0.915 | 28.140 | <0.0001 |
| A-Pyrolysis Temperature | 0.258 | 1 | 0.258 | 5.820 | 0.030 | 0.369 | 1 | 0.369 | 11.350 | 0.005 |
| B-Particle Size | 0.473 | 1 | 0.473 | 10.680 | 0.006 | |||||
| C-Filler loading | 0.597 | 1 | 0.597 | 13.490 | 0.003 | 2.630 | 1 | 2.630 | 80.970 | <0.0001 |
| AB | 0.369 | 1 | 0.369 | 11.350 | 0.005 | |||||
| BC | 0.289 | 1 | 0.289 | 8.890 | 0.0106 | |||||
| Residual | 0.619 | 14 | 0.044 | 0.423 | 13 | 0.033 | ||||
| Lack of Fit | 0.249 | 5 | 0.050 | 1.210 | 0.378 | 0.088 | 4 | 0.022 | 0.592 | 0.6775 |
| Pure Error | 0.370 | 9 | 0.041 | 0.335 | 9 | 0.037 | ||||
| Total | 1.950 | 17 | 4.080 | 17 | ||||||
| Samples | SD | Mean | C.V. % | R2 | Adjusted R2 | Predicted R2 | PRESS |
|---|---|---|---|---|---|---|---|
| Hemp biocarbon composites | 0.2103 | 1.47 | 14.28 | 0.6818 | 0.6136 | 0.4728 | 1.03 |
| Switchgrass biocarbon composites | 0.1803 | 1.2 | 14.99 | 0.8965 | 0.8646 | 0.8329 | 0.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dahal, R.K.; Acharya, B.; Dutta, A. The Interaction Effect of the Design Parameters on the Water Absorption of the Hemp-Reinforced Biocarbon-Filled Bio-Epoxy Composites. Int. J. Mol. Sci. 2023, 24, 6093. https://doi.org/10.3390/ijms24076093
Dahal RK, Acharya B, Dutta A. The Interaction Effect of the Design Parameters on the Water Absorption of the Hemp-Reinforced Biocarbon-Filled Bio-Epoxy Composites. International Journal of Molecular Sciences. 2023; 24(7):6093. https://doi.org/10.3390/ijms24076093
Chicago/Turabian StyleDahal, Raj Kumar, Bishnu Acharya, and Animesh Dutta. 2023. "The Interaction Effect of the Design Parameters on the Water Absorption of the Hemp-Reinforced Biocarbon-Filled Bio-Epoxy Composites" International Journal of Molecular Sciences 24, no. 7: 6093. https://doi.org/10.3390/ijms24076093
APA StyleDahal, R. K., Acharya, B., & Dutta, A. (2023). The Interaction Effect of the Design Parameters on the Water Absorption of the Hemp-Reinforced Biocarbon-Filled Bio-Epoxy Composites. International Journal of Molecular Sciences, 24(7), 6093. https://doi.org/10.3390/ijms24076093








