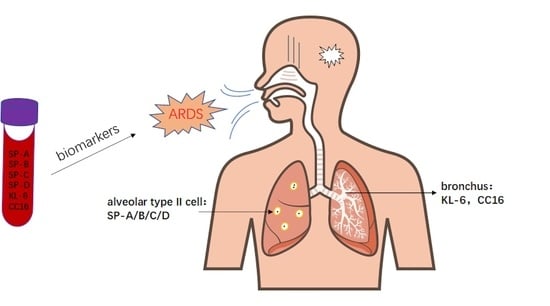
Circulating Pulmonary-Originated Epithelial Biomarkers for Acute Respiratory Distress Syndrome: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Search Strategy
2.3. Study Selection
2.4. Data Extraction
2.5. Risk of Bias
2.6. Statistical Analysis
2.6.1. Meta-Analysis of Continuous Variables
2.6.2. Meta-Analysis of Diagnostic Test Accuracy
3. Results
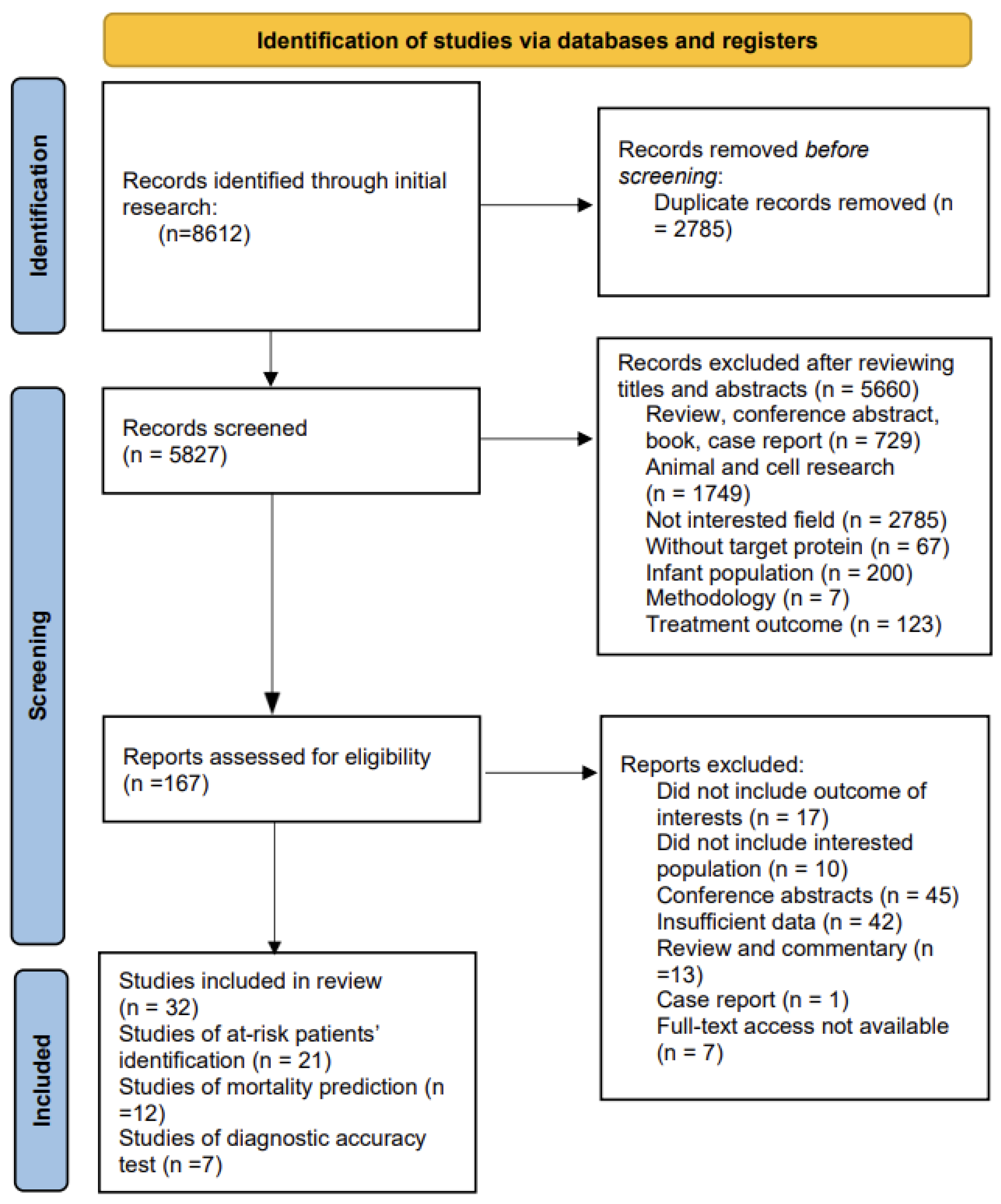
3.1. Literature Search
3.2. Study Characteristics and Quality Assessment
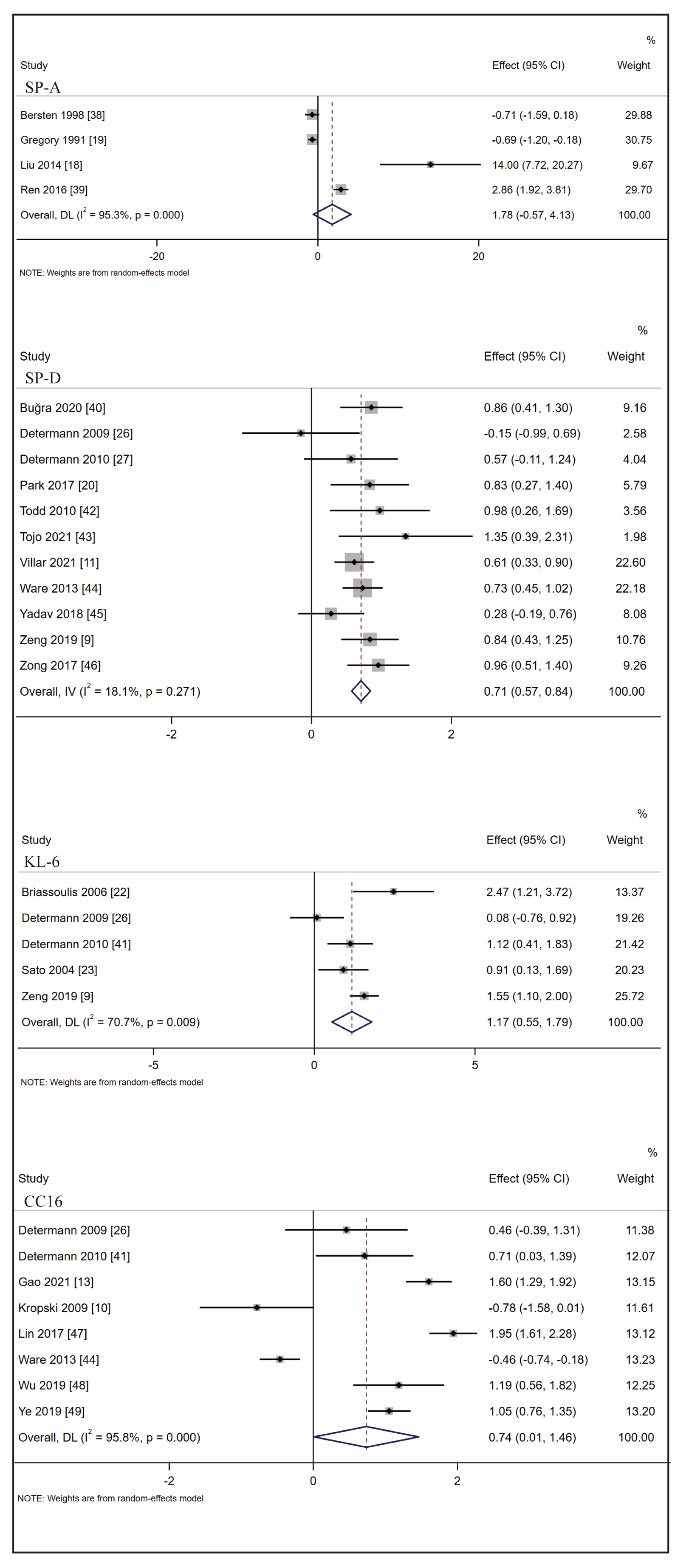
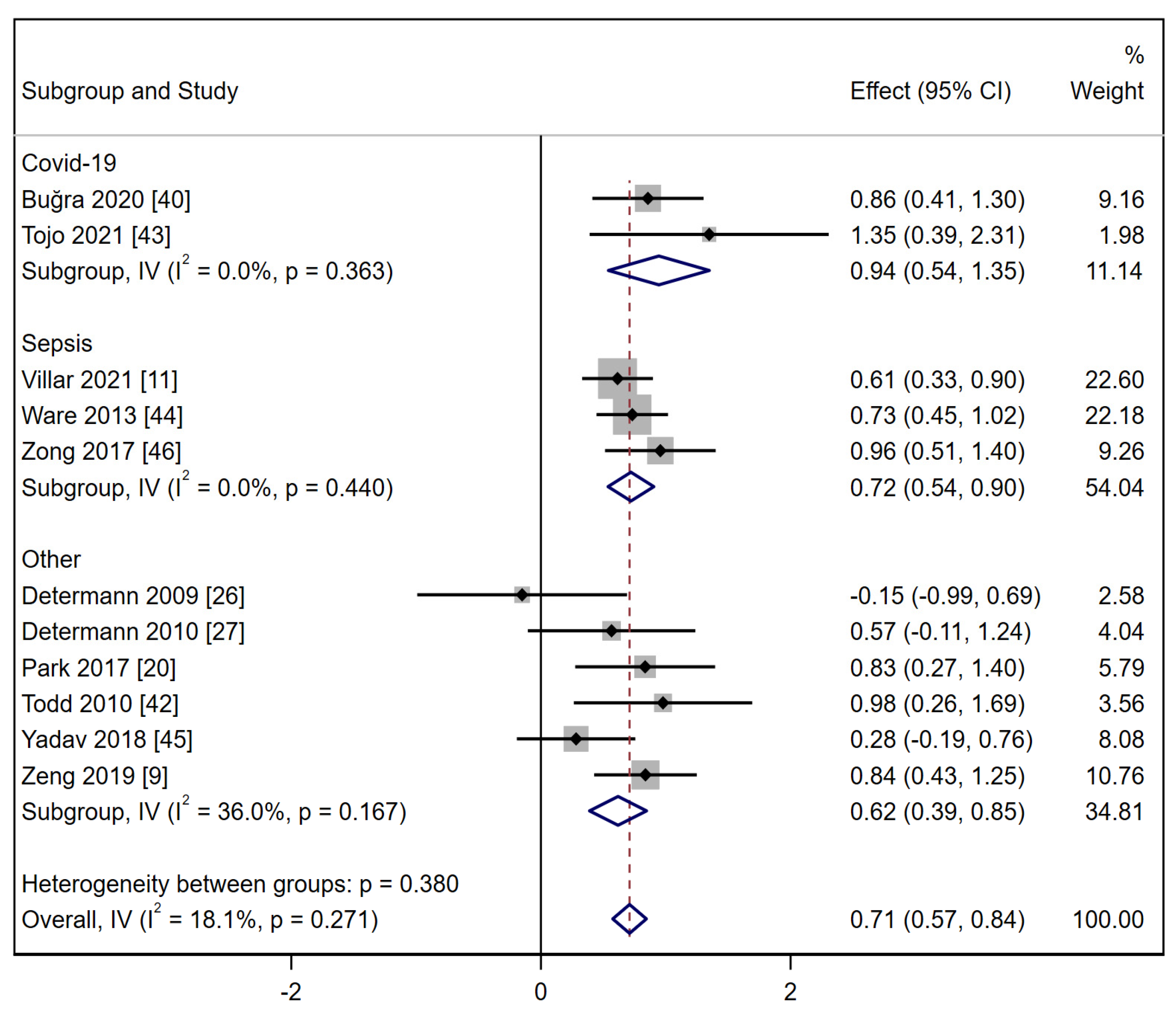
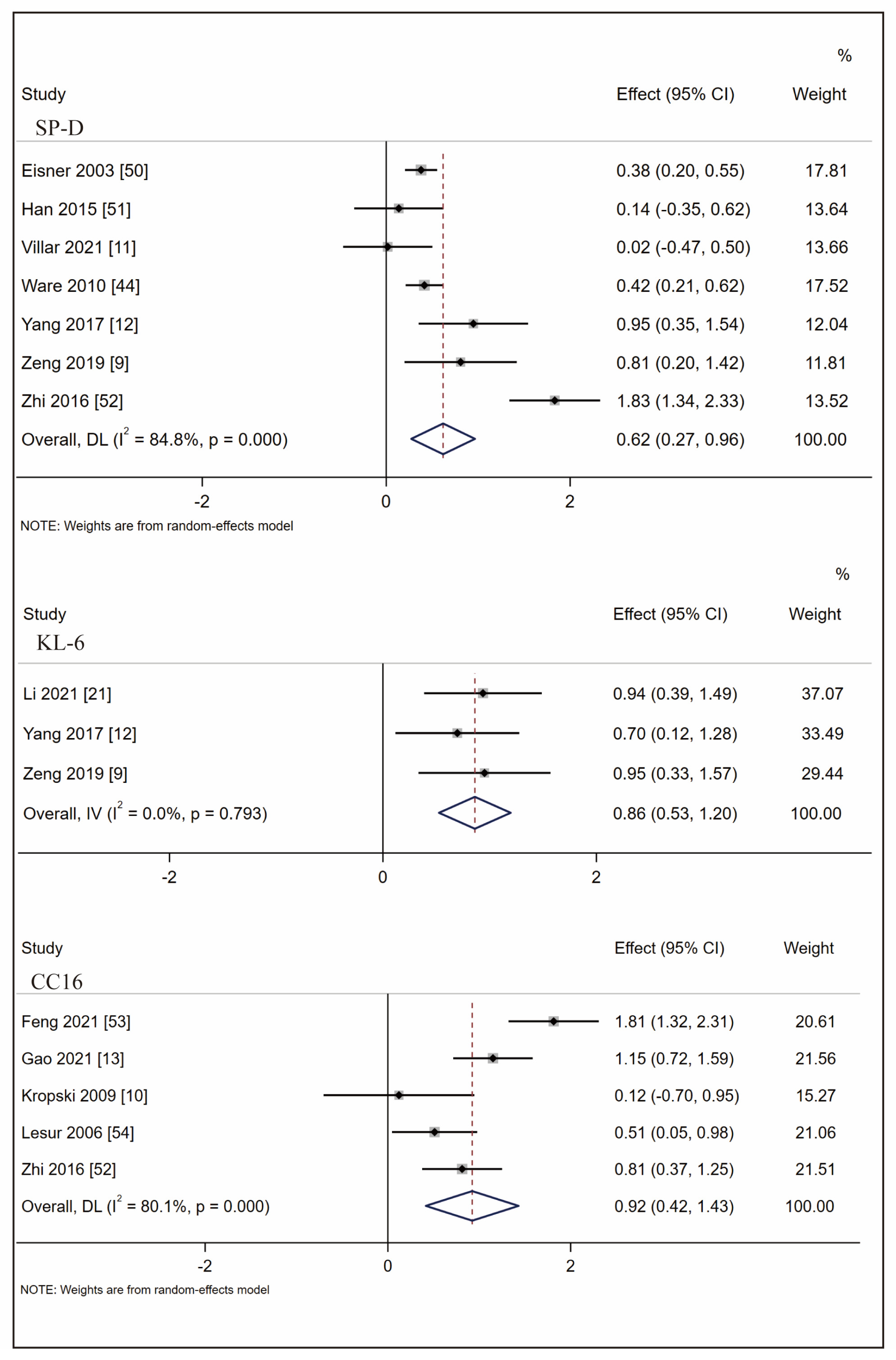
3.3. Biomarkers Associated with ARDS at-Risk Patients’ Identification
3.4. Biomarkers Associated with ARDS Mortality Prediction
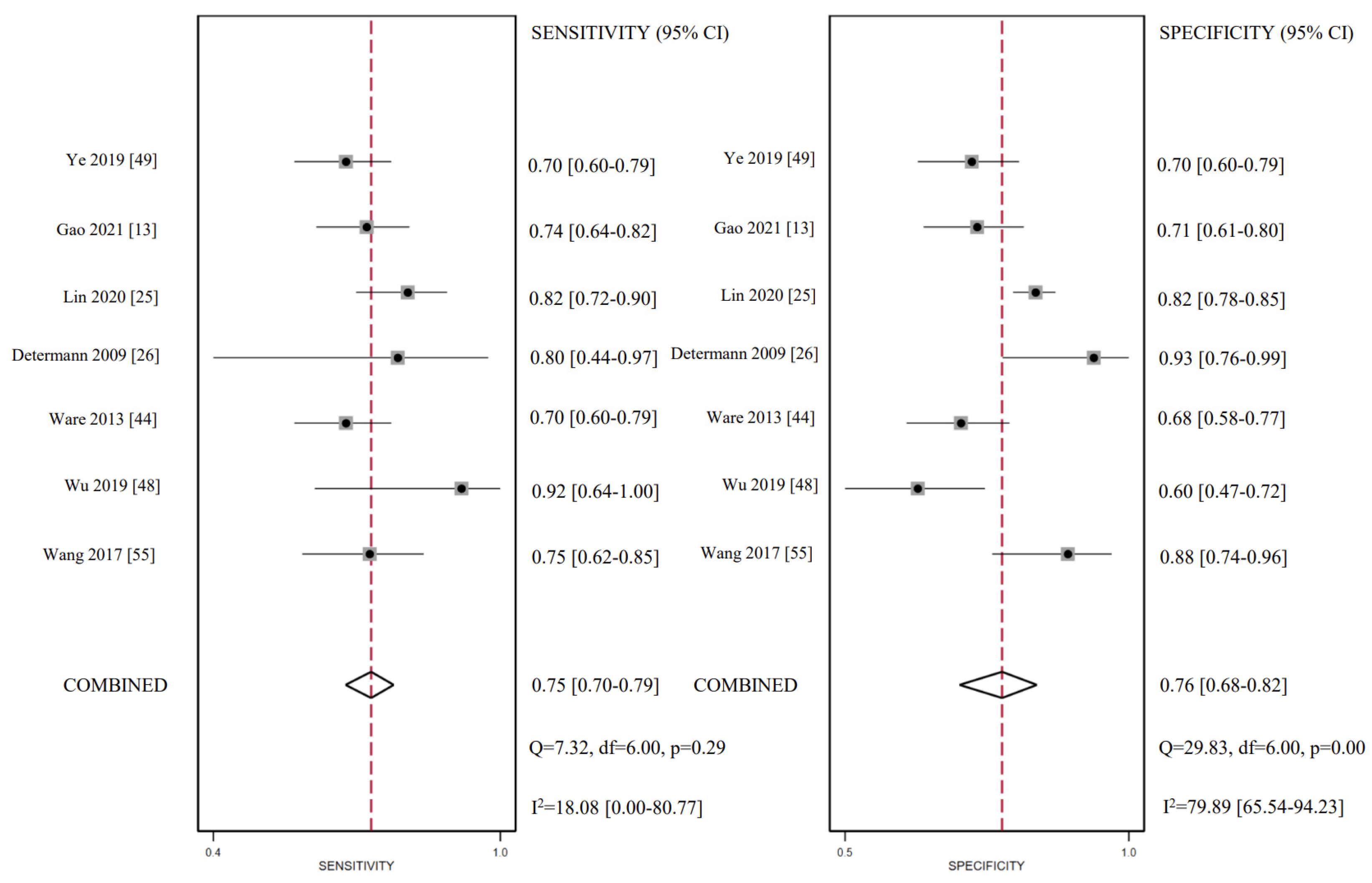
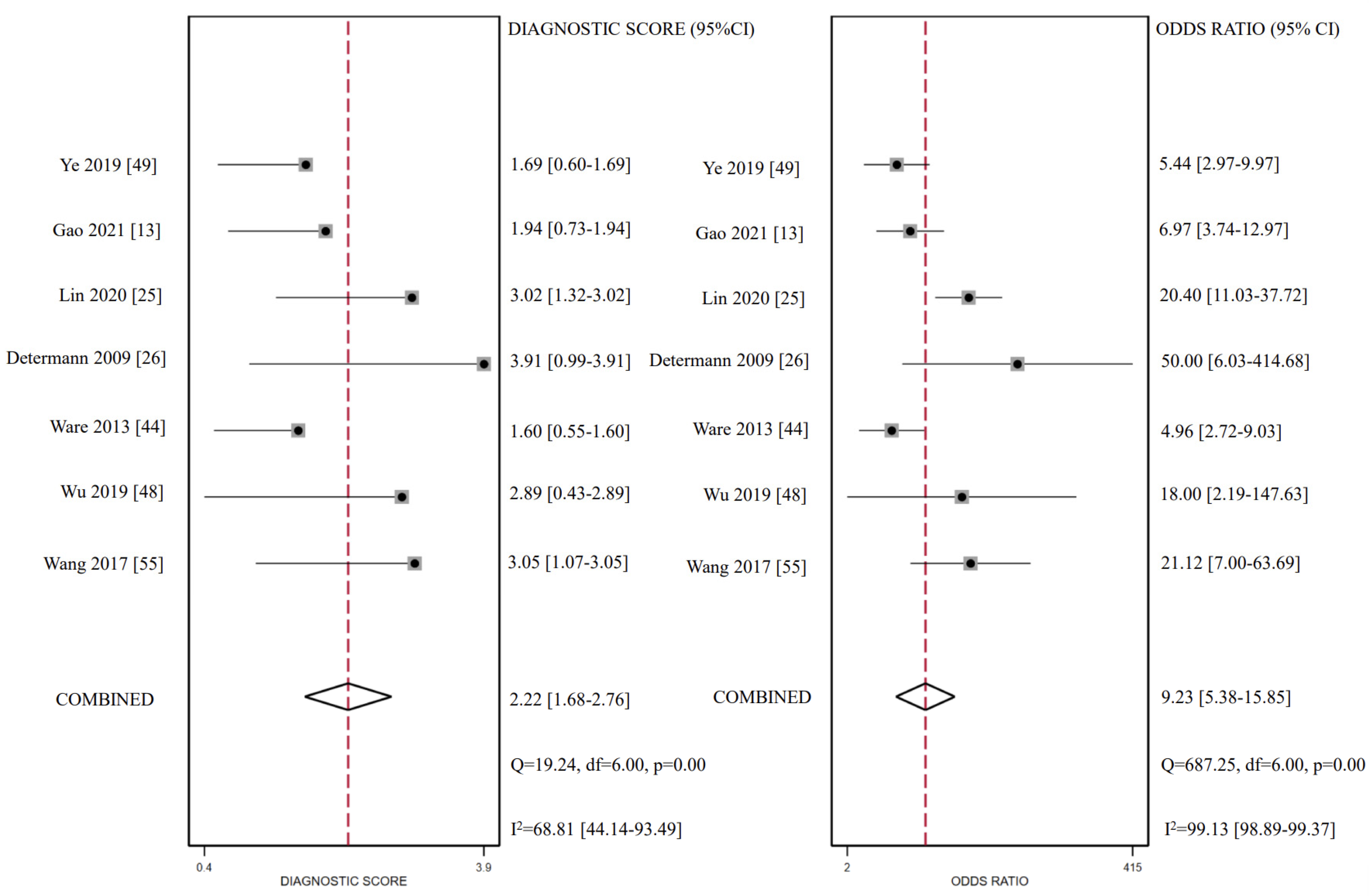
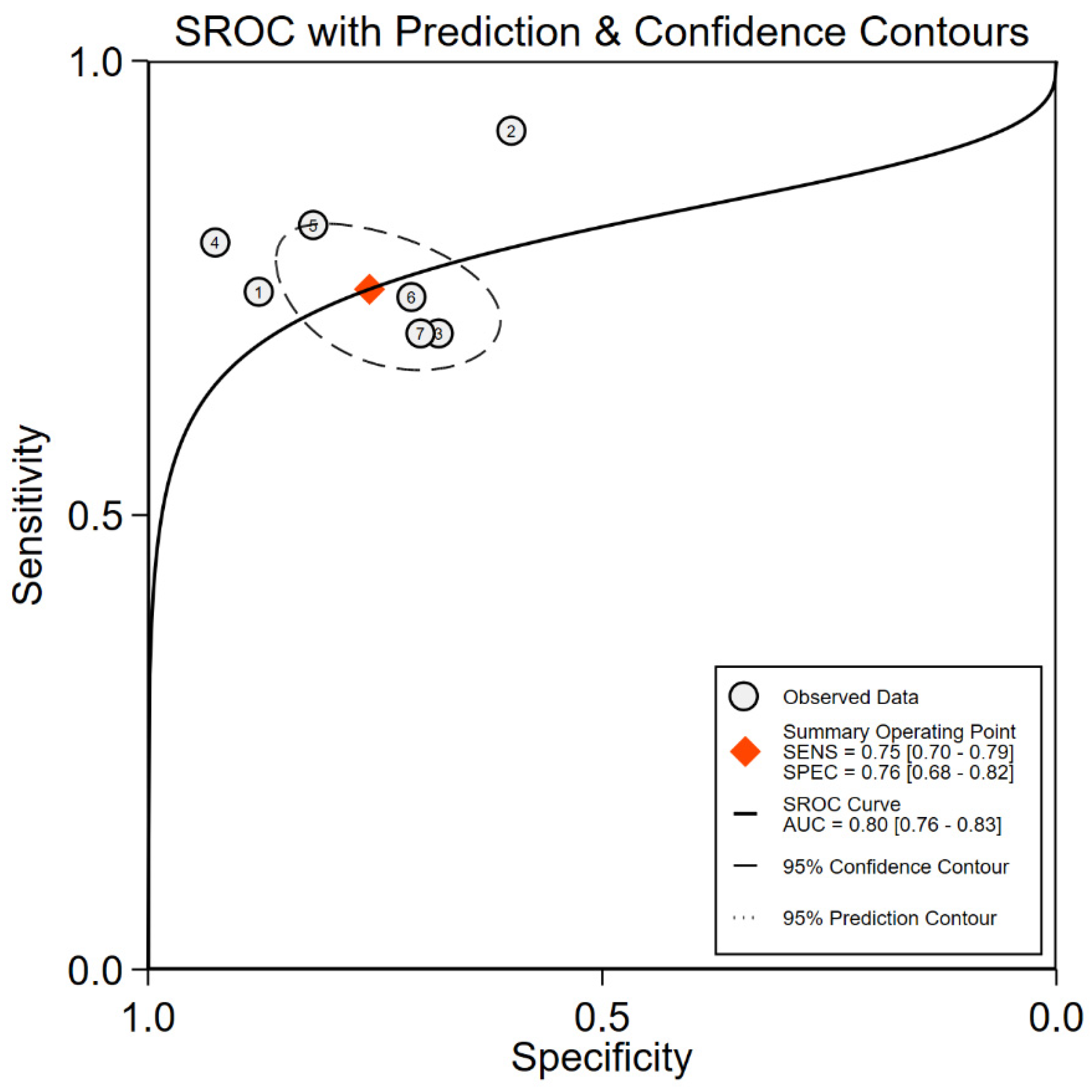
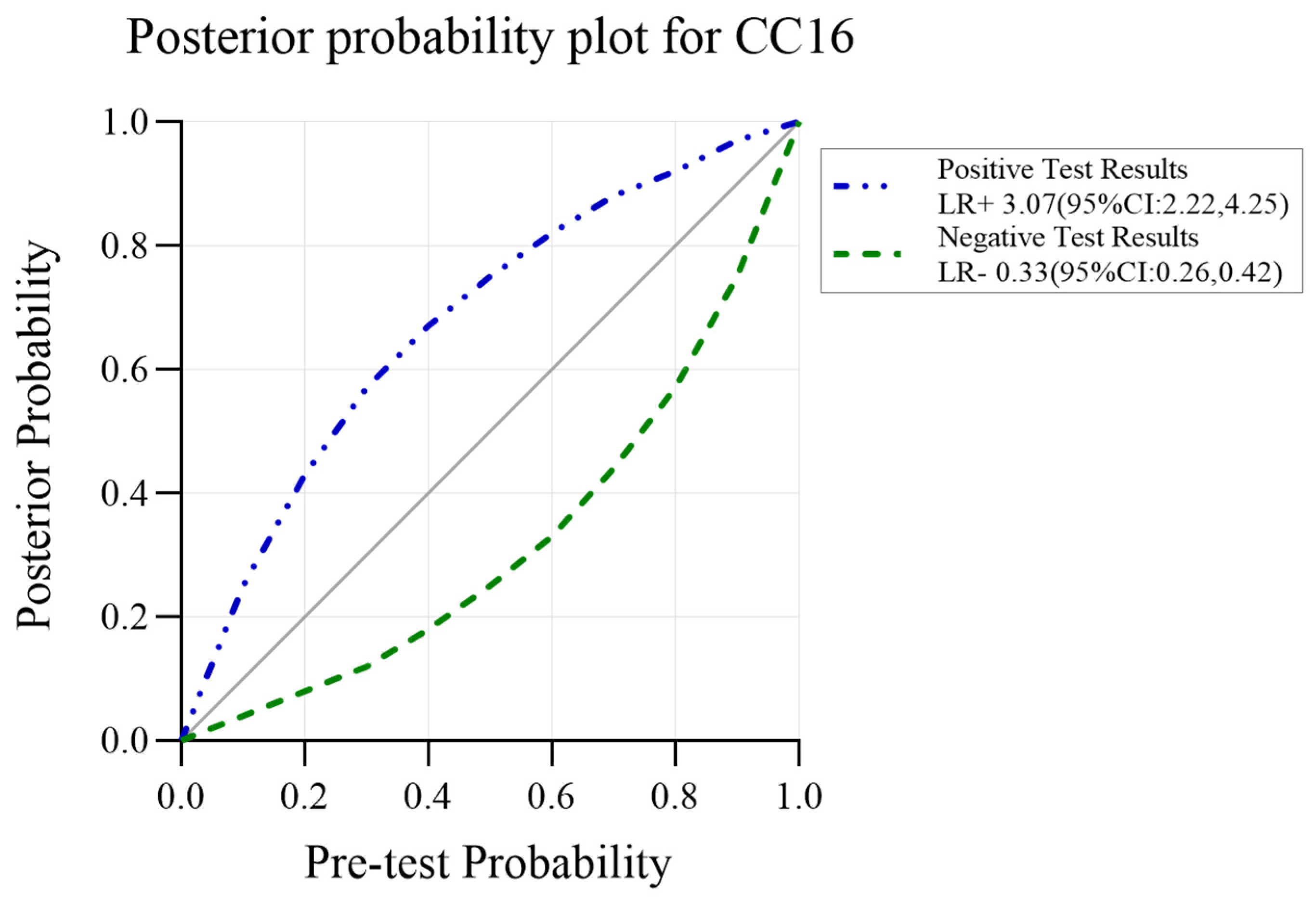
3.5. CC16 Diagnosis Test Accuracy
3.6. Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bellani, G.; Laffey, J.G.; Pham, T.; Fan, E.; Brochard, L.; Esteban, A.; Gattinoni, L.; Van Haren, F.; Larsson, A.; McAuley, D.F.; et al. Epidemiology, Patterns of Care, and Mortality for Patients with Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016, 315, 788–800. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.N.; Aggarwal, N.R.; Thompson, B.T.; Schmidt, E.P. Advancing Precision Medicine for the Diagnosis and Treatment of Acute Respiratory Distress Syndrome. J. Clin. Med. 2023, 12, 1563. [Google Scholar] [CrossRef] [PubMed]
- Pelosi, P.; Tonelli, R.; Torregiani, C.; Baratella, E.; Confalonieri, M.; Battaglini, D.; Marchioni, A.; Confalonieri, P.; Clini, E.; Salton, F.; et al. Different Methods to Improve the Monitoring of Noninvasive Respiratory Support of Patients with Severe Pneumonia/ARDS Due to COVID-19: An Update. J. Clin. Med. 2022, 11, 1704. [Google Scholar] [CrossRef]
- Umbrello, M.; Formenti, P.; Bolgiaghi, L.; Chiumello, D. Current Concepts of ARDS: A Narrative Review. Int. J. Mol. Sci. 2016, 18, 64. [Google Scholar] [CrossRef] [PubMed]
- Bernard, G.R.; Artigas, A.; Brigham, K.L.; Carlet, J.; Falke, K.; Hudson, L.; Lamy, M.; Legall, J.R.; Morris, A.; Spragg, R. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am. J. Respir. Crit. Care Med. 1994, 149 Pt 1, 818–824. [Google Scholar] [CrossRef]
- Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.; Ferguson, N.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute respiratory distress syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar]
- Bellani, G.; Pham, T.; Laffey, J.G. Missed or delayed diagnosis of ARDS: A common and serious problem. Intensiv. Care Med. 2020, 46, 1180–1183. [Google Scholar] [CrossRef]
- Fan, E.; Brodie, D.; Slutsky, A. Acute Respiratory Distress Syndrome: Advances in Diagnosis and Treatment. JAMA 2018, 319, 698–710. [Google Scholar] [CrossRef]
- Zeng, X.; Lu, D.; Zhang, X.; Li, J. Predictive and prognostic evaluation of five serum markers for acute respiratory distress syndrome. J. Xi’an Jiaotong Univ. Med. Sci. 2019, 40, 588–592+618. [Google Scholar]
- Kropski, J.A.; Fremont, R.D.; Calfee, C.S.; Ware, L.B. Clara Cell Protein (CC16), a Marker of Lung Epithelial Injury, Is Decreased in Plasma and Pulmonary Edema Fluid from Patients with Acute Lung Injury. Chest 2009, 135, 1440–1447. [Google Scholar] [CrossRef]
- Villar, J.; Herrán-Monge, R.; González-Higueras, E.; Prieto-González, M.; Ambrós, A.; Rodríguez-Pérez, A.; Muriel-Bombín, A.; Solano, R.; Cuenca-Rubio, C.; Vidal, A.; et al. Clinical and biological markers for predicting ARDS and outcome in septic patients. Sci. Rep. 2021, 11, 22702. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Li, Z.Q.; Lan, H.B.; Xiong, S.S.; Wang, S.S.; Yan, C.S. Research of the biomarkers in pulmonary and extrapulmonary acute respiratory distress syndrome. Natl. Med. J. China 2017, 97, 2023–2027. [Google Scholar] [CrossRef]
- Gao, Y.; Li, J. Significance of combined application of biomarkers in the diagnosis and prognosis assessment of patients with acute respiratory distress syndrome. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2021, 33, 69–73. [Google Scholar] [PubMed]
- Terpstra, M.L.; Aman, J.; van Nieuw Amerongen, G.P.; Groeneveld, A.J. Plasma biomarkers for acute respiratory distress syndrome: A systematic review and meta-analysis. Crit. Care Med. 2014, 42, 691–700. [Google Scholar] [CrossRef]
- Van Der Zee, P.; Rietdijk, W.; Somhorst, P.; Endeman, H.; Gommers, D. A systematic review of biomarkers multivariately associated with acute respiratory distress syndrome development and mortality. Crit. Care 2020, 24, 243. [Google Scholar] [CrossRef]
- Mokra, D.; Kosutova, P. Biomarkers in acute lung injury. Respir. Physiol. Neurobiol. 2015, 209, 52–58. [Google Scholar] [CrossRef]
- Cross, L.M.; Matthay, M.A. Biomarkers in Acute Lung Injury: Insights into the Pathogenesis of Acute Lung Injury. Crit. Care Clin. 2011, 27, 355–377. [Google Scholar] [CrossRef]
- Liu, X.; Shao, Q.; Bai, X.; Sun, R.; Liu, P. The early diagnosis value of the serum surfactant protein a in critically ill acute lung injury of HFMD. Chongqing Med. 2014, 43, 663–665. [Google Scholar]
- Gregory, T.J.; Longmore, W.J.; A Moxley, M.; A Whitsett, J.; Reed, C.R.; A Fowler, A.; Hudson, L.D.; Maunder, R.J.; Crim, C.; Hyers, T.M. Surfactant chemical composition and biophysical activity in acute respiratory distress syndrome. J. Clin. Investig. 1991, 88, 1976–1981. [Google Scholar] [CrossRef]
- Park, J.; Pabon, M.; Choi, A.M.K.; Siempos, I.I.; Fredenburgh, L.E.; Baron, R.M.; Jeon, K.; Chung, C.R.; Yang, J.H.; Park, C.-M.; et al. Plasma surfactant protein-D as a diagnostic biomarker for acute respiratory distress syndrome: Validation in US and Korean cohorts. BMC Pulm. Med. 2017, 17, 204. [Google Scholar] [CrossRef]
- Li, S.; Gao, Y.; Zhou, L.; Li, X.; Zhang, L.; Dong, R.; Zhang, R.; Zhang, G.; Shang, M. The prognosis evaluation of sICAM-1,KL-6 combined with EVLWI in severe pneumonia patients with acute respiratory distress syndrome. Chin. J. Emerg. Med. 2021, 30, 730–736. [Google Scholar]
- Briassoulis, G.; Mavrikiou, M.; Margeli, A.; Bsc, C.L.; Natsi, L.; Papassotiriou, I.; Hatzis, T. Circulating levels of KL-6 in acute respiratory distress syndrome sepsis or traumatic brain injury in critically ill children. Pediatr. Pulmonol. 2006, 41, 790–795. [Google Scholar] [CrossRef]
- Sato, H.; Callister, M.; Mumby, S.; Quinlan, G.; Welsh, K.; Dubois, R.; Evans, T. KL-6 levels are elevated in plasma from patients with acute respiratory distress syndrome. Eur. Respir. J. 2004, 23, 142–145. [Google Scholar] [CrossRef]
- Arsalane, K.; Broeckaert, F.; Knoops, B.; Wiedig, M.; Toubeau, G.; Bernard, A. Clara Cell Specific Protein (CC16) Expression after Acute Lung Inflammation Induced by Intratracheal Lipopolysaccharide Administration. Am. J. Respir. Crit. Care Med. 2000, 161, 1624–1630. [Google Scholar] [CrossRef]
- Lin, J.; Tao, W.; Wei, J.; Wu, J.; Zhang, W.; Ye, J.; Fu, X.; Zeng, S.; Dou, Q.; Wang, L.; et al. Renal dysfunction reduces the diagnostic and prognostic value of serum CC16 for acute respiratory distress syndrome in intensive care patients. BMC Pulm. Med. 2020, 20, 212. [Google Scholar] [CrossRef]
- Determann, R.M.; Millo, J.L.; Waddy, S.; Lutter, R.; Garrard, C.S.; Schultz, M.J. Plasma CC16 levels are associated with development of ALI/ARDS in patients with ventilator-associated pneumonia: A retrospective observational study. BMC Pulm. Med. 2009, 9, 49. [Google Scholar] [CrossRef]
- Baker, C.S.; Evans, T.W.; Randle, B.J.; Haslam, P.L. Damage to surfactant-specific protein in acute respiratory distress syndrome. Lancet 1999, 353, 1232–1237. [Google Scholar] [CrossRef]
- Kondo, T.; Hattori, N.; Ishikawa, N.; Murai, H.; Haruta, Y.; Hirohashi, N.; Tanigawa, K.; Kohno, N. KL-6 concentration in pulmonary epithelial lining fluid is a useful prognostic indicator in patients with acute respiratory distress syndrome. Respir. Res. 2011, 12, 32. [Google Scholar] [CrossRef]
- Jabaudon, M.; Blondonnet, R.; Pereira, B.; Cartin-Ceba, R.; Lichtenstern, C.; Mauri, T.; Determann, R.M.; Drabek, T.; Hubmayr, R.D.; Gajic, O.; et al. Plasma sRAGE is independently associated with increased mortality in ARDS: A meta-analysis of individual patient data. Intensiv. Care Med. 2018, 44, 1388–1399. [Google Scholar] [CrossRef]
- Khan, K.S.; Bachmann, L.M.; ter Riet, G. Systematic reviews with individual patient data meta-analysis to evaluate diagnostic tests. Eur. J. Obstet. Gynecol. Reprod. Biol. 2003, 108, 121–125. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Zhang, C.; Zhang, C.; Yang, H.; Gao, R.; Tong, Z. Lung fluid biomarkers for acute respiratory distress syndrome: A systematic review and meta-analysis. Crit. Care 2019, 23, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Wan, X.; Liu, J.; Tong, T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat. Methods Med. Res. 2018, 27, 1785–1805. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- Andrade, C. Mean Difference, Standardized Mean Difference (SMD), and Their Use in Meta-Analysis: As Simple as It Gets. J. Clin. Psychiatry 2020, 81, 11349. [Google Scholar] [CrossRef]
- Melsen, W.G.; Bootsma, M.C.J.; Rovers, M.M.; Bonten, M.J.M. The effects of clinical and statistical heterogeneity on the predictive values of results from meta-analyses. Clin. Microbiol. Infect. 2014, 20, 123–129. [Google Scholar] [CrossRef]
- Bersten, A.; Doyle, I.; Davidson, K.; Barr, H.; Nicholas, T.; Kermeen, F. Surfactant composition reflects lung overinflation and arterial oxygenation in patients with acute lung injury. Eur. Respir. J. 1998, 12, 301–308. [Google Scholar] [CrossRef]
- Ren, S.; Chen, X.; Jiang, L.; Zhu, B.; Jiang, Q.; Xi, X. Deleted in malignant brain tumors 1 protein is a potential biomarker of acute respiratory distress syndrome induced by pneumonia. Biochem. Biophys. Res. Commun. 2016, 478, 1344–1349. [Google Scholar] [CrossRef]
- Kerget, B.; Kerget, F.; Koçak, A.O.; Kızıltunç, A.; Araz, Ö.; Uçar, E.Y.; Akgün, M. Are Serum Interleukin 6 and Surfactant Protein D Levels Associated with the Clinical Course of COVID-19? Lung 2020, 198, 777–784. [Google Scholar] [CrossRef]
- Determann, R.M.; Royakkers, A.A.N.M.; Haitsma, J.J.; Zhang, H.; Slutsky, A.S.; Ranieri, V.M.; Schultz, M.J. Plasma levels of surfactant protein D and KL-6 for evaluation of lung injury in critically ill mechanically ventilated patients. BMC Pulm. Med. 2010, 10, 6. [Google Scholar] [CrossRef]
- Todd, D.A.; Marsh, M.J.; George, A.; Henderson, N.G.; Barr, H.; Sebastian, S.; Clark, G.T.; Koster, G.; Clark, H.W.; Postle, A.D. Surfactant phospholipids, surfactant proteins, and inflammatory markers during acute lung injury in children. Pediatr. Crit. Care Med. 2010, 11, 82–91. [Google Scholar] [CrossRef]
- Tojo, K.; Yamamoto, N.; Mihara, T.; Abe, M.; Goto, T. Distinct temporal characteristics of circulating alveolar epithelial and endothelial injury markers in ARDS with COVID-19. Crit. Care 2021, 25, 169. [Google Scholar] [CrossRef]
- Ware, L.B.; Koyama, T.; Zhao, Z.; Janz, D.R.; Wickersham, N.; Bernard, G.R.; May, A.K.; Calfee, C.S.; A Matthay, M. Biomarkers of lung epithelial injury and inflammation distinguish severe sepsis patients with acute respiratory distress syndrome. Crit. Care 2013, 17, R253. [Google Scholar] [CrossRef]
- Yadav, H.; Bartley, A.; Keating, S.; A Meade, L.; Norris, P.J.; E Carter, R.; Gajic, O.; Kor, D.J. Evolution of Validated Biomarkers and Intraoperative Parameters in the Development of Postoperative ARDS. Respir. Care 2018, 63, 1331–1340. [Google Scholar] [CrossRef]
- Zong, X.; Li, Z.; Wei, D.; Chen, Y.; Sun, G. Predictive and prognostic evaluation of surfactant protein D,von Willebrand factor and interleukin-8 for sepsis-induced acute respiratory distress syndrome. Chin. J. Clin. Lab. Sci. 2017, 35, 118–121. [Google Scholar]
- Lin, J.; Zhang, W.; Wang, L.; Tian, F. Diagnostic and prognostic values of Club cell protein 16 (CC16) in critical care patients with acute respiratory distress syndrome. J. Clin. Lab. Anal. 2017, 32, e22262. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Cheng, Y.-J.; Hung, M.-H.; Lin, I.-J.; Sun, W.-Z.; Chan, K.-C. Association between Early Acute Respiratory Distress Syndrome after Living-Donor Liver Transplantation and Perioperative Serum Biomarkers: The Role of Club Cell Protein 16. BioMed Res. Int. 2019, 2019, 8958069. [Google Scholar] [CrossRef]
- Ye, J.; Lin, J.; Zhong, C.; Ye, J.; Shi, M.; Wei, J.; Fu, X.; Zeng, S.; Tao, W.; Dou, Q.; et al. The clinical value of combined detection of serum angiopoietin 2 and Clara cell protein 16 in the early diagnosis of acute respiratory distress syndrome. Chin. J. Emerg. Med. 2019, 28, 1112–1117. [Google Scholar]
- Eisner, M.D.; Parsons, P.; Matthay, M.A.; Ware, L.; Greene, K. Plasma surfactant protein levels and clinical outcomes in patients with acute lung injury. Thorax 2003, 58, 983–988. [Google Scholar] [CrossRef]
- Han, F.; Wang, H.; Ni, Y. Dynamic Changes of Lung Surfactant Protein D in Patients with Acute Respiratory Distress Syndrome. Chin. Gen. Pract. 2015, 18, 1541–1544. [Google Scholar]
- Zhi, D.; Duan, M.; Liu, Z.; Li, A. Surfactant, pulmonary-associated protein D may serve as a biomarker in addition to APACHE II score in predicting mortality rate of ARDS. Int. J. Clin. Exp. Pathol. 2016, 9, 2127–2133. [Google Scholar]
- Feng, X.; Huang, Y.; Chen, Y.; Fu, Z.; Xu, J.; Hao, J. Prognostic value of serum Activin-A CC-16 and IL-18 levels in the patients with acute respiratory distress syndrome. Chin. J. Crit. Care Med. 2021, 41, 41–45. [Google Scholar]
- Lesur, O.; Critical Care Research Group of the Québec Respiratory Health Network; Langevin, S.; Berthiaume, Y.; Légaré, M.; Skrobik, Y.; Bellemare, J.-F.; Lévy, B.; Fortier, Y.; Lauzier, F.; et al. Outcome value of Clara cell protein in serum of patients with acute respiratory distress syndrome. Intensiv. Care Med. 2006, 32, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhang, Q.; Qianli, M.A.; Yang, X.; Zhao, S.; Wang, Y.; Deng, Z.; Liu, M.; Jiang, D.; Zhou, J. Predictive values of serological biomarkers in progress and prognosis of acute respiratory distress syndrome. J. Third Mil. Med. Univ. 2017, 39, 1926–1932. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Lawrence Erlbaum Associates: Mahwah, NJ, USA, 1988. [Google Scholar]
- LaKind, J.S.; Holgate, S.T.; Ownby, D.R.; Mansur, A.H.; Helms, P.J.; Pyatt, D.; Hays, S.M. A critical review of the use of Clara cell secretory protein (CC16) as a biomarker of acute or chronic pulmonary effects. Biomarkers 2007, 12, 445–467. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, D.; Wang, Z.; Zou, Y.; Wang, H.; Li, X.; Zheng, D.; Zhou, G. Association between inflammatory biomarkers and acute respiratory distress syndrome or acute lung injury risk: A systematic review and meta-analysis. Wien. Klin. Wochenschr. 2022, 134, 24–38. [Google Scholar] [CrossRef]
- Mizgerd, J.P. Inflammation and Pneumonia: Why Are Some More Susceptible than Others? Clin. Chest. Med. 2018, 39, 669–676. [Google Scholar] [CrossRef]
- Huang, M.; Cai, S.; Su, J. The Pathogenesis of Sepsis and Potential Therapeutic Targets. Int. J. Mol. Sci. 2019, 20, 5376. [Google Scholar] [CrossRef]
- Endo, S.; Nishimura, N.; Kawano, Y.; Ueno, N.; Ueno, S.; Tatetsu, H.; Komohara, Y.; Takeya, M.; Hata, H.; Mitsuya, H.; et al. MUC1/KL-6 expression confers an aggressive phenotype upon myeloma cells. Biochem. Biophys. Res. Commun. 2018, 507, 246–252. [Google Scholar] [CrossRef]
- Li, L.; Zhang, W.; Wei, Y.; Yu, L.; Feng, D. A Sensitive Fluorescent Immunoassay for Prostate Specific Antigen Detection Based on Signal Amplify Strategy of Horseradish Peroxidase and Silicon Dioxide Nanospheres. J. Anal. Methods Chem. 2022, 2022, 6209731. [Google Scholar] [CrossRef]
- Tighe, P.J.; Ryder, R.R.; Todd, I.; Fairclough, L.C. ELISA in the multiplex era: Potentials and pitfalls. Proteom. Clin. Appl. 2015, 9, 406–422. [Google Scholar] [CrossRef]
| Study | Biomarkers | Study Design | Setting | Study Population | ARDS Definition | Study Size | ARDS/ ALI (n) | Age | Male (%) | Plasma Sample Moment |
|---|---|---|---|---|---|---|---|---|---|---|
| Bersten 1998 [38] | SP-A/SP-B | Observational study | Single center | NM | NM | NM | 10 | NM | NM | NM |
| Briassoulis 2006 [22] | KL-6 | Observational study | NM | Critically ill | AECC | 36 | 9 | 8.4 | NM | Within 8 H Of Admission |
| Buğra 2020 [40] | SP-D/KL-6 | Observational study | Two centers | COVID-19 patient | Berlin | 88 | 35 | 49 | 47 | At admission |
| Determann 2009 [26] | SP-D/KL-6/CC16 | Retrospective observational cohort | Single center | Critically ill | AECC | 37 | 10 | 67 | 70 | Baseline characteristics |
| Determann 2010 [41] | SP-D/KL-6/CC16 | RCT | Multicenter | NM | AECC | 36 | 16 | 58 | 50 | At admission |
| Gao 2021 [13] | CC16 | Case-control study | Single center | Critically ill | Berlin | 200 | 100 | 56 | 58 | NM |
| Gregory 1991 [19] | SP-A/SP-B | Observational study | Multicenter | NM | PaO2/ FiO2 ratio + clinic criteria | 116 | 67 | 45 | 61 | NM |
| Kropski 2009 [10] | CC16 | Observational cohort | Single center | Critically ill | AECC | 32 | 23 | 40 | 48 | NM |
| Lin 2017 [47] | CC16 | Retrospective observation cohort | Single center | Critically ill | Berlin | 212 | 83 | 54 | 64 | Within 2 h of admission |
| Liu 2014 [18] | SP-A | Observational study | Single center | Critically ill | AECC | 60 | 9 | 1.9 | NM | Within 24 h of diagnosis |
| Park 2017 [20] | SP-D | Retrospective observation cohort | Multicenter | Critically ill | Berlin | 407 | 39 | 57 | 67 | Within 48 h of ICU admission |
| Ren 2016 [39] | SP-A/CC16 | prospective observational study | Single center | Critically ill | Berlin | 212 | 83 | 54 | 67 | Within 2 h of admission |
| Sato 2004 [23] | KL-6 | Observational study | NM | Critically ill | AECC | 47 | 28 | 40 | 36 | At ICU admission or at the time of diagnosis |
| Todd 2010 [42] | SP-D | Prospective cohort study | NM | PICU | AECC + radiograph | 1165 | 18 | 7 | NM | A total of 12–18 h after ALI diagnosis |
| Tojo 2021 [43] | SP-D | Retrospective observational study | Single center | COVID-19 patient | Berlin | 21 | 11 | 69 | 91 | Day 1 in hospital |
| Villar 2021 [11] | SP-D | Observational study | Multicenter | Critically ill sepsis | Berlin | 232 | 72 | 60 | 58 | Within 24 h of diagnosis |
| Ware 2013 [44] | SP-D/CC16 | Retrospective nested case-control study | Single center | Critically ill sepsis | AECC | 200 | 100 | 56 | 52 | On the morning of ICU day 2 |
| Wu 2019 [48] | CC16 | Clinical trial | Single center | Living-donor liver transplantation patient | Bilateral infiltrates + radiograph | 73 | 13 | 59 | 46 | At postoperative day 1 |
| Yadav 2018 [45] | SP-D | Prospective observational case-control study | Single center | Adults undergoing elective thoracic, aortic vascular, or cardiac surgery | Berlin | 467 | 26 | 63 | 77 | Immediately follow the major intraoperative insult believed associated with development of lung injury |
| Ye 2019 [49] | CC16 | Case-control study | Single center | Critically ill | Berlin | 200 | 100 | 57 | 59 | First day of ARDS diagnosis |
| Zeng 2019 [9] | SP-D/KL-6 | Prospective cohort | Two centers | Critically ill | Berlin | 99 | 49 | 53 | 73 | At ICU admission |
| Zong 2017 [46] | SP-D | Prospective study | Single center | Critically ill sepsis | Berlin | 88 | 48 | NM | 65 | Within 24 h of ICU admission |
| Study | Mortality | Biomarkers | Study Design | Setting | Study Population | ARDS Definition | Study Size | ARDS/ALI (n) | Age | Male (%) | Plasma Sample Moment |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Eisner 2003 [50] | 38.5 (180D) | SP-A/D | RCT | Multicenter | NM | AECC + radiograph | 565 | 565 | 51 | 59 | Before implementing the ventilator protocol |
| Feng 2021 [53] | 32.7 (28D) | CC16 | Observational study | Single center | NM | Berlin | 98 | 98 | 55 | 63 | First day of ARDS diagnosis |
| Gao 2021 [13] | 38 (hosp. mort.) | CC16 | Case-control study | Single center | Critically ill | Berlin | 200 | 100 | 56 | 58 | NM |
| Han 2015 [51] | 29.1 (28D) | SP-D | Observational study | Single center | Critically ill | AECC | 79 | 79 | NM | NM | Within 24 h of ARDS diagnosis |
| Kropski 2009 [10] | 56.5 (ICU) | CC16 | Observational cohort | Single center | Critically ill | AECC | 32 | 23 | 40 | 48 | At emergency department |
| Lesur 2006 [54] | 37.2 (28D) | CC16 | Prospective observational study | Multicenter | Critically ill | AECC | 78 | 78 | 63 | 62 | Within 48 h of ARDS diagnosis |
| Li 2021 [21] | 30.8 (28D) | KL-6 | Prospective cohort | Single center | Critically ill | Berlin | 65 | 65 | 74 | 63 | Day1 of RICU admission |
| Villar 2021 [11] | 34.7 (28D) | SP-D/KL-6 | Observational study | Multicenter | Critically ill sepsis | Berlin | 232 | 72 | 60 | 58 | Within 24 h of diagnosis |
| Ware 2013 [44] | 27.3 (hosp. mort.) | SP-D | RCT | Multicenter | NM | AECC + radiograph | 528 | 528 | 50 | 45 | At time of enrolment (prior to randomization) |
| Yang 2017 [12] | 44.9 (28D) | SP-D/KL-6 | Observational study | Single center | Critically ill | Berlin | 49 | 49 | NM | NM | The day after ARDS diagnosis |
| Zeng 2019 [9] | 34.7 (28D) | SP-D/KL-6 | Prospective cohort | Two centers | Critically ill | Berlin | 99 | 49 | 53 | 73 | At time of ARDS diagnosis |
| Zhi 2016 [52] | 30.7 (28D) | SP-A/SP-D/CC16 | Retrospective study | Single center | Critically ill | AECC | 101 | 101 | 56 | 46 | Within 24 h of admission |
| Study | AUC | Study Design | Setting | Study Population | ARDS Definition | Study Size | ARDS/ ALI (n) | Age | Male (%) | Plasma Sample Moment |
|---|---|---|---|---|---|---|---|---|---|---|
| Determann 2009 [26] | 0.91 | Retrospective observational cohort | Single center | Critically ill | AECC | 37 | 10 | 67 | 70 | Baseline characteristics |
| Gao 2021 [13] | 0.75 | Case-control study | Single center | Critically ill | Berlin | 200 | 100 | 56 | 58 | NM |
| Lin 2020 [25] | 0.87 | Observational cohort | Single center | Critically ill | Berlin | 479 | 83 | 52 | 58 | Within 3 h of admission |
| Wang 2017 [55] | 0.86 | Prospective cohort | Multicenter | Critically ill sepsis | Berlin | 100 | 59 | NM | 67 | At admission |
| Ware 2013 [44] | 0.60 | Retrospective nested case-control study | Single center | Critically ill sepsis | AECC | 200 | 100 | 56 | 52 | On the morning of ICU day 2 |
| Wu 2019 [48] | 0.80 | Clinical trial | Single center | Living-donor liver transplantation patient | Berlin | 73 | 13 | 59 | 46 | At postoperative day 1 |
| Ye 2019 [49] | 0.74 | Case-control study | Single center | Critically ill | Berlin | 200 | 100 | 57 | 59 | First day of ARDS diagnosis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, H.; Liu, Q.; Zhao, L.; Liu, Z.; Cui, H.; Li, P.; Fan, H.; Guo, L. Circulating Pulmonary-Originated Epithelial Biomarkers for Acute Respiratory Distress Syndrome: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2023, 24, 6090. https://doi.org/10.3390/ijms24076090
Lin H, Liu Q, Zhao L, Liu Z, Cui H, Li P, Fan H, Guo L. Circulating Pulmonary-Originated Epithelial Biomarkers for Acute Respiratory Distress Syndrome: A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences. 2023; 24(7):6090. https://doi.org/10.3390/ijms24076090
Chicago/Turabian StyleLin, Huishu, Qisijing Liu, Lei Zhao, Ziquan Liu, Huanhuan Cui, Penghui Li, Haojun Fan, and Liqiong Guo. 2023. "Circulating Pulmonary-Originated Epithelial Biomarkers for Acute Respiratory Distress Syndrome: A Systematic Review and Meta-Analysis" International Journal of Molecular Sciences 24, no. 7: 6090. https://doi.org/10.3390/ijms24076090
APA StyleLin, H., Liu, Q., Zhao, L., Liu, Z., Cui, H., Li, P., Fan, H., & Guo, L. (2023). Circulating Pulmonary-Originated Epithelial Biomarkers for Acute Respiratory Distress Syndrome: A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences, 24(7), 6090. https://doi.org/10.3390/ijms24076090