MPEP Attenuates Intrahepatic Fat Accumulation in Obese Mice
Abstract
1. Introduction
2. Results
2.1. Model Characterization and Liver Injury Evaluation
MPEP Reduces Body and Liver Weight
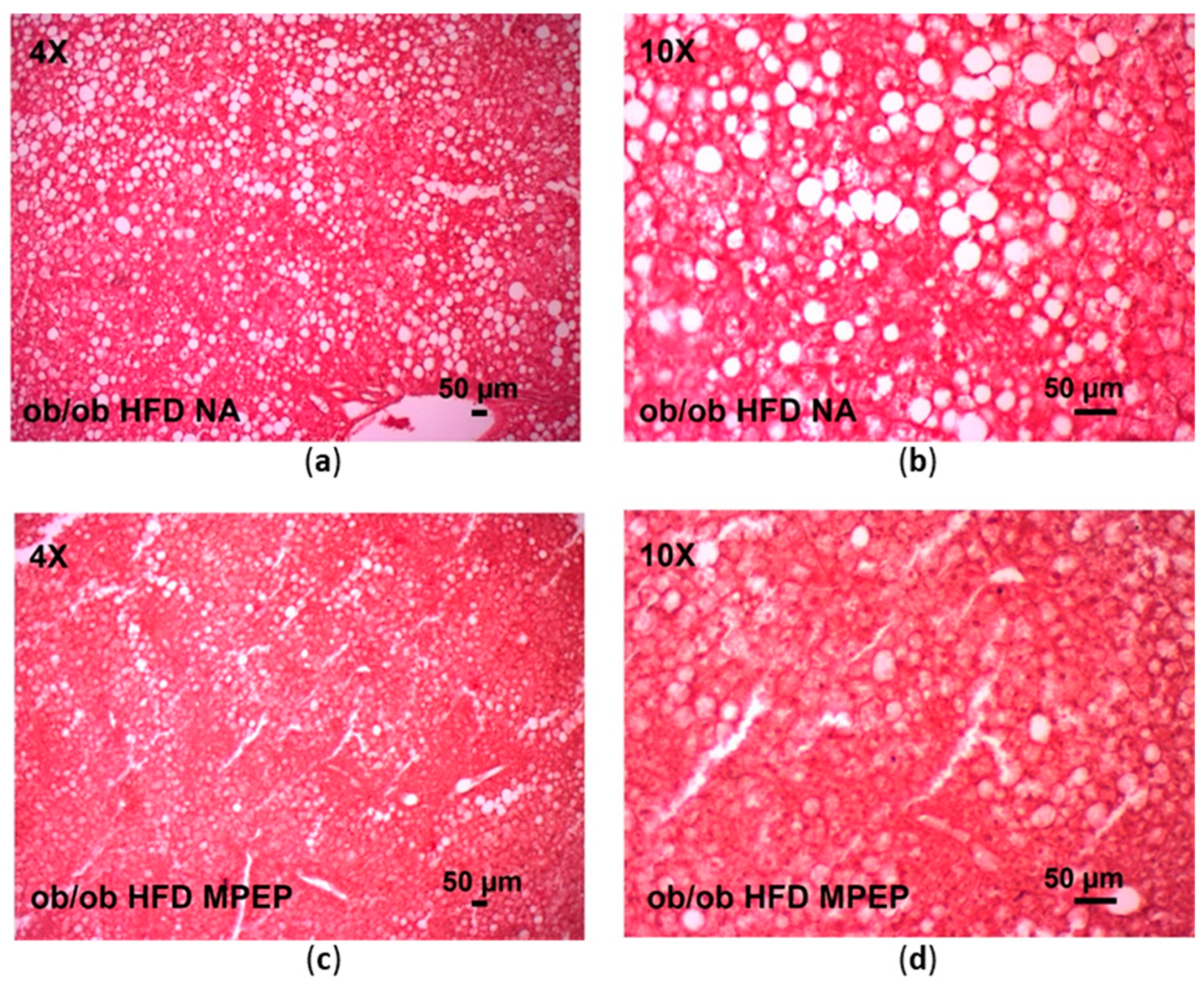
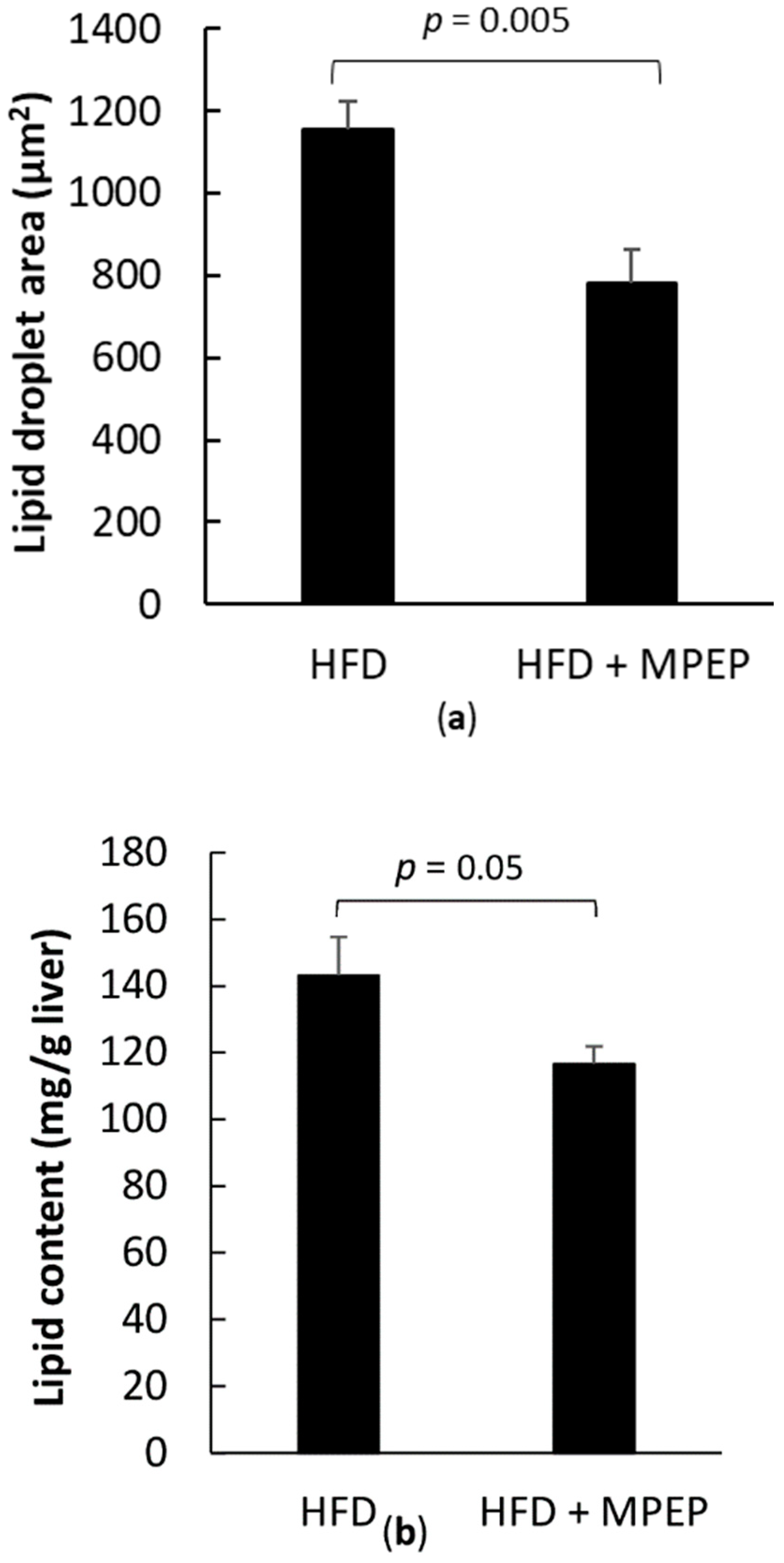
2.2. MPEP Reduces Macrosteatosis, Hepatic Lipid Accumulation and Serum Triglycerides
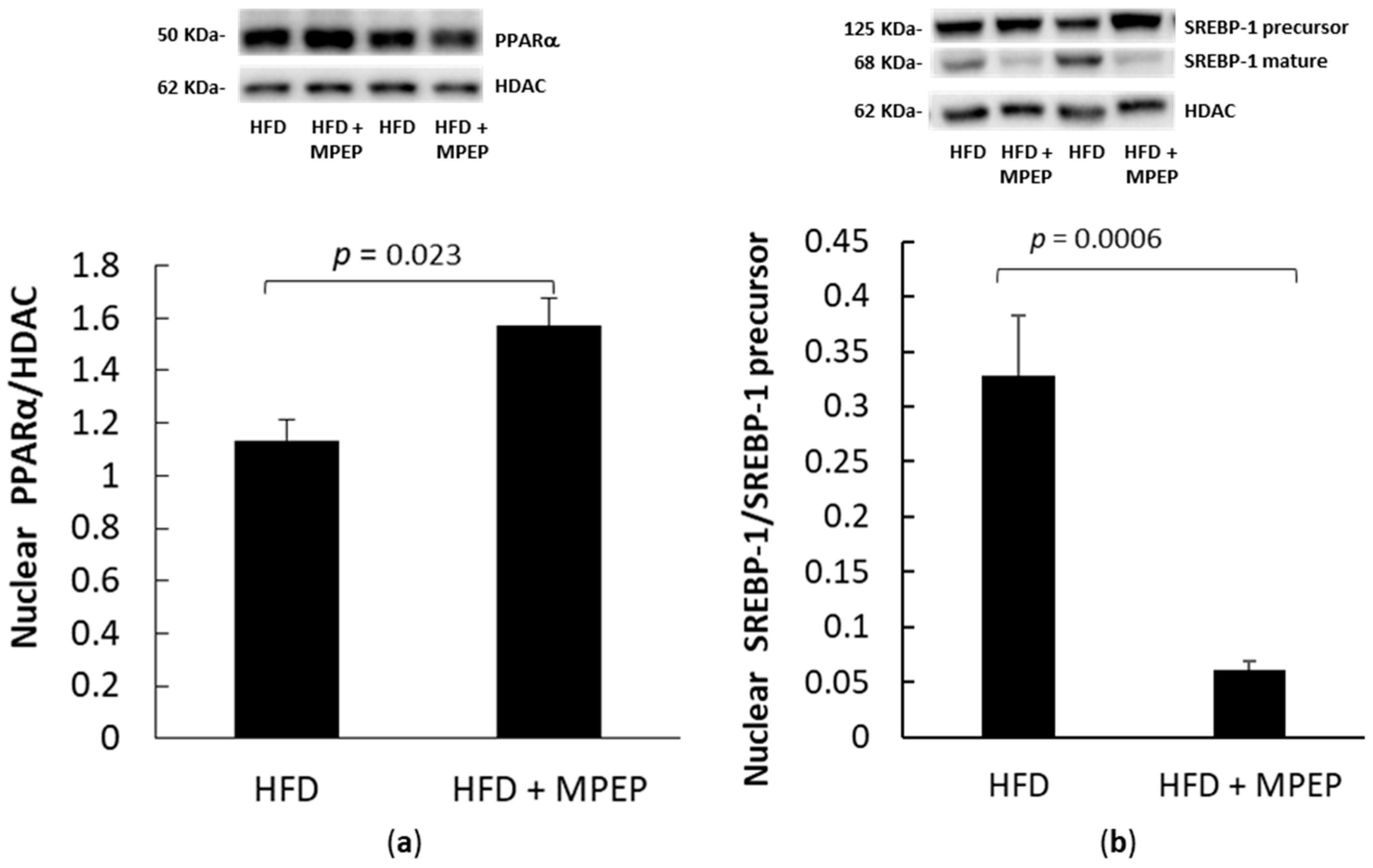
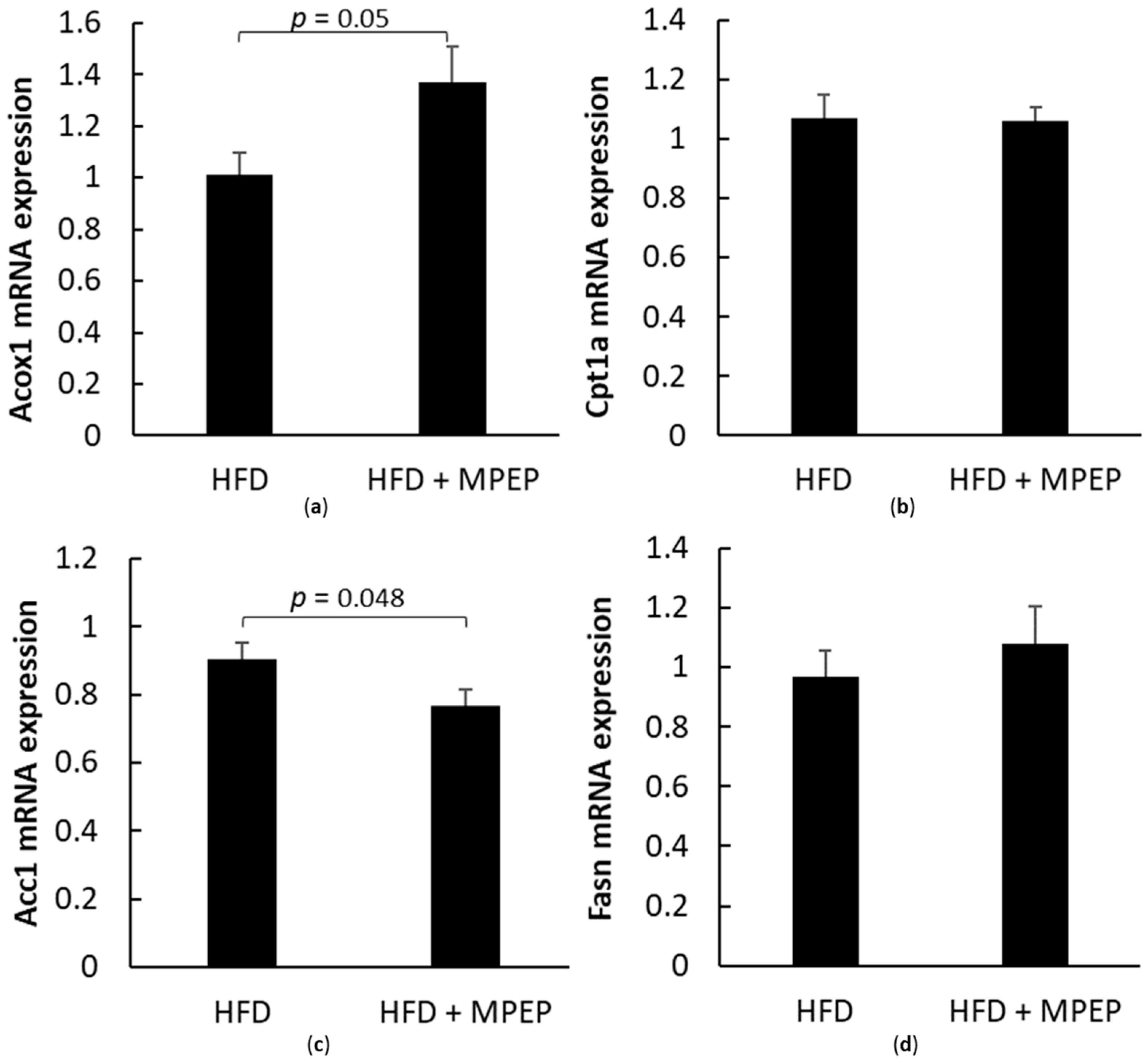
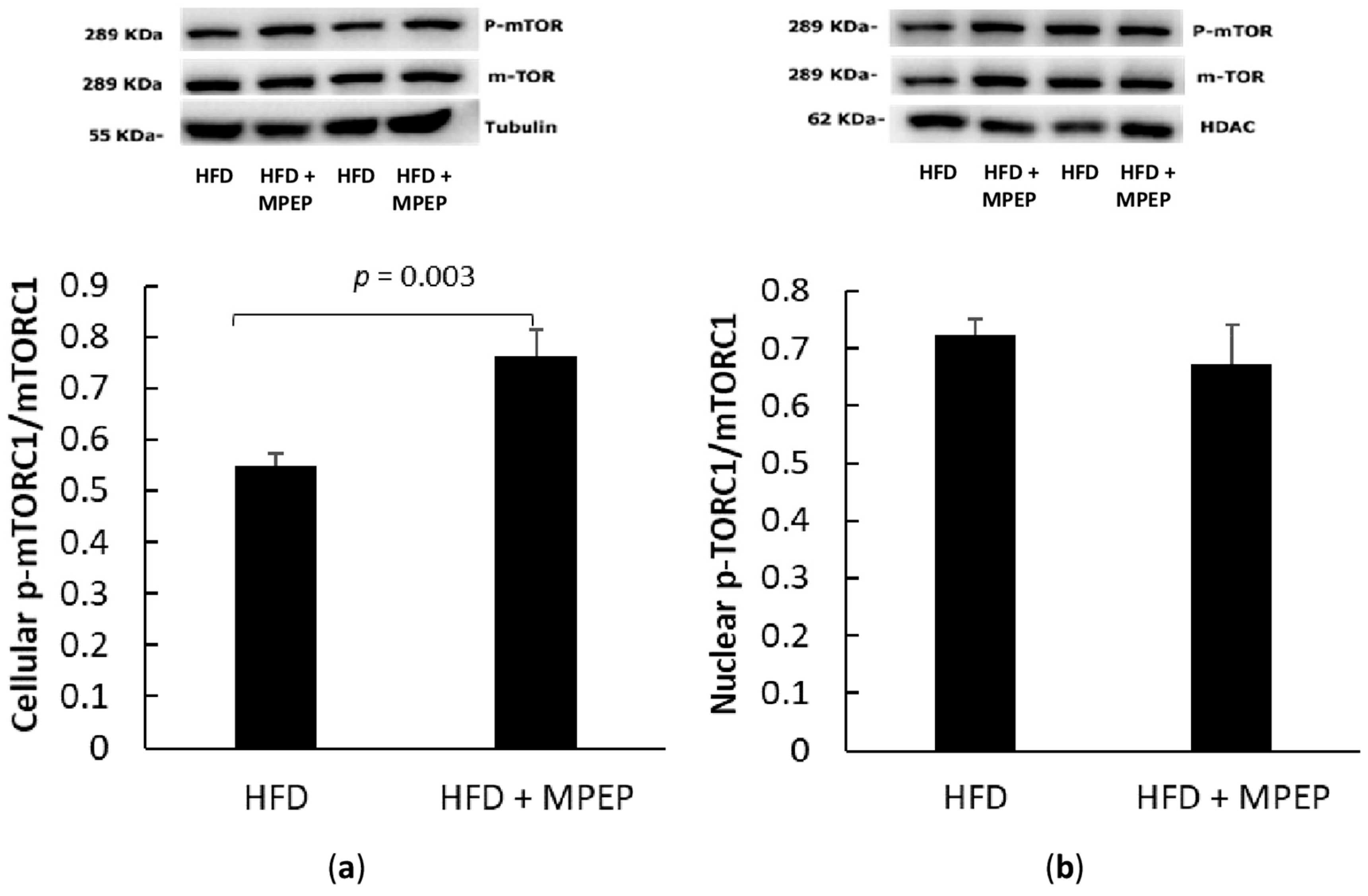
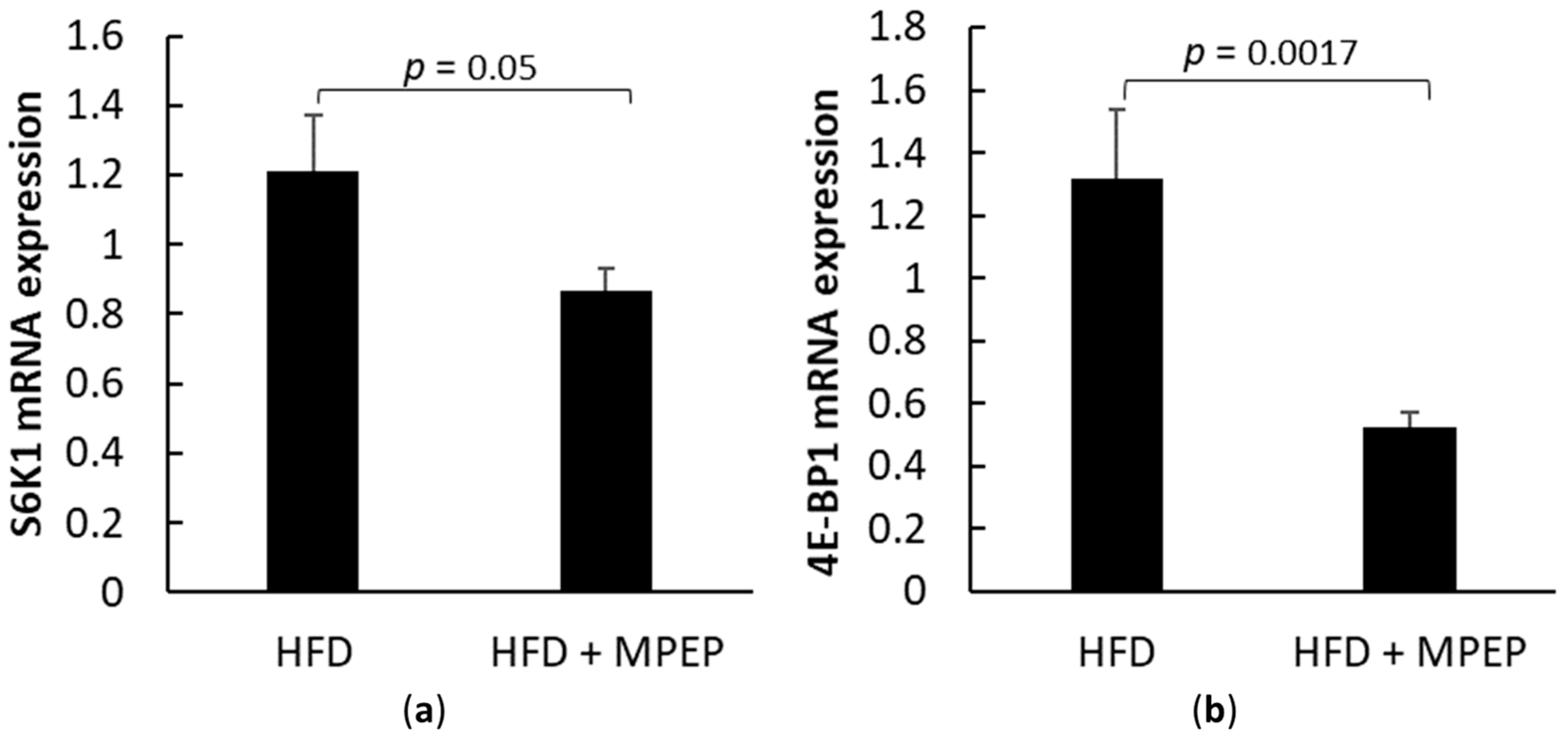
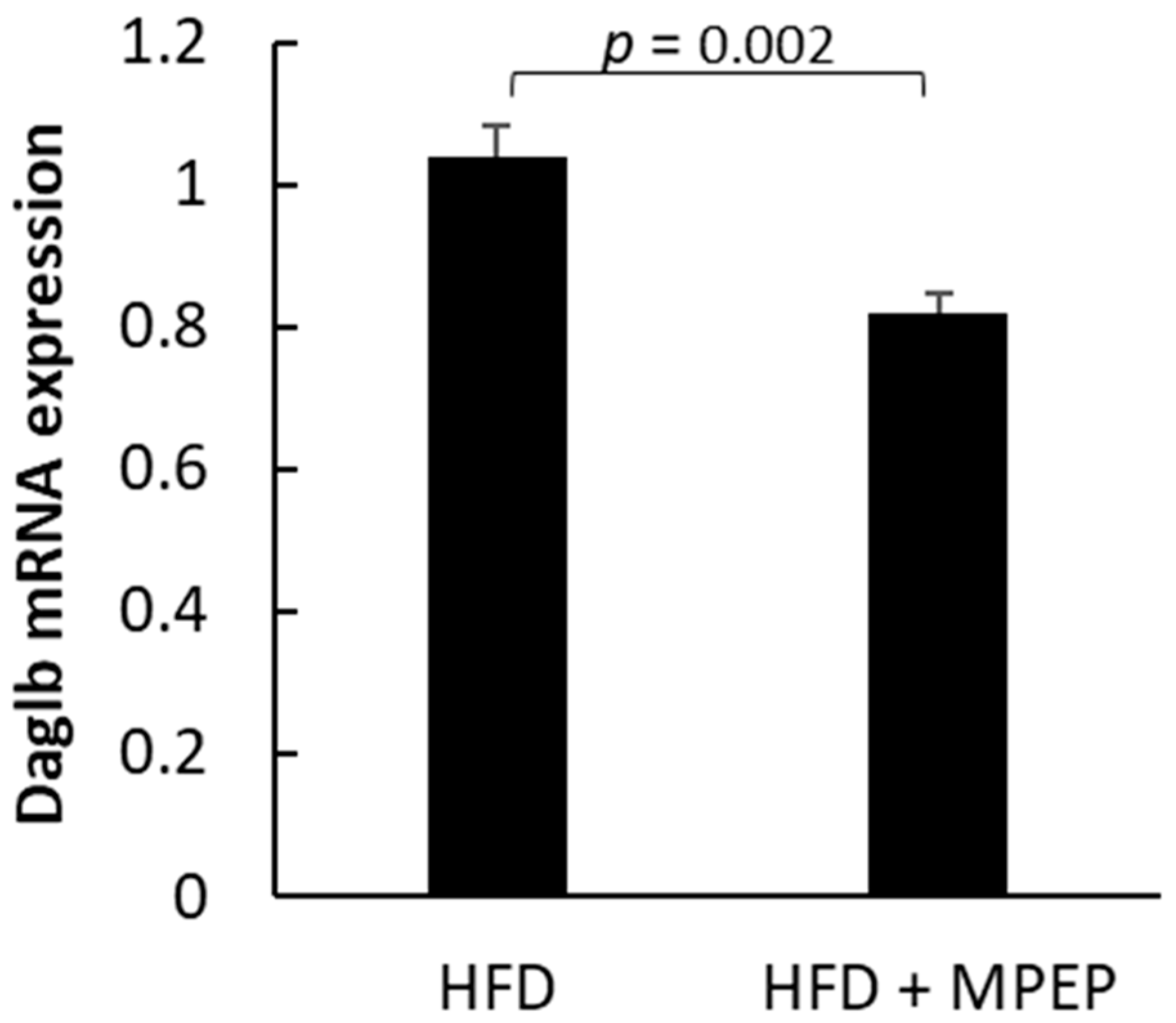
2.3. MPEP Modulates Lipid Metabolism through SREBP-1 and PPARα in an mTOR-Independent Way
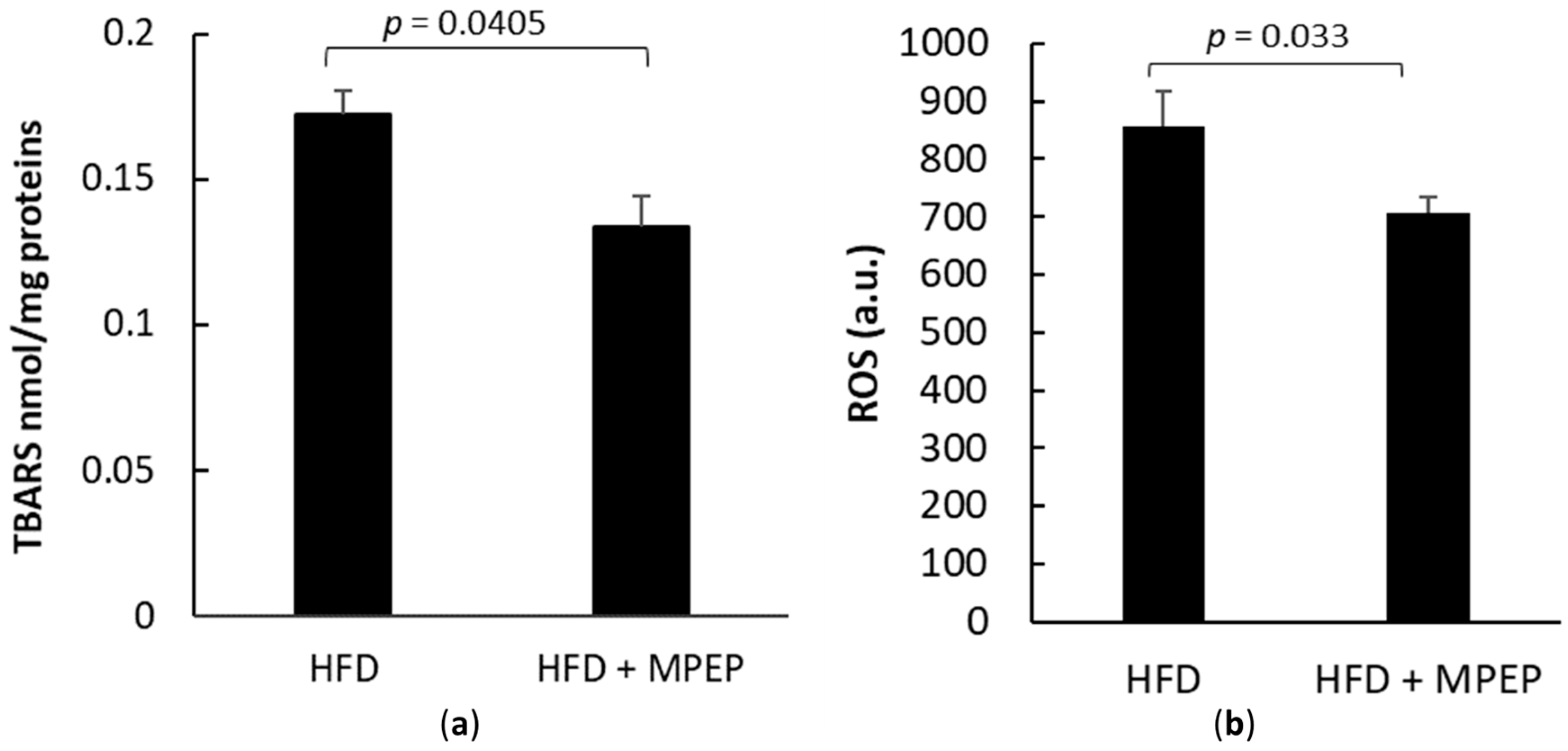
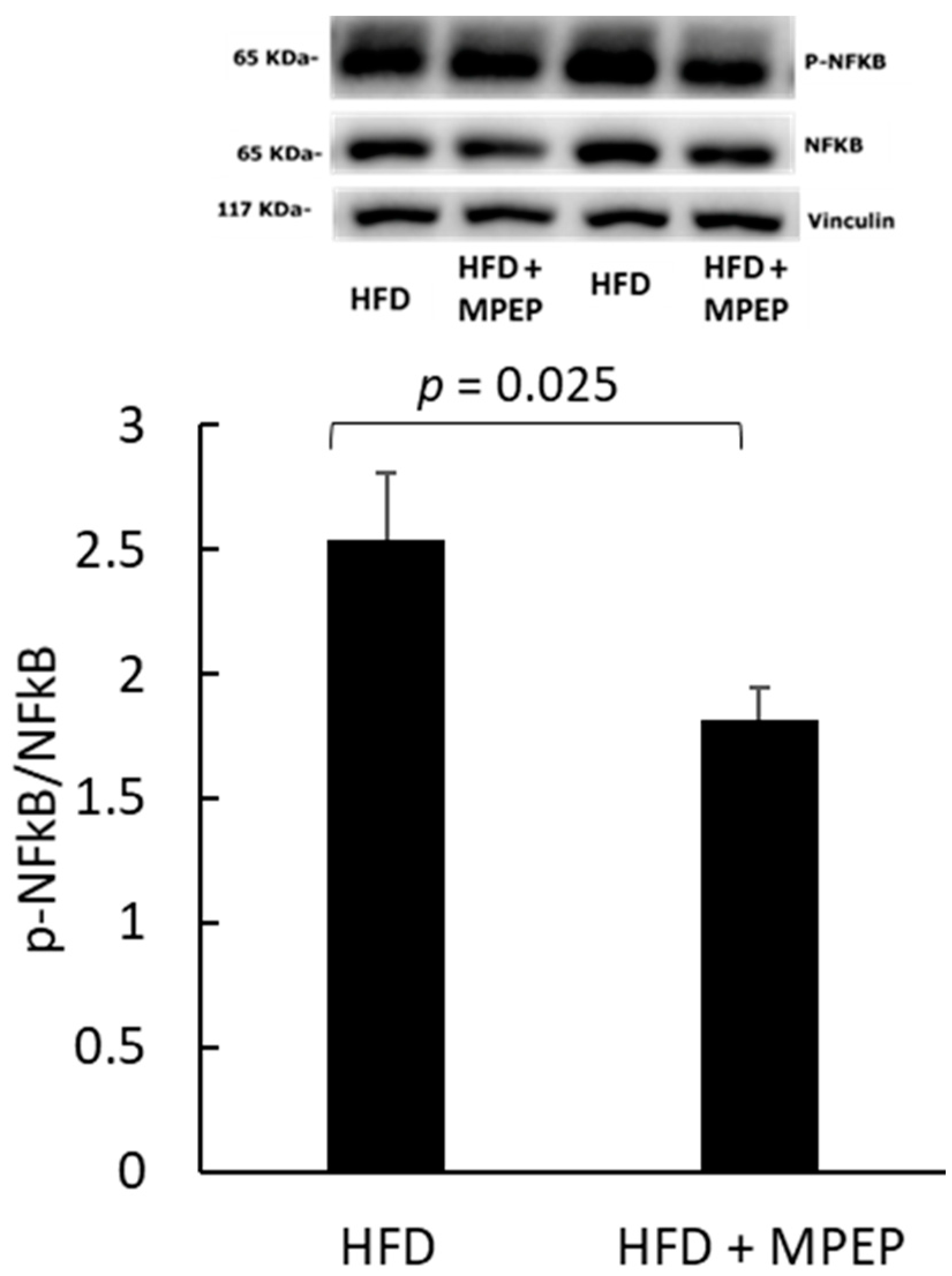
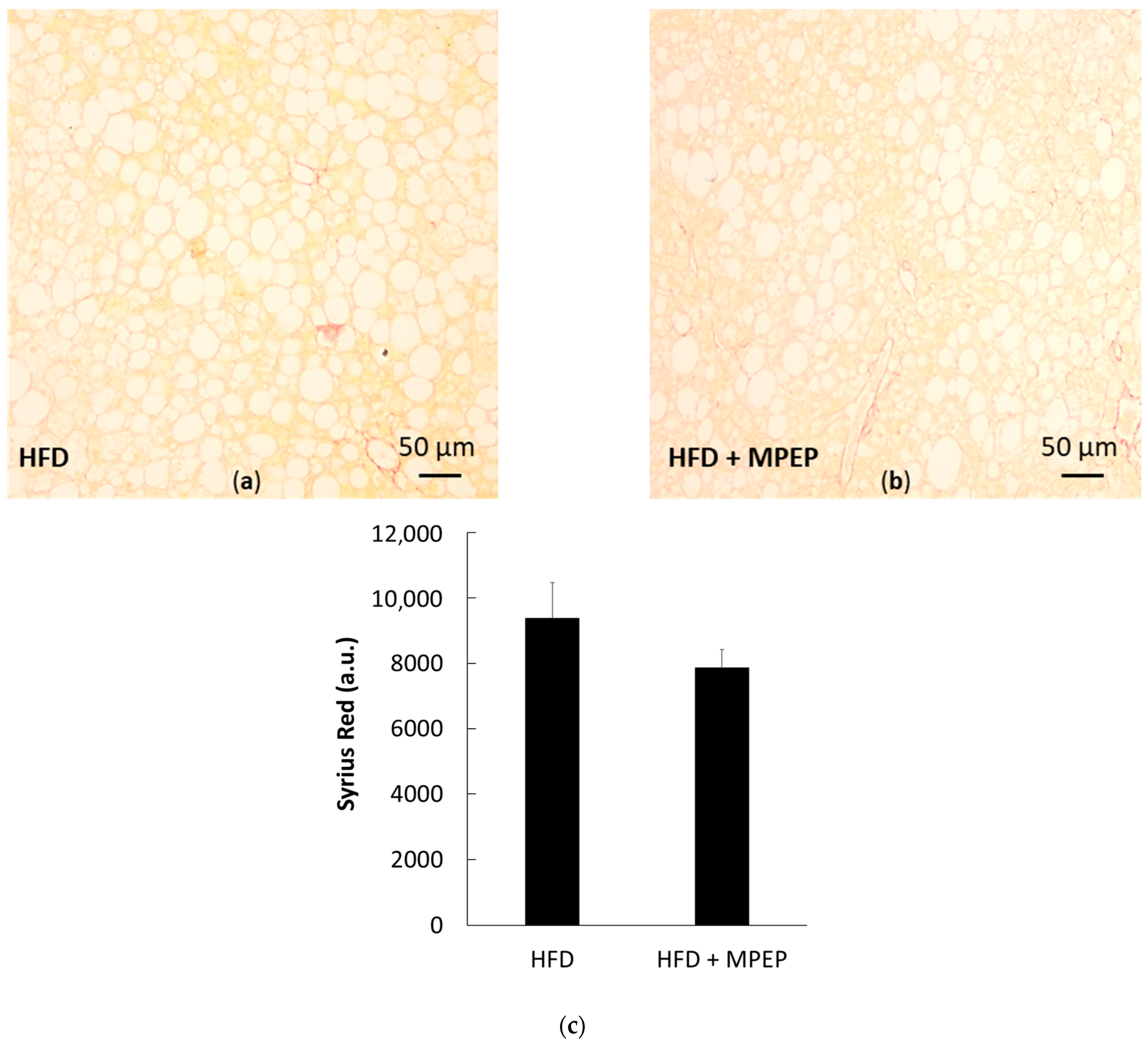
2.4. MPEP Reduces Hepatic Stellate Cells (HSC) Activation
3. Discussion
4. Materials and Methods
4.1. Animals and Experimental Model
4.2. Materials
4.3. Tissue Histology and Staining
4.4. Blood Sample Preparation and Plasma Cholesterol and Triglycerides Evaluation
4.5. Hepatic Lipid Extraction and Quantification
4.6. Analysis of Oxidative Stress and Radical Oxygen Species
4.7. Nuclear Extracts
4.8. Western Blot
4.9. RT-PCR Analysis
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berardo, C.; Di Pasqua, L.G.; Cagna, M.; Richelmi, P.; Vairetti, M.; Ferrigno, A. Nonalcoholic Fatty Liver Disease and Non-Alcoholic Steatohepatitis: Current Issues and Future Perspectives in Preclinical and Clinical Research. Int. J. Mol. Sci. 2020, 21, 9646. [Google Scholar] [CrossRef]
- Huh, Y.; Cho, Y.J.; Nam, G.E. Recent Epidemiology and Risk Factors of Nonalcoholic Fatty Liver Disease. J. Obes. Metab. Syndr. 2022, 31, 17–27. [Google Scholar] [CrossRef] [PubMed]
- LaBrecque, D.R.; Abbas, Z.; Anania, F.; Ferenci, P.; Khan, A.G.; Goh, K.-L.; Hamid, S.S.; Isakov, V.; Lizarzabal, M.; Peñaranda, M.M.; et al. World Gastroenterology Organisation Global Guidelines. J. Clin. Gastroenterol. 2014, 48, 467–473. [Google Scholar] [CrossRef]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 2016, 65, 1038–1048. [Google Scholar] [CrossRef] [PubMed]
- Han, A.L. Association between Non-Alcoholic Fatty Liver Disease and Dietary Habits, Stress, and Health-Related Quality of Life in Korean Adults. Nutrients 2020, 12, 1555. [Google Scholar] [CrossRef]
- Kitade, H.; Chen, G.; Ni, Y.; Ota, T. Nonalcoholic Fatty Liver Disease and Insulin Resistance: New Insights and Potential New Treatments. Nutrients 2017, 9, 387. [Google Scholar] [CrossRef]
- Hrncir, T.; Hrncirova, L.; Kverka, M.; Hromadka, R.; Machova, V.; Trckova, E.; Kostovcikova, K.; Kralickova, P.; Krejsek, J.; Tlaskalova-Hogenova, H. Gut Microbiota and NAFLD: Pathogenetic Mechanisms, Microbiota Signatures, and Therapeutic Interventions. Microorganisms 2021, 9, 957. [Google Scholar] [CrossRef] [PubMed]
- Prasun, P.; Ginevic, I.; Oishi, K. Mitochondrial dysfunction in nonalcoholic fatty liver disease and alcohol related liver disease. Transl. Gastroenterol. Hepatol. 2021, 6, 854–868. [Google Scholar] [CrossRef]
- Flessa, C.M.; Kyrou, I.; Nasiri-Ansari, N.; Kaltsas, G.; Papavassiliou, A.G.; Kassi, E.; Randeva, H.S. Endoplasmic Reticulum Stress and Autophagy in the Pathogenesis of Non-alcoholic Fatty Liver Disease (NAFLD): Current Evidence and Perspectives. Curr. Obes. Rep. 2021, 10, 134–161. [Google Scholar] [CrossRef]
- Lee, Y.A.; Friedman, S.L. Inflammatory and fibrotic mechanisms in NAFLD-Implications for new treatment strategies. J. Intern. Med. 2022, 291, 11–31. [Google Scholar] [CrossRef]
- Eslam, M.; Valenti, L.; Romeo, S. Genetics and epigenetics of NAFLD and NASH: Clinical impact. J. Hepatol. 2018, 68, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Di Pasqua, L.G.; Cagna, M.; Berardo, C.; Vairetti, M.; Ferrigno, A. Detailed Molecular Mechanisms Involved in Drug-Induced Non-Alcoholic Fatty Liver Disease and Non-Alcoholic Steatohepatitis: An Update. Biomedicines 2022, 10, 194. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver (EASL). EASL–EASD–EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. Diabetologia 2016, 59, 1121–1140. [Google Scholar] [CrossRef]
- Storto, M.; de Grazia, U.; Knöpfel, T.; Canonico, P.L.; Copani, A.; Richelmi, P.; Nicoletti, F.; Vairetti, M. Selective blockade of mGlu5 metabotropic glutamate receptors protects rat hepatocytes against hypoxic damage. Hepatology 2000, 31, 649–655. [Google Scholar] [CrossRef]
- Storto, M.; Battaglia, G.; Gradini, R.; Bruno, V.; Nicoletti, F.; Vairetti, M. Mouse hepatocytes lacking mGlu5 metabotropic glutamate receptors are less sensitive to hypoxic damage. Eur. J. Pharmacol. 2004, 497, 25–27. [Google Scholar] [CrossRef]
- Storto, M.; Ngomba, R.T.; Battaglia, G.; Freitas, I.; Griffini, P.; Richelmi, P.; Nicoletti, F.; Vairetti, M. Selective blockade of mGlu5 metabotropic glutamate receptors is protective against acetaminophen hepatotoxicity in mice. J. Hepatol. 2003, 38, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Ferrigno, A.; Berardo, C.; Di Pasqua, L.G.; Siciliano, V.; Richelmi, P.; Nicoletti, F.; Vairetti, M. Selective blockade of the metabotropic glutamate receptor mGluR5 protects mouse livers in in vitro and ex vivo models of ischemia reperfusion injury. Int. J. Mol. Sci. 2018, 19, 314. [Google Scholar] [CrossRef]
- Di Pasqua, L.G.; Berardo, C.; Cagna, M.; Verta, R.; Collotta, D.; Nicoletti, F.; Ferrigno, A.; Collino, M.; Vairetti, M. Metabotropic Glutamate Receptor Blockade Reduces Preservation Damage in Livers from Donors after Cardiac Death. Int. J. Mol. Sci. 2021, 22, 2234. [Google Scholar] [CrossRef]
- Ferrigno, A.; Berardo, C.; Di Pasqua, L.G.; Cagna, M.; Siciliano, V.; Richelmi, P.; Vairetti, M. The selective blockade of metabotropic glutamate receptor-5 attenuates fat accumulation in an in vitro model of benign steatosis. Eur. J. Histochem. 2020, 64, 285–293. [Google Scholar] [CrossRef]
- Paquette, A.; Wang, D.; Jankowski, M.; Gutkowska, J.; Lavoie, J.M. Effects of ovariectomy on PPAR alpha, SREBP-1c, and SCD-1 gene expression in the rat liver. Menopause 2008, 15, 1169–1175. [Google Scholar] [CrossRef]
- Chan, T.S.; Cassim, S.; Raymond, V.-A.; Gottschalk, S.; Merlen, G.; Zwingmann, C.; Lapierre, P.; Darby, P.; Mazer, C.D.; Bilodeau, M. Upregulation of Krebs cycle and anaerobic glycolysis activity early after onset of liver ischemia. PLoS ONE 2018, 13, e0199177. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.M.; Kim, H.H.; Kim, M.H.; Cinar, R.; Yi, H.S.; Eun, H.S.; Kim, S.H.; Choi, Y.J.; Lee, Y.S.; Kim, S.Y.; et al. Glutamate Signaling in Hepatic Stellate Cells Drives Alcoholic Steatosis. Cell Metab. 2019, 30, 877–889.e7. [Google Scholar] [CrossRef]
- Choi, W.M.; Ryu, T.; Lee, J.H.; Shim, Y.R.; Kim, M.H.; Kim, H.H.; Kim, Y.E.; Yang, K.; Kim, K.; Choi, S.E.; et al. Metabotropic Glutamate Receptor 5 in Natural Killer Cells Attenuates Liver Fibrosis by Exerting Cytotoxicity to Activated Stellate Cells. Hepatology 2021, 74, 2170–2185. [Google Scholar] [CrossRef] [PubMed]
- Kristiansen, M.N.B.; Veidal, S.S.; Rigbolt, K.T.G.; Tølbøl, K.S.; Roth, J.D.; Jelsing, J.; Vrang, N.; Feigh, M. Obese diet-induced mouse models of nonalcoholic steatohepatitis-tracking disease by liver biopsy. World J. Hepatol. 2016, 8, 673–684. [Google Scholar] [CrossRef]
- Yan, Q.J.; Rammal, M.; Tranfaglia, M.; Bauchwitz, R.P. Suppression of two major Fragile X Syndrome mouse model phenotypes by the mGluR5 antagonist MPEP. Neuropharmacology 2005, 49, 1053–1066. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Qiu, S.; Zhou, S.; Tan, Y.; Bai, Y.; Cao, H.; Guo, J.; Su, Z. mTOR: A Potential New Target in Nonalcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2022, 23, 9196. [Google Scholar] [CrossRef] [PubMed]
- Wandrer, F.; Liebig, S.; Marhenke, S.; Vogel, A.; John, K.; Manns, M.P.; Teufel, A.; Itzel, T.; Longerich, T.; Maier, O.; et al. TNF-Receptor-1 inhibition reduces liver steatosis, hepatocellular injury and fibrosis in NAFLD mice. Cell Death Dis. 2020, 11, 1–9. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, W.; Zeng, L.Q.; Bai, H.; Li, J.; Zhou, J.; Zhou, G.Y.; Fang, C.W.; Wang, F.; Qin, X.J. Exercise and dietary intervention ameliorate high-fat diet-induced NAFLD and liver aging by inducing lipophagy. Redox Biol. 2020, 36, 101635. [Google Scholar] [CrossRef]
- Richards, S.A.; Fu, J.; Romanelli, A.; Shimamura, A.; Blenis, J. Ribosomal S6 kinase 1 (RSK1) activation requires signals dependent on and independent of the MAP kinase ERK. Curr. Biol. 1999, 9, 810–820. [Google Scholar] [CrossRef]
- Iwamaru, A.; Kondo, Y.; Iwado, E.; Aoki, H.; Fujiwara, K.; Yokoyama, T.; Mills, G.B.; Kondo, S. Silencing mammalian target of rapamycin signaling by small interfering RNA enhances rapamycin-induced autophagy in malignant glioma cells. Oncogene 2006, 26, 1840–1851. [Google Scholar] [CrossRef]
- Willett, M.; Cowan, J.L.; Vlasak, M.; Coldwell, M.J.; Morley, S.J. Inhibition of mammalian target of rapamycin (mTOR) signalling in C2C12 myoblasts prevents myogenic differentiation without affecting the hyperphosphorylation of 4E-BP1. Cell. Signal. 2009, 21, 1504–1512. [Google Scholar] [CrossRef] [PubMed]
- Chapuis, N.; Tamburini, J.; Green, A.S.; Vignon, C.; Bardet, V.; Neyret, A.; Pannetier, M.; Willems, L.; Park, S.; Macone, A.; et al. Dual inhibition of PI3K and mTORC1/2 signaling by NVP-BEZ235 as a new therapeutic strategy for acute myeloid leukemia. Clin. Cancer Res. 2010, 16, 5424–5435. [Google Scholar] [CrossRef] [PubMed]
- Day, C.P.; Saksena, S. Non-alcoholic steatohepatitis: Definitions and pathogenesis. J. Gastroenterol. Hepatol. 2002, 17, S377–S384. [Google Scholar] [CrossRef]
- Zhang, Q.; Yu, K.; Cao, Y.; Luo, Y.; Liu, Y.; Zhao, C. miR-125b promotes the NF-κB-mediated inflammatory response in NAFLD via directly targeting TNFAIP3. Life Sci. 2021, 270, 119071. [Google Scholar] [CrossRef]
- Ho, E.; Karimi Galougahi, K.; Liu, C.C.; Bhindi, R.; Figtree, G.A. Biological markers of oxidative stress: Applications to cardiovascular research and practice. Redox Biol. 2013, 1, 483–491. [Google Scholar] [CrossRef]
- Smith, G.I.; Shankaran, M.; Yoshino, M.; Schweitzer, G.G.; Chondronikola, M.; Beals, J.W.; Okunade, A.L.; Patterson, B.W.; Nyangau, E.; Field, T.; et al. Insulin resistance drives hepatic de novo lipogenesis in nonalcoholic fatty liver disease. J. Clin. Invest. 2020, 130, 1453. [Google Scholar] [CrossRef]
- Guilherme, A.; Virbasius, J.V.; Puri, V.; Czech, M.P. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat. Rev. Mol. Cell Biol. 2008, 9, 367–377. [Google Scholar] [CrossRef]
- Kucera, O.; Cervinkova, Z. Experimental models of non-alcoholic fatty liver disease in rats. World J. Gastroenterol. 2014, 20, 8364–8376. [Google Scholar] [CrossRef]
- Friedman, S.L.; Neuschwander-Tetri, B.A.; Rinella, M.; Sanyal, A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 2018, 24, 908–922. [Google Scholar] [CrossRef] [PubMed]
- Lindström, P. The physiology of obese-hyperglycemic mice [ob/ob mice]. Sci. World J. 2007, 7, 666–685. [Google Scholar] [CrossRef]
- Moslehi, A.; Hamidi-zad, Z. Role of SREBPs in Liver Diseases: A Mini-review. J. Clin. Transl. Hepatol. 2018, 6, 1–7. [Google Scholar] [CrossRef]
- Yahagi, N.; Shimano, H.; Hasty, A.H.; Matsuzaka, T.; Ide, T.; Yoshikawa, T.; Amemiya-Kudo, M.; Tomita, S.; Okazaki, H.; Tamura, Y.; et al. Absence of Sterol Regulatory Element-binding Protein-1 (SREBP-1) Ameliorates Fatty Livers but Not Obesity or Insulin Resistance in Lepob/Lepob Mice. J. Biol. Chem. 2002, 277, 19353–19357. [Google Scholar] [CrossRef]
- Todisco, S.; Santarsiero, A.; Convertini, P.; De Stefano, G.; Gilio, M.; Iacobazzi, V.; Infantino, V. PPAR Alpha as a Metabolic Modulator of the Liver: Role in the Pathogenesis of Nonalcoholic Steatohepatitis (NASH). Biology 2022, 11, 792. [Google Scholar] [CrossRef] [PubMed]
- Montagner, A.; Polizzi, A.; Fouché, E.; Ducheix, S.; Lippi, Y.; Lasserre, F.; Barquissau, V.; Régnier, M.; Lukowicz, C.; Benhamed, F.; et al. Liver PPARα is crucial for whole-body fatty acid homeostasis and is protective against NAFLD. Gut 2016, 65, 1202–1214. [Google Scholar] [CrossRef] [PubMed]
- Régnier, M.; Polizzi, A.; Smati, S.; Lukowicz, C.; Fougerat, A.; Lippi, Y.; Fouché, E.; Lasserre, F.; Naylies, C.; Bétoulières, C.; et al. Hepatocyte-specific deletion of Pparα promotes NAFLD in the context of obesity. Sci. Rep. 2020, 10, 6489. [Google Scholar] [CrossRef]
- Huang, X.T.; Yang, J.X.; Wang, Z.; Zhang, C.Y.; Luo, Z.Q.; Liu, W.; Tang, S.Y. Activation of N-methyl-D-aspartate receptor regulates insulin sensitivity and lipid metabolism. Theranostics 2021, 11, 2247. [Google Scholar] [CrossRef] [PubMed]
- O’leary, D.M.; Movsesyan, V.; Vicini, S.; Faden, A.I. Selective mGluR5 antagonists MPEP and SIB-1893 decrease NMDA or glutamate-mediated neuronal toxicity through actions that reflect NMDA receptor antagonism. Br. J. Pharmacol. 2000, 131, 1429–1437. [Google Scholar] [CrossRef]
- Lee, J.H.; Kang, H.S.; Park, H.Y.; Moon, Y.A.; Kang, Y.N.; Oh, B.C.; Song, D.K.; Bae, J.H.; Im, S.S. PPARα-dependent Insig2a overexpression inhibits SREBP-1c processing during fasting. Sci. Rep. 2017, 7, 9958. [Google Scholar] [CrossRef]
- Yoon, M. The role of PPARα in lipid metabolism and obesity: Focusing on the effects of estrogen on PPARα actions. Pharmacol. Res. 2009, 60, 151–159. [Google Scholar] [CrossRef]
- Liang, C.P.; Tall, A.R. Transcriptional Profiling Reveals Global Defects in Energy Metabolism, Lipoprotein, and Bile Acid Synthesis and Transport with Reversal by Leptin Treatment in Ob/ob Mouse Liver. J. Biol. Chem. 2001, 276, 49066–49076. [Google Scholar] [CrossRef] [PubMed]
- Muoio, D.M.; Dohm, G.L. Peripheral metabolic actions of leptin. Best Pract. Res. Clin. Endocrinol. Metab. 2002, 16, 653–666. [Google Scholar] [CrossRef]
- König, B.; Koch, A.; Spielmann, J.; Hilgenfeld, C.; Hirche, F.; Stangl, G.I.; Eder, K. Activation of PPARα and PPARγ reduces triacylglycerol synthesis in rat hepatoma cells by reduction of nuclear SREBP-1. Eur. J. Pharmacol. 2009, 605, 23–30. [Google Scholar] [CrossRef]
- Shimomura, I.; Bashmakov, Y.; Horton, J.D. Increased levels of nuclear SREBP-1c associated with fatty livers in two mouse models of diabetes mellitus. J. Biol. Chem. 1999, 274, 30028–30032. [Google Scholar] [CrossRef] [PubMed]
- Nawroth, R.; Stellwagen, F.; Schulz, W.A.; Stoehr, R.; Hartmann, A.; Krause, B.J.; Gschwend, J.E.; Retz, M. S6K1 and 4E-BP1 Are Independent Regulated and Control Cellular Growth in Bladder Cancer. PLoS ONE 2011, 6, e27509. [Google Scholar] [CrossRef]
- Gosis, B.S.; Wada, S.; Thorsheim, C.; Li, K.; Jung, S.; Rhoades, J.H.; Yang, Y.; Brandimarto, J.; Li, L.; Uehara, K.; et al. Inhibition of nonalcoholic fatty liver disease in mice by selective inhibition of mTORC1. Science 2022, 376, eabf8271. [Google Scholar] [CrossRef] [PubMed]
- Peterson, T.R.; Sengupta, S.S.; Harris, T.E.; Carmack, A.E.; Kang, S.A.; Balderas, E.; Guertin, D.A.; Madden, K.L.; Carpenter, A.E.; Finck, B.N.; et al. mTOR Complex 1 Regulates Lipin 1 Localization to Control the SREBP Pathway. Cell 2011, 146, 408–420. [Google Scholar] [CrossRef]
- Quinn, W.J.; Wan, M.; Shewale, S.V.; Gelfer, R.; Rader, D.J.; Birnbaum, M.J.; Titchenell, P.M. mTORC1 stimulates phosphatidylcholine synthesis to promote triglyceride secretion. J. Clin. Investig. 2017, 127, 4207–4215. [Google Scholar] [CrossRef]
- Umemura, A.; Park, E.J.; Taniguchi, K.; Lee, J.H.; Shalapour, S.; Valasek, M.A.; Aghajan, M.; Nakagawa, H.; Seki, E.; Hall, M.N.; et al. Liver Damage, Inflammation, and Enhanced Tumorigenesis after Persistent mTORC1 Inhibition. Cell Metab. 2014, 20, 133–144. [Google Scholar] [CrossRef]
- Fouad, Y.; Waked, I.; Bollipo, S.; Gomaa, A.; Ajlouni, Y.; Attia, D. What’s in a name? Renaming ‘NAFLD’ to ‘MAFLD’. Liver Int. 2020, 40, 1254–1261. [Google Scholar] [CrossRef]
- Du, J.; Zhang, X.; Han, J.; Man, K.; Zhang, Y.; Chu, E.S.; Nan, Y.; Yu, J. Pro-Inflammatory CXCR3 Impairs Mitochondrial Function in Experimental Non-Alcoholic Steatohepatitis. Theranostics 2017, 7, 4192–4203. [Google Scholar] [CrossRef] [PubMed]
- Vineeth Daniel, P.; Dogra, S.; Rawat, P.; Choubey, A.; Khan, A.S.; Rajak, S.; Kamthan, M.; Mondal, P. NF-κB p65 regulates hepatic lipogenesis by promoting nuclear entry of ChREBP in response to a high carbohydrate diet. J. Biol. Chem. 2021, 296, 100714. [Google Scholar] [CrossRef] [PubMed]
- O’Riordan, K.J.; Huang, I.C.; Pizzi, M.; Spano, P.F.; Boroni, F.; Egli, R.; Desai, P.; Fitch, O.; Malone, L.; Hyung, J.A.; et al. Regulation of Nuclear Factor κB in the Hippocampus by Group I Metabotropic Glutamate Receptors. J. Neurosci. 2006, 26, 4870. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Silverstein, P.S.; Singh, D.P.; Kumar, A. Involvement of metabotropic glutamate receptor 5, AKT/PI3K Signaling and NF-κB pathway in methamphetamine-mediated increase in IL-6 and IL-8 expression in astrocytes. J. Neuroinflamm. 2012, 9, 52. [Google Scholar] [CrossRef]
- Feng, L.R.; Fernández-Martínez, J.L.; Zaal, K.J.M.; Deandrés-Galiana, E.J.; Wolff, B.S.; Saligan, L.N. mGluR5 mediates post-radiotherapy fatigue development in cancer patients. Transl. Psychiatry 2018, 8, 110. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Buttari, B.; Panieri, E.; Profumo, E.; Saso, L. An Overview of Nrf2 Signaling Pathway and Its Role in Inflammation. Molecules 2020, 25, 5474. [Google Scholar] [CrossRef]
- Berardo, C.; Cagna, M.; Di Pasqua, L.G.; Croce, A.C.; Nicoletti, F.; Vairetti, M.; Ferrigno, A. mGluR5 Selective Blockade Reduces Oxidative Stress and Lipid Accumulation in Obese-High Fat Diet Mice. In Proceedings of the Taormina 2021-10th International Meeting on Metabotropic Glutamate Receptors, Taormina, Italy, 1–6 October 2021. [Google Scholar]
- Lyn-Cook, L.E.; Lawton, M.; Tong, M.; Silbermann, E.; Longato, L.; Jiao, P.; Mark, P.; Wands, J.R.; Xu, H.; de la Monte, S.M. Hepatic ceramide may mediate brain insulin resistance and neurodegeneration in type 2 diabetes and non-alcoholic steatohepatitis. J. Alzheimer’s Dis. 2009, 16, 715–729. [Google Scholar] [CrossRef]
- Esterbauer, H.; Cheeseman, K.H. Determination of aldehydic lipid peroxidation products: Malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990, 186, 407–421. [Google Scholar] [PubMed]
- Palladini, G.; Di Pasqua, L.G.; Berardo, C.; Siciliano, V.; Richelmi, P.; Mannucci, B.; Croce, A.C.; Rizzo, V.; Perlini, S.; Vairetti, M.; et al. Fatty acid desaturase involvement in non-alcoholic fatty liver disease rat models: Oxidative stress versus metalloproteinases. Nutrients 2019, 11, 799. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Nagata, T.; Redman, R.S.; Lakshman, R. Isolation of intact nuclei of high purity from mouse liver. Anal. Biochem. 2010, 398, 178–184. [Google Scholar] [CrossRef]
- Chomczynski, P.; Mackey, K. Substitution of chloroform by bromo-chloropropane in the single-step method of RNA isolation. Anal. Biochem. 1995, 225, 163–164. [Google Scholar] [CrossRef] [PubMed]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed]


| HFD | HFD + MPEP | |
|---|---|---|
| Body weight (g) | 46 ± 0.97 | 40.83 ± 1.51 *** |
| Liver weight (g) | 5.37 ± 0.32 | 4.26 ± 0.17 *** |
| AST (mg/dL) | 537 ± 77.93 | 520.33 ± 30.67 |
| ALT (mg/dL) | 523.2 ± 49.47 | 485.6 ± 17.07 |
| Glucose (mg/dL) | 196.5 ± 35.22 | 199.33 ± 32.59 |
| Gene | Amplicon Sequence |
|---|---|
| Acc1 (Acaca) | GAGAAGGAGGGCTCCCTGTCACCAGCCTCCGTCAGCTCAGATACACTTTCTGAT TTGGGGATCTCTGGCTTACAGGATGGTTTGGCCTTTCACATGAGATCCAGCATGT CTGGCTTGCACCTAGTAA |
| Acox1 | TACGACACCATACCACCCACCAGCTTCCCCGACTGAACCTGGTCATAGATTTTCA TCAAGAACCTGGCCGTCTGCAGCATCATAACAGTGTTCTCCC |
| Cpt1a | TACATCTACCTGCGGGGCCGAGGGCCGATCATGGTTAACAGCAACTACTACGCC ATGGAGATGCTCTACATCACCCCAACCCATATTCAGGCAGCGAGAGCTGGCAAC ACCATCCACGCCATACTGC |
| Daglb | GGAGGACCTCAAGAGGAGGATCCTGAGAGTGATCGCTAACTGCAATAAGCCGAA GTACAAGATCTTGCTGCATGGCTGTTGGTACGGACTGTTCGGAGGAAGCCCTG |
| 4E-BP1 (Eif4ebp1) | GAACCAGGATTATCTATGACCGGAAATTTCTGATGGAGTGTCGGAACTCACCTGT GGCCAAAACACCCCCAAAGGACCTGCCAGCCATTCCTGGGGTCACTAGCCCTAC CAGCGATGAGCCTCCCATGCAAGCCAGCCAGAGCCAACTGCCCAGCAGCCCGG AAGATAAGCGGGCAGGCGGTGAAGAGTCACAATTTGAGATGGACATTTAAGGGA CCAGCCGTAGGAC |
| Fasn | ACTAGAGCCAACCAGATGCTTCAGTCTTAGGCTAATCACTCAGACTGGGATCCTC TGAACACCTTTGTACCCTACCAATGCAGTTGTCCTCTGGATGCTCCTCTCTGGAT GTGATCGAATGCTGCG |
| Gapdh | TGGGAGTTGCTGTTGAAGTCGCAGGAGACAACCTGGTCCTCAGTGTAGCCCAAG ATGCCCTTCAGTGGGCCCTCAGATGCCTGCTTCACCACCTTCTTGATGTCA |
| Il1β | TATTTTGTCGTTGCTTGGTTCTCCTTGTACAAAGCTCATGGAGAATATCACTTGTT GGTTGATATTCTGTCCATTGAGGTGGAGAGCTTTCAGCTCATATGGGTCC |
| Il-6 | ACAAGAAAGACAAAGCCAGAGTCCTTCAGAGAGATACAGAAACTCTAATTCATAT CTTCAACCAAGAGGTAAAAGATTTACATAAAAT |
| Nrf2 (Nfe2l2) | ACTTACTCCAAGATCTATGTCTTGCCTCCAAAGGATGTCAATCAAATCCATGTCCT GCTGGGACTGTAGTCCTGGCGGTGGCAACTCCAAGTCCATCATGCTGAGGGCG GACGCTGTGGTAGGGC |
| Rps9 | CGTCCAGGCCGAGTGAAGAGGAAGAATGCCAAGAAAGGCCAGGGCGGGGCTG GAGCTGGTGATGATGAGGAAGAGGATTAATTAATACTTGGCTGAACTGGAGGAT TGTCTAGTTTTCC |
| S6K1 (Rps6kb1) | TTTTTAAGCACCTTCATGGCAAATATCTTCCCAGTATTTGCTCCTGTTACTTTTCG TACTTGAAAAACCTTTCCATAGCCCCCTTTACCAAGTACCCGAAGTAGCTCAAAA CATTCTGGTCTGATTTTTTCTGGCCCTCTGTTCACACTAGTTTCTGAGATTTCAAA TTTCTCACAATGTTCCATGCCAAGTTCATATGGTCCAACTCCC |
| Tnf-α | CCACAAGCAGGAATGAGAAGAGGCTGAGACATAGGCACCGCCTGGAGTTCTGG AAGCCCCCCATCTTTTGGGGGAGTGCCTCTTCTGCCAGTTCCACGTCGCGGATC ATGCTTTCTGTGCTCATGGTGTCTTTTCTGGAGGGAGATGTG |
| Ubc | GATGCCCTCCTTGTCCTGGATCTTTGCCTTGACATTCTCAATGGTGTCACTGGGC TCGACCTCCAGGGTGATGGTCTTACCAGTTAAGGTTTTCACAAAGATCTGCATCG TCTCTCTCACGGAGTTGTTTCACGGTGGCGTCCAGA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrigno, A.; Cagna, M.; Bosco, O.; Trucchi, M.; Berardo, C.; Nicoletti, F.; Vairetti, M.; Di Pasqua, L.G. MPEP Attenuates Intrahepatic Fat Accumulation in Obese Mice. Int. J. Mol. Sci. 2023, 24, 6076. https://doi.org/10.3390/ijms24076076
Ferrigno A, Cagna M, Bosco O, Trucchi M, Berardo C, Nicoletti F, Vairetti M, Di Pasqua LG. MPEP Attenuates Intrahepatic Fat Accumulation in Obese Mice. International Journal of Molecular Sciences. 2023; 24(7):6076. https://doi.org/10.3390/ijms24076076
Chicago/Turabian StyleFerrigno, Andrea, Marta Cagna, Oriana Bosco, Michelangelo Trucchi, Clarissa Berardo, Ferdinando Nicoletti, Mariapia Vairetti, and Laura G. Di Pasqua. 2023. "MPEP Attenuates Intrahepatic Fat Accumulation in Obese Mice" International Journal of Molecular Sciences 24, no. 7: 6076. https://doi.org/10.3390/ijms24076076
APA StyleFerrigno, A., Cagna, M., Bosco, O., Trucchi, M., Berardo, C., Nicoletti, F., Vairetti, M., & Di Pasqua, L. G. (2023). MPEP Attenuates Intrahepatic Fat Accumulation in Obese Mice. International Journal of Molecular Sciences, 24(7), 6076. https://doi.org/10.3390/ijms24076076









