Enhanced Performance of WO3/SnO2 Nanocomposite Electrodes with Redox-Active Electrolytes for Supercapacitors
Abstract
1. Introduction
2. Results
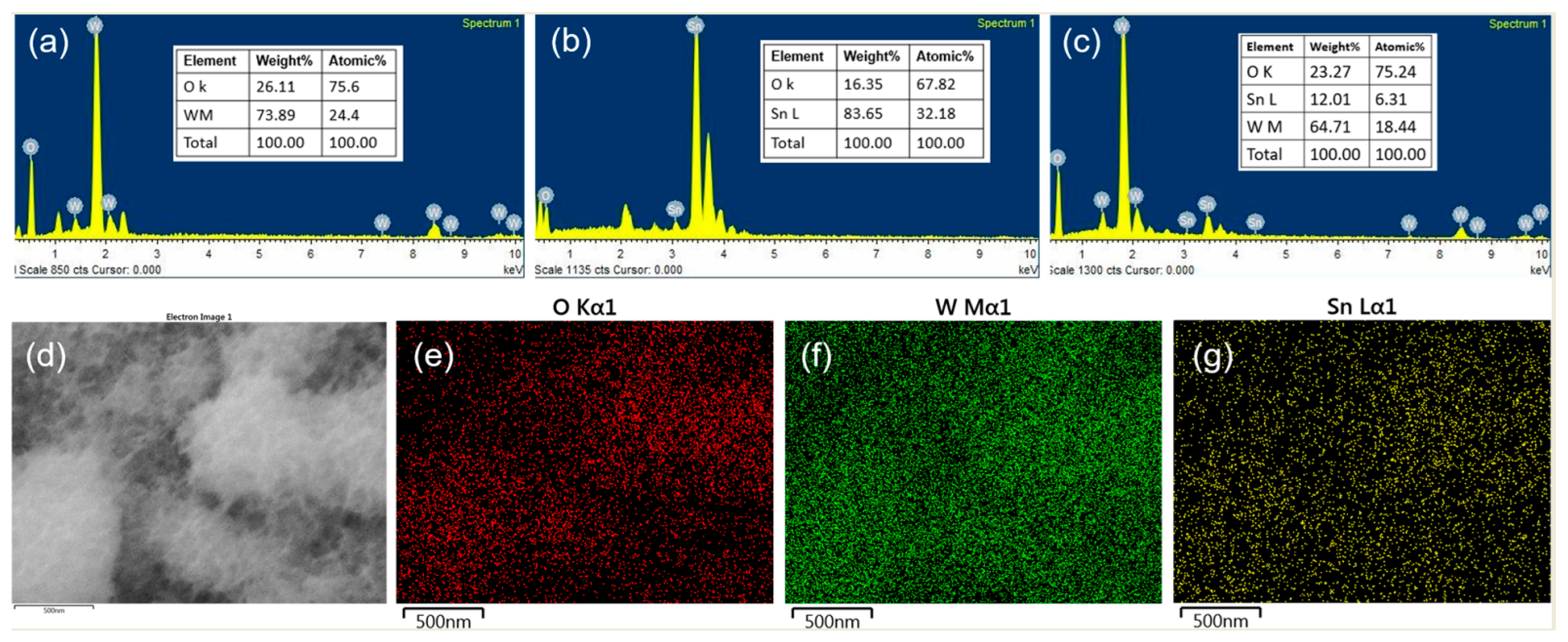
2.1. Characterization of WO3, SnO2, and WO3/SnO2 Nanocomposite Thin Films
2.2. Electrochemical Measurements
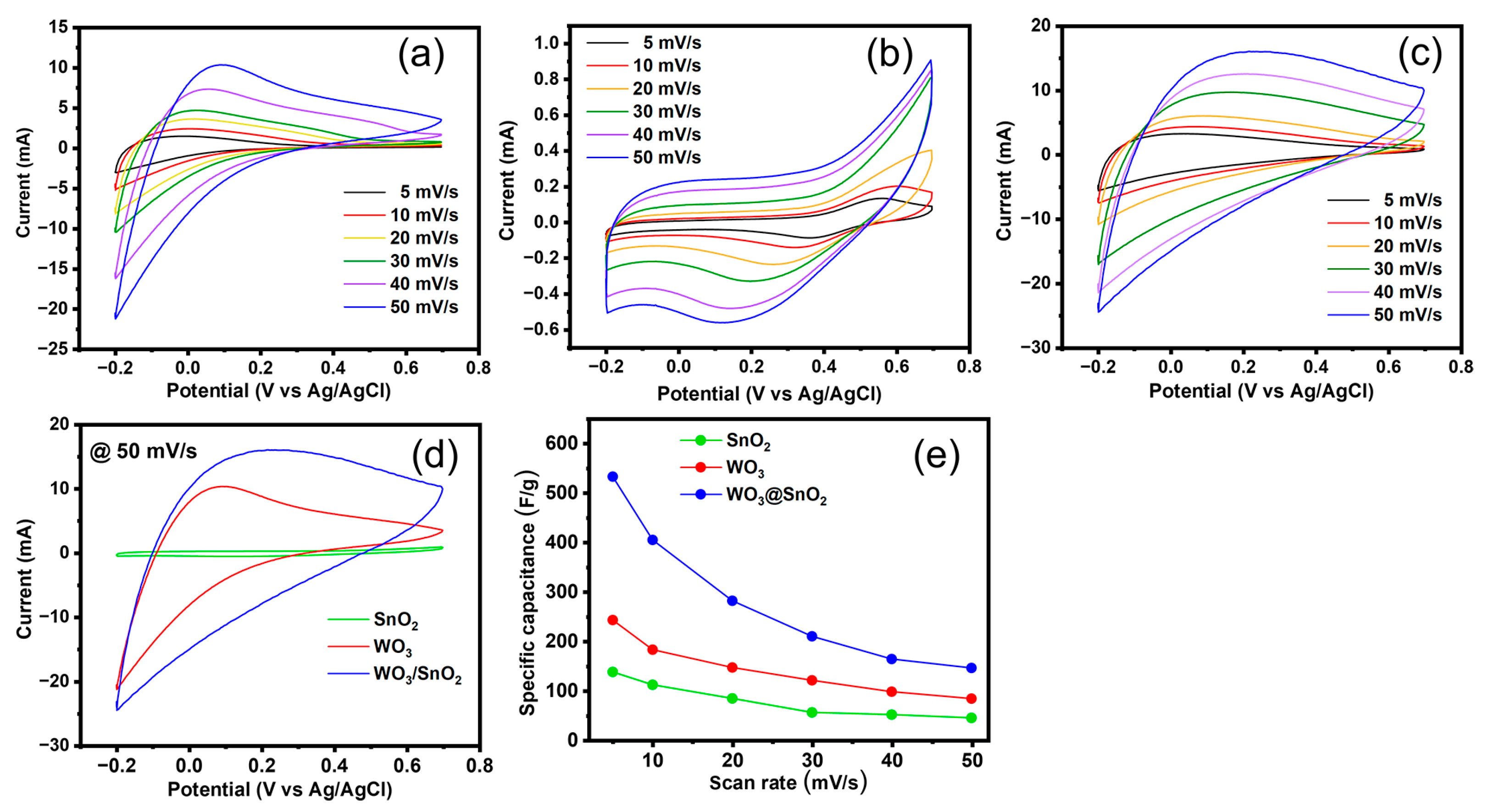
2.2.1. Electrochemical Measurements without a Redox-Active Electrolyte
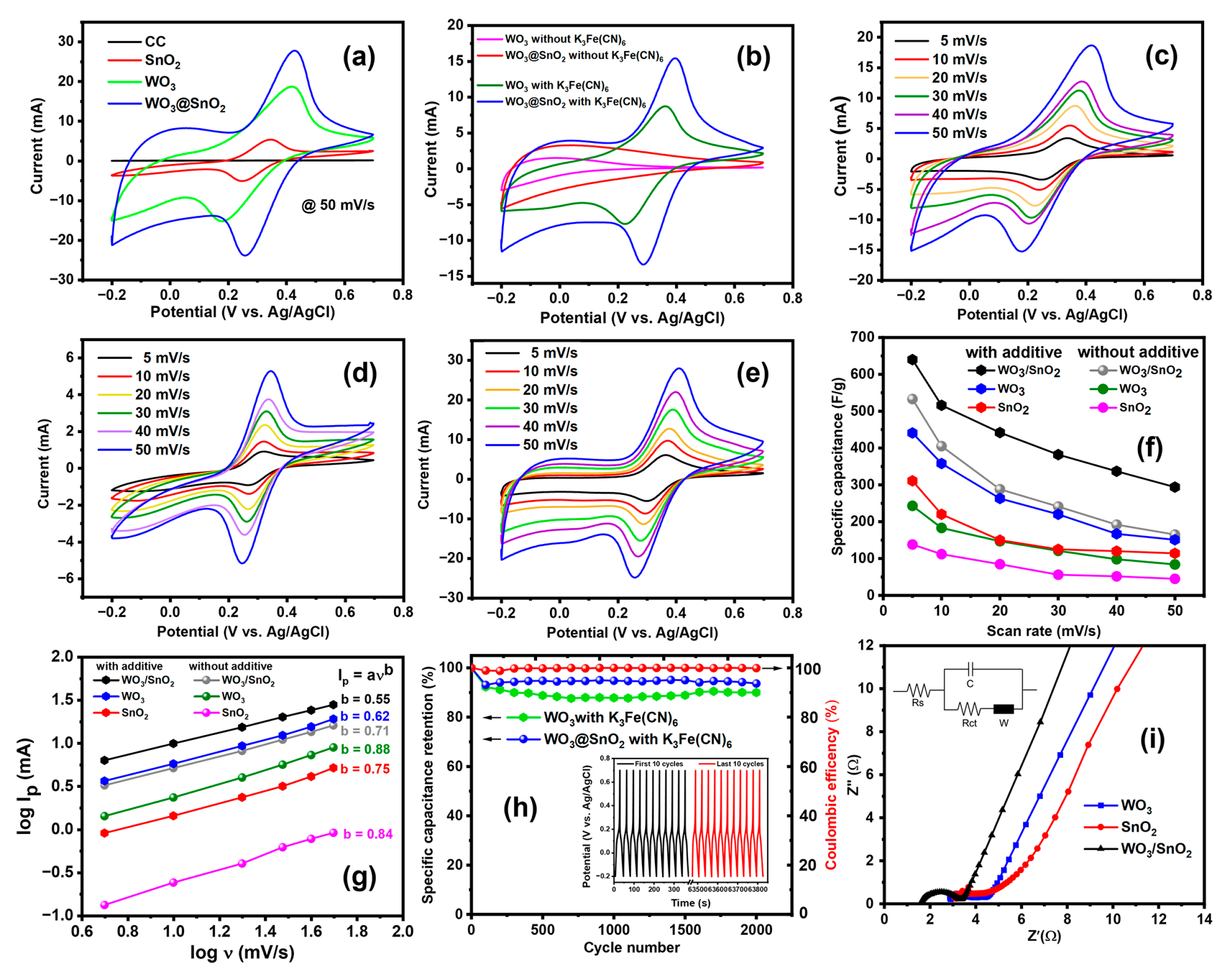
2.2.2. Effects of Redox-Active Electrolyte
2.2.3. Electrochemical Performance of Symmetric Systems
3. Discussion
4. Materials and Methods
4.1. Chemical Bath Deposition of WO3 Nanosphere Thin Films
4.2. Electrochemical Deposition of SnO2 Nanorods
4.3. Synthesis of a WO3/SnO2 Nanocomposite Electrode
4.4. Electrochemical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shinde, P.A.; Lokhande, A.C.; Patil, A.M.; Lokhande, C.D. Facile synthesis of self-assembled WO3 nanorods for high-performance electrochemical capacitor. J. Alloys Compd. 2019, 770, 1130–1137. [Google Scholar] [CrossRef]
- Barik, R.; Yadav, A.K.; Jha, S.N.; Bhattacharyya, D.; Ingole, P.P. Two-dimensional tungsten oxide/selenium nanocomposite fabricated for flexible supercapacitors with higher operational voltage and their charge storage mechanism. ACS Appl. Mater. Interfaces 2021, 13, 8102–8119. [Google Scholar] [CrossRef] [PubMed]
- Debelo, T.T.; Ujihara, M. Effect of simultaneous electrochemical deposition of manganese hydroxide and polypyrrole on structure and capacitive behavior. J. Electroanal. Chem. 2020, 859, 113825. [Google Scholar] [CrossRef]
- Mevada, C.; Mukhopadhyay, M. High mass loading tin oxide-ruthenium oxide-based nanocomposite electrode for supercapacitor application. J. Energy Storage 2020, 31, 101587. [Google Scholar] [CrossRef]
- Kariper, İ.; Tezel, F.M.; Gökkuş, Ö. Synthesize of WO3 thin film supercapacitor and its characterization. Phys. Lett. A 2021, 388, 127059. [Google Scholar] [CrossRef]
- Abebe, E.M.; Ujihara, M. Influence of Temperature on ZnO/Co3O4 Nanocomposites for High Energy Storage Supercapacitors. ACS Omega 2021, 6, 23750. [Google Scholar] [CrossRef]
- Abebe, E.M.; Ujihara, M. Simultaneous Electrodeposition of Ternary Metal Oxide Nanocomposites for High-Efficiency Supercapacitor Applications. ACS Omega 2022, 7, 17161–17174. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Li, Z.; Xu, K.; He, X.; Wei, S.; Cao, Y. Hollow SnO2 nanospheres with single-shelled structure and the application for supercapacitors. J. Alloys Compd. 2019, 779, 728–734. [Google Scholar] [CrossRef]
- Debelo, T.T.; Ujihara, M. Electrodeposition of binder-free polypyrrole on a three-dimensional flower-like nanosheet of manganese oxides containing pyrrole derivative for supercapacitor electrode. J. Mater. Chem. A 2022, 278, 125690. [Google Scholar] [CrossRef]
- Poudel, M.B.; Lohani, P.C.; Kim, A.A. Synthesis of silver nanoparticles decorated tungsten oxide nanorods as high-performance supercapacitor electrode. Chem. Phys. Lett. 2022, 804, 139884. [Google Scholar] [CrossRef]
- Nithya, V.; Arul, N.S. Review on α-Fe2O3 based negative electrode for high performance supercapacitors. J. Power Sources 2016, 327, 297–318. [Google Scholar] [CrossRef]
- Shinde, P.A.; Lokhande, A.C.; Chodankar, N.R.; Patil, A.M.; Kim, J.H.; Lokhande, C.D. Temperature dependent surface morphological modifications of hexagonal WO3 thin films for high performance supercapacitor application. Electrochim. Acta 2017, 224, 397–404. [Google Scholar] [CrossRef]
- Ji, S.-H.; Chodankar, N.R.; Kim, D.-H. Aqueous asymmetric supercapacitor based on RuO2-WO3 electrodes. Electrochim. Acta 2019, 325, 134879. [Google Scholar] [CrossRef]
- Chu, J.; Lu, D.; Wang, X.; Wang, X.; Xiong, S. WO3 nanoflower coated with graphene nanosheet: Synergetic energy storage composite electrode for supercapacitor application. J. Alloys Compd. 2017, 702, 568–572. [Google Scholar] [CrossRef]
- Shinde, P.A.; Lokhande, V.C.; Patil, A.M.; Ji, T.; Lokhande, C.D. Single-step hydrothermal synthesis of WO3-MnO2 composite as an active material for all-solid-state flexible asymmetric supercapacitor. Int. J. Hydrogen Energy 2018, 43, 2869–2880. [Google Scholar] [CrossRef]
- Kumar, R.D.; Andou, Y.; Karuppuchamy, S. Synthesis and characterization of nanostructured Ni-WO3 and NiWO4 for supercapacitor applications. J. Alloys Compd. 2016, 654, 349–356. [Google Scholar] [CrossRef]
- Kumar, R.D.; Karuppuchamy, S. Microwave mediated synthesis of nanostructured Co-WO3 and CoWO4 for supercapacitor applications. J. Alloys Compd. 2016, 674, 384–391. [Google Scholar] [CrossRef]
- Hai, Z.; Akbari, M.K.; Xue, C.; Xu, H.; Solano, E.; Detavernier, C.; Hu, J.; Zhuiykov, S. Atomically-thin WO3/TiO2 heterojunction for supercapacitor electrodes developed by atomic layer deposition. Compos. Commun. 2017, 5, 31–35. [Google Scholar] [CrossRef]
- Periasamy, P.; Krishnakumar, T.; Sandhiya, M.; Sathish, M.; Chavali, M.; Siril, P.F.; Devarajan, V. Preparation and comparison of hybridized WO3–V2O5 nanocomposites electrochemical supercapacitor performance in KOH and H2SO4 electrolyte. Mater. Lett. 2019, 236, 702–705. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, L.; Zhou, Y.; Guo, J.; Zhang, S.; Lu, Y. Synthesis of heterostructure SnO2/graphitic carbon nitride composite for high-performance electrochemical supercapacitor. J. Electroanal. Chem. 2019, 852, 113507. [Google Scholar] [CrossRef]
- Shinde, P.A.; Lokhande, V.C.; Ji, T.; Lokhande, C.D. Facile synthesis of hierarchical mesoporous weirds-like morphological MnO2 thin films on carbon cloth for high performance supercapacitor application. J. Colloid Interface Sci. 2017, 498, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Shinde, P.A.; Lokhande, V.C.; Patil, A.M.; Ji, T.; Lokhande, C.D. Design and synthesis of hierarchical mesoporous WO3-MnO2 composite nanostructures on carbon cloth for high-performance supercapacitors. J. Electrochem. Sci. Technol. 2017, 3, 35–48. [Google Scholar] [CrossRef]
- Veerasubramani, G.K.; Krishnamoorthy, K.; Kim, S.J. Improved electrochemical performances of binder-free CoMoO4 nanoplate arrays@ Ni foam electrode using redox additive electrolyte. J. Power Sources 2016, 306, 378–386. [Google Scholar] [CrossRef]
- Ojha, G.P.; Pant, B.; Acharya, J.; Park, M. Prussian red anions immobilized freestanding three-dimensional porous carbonaceous networks: A new avenue to attain capacitor-and faradic-type electrodes in a single frame for 2.0 V hybrid supercapacitors. ACS Sustain. Chem. Eng. 2022, 10, 2994–3006. [Google Scholar] [CrossRef]
- Sankar, K.V.; Selvan, R.K. Improved electrochemical performances of reduced graphen oxide based supercapacitor using redox additive electrolyte. Carbon 2015, 90, 260–273. [Google Scholar] [CrossRef]
- Huang, X.; Wang, Q.; Chen, X.Y.; Zhang, Z.J. The effects of amine/nitro/hydroxyl groups on the benzene rings of redox additives on the electrochemical performance of carbon-based supercapacitors. Phys. Chem. Chem. Phys. 2016, 18, 10438–10452. [Google Scholar] [CrossRef]
- Han, X.; Jiang, T.; Chen, X.; Jiang, D.; Xie, K.; Jiang, Y.; Wang, Y. Electrolyte additive induced fast-charge/slow-discharge process: Potassium ferricyanide and potassium persulfate for CoO-based supercapacitors. J. Colloid Interface Sci. 2020, 576, 505–513. [Google Scholar] [CrossRef]
- Lee, J.; Choudhury, S.; Weingarth, D.; Kim, D.; Presser, V. High performance hybrid energy storage with potassium ferricyanide redox electrolyte. ACS Appl. Mater. Interfaces 2016, 8, 23676–23687. [Google Scholar] [CrossRef]
- Lokhande, V.; Lokhande, A.; Namkoong, G.; Kim, J.H.; Ji, T. Charge storage in WO3 polymorphs and their application as supercapacitor electrode material. Results Phys. 2019, 12, 2012–2020. [Google Scholar] [CrossRef]
- Zheng, F.; Song, S.; Lu, F.; Li, R.; Bu, N.; Liu, J.; Li, Y.; Hu, P.; Zhen, Q. Hydrothermal preparation, growth mechanism and supercapacitive properties of WO 3 nanorod arrays grown directly on a Cu substrate. CrystEngComm 2016, 18, 3891–3904. [Google Scholar] [CrossRef]
- Gao, L.; Wang, X.; Xie, Z.; Song, W.; Wang, L.; Wu, X.; Qu, F.; Chen, D.; Shen, G. High-performance energy-storage devices based on WO3 nanowire arrays/carbon cloth integrated electrodes. J. Mater. Chem. A 2013, 1, 7167–7173. [Google Scholar] [CrossRef]
- Nayak, A.K.; Ghosh, R.; Santra, S.; Guha, P.K.; Pradhan, D. Hierarchical nanostructured WO 3–SnO 2 for selective sensing of volatile organic compounds. Nanoscale 2015, 7, 12460–12473. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, H.; Liu, J.; Yin, M.; Yu, L.; Zhou, J.; Liu, Y.; Qiao, F. High-performance gas sensors based on the WO3-SnO2 nanosphere composites. J. Alloys Compd. 2019, 782, 789–795. [Google Scholar] [CrossRef]
- Darwish, A.M.; Eisa, W.H.; Shabaka, A.A.; Talaat, M.H. Investigation of factors affecting the synthesis of nano-cadmium sulfide by pulsed laser ablation in liquid environment. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 153, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Hasanin, M.S.; Mostafa, A.M.; Mwafy, E.A.; Darwesh, O.M. Eco-friendly cellulose nano fibers via first reported Egyptian Humicola fuscoatra Egyptia X4: Isolation and characterization. Environ. Nanotechnol. Monit. Manag. 2018, 10, 409–418. [Google Scholar] [CrossRef]
- Geleta, T.A.; Imae, T. Influence of additives on zinc oxide-based dye sensitized solar cells. Bull. Chem. Soc. Jpn. 2020, 93, 611. [Google Scholar] [CrossRef]
- Shao, H.; Huang, M.; Fu, H.; Wang, S.; Wang, L.; Lu, J.; Wang, Y.; Yu, K. Hollow WO3/SnO2 hetero-nanofibers: Controlled synthesis and high efficiency of acetone vapor detection. Front. Chem. 2019, 7, 785. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-M.; Motora, K.G.; Chen, G.-Y.; Kuo, D.-H.; Gultom, N.S. Highly efficient MoS2@CsxWO3 nanocomposite hydrogen gas sensors. Front. Mater. 2022, 9, 18. [Google Scholar] [CrossRef]
- Claros, M.; Setka, M.; Jimenez, Y.P.; Vallejos, S. AACVD synthesis and characterization of iron and copper oxides modified ZnO structured films. J. Nanomater. 2020, 10, 471. [Google Scholar] [CrossRef]
- Motsoeneng, R.G.; Kortidis, I.; Ray, S.S.; Motaung, D.E. Designing SnO2 nanostructure-based sensors with tailored selectivity toward propanol and ethanol vapors. J. Alloys Compd. 2019, 4, 13696–13709. [Google Scholar] [CrossRef]
- Ciftyürek, E.; Šmíd, B.; Li, Z.; Matolín, V.; Schierbaum, K. Spectroscopic understanding of SnO2 and WO3 metal oxide surfaces with advanced synchrotron based; XPS-UPS and near ambient pressure (NAP) XPS surface sensitive techniques for gas sensor applications under operational conditions. J. Sens. 2019, 19, 4737. [Google Scholar] [CrossRef] [PubMed]
- Farhadian, M.; Sangpour, P.; Hosseinzadeh, G. Morphology dependent photocatalytic activity of WO3 nanostructures. J. Energy Chem. 2015, 24, 171–177. [Google Scholar] [CrossRef]
- Han, C.; Zhang, B.; Zhao, K.; Meng, J.; He, Q.; He, P.; Yang, W.; Li, Q.; Mai, L. Oxalate-assisted formation of uniform carbon-confined SnO2 nanotubes with enhanced lithium storage. Chem. Commun. 2017, 53, 9542–9545. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Xu, P.; Wang, G.; Yan, J. SnO2 Nanorod arrays grown on carbon cloth as a flexible binder-free electrode for high-performance lithium batteries. J. Electron. Mater. 2019, 48, 8206–8211. [Google Scholar] [CrossRef]
- Zhao, Y.-F.; Sun, Y.-P.; Yin, X.; Yin, G.-C.; Wang, X.-M.; Jia, F.-C.; Liu, B. Effect of surfactants on the microstructures of hierarchical SnO2 blooming nanoflowers and their gas-sensing properties. Nanoscale Res. Lett. 2018, 13, 54. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.P.; Nishad, H.H.; Chakane, S.D.; Gosavi, S.W.; Late, D.J.; Walke, P.S. Phase transformation in tungsten oxide nanoplates as a function of post-annealing temperature and its electrochemical influence on energy storage. Nanoscale Adv. 2020, 2, 4689–4701. [Google Scholar] [CrossRef]
- Huang, H.; Tan, O.; Lee, Y.; Tse, M.; Guo, J.; White, T. In situ growth of SnO2 nanorods by plasma treatment of SnO2 thin films. J. Nanotechnol. 2006, 17, 3668. [Google Scholar] [CrossRef]
- Li, K.-D.; Chen, P.-W.; Chang, K.-S.; Hsu, S.-C.; Jan, D.-J. Indium-Zinc-Tin-Oxide Film Prepared by Reactive Magnetron Sputtering for Electrochromic Applications. Materials 2018, 11, 2221. [Google Scholar] [CrossRef]
- Yang, F.; Jia, J.; Mi, R.; Liu, X.; Fu, Z.; Wang, C.; Liu, X.; Tang, Y. Fabrication of WO3· 2H2O/BC hybrids by the radiation method for enhanced performance supercapacitors. Front. Chem. 2018, 6, 290. [Google Scholar] [CrossRef]
- Wang, S.; Xu, H.; Hao, T.; Wang, P.; Zhang, X.; Zhang, H.; Xue, J.; Zhao, J.; Li, Y. In situ XRD and operando spectra-electrochemical investigation of tetragonal WO3-x nanowire networks for electrochromic supercapacitors. NPG Asia Mater. 2021, 13, 51. [Google Scholar] [CrossRef]
- Ashraf, M.; Shah, S.S.; Khan, I.; Aziz, M.A.; Ullah, N.; Khan, M.; Adil, S.F.; Liaqat, Z.; Usman, M.; Tremel, W. A High-Performance Asymmetric Supercapacitor Based on Tungsten Oxide Nanoplates and Highly Reduced Graphene Oxide Electrodes. Chem. A Eur. J. 2021, 27, 6973–6984. [Google Scholar] [CrossRef] [PubMed]
- Angelin, M.D.; Rajkumar, S.; Merlin, J.P.; Xavier, A.R.; Franklin, M.; Ravichandran, A. Electrochemical investigation of Zr-doped ZnO nanostructured electrode material for high-performance supercapacitor. Ionics 2020, 26, 5757–5772. [Google Scholar] [CrossRef]
- Mathis, T.S.; Kurra, N.; Wang, X.; Pinto, D.; Simon, P.; Gogotsi, Y. Energy storage data reporting in perspective—Guidelines for interpreting the performance of electrochemical energy storage systems. Adv. Energy Mater. 2019, 9, 1902007. [Google Scholar] [CrossRef]
- Pham, D.T.; Baboo, J.P.; Song, J.; Kim, S.; Jo, J.; Mathew, V.; Alfaruqi, M.H.; Sambandam, B.; Kim, J. Facile synthesis of pyrite (FeS2/C) nanoparticles as an electrode material for non-aqueous hybrid electrochemical capacitors. Nanoscale 2018, 10, 5938–5949. [Google Scholar] [CrossRef] [PubMed]
- Nagaraju, P.; Alsalme, A.; Alkathiri, A.M.; Jayavel, R. Rapid synthesis of WO3/graphene nanocomposite via in-situ microwave method with improved electrochemical properties. J. Phys. Chem. Solids 2018, 120, 250–260. [Google Scholar] [CrossRef]
- Huang, Y.; Li, M.; Chen, S.; Sun, P.; Lv, X.; Li, B.; Fang, L.; Sun, X. Constructing aqueous Zn//Ni hybrid battery with NiSe nanorod array on nickel foam and redox electrolytes for high-performance electrochemical energy storage. Appl. Surf. Sci. 2021, 562, 150222. [Google Scholar] [CrossRef]
- Chowdhury, A.; Biswas, S.; Singh, T.; Chandra, A. Redox mediator induced electrochemical reactions at the electrode-electrolyte interface: Making sodium-ion supercapacitors a competitive technology. Electrochem. Sci. Adv. 2022, 2, e2100030. [Google Scholar] [CrossRef]
- Kim, H.-S.; Cook, J.B.; Lin, H.; Ko, J.S.; Tolbert, S.H.; Ozolins, V.; Dunn, B. Oxygen vacancies enhance pseudocapacitive charge storage properties of MoO3−x. Nat. Mater. 2017, 16, 454–460. [Google Scholar] [CrossRef]
- Dong, X.; Chen, L.; Liu, J.; Haller, S.; Wang, Y.; Xia, Y. Environmentally-friendly aqueous Li (or Na)-ion battery with fast electrode kinetics and super-long life. Sci. Adv. 2016, 2, e1501038. [Google Scholar] [CrossRef]
- Teli, A.M.; Beknalkar, S.A.; Pawar, S.A.; Dubal, D.P.; Dongale, T.D.; Patil, D.S.; Patil, P.S.; Shin, J.C. Effect of concentration on the charge storage kinetics of nanostructured MnO2 thin-film supercapacitors synthesized by the hydrothermal method. Energies 2020, 13, 6124. [Google Scholar] [CrossRef]
- Sahoo, M.K.; Rao, G.R. A high energy flexible symmetric supercapacitor fabricated using N-doped activated carbon derived from palm flowers. Nanoscale Adv. 2021, 3, 5417–5429. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Lin, B.; Sun, Y.; Zhang, X.; Yang, H. Porous WO3@CuO composites derived from polyoxometalates@ metal organic frameworks for supercapacitor. Mater. Lett. 2017, 206, 91–94. [Google Scholar] [CrossRef]
- Gao, L.; Qu, F.; Wu, X. Hierarchical WO3@SnO2 core–shell nanowire arrays on carbon cloth: A new class of anode for high-performance lithium-ion batteries. J. Mater. Chem. A 2014, 2, 7367–7372. [Google Scholar] [CrossRef]





| W 4f7/2 | W 4f5/2 | Doublet Separation | ||
|---|---|---|---|---|
| WO3 | 36.08 | 38.21 | 2.13 | |
| WO3/SnO2 | 35.84 | 38.08 | 2.24 | |
| Sn 3d5/2 | Sn 3d3/2 | |||
| SnO2 | 487.60 | 496.05 | 8.45 | |
| WO3/SnO2 | 487.69 | 496.12 | 8.43 | |
| O in Lattice Metal Oxide | O Deficiency (Defects) | Chemisorbed O | O in Water, Hydroxide (OH−/H2O) | |
| WO3 | 531.43 | 532.01 | 532.72 | 533.60 |
| SnO2 | 531.50 | - | 532.70 | 534.38 |
| WO3/SnO2 | 531.09 | 531.76 | 532.80 | 534.30 |
| Electrode | With K3Fe(CN)6 | Without K3Fe(CN)6 | ||
|---|---|---|---|---|
| Rs (Ω) | Rct (Ω) | Rs (Ω) | Rct (Ω) | |
| WO3 | 2.78 | 1.71 | 2.37 | 2.12 |
| SnO2 | 2.13 | 1.69 | 2.32 | 1.94 |
| WO3/SnO2 | 1.65 | 1.56 | 1.87 | 1.72 |
| Electrode | Specific Capacitance (Fg−1) | Current Density (Ag−1) | Energy Density (Whkg−1) | Power Density (Wkg−1) | Stability (Retention, Cycles) | Electrolyte | Ref. |
|---|---|---|---|---|---|---|---|
| MnO2-WO3 | 657@5 mVs−1 | NA | 10.8 | 650 | 92%, 2000 | Na2SO4 | [21] |
| RuO2-WO3 | NA | NA | 16.92 | 540 | NA | NA | [12] |
| WO3-MnO2/WO3 | 88.6 | 4 mAcm−2 | 24.13 | 915 | 95%, 2500 | CMC-Na2SO4 | [14] |
| WO3-RGO | 495 | 1 | NA | NA | 85%, 1000 | 0.5 M H2SO4 | [13] |
| WO3@CuO | 248.2 | 1 | NA | NA | 85.2%, 1500 | 6 M KOH | [60] |
| WO3/Se(ASC) | 0.858 | 0.2 | 0.047 | 0.345 | 74%, 4000 | PVA-H2SO4 | [2] |
| WO3/SnO2 | 530@5 mVs−1 | NA | 35 | 468 | NA | 1 M Na2SO4 | This work |
| WO3/SnO2 | 640@5 mVs−1 | NA | 64 | 542 | 94.7%, 2000 | 0.02 M K3Fe(CN)6/1 M Na2SO4 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morka, T.D.; Ujihara, M. Enhanced Performance of WO3/SnO2 Nanocomposite Electrodes with Redox-Active Electrolytes for Supercapacitors. Int. J. Mol. Sci. 2023, 24, 6045. https://doi.org/10.3390/ijms24076045
Morka TD, Ujihara M. Enhanced Performance of WO3/SnO2 Nanocomposite Electrodes with Redox-Active Electrolytes for Supercapacitors. International Journal of Molecular Sciences. 2023; 24(7):6045. https://doi.org/10.3390/ijms24076045
Chicago/Turabian StyleMorka, Tamiru Deressa, and Masaki Ujihara. 2023. "Enhanced Performance of WO3/SnO2 Nanocomposite Electrodes with Redox-Active Electrolytes for Supercapacitors" International Journal of Molecular Sciences 24, no. 7: 6045. https://doi.org/10.3390/ijms24076045
APA StyleMorka, T. D., & Ujihara, M. (2023). Enhanced Performance of WO3/SnO2 Nanocomposite Electrodes with Redox-Active Electrolytes for Supercapacitors. International Journal of Molecular Sciences, 24(7), 6045. https://doi.org/10.3390/ijms24076045







