Hypoxia Promotes Cartilage Regeneration in Cell-Seeded 3D-Printed Bioscaffolds Cultured with a Bespoke 3D Culture Device
Abstract
1. Introduction
2. Results
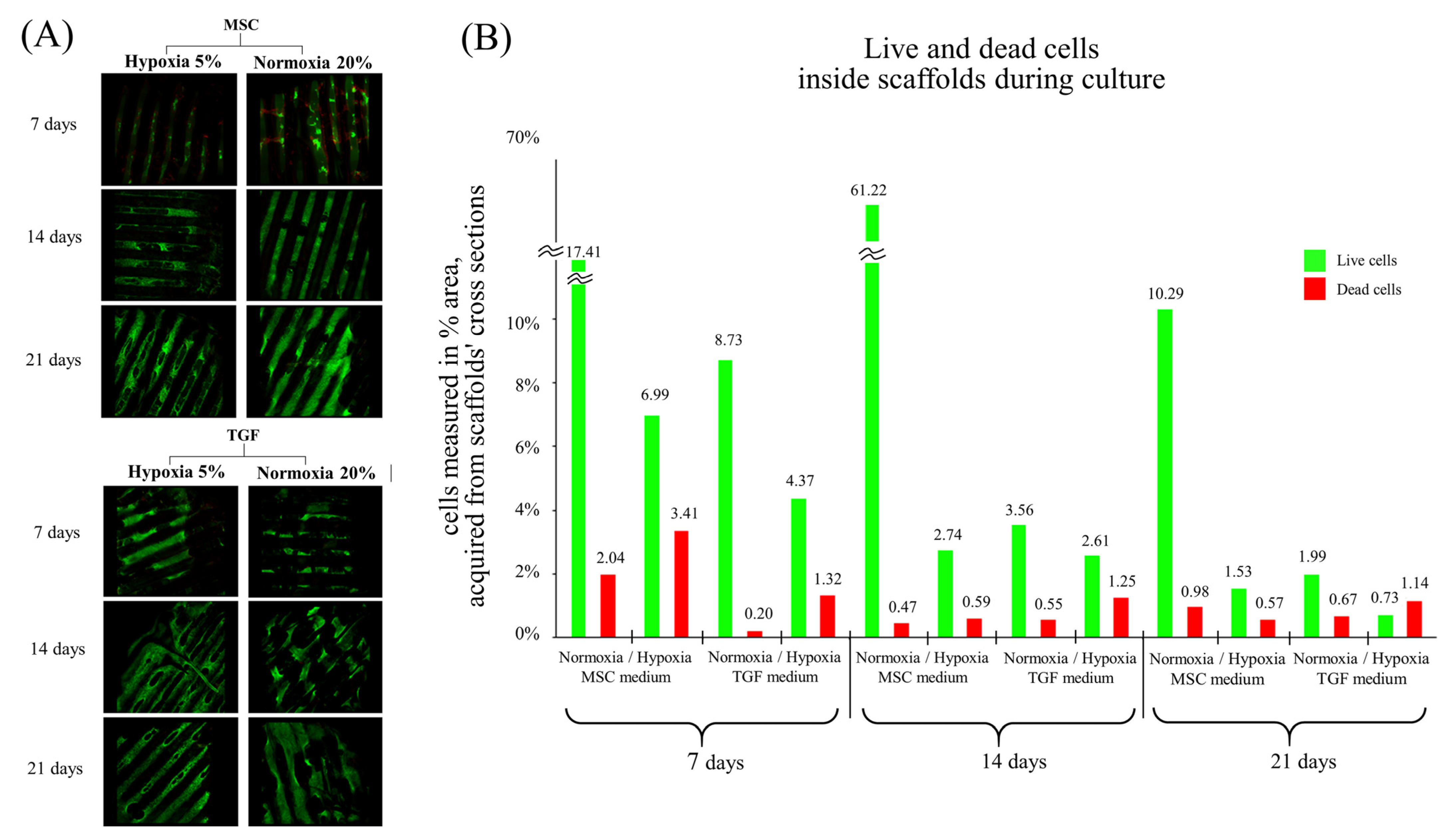
2.1. Assessment of Live/Dead Cells
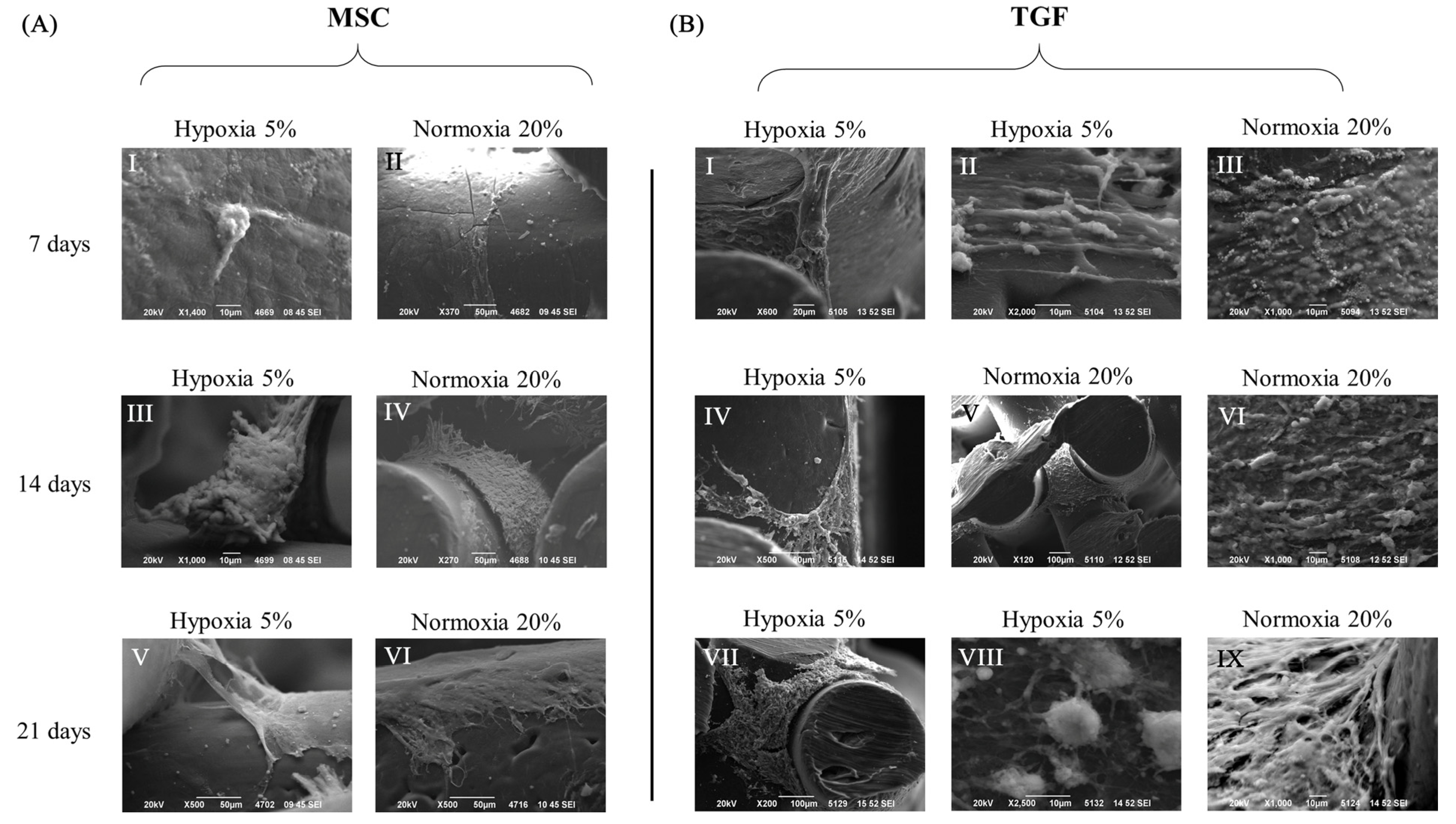
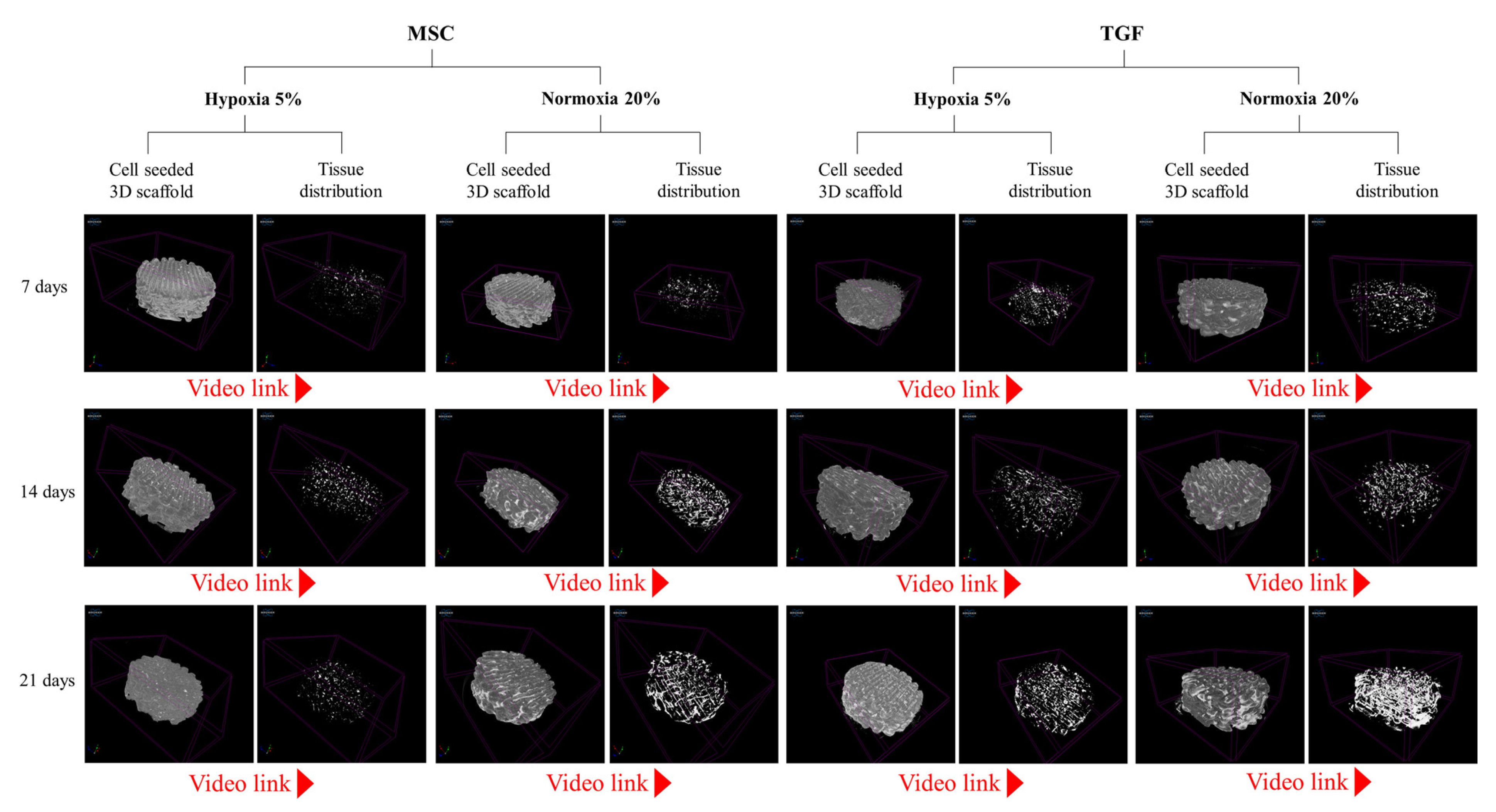
2.2. Cell and Tissue Distribution, Gross Morphology and Macroscopic Characteristics of the Engineered Cartilage Tissue
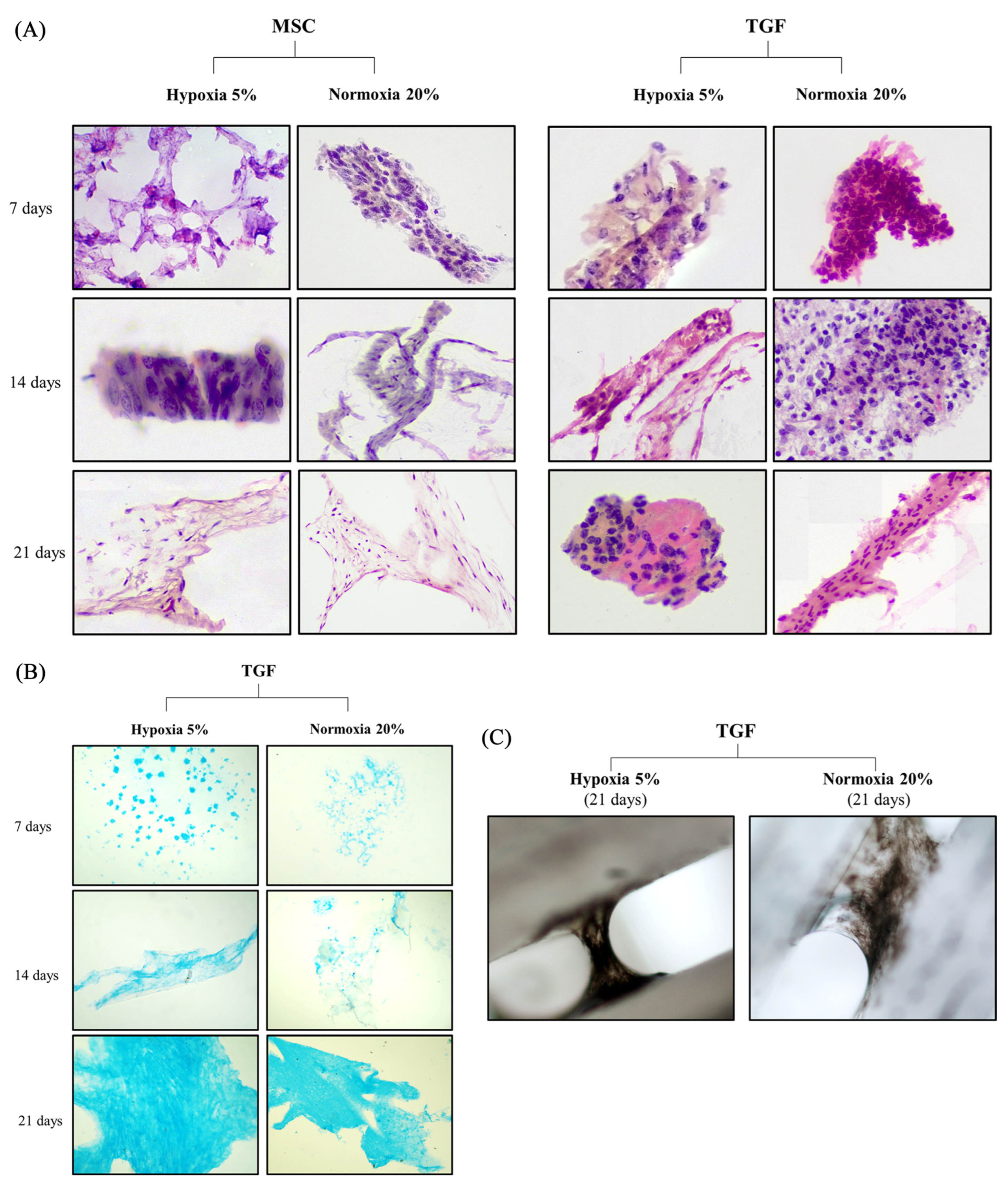
2.3. Histochemical Evaluation of the Engineered Cartilage Tissue
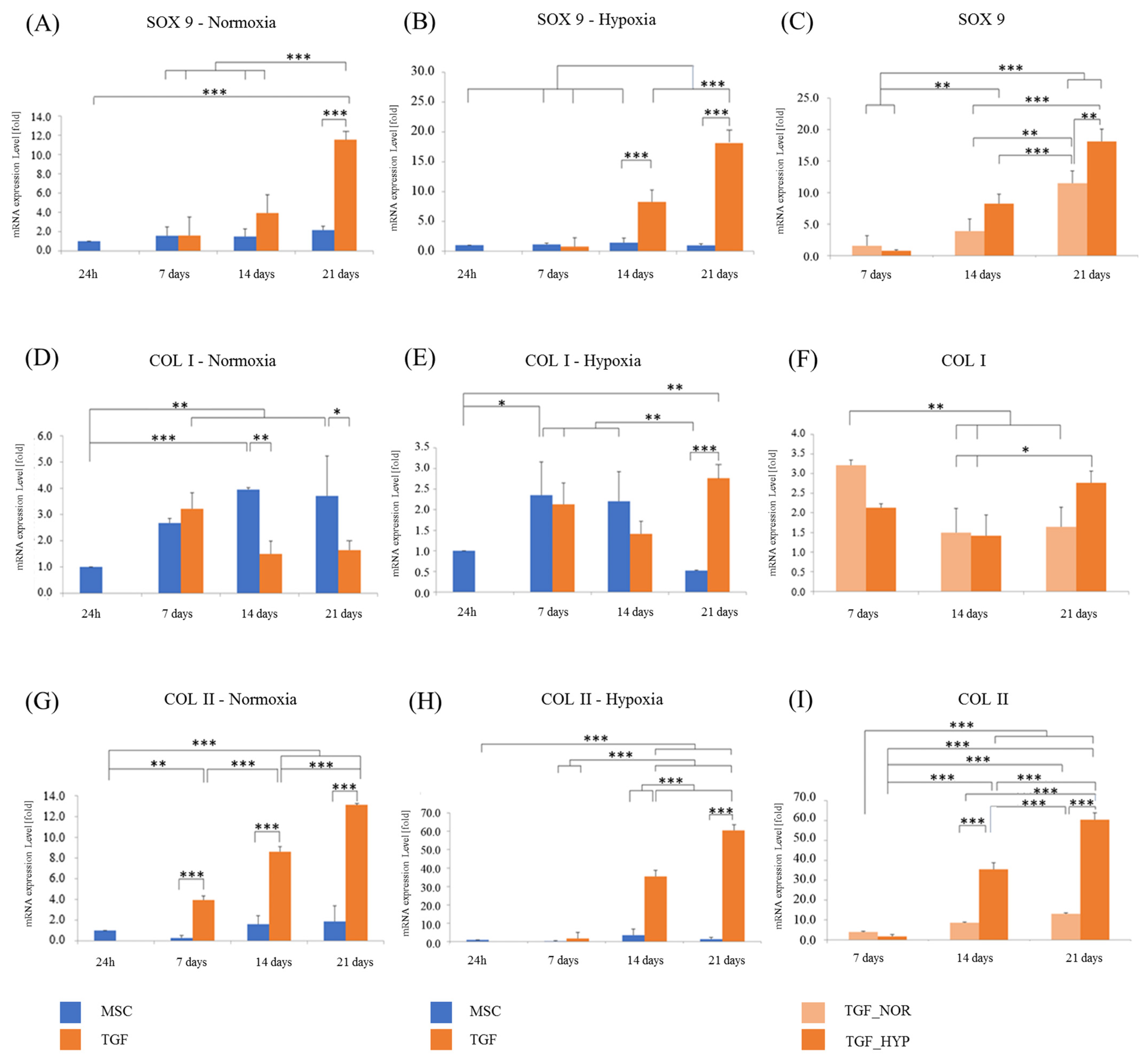
2.4. Chondrogenic Evaluation of the Engineered Cartilage Tissue
2.5. Biomechanical Analysis of the Engineered Cartilage Tissue
3. Discussion
3.1. Hypoxia and Chondrogenesis
3.2. Hypoxia and Cell Expansion
3.3. Biomechanical Properties
4. Materials and Methods
4.1. Cell Isolation
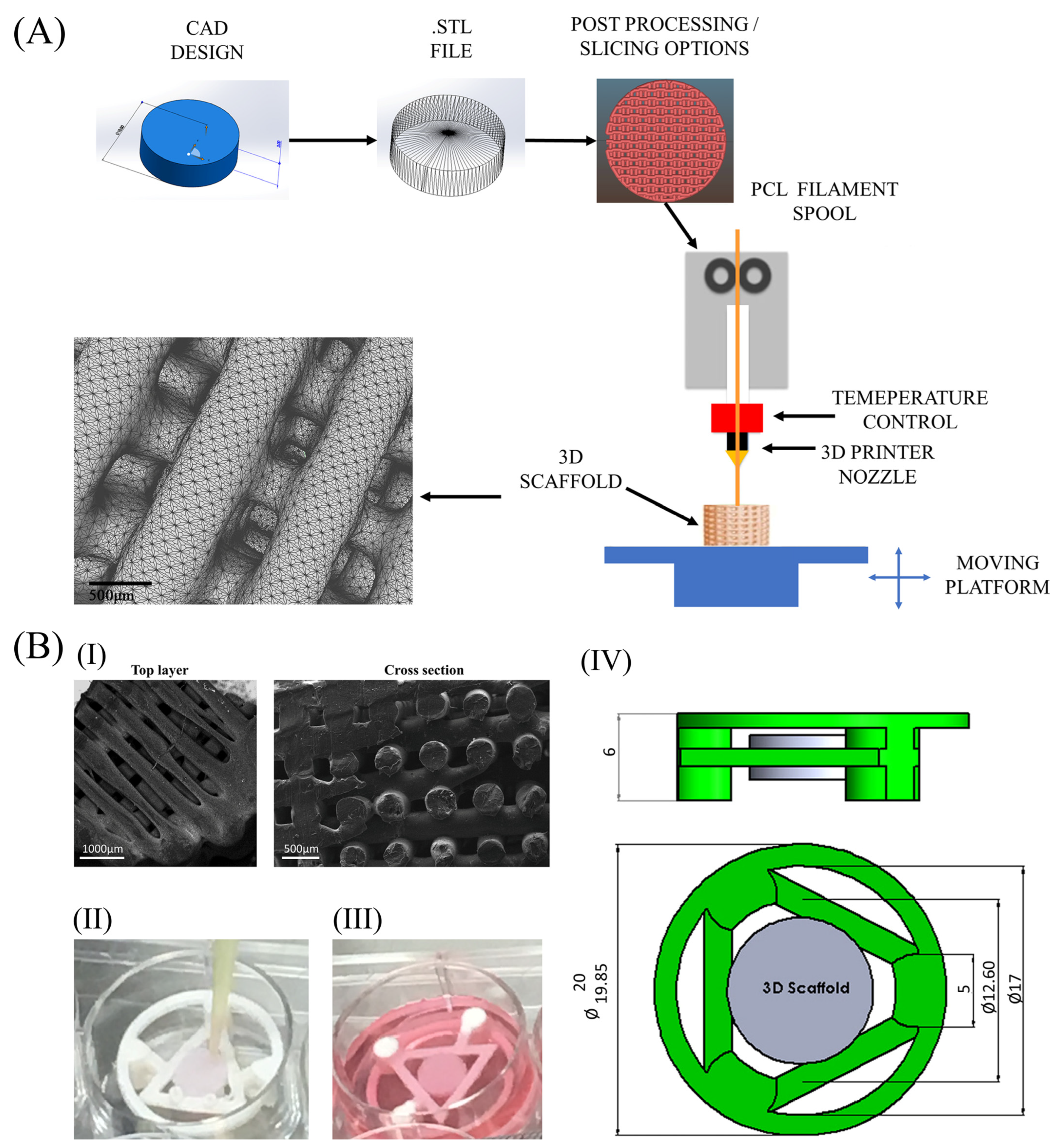
4.2. Scaffolds and Culturing Device
4.3. Cell Culturing Conditions
4.4. Cell viability and Confocal Microscopy
4.5. Scanning Electron Microscopy
4.6. Micro-Computed Tomography
4.7. Histochemical Evaluation
4.8. Quantitative Real-Time Reverse Transcription Polymerase Chain Reaction
4.9. Biomechanical Properties
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Darling, E.; Athanasiou, K.A. Biomechanical Strategies for Articular Cartilage Regeneration. Ann. Biomed. Eng. 2003, 31, 1114–1124. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Klein, J. Recent Progress in Cartilage Lubrication. Adv. Mater. 2021, 33, e2005513. [Google Scholar] [CrossRef] [PubMed]
- Dawson, J.; Linsell, L.; Zondervan, K.; Rose, P.; Randall, T.; Carr, A.; Fitzpatrick, R. Epidemiology of hip and knee pain and its impact on overall health status in older adults. Rheumatology 2004, 43, 497–504. [Google Scholar] [CrossRef]
- Shane Anderson, A.; Loeser, R.F. Why is osteoarthritis an age-related disease? Best Pract. Res. Clin. Rheumatol. 2010, 24, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Hootman, J.M.; Helmick, C.G.; Barbour, K.E.; Theis, K.A.; Boring, M.A. Updated Projected Prevalence of Self-Reported Doctor-Diagnosed Arthritis and Arthritis-Attributable Activity Limitation Among US Adults, 2015–2040. Arthritis Rheumatol. 2016, 68, 1582–1587. [Google Scholar] [CrossRef]
- Minas, T.; Ogura, T.; Headrick, J.; Bryant, T. Autologous Chondrocyte Implantation “Sandwich” Technique Compared With Autologous Bone Grafting for Deep Osteochondral Lesions in the Knee. Am. J. Sport. Med. 2018, 46, 322–332. [Google Scholar] [CrossRef]
- Bansal, H.; Comella, K.; Leon, J.; Verma, P.; Agrawal, D.; Koka, P.; Ichim, T. Intra-articular injection in the knee of adipose derived stromal cells (stromal vascular fraction) and platelet rich plasma for osteoarthritis. J. Transl. Med. 2017, 15, 141. [Google Scholar] [CrossRef]
- Theodoridis, K.; Manthou, M.E.; Aggelidou, E.; Kritis, A. In Vivo Cartilage Regeneration with Cell-Seeded Natural Biomaterial Scaffold Implants: 15-Year Study. Tissue Eng. Part B Rev. 2021, 28, 206–245. [Google Scholar] [CrossRef]
- Schulze-Tanzil, G.; Mobasheri, A.; de Souza, P.; John, T.; Shakibaei, M. Loss of chondrogenic potential in dedifferentiated chondrocytes correlates with deficient Shc–Erk interaction and apoptosis. Osteoarthr. Cartil. 2004, 12, 448–458. [Google Scholar] [CrossRef]
- Cavalli, E.; Levinson, C.; Hertl, M.; Broguiere, N.; Brück, O.; Mustjoki, S.; Gerstenberg, A.; Weber, D.; Salzmann, G.; Steinwachs, M.; et al. Characterization of polydactyly chondrocytes and their use in cartilage engineering. Sci. Rep. 2019, 9, 4275. [Google Scholar] [CrossRef]
- Huey, D.J.; Hu, J.C.; Athanasiou, K.A. Unlike Bone, Cartilage Regeneration Remains Elusive. Science 2012, 338, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Kokai, L.E.; Marra, K.; Rubin, J.P. Adipose stem cells: Biology and clinical applications for tissue repair and regeneration. Transl. Res. J. Lab. Clin. Med. 2014, 163, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Zubillaga, V.; Alonso-Varona, A.; Fernandes, S.C.M.; Salaberria, A.M.; Palomares, T. Adipose-Derived Mesenchymal Stem Cell Chondrospheroids Cultured in Hypoxia and a 3D Porous Chitosan/Chitin Nanocrystal Scaffold as a Platform for Cartilage Tissue Engineering. Int. J. Mol. Sci. 2020, 21, 1004. [Google Scholar] [CrossRef] [PubMed]
- Lam, A.T.; Reuveny, S.; Oh, S.K.-W. Human mesenchymal stem cell therapy for cartilage repair: Review on isolation, expansion, and constructs. Stem Cell Res. 2020, 44, 101738. [Google Scholar] [CrossRef] [PubMed]
- Bakopoulou, A.; Apatzidou, D.; Aggelidou, E.; Gousopoulou, E.; Leyhausen, G.; Volk, J.; Kritis, A.; Koidis, P.; Geurtsen, W. Isolation and prolonged expansion of oral mesenchymal stem cells under clinical-grade, GMP-compliant conditions differentially affects “stemness” properties. Stem Cell Res. Ther. 2017, 8, 247. [Google Scholar] [CrossRef]
- Tuli, R.; Tuli, S.; Nandi, S.; Huang, X.; Manner, P.A.; Hozack, W.J.; Danielson, K.G.; Hal, D.J.; Tuan, R.S. Transforming growth factor-beta-mediated chondrogenesis of human mesenchymal progenitor cells involves N-cadherin and mitogen-activated protein kinase and Wnt signaling cross-talk. J. Biol. Chem. 2003, 278, 41227–41236. [Google Scholar] [CrossRef]
- Yu, D.-A.; Han, J.; Kim, B.-S. Stimulation of Chondrogenic Differentiation of Mesenchymal Stem Cells. Int. J. Stem Cells 2012, 5, 16–22. [Google Scholar] [CrossRef]
- Neybecker, P.; Henrionnet, C.; Pape, E.; Mainard, D.; Galois, L.; Loeuille, D.; Gillet, P.; Pinzano, A. In vitro and in vivo potentialities for cartilage repair from human advanced knee osteoarthritis synovial fluid-derived mesenchymal stem cells. Stem Cell Res. Ther. 2018, 9, 329. [Google Scholar] [CrossRef]
- Fahy, N.; Alini, M.; Stoddart, M.J. Mechanical stimulation of mesenchymal stem cells: Implications for cartilage tissue engineering. J. Orthop. Res. 2018, 36, 52–63. [Google Scholar] [CrossRef]
- Shi, Y.; Ma, J.; Zhang, X.; Li, H.; Jiang, L.; Qin, J. Hypoxia combined with spheroid culture improves cartilage specific function in chondrocytes. Integr. Biol. 2015, 7, 289–297. [Google Scholar] [CrossRef]
- Demoor, M.; Ollitrault, D.; Gomez-Leduc, T.; Bouyoucef, M.; Hervieu, M.; Fabre, H.; Lafont, J.; Denoix, J.-M.; Audigié, F.; Mallein-Gerin, F.; et al. Cartilage tissue engineering: Molecular control of chondrocyte differentiation for proper cartilage matrix reconstruction. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2014, 1840, 2414–2440. [Google Scholar] [CrossRef]
- Adesida, A.B.; Mulet-Sierra, A.; Jomha, N.M. Hypoxia mediated isolation and expansion enhances the chondrogenic capacity of bone marrow mesenchymal stromal cells. Stem Cell Res. Ther. 2012, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.R.; Yong, K.W.; Wan Safwani, W.K.Z. Effect of hypoxia on human adipose-derived mesenchymal stem cells and its potential clinical applications. Cell. Mol. Life Sci. CMLS 2017, 74, 2587–2600. [Google Scholar] [CrossRef]
- Gawlitta, D.; Van Rijen, M.H.; Schrijver, E.J.; Alblas, J.; Dhert, W. Hypoxia Impedes Hypertrophic Chondrogenesis of Human Multipotent Stromal Cells. Tissue Eng. Part A 2012, 18, 1957–1966. [Google Scholar] [CrossRef]
- Mwale, F.; Ciobanu, I.; Giannitsios, D.; Roughley, P.; Steffen, T.; Antoniou, J. Effect of Oxygen Levels on Proteoglycan Synthesis by Intervertebral Disc Cells. Spine 2011, 36, E131–E138. [Google Scholar] [CrossRef] [PubMed]
- Sheehy, E.J.; Buckley, C.T.; Kelly, D.J. Oxygen tension regulates the osteogenic, chondrogenic and endochondral phenotype of bone marrow derived mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2012, 417, 305–310. [Google Scholar] [CrossRef]
- Cicione, C.; Muiños-López, E.; Hermida-Gómez, T.; Fuentes-Boquete, I.; Díaz-Prado, S.; Blanco, F.J. Effects of Severe Hypoxia on Bone Marrow Mesenchymal Stem Cells Differentiation Potential. Stem Cells Int. 2013, 2013, 232896. [Google Scholar] [CrossRef] [PubMed]
- Wan Safwani, W.K.Z.; Choi, J.R.; Yong, K.W.; Ting, I.; Mat Adenan, N.A.; Pingguan-Murphy, B. Hypoxia enhances the viability, growth and chondrogenic potential of cryopreserved human adipose-derived stem cells. Cryobiology 2017, 75, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Malladi, P.; Chiou, M.; Bekerman, E.; Giaccia, A.J.; Longaker, M.T. In Vitro Expansion of Adipose-Derived Adult Stromal Cells in Hypoxia Enhances Early Chondrogenesis. Tissue Eng. 2007, 13, 2981–2993. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Jin, X.; Hu, J.; Ma, H.; Gupte, M.J.; Liu, H.; Ma, P.X. Effects of hypoxias and scaffold architecture on rabbit mesenchymal stem cell differentiation towards a nucleus pulposus-like phenotype. Biomaterials 2011, 32, 8182–8189. [Google Scholar] [CrossRef]
- Baumgartner, L.; Arnhold, S.; Brixius, K.; Addicks, K.; Bloch, W. Human mesenchymal stem cells: Influence of oxygen pressure on proliferation and chondrogenic differentiation in fibrin glue in vitro. J. Biomed. Mater. Res. Part A 2010, 93, 930–940. [Google Scholar] [CrossRef]
- Gómez-Leduc, T.; Desancé, M.; Hervieu, M.; Legendre, F.; Ollitrault, D.; De Vienne, C.; Herlicoviez, M.; Galéra, P.; Demoor, M. Hypoxia Is a Critical Parameter for Chondrogenic Differentiation of Human Umbilical Cord Blood Mesenchymal Stem Cells in Type I/III Collagen Sponges. Int. J. Mol. Sci. 2017, 18, 1933. [Google Scholar] [CrossRef] [PubMed]
- Kalpakci, K.N.; Brown, W.E.; Hu, J.C.; Athanasiou, K.A. Cartilage Tissue Engineering Using Dermis Isolated Adult Stem Cells: The Use of Hypoxia during Expansion versus Chondrogenic Differentiation. PLoS ONE 2014, 9, e98570. [Google Scholar]
- Koay, E.; Athanasiou, K. Hypoxic chondrogenic differentiation of human embryonic stem cells enhances cartilage protein synthesis and biomechanical functionality. Osteoarthr. Cartil. 2008, 16, 1450–1456. [Google Scholar] [CrossRef] [PubMed]
- Rodenas-Rochina, J.; Kelly, D.J.; Ribelles, J.L.G.; Lebourg, M. Influence of oxygen levels on chondrogenesis of porcine mesenchymal stem cells cultured in polycaprolactone scaffolds. J. Biomed. Mater. Res. Part A 2017, 105, 1684–1691. [Google Scholar] [CrossRef]
- Foyt, D.A.; Taheem, D.K.; Ferreira, S.A.; Norman, M.D.A.; Petzold, J.; Jell, G.; Grigoriadis, A.E.; Gentleman, E. Hypoxia impacts human MSC response to substrate stiffness during chondrogenic differentiation. Acta Biomater. 2019, 89, 73–83. [Google Scholar] [CrossRef]
- Buizer, A.T.; Bulstra, S.K.; Veldhuizen, A.G.; Kuijer, R. The balance between proliferation and transcription of angiogenic factors of mesenchymal stem cells in hypoxia. Connect. Tissue Res. 2018, 59, 12–20. [Google Scholar] [CrossRef]
- Shi, S.; Xie, J.; Zhong, J.; Lin, S.; Zhang, T.; Sun, K.; Fu, N.; Shao, X.; Lin, Y. Effects of low oxygen tension on gene profile of soluble growth factors in co-cultured adipose-derived stromal cells and chondrocytes. Cell Prolif. 2016, 49, 341–351. [Google Scholar] [CrossRef]
- Kakudo, N.; Morimoto, N.; Ogawa, T.; Taketani, S.; Kusumoto, K. Hypoxia Enhances Proliferation of Human Adipose-Derived Stem Cells via HIF-1ɑ Activation. PLoS ONE 2015, 10, e0139890. [Google Scholar] [CrossRef]
- Stubbs, S.L.; Hsiao, S.T.; Peshavariya, H.M.; Lim, S.Y.; Dusting, G.J.; Dilley, R.J. Hypoxic Preconditioning Enhances Survival of Human Adipose-Derived Stem Cells and Conditions Endothelial Cells In Vitro. Stem Cells Dev. 2012, 21, 1887–1896. [Google Scholar] [CrossRef]
- Grayson, W.L.; Zhao, F.; Bunnell, B.; Ma, T. Hypoxia enhances proliferation and tissue formation of human mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2007, 358, 948–953. [Google Scholar] [PubMed]
- Lennon, D.P.; Edmison, J.M.; Caplan, A.I. Cultivation of rat marrow-derived mesenchymal stem cells in reduced oxygen tension: Effects on in vitro and in vivo osteochondrogenesis. J. Cell. Physiol. 2001, 187, 345–355. [Google Scholar] [PubMed]
- Kaukinen, A.L.M.; Lammentausta, E.; Halmesmäki, E.; Helminen, H.; Jurvelin, J.; Rieppo, J. Destructive testing of articular cartilage in compression-effect of collagen network. In Proceedings of the 51st Annual Meeting of the Orthopaedic Research Society 2005, Washington, DC, USA, 20–23 February 2005. [Google Scholar]
- Shepherd, D.E.; Seedhom, B.B. The ‘instantaneous’ compressive modulus of human articular cartilage in joints of the lower limb. Rheumatology 1999, 38, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.; Carter, D. Articular cartilage functional histomorphology and mechanobiology: A research perspective. Bone 2003, 33, 1–13. [Google Scholar]
- Maroudas, A. Biophysical chemistry of cartilaginous tissues with special reference to solute and fluid transport. Biorheology 1975, 12, 233–248. [Google Scholar] [CrossRef]
- Zhu, W.; Mow, V.C.; Koob, T.J.; Eyre, D.R. Viscoelastic shear properties of articular cartilage and the effects of glycosidase treatments. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 1993, 11, 771–781. [Google Scholar]
- Bader, D.L.; Kempson, G.E.; Egan, J.; Gilbey, W.; Barrett, A.J. The effects of selective matrix degradation on the short-term compressive properties of adult human articular cartilage. Biochim. Biophys. Acta (BBA)-Gen. Subj. 1992, 1116, 147–154. [Google Scholar]
- Urban, J.P.; Maroudas, A.; Bayliss, M.T.; Dillon, J. Swelling pressures of proteoglycans at the concentrations found in cartilaginous tissues. Biorheology 1979, 16, 447–464. [Google Scholar] [CrossRef]
- Theodoridis, K.; Aggelidou, E.; Manthou, M.; Demiri, E.; Bakopoulou, A.; Kritis, A. Assessment of cartilage regeneration on 3D collagen-polycaprolactone scaffolds: Evaluation of growth media in static and in perfusion bioreactor dynamic culture. Colloids Surf. B: Biointerfaces 2019, 183, 110403. [Google Scholar]
- Theodoridis, K.; Aggelidou, E.; Vavilis, T.; Manthou, M.E.; Tsimponis, A.; Demiri, E.C.; Boukla, A.; Salpistis, C.; Bakopoulou, A.; Mihailidis, A.; et al. Hyaline cartilage next generation implants from adipose-tissue-derived mesenchymal stem cells: Comparative study on 3D-printed polycaprolactone scaffold patterns. J. Tissue Eng. Regen. Med. 2019, 13, 342–355. [Google Scholar] [CrossRef]
- Theodoridis, K.; Aggelidou, E.; Manthou, M.-E.; Keklikoglou, K.; Tsimponis, A.; Demiri, E.; Bakopoulou, A.; Mihailidis, A.; Kritis, A. An effective device and method for enhanced cell growth in 3D scaffolds: Investigation of cell seeding and proliferation under static and dynamic conditions. Mater. Sci. Eng. C 2020, 114, 111060. [Google Scholar]
- Shihan, M.H.; Novo, S.G.; Le Marchand, S.J.; Wang, Y.; Duncan, M.K. A simple method for quantitating confocal fluorescent images. Biochem. Biophys. Rep. 2021, 25, 100916. [Google Scholar] [PubMed]
- Baviskar, S.N. A Quick & Automated Method for Measuring Cell Area Using ImageJ. Am. Biol. Teach. 2011, 73, 554–556. [Google Scholar]
- Arampatzis, A.S.; Giannakoula, K.; Kontogiannopoulos, K.N.; Theodoridis, K.; Aggelidou, E.; Rat, A.; Kampasakali, E.; Willems, A.; Christofilos, D.; Kritis, A.; et al. Novel electrospun poly-hydroxybutyrate scaffolds as carriers for the wound healing agents alkannins and shikonins. Regen. Biomater. 2021, 8, rbab011. [Google Scholar]
- Chang, D.G.; Iverson, E.P.; Schinagl, R.M.; Sonoda, M.; Amiel, D.; Coutts, R.D.; Sah, R.L. Quantitation and localization of cartilage degeneration following the induction of osteoarthritis in the rabbit knee. Osteoarthr. Cartil. 1997, 5, 357–372. [Google Scholar]
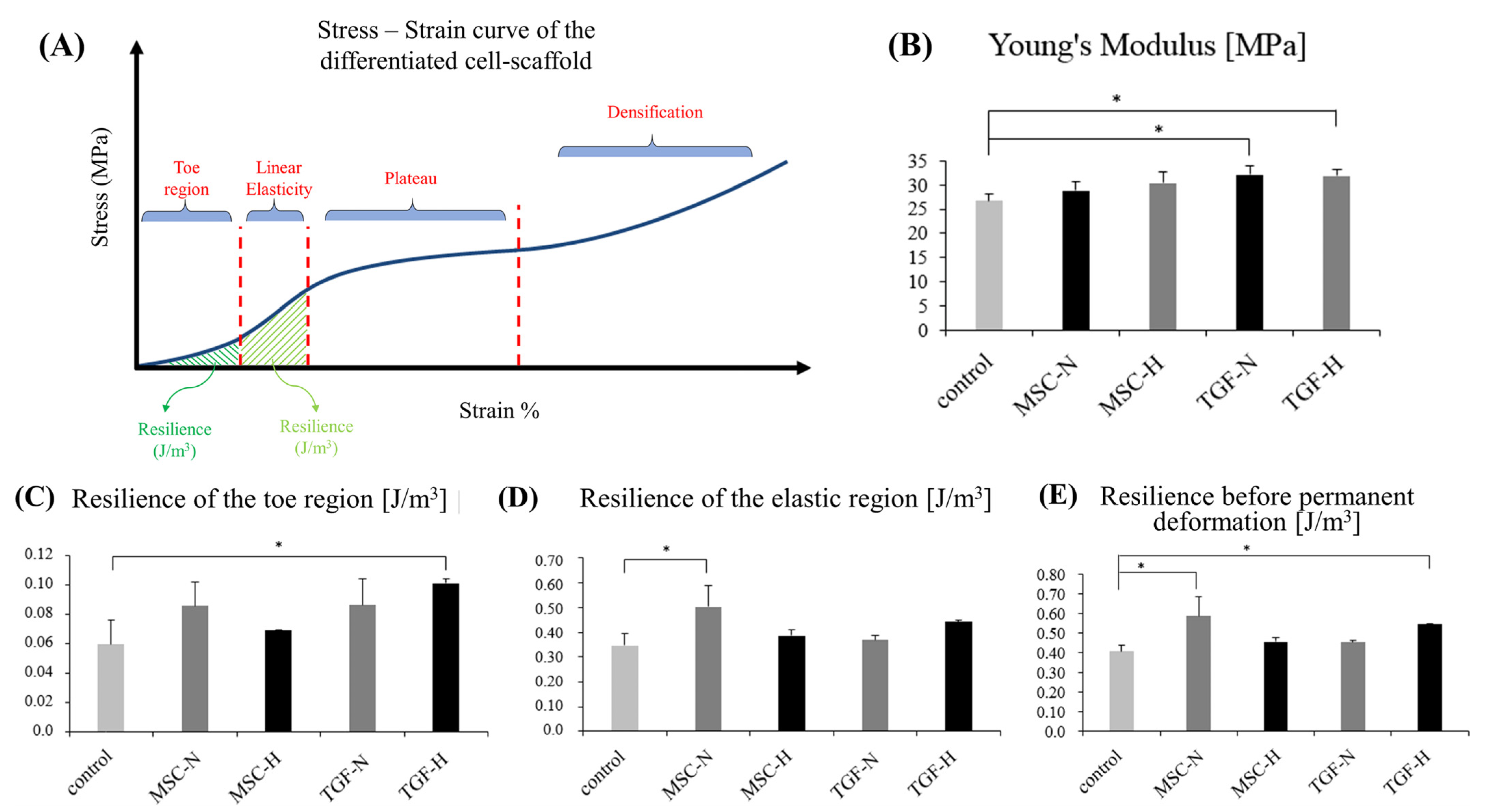
| Scaffold Groups | Young’s Modulus (MPa) | Yield Strength (MPa) | Resilience of the Toe Region J/m3 | Resilience of the Elastic Region J/m3 | Resilience before Permanent Deformation J/m3 |
|---|---|---|---|---|---|
| Control | 26.66 (±1.54) | 4.47 (±0.21) | 0.059 (±0.016) | 0.347 (±0.047) | 0.406 (±0.030) |
| MSC-N | 28.83 (±1.81) | 5.41 (±0.62) | 0.085 (±0.017) | 0.501 (±0.086) | 0.587 (±0.100) |
| MSC-H | 30.38 (±2.39) | 4.83 (±0.06) | 0.069 (±0.000) | 0.386 (±0.023) | 0.455 (±0.023) |
| TGF-N | 32.13 (±1.74) | 4.98 (±0.24) | 0.086 (±0.018) | 0.368 (±0.018) | 0.454 (±0.009) |
| TGF-H | 31.82 (±1.26) | 5.39 (±0.05) | 0.101 (±0.003) | 0.443 (±0.005) | 0.544 (±0.002) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Theodoridis, K.; Aggelidou, E.; Manthou, M.-E.; Kritis, A. Hypoxia Promotes Cartilage Regeneration in Cell-Seeded 3D-Printed Bioscaffolds Cultured with a Bespoke 3D Culture Device. Int. J. Mol. Sci. 2023, 24, 6040. https://doi.org/10.3390/ijms24076040
Theodoridis K, Aggelidou E, Manthou M-E, Kritis A. Hypoxia Promotes Cartilage Regeneration in Cell-Seeded 3D-Printed Bioscaffolds Cultured with a Bespoke 3D Culture Device. International Journal of Molecular Sciences. 2023; 24(7):6040. https://doi.org/10.3390/ijms24076040
Chicago/Turabian StyleTheodoridis, Konstantinos, Eleni Aggelidou, Maria-Eleni Manthou, and Aristeidis Kritis. 2023. "Hypoxia Promotes Cartilage Regeneration in Cell-Seeded 3D-Printed Bioscaffolds Cultured with a Bespoke 3D Culture Device" International Journal of Molecular Sciences 24, no. 7: 6040. https://doi.org/10.3390/ijms24076040
APA StyleTheodoridis, K., Aggelidou, E., Manthou, M.-E., & Kritis, A. (2023). Hypoxia Promotes Cartilage Regeneration in Cell-Seeded 3D-Printed Bioscaffolds Cultured with a Bespoke 3D Culture Device. International Journal of Molecular Sciences, 24(7), 6040. https://doi.org/10.3390/ijms24076040








