Unlocking the Full Potential of SGLT2 Inhibitors: Expanding Applications beyond Glycemic Control
Abstract
1. Introduction
2. Sodium–Glucose Transporters (SGLTs)
2.1. SLC5A1 (SGLT1)
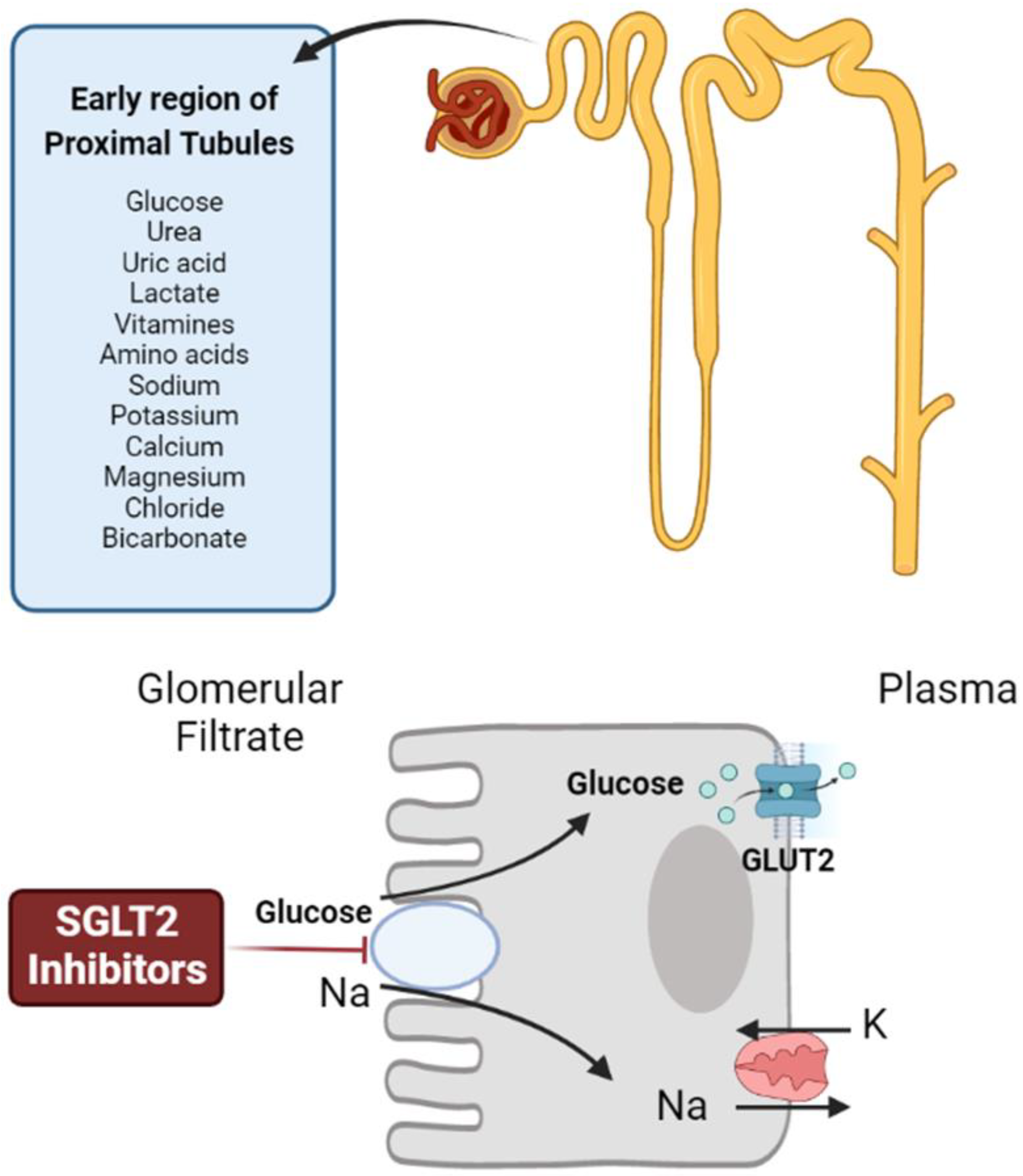
2.2. SLC5A2 (SGLT2)
2.3. SLC5A4 (SGLT3)
2.4. SLC5A9 (SGLT4)
2.5. SLC5A10 (SGLT5)
2.6. SLC5A11 (SGLT6)
2.7. SLC5A3 (SMIT)
2.8. SLC5A7 (CHT)
2.9. SLC5A6 (SMVT)
2.10. SLC5A8 (SMCT1)
2.11. SLC5A12 (SMCT2)
2.12. SLC5A5 (NIS)
3. Antidiabetic Medications
3.1. α-Glucosidases Inhibitors
3.2. Biguanides
3.3. Sulfonylureas
3.4. Meglitinides
3.5. GLP-1 Receptor Agonists
3.6. Peroxisome Proliferator-Activated Receptor γ (PPAR-γ) Agonists
3.7. Dipeptidyl Peptidase 4 (DDP4) Inhibitors
3.8. Sodium–Glucose Transporter 2 (SGLT2) Inhibitors
4. Cardiovascular Effects of SGLT2 Inhibitors
4.1. Dapagliflozin
4.2. Empagliflozin
| Dapagliflozin | Empagliflozin | Canagliflozin | Ertugliflozin | |
|---|---|---|---|---|
| Cardiovascular effects |
|
| ||
| Metabolic effects | ||||
| Cancer |
| |||
| Bone metabolism |
|
|
| |
| Neuroprotective effect |
|
4.3. Canagliflozin
5. Cardiorenal Effects of SGLT2 Inhibitors
6. Metabolic Effects of Antidiabetic Drugs on Adipocytes
6.1. The Role of Adipose Tissue in Diabetic Complications
6.2. Adipocyte Metabolism the Presence of SGLT2 Inhibitors
6.2.1. Dapagliflozin
6.2.2. Empagliflozin
7. SGLT2 Inhibitors and Cancer
7.1. Dapagliflozin
7.2. Empagliflozin
7.3. Canagliflozin
7.4. Ertugliflozin
8. Effect of SGLT2 Inhibitors on Bone Metabolism and Fracture Risk
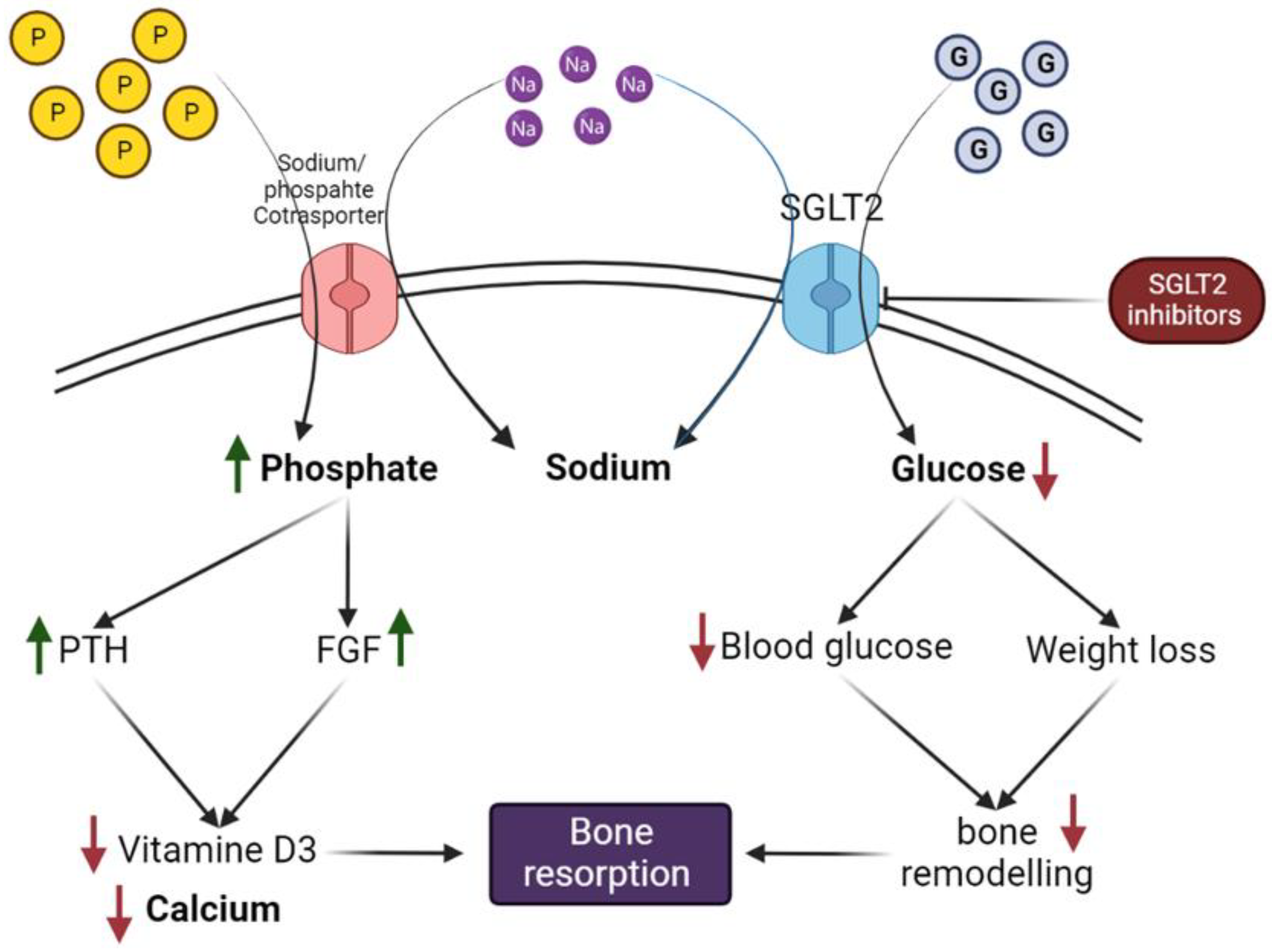
8.1. Potential Mechanism of SGLT2 Inhibitors on Bone Metabolism, Turnover, Microarchitecture, and Calcium and Phosphate Hemostasis
8.1.1. Dapagliflozin
8.1.2. Empagliflozin
8.1.3. Canagliflozin
9. Cognitive Impairment and SGLT2 Inhibitors
9.1. SGLTs in Brain
9.2. Neuroprotective and Neuro-Cognitive Effects of SGLT2 Inhibitors
9.2.1. Dapagliflozin
9.2.2. Empagliflozin
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Abbreviations
References
- Kelly, S.J.; Ismail, M. Stress and type 2 diabetes: A review of how stress contributes to the development of type 2 diabetes. Annu. Rev. Public Health 2015, 36, 441–462. [Google Scholar] [CrossRef] [PubMed]
- Ahmadieh, H.; Sawaya, M.-T.; Azar, S.T. Management and control of type 2 diabetes mellitus in Lebanon: Results from the International Diabetes Management Practices Study Wave 6. World J. Diabetes 2019, 10, 249. [Google Scholar] [CrossRef] [PubMed]
- Eyre, H.; Kahn, R.; Robertson, R.M.; ACS/ADA/AHA Collaborative Writing Committee; ACS/ADA/AHA Collaborative Writing Committee Members; Clark, N.G.; Doyle, C.; Hong, Y.; Gansler, T.; Glynn, T. Preventing cancer, cardiovascular disease, and diabetes: A common agenda for the American Cancer Society, the American Diabetes Association, and the American Heart Association. Circulation 2004, 109, 3244–3255. [Google Scholar] [CrossRef]
- Campbell-Tofte, J.I.; Mølgaard, P.; Winther, K. Harnessing the potential clinical use of medicinal plants as anti-diabetic agents. Bot. Targets Ther. 2012, 2, 7–19. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Katsiki, N.; Behnam, B.; Iranpanah, H.; Sahebkar, A. MicroRNAs and type 2 diabetes mellitus: Molecular mechanisms and the effect of antidiabetic drug treatment. Metabolism 2018, 87, 48–55. [Google Scholar] [CrossRef]
- Rieg, T.; Vallon, V. Development of SGLT1 and SGLT2 inhibitors. Diabetologia 2018, 61, 2079–2086. [Google Scholar] [CrossRef]
- Wright, E.M.; Loo, D.D.; Hirayama, B.A. Biology of human sodium glucose transporters. Physiol. Rev. 2011, 91, 733–794. [Google Scholar] [CrossRef]
- Vallon, V. The mechanisms and therapeutic potential of SGLT2 inhibitors in diabetes mellitus. Annu. Rev. Med. 2015, 66, 255–270. [Google Scholar] [CrossRef]
- Saisho, Y. SGLT2 Inhibitors: The Star in the Treatment of Type 2 Diabetes? Diseases 2020, 8, 14. [Google Scholar] [CrossRef]
- Soták, M.; Casselbrant, A.; Rath, E.; Zietek, T.; Strömstedt, M.; Adingupu, D.D.; Karlsson, D.; Fritsch Fredin, M.; Ergang, P.; Pácha, J.; et al. Intestinal sodium/glucose cotransporter 3 expression is epithelial and downregulated in obesity. Life Sci. 2021, 267, 118974. [Google Scholar] [CrossRef]
- Bianchi, L.; Díez-Sampedro, A. A single amino acid change converts the sugar sensor SGLT3 into a sugar transporter. PLoS ONE 2010, 5, e10241. [Google Scholar] [CrossRef] [PubMed]
- Wright, E.M.; Ghezzi, C.; Loo, D.D.F. Novel and Unexpected Functions of SGLTs. Physiology 2017, 32, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Gyimesi, G.; Pujol-Giménez, J.; Kanai, Y.; Hediger, M.A. Sodium-coupled glucose transport, the SLC5 family, and therapeutically relevant inhibitors: From molecular discovery to clinical application. Pflugers Arch. 2020, 472, 1177–1206. [Google Scholar] [CrossRef] [PubMed]
- Sano, R.; Shinozaki, Y.; Ohta, T. Sodium–glucose cotransporters: Functional properties and pharmaceutical potential. J. Diabetes Investig. 2020, 11, 770–782. [Google Scholar] [CrossRef] [PubMed]
- Boulanger, C.; Stephenne, X.; Diederich, J.; Mounkoro, P.; Chevalier, N.; Ferster, A.; Van Schaftingen, E.; Veiga-da-Cunha, M. Successful use of empagliflozin to treat neutropenia in two G6PC3-deficient children: Impact of a mutation in SGLT5. J. Inherit. Metab. Dis. 2022, 45, 759–768. [Google Scholar] [CrossRef]
- Wood, I.S.; Trayhurn, P. Glucose transporters (GLUT and SGLT): Expanded families of sugar transport proteins. Br. J. Nutr. 2003, 89, 3–9. [Google Scholar] [CrossRef]
- Baader-Pagler, T.; Eckhardt, M.; Himmelsbach, F.; Sauer, A.; Stierstorfer, B.E.; Hamilton, B.S. SGLT6-A pharmacological target for the treatment of obesity? Adipocyte 2018, 7, 277–284. [Google Scholar] [CrossRef]
- Munoz, C.; Sopjani, M.; Dërmaku-Sopjani, M.; Almilaji, A.; Föller, M.; Lang, F. Downregulation of the osmolyte transporters SMIT and BGT1 by AMP-activated protein kinase. Biochem. Biophys. Res. Commun. 2012, 422, 358–362. [Google Scholar] [CrossRef]
- Van Steenbergen, A.; Balteau, M.; Ginion, A.; Ferté, L.; Battault, S.; Ravenstein, C.d.M.d.; Balligand, J.-L.; Daskalopoulos, E.-P.; Gilon, P.; Despa, F. Sodium-myoinositol cotransporter-1, SMIT1, mediates the production of reactive oxygen species induced by hyperglycemia in the heart. Sci. Rep. 2017, 7, 41166. [Google Scholar] [CrossRef]
- Donovan, E.; Avila, C.; Klausner, S.; Parikh, V.; Fenollar-Ferrer, C.; Blakely, R.D.; Sarter, M. Disrupted Choline Clearance and Sustained Acetylcholine Release In Vivo by a Common Choline Transporter Coding Variant Associated with Poor Attentional Control in Humans. J. Neurosci. 2022, 42, 3426–3444. [Google Scholar] [CrossRef]
- Prasad, P.D.; Wang, H.; Kekuda, R.; Fujita, T.; Fei, Y.J.; Devoe, L.D.; Leibach, F.H.; Ganapathy, V. Cloning and functional expression of a cDNA encoding a mammalian sodium-dependent vitamin transporter mediating the uptake of pantothenate, biotin, and lipoate. J. Biol. Chem. 1998, 273, 7501–7506. [Google Scholar] [CrossRef] [PubMed]
- Vadlapudi, A.D.; Vadlapatla, R.K.; Mitra, A.K. Sodium dependent multivitamin transporter (SMVT): A potential target for drug delivery. Curr. Drug Targets 2012, 13, 994–1003. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Myeroff, L.; Smiraglia, D.; Romero, M.F.; Pretlow, T.P.; Kasturi, L.; Lutterbaugh, J.; Rerko, R.M.; Casey, G.; Issa, J.P.; et al. SLC5A8, a sodium transporter, is a tumor suppressor gene silenced by methylation in human colon aberrant crypt foci and cancers. Proc. Natl. Acad. Sci. USA 2003, 100, 8412–8417. [Google Scholar] [CrossRef]
- Martin, P.M.; Gopal, E.; Ananth, S.; Zhuang, L.; Itagaki, S.; Prasad, B.M.; Smith, S.B.; Prasad, P.D.; Ganapathy, V. Identity of SMCT1 (SLC5A8) as a neuron-specific Na+-coupled transporter for active uptake of l-lactate and ketone bodies in the brain. J. Neurochem. 2006, 98, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Gill, R.K.; Alrefai, W.A.; Borthakur, A.; Dudeja, P.K. Chapter 67—Intestinal Anion Absorption. In Physiology of the Gastrointestinal Tract, 5th ed.; Johnson, L.R., Ghishan, F.K., Kaunitz, J.D., Merchant, J.L., Said, H.M., Wood, J.D., Eds.; Academic Press: Boston, MA, USA, 2012; pp. 1819–1847. [Google Scholar]
- Gopal, E.; Umapathy, N.S.; Martin, P.M.; Ananth, S.; Gnana-Prakasam, J.P.; Becker, H.; Wagner, C.A.; Ganapathy, V.; Prasad, P.D. Cloning and functional characterization of human SMCT2 (SLC5A12) and expression pattern of the transporter in kidney. Biochim. Biophys. Acta 2007, 1768, 2690–2697. [Google Scholar] [CrossRef]
- Nicola, J.P.; Nazar, M.; Serrano-Nascimento, C.; Goulart-Silva, F.; Sobrero, G.; Testa, G.; Nunes, M.T.; Muñoz, L.; Miras, M.; Masini-Repiso, A.M. Iodide transport defect: Functional characterization of a novel mutation in the Na+/I− symporter 5’-untranslated region in a patient with congenital hypothyroidism. J. Clin. Endocrinol. Metab. 2011, 96, E1100–E1107. [Google Scholar] [CrossRef]
- De Morais, R.M.; Sobrinho, A.B.; de Souza Silva, C.M.; de Oliveira, J.R.; da Silva, I.C.R.; de Toledo Nóbrega, O. The Role of the NIS (SLC5A5) Gene in Papillary Thyroid Cancer: A Systematic Review. Int. J. Endocrinol. 2018, 2018, 9128754. [Google Scholar] [CrossRef]
- Pokusaeva, K.; Fitzgerald, G.F.; van Sinderen, D. Carbohydrate metabolism in Bifidobacteria. Genes Nutr. 2011, 6, 285–306. [Google Scholar] [CrossRef]
- Meneses, M.J.; Silva, B.M.; Sousa, M.; Sa, R.; Oliveira, P.F.; Alves, M.G. Antidiabetic drugs: Mechanisms of action and potential outcomes on cellular metabolism. Curr. Pharm. Des. 2015, 21, 3606–3620. [Google Scholar] [CrossRef]
- Bell, D.S. Type 2 diabetes mellitus: What is the optimal treatment regimen? Am. J. Med. 2004, 116, 23–29. [Google Scholar] [CrossRef]
- Bailey, C.J. Metformin: Historical overview. Diabetologia 2017, 60, 1566–1576. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, G.R.; Carling, D. AMP-activated protein kinase: The current landscape for drug development. Nat. Rev. Drug Discov. 2019, 18, 527–551. [Google Scholar] [CrossRef] [PubMed]
- Winder, W.A.; Hardie, D. AMP-activated protein kinase, a metabolic master switch: Possible roles in type 2 diabetes. Am. J. Physiol. -Endocrinol. Metab. 1999, 277, E1–E10. [Google Scholar] [CrossRef] [PubMed]
- Seltzer, H.S. Drug-induced hypoglycemia: A review of 1418 cases. Endocrinol. Metab. Clin. North Am. 1989, 18, 163–183. [Google Scholar] [CrossRef]
- Rendell, M. The role of sulphonylureas in the management of type 2 diabetes mellitus. Drugs 2004, 64, 1339–1358. [Google Scholar] [CrossRef]
- Donley, V.R.; Hiskett, E.K.; Kidder, A.C.; Schermerhorn, T. ATP-sensitive potassium channel (KATPchannel) expression in the normal canine pancreas and in canine insulinomas. BMC Vet. Res. 2005, 1, 8. [Google Scholar] [CrossRef]
- De Wet, H.; Proks, P. Molecular action of sulphonylureas on KATP channels: A real partnership between drugs and nucleotides. Biochem. Soc. Trans. 2015, 43, 901–907. [Google Scholar] [CrossRef]
- Guardado-Mendoza, R.; Prioletta, A.; Jiménez-Ceja, L.M.; Sosale, A.; Folli, F. The role of nateglinide and repaglinide, derivatives of meglitinide, in the treatment of type 2 diabetes mellitus. Arch. Med. Sci. AMS 2013, 9, 936. [Google Scholar] [CrossRef]
- Culy, C.R.; Jarvis, B. Repaglinide. Drugs 2001, 61, 1625–1660. [Google Scholar] [CrossRef]
- Holst, J.J. From the incretin concept and the discovery of GLP-1 to today’s diabetes therapy. Front. Endocrinol. 2019, 10, 260. [Google Scholar] [CrossRef]
- Doyle, M.E.; Egan, J.M. Mechanisms of action of glucagon-like peptide 1 in the pancreas. Pharmacol. Ther. 2007, 113, 546–593. [Google Scholar] [CrossRef] [PubMed]
- McLean, B.A.; Wong, C.K.; Campbell, J.E.; Hodson, D.J.; Trapp, S.; Drucker, D.J. Revisiting the complexity of GLP-1 action from sites of synthesis to receptor activation. Endocr. Rev. 2021, 42, 101–132. [Google Scholar] [CrossRef] [PubMed]
- Forman, B.M.; Tontonoz, P.; Chen, J.; Brun, R.P.; Spiegelman, B.M.; Evans, R.M. 15-deoxy-Δ12, 14-prostaglandin J2 is a ligand for the adipocyte determination factor PPARγ. Cell 1995, 83, 803–812. [Google Scholar] [CrossRef]
- Kurtz, T.W.; Pravenec, M. Antidiabetic mechanisms of angiotensin-converting enzyme inhibitors and angiotensin II receptor antagonists: Beyond the renin–angiotensin system. J. Hypertens. 2004, 22, 2253–2261. [Google Scholar] [CrossRef] [PubMed]
- Van Genugten, R.; Van Raalte, D.; Diamant, M. Dipeptidyl peptidase-4 inhibitors and preservation of pancreatic islet-cell function: A critical appraisal of the evidence. Diabetes Obes. Metab. 2012, 14, 101–111. [Google Scholar] [CrossRef]
- Omar, B.; Ahrén, B. Pleiotropic mechanisms for the glucose-lowering action of DPP-4 inhibitors. Diabetes 2014, 63, 2196–2202. [Google Scholar] [CrossRef]
- Karagiannis, T.; Paschos, P.; Paletas, K.; Matthews, D.R.; Tsapas, A. Dipeptidyl peptidase-4 inhibitors for treatment of type 2 diabetes mellitus in the clinical setting: Systematic review and meta-analysis. BMJ 2012, 344, e1369. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, K.B.; Delpire, E. Sodium transporters in human health and disease. Front. Physiol. 2021, 1382, 588664. [Google Scholar] [CrossRef] [PubMed]
- Flores, E.; Santos-Gallego, C.G.; Diaz-Mejía, N.; Badimon, J.J. Do the SGLT-2 inhibitors offer more than hypoglycemic activity? Cardiovasc. Drugs Ther. 2018, 32, 213–222. [Google Scholar] [CrossRef]
- Gallo, L.A.; Wright, E.M.; Vallon, V. Probing SGLT2 as a therapeutic target for diabetes: Basic physiology and consequences. Diabetes Vasc. Dis. Res. 2015, 12, 78–89. [Google Scholar] [CrossRef]
- Kaushal, S.; Singh, H.; Thangaraju, P.; Singh, J. Canagliflozin: A Novel SGLT2 Inhibitor for Type 2 Diabetes Mellitus. N. Am. J. Med. Sci. 2014, 6, 107–113. [Google Scholar] [PubMed]
- Sano, M. A new class of drugs for heart failure: SGLT2 inhibitors reduce sympathetic overactivity. J. Cardiol. 2018, 71, 471–476. [Google Scholar] [CrossRef] [PubMed]
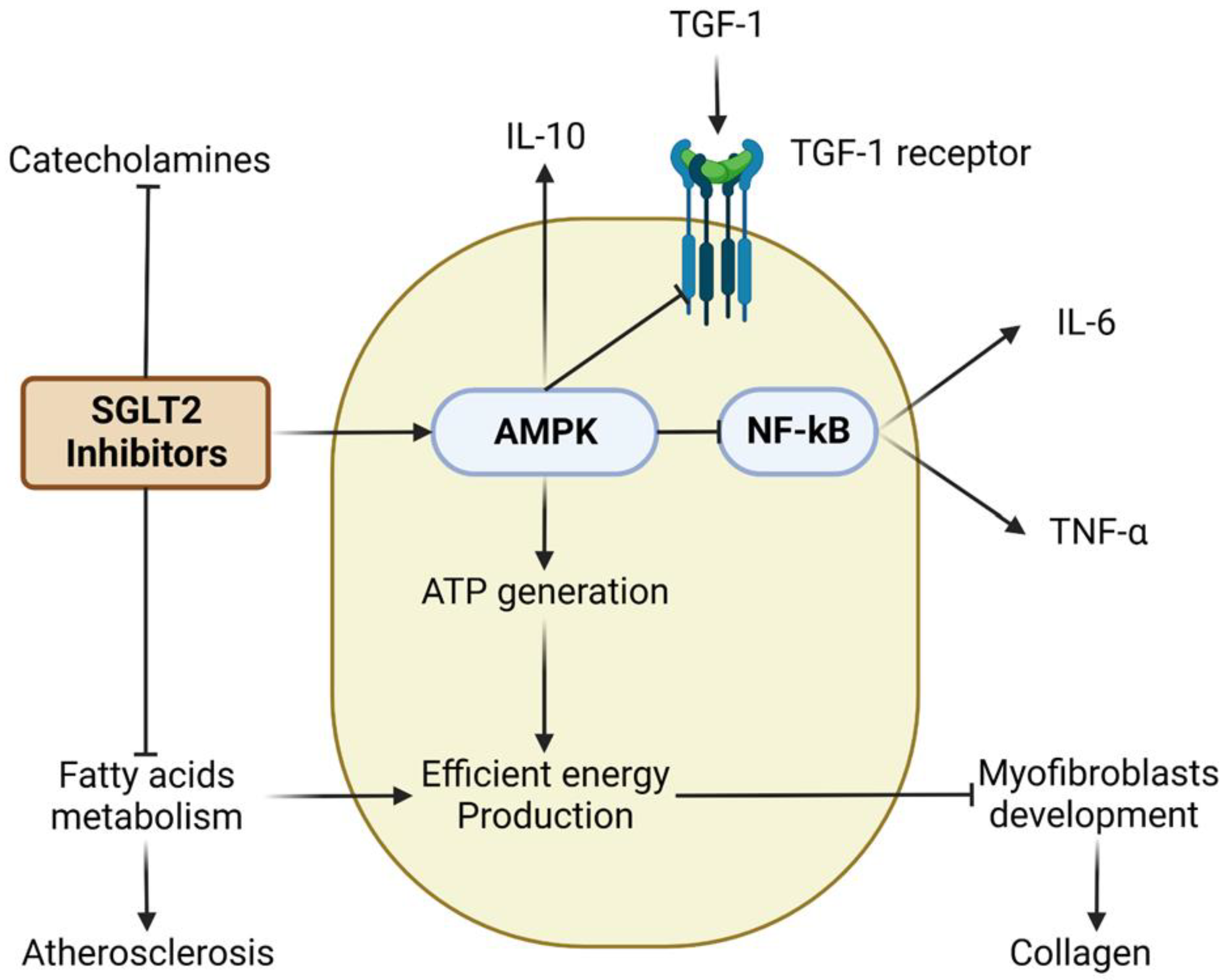
- Tian, J.; Zhang, M.; Suo, M.; Liu, D.; Wang, X.; Liu, M.; Pan, J.; Jin, T.; An, F. Dapagliflozin alleviates cardiac fibrosis through suppressing EndMT and fibroblast activation via AMPKα/TGF-β/Smad signalling in type 2 diabetic rats. J. Cell Mol. Med. 2021, 25, 7642–7659. [Google Scholar] [CrossRef] [PubMed]
- Puglisi, S.; Rossini, A.; Poli, R.; Dughera, F.; Pia, A.; Terzolo, M.; Reimondo, G. Effects of SGLT2 inhibitors and GLP-1 receptor agonists on renin-angiotensin-aldosterone system. Front. Endocrinol. 2021, 12, 738848. [Google Scholar] [CrossRef] [PubMed]
- Feder, D.; de Fatima Veiga Gouveia, M.R.; Govato, T.C.P.; Nassis, C.D.Z. SGLT2 Inhibitors and the Mechanisms Involved in Weight Loss. Curr. Pharmacol. Rep. 2020, 6, 346–353. [Google Scholar] [CrossRef]
- Durante, W.; Behnammanesh, G.; Peyton, K.J. Effects of Sodium-Glucose Co-Transporter 2 Inhibitors on Vascular Cell Function and Arterial Remodeling. Int. J. Mol. Sci. 2021, 22, 8786. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Huang, X.; Akgün, E.E.; Mehmood, K.; Zhang, H.; Tang, Z.; Li, Y. Mechanism of Hypoxia-Mediated Smooth Muscle Cell Proliferation Leading to Vascular Remodeling. BioMed Res. Int. 2022, 2022, 3959845. [Google Scholar] [CrossRef]
- García-Ropero, Á.; Vargas-Delgado, A.P.; Santos-Gallego, C.G.; Badimon, J.J. Inhibition of sodium glucose cotransporters improves cardiac performance. Int. J. Mol. Sci. 2019, 20, 3289. [Google Scholar] [CrossRef]
- Layton, A.T.; Vallon, V. Cardiovascular benefits of SGLT2 inhibition in diabetes and chronic kidney diseases. Acta Physiol. 2018, 222, e13050. [Google Scholar] [CrossRef]
- Heidrich, F.; Schotola, H.; Popov, A.F.; Sohns, C.; Schuenemann, J.; Friedrich, M.; Coskun, K.O.; von Lewinski, D.; Hinz, J.; Bauer, M. AMPK-activated protein kinase and its role in energy metabolism of the heart. Curr. Cardiol. Rev. 2010, 6, 337–342. [Google Scholar] [CrossRef]
- Hoong, C.W.S.; Chua, M.W.J. SGLT2 Inhibitors as Calorie Restriction Mimetics: Insights on Longevity Pathways and Age-Related Diseases. Endocrinology 2021, 162, bqab079. [Google Scholar] [CrossRef]
- Ke, R.; Xu, Q.; Li, C.; Luo, L.; Huang, D. Mechanisms of AMPK in the maintenance of ATP balance during energy metabolism. Cell Biol. Int. 2018, 42, 384–392. [Google Scholar] [CrossRef]
- Abd El-Fattah, E.E.; Saber, S.; Mourad, A.A.E.; El-Ahwany, E.; Amin, N.A.; Cavalu, S.; Yahya, G.; Saad, A.S.; Alsharidah, M.; Shata, A.; et al. The dynamic interplay between AMPK/NFκB signaling and NLRP3 is a new therapeutic target in inflammation: Emerging role of dapagliflozin in overcoming lipopolysaccharide-mediated lung injury. Biomed. Pharmacother. 2022, 147, 112628. [Google Scholar] [CrossRef]
- Abdelhamid, A.M.; Saber, S.; Youssef, M.E.; Gaafar, A.G.A.; Eissa, H.; Abd-Eldayem, M.A.; Alqarni, M.; Batiha, G.E.-S.; Obaidullah, A.J.; Shahien, M.A.; et al. Empagliflozin adjunct with metformin for the inhibition of hepatocellular carcinoma progression: Emerging approach for new application. Biomed. Pharmacother. 2022, 145, 112455. [Google Scholar] [CrossRef]
- Abdelhamid, A.M.; Youssef, M.E.; Abd El-Fattah, E.E.; Gobba, N.A.; Gaafar, A.G.A.; Girgis, S.; Shata, A.; Hafez, A.-M.; El-Ahwany, E.; Amin, N.A.; et al. Blunting p38 MAPKα and ERK1/2 activities by empagliflozin enhances the antifibrotic effect of metformin and augments its AMPK-induced NF-κB inactivation in mice intoxicated with carbon tetrachloride. Life Sci. 2021, 286, 120070. [Google Scholar] [CrossRef]
- Nasr, M.; Cavalu, S.; Saber, S.; Youssef, M.E.; Abdelhamid, A.M.; Elagamy, H.I.; Kamal, I.; Gaafar, A.G.A.; El-Ahwany, E.; Amin, N.A.; et al. Canagliflozin-loaded chitosan-hyaluronic acid microspheres modulate AMPK/NF-κB/NLRP3 axis: A new paradigm in the rectal therapy of ulcerative colitis. Biomed. Pharmacother. 2022, 153, 113409. [Google Scholar] [CrossRef]
- Youssef, M.E.; Abd El-Fattah, E.E.; Abdelhamid, A.M.; Eissa, H.; El-Ahwany, E.; Amin, N.A.; Hetta, H.F.; Mahmoud, M.H.; Batiha, G.E.; Gobba, N.; et al. Interference with the AMPKα/mTOR/NLRP3 Signaling and the IL-23/IL-17 Axis Effectively Protects Against the Dextran Sulfate Sodium Intoxication in Rats: A New Paradigm in Empagliflozin and Metformin Reprofiling for the Management of Ulcerative Colitis. Front. Pharmacol. 2021, 12, 719984. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Karwi, Q.G.; Ho, K.L.; Pherwani, S.; Ketema, E.B. Ketone metabolism in the failing heart. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2020, 1865, 158813. [Google Scholar]
- Li, Z.; Agrawal, V.; Ramratnam, M.; Sharma, R.K.; D’Auria, S.; Sincoular, A.; Jakubiak, M.; Music, M.L.; Kutschke, W.J.; Huang, X.N.; et al. Cardiac sodium-dependent glucose cotransporter 1 is a novel mediator of ischaemia/reperfusion injury. Cardiovasc. Res. 2019, 115, 1646–1658. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Verma, S. Mechanisms of cardiovascular benefits of sodium glucose co-transporter 2 (SGLT2) inhibitors: A state-of-the-art review. Basic Transl. Sci. 2020, 5, 632–644. [Google Scholar]
- Lee, T.-M.; Chang, N.-C.; Lin, S.-Z. Dapagliflozin, a selective SGLT2 Inhibitor, attenuated cardiac fibrosis by regulating the macrophage polarization via STAT3 signaling in infarcted rat hearts. Free. Radic. Biol. Med. 2017, 104, 298–310. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wang, Y.; Zhang, Y.; Yan, B. Mechanisms of protective effects of SGLT2 inhibitors in cardiovascular disease and renal dysfunction. Curr. Top. Med. Chem. 2019, 19, 1818–1849. [Google Scholar] [CrossRef]
- García Ropero, A. Empagliflozine Improves Myocardial Fibrosis and Diastolic Function in a Non-Diabetic Porcine Model of Ischaemic Heart Failure; Universidad Autónoma de Madrid: Madrid, Spain, 2020. [Google Scholar]
- Dimitriadis, G.K.; Nasiri-Ansari, N.; Agrogiannis, G.; Kostakis, I.D.; Randeva, M.S.; Nikiteas, N.; Patel, V.H.; Kaltsas, G.; Papavassiliou, A.G.; Randeva, H.S. Empagliflozin improves primary haemodynamic parameters and attenuates the development of atherosclerosis in high fat diet fed APOE knockout mice. Mol. Cell. Endocrinol. 2019, 494, 110487. [Google Scholar] [CrossRef]
- Bonnet, F.; Scheen, A. Effects of SGLT2 inhibitors on systemic and tissue low-grade inflammation: The potential contribution to diabetes complications and cardiovascular disease. Diabetes Metab. 2018, 44, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Hupa-Breier, K.L.; Dywicki, J.; Hartleben, B.; Wellhöner, F.; Heidrich, B.; Taubert, R.; Mederacke, Y.-S.E.; Lieber, M.; Iordanidis, K.; Manns, M.P. Dulaglutide Alone and in Combination with Empagliflozin Attenuate Inflammatory Pathways and Microbiome Dysbiosis in a Non-Diabetic Mouse Model of NASH. Biomedicines 2021, 9, 353. [Google Scholar] [CrossRef]
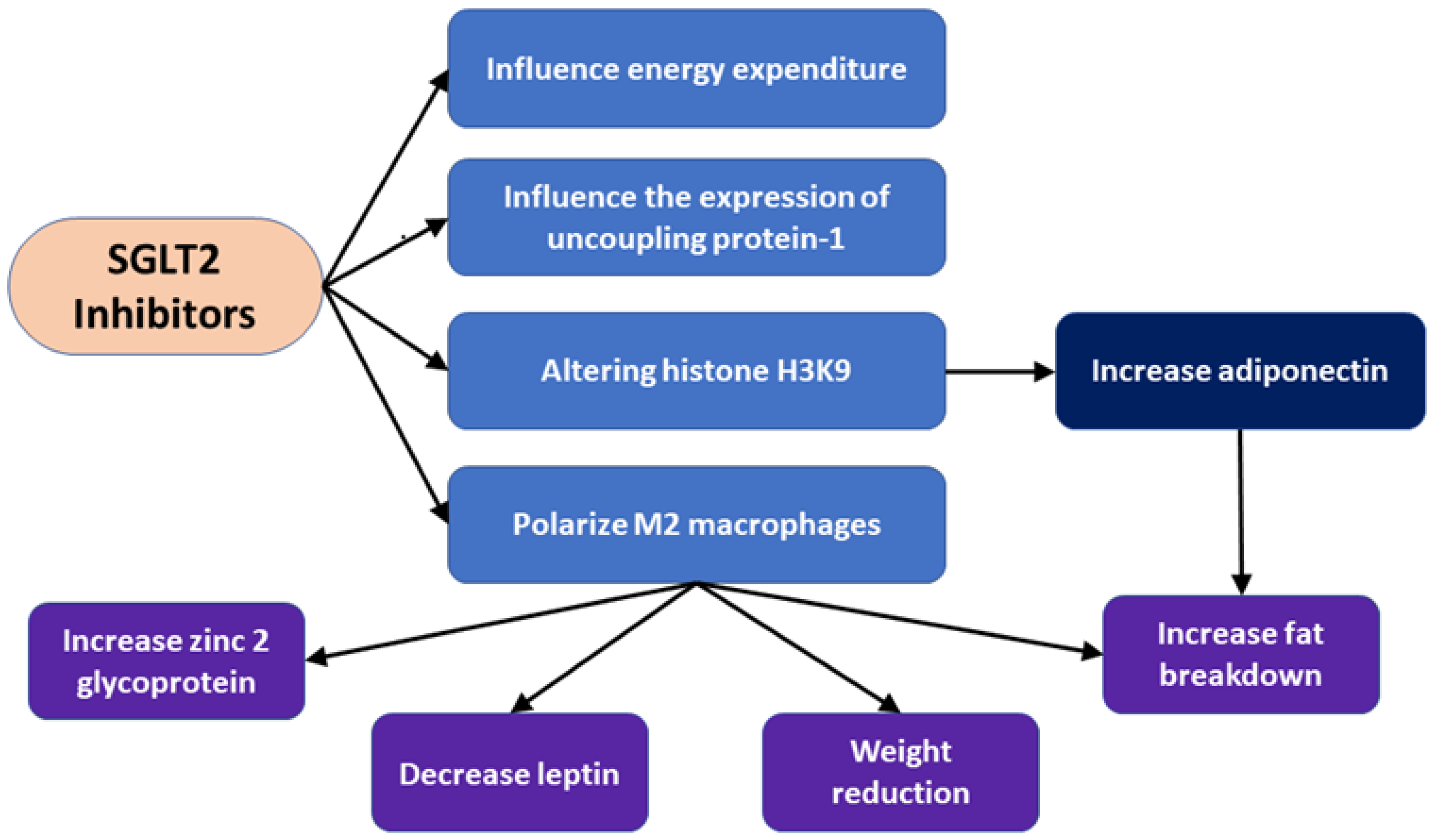
- Nishitani, S.; Fukuhara, A.; Shin, J.; Okuno, Y.; Otsuki, M.; Shimomura, I. Metabolomic and microarray analyses of adipose tissue of dapagliflozin-treated mice, and effects of 3-hydroxybutyrate on induction of adiponectin in adipocytes. Sci. Rep. 2018, 8, 8805. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Simental-Mendía, L.E.; Barreto, G.E.; Sahebkar, A. Metabolic effects of antidiabetic drugs on adipocytes and adipokine expression. J. Cell. Physiol. 2019, 234, 16987–16997. [Google Scholar] [CrossRef]
- Sawada, Y.; Izumida, Y.; Takeuchi, Y.; Aita, Y.; Wada, N.; Li, E.; Murayama, Y.; Piao, X.; Shikama, A.; Masuda, Y. Effect of sodium-glucose cotransporter 2 (SGLT2) inhibition on weight loss is partly mediated by liver-brain-adipose neurocircuitry. Biochem. Biophys. Res. Commun. 2017, 493, 40–45. [Google Scholar] [CrossRef]
- Lau, K.T.K.; Ng, L.; Wong, J.W.H.; Loong, H.H.F.; Chan, W.W.L.; Lee, C.H.; Wong, C.K.H. Repurposing sodium-glucose co-transporter 2 inhibitors (SGLT2i) for cancer treatment—A Review. Rev. Endocr. Metab. Disord. 2021, 22, 1121–1136. [Google Scholar] [CrossRef]
- Okada, J.; Yamada, E.; Saito, T.; Yokoo, H.; Osaki, A.; Shimoda, Y.; Ozawa, A.; Nakajima, Y.; Pessin, J.E.; Okada, S. Dapagliflozin inhibits cell adhesion to collagen I and IV and increases ectodomain proteolytic cleavage of DDR1 by increasing ADAM10 activity. Molecules 2020, 25, 495. [Google Scholar] [CrossRef] [PubMed]
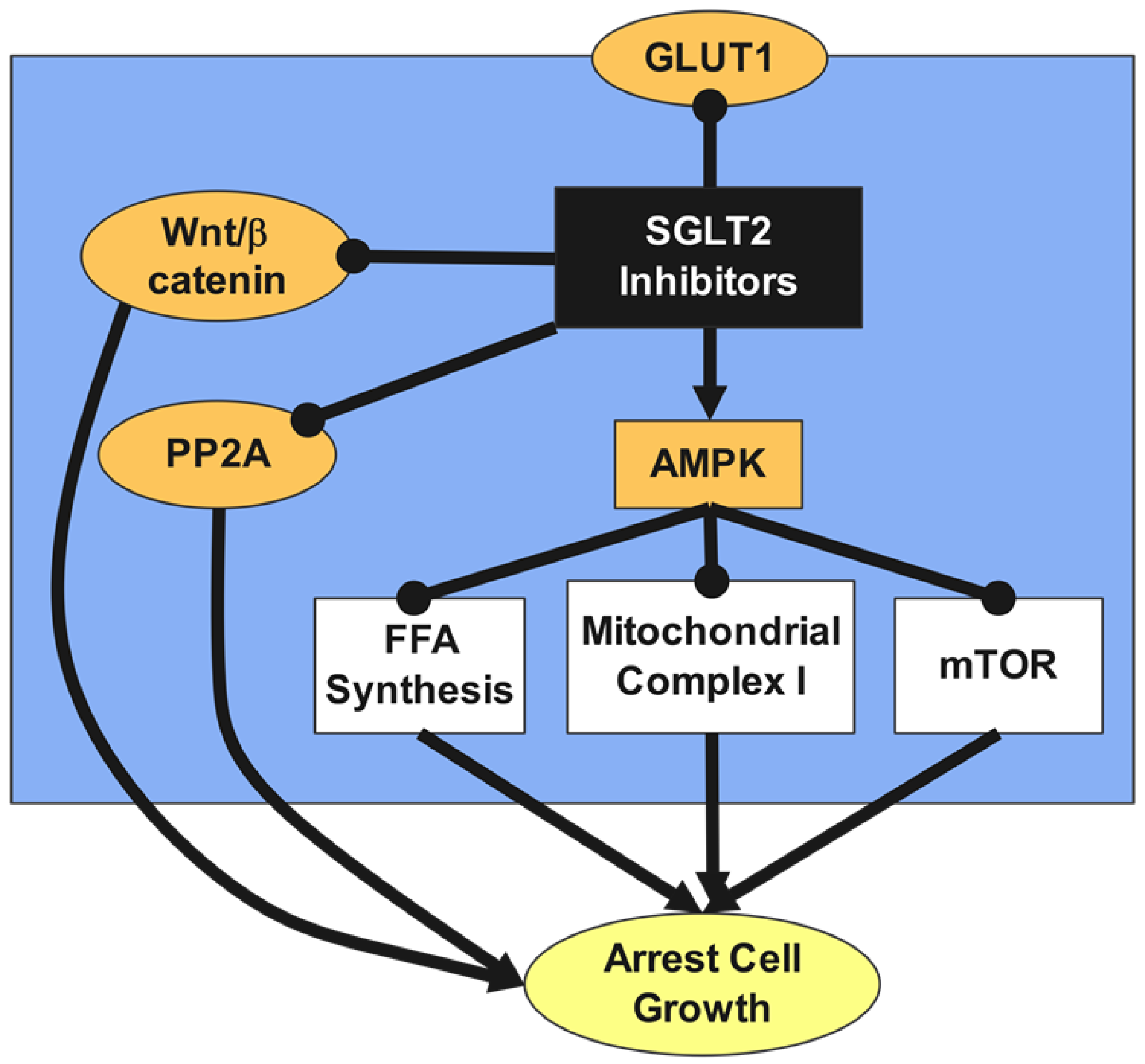
- Villani, L.A.; Smith, B.K.; Marcinko, K.; Ford, R.J.; Broadfield, L.A.; Green, A.E.; Houde, V.P.; Muti, P.; Tsakiridis, T.; Steinberg, G.R. The diabetes medication Canagliflozin reduces cancer cell proliferation by inhibiting mitochondrial complex-I supported respiration. Mol. Metab. 2016, 5, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.H.; Lee, T.-J.; Sung, E.-G.; Song, I.-H.; Kim, J.-Y. Dapagliflozin induces apoptosis by downregulating cFILP L and increasing cFILP S instability in Caki-1 cells. Oncol. Lett. 2022, 24, 401. [Google Scholar] [CrossRef]
- Herat, L.Y.; Magno, A.L.; Rudnicka, C.; Hricova, J.; Carnagarin, R.; Ward, N.C.; Arcambal, A.; Kiuchi, M.G.; Head, G.A.; Schlaich, M.P.; et al. SGLT2 Inhibitor-Induced Sympathoinhibition: A Novel Mechanism for Cardiorenal Protection. JACC Basic Transl. Sci. 2020, 5, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Dai, Q.; Shi, W.; Zhai, S.; Song, Y.; Han, J. SGLT2 inhibitors and risk of cancer in type 2 diabetes: A systematic review and meta-analysis of randomised controlled trials. Diabetologia 2017, 60, 1862–1872. [Google Scholar] [CrossRef]
- Dąbrowski, M. Diabetes, Antidiabetic Medications and Cancer Risk in Type 2 Diabetes: Focus on SGLT-2 Inhibitors. Int. J. Mol. Sci. 2021, 22, 1680. [Google Scholar] [CrossRef]
- Rosenwasser, R.F.; Sultan, S.; Sutton, D.; Choksi, R.; Epstein, B.J. SGLT-2 inhibitors and their potential in the treatment of diabetes. Diabetes Metab. Syndr. Obes. Targets Ther. 2013, 6, 453. [Google Scholar]
- Bolinder, J.; Ljunggren, Ö.; Johansson, L.; Wilding, J.; Langkilde, A.; Sjöström, C.; Sugg, J.; Parikh, S. Dapagliflozin maintains glycaemic control while reducing weight and body fat mass over 2 years in patients with type 2 diabetes mellitus inadequately controlled on metformin. Diabetes Obes. Metab. 2014, 16, 159–169. [Google Scholar] [CrossRef]
- Cianciolo, G.; De Pascalis, A.; Capelli, I.; Gasperoni, L.; Di Lullo, L.; Bellasi, A.; La Manna, G. Mineral and electrolyte disorders with SGLT2i therapy. JBMR Plus 2019, 3, e10242. [Google Scholar] [CrossRef]
- Thrailkill, K.M.; Clay Bunn, R.; Nyman, J.S.; Rettiganti, M.R.; Cockrell, G.E.; Wahl, E.C.; Uppuganti, S.; Lumpkin, C.K., Jr.; Fowlkes, J.L. SGLT2 inhibitor therapy improves blood glucose but does not prevent diabetic bone disease in diabetic DBA/2J male mice. Bone 2016, 82, 101–107. [Google Scholar] [CrossRef]
- Ye, Y.; Zhao, C.; Liang, J.; Yang, Y.; Yu, M.; Qu, X. Effect of sodium-glucose co-transporter 2 inhibitors on bone metabolism and fracture risk. Front. Pharmacol. 2019, 9, 1517. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.E.; Coleman, C.M. Impact of diabetes mellitus on bone health. Int. J. Mol. Sci. 2019, 20, 4873. [Google Scholar] [CrossRef] [PubMed]
- Bilezikian, J.P.; Watts, N.B.; Usiskin, K.; Polidori, D.; Fung, A.; Sullivan, D.; Rosenthal, N. Evaluation of bone mineral density and bone biomarkers in patients with type 2 diabetes treated with canagliflozin. J. Clin. Endocrinol. 2016, 101, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Rosenstock, J.; Frias, J.; Páll, D.; Charbonnel, B.; Pascu, R.; Saur, D.; Darekar, A.; Huyck, S.; Shi, H.; Lauring, B.; et al. Effect of ertugliflozin on glucose control, body weight, blood pressure and bone density in type 2 diabetes mellitus inadequately controlled on metformin monotherapy (VERTIS MET). Diabetes Obes. Metab. 2018, 20, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Tharmaraja, T.; Ho, J.S.; Sia, C.-H.; Lim, N.-A.; Chong, Y.F.; Lim, A.Y.; Rathakrishnan, R.R.; Yeo, L.L.; Sharma, V.K.; Tan, B.Y. Sodium-glucose cotransporter 2 inhibitors and neurological disorders: A scoping review. Ther. Adv. Chronic Dis. 2022, 13, 20406223221086996. [Google Scholar] [CrossRef]
- Shaikh, S.; Rizvi, S.M.D.; Shakil, S.; Riyaz, S.; Biswas, D.; Jahan, R. Forxiga (dapagliflozin): Plausible role in the treatment of diabetes-associated neurological disorders. Biotechnol. Appl. Biochem. 2016, 63, 145–150. [Google Scholar] [CrossRef]
- Wiciński, M.; Wódkiewicz, E.; Górski, K.; Walczak, M.; Malinowski, B. Perspective of SGLT2 inhibition in treatment of conditions connected to neuronal loss: Focus on Alzheimer’s disease and ischemia-related brain injury. Pharmaceuticals 2020, 13, 379. [Google Scholar] [CrossRef]
- Nikolajević Starčević, J.; Janić, M.; Šabovič, M. Molecular mechanisms responsible for diastolic dysfunction in diabetes mellitus patients. Int. J. Mol. Sci. 2019, 20, 1197. [Google Scholar] [CrossRef]
- Heerspink, H.J.; Perco, P.; Mulder, S.; Leierer, J.; Hansen, M.K.; Heinzel, A.; Mayer, G. Canagliflozin reduces inflammation and fibrosis biomarkers: A potential mechanism of action for beneficial effects of SGLT2 inhibitors in diabetic kidney disease. Diabetologia 2019, 62, 1154–1166. [Google Scholar] [CrossRef]
- Romero, J.C.; Knox, F.G. Mechanisms underlying pressure-related natriuresis: The role of the renin-angiotensin and prostaglandin systems. State of the art lecture. Hypertension 1988, 11, 724–738. [Google Scholar] [CrossRef]
- Ilias, I.; Thomopoulos, C.; Michalopoulou, H.; Bazoukis, G.; Tsioufis, C.; Makris, T. Antidiabetic drugs and blood pressure changes. Pharmacol. Res. 2020, 161, 105108. [Google Scholar] [CrossRef] [PubMed]
- Dandona, P.; Chaudhuri, A. Sodium-glucose co-transporter 2 inhibitors for type 2 diabetes mellitus: An overview for the primary care physician. Int. J. Clin. Pract. 2017, 71, e12937. [Google Scholar] [CrossRef] [PubMed]
- Adam, C.A.; Anghel, R.; Marcu, D.T.M.; Mitu, O.; Roca, M.; Mitu, F. Impact of Sodium-Glucose Cotransporter 2 (SGLT2) Inhibitors on Arterial Stiffness and Vascular Aging-What Do We Know So Far? (A Narrative Review). Life 2022, 12, 803. [Google Scholar] [CrossRef]
- Yang, F.; Meng, R.; Zhu, D.L. Cardiovascular effects and mechanisms of sodium-glucose cotransporter-2 inhibitors. Chronic Dis. Transl. Med. 2020, 6, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Lupsa, B.C.; Inzucchi, S.E. Use of SGLT2 inhibitors in type 2 diabetes: Weighing the risks and benefits. Diabetologia 2018, 61, 2118–2125. [Google Scholar] [CrossRef] [PubMed]
- Nelinson, D.S.; Sosa, J.M.; Chilton, R.J. SGLT2 inhibitors: A narrative review of efficacy and safety. J. Osteopath. Med. 2021, 121, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Yaribeygi, H.; Atkin, S.L.; Sahebkar, A. Interleukin-18 and diabetic nephropathy: A review. J. Cell. Physiol. 2019, 234, 5674–5682. [Google Scholar] [CrossRef]
- Kusminski, C.M.; Bickel, P.E.; Scherer, P.E. Targeting adipose tissue in the treatment of obesity-associated diabetes. Nat. Rev. Drug Discov. 2016, 15, 639–660. [Google Scholar] [CrossRef]
- Robles, H.; Park, S.; Joens, M.S.; Fitzpatrick, J.A.; Craft, C.S.; Scheller, E.L. Characterization of the bone marrow adipocyte niche with three-dimensional electron microscopy. Bone 2019, 118, 89–98. [Google Scholar] [CrossRef]
- Jeremic, N.; Chaturvedi, P.; Tyagi, S.C. Browning of white fat: Novel insight into factors, mechanisms, and therapeutics. J. Cell. Physiol. 2017, 232, 61–68. [Google Scholar] [CrossRef]
- Bartelt, A.; Heeren, J. Adipose tissue browning and metabolic health. Nat. Rev. Endocrinol. 2014, 10, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G.F.; Carpentier, A.; Adeli, K.; Giacca, A. Disordered fat storage and mobilization in the pathogenesis of insulin resistance and type 2 diabetes. Endocr. Rev. 2002, 23, 201–229. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, I.; Yoshida, Y.; Minamino, T. Maintenance of Subcutaneous Fat Homeostasis Improves Systemic Metabolic Dysfunction in Obesity. Diabetes 2015, 64, 3984–3986. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.M. Subcutaneous and visceral adipose tissue: Structural and functional differences. Obes. Rev. 2010, 11, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Fasshauer, M.; Blüher, M. Adipokines in health and disease. Trends Pharmacol. Sci. 2015, 36, 461–470. [Google Scholar] [CrossRef]
- Reinehr, T.; Roth, C.L. Inflammation markers in type 2 diabetes and the metabolic syndrome in the pediatric population. Curr. Diabetes Rep. 2018, 18, 131. [Google Scholar] [CrossRef]
- Ruggiero, A.D.; Key, C.-C.C.; Kavanagh, K. Adipose tissue macrophage polarization in healthy and unhealthy obesity. Front. Nutr. 2021, 8, 625331. [Google Scholar] [CrossRef]
- Małodobra-Mazur, M.; Cierzniak, A.; Myszczyszyn, A.; Kaliszewski, K.; Dobosz, T. Histone modifications influence the insulin-signaling genes and are related to insulin resistance in human adipocytes. Int. J. Biochem. Cell Biol. 2021, 137, 106031. [Google Scholar] [CrossRef]
- Balaz, M.; Vician, M.; Janakova, Z.; Kurdiova, T.; Surova, M.; Imrich, R.; Majercikova, Z.; Penesova, A.; Vlcek, M.; Kiss, A.; et al. Subcutaneous adipose tissue zinc-α2-glycoprotein is associated with adipose tissue and whole-body insulin sensitivity. Obesity 2014, 22, 1821–1829. [Google Scholar] [CrossRef]
- Brown, E.; Rajeev, S.P.; Cuthbertson, D.J.; Wilding, J.P. A review of the mechanism of action, metabolic profile and haemodynamic effects of sodium-glucose co-transporter-2 inhibitors. Diabetes Obes. Metab. 2019, 21, 9–18. [Google Scholar] [CrossRef]
- Thomas, M.C.; Cherney, D.Z. The actions of SGLT2 inhibitors on metabolism, renal function and blood pressure. Diabetologia 2018, 61, 2098–2107. [Google Scholar] [CrossRef] [PubMed]
- Dutka, M.; Bobiński, R.; Francuz, T.; Garczorz, W.; Zimmer, K.; Ilczak, T.; Ćwiertnia, M.; Hajduga, M.B. SGLT-2 Inhibitors in Cancer Treatment—Mechanisms of Action and Emerging New Perspectives. Cancers 2022, 14, 5811. [Google Scholar] [CrossRef] [PubMed]
- Nasiri, A.R.; Rodrigues, M.R.; Li, Z.; Leitner, B.P.; Perry, R.J. SGLT2 inhibition slows tumor growth in mice by reversing hyperinsulinemia. Cancer Metab. 2019, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Heydarzadeh, S.; Moshtaghie, A.A.; Daneshpoor, M.; Hedayati, M. Regulators of glucose uptake in thyroid cancer cell lines. Cell Commun. Signal. 2020, 18, 83. [Google Scholar] [CrossRef]
- Luo, J.; Hendryx, M.; Dong, Y. Sodium-glucose cotransporter 2 (SGLT2) inhibitors and non-small cell lung cancer survival. Br. J. Cancer 2023. [Google Scholar] [CrossRef]
- Scafoglio, C.R.; Villegas, B.; Abdelhady, G.; Bailey, S.T.; Liu, J.; Shirali, A.S.; Wallace, W.D.; Magyar, C.E.; Grogan, T.R.; Elashoff, D. Sodium-glucose transporter 2 is a diagnostic and therapeutic target for early-stage lung adenocarcinoma. Sci. Transl. Med. 2018, 10, eaat5933. [Google Scholar] [CrossRef]
- Scafoglio, C.; Hirayama, B.A.; Kepe, V.; Liu, J.; Ghezzi, C.; Satyamurthy, N.; Moatamed, N.A.; Huang, J.; Koepsell, H.; Barrio, J.R.; et al. Functional expression of sodium-glucose transporters in cancer. Proc. Natl. Acad. Sci. USA 2015, 112, E4111–E4119. [Google Scholar] [CrossRef]
- García, M.; Arteche-Martinez, U.; Lertxundi, U.; Aguirre, C. SGLT2 inhibitors and bladder cancer: Analysis of cases reported in the European Pharmacovigilance Database. J. Clin. Pharmacol. 2021, 61, 187–192. [Google Scholar] [CrossRef]
- Kohler, S.; Zeller, C.; Iliev, H.; Kaspers, S. Safety and tolerability of empagliflozin in patients with type 2 diabetes: Pooled analysis of phase I–III clinical trials. Adv. Ther. 2017, 34, 1707–1726. [Google Scholar] [CrossRef]
- Hung, M.-H.; Chen, Y.-L.; Chen, L.-J.; Chu, P.-Y.; Hsieh, F.-S.; Tsai, M.-H.; Shih, C.-T.; Chao, T.-I.; Huang, C.-Y.; Chen, K.-F. Canagliflozin inhibits growth of hepatocellular carcinoma via blocking glucose-influx-induced β-catenin activation. Cell Death Dis. 2019, 10, 420. [Google Scholar] [CrossRef]
- Arvanitakis, K.; Koufakis, T.; Kotsa, K.; Germanidis, G. The effects of sodium-glucose cotransporter 2 inhibitors on hepatocellular carcinoma: From molecular mechanisms to potential clinical implications. Pharmacol. Res. 2022, 181, 106261. [Google Scholar] [CrossRef] [PubMed]
- Nakano, D.; Kawaguchi, T.; Iwamoto, H.; Hayakawa, M.; Koga, H.; Torimura, T. Effects of canagliflozin on growth and metabolic reprograming in hepatocellular carcinoma cells: Multi-omics analysis of metabolomics and absolute quantification proteomics (iMPAQT). PLoS ONE 2020, 15, e0232283. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Zhou, Y.; Xie, X.; He, L.; Ding, J.; Pang, S.; Shen, B.; Zhou, C. Inhibitory effects of canagliflozin on pancreatic cancer are mediated via the downregulation of glucose transporter-1 and lactate dehydrogenase A. Int. J. Oncol. 2020, 57, 1223–1233. [Google Scholar] [CrossRef]
- Sabaa, M.; Sharawy, M.H.; El-Sherbiny, M.; Said, E.; Salem, H.A.; Ibrahim, T.M. Canagliflozin interrupts mTOR-mediated inflammatory signaling and attenuates DMBA-induced mammary cell carcinoma in rats. Biomed. Pharmacother. 2022, 155, 113675. [Google Scholar] [CrossRef] [PubMed]
- Dicembrini, I.; Nreu, B.; Mannucci, E.; Monami, M. Sodium-glucose co-transporter-2 (SGLT-2) inhibitors and cancer: A meta-analysis of randomized controlled trials. Diabetes Obes. Metab. 2019, 21, 1871–1877. [Google Scholar] [CrossRef] [PubMed]
- Shi, N.; Shi, Y.; Xu, J.; Si, Y.; Yang, T.; Zhang, M.; Ng, D.M.; Li, X.; Xie, F. SGLT-2i and risk of malignancy in type 2 diabetes: A meta-analysis of randomized controlled trials. Front. Public Health 2021, 9, 652. [Google Scholar] [CrossRef] [PubMed]
- Andrade, C. Sample Size and its Importance in Research. Indian J. Psychol. Med. 2020, 42, 102–103. [Google Scholar] [CrossRef]
- Patel, S.; Hickman, A.; Frederich, R.; Johnson, S.; Huyck, S.; Mancuso, J.P.; Gantz, I.; Terra, S.G. Safety of ertugliflozin in patients with type 2 diabetes mellitus: Pooled analysis of seven phase 3 randomized controlled trials. Diabetes Ther. 2020, 11, 1347–1367. [Google Scholar] [CrossRef]
- Wawrzyniak, A.; Balawender, K. Structural and Metabolic Changes in Bone. Animals 2022, 12, 1946. [Google Scholar] [CrossRef]
- Siddiqui, J.A.; Partridge, N.C. Physiological Bone Remodeling: Systemic Regulation and Growth Factor Involvement. Physiology 2016, 31, 233–245. [Google Scholar] [CrossRef]
- Charoenngam, N.; Holick, M.F. Immunologic Effects of Vitamin D on Human Health and Disease. Nutrients 2020, 12, 2097. [Google Scholar] [CrossRef] [PubMed]
- Razzaque, M.S. Bone–kidney axis in systemic phosphate turnover. Arch. Biochem. Biophys. 2014, 561, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Cipriani, C.; Colangelo, L.; Santori, R.; Renella, M.; Mastrantonio, M.; Minisola, S.; Pepe, J. The Interplay Between Bone and Glucose Metabolism. Front. Endocrinol. 2020, 11, 122. [Google Scholar] [CrossRef]
- Wallander, M.; Axelsson, K.F.; Nilsson, A.G.; Lundh, D.; Lorentzon, M. Type 2 diabetes and risk of hip fractures and non-skeletal fall injuries in the elderly: A study from the fractures and fall injuries in the elderly cohort (FRAILCO). J. Bone Miner. Res. 2017, 32, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Zeitoun, D.; Caliaperoumal, G.; Bensidhoum, M.; Constans, J.M.; Anagnostou, F.; Bousson, V. Microcomputed tomography of the femur of diabetic rats: Alterations of trabecular and cortical bone microarchitecture and vasculature—A feasibility study. Eur. Radiol. Exp. 2019, 3, 17. [Google Scholar] [CrossRef] [PubMed]
- Farr, J.N.; Drake, M.T.; Amin, S.; Melton III, L.J.; McCready, L.K.; Khosla, S. In vivo assessment of bone quality in postmenopausal women with type 2 diabetes. J. Bone Miner. Res. 2014, 29, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Tonks, K.; Center, J.; Samocha-Bonet, D.; Greenfield, J. Complex interplay among adiposity, insulin resistance and bone health. Clin. Obes. 2018, 8, 131–139. [Google Scholar] [CrossRef]
- Mohsin, S.; Baniyas, M.M.; AlDarmaki, R.S.; Tekes, K.; Kalász, H.; Adeghate, E.A. An update on therapies for the treatment of diabetes-induced osteoporosis. Expert Opin. Biol. Ther. 2019, 19, 937–948. [Google Scholar] [CrossRef]
- Masajtis-Zagajewska, A.; Hołub, T.; Pęczek, K.; Makówka, A.; Nowicki, M. Different Effects of Empagliflozin on Markers of Mineral-Bone Metabolism in Diabetic and Non-Diabetic Patients with Stage 3 chronic kidney disease. Medicina 2021, 57, 1352. [Google Scholar] [CrossRef]
- Vianna, A.; Sanches, C.; Barreto, F. Review article: Effects of type 2 diabetes therapies on bone metabolism. Diabetol. Metab. Syndr. 2017, 9, 75. [Google Scholar] [CrossRef]
- Adil, M.; Khan, R.A.; Kalam, A.; Venkata, S.K.; Kandhare, A.D.; Ghosh, P.; Sharma, M. Effect of anti-diabetic drugs on bone metabolism: Evidence from preclinical and clinical studies. Pharmacol. Rep. 2017, 69, 1328–1340. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Wu, F.; Zhuang, B.; Ou, Q.; Peng, X.; Shi, N.; Peng, L.; Li, Z.; Wang, J.; Cai, S. Empagliflozin activates Wnt/β-catenin to stimulate FUNDC1-dependent mitochondrial quality surveillance against type-3 cardiorenal syndrome. Mol. Metab. 2022, 64, 101553. [Google Scholar] [CrossRef] [PubMed]
- Frent, I.; Bucsa, C.; Leucuta, D.; Farcas, A.; Mogosan, C. An investigation on the association between sodium glucose co-transporter 2 inhibitors use and acute pancreatitis: A VigiBase study. Pharmacoepidemiol. Drug Saf. 2021, 30, 1428–1440. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.R.; Long, F.; Zemel, B.S.; Kindler, J.M. Glycemic Control and Bone in Diabetes. Curr. Osteoporos. Rep. 2022, 20, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Faillie, J.-L. Pharmacological aspects of the safety of gliflozins. Pharmacol. Res. 2017, 118, 71–81. [Google Scholar] [CrossRef]
- Van der Vaart, A.; Yeung, S.M.; van Dijk, P.R.; Bakker, S.J.; de Borst, M.H. Phosphate and fibroblast growth factor 23 in diabetes. Clin. Sci. 2021, 135, 1669–1687. [Google Scholar] [CrossRef]
- Liu, S.; Tang, W.; Zhou, J.; Stubbs, J.R.; Luo, Q.; Pi, M.; Quarles, L.D. Fibroblast growth factor 23 is a counter-regulatory phosphaturic hormone for vitamin D. J. Am. Soc. Nephrol. 2006, 17, 1305–1315. [Google Scholar] [CrossRef]
- Musicco, M.; Palmer, K.; Salamone, G.; Lupo, F.; Perri, R.; Mosti, S.; Spalletta, G.; Di Iulio, F.; Pettenati, C.; Cravello, L. Predictors of progression of cognitive decline in Alzheimer’s disease: The role of vascular and sociodemographic factors. J. Neurol. 2009, 256, 1288–1295. [Google Scholar] [CrossRef]
- Feinkohl, I.; Price, J.F.; Strachan, M.W.; Frier, B.M. The impact of diabetes on cognitive decline: Potential vascular, metabolic, and psychosocial risk factors. Alzheimers Res. Ther. 2015, 7, 46. [Google Scholar] [CrossRef]
- Erdogan, M.A.; Yusuf, D.; Christy, J.; Solmaz, V.; Erdogan, A.; Taskiran, E.; Erbas, O. Highly selective SGLT2 inhibitor dapagliflozin reduces seizure activity in pentylenetetrazol-induced murine model of epilepsy. BMC Neurol. 2018, 18, 81. [Google Scholar] [CrossRef]
- Ahmed, S.; El-Sayed, M.M.; Kandeil, M.A.; Khalaf, M.M. Empagliflozin attenuates neurodegeneration through antioxidant, anti-inflammatory, and modulation of α-synuclein and Parkin levels in rotenone-induced Parkinson’s disease in rats. Saudi Pharm. J. 2022, 30, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, M.R.; Di Meo, I.; Polito, R.; Auriemma, M.C.; Gambardella, A.; di Mauro, G.; Capuano, A.; Paolisso, G. Cognitive impairment and Type 2 Diabetes Mellitus: Focus of SGLT2 Inhibitors Treatment. Pharmacol. Res. 2022, 176, 106062. [Google Scholar] [CrossRef] [PubMed]
- Sebastiani, A.; Greve, F.; Gölz, C.; Förster, C.Y.; Koepsell, H.; Thal, S.C. RS 1 (Rsc1A1) deficiency limits cerebral SGLT 1 expression and delays brain damage after experimental traumatic brain injury. J. Neurochem. 2018, 147, 190–203. [Google Scholar] [CrossRef] [PubMed]
- Sim, A.Y.; Barua, S.; Kim, J.Y.; Lee, Y.H.; Lee, J.E. Role of DPP-4 and SGLT2 Inhibitors Connected to Alzheimer Disease in Type 2 Diabetes Mellitus. Front. Neurosci. 2021, 15, 708547. [Google Scholar] [CrossRef]
- Ferrari, F.; Moretti, A.; Villa, R.F. Hyperglycemia in acute ischemic stroke: Physiopathological and therapeutic complexity. Neural Regen. Res. 2022, 17, 292. [Google Scholar]
- Sripetchwandee, J.; Chattipakorn, N.; Chattipakorn, S.C. Links between obesity-induced brain insulin resistance, brain mitochondrial dysfunction, and dementia. Front. Endocrinol. 2018, 9, 496. [Google Scholar] [CrossRef]
- Arab, H.H.; Safar, M.M.; Shahin, N.N. Targeting ROS-Dependent AKT/GSK-3β/NF-κB and DJ-1/Nrf2 Pathways by Dapagliflozin Attenuates Neuronal Injury and Motor Dysfunction in Rotenone-Induced Parkinson’s Disease Rat Model. ACS Chem. Neurosci. 2021, 12, 689–703. [Google Scholar] [CrossRef]
- Jiang, T.; Gao, L.; Guo, J.; Lu, J.; Wang, Y.; Zhang, Y. Suppressing inflammation by inhibiting the NF-κB pathway contributes to the neuroprotective effect of angiotensin-(1-7) in rats with permanent cerebral ischaemia. Br. J. Pharmacol. 2012, 167, 1520–1532. [Google Scholar] [CrossRef]
- Sa-Nguanmoo, P.; Tanajak, P.; Kerdphoo, S.; Jaiwongkam, T.; Pratchayasakul, W.; Chattipakorn, N.; Chattipakorn, S.C. SGLT2-inhibitor and DPP-4 inhibitor improve brain function via attenuating mitochondrial dysfunction, insulin resistance, inflammation, and apoptosis in HFD-induced obese rats. Toxicol. Appl. Pharmacol. 2017, 333, 43–50. [Google Scholar] [CrossRef]
- Sharma, V.K.; Singh, T.G. Insulin resistance and bioenergetic manifestations: Targets and approaches in Alzheimer’s disease. Life Sci. 2020, 262, 118401. [Google Scholar] [CrossRef]
- Khan, T.; Khan, S.; Akhtar, M.; Ali, J.; Najmi, A.K. Empagliflozin nanoparticles attenuates type2 diabetes induced cognitive impairment via oxidative stress and inflammatory pathway in high fructose diet induced hyperglycemic mice. Neurochem. Int. 2021, 150, 105158. [Google Scholar] [CrossRef] [PubMed]
- Cignarelli, A.; Genchi, V.A.; Le Grazie, G.; Caruso, I.; Marrano, N.; Biondi, G.; D’Oria, R.; Sorice, G.P.; Natalicchio, A.; Perrini, S. Minireview: Effect of GLP-1 Receptor Agonists and SGLT-2 Inhibitors on the Growth Hormone/IGF axis. Front. Endocrinol. 2022, 231. [Google Scholar]
| Antidiabetic Drug | Mechanism of Action |
|---|---|
| α-Glucosidase inhibitors |
|
| Biguanides |
|
| Sulfonylureas |
|
| Meglitinides |
|
| GLP-1 Agonists |
|
| PPAR-γ agonists |
|
| DDP4 inhibitors |
|
| SGLT2 inhibitors |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Youssef, M.E.; Yahya, G.; Popoviciu, M.S.; Cavalu, S.; Abd-Eldayem, M.A.; Saber, S. Unlocking the Full Potential of SGLT2 Inhibitors: Expanding Applications beyond Glycemic Control. Int. J. Mol. Sci. 2023, 24, 6039. https://doi.org/10.3390/ijms24076039
Youssef ME, Yahya G, Popoviciu MS, Cavalu S, Abd-Eldayem MA, Saber S. Unlocking the Full Potential of SGLT2 Inhibitors: Expanding Applications beyond Glycemic Control. International Journal of Molecular Sciences. 2023; 24(7):6039. https://doi.org/10.3390/ijms24076039
Chicago/Turabian StyleYoussef, Mahmoud E., Galal Yahya, Mihaela Simona Popoviciu, Simona Cavalu, Marwa A. Abd-Eldayem, and Sameh Saber. 2023. "Unlocking the Full Potential of SGLT2 Inhibitors: Expanding Applications beyond Glycemic Control" International Journal of Molecular Sciences 24, no. 7: 6039. https://doi.org/10.3390/ijms24076039
APA StyleYoussef, M. E., Yahya, G., Popoviciu, M. S., Cavalu, S., Abd-Eldayem, M. A., & Saber, S. (2023). Unlocking the Full Potential of SGLT2 Inhibitors: Expanding Applications beyond Glycemic Control. International Journal of Molecular Sciences, 24(7), 6039. https://doi.org/10.3390/ijms24076039








