Efficient Knocking Out of the Organophosphorus Insecticides Degradation Gene opdB in Cupriavidus nantongensis X1T via CRISPR/Cas9 with Red System
Abstract
1. Introduction
2. Results
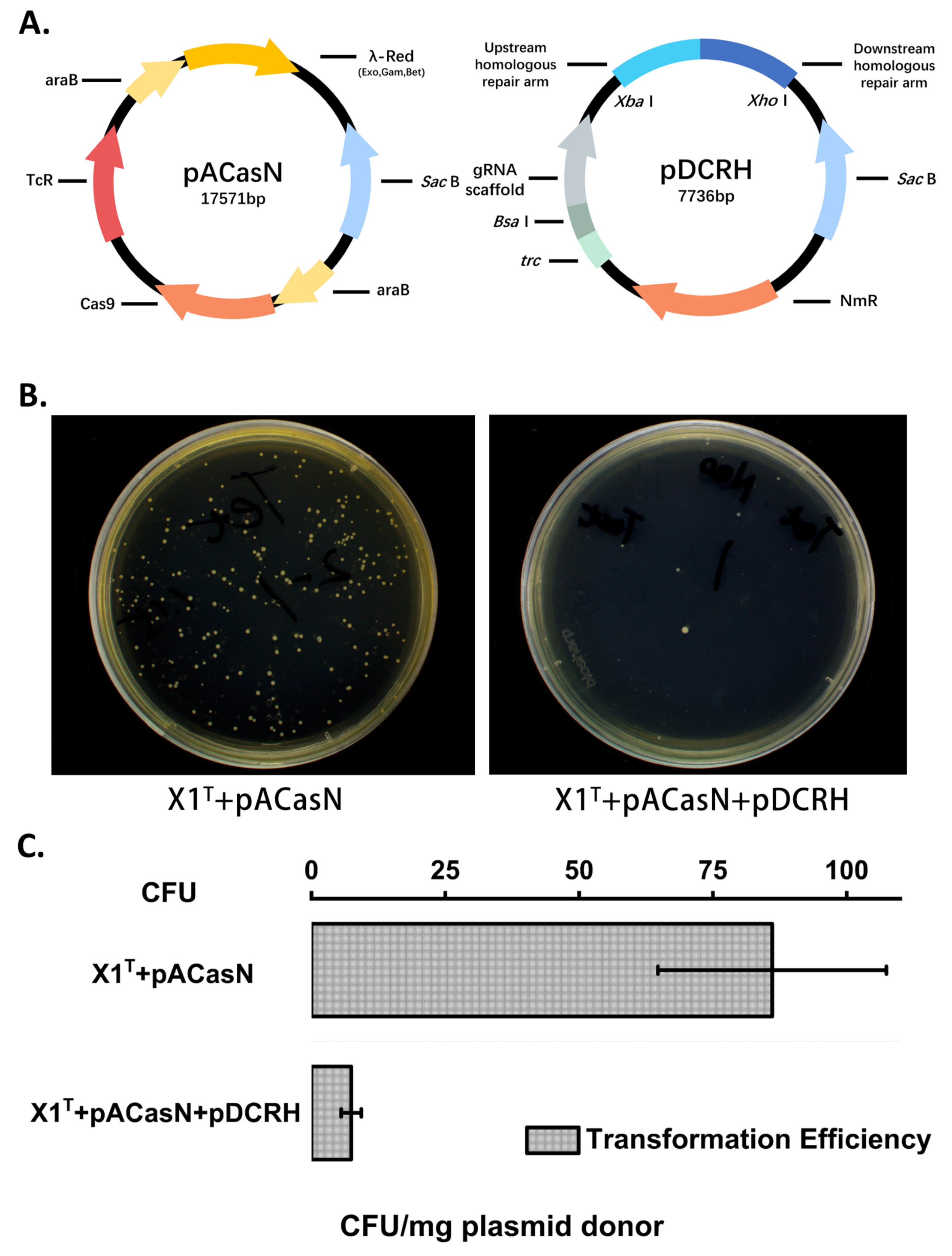
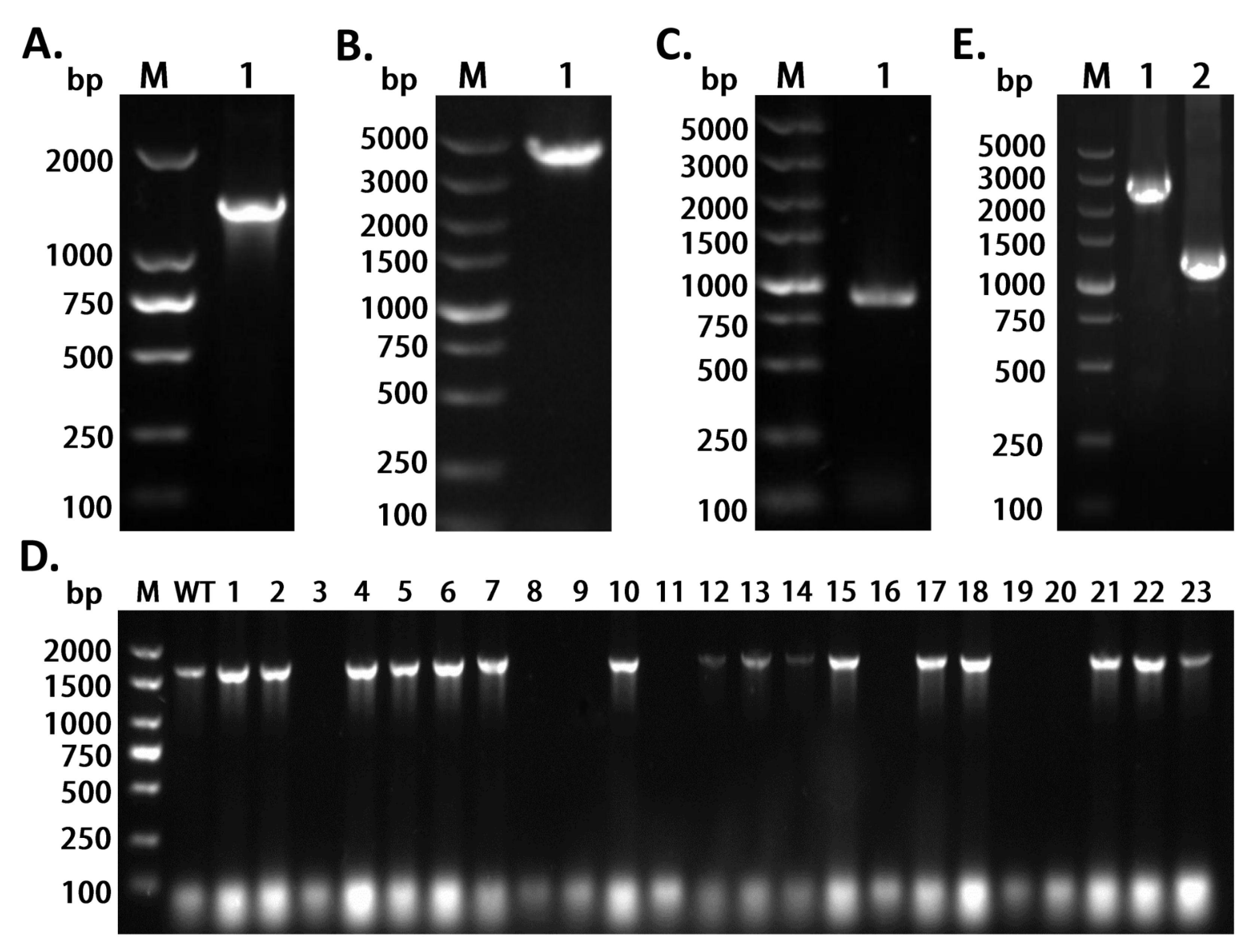
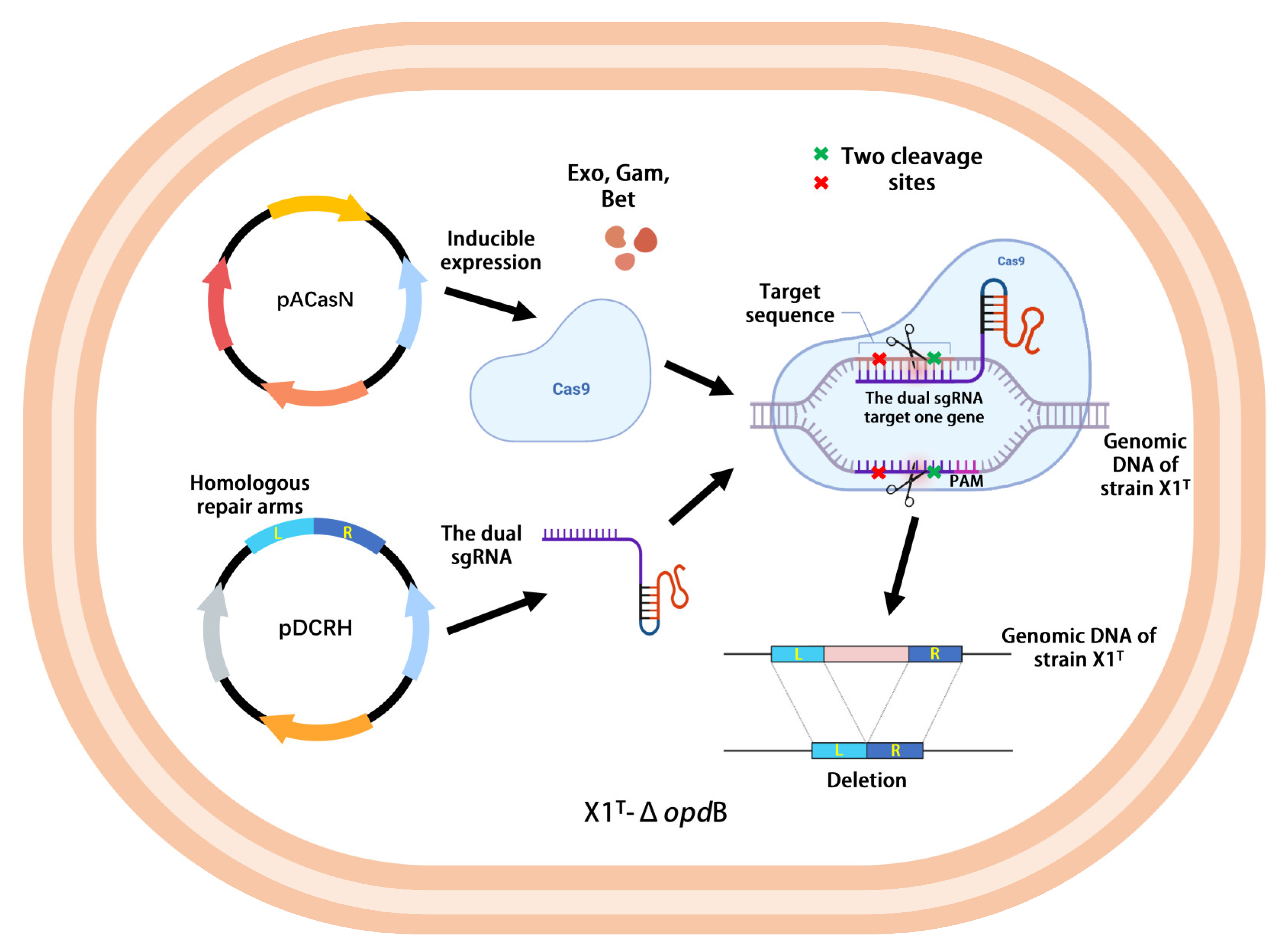
2.1. Construction and Screening of the CRISPR/Cas9-Based Genome Editing System in the X1T Strain
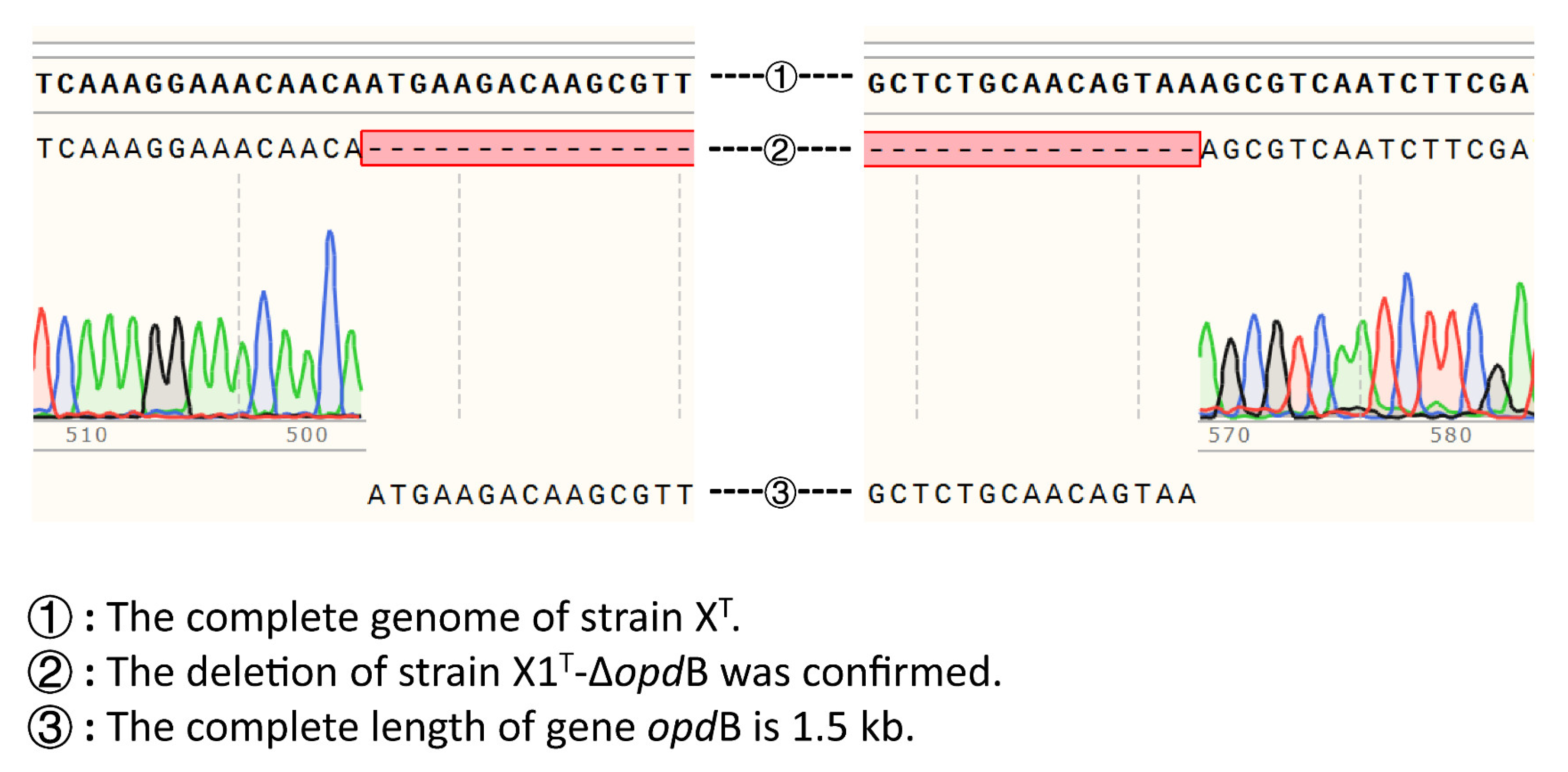
2.2. Evaluation of the Efficiency of Gene Knockout of opdB in the X1T Strain
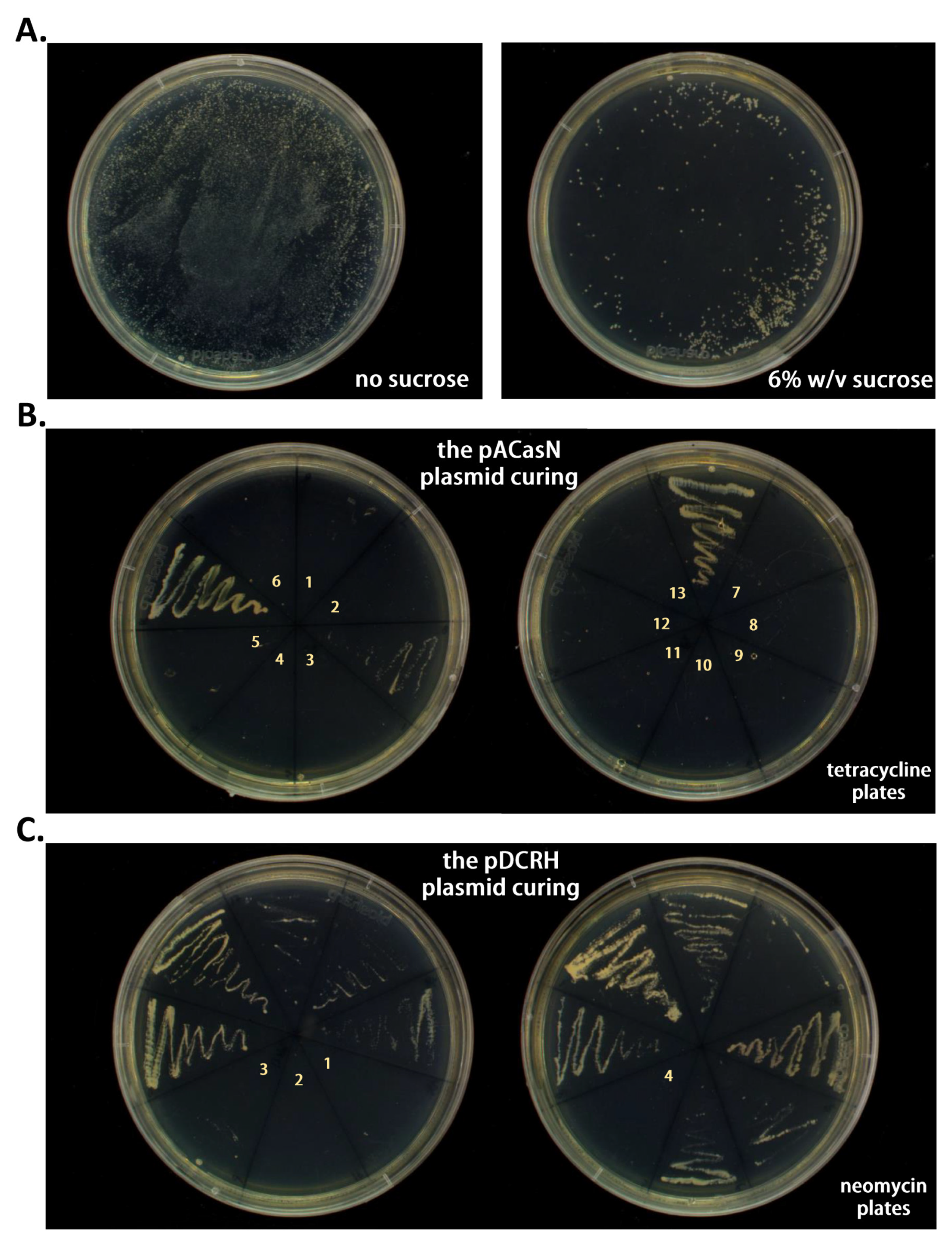
2.3. Plasmid Curing
2.4. Assessment of the Degradation Capacity of OPs in the X1T-ΔopdB Strain
3. Discussion
4. Material and Methods
4.1. Strains and Plasmids
4.2. Culture and Reagents
4.3. Construction of the Prokaryotic Expression Vector
4.4. Genome Editing
4.5. Plasmid Curing
4.6. Degradation Experiments
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Name | Sequence (5′-3′) | Purpose | |
|---|---|---|---|
| Oligos | dsgRNA-F | gtgg ATTGAAGCCGTTCGCCCGGA GGTCGCGGGCAAGTCGTATC | The dual sgRNA in CRISPR vector |
| dsgRNA-R | aaac GATACGACTTGCCCGCGACC TCCGGGCGAACGGCTTCAAT | ||
| T600-F | tgtccatacccatgg TCTAGA TCCGAGTCCGCCAGGACAATGT | The upstream homology arm for opdB deletion | |
| T600-R | actgaagaacttgaac TGTTGTTTCCTTTGAATTTAGGAAAGG | ||
| B600-F | ttcaaaggaaacaaca GTTCAAGTTCTTCAGTTCGCTCC | The downstream homology arm for opdB deletion | |
| B600-R | gggagtatgaaaagt CTCGAG GTTTTCCCGCAAAGGAAACTCCGA | ||
| P1 | AGTAGAAACAGACGAAGAATCCAT | Detection of homology arms and sgRNA | |
| P2 | TCTGAATGGCGGGAGTATGAAAAGT | ||
| opdB-F | ATGAAGACAAGCGTTCATCTCAGTT | The degradation gene of organophosphorus insecticides | |
| opdB-R | TTACTGTTGCAGAGCAGATGCCACA | ||
| Neo-F | ATGATTGAACAAGATGGATTGCA | Neomycin resistance gene in CRISPR vector | |
| Neo-R | TCAGAAGAACTCGTCAAGAAGGC | ||
| Cas9-F | ATGGATAAGAAATACTCAATAGGCTTAG | Expression of Cas9 protein encoded by cas9 gene | |
| Cas9-R | TCAGTCACCTCCTAGCTGACTCAAA |
| Name | Sequence (5′-3′) | Position in an Original Plasmid pX1 | |
|---|---|---|---|
| Spacers | sgRNA-188rev | ATTGAAGCCGTTCGCCCGGA GGG | 7488–7466 |
| sgRNA-1016fw | GGTCGCGGGCAAGTCGTATC TGG | 8274–8296 |
References
- Vandamme, P.; Coenye, T. Taxonomy of the genus Cupriavidus: A tale of lost and found. Int. J. Syst. Evol. Microbiol. 2004, 54, 2285–2289. [Google Scholar] [CrossRef] [PubMed]
- Ray, J.; Waters, R.J.; Skerker, J.M.; Kuehl, J.V.; Price, M.N.; Huang, J.W.; Chakraborty, R.; Arkin, A.P.; Deutschbauer, A. Complete Genome Sequence of Cupriavidus basilensis 4G11, Isolated from the Oak Ridge Field Research Center Site. Genome Announc. 2015, 3, e00322-15. [Google Scholar] [CrossRef] [PubMed]
- Poehlein, A.; Kusian, B.; Friedrich, B.; Daniel, R.; Bowien, B. Complete genome sequence of the type strain Cupriavidus necator N-1. J. Bacteriol. 2011, 193, 5017. [Google Scholar] [CrossRef] [PubMed]
- Li, L.H.; Cai, L.; Zhang, T. Genome of Cupriavidus sp. HMR-1, a Heavy Metal-Resistant Bacterium. Genome Announc. 2013, 1, e00202-12. [Google Scholar] [CrossRef]
- Monsieurs, P.; Provoost, A.; Mijnendonckx, K.; Leys, N.; Gaudreau, C.; Houdt, R.V. Genome Sequence of Cupriavidus metallidurans Strain H1130, Isolated from an Invasive Human Infection. Genome Announc. 2013, 1, e00202-12. [Google Scholar] [CrossRef]
- Amadou, C.; Pascal, G.; Mangenot, S.; Glew, M.; Bontemps, C.; Capela, D.; Carrere, S.; Cruveiller, S.; Dossat, C.; Lajus, A.; et al. Genome sequence of the beta-rhizobium Cupriavidus taiwanensis and comparative genomics of rhizobia. Genome Res. 2008, 18, 1472–1483. [Google Scholar] [CrossRef]
- Monsieurs, P.; Mijnendonckx, K.; Provoost, A.; Venkateswaran, K.; Ott, C.M.; Leys, N.; Van Houdt, R. Genome Sequences of Cupriavidus metallidurans Strains NA1, NA4, and NE12, Isolated from Space Equipment. Genome Announc. 2014, 2, e00719-14. [Google Scholar] [CrossRef]
- Abbaszade, G.; Szabo, A.; Vajna, B.; Farkas, R.; Szabo, C.; Toth, E. Whole genome sequence analysis of Cupriavidus campinensis S14E4C, a heavy metal resistant bacterium. Mol. Biol. Rep. 2020, 47, 3973–3985. [Google Scholar] [CrossRef]
- AL-Nussairawi, M.; Risa, A.; Garai, E.; Varga, E.; Szabo, I.; Csenki-Bakos, Z.; Kriszt, B.; Cserhati, M. Mycotoxin Biodegradation Ability of the Cupriavidus Genus. Curr. Microbiol. 2020, 77, 2430–2440. [Google Scholar] [CrossRef]
- Butof, L.; Wiesemann, N.; Herzberg, M.; Altzschner, M.; Holleitner, A.; Reith, F.; Nies, D.H. Synergistic gold-copper detoxification at the core of gold biomineralisation in Cupriavidus metallidurans. Metallomics 2018, 10, 278–286. [Google Scholar] [CrossRef]
- Deshmukh, A.D.; Pawar, S.V.; Rathod, V.K. Ultrasound-assisted fermentative production of Polyhydroxybutyrate (PHB) in Cupriavidus necator. Chem. Eng. Process. Process Intensif. 2020, 153, 107923. [Google Scholar] [CrossRef]
- Tiwari, J.; Naoghare, P.; Sivanesan, S.; Bafana, A. Biodegradation and detoxification of chloronitroaromatic pollutant by Cupriavidus. Bioresour. Technol. 2017, 223, 184–191. [Google Scholar] [CrossRef]
- Lerch, T.Z.; Chenu, C.; Dignac, M.F.; Barriuso, E.; Mariotti, A. Biofilm vs. Planktonic Lifestyle: Consequences for Pesticide 2,4-D Metabolism by Cupriavidus necator JMP134. Front. Microbiol. 2017, 8, 904. [Google Scholar] [CrossRef]
- Min, J.; Xu, L.X.; Fang, S.Y.; Chen, W.W.; Hu, X.K. Microbial degradation kinetics and molecular mechanism of 2,6-dichloro-4-nitrophenol by a Cupriavidus strain. Environ. Pollut. 2020, 258, 113703. [Google Scholar] [CrossRef]
- Ledger, T.; Aceituno, F.; Gonzalez, B. 3-Chlorobenzoate is taken up by a chromosomally encoded transport system in Cupriavidus necator JMP134. Microbiology 2009, 155, 2757–2765. [Google Scholar] [CrossRef]
- Min, J.; Chen, W.W.; Hu, X.K. Biodegradation of 2,6-dibromo-4-nitrophenol by Cupriavidus sp. strain CNP-8: Kinetics, pathway, genetic and biochemical characterization. J. Hazard. Mater. 2019, 361, 10–18. [Google Scholar] [CrossRef]
- Basu, S.; Pal Chowdhury, P.; Deb, S.; Dutta, T.K. Degradation Pathways of 2- and 4-Nitrobenzoates in Cupriavidus sp. Strain ST-14 and Construction of a Recombinant Strain, ST-14::3NBA, Capable of Degrading 3-Nitrobenzoate. Appl. Environ. Microbiol. 2016, 82, 4253–4263. [Google Scholar] [CrossRef]
- Min, J.; Chen, W.W.; Wang, J.P.; Hu, X.K. Genetic and Biochemical Characterization of 2-Chloro-5-Nitrophenol Degradation in a Newly Isolated Bacterium, Cupriavidus sp. Strain CNP-8. Front. Microbiol. 2017, 8, 1778. [Google Scholar] [CrossRef]
- Arenas-Lopez, C.; Locker, J.; Orol, D.; Walter, F.; Busche, T.; Kalinowski, J.; Minton, N.P.; Kovacs, K.; Winzer, K. The genetic basis of 3-hydroxypropanoate metabolism in Cupriavidus necator H16. Biotechnol. Biofuels 2019, 12, 150. [Google Scholar] [CrossRef]
- Horvath, P.; Barrangou, R. CRISPR/Cas, the Immune System of Bacteria and Archaea. Science 2010, 327, 167–170. [Google Scholar] [CrossRef]
- Dalvie, N.C.; Lorgeree, T.; Biedermann, A.M.; Love, K.R.; Love, J.C. Simplified Gene Knockout by CRISPR-Cas9-Induced Homologous Recombination. ACS Synth. Biol. 2022, 11, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Wood, A.J.; Lo, T.W.; Zeitler, B.; Pickle, C.S.; Ralston, E.J.; Lee, A.H.; Amora, R.; Miller, J.C.; Leung, E.; Meng, X.D.; et al. Targeted Genome Editing Across Species Using ZFNs and TALENs. Science 2011, 333, 307. [Google Scholar] [CrossRef] [PubMed]
- Deltcheva, E.; Chylinski, K.; Sharma, C.M.; Gonzales, K.; Chao, Y.; Pirzada, Z.A.; Eckert, M.R.; Vogel, J.; Charpentier, E. CRISPR RNA maturation by trans -encoded small RNA and host factor RNase III. Nature 2011, 471, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Charpentier, E.; Doudna, J.A.; Charpentier, E. A Programmable Dual-RNA-Guided DNA Endonuclease in Adaptive Bacterial Immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef]
- Chen, X.Y.; Xu, F.; Zhu, C.M.; Ji, J.J.; Zhou, X.F.; Feng, X.Z.; Guang, S.H. Dual sgRNA-directed gene knockout using CRISPR/Cas9 technology in Caenorhabditis elegans. Sci. Rep. 2014, 4, 7581. [Google Scholar] [CrossRef]
- Pauwels, L.; De Clercq, R.; Goossens, J.; Inigo, S.; Williams, C.; Ron, M.; Britt, A.; Goossens, A. A Dual sgRNA Approach for Functional Genomics in Arabidopsis thaliana. G3 Genes Genomes Genet. 2018, 8, 2603–2615. [Google Scholar]
- Wang, L.; Xu, F.; Wang, G.S.; Wang, X.R.; Liang, A.J.; Huang, H.F.; Sun, F. C30F12.4 influences oogenesis, fat metabolism, and lifespan in C. elegans. Protein Cell 2016, 7, 714–721. [Google Scholar] [CrossRef]
- Oh, J.H.; van Pijkeren, J.P. CRISPR–Cas9-assisted recombineering in Lactobacillus reuteri. Nucleic Acids Res. 2014, 42, e131. [Google Scholar] [CrossRef]
- Cobb, R.E.; Wang, Y.J.; Zhao, H.M. High-Efficiency Multiplex Genome Editing of Streptomyces Species Using an Engineered CRISPR/Cas System. ACS Synth. Biol. 2015, 4, 723–728. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.T.; Seo, S.O.; Choi, K.; Lu, T.; Jin, Y.S.; Blaschek, H.P. Markerless chromosomal gene deletion in Clostridium beijerinckii using CRISPR/Cas9 system. J. Biotechnol. 2015, 200, 1–5. [Google Scholar] [CrossRef]
- Shuman, S.; Glickman, M.S. Bacterial DNA repair by non-homologous end joining. Nat. Rev. Microbiol. 2007, 5, 852–861. [Google Scholar] [CrossRef]
- Cui, L.; Bikard, D. Consequences of Cas9 cleavage in the chromosome of Escherichia coli. Nucleic Acids Res. 2016, 44, 4243–4251. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, B.; Duan, C.L.; Sun, B.B.; Yang, J.J.; Yang, S. Multigene Editing in the Escherichia coli Genome via the CRISPR-Cas9 System. Appl. Environ. Microbiol. 2015, 81, 2506–2514. [Google Scholar] [CrossRef]
- Yu, D.G.; Ellis, H.M.; Lee, E.C.; Jenkins, N.A.; Copeland, N.G.; Court, D.L. An efficient recombination system for chromosome engineering in Escherichia coli. Proc. Natl. Acad. Sci. USA 2000, 97, 5978–5983. [Google Scholar] [CrossRef]
- Mosberg, J.A.; Gregg, C.J.; Lajoie, M.J.; Wang, H.H.; Church, G.M. Improving Lambda Red Genome Engineering in Escherichia coli via Rational Removal of Endogenous Nucleases. PLoS ONE 2012, 7, e44638. [Google Scholar] [CrossRef]
- Thomason, L.C.; Costantino, N.; Shaw, D.V.; Court, D.L. Multicopy Plasmid Modification with Phage lambda Red Recombineering. Plasmid 2007, 58, 148–158. [Google Scholar] [CrossRef]
- Murphy, K.C. Use of Bacteriophage λ Recombination Functions To Promote Gene Replacement in Escherichia coli. J. Bacteriol. 1998, 180, 2063–2071. [Google Scholar] [CrossRef]
- Takahashi, N.; Kobayashi, I. Evidence for the double-strand break repair model of bacteriophage λ recombination. Proc. Natl. Acad. Sci. USA 1990, 87, 2790–2794. [Google Scholar] [CrossRef]
- Fang, L.C.; Chen, Y.F.; Zhou, Y.L.; Wang, D.S.; Sun, L.N.; Tang, X.Y.; Hua, R.M. Complete genome sequence of a novel chlorpyrifos degrading bacterium, Cupriavidus nantongensis X1. J. Biotechnol. 2016, 227, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.Z.; Fang, L.C.; Qin, H.; Wu, X.W.; Li, Q.X.; Hua, R.M. Minute-Speed Biodegradation of Organophosphorus Insecticides by Cupriavidus nantongensis X1T. J. Agric. Food Chem. 2019, 67, 13558–13567. [Google Scholar] [CrossRef]
- Fang, L.C.; Shi, Q.Y.; Xu, L.Y.; Shi, T.Z.; Wu, X.W.; Li, Q.X.; Hua, R.M. Enantioselective Uptake Determines Degradation Selectivity of Chiral Profenofos in Cupriavidus nantongensis X1T. J. Agric. Food Chem. 2020, 68, 6493–6501. [Google Scholar] [CrossRef] [PubMed]
- Altenbuchner, J. Editing of the Bacillus subtilis Genome by the CRISPR-Cas9 System. Appl. Environ. Microbiol. 2016, 82, 5421–5427. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Z.T.; Seo, S.O.; Lynn, P.; Lu, T.; Jin, Y.S.; Blaschek, H.P. Bacterial Genome Editing with CRISPR-Cas9: Deletion, Integration, Single Nucleotide Modification, and Desirable “Clean” Mutant Selection in Clostridium beijerinckii as an Example. ACS Synth. Biol. 2016, 5, 721–732. [Google Scholar] [CrossRef] [PubMed]
- Pyne, M.E.; Moo-Young, M.; Chung, D.A.; Chou, C.P. Coupling the CRISPR/Cas9 System with Lambda Red Recombineering Enables Simplified Chromosomal Gene Replacement in Escherichia coli. Appl. Environ. Microbiol. 2015, 81, 5103–5114. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Chai, C.; Li, N.; Rowe, P.; Minton, N.P.; Yang, S.; Jiang, W.; Gu, Y. CRISPR/Cas9-Based Efficient Genome Editing in Clostridium ljungdahlii, an Autotrophic Gas-Fermenting Bacterium. ACS Synth. Biol. 2016, 5, 1355–1361. [Google Scholar] [CrossRef]
- Fang, L.C.; Xu, L.Y.; Zhang, N.; Shi, Q.Y.; Shi, T.Z.; Ma, X.; Wu, X.W.; Li, Q.X.; Hua, R.M. Enantioselective degradation of the organophosphorus insecticide isocarbophos in Cupriavidus nantongensis X1T: Characteristics, enantioselective regulation, degradation pathways, and toxicity assessment. J. Hazard. Mater. 2021, 417, 126024. [Google Scholar] [CrossRef]
- Schenk, G.; Mateen, I.; Ng, T.-K.; Pedroso, M.M.; Mitić, N.; Jafelicci, M.; Marques, R.F.C.; Gahan, L.R.; Ollis, D.L. Organophosphate-degrading metallohydrolases: Structure and function of potent catalysts for applications in bioremediation. Coord. Chem. Rev. 2016, 317, 122–131. [Google Scholar] [CrossRef]
- Jiang, W.; Bikard, D.; Cox, D.; Zhang, F.; Marraffini, L.A. RNA-guided editing of bacterial genomes using CRISPR-Cas systems. Nat. Biotechnol. 2013, 31, 233–239. [Google Scholar] [CrossRef]
- Datsenko, K.A.; Wanner, B.L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 2000, 97, 6640–6645. [Google Scholar] [CrossRef]
- Li, K.W.; Xu, C.; Jin, Y.X.; Sun, Z.Y.; Liu, C.; Shi, J.; Chen, G.K.; Chen, R.H.; Jin, S.G.; Wu, W. SuhB is a regulator of multiple virulence genes and essential for pathogenesis of Pseudomonas aeruginosa. mBio 2013, 4, e00419-13. [Google Scholar] [CrossRef]
- Schweizer, H.P.; Hoang, T.T. An improved system for gene replacement and xylE fusion analysis in Pseudomonas aeruginosa. Gene 1995, 158, 15–22. [Google Scholar] [CrossRef]
- Lightfoot, J.; Lam, J.S. Chromosomal mapping, expression and synthesis of lipopolysaccharide in Pseudomonas aeruginosa: A role for guanosine diphospho (GDP)-D-mannose. Mol. Microbiol. 1993, 8, 771–782. [Google Scholar] [CrossRef]
- Kaczmarczyk, A.; Vorholt, J.A.; Francez-Charlot, A. Markerless gene deletion system for sphingomonads. Appl. Environ. Microbiol. 2012, 78, 3774–3777. [Google Scholar] [CrossRef]
- Concordet, J.P.; Haeussler, M. CRISPOR: Intuitive guide selection for CRISPR/Cas9 genome editing experiments and screens. Nucleic Acids Res. 2018, 46, W242–W245. [Google Scholar] [CrossRef]
| OPs | Concentration of OPs after 24 h of Treatment (mg/L) | ||
|---|---|---|---|
| Control | C. nantongensis X1T | C. nantongensis X1T-ΔopdB | |
| Profenofos | 17.1 ± 0.4 c | 9.7 ± 0.2 a | 17.7 ± 0.3 c |
| Chlorpyrifos | 19.1 ± 0.1 ab | ND | 19.4 ± 0.4 ab |
| Methyl parathion | 19.6 ± 0.0 a | ND | 19.7 ± 0.4 a |
| Parathion | 18.7 ± 0.5 b | ND | 18.7 ± 0.1 b |
| Triazophos | 18.6 ± 0.2 b | 1.4 ± 0.1 c | 18.6 ± 0.2 b |
| Phoxim | 18.5 ± 0.4 b | 1.0 ± 0.0 d | 18.7 ± 0.3 b |
| Fenitrothion | 17.6 ± 0.4 c | ND | 17.0 ± 0.4 c |
| Isocarbophos | 14.2 ± 0.3 d | 6.2 ± 0.1 b | 14.0 ± 0.2 d |
| Strains and Plasmids | Description | Source |
|---|---|---|
| E. coli DH5α | supE44ΔlacU169 (φ80 lacZΔM15) hsdR17 recA1 end A1 gyrA96 thi-1 relA1 | Solarbio |
| pMD19-T | Apr | Takara |
| Cupriavidus nantongensis X1T | an aerobic, Gram-negative, motile Proteobacterium that forms circular colonies | Lab store |
| pCas9 | Cmr, bacterial expression of Cas9 nuclease; a temperature sensitive vector in E. coli for genome editing | BioSci |
| pKD20 | the Red recombination system and the arabinose-inducible ParaB promoter | BioSci |
| pDN19 | Tcr, the P. aeruginosa shuttle cloning vector | BioSci |
| pALB2 | Tcr, SacB | BioSci |
| pAK1900 | Apr, broad-host-range cloning vector | BioSci |
| pAK405 | Plasmid for allelic exchange and seamless gene deletions; Nmr | BioSci |
| pACasN | A shuttle vector; Tcr, SacB, Cas9 nuclease and the Red recombination system | This study |
| pDCRH | A shuttle vector; Nmr; SacB | This study |
| pDCRH-spacer | The pDCRH plasmid with the dual sgRNA | This study |
| pDCRH-ΔUD | Digested pDCRH-spacer plasmid | This study |
| pDCRH-UD | The pDCRH plasmid with the dual sgRNA and homologous arms | This study |
| X1-pACasN | Tcr | This study |
| X1-ΔopdB | The opdB gene deleted in Cupriavidus nantongensis X1T | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Geng, Y.; Li, S.; Shi, T.; Ma, X.; Hua, R.; Fang, L. Efficient Knocking Out of the Organophosphorus Insecticides Degradation Gene opdB in Cupriavidus nantongensis X1T via CRISPR/Cas9 with Red System. Int. J. Mol. Sci. 2023, 24, 6003. https://doi.org/10.3390/ijms24066003
Zhang Y, Geng Y, Li S, Shi T, Ma X, Hua R, Fang L. Efficient Knocking Out of the Organophosphorus Insecticides Degradation Gene opdB in Cupriavidus nantongensis X1T via CRISPR/Cas9 with Red System. International Journal of Molecular Sciences. 2023; 24(6):6003. https://doi.org/10.3390/ijms24066003
Chicago/Turabian StyleZhang, Yufei, Yuehan Geng, Shengyang Li, Taozhong Shi, Xin Ma, Rimao Hua, and Liancheng Fang. 2023. "Efficient Knocking Out of the Organophosphorus Insecticides Degradation Gene opdB in Cupriavidus nantongensis X1T via CRISPR/Cas9 with Red System" International Journal of Molecular Sciences 24, no. 6: 6003. https://doi.org/10.3390/ijms24066003
APA StyleZhang, Y., Geng, Y., Li, S., Shi, T., Ma, X., Hua, R., & Fang, L. (2023). Efficient Knocking Out of the Organophosphorus Insecticides Degradation Gene opdB in Cupriavidus nantongensis X1T via CRISPR/Cas9 with Red System. International Journal of Molecular Sciences, 24(6), 6003. https://doi.org/10.3390/ijms24066003






