Long Non-Coding RNA Malat1 Increases the Rescuing Effect of Quercetin on TNFα-Impaired Bone Marrow Stem Cell Osteogenesis and Ovariectomy-Induced Osteoporosis
Abstract
1. Introduction
2. Result
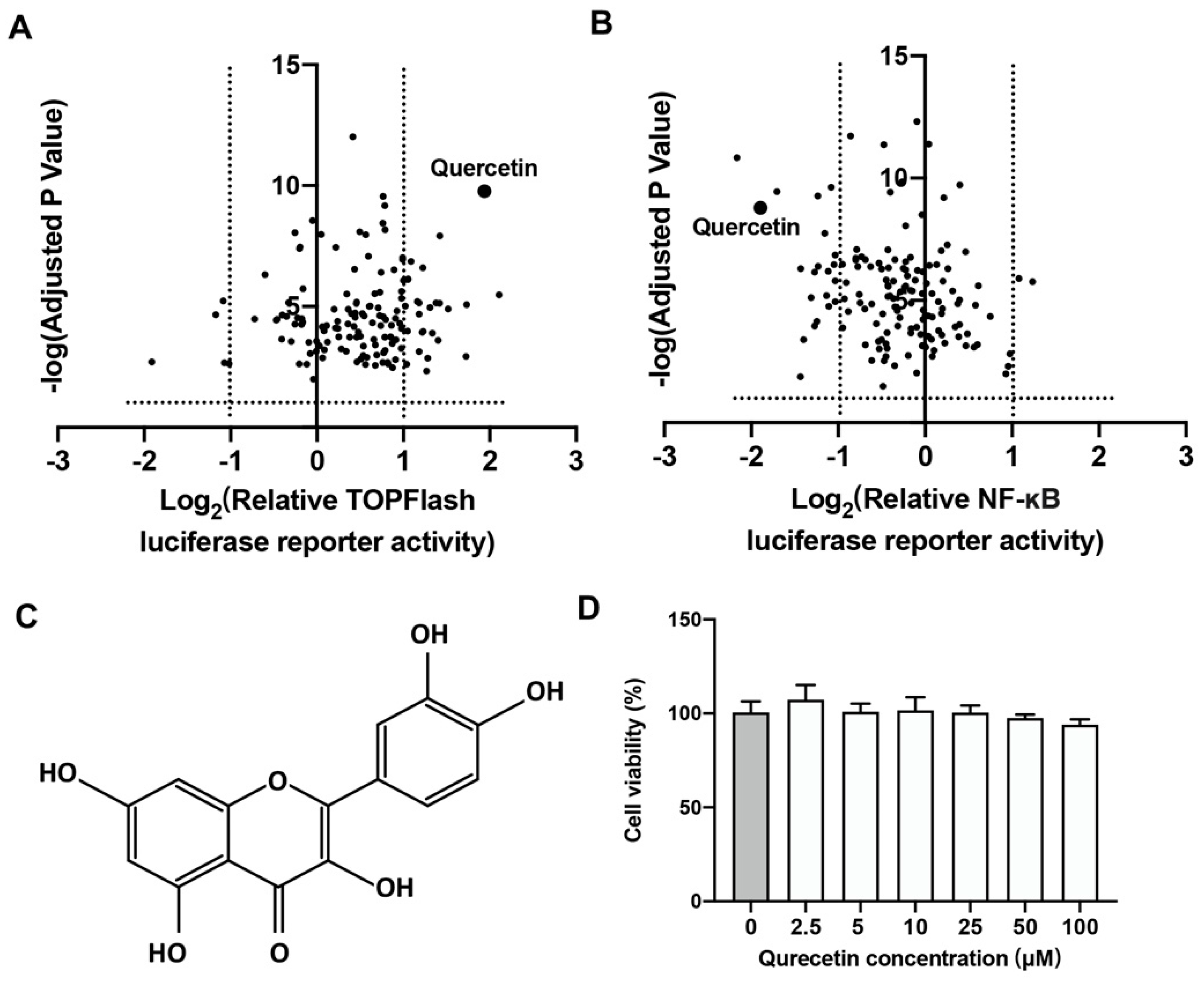
2.1. Quercetin Could Upregulate Wnt/β-Catenin and Downregulate NF-κB Signaling
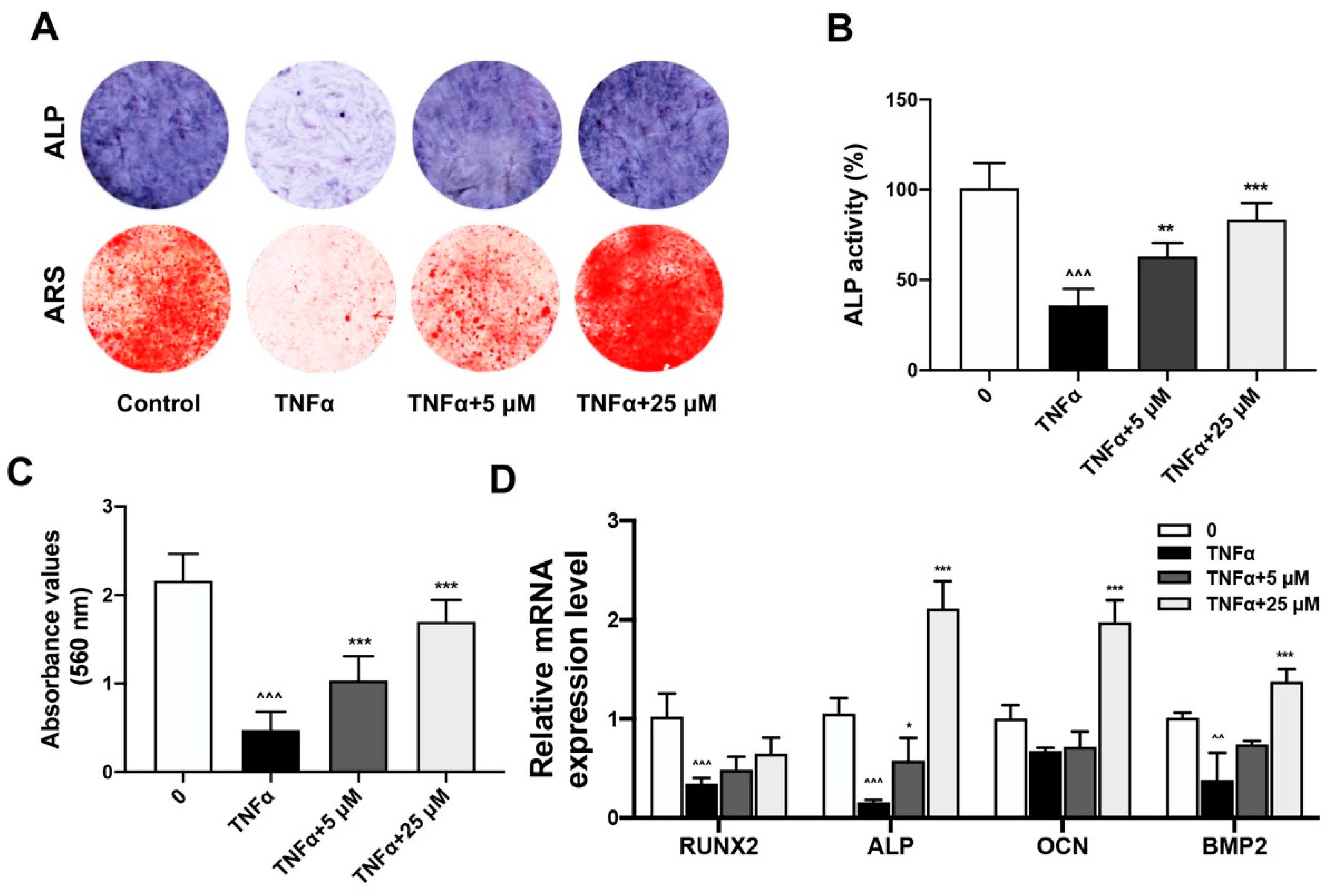
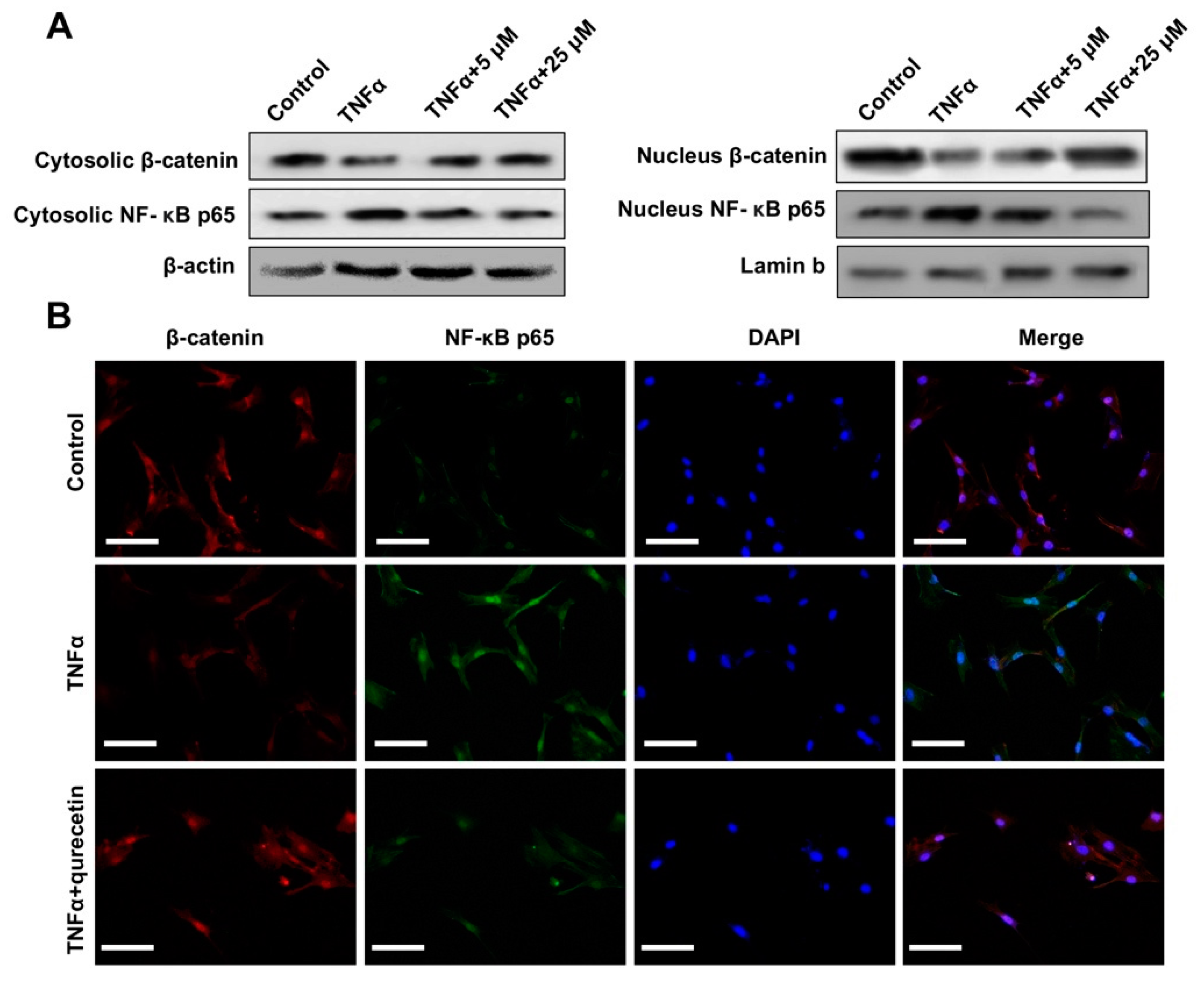
2.2. Quercetin Rescued TNFα-Impaired BMSCs Osteogenesis via Increasing Wnt/β-Catenin and Decreasing NF-κB Signaling
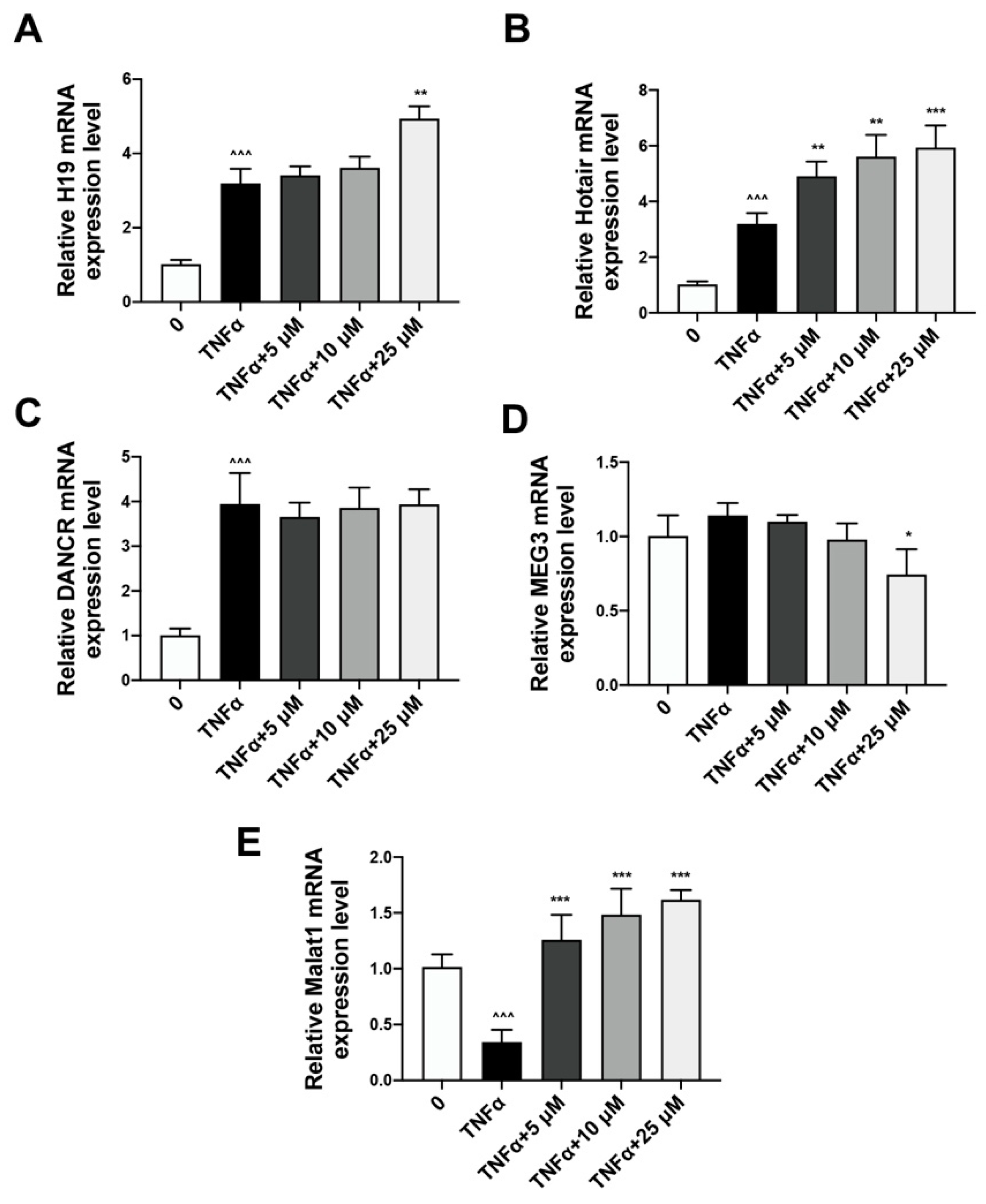
2.3. Quercetin Could Reverse the Expression of lncRNA Malat1 Downregulated by TNFα Treatment
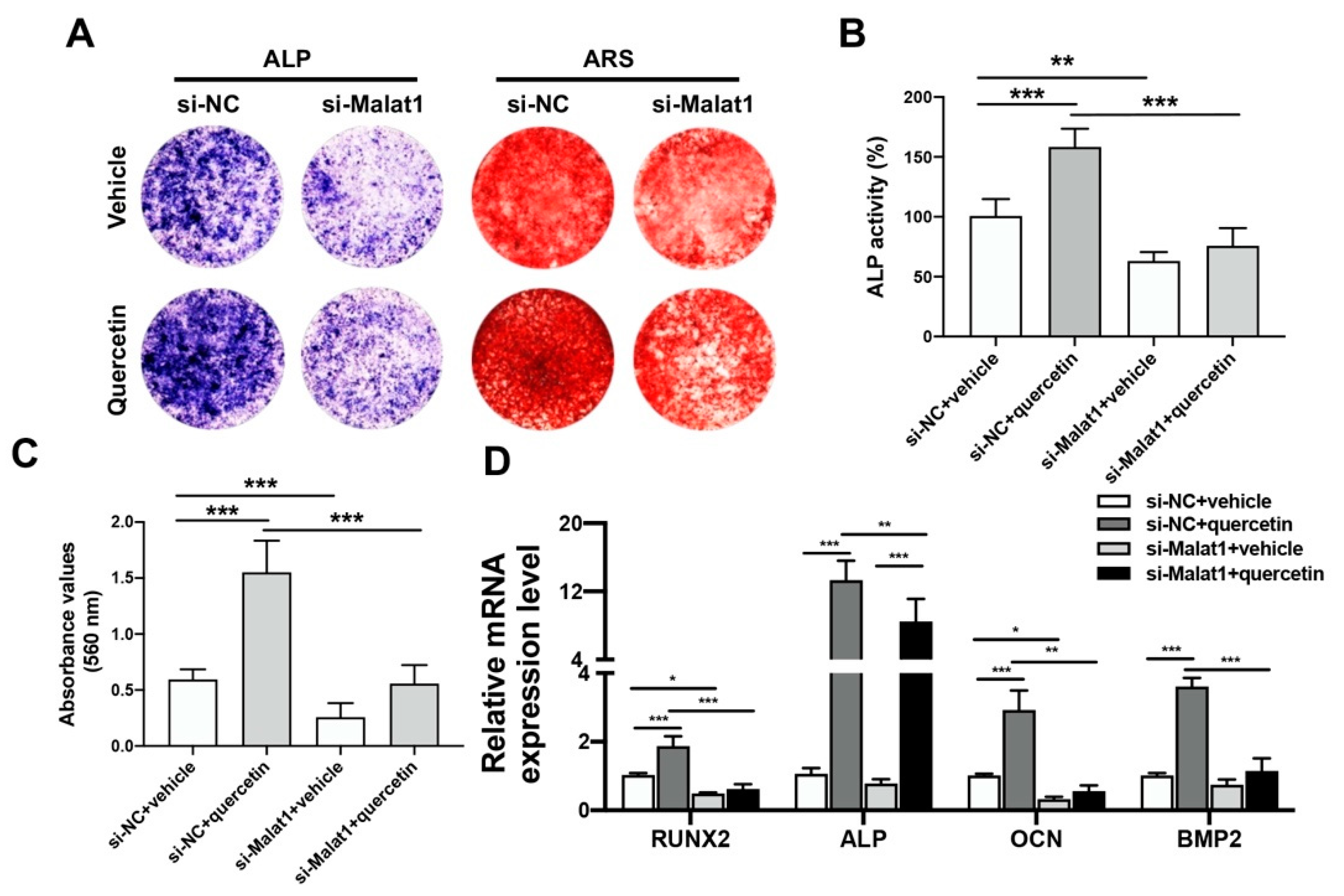
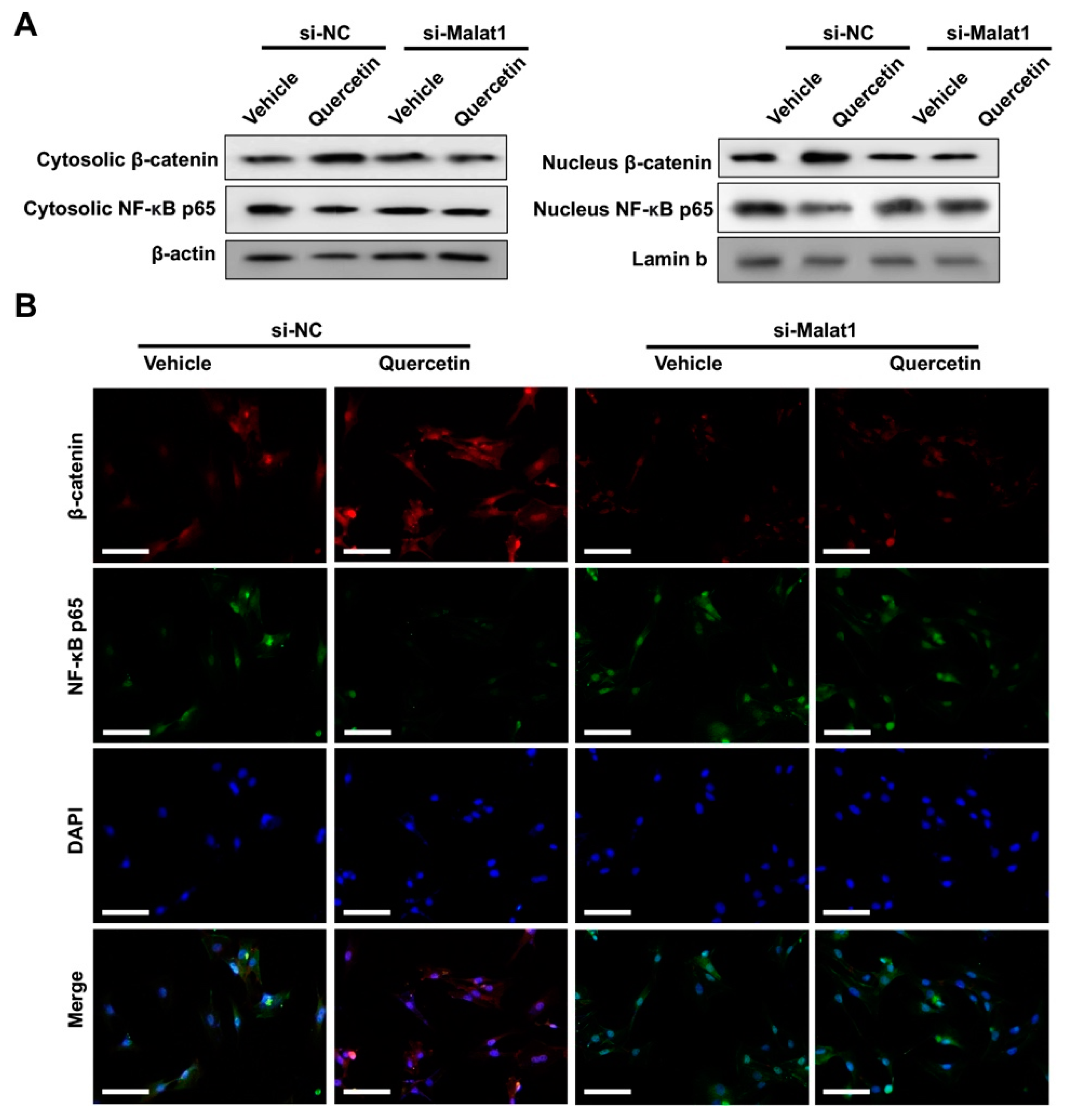
2.4. Knockdown of Malat1 Ameliorated the Rescuing Effect of Quercetin on TNFα-Impaired BMSCs Osteogenesis
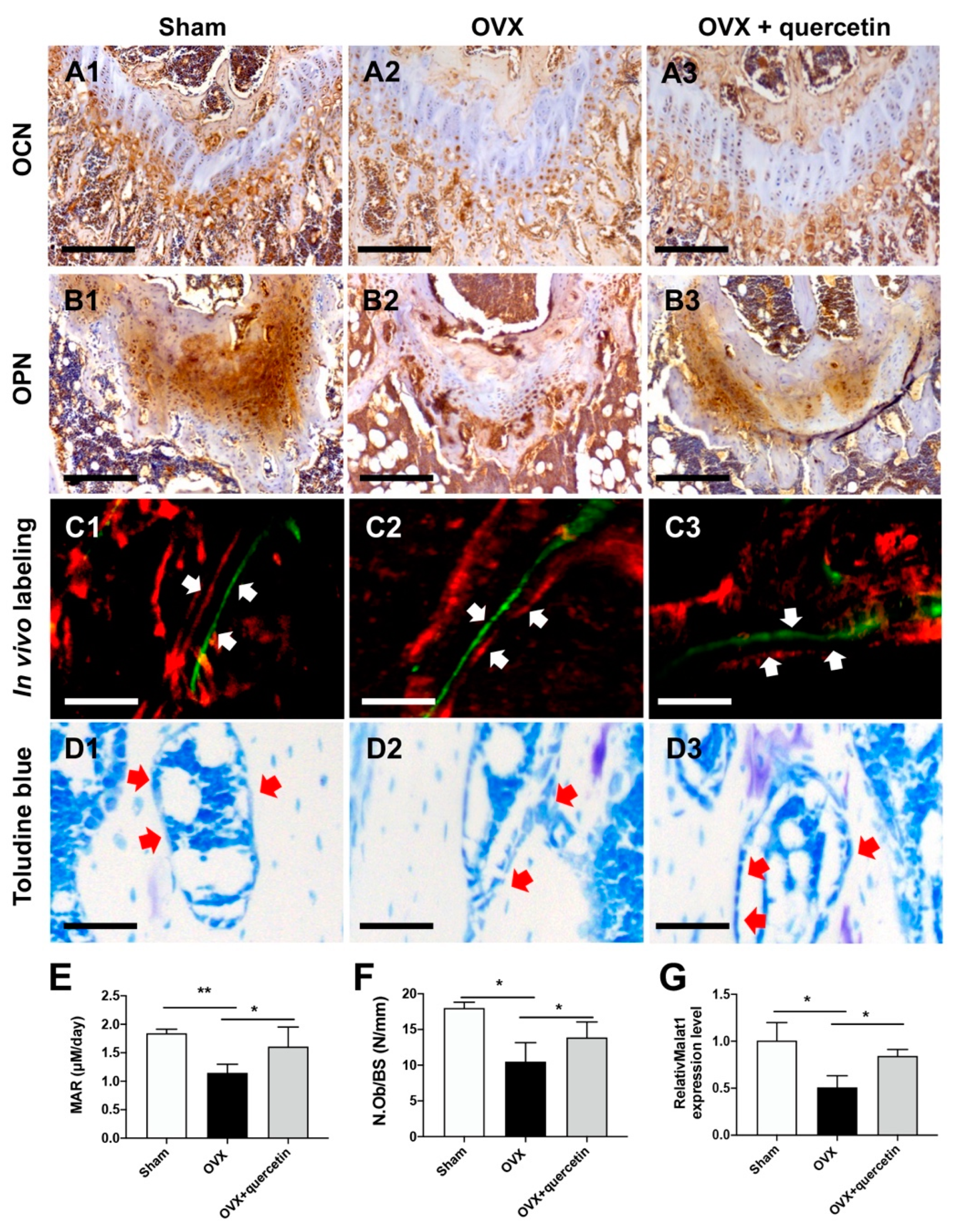
2.5. Quercetin Attenuates Bone Loss in the OVX Mouse Osteoporosis Model via Upregulating Malat1 Expression
3. Discussion
4. Material and Methods
4.1. Herb-Derived Small Molecule Library
4.2. Transient Cell Transfection and Dual-Luciferase Reporter Assay
4.3. Cell Viability Assay
4.4. Osteogenesis Induction
4.5. RNA Isolation and Real-Time PCR
4.6. Western Blot
4.7. Ovariectomized Osteoporotic Model Mice Experiment
4.8. Micro-Computed Tomography Analysis
4.9. Immunohistochemistry and Histology
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Wong, R.M.Y.; Cheung, W.-H.; Chow, S.K.H.; Ng, R.W.K.; Li, W.; Hsu, A.Y.-C.; Wong, K.K.; Ho, A.W.-H.; Choi, S.-H.; Fang, C.X.; et al. Recommendations on the post-acute management of the osteoporotic fracture—Patients with “very-high” Re-fracture risk. J. Orthop. Transl. 2022, 37, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Xavier, A.; Toumi, H.; Lespessailles, E. Animal Model for Glucocorticoid Induced Osteoporosis: A Systematic Review from 2011 to 2021. Int. J. Mol. Sci. 2021, 23, 377. [Google Scholar] [CrossRef] [PubMed]
- Shan, Z.; Xie, X.; Wu, X.; Zhuang, S.; Zhang, C. Development of degradable magnesium-based metal implants and their function in promoting bone metabolism (A review). J. Orthop. Transl. 2022, 36, 184–193. [Google Scholar] [CrossRef]
- Iwamoto, N.; Kawakami, A. The monocyte-to-osteoclast transition in rheumatoid arthritis: Recent findings. Front. Immunol. 2022, 13, 998554. [Google Scholar] [CrossRef]
- Kim, H.-H.; Kim, J.H.; Kwak, H.B.; Huang, H.; Han, S.-H.; Ha, H.; Lee, S.W.; Woo, E.-R.; Lee, Z.H. Inhibition of osteoclast differentiation and bone resorption by tanshinone IIA isolated from Salvia miltiorrhiza Bunge. Biochem. Pharmacol. 2004, 67, 1647–1656. [Google Scholar] [CrossRef]
- Amarasekara, D.; Kim, S.; Rho, J. Regulation of Osteoblast Differentiation by Cytokine Networks. Int. J. Mol. Sci. 2021, 22, 2851. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/beta-catenin signalling: Function, biological mechanisms, and therapeutic opportunities. Signal Transduct. Target. Ther. 2022, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, H.; Ding, X.; Hu, J.; Li, Y.; Zhang, J.; Wang, H.; Qi, S.; Xie, A.; Shi, J.; et al. Crosstalk between macrophage-derived PGE2 and tumor UHRF1 drives hepatocellular carcinoma progression. Theranostics 2022, 12, 3776–3793. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Feng, L.; Wang, H.; Li, Y.; Lo, J.H.T.; Zhang, X.; Lu, X.; Wang, Y.; Lin, S.; Tortorella, M.D.; et al. DANCR Mediates the Rescuing Effects of Sesamin on Postmenopausal Osteoporosis Treatment via Orchestrating Osteogenesis and Osteoclastogenesis. Nutrients 2021, 13, 4455. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wang, H.; Huang, M.; Zong, Z.; Wu, X.; Xu, J.; Lan, H.; Zheng, J.; Zhang, X.; Lee, Y.W.; et al. Asiatic Acid Attenuates Bone Loss by Regulating Osteoclastic Differentiation. Calcif. Tissue Int. 2019, 105, 531–545. [Google Scholar] [CrossRef]
- Salehi, B.; Machin, L.; Monzote, L.; Sharifi-Rad, J.; Ezzat, S.M.; Salem, M.A.; Merghany, R.M.; El Mahdy, N.M.; Kılıç, C.S.; Sytar, O.; et al. Therapeutic Potential of Quercetin: New Insights and Perspectives for Human Health. ACS Omega 2020, 5, 11849–11872. [Google Scholar] [CrossRef]
- Bian, W.; Xiao, S.; Yang, L.; Chen, J.; Deng, S. Quercetin promotes bone marrow mesenchymal stem cell proliferation and osteogenic differentiation through the H19/miR-625-5p axis to activate the Wnt/beta-catenin pathway. BMC Complement. Med. Ther. 2021, 21, 243. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Cao, L.; He, S.; Zhong, Y.; Ma, H.; Zhang, Y.; Shuai, C. An Overview of Long Noncoding RNAs Involved in Bone Regeneration from Mesenchymal Stem Cells. Stem Cells Int. 2018, 2018, 8273648. [Google Scholar] [CrossRef] [PubMed]
- Tabacco, G.; Bilezikian, J.P. Osteoanabolic and dual action drugs. Br. J. Clin. Pharmacol. 2018, 85, 1084–1094. [Google Scholar] [CrossRef]
- Russow, G.; Jahn, D.; Appelt, J.; Märdian, S.; Tsitsilonis, S.; Keller, J. Anabolic Therapies in Osteoporosis and Bone Regeneration. Int. J. Mol. Sci. 2018, 20, 83. [Google Scholar] [CrossRef]
- He, J.; Li, X.; Wang, Z.; Bennett, S.; Chen, K.; Xiao, Z.; Zhan, J.; Chen, S.; Hou, Y.; Chen, J.; et al. Therapeutic Anabolic and Anticatabolic Benefits of Natural Chinese Medicines for the Treatment of Osteoporosis. Front. Pharmacol. 2019, 10, 1344. [Google Scholar] [CrossRef]
- Pelusi, L.; Mandatori, D.; Pietrantonio, N.; Pizzo, F.; Tomo, P.; Pietro, N.; Buda, R.; Genovese, S.; Epifano, F.; Pandolfi, A.; et al. Estrogen Receptor 1 (ESR1) and the Wnt/β-Catenin Pathway Mediate the Effect of the Coumarin Derivative Umbelliferon on Bone Mineralization. Nutrients 2022, 14, 3209. [Google Scholar] [CrossRef]
- Crisostomo, P.R.; Wang, Y.; Markel, T.A.; Wang, M.; Lahm, T.; Meldrum, D.R. Human mesenchymal stem cells stimulated by TNF-alpha, LPS, or hypoxia produce growth factors by an NF kappa B- but not JNK-dependent mechanism. Am. J. Physiol. Cell Physiol. 2008, 294, C675–C682. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Liu, F.; Lee, M.; Wu, B.; Ting, K.; Zara, J.N.; Soo, C.; Al Hezaimi, K.; Zou, W.; Chen, X.; et al. NF-kappaB inhibits osteogenic differentiation of mesenchymal stem cells by promoting beta-catenin degradation. Proc. Natl. Acad. Sci. USA 2013, 110, 9469–9474. [Google Scholar] [CrossRef]
- Osta, B.; Benedetti, G.; Miossec, P. Classical and Paradoxical Effects of TNF-α on Bone Homeostasis. Front. Immunol. 2014, 5, 48. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhou, Z.; Wu, T.; Liu, W.; Yin, P.; Pan, C.; Yu, X. TNF-alpha-induced NF-kappaB activation upregulates microRNA-150-3p and inhibits osteogenesis of mesenchymal stem cells by targeting beta-catenin. Open Biol. 2016, 6, 150258. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.M.; Moura, S.R.; Teixeira, J.H.; Barbosa, M.A.; Santos, S.G.; Almeida, M.I. Long noncoding RNAs: A missing link in osteoporosis. Bone Res. 2019, 7, 10. [Google Scholar] [CrossRef]
- Yin, J.; Zheng, Z.; Zeng, X.; Zhao, Y.; Ai, Z.; Yu, M.; Wu, Y.; Jiang, J.; Li, J.; Li, S. lncRNA MALAT1 mediates osteogenic differentiation of bone mesenchymal stem cells by sponging miR-129-5p. PeerJ 2022, 10, e13355. [Google Scholar] [CrossRef]
- Liang, W.-C.; Fu, W.-M.; Wang, Y.-B.; Sun, Y.-X.; Xu, L.-L.; Wong, C.-W.; Chan, K.-M.; Li, G.; Waye, M.M.-Y.; Zhang, J.-F. H19 activates Wnt signaling and promotes osteoblast differentiation by functioning as a competing endogenous RNA. Sci. Rep. 2016, 6, 20121. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Qi, X.; Wang, X.H.; Miao, H.S.; Xue, Z.C.; Zhang, L.L.; Zhao, S.H.; Wu, L.H.; Gao, G.Y.; Lou, M.Q.; et al. Downregulation of the LncRNA MEG3 Promotes Osteogenic Differentiation of BMSCs and Bone Repairing by Activating Wnt/beta-Catenin Signaling Pathway. J. Clin. Med. 2022, 11, 395. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.J.; Zhang, C.H.; Chen, Z.W.; Wang, Z.X.; Yang, D.C.; Zhang, F.L.; Feng, K.H. LncRNA HOTAIR inhibited osteogenic differentiation of BMSCs by regulating Wnt/beta-catenin pathway. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 7232–7246. [Google Scholar]
- Zhao, G.; Su, Z.; Song, D.; Mao, Y.; Mao, X. The long noncoding RNA MALAT1 regulates the lipopolysaccharide-induced inflammatory response through its interaction with NF-kappaB. FEBS Lett. 2016, 590, 2884–2895. [Google Scholar] [CrossRef]
- Niu, Y.-B.; Yang, Y.-Y.; Xiao, X.; Sun, Y.; Zhou, Y.-M.; Zhang, Y.-H.; Dong, D.; Li, C.-R.; Wu, X.-L.; Li, Y.-H.; et al. Quercetin prevents bone loss in hindlimb suspension mice via stanniocalcin 1-mediated inhibition of osteoclastogenesis. Acta Pharmacol. Sin. 2020, 41, 1476–1486. [Google Scholar] [CrossRef]
- Qian, T.-Y.; Wan, H.; Huang, C.-Y.; Hu, X.-J.; Yao, W.-F. Plasma LncRNA MALAT1 Expressions Are Negatively Associated with Disease Severity of Postmenopausal Osteoporosis. Lab. Med. 2022, 53, 446–452. [Google Scholar] [CrossRef]
- Feng, L.; Yang, Z.; Li, Y.; Pan, Q.; Zhang, X.; Wu, X.; Lo, J.H.T.; Wang, H.; Bai, S.; Lu, X.; et al. MicroRNA-378 contributes to osteoarthritis by regulating chondrocyte autophagy and bone marrow mesenchymal stem cell chondrogenesis. Mol. Ther.-Nucleic Acids 2022, 28, 328–341. [Google Scholar] [CrossRef]
- Feng, L.; Shi, L.; Lu, Y.-F.; Wang, B.; Tang, T.; Fu, W.-M.; He, W.; Li, G.; Zhang, J.-F. Linc-ROR Promotes Osteogenic Differentiation of Mesenchymal Stem Cells by Functioning as a Competing Endogenous RNA for miR-138 and miR-145. Mol. Ther.-Nucleic Acids 2018, 11, 345–353. [Google Scholar] [CrossRef]
- Shi, L.; Feng, L.; Zhu, M.L.; Yang, Z.M.; Wu, T.Y.; Xu, J.; Liu, Y.; Lin, W.P.; Lo, J.H.T.; Zhang, J.F.; et al. Vasoactive Intestinal Peptide Stimulates Bone Marrow-Mesenchymal Stem Cells Osteogenesis Differentiation by Activating Wnt/beta-Catenin Signaling Pathway and Promotes Rat Skull Defect Repair. Stem. Cells Dev. 2020, 29, 655–666. [Google Scholar] [CrossRef]
- Cunningham, P.; Patton, E.; VanderVeen, B.N.; Unger, C.; Aladhami, A.; Enos, R.T.; Madero, S.; Chatzistamou, I.; Fan, D.; Murphy, E.A.; et al. Sub-chronic oral toxicity screening of quercetin in mice. BMC Complement. Med. Ther. 2022, 22, 279. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Liu, Y.; Yang, Z.; Wu, T.; Lo, H.T.; Xu, J.; Zhang, J.; Lin, W.; Zhang, J.; Feng, L.; et al. Vasoactive Intestinal Peptide Promotes Fracture Healing in Sympathectomized Mice. Calcif. Tissue Int. 2021, 109, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Feng, L.; Wang, M.; Li, Y.; Bai, S.; Lu, X.; Wang, H.; Zhang, X.; Wang, Y.; Lin, S.; et al. Sesamin Promotes Osteoporotic Fracture Healing by Activating Chondrogenesis and Angiogenesis Pathways. Nutrients 2022, 14, 2106. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, L.; Yang, Z.; Hou, N.; Wang, M.; Lu, X.; Li, Y.; Wang, H.; Wang, Y.; Bai, S.; Zhang, X.; et al. Long Non-Coding RNA Malat1 Increases the Rescuing Effect of Quercetin on TNFα-Impaired Bone Marrow Stem Cell Osteogenesis and Ovariectomy-Induced Osteoporosis. Int. J. Mol. Sci. 2023, 24, 5965. https://doi.org/10.3390/ijms24065965
Feng L, Yang Z, Hou N, Wang M, Lu X, Li Y, Wang H, Wang Y, Bai S, Zhang X, et al. Long Non-Coding RNA Malat1 Increases the Rescuing Effect of Quercetin on TNFα-Impaired Bone Marrow Stem Cell Osteogenesis and Ovariectomy-Induced Osteoporosis. International Journal of Molecular Sciences. 2023; 24(6):5965. https://doi.org/10.3390/ijms24065965
Chicago/Turabian StyleFeng, Lu, Zhengmeng Yang, Nan Hou, Ming Wang, Xuan Lu, Yucong Li, Haixing Wang, Yaofeng Wang, Shanshan Bai, Xiaoting Zhang, and et al. 2023. "Long Non-Coding RNA Malat1 Increases the Rescuing Effect of Quercetin on TNFα-Impaired Bone Marrow Stem Cell Osteogenesis and Ovariectomy-Induced Osteoporosis" International Journal of Molecular Sciences 24, no. 6: 5965. https://doi.org/10.3390/ijms24065965
APA StyleFeng, L., Yang, Z., Hou, N., Wang, M., Lu, X., Li, Y., Wang, H., Wang, Y., Bai, S., Zhang, X., Lin, Y., Yan, X., Lin, S., Tortorella, M. D., & Li, G. (2023). Long Non-Coding RNA Malat1 Increases the Rescuing Effect of Quercetin on TNFα-Impaired Bone Marrow Stem Cell Osteogenesis and Ovariectomy-Induced Osteoporosis. International Journal of Molecular Sciences, 24(6), 5965. https://doi.org/10.3390/ijms24065965






