dCas9-Based PDGFR–β Activation ADSCs Accelerate Wound Healing in Diabetic Mice through Angiogenesis and ECM Remodeling
Abstract
:1. Introduction
2. Results
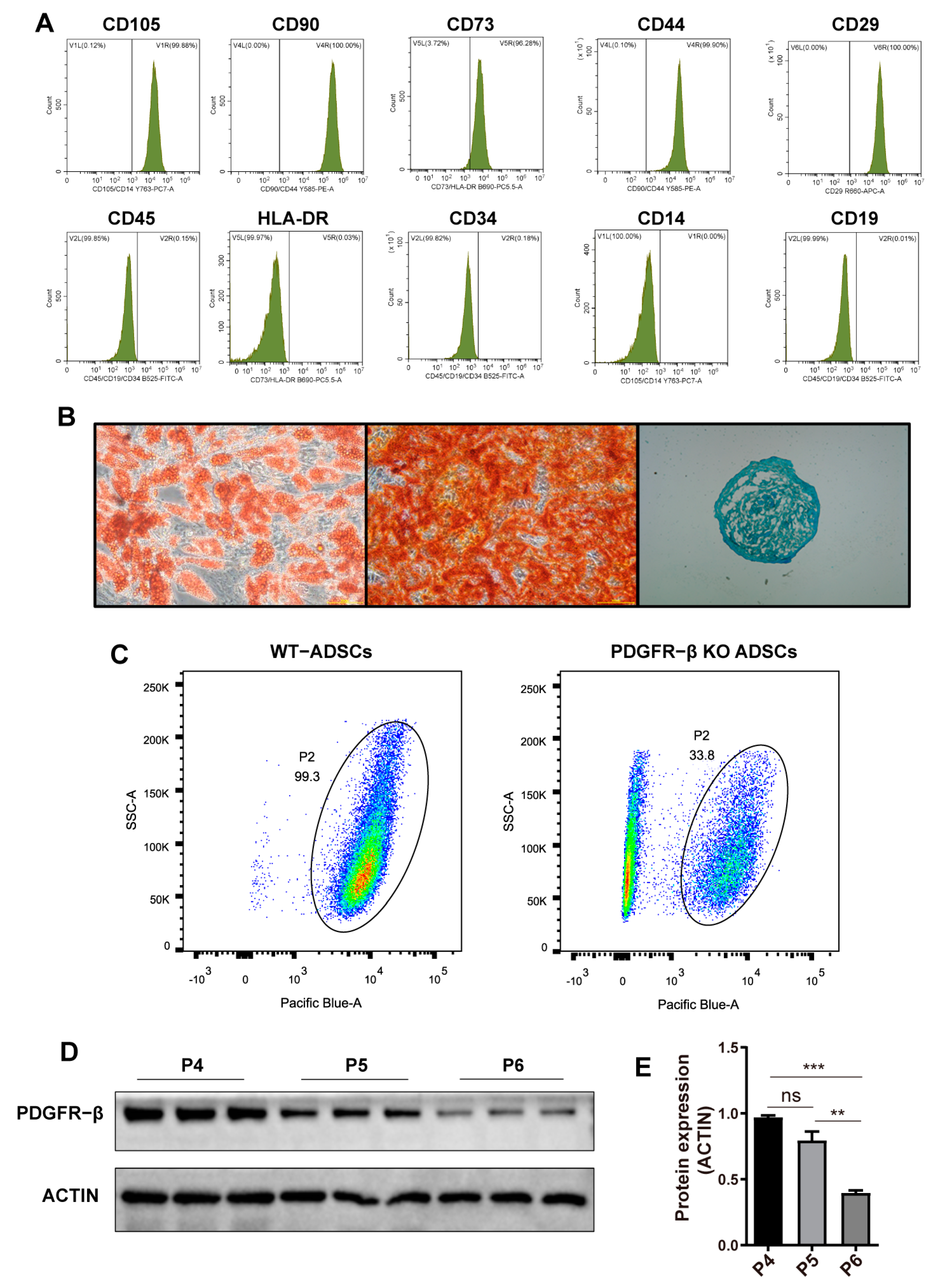
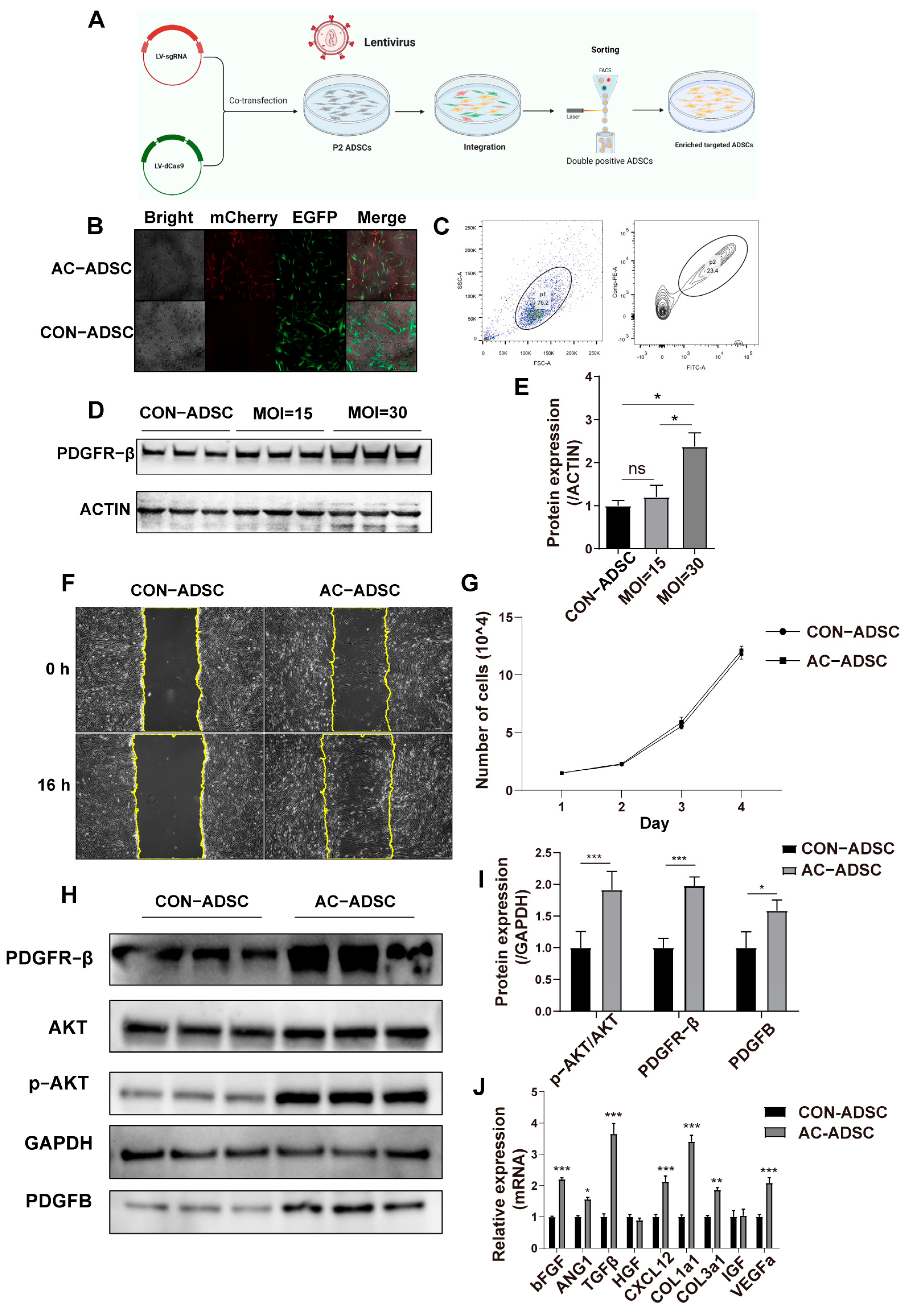
2.1. Characterization of ADSCs and the Dynamic Expression of PDGFR–β in ADSCs
2.2. PDGFR–β Enhances Migration and Paracrine of ADSCs
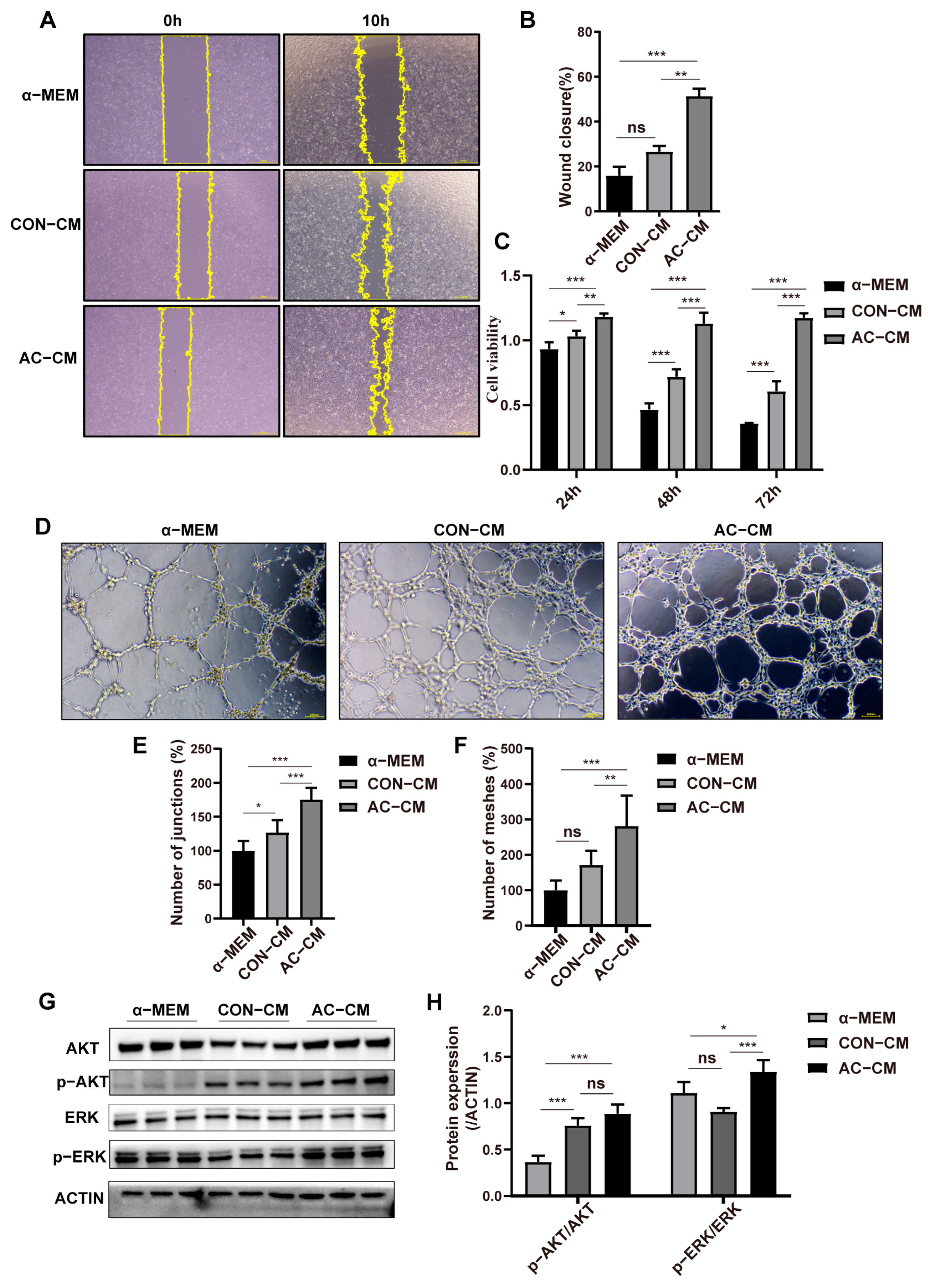
2.3. PDGFR–β–ADSCs Conditioned Medium Promotes the Function of HUVEC In Vitro
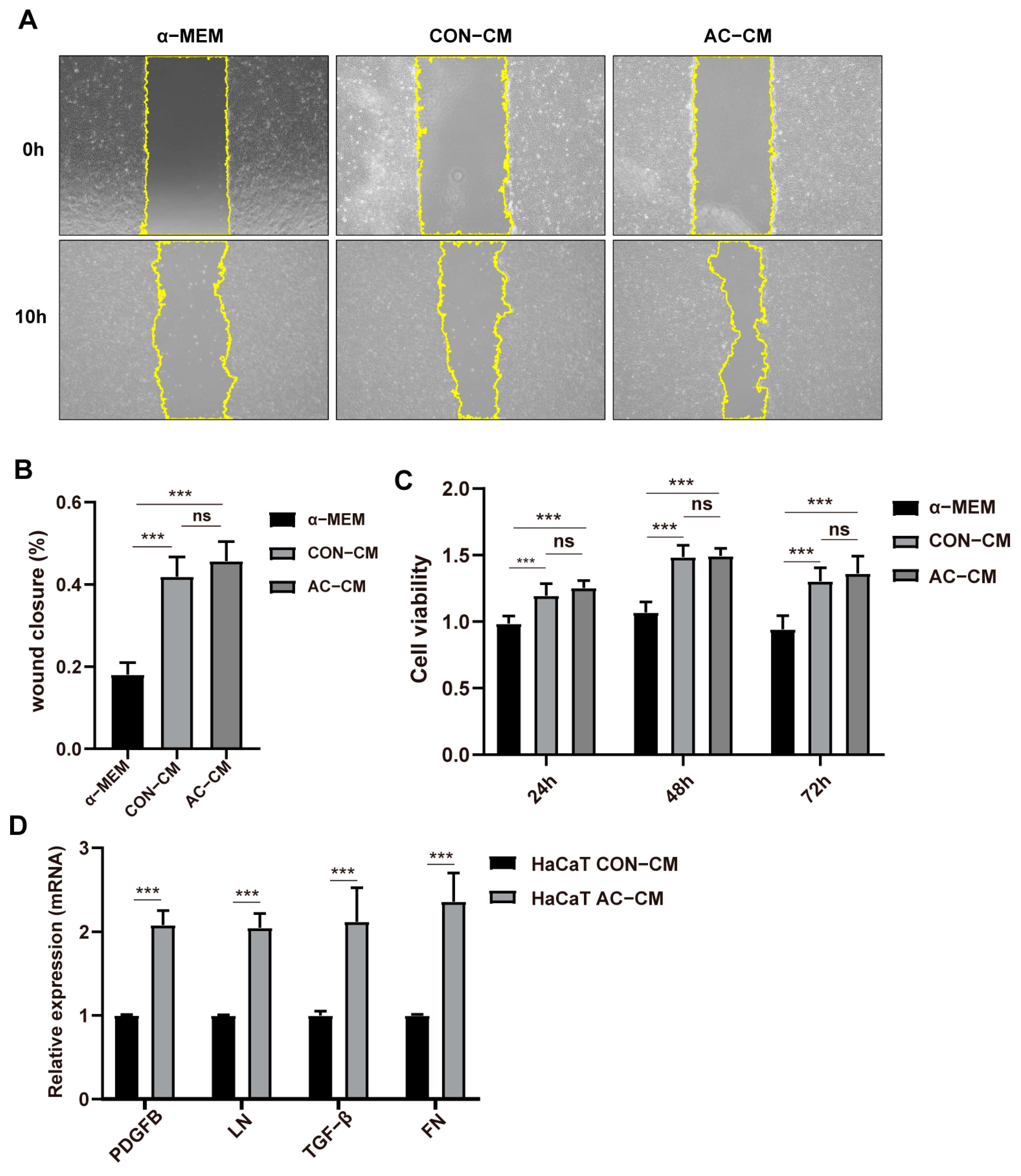
2.4. PDGFR–β Overexpressed ADSCs Conditioned Medium Promoted the Expression of ECM Related Genes in HaCaT Cells
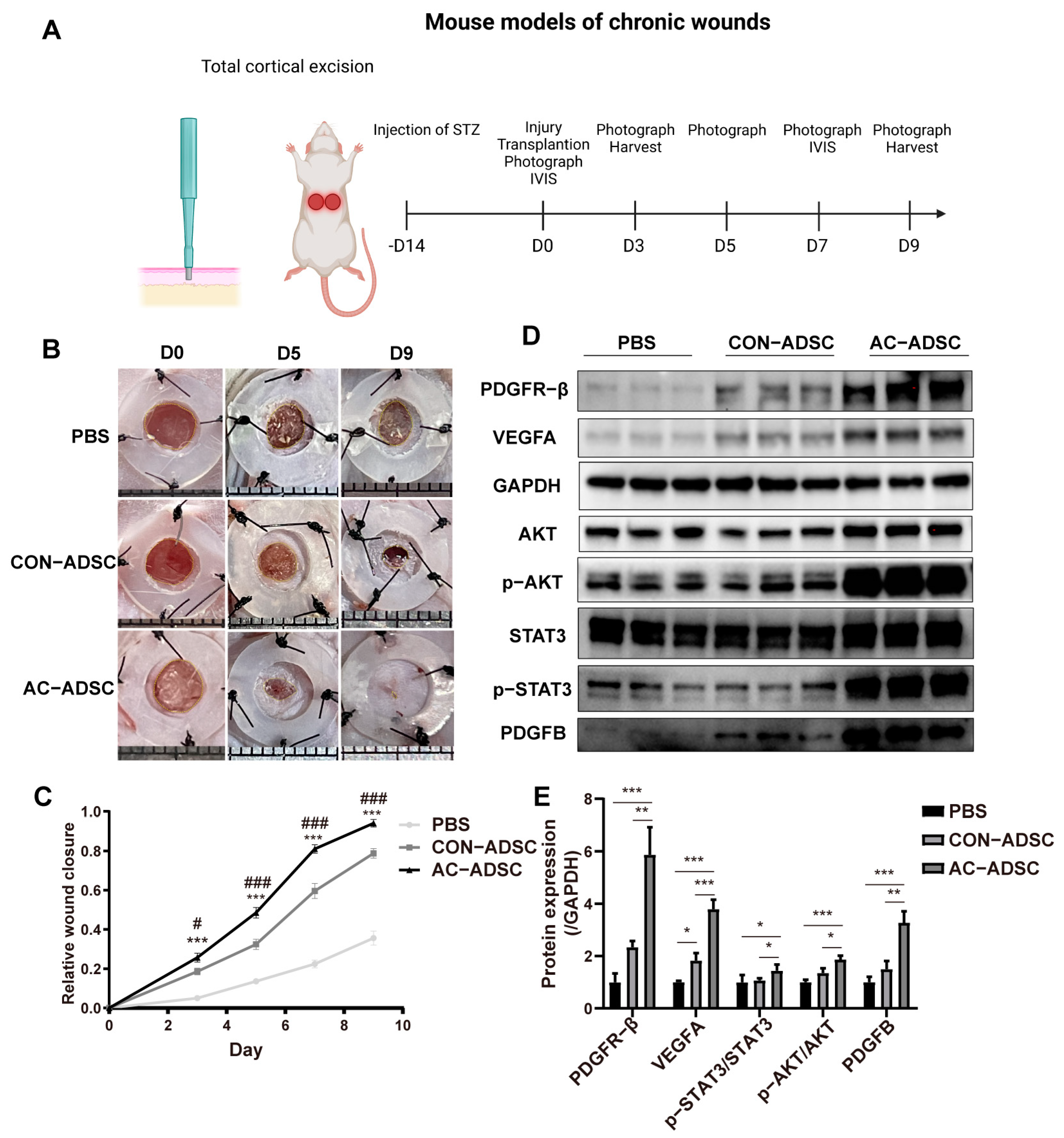
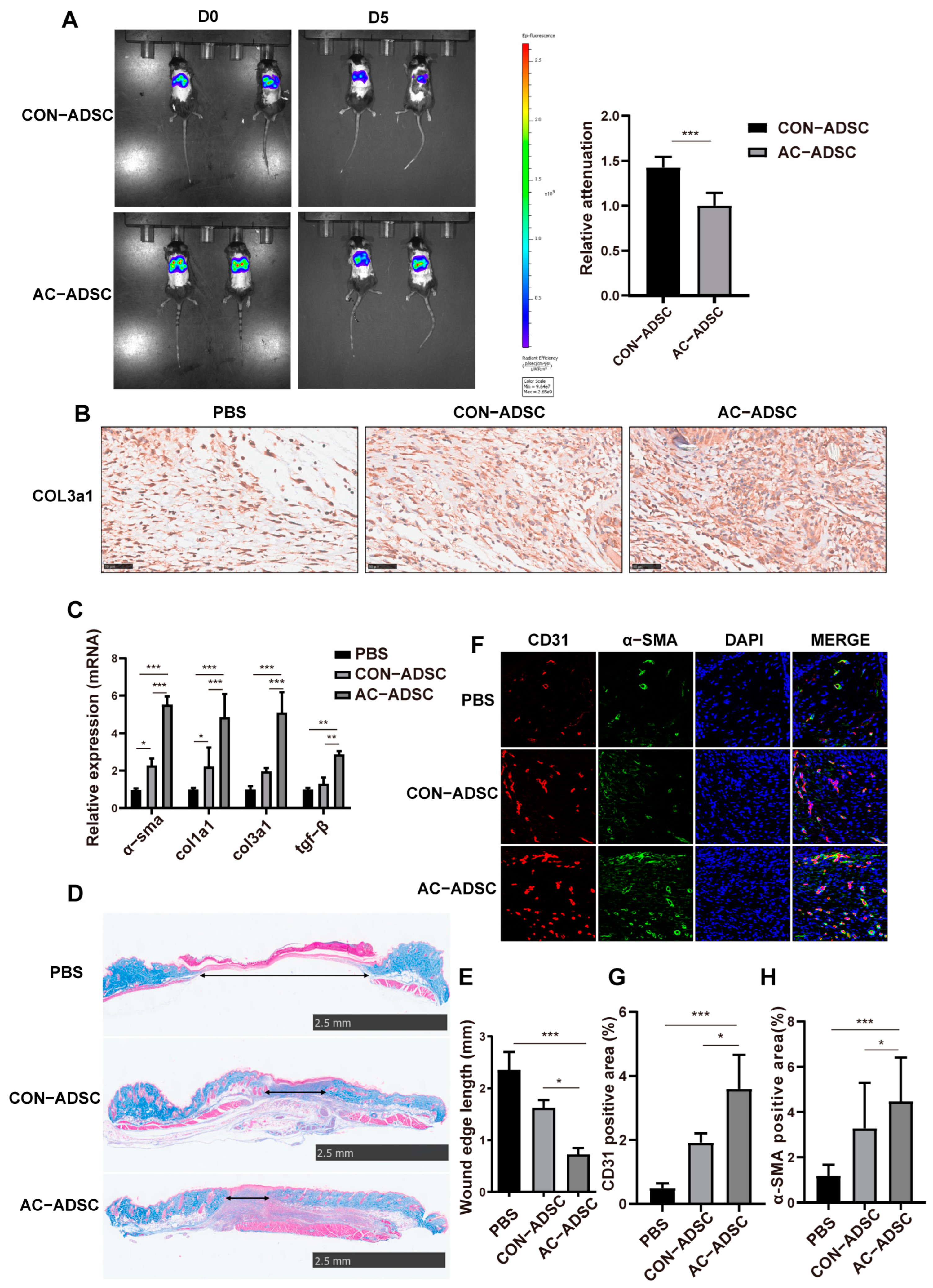
2.5. PDGFR–β Overexpressed ADSCs Accelerated Wound Healing in Diabetic Mice via ECM Remodeling and Angiogenesis
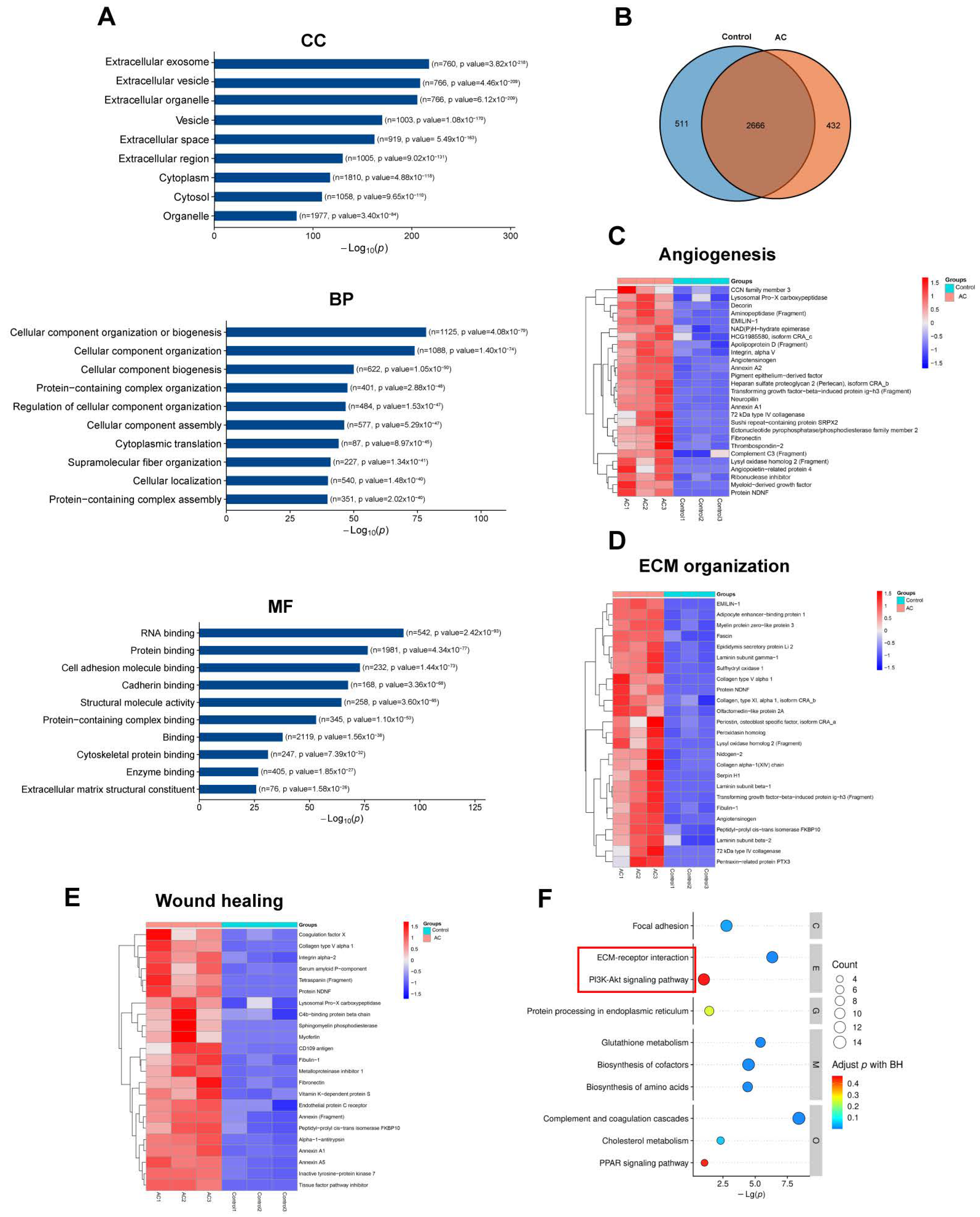
2.6. Proteomics of CON–ADSCs CM and AC–ADSCs CM
3. Discussion
4. Materials and Methods
4.1. Isolation of ADSCs
4.2. Phenotypic Analysis and PDGFR–β Expression by Flow Cytometry
4.3. Cell Differentiation Capacity Analysis
4.4. Ex Vivo Genome Editing of ADSCs
4.5. Lentivirus Transduction and FACS
4.6. Flow Cytometry and FACS
4.7. Real Time Quantitative Reverse Transcription Polymerase Chain Reaction (RT–qPCR)
4.8. Conditioned Medium of ADSCs
4.9. ELISA Analysis
4.10. Western Blot Analysis
4.11. Diabetic Wound Healing Evaluation
4.12. Cell Labeling with DIR
4.13. IVIS Observation
4.14. Histological Observation
4.15. Proteomic Analysis
4.16. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MSCs | Mesenchymal stem cells |
| ADSCs | Adipose–derived stem cells |
| AC–ADSCs | PDGFR–β activation adipose–derived stem cells |
| CON–ADSCs | ADSCs with control lentivirus transduction |
| PDGFR–β | Platelet–derived growth factor β |
| PDGFR–α | Platelet–derived growth factor α |
| SAM | SAM: Synergistic Activation Mediator |
| RT–qPCR | quantitative reverse transcription PCR |
| IVIS | in vivo imaging system |
| EGFP | Enhanced green fluorescent protein |
| mCherry | a red fluorescent protein |
| IL–10 | Interleukin 10 |
| TGF–β | transforming growth factor β |
| VEGFA | Vascular endothelial growth factor A |
| IGF | Insulin–like growth factor |
| PDGFB | Platelet–derived growth factor subunit B |
| Col1a1 | collagen type I alpha 1 chain |
| Col3a1 | collagen type 3 alpha 1 chain |
| ELISA | Enzyme linked immunosorbent assay |
| ECM | extracellular matrix |
| EV | extracellular vesicle. |
References
- Rani, S.; Ritter, T. The Exosome—A Naturally Secreted Nanoparticle and its Application to Wound Healing. Adv. Mater. 2016, 28, 5542–5552. [Google Scholar] [CrossRef]
- Lin, F.; Yang, Y.; Wei, S.; Huang, X.; Peng, Z.; Ke, X.; Zeng, Z.; Song, Y. Hydrogen Sulfide Protects Against High Glucose-Induced Human Umbilical Vein Endothelial Cell Injury Through Activating PI3K/Akt/eNOS Pathway. Drug Des. Dev. Ther. 2020, 14, 621–633. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Ullah, M.W.; Li, B.; Chen, H. Recent Progress in Advanced Hydrogel-Based Embolic Agents: From Rational Design Strategies to Improved Endovascular Embolization. Adv. Healthc. Mater. 2023, e2202787. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef]
- Zhang, P.; Lu, J.; Jing, Y.; Tang, S.; Zhu, D.; Bi, Y. Global epidemiology of diabetic foot ulceration: A systematic review and meta-analysis. Ann. Med. 2017, 49, 106–116. [Google Scholar] [CrossRef]
- Marquez-Curtis, L.A.; Janowska-Wieczorek, A.; McGann, L.E.; Elliott, J.A. Mesenchymal stromal cells derived from various tissues: Biological, clinical and cryopreservation aspects. Cryobiology 2015, 71, 181–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.E.; Ayoub, N.; Agrawal, D.K. Mesenchymal stem cells and cutaneous wound healing: Novel methods to increase cell delivery and therapeutic efficacy. Stem Cell Res. Ther. 2016, 7, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabatabaei, Q.R.; Sheykhhasan, M. Adipose-derived stromal cell in regenerative medicine: A review. World J. Stem Cells 2017, 9, 107–117. [Google Scholar]
- Qiu, H.; Liu, S.; Wu, K.; Zhao, R.; Cao, L.; Wang, H. Prospective application of exosomes derived from adipose-derived stem cells in skin wound healing: A review. J. Cosmet. Dermatol. 2020, 19, 574–581. [Google Scholar] [CrossRef]
- Fang, J.; Chen, F.; Liu, D.; Gu, F.; Wang, Y. Adipose tissue-derived stem cells in breast reconstruction: A brief review on biology and translation. Stem Cell Res. Ther. 2021, 12, 8. [Google Scholar] [CrossRef]
- Geng, Y.; Yang, J.; Li, S.; Chen, M. Chyloid Fat Carried Adipose-Derived Mesenchymal Stem Cells Accelerate Wound Healing Via Promoting Angiogenesis. Ann. Plast. Surg. 2021, 87, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yuan, Y.; Liu, D. Extracellular Vesicles from Adipose-Derived Stem Cells Promote Diabetic Wound Healing via the PI3K-AKT-mTOR-HIF-1alpha Signaling Pathway. Tissue Eng. Regen. Med. 2021, 18, 1035–1044. [Google Scholar] [CrossRef]
- Xiong, J.; Qiang, H.; Li, T.; Zhao, J.; Wang, Z.; Li, F.; Xu, J. Human adipose-derived stem cells promote seawater-immersed wound healing via proangiogenic effects. Aging (Albany NY) 2021, 13, 17118–17136. [Google Scholar] [CrossRef] [PubMed]
- Roshangar, L.; Rad, J.S.; Kheirjou, R.; Khosroshahi, A.F. Using 3D-bioprinting scaffold loaded with adipose-derived stem cells to burns wound healing. J. Tissue Eng. Regen. Med. 2021, 15, 546–555. [Google Scholar] [CrossRef]
- Koenen, P.; Spanholtz, T.A.; Maegele, M.; Sturmer, E.; Brockamp, T.; Neugebauer, E.; Thamm, O.C. Acute and chronic wound fluids inversely influence adipose-derived stem cell function: Molecular insights into impaired wound healing. Int. Wound J. 2015, 12, 10–16. [Google Scholar] [CrossRef]
- Ferrer-Lorente, R.; Bejar, M.T.; Tous, M.; Vilahur, G.; Badimon, L. Systems biology approach to identify alterations in the stem cell reservoir of subcutaneous adipose tissue in a rat model of diabetes: Effects on differentiation potential and function. Diabetologia 2014, 57, 246–256. [Google Scholar] [CrossRef] [Green Version]
- Galipeau, J.; Sensebe, L. Mesenchymal Stromal Cells: Clinical Challenges and Therapeutic Opportunities. Cell Stem Cell 2018, 22, 824–833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levy, O.; Kuai, R.; Siren, E.; Bhere, D.; Milton, Y.; Nissar, N.; De Biasio, M.; Heinelt, M.; Reeve, B.; Abdi, R.; et al. Shattering barriers toward clinically meaningful MSC therapies. Sci. Adv. 2020, 6, a6884. [Google Scholar] [CrossRef] [PubMed]
- Hellstrom, M.; Kalen, M.; Lindahl, P.; Abramsson, A.; Betsholtz, C. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development 1999, 126, 3047–3055. [Google Scholar] [CrossRef]
- Gao, Z.; Sasaoka, T.; Fujimori, T.; Oya, T.; Ishii, Y.; Sabit, H.; Kawaguchi, M.; Kurotaki, Y.; Naito, M.; Wada, T.; et al. Deletion of the PDGFR-beta gene affects key fibroblast functions important for wound healing. J. Biol. Chem. 2005, 280, 9375–9389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mo, M.; Wang, S.; Zhou, Y.; Li, H.; Wu, Y. Mesenchymal stem cell subpopulations: Phenotype, property and therapeutic potential. Cell. Mol. Life Sci. 2016, 73, 3311–3321. [Google Scholar] [CrossRef]
- Alfaro, M.P.; Vincent, A.; Saraswati, S.; Thorne, C.A.; Hong, C.C.; Lee, E.; Young, P.P. sFRP2 suppression of bone morphogenic protein (BMP) and Wnt signaling mediates mesenchymal stem cell (MSC) self-renewal promoting engraftment and myocardial repair. J. Biol. Chem. 2010, 285, 35645–35653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gnecchi, M.; Zhang, Z.; Ni, A.; Dzau, V.J. Paracrine mechanisms in adult stem cell signaling and therapy. Circ. Res. 2008, 103, 1204–1219. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, X.; Zhang, H.; Chen, Q.; Cui, H. Low anticoagulant heparin-iron complex targeting inhibition of hepcidin ameliorates anemia of chronic disease in rodents. Eur. J. Pharmacol. 2021, 897, 173958. [Google Scholar] [CrossRef]
- Binato, R.; de Souza, F.T.; Lazzarotto-Silva, C.; Du Rocher, B.; Mencalha, A.; Pizzatti, L.; Bouzas, L.F.; Abdelhay, E. Stability of human mesenchymal stem cells during in vitro culture: Considerations for cell therapy. Cell Prolif. 2013, 46, 10–22. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, X.; Zhao, L.; Zuo, S.; Chen, X.; Zhang, L.; Lin, Z.; Zhao, X.; Qin, Y.; Zhou, X.; et al. Lineage reprogramming of fibroblasts into induced cardiac progenitor cells by CRISPR/Cas9-based transcriptional activators. Acta Pharm. Sin. B 2020, 10, 313–326. [Google Scholar] [CrossRef]
- Wang, C.H.; Lundh, M.; Fu, A.; Kriszt, R.; Huang, T.L.; Lynes, M.D.; Leiria, L.O.; Shamsi, F.; Darcy, J.; Greenwood, B.P.; et al. CRISPR-engineered human brown-like adipocytes prevent diet-induced obesity and ameliorate metabolic syndrome in mice. Sci. Transl. Med. 2020, 12, eaaz8664. [Google Scholar] [CrossRef]
- Baloh, R.H.; Johnson, J.P.; Avalos, P.; Allred, P.; Svendsen, S.; Gowing, G.; Roxas, K.; Wu, A.; Donahue, B.; Osborne, S.; et al. Transplantation of human neural progenitor cells secreting GDNF into the spinal cord of patients with ALS: A phase 1/2a trial. Nat. Med. 2022, 28, 1813–1822. [Google Scholar] [CrossRef] [PubMed]
- von Einem, J.C.; Guenther, C.; Volk, H.D.; Grutz, G.; Hirsch, D.; Salat, C.; Stoetzer, O.; Nelson, P.J.; Michl, M.; Modest, D.P.; et al. Treatment of advanced gastrointestinal cancer with genetically modified autologous mesenchymal stem cells: Results from the phase 1/2 TREAT-ME-1 trial. Int. J. Cancer 2019, 145, 1538–1546. [Google Scholar] [CrossRef]
- Fumagalli, F.; Calbi, V.; Natali, S.M.; Sessa, M.; Baldoli, C.; Rancoita, P.; Ciotti, F.; Sarzana, M.; Fraschini, M.; Zambon, A.A.; et al. Lentiviral haematopoietic stem-cell gene therapy for early-onset metachromatic leukodystrophy: Long-term results from a non-randomised, open-label, phase 1/2 trial and expanded access. Lancet 2022, 399, 372–383. [Google Scholar] [CrossRef]
- Veceric-Haler, Z.; Sever, M.; Kojc, N.; Halloran, P.F.; Bostjancic, E.; Mlinsek, G.; Oblak, M.; Pozenel, P.; Svajger, U.; Hartman, K.; et al. Autologous Mesenchymal Stem Cells for Treatment of Chronic Active Antibody-Mediated Kidney Graft Rejection: Report of the Phase I/II Clinical Trial Case Series. Transpl. Int. 2022, 35, 10772. [Google Scholar] [CrossRef] [PubMed]
- Bowdish, M.E.; Barkauskas, C.E.; Overbey, J.R.; Gottlieb, R.L.; Osman, K.; Duggal, A.; Marks, M.E.; Hupf, J.; Fernandes, E.; Leshnower, B.G.; et al. A Randomized Trial of Mesenchymal Stromal Cells for Moderate to Severe Acute Respiratory Distress Syndrome from COVID-19. Am. J. Respir. Crit. Care Med. 2023, 207, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Aghayan, H.R.; Salimian, F.; Abedini, A.; Fattah, G.S.; Yunesian, M.; Alavi-Moghadam, S.; Makarem, J.; Majidzadeh-A, K.; Hatamkhani, A.; Moghri, M.; et al. Human placenta-derived mesenchymal stem cells transplantation in patients with acute respiratory distress syndrome (ARDS) caused by COVID-19 (phase I clinical trial): Safety profile assessment. Stem Cell Res. Ther. 2022, 13, 365. [Google Scholar] [CrossRef]
- Bakogiannis, C.; Sachse, M.; Stamatelopoulos, K.; Stellos, K. Platelet-derived chemokines in inflammation and atherosclerosis. Cytokine 2019, 122, 154157. [Google Scholar] [CrossRef]
- Rajkumar, V.S.; Shiwen, X.; Bostrom, M.; Leoni, P.; Muddle, J.; Ivarsson, M.; Gerdin, B.; Denton, C.P.; Bou-Gharios, G.; Black, C.M.; et al. Platelet-derived growth factor-beta receptor activation is essential for fibroblast and pericyte recruitment during cutaneous wound healing. Am. J. Pathol. 2006, 169, 2254–2265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owen, A.; Patten, D.; Vigneswara, V.; Frampton, J.; Newsome, P.N. PDGFRalpha/Sca-1 Sorted Mesenchymal Stromal Cells Reduce Liver Injury in Murine Models of Hepatic Ischemia-Reperfusion Injury. Stem Cells 2022, 40, 1056–1070. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Jin, Q.; Ming-Yu, Y.; Yang, H.; Xu, T.; You-Xing, S.; Xu-Ting, B.; Wan, C.; Yun-Jiao, W.; Huan, W.; et al. MiR-6924-5p-rich exosomes derived from genetically modified Scleraxis-overexpressing PDGFRalpha(+) BMMSCs as novel nanotherapeutics for treating osteolysis during tendon-bone healing and improving healing strength. Biomaterials 2021, 279, 121242. [Google Scholar] [CrossRef]
- Ball, S.G.; Shuttleworth, A.; Kielty, C.M. Inhibition of platelet-derived growth factor receptor signaling regulates Oct4 and Nanog expression, cell shape, and mesenchymal stem cell potency. Stem Cells 2012, 30, 548–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ball, S.G.; Worthington, J.J.; Canfield, A.E.; Merry, C.L.; Kielty, C.M. Mesenchymal stromal cells: Inhibiting PDGF receptors or depleting fibronectin induces mesodermal progenitors with endothelial potential. Stem Cells 2014, 32, 694–705. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Crawford, R.; Chen, C.; Xiao, Y. The key regulatory roles of the PI3K/Akt signaling pathway in the functionalities of mesenchymal stem cells and applications in tissue regeneration. Tissue Eng. Part B-Rev. 2013, 19, 516–528. [Google Scholar] [CrossRef] [Green Version]
- Yan, W.; Lin, C.; Guo, Y.; Chen, Y.; Du, Y.; Lau, W.B.; Xia, Y.; Zhang, F.; Su, R.; Gao, E.; et al. N-Cadherin Overexpression Mobilizes the Protective Effects of Mesenchymal Stromal Cells Against Ischemic Heart Injury Through a beta-Catenin-Dependent Manner. Circ. Res. 2020, 126, 857–874. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Xu, Y.; Zhong, Z.; Wu, Y.; Zhao, J.; Wang, Y.; Cheng, H.; Kong, M.; Zhang, F.; Chen, Q.; et al. A Large-Scale Investigation of Hypoxia-Preconditioned Allogeneic Mesenchymal Stem Cells for Myocardial Repair in Nonhuman Primates: Paracrine Activity without Remuscularization. Circ. Res. 2016, 118, 970–983. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.D.; Krasnodembskaya, A.D. Investigation of the MSC Paracrine Effects on Alveolar-Capillary Barrier Integrity in the In Vitro Models of ARDS. Methods Mol. Biol. 2021, 2269, 63–81. [Google Scholar]
- Werner, S.; Krieg, T.; Smola, H. Keratinocyte-fibroblast interactions in wound healing. J. Invest. Dermatol. 2007, 127, 998–1008. [Google Scholar] [CrossRef] [Green Version]
- Veith, A.P.; Henderson, K.; Spencer, A.; Sligar, A.D.; Baker, A.B. Therapeutic strategies for enhancing angiogenesis in wound healing. Adv. Drug Deliv. Rev. 2019, 146, 97–125. [Google Scholar] [CrossRef]
- Lou, P.; Liu, S.; Xu, X.; Pan, C.; Lu, Y.; Liu, J. Extracellular vesicle-based therapeutics for the regeneration of chronic wounds: Current knowledge and future perspectives. Acta Biomater. 2021, 119, 42–56. [Google Scholar] [CrossRef]
- Mao, J.; Li, C.; Wu, F.; She, Z.; Luo, S.; Chen, X.; Wen, C.; Tian, J. MSC-EVs transferring mitochondria and related components: A new hope for the treatment of kidney disease. Front. Immunol. 2022, 13, 978571. [Google Scholar] [CrossRef]
- Zhuang, Y.; Jiang, S.; Yuan, C.; Lin, K. The potential therapeutic role of extracellular vesicles in osteoarthritis. Front. Bioeng. Biotechnol. 2022, 10, 1022368. [Google Scholar] [CrossRef]
- Bray, E.R.; Kirsner, R.S.; Badiavas, E.V. Mesenchymal Stem Cell-Derived Extracellular Vesicles as an Advanced Therapy for Chronic Wounds. Cold Spring Harbor Perspect. Biol. 2022, 14, a041227. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Li, D.; You, L.; Deng, T.; Pang, Q.; Meng, X.; Zhu, B. dCas9-Based PDGFR–β Activation ADSCs Accelerate Wound Healing in Diabetic Mice through Angiogenesis and ECM Remodeling. Int. J. Mol. Sci. 2023, 24, 5949. https://doi.org/10.3390/ijms24065949
Li Y, Li D, You L, Deng T, Pang Q, Meng X, Zhu B. dCas9-Based PDGFR–β Activation ADSCs Accelerate Wound Healing in Diabetic Mice through Angiogenesis and ECM Remodeling. International Journal of Molecular Sciences. 2023; 24(6):5949. https://doi.org/10.3390/ijms24065949
Chicago/Turabian StyleLi, Yumeng, Deyong Li, Lu You, Tian Deng, Qiuyu Pang, Xiangmin Meng, and Bingmei Zhu. 2023. "dCas9-Based PDGFR–β Activation ADSCs Accelerate Wound Healing in Diabetic Mice through Angiogenesis and ECM Remodeling" International Journal of Molecular Sciences 24, no. 6: 5949. https://doi.org/10.3390/ijms24065949
APA StyleLi, Y., Li, D., You, L., Deng, T., Pang, Q., Meng, X., & Zhu, B. (2023). dCas9-Based PDGFR–β Activation ADSCs Accelerate Wound Healing in Diabetic Mice through Angiogenesis and ECM Remodeling. International Journal of Molecular Sciences, 24(6), 5949. https://doi.org/10.3390/ijms24065949





