Oxygen-Releasing Hyaluronic Acid-Based Dispersion with Controlled Oxygen Delivery for Enhanced Periodontal Tissue Engineering
Abstract
1. Introduction
2. Results
2.1. Oxygen Release Kinetics of the O2-Releasing HA-Based Dispersion
2.2. Detection of Calcium Peroxide Particles in HA-CaO2 Dispersions
2.3. FTIR of the O2-Releasing HA-Based Dispersion
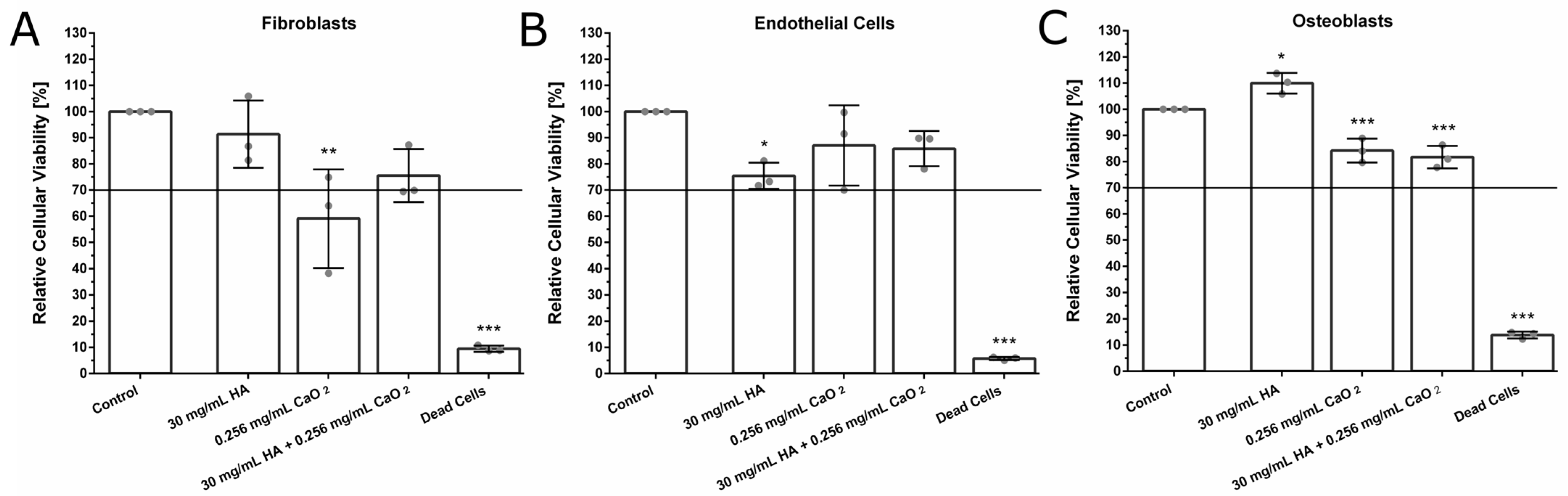
2.4. Biocompatibility of the O2-Releasing HA-Based Dispersion
2.5. Broth Microdilution Assay
3. Discussion
4. Materials and Methods
4.1. Preparation of O2-Releasing HA-Based Dispersion
4.2. Energy-Dispersive X-ray Analysis (EDX)
4.3. Fourier-Transform Infrared Spectroscopy (FTIR)
4.4. Oxygen Release Measurements
4.5. Cell Isolation
4.5.1. Fibroblasts
4.5.2. Osteoblasts
4.5.3. Primary Human Umbilical Vein Endothelial Cells (HUVEC)
4.6. Cellular Viability Assays
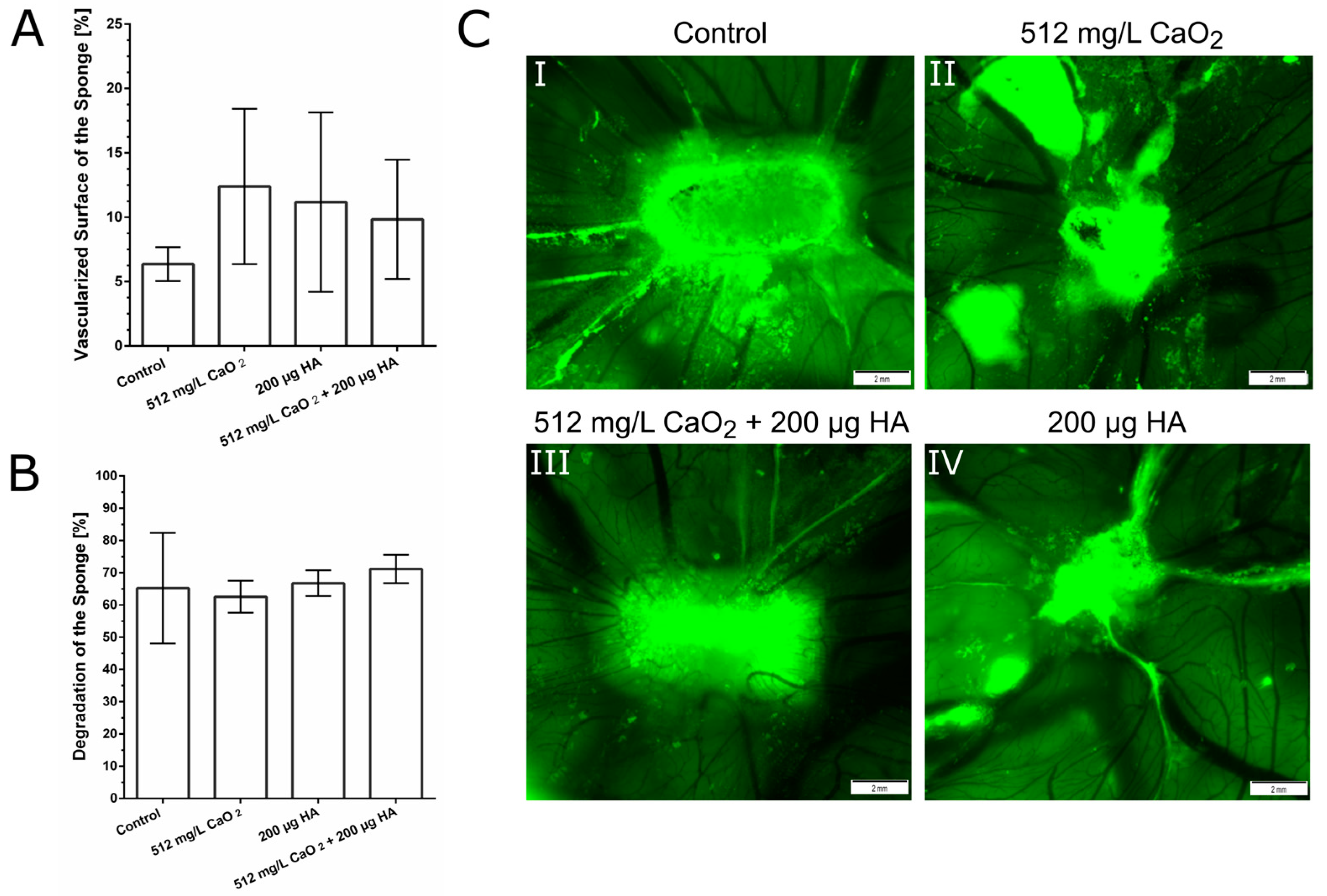
4.7. Chorioallantoic Membrane Assay (CAM Assay)
4.8. Broth Microdilution Assay
4.9. Statistical Analysis
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89 (Suppl. 1), S173–S182. [Google Scholar] [CrossRef] [PubMed]
- Caton, J.G.; Armitage, G.; Berglundh, T.; Chapple, I.L.; Jepsen, S.; Kornman, K.S.; Mealey, B.L.; Papapanou, P.N.; Sanz, M.; Tonetti, M.S. A new classification scheme for periodontal and peri-implant diseases and conditions—Introduction and key changes from the 1999 classification. J. Periodontol. 2018, 89, S1–S8. [Google Scholar] [CrossRef]
- Botelho, J.; Machado, V.; Leira, Y.; Proença, L.; Chambrone, L.; Mendes, J.J. Economic burden of periodontitis in the United States and Europe: An updated estimation. J. Periodontol. 2022, 93, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Ceriello, A.; Buysschaert, M.; Chapple, I.; Demmer, R.T.; Graziani, F.; Herrera, D.; Jepsen, S.; Lione, L.; Madianos, P.; et al. Scientific evidence on the links between periodontal diseases and diabetes: Consensus report and guidelines of the joint workshop on periodontal diseases and diabetes by the International Diabetes Federation and the European Federation of Periodontology. J. Clin. Periodontol. 2018, 45, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Marco Del Castillo, A.; Jepsen, S.; Gonzalez-Juanatey, J.R.; D’Aiuto, F.; Bouchard, P.; Chapple, I.; Dietrich, T.; Gotsman, I.; Graziani, F.; et al. Periodontitis and cardiovascular diseases: Consensus report. J. Clin. Periodontol. 2020, 47, 268–288. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yang, Y.; Yang, P.; Gu, Y.; Zhao, Z.; Tan, L.; Zhao, L.; Tang, T.; Li, Y. The osteogenic differentiation of PDLSCs is mediated through MEK/ERK and p38 MAPK signalling under hypoxia. Arch. Oral Biol. 2013, 58, 1357–1368. [Google Scholar] [CrossRef]
- Celik, D.; Kantarci, A. Vascular Changes and Hypoxia in Periodontal Disease as a Link to Systemic Complications. Pathogens 2021, 10, 1280. [Google Scholar] [CrossRef]
- Song, Z.C.; Zhou, W.; Shu, R.; Ni, J. Hypoxia induces apoptosis and autophagic cell death in human periodontal ligament cells through HIF-1α pathway. Cell Prolif. 2012, 45, 239–248. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Liu, R.; Xing, Y.J.; Xu, P.; Li, Y.; Li, C.J. Effects of hypoxia on the proliferation, mineralization and ultrastructure of human periodontal ligament fibroblasts in vitro. Exp. Ther. Med. 2013, 6, 1553–1559. [Google Scholar] [CrossRef]
- Krishnan, V.; Davidovitch, Z. Cellular, molecular, and tissue-level reactions to orthodontic force. Am. J. Orthod. Dentofac. Orthop. 2006, 129, 469.E1–469.E3. [Google Scholar] [CrossRef]
- Özcan, E.; Işıl Saygun, N.; Serdar, M.A.; Umut Bengi, V.; Kantarcı, A. Non-Surgical Periodontal Therapy Reduces Saliva Adipokine and Matrix Metalloproteinase Levels in Periodontitis. J. Periodontol. 2016, 87, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Butera, A.; Gallo, S.; Pascadopoli, M.; Maiorani, C.; Milone, A.; Alovisi, M.; Scribante, A. Paraprobiotics in Non-Surgical Periodontal Therapy: Clinical and Microbiological Aspects in a 6-Month Follow-Up Domiciliary Protocol for Oral Hygiene. Microorganisms 2022, 10, 337. [Google Scholar] [CrossRef] [PubMed]
- Butera, A.; Folini, E.; Cosola, S.; Russo, G.; Scribante, A.; Gallo, S.; Stablum, G.; Fabris, G.B.M.; Covani, U.; Genovesi, A. Evaluation of the Efficacy of Probiotics Domiciliary Protocols for the Management of Periodontal Disease, in Adjunction of Non-Surgical Periodontal Therapy (NSPT): A Systematic Literature Review. Appl. Sci. 2023, 13, 663. [Google Scholar] [CrossRef]
- Gheisary, Z.; Mahmood, R.; Harri Shivanantham, A.; Liu, J.; Lieffers, J.R.L.; Papagerakis, P.; Papagerakis, S. The Clinical, Microbiological, and Immunological Effects of Probiotic Supplementation on Prevention and Treatment of Periodontal Diseases: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 1036. [Google Scholar] [CrossRef]
- Butera, A.; Gallo, S.; Pascadopoli, M.; Taccardi, D.; Scribante, A. Home Oral Care of Periodontal Patients Using Antimicrobial Gel with Postbiotics, Lactoferrin, and Aloe Barbadensis Leaf Juice Powder vs. Conventional Chlorhexidine Gel: A Split-Mouth Randomized Clinical Trial. Antibiotics 2022, 1, 118. [Google Scholar] [CrossRef]
- Schlagenhauf, U.; Jockel-Schneider, Y. Probiotics in the Management of Gingivitis and Periodontitis. A Review. Front. Dent. Med. 2021, 2, 708666. [Google Scholar] [CrossRef]
- Xiao, Z.; Han, Y.; Zhang, Y.; Zhang, X. Hypoxia-regulated human periodontal ligament cells via Wnt/β-catenin signaling pathway. Medicine 2017, 96, e6562. [Google Scholar] [CrossRef]
- Mettraux, G.R.; Gusberti, F.A.; Graf, H. Oxygen tension (pO2) in untreated human periodontal pockets. J. Periodontol. 1984, 55, 516–521. [Google Scholar] [CrossRef]
- Seo, B.M.; Miura, M.; Gronthos, S.; Bartold, P.M.; Batouli, S.; Brahim, J.; Young, M.; Robey, P.G.; Wang, C.Y.; Shi, S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 2004, 364, 149–155. [Google Scholar] [CrossRef]
- Li, Q.; Luo, T.; Lu, W.; Yi, X.; Zhao, Z.; Liu, J. Proteomic analysis of human periodontal ligament cells under hypoxia. Proteome Sci. 2019, 17, 3. [Google Scholar] [CrossRef]
- Liu, J.; Ruan, J.; Weir, M.D.; Ren, K.; Schneider, A.; Wang, P.; Oates, T.W.; Chang, X.; Xu, H.H.K. Periodontal Bone-Ligament-Cementum Regeneration via Scaffolds and Stem Cells. Cells 2019, 8, 537. [Google Scholar] [CrossRef] [PubMed]
- Dangaria, S.J.; Ito, Y.; Luan, X.; Diekwisch, T.G. Successful periodontal ligament regeneration by periodontal progenitor preseeding on natural tooth root surfaces. Stem Cells Dev. 2011, 20, 1659–1668. [Google Scholar] [CrossRef] [PubMed]
- Son, H.; Jeon, M.; Choi, H.-J.; Lee, H.-S.; Kim, I.-H.; Kang, C.-M.; Song, J.S. Decellularized human periodontal ligament for periodontium regeneration. PLoS ONE 2019, 14, e0221236. [Google Scholar] [CrossRef] [PubMed]
- Utting, J.C.; Robins, S.P.; Brandao-Burch, A.; Orriss, I.R.; Behar, J.; Arnett, T.R. Hypoxia inhibits the growth, differentiation and bone-forming capacity of rat osteoblasts. Exp. Cell Res. 2006, 312, 1693–1702. [Google Scholar] [CrossRef] [PubMed]
- Signoretto, C.; Bianchi, F.; Burlacchini, G.; Canepari, P. Microbiological evaluation of the effects of hyperbaric oxygen on periodontal disease. New Microbiol. 2007, 30, 431–437. [Google Scholar]
- Burcea, A.; Mihai, L.L.; Bechir, A.; Suciu, M.; Bechir, E.S. Clinical Assessment of the Hyperbaric Oxygen Therapy Efficacy in Mild to Moderate Periodontal Affections: A Simple Randomised Trial. Medicina 2022, 58, 234. [Google Scholar] [CrossRef]
- Chen, T.L.; Xu, B.; Liu, J.C.; Li, S.G.; Li, D.Y.; Gong, G.C.; Wu, Z.F.; Lin, S.L.; Zhou, Y.J. Effects of hyperbaric oxygen on aggressive periodontitis and subgingival anaerobes in Chinese patients. J. Indian Soc. Periodontol. 2012, 16, 492–497. [Google Scholar] [CrossRef]
- Mesa, F.L.; Aneiros, J.; Cabrera, A.; Bravo, M.; Caballero, T.; Revelles, F.; del Moral, R.G.; O’Valle, F. Antiproliferative effect of topic hyaluronic acid gel. Study in gingival biopsies of patients with periodontal disease. Histol. Histopathol. 2002, 17, 747–753. [Google Scholar] [CrossRef]
- Johannsen, A.; Tellefsen, M.; Wikesjö, U.; Johannsen, G. Local delivery of hyaluronan as an adjunct to scaling and root planing in the treatment of chronic periodontitis. J. Periodontol. 2009, 80, 1493–1497. [Google Scholar] [CrossRef]
- Polepalle, T.; Srinivas, M.; Swamy, N.; Aluru, S.; Chakrapani, S.; Chowdary, B.A. Local delivery of hyaluronan 0.8% as an adjunct to scaling and root planing in the treatment of chronic periodontitis: A clinical and microbiological study. J. Indian Soc. Periodontol. 2015, 19, 37–42. [Google Scholar] [CrossRef]
- Xu, Y.; Höfling, K.; Fimmers, R.; Frentzen, M.; Jervøe-Storm, P.M. Clinical and microbiological effects of topical subgingival application of hyaluronic acid gel adjunctive to scaling and root planing in the treatment of chronic periodontitis. J. Periodontol. 2004, 75, 1114–1118. [Google Scholar] [CrossRef]
- Eick, S.; Renatus, A.; Heinicke, M.; Pfister, W.; Stratul, S.I.; Jentsch, H. Hyaluronic Acid as an adjunct after scaling and root planing: A prospective randomized clinical trial. J. Periodontol. 2013, 84, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.S.; Bains, V.K.; Gupta, V.; Singh, G.P.; Patil, S.S. Comparative analysis of hyaluronan gel and xanthan-based chlorhexidine gel, as adjunct to scaling and root planing with scaling and root planing alone in the treatment of chronic periodontitis: A preliminary study. Contemp. Clin. Dent. 2013, 4, 54–61. [Google Scholar] [CrossRef]
- Rajan, P.; Baramappa, R.; Rao, N.M.; Pavaluri, A.K.; Indeevar, P.; Rahaman, S.M.U. Hyaluronic Acid as an adjunct to scaling and root planing in chronic periodontitis. A randomized clinical trail. J. Clin. Diagn. Res. 2014, 8, ZC11–ZC14. [Google Scholar] [CrossRef]
- Camci-Unal, G.; Alemdar, N.; Annabi, N.; Khademhosseini, A. Oxygen Releasing Biomaterials for Tissue Engineering. Polym. Int. 2013, 62, 843–848. [Google Scholar] [CrossRef]
- Suvarnapathaki, S.; Wu, X.; Lantigua, D.; Nguyen, M.A.; Camci-Unal, G. Breathing life into engineered tissues using oxygen-releasing biomaterials. NPG Asia Mater. 2019, 11, 65. [Google Scholar] [CrossRef]
- Shiekh, P.A.; Singh, A.; Kumar, A. Oxygen-Releasing Antioxidant Cryogel Scaffolds with Sustained Oxygen Delivery for Tissue Engineering Applications. ACS Appl. Mater. Interfaces 2018, 10, 18458–18469. [Google Scholar] [CrossRef]
- Agarwal, T.; Kazemi, S.; Costantini, M.; Perfeito, F.; Correia, C.R.; Gaspar, V.; Montazeri, L.; De Maria, C.; Mano, J.F.; Vosough, M.; et al. Oxygen releasing materials: Towards addressing the hypoxia-related issues in tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 122, 111896. [Google Scholar] [CrossRef]
- Zhang, M.; Kiratiwongwan, T.; Shen, W. Oxygen-releasing polycaprolactone/calcium peroxide composite microspheres. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 1097–1106. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.E.; Son, J.S.; Park, K.; Han, D.K. Fabrication of covered porous PLGA microspheres using hydrogen peroxide for controlled drug delivery and regenerative medicine. J. Control. Release 2009, 133, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, Y.; Bawa, H.K.; Ng, G.; Wu, Y.; Libera, M.; van der Mei, H.C.; Busscher, H.J.; Yu, X. Oxygen-generating nanofiber cell scaffolds with antimicrobial properties. ACS Appl. Mater. Interfaces 2011, 3, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Daneshmandi, L.; Laurencin, C.T. Regenerative engineered vascularized bone mediated by calcium peroxide. J. Biomed. Mater. Res. A 2020, 108, 1045–1057. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Li, Z.; Chen, S.; Xie, M.; Huang, J.; Peng, X.; Yang, R.; Wang, H.; Xu, Y.; Feng, C. Structural and functional evaluation of oxygenating keratin/silk fibroin scaffold and initial assessment of their potential for urethral tissue engineering. Biomaterials 2016, 84, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Pandit, A.H.; Mazumdar, N.; Ahmad, S. Periodate oxidized hyaluronic acid-based hydrogel scaffolds for tissue engineering applications. Int. J. Biol. Macromol. 2019, 137, 853–869. [Google Scholar] [CrossRef] [PubMed]
- Colares, V.L.P.; Lima, S.N.L.; Sousa, N.C.F.; Araújo, M.C.; Pereira, D.M.S.; Mendes, S.J.F.; Teixeira, S.A.; Monteiro, C.A.; Bandeca, M.C.; Siqueira, W.L.; et al. Hydrogen peroxide-based products alter inflammatory and tissue damage-related proteins in the gingival crevicular fluid of healthy volunteers: A randomized trial. Sci. Rep. 2019, 9, 3457. [Google Scholar] [CrossRef] [PubMed]
- Bladier, C.; Wolvetang, E.J.; Hutchinson, P.; de Haan, J.B.; Kola, I. Response of a primary human fibroblast cell line to H2O2: Senescence-like growth arrest or apoptosis? Cell Growth Differ. 1997, 8, 589–598. [Google Scholar]
- Liang, J.; Shen, Y.C.; Zhang, X.Y.; Chen, C.; Zhao, H.; Hu, J. Circular RNA HIPK3 downregulation mediates hydrogen peroxide-induced cytotoxicity in human osteoblasts. Aging 2020, 12, 1159–1170. [Google Scholar] [CrossRef]
- Lin, X.L.; Liu, Y.; Liu, M.; Hu, H.; Pan, Y.; Fan, X.J.; Hu, X.M.; Zou, W.W. Inhibition of Hydrogen Peroxide-Induced Human Umbilical Vein Endothelial Cells Aging by Allicin Depends on Sirtuin1 Activation. Med. Sci. Monit. 2017, 23, 563–570. [Google Scholar] [CrossRef]
- Lu, Z.; Imlay, J.A. When anaerobes encounter oxygen: Mechanisms of oxygen toxicity, tolerance and defence. Nat. Rev. Microbiol. 2021, 19, 774–785. [Google Scholar] [CrossRef]
- Kommerein, N.; Vierengel, N.; Groß, J.; Opatz, T.; Al-Nawas, B.; Müller-Heupt, L.K. Antiplanktonic and antibiofilm activity of Rheum palmatum against Streptococcus oralis and Porphyromonas gingivalis. Microorganisms 2022, 10, 965. [Google Scholar] [CrossRef] [PubMed]
- Elashiry, M.; Morandini, A.C.; Cornelius Timothius, C.J.; Ghaly, M.; Cutler, C.W. Selective Antimicrobial Therapies for Periodontitis: Win the “Battle and the War”. Int. J. Mol. Sci. 2021, 22, 6459. [Google Scholar] [CrossRef] [PubMed]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The oral microbiota: Dynamic communities and host interactions. Nat. Rev. Microbiol. 2018, 16, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Müller-Heupt, L.K.; Vierengel, N.; Groß, J.; Opatz, T.; Deschner, J.; von Loewenich, F.D. Antimicrobial activity of Eucalyptus globulus, Azadirachta indica, Glycyrrhiza glabra, Rheum palmatum extracts and Rhein against Porphyromonas gingivalis. Antibiotics 2022, 11, 186. [Google Scholar] [CrossRef]
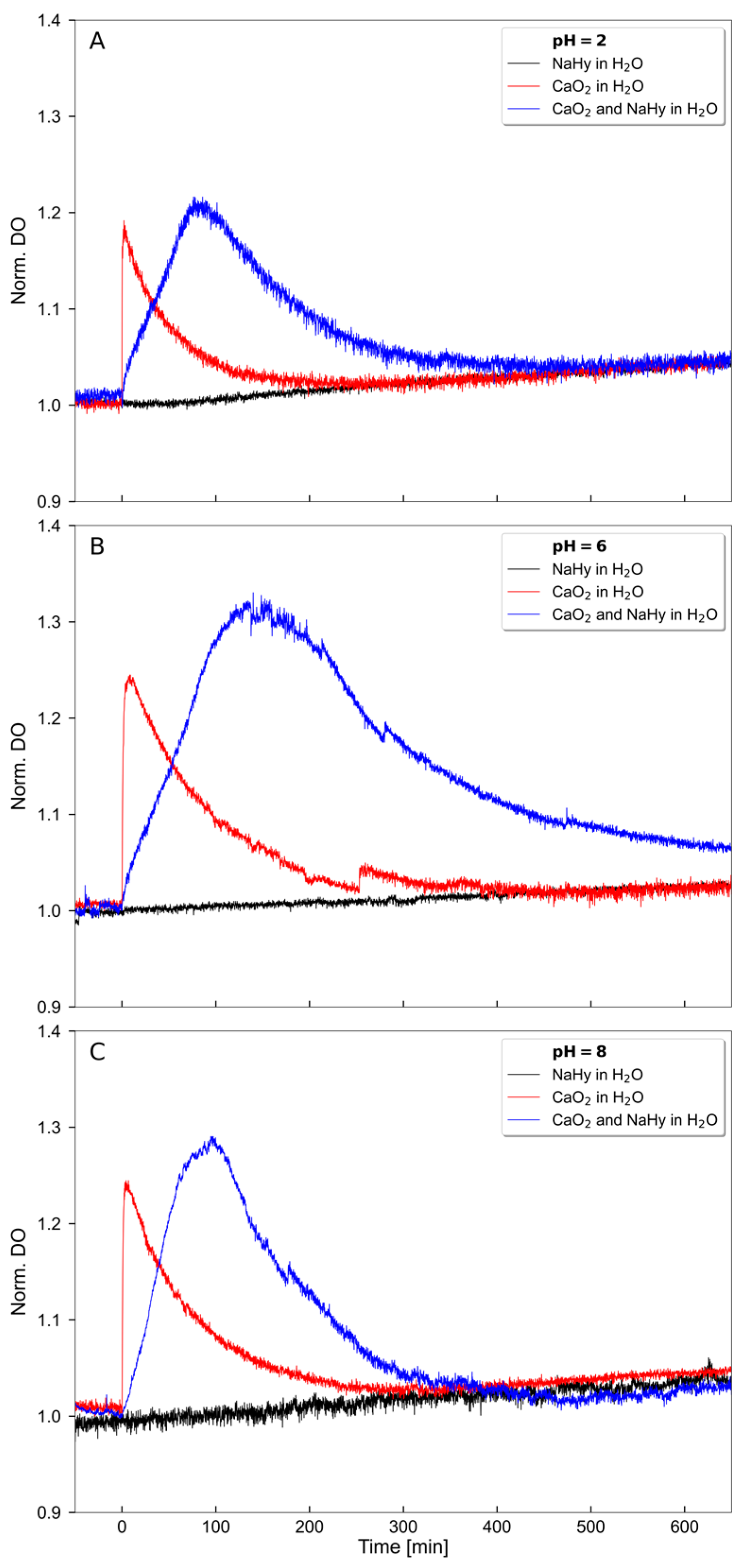

| pH | CaO2 in H2O (mg/L × min) | CaO2 and HA in H2O (mg/L × min) |
|---|---|---|
| 2 | 11.67 | 34.24 |
| 6 | 25.83 | 98.52 |
| 8 | 23.45 | 45.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Müller-Heupt, L.K.; Wiesmann-Imilowski, N.; Schröder, S.; Groß, J.; Ziskoven, P.C.; Bani, P.; Kämmerer, P.W.; Schiegnitz, E.; Eckelt, A.; Eckelt, J.; et al. Oxygen-Releasing Hyaluronic Acid-Based Dispersion with Controlled Oxygen Delivery for Enhanced Periodontal Tissue Engineering. Int. J. Mol. Sci. 2023, 24, 5936. https://doi.org/10.3390/ijms24065936
Müller-Heupt LK, Wiesmann-Imilowski N, Schröder S, Groß J, Ziskoven PC, Bani P, Kämmerer PW, Schiegnitz E, Eckelt A, Eckelt J, et al. Oxygen-Releasing Hyaluronic Acid-Based Dispersion with Controlled Oxygen Delivery for Enhanced Periodontal Tissue Engineering. International Journal of Molecular Sciences. 2023; 24(6):5936. https://doi.org/10.3390/ijms24065936
Chicago/Turabian StyleMüller-Heupt, Lena Katharina, Nadine Wiesmann-Imilowski, Sofia Schröder, Jonathan Groß, Pablo Cores Ziskoven, Philipp Bani, Peer Wolfgang Kämmerer, Eik Schiegnitz, Anja Eckelt, John Eckelt, and et al. 2023. "Oxygen-Releasing Hyaluronic Acid-Based Dispersion with Controlled Oxygen Delivery for Enhanced Periodontal Tissue Engineering" International Journal of Molecular Sciences 24, no. 6: 5936. https://doi.org/10.3390/ijms24065936
APA StyleMüller-Heupt, L. K., Wiesmann-Imilowski, N., Schröder, S., Groß, J., Ziskoven, P. C., Bani, P., Kämmerer, P. W., Schiegnitz, E., Eckelt, A., Eckelt, J., Ritz, U., Opatz, T., Al-Nawas, B., Synatschke, C. V., & Deschner, J. (2023). Oxygen-Releasing Hyaluronic Acid-Based Dispersion with Controlled Oxygen Delivery for Enhanced Periodontal Tissue Engineering. International Journal of Molecular Sciences, 24(6), 5936. https://doi.org/10.3390/ijms24065936






