Time Dependent Changes in the Ovine Neurovascular Unit; A Potential Neuroprotective Role of Annexin A1 in Neonatal Hypoxic-Ischemic Encephalopathy
Abstract
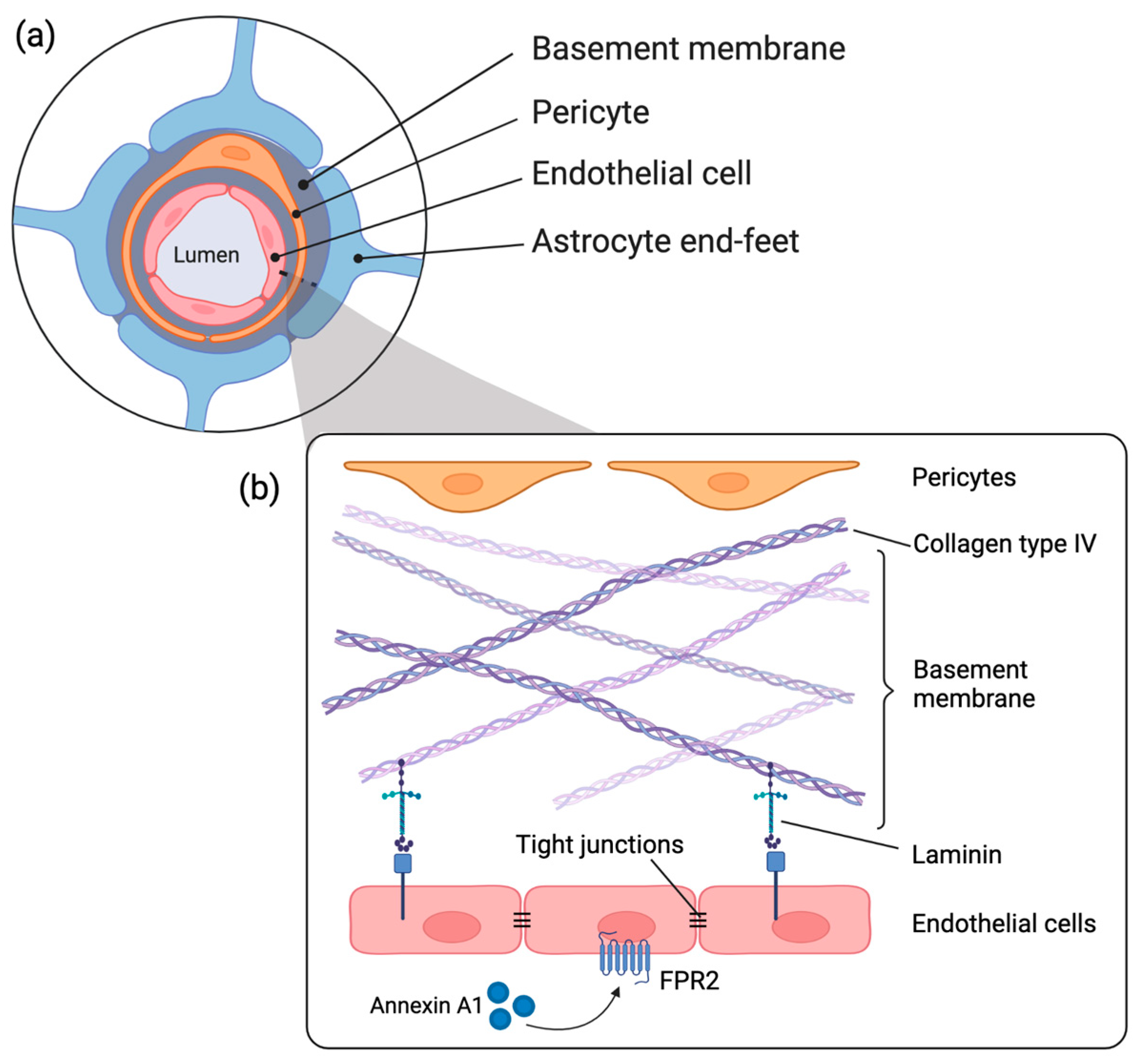
1. Introduction
2. Results
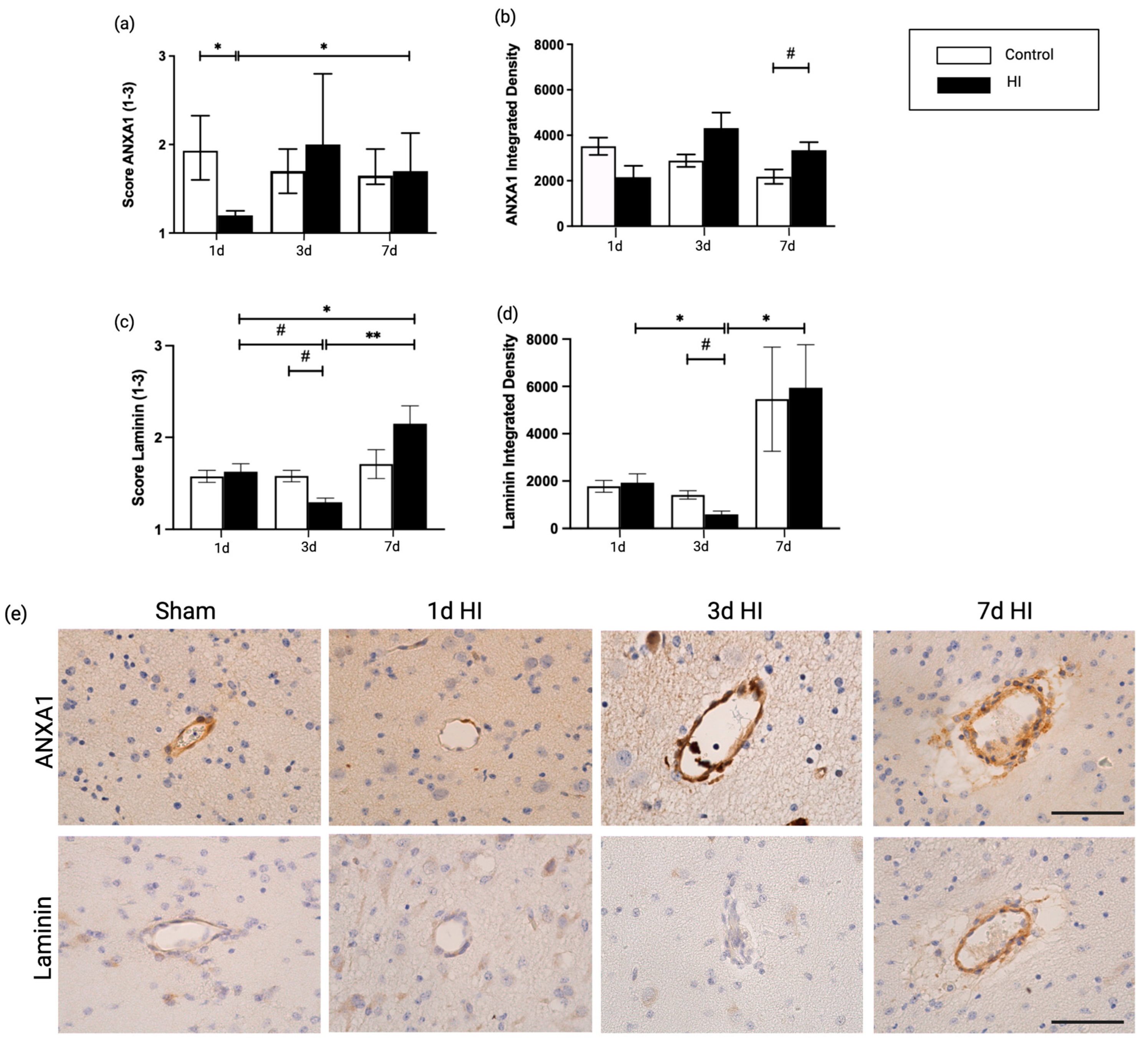
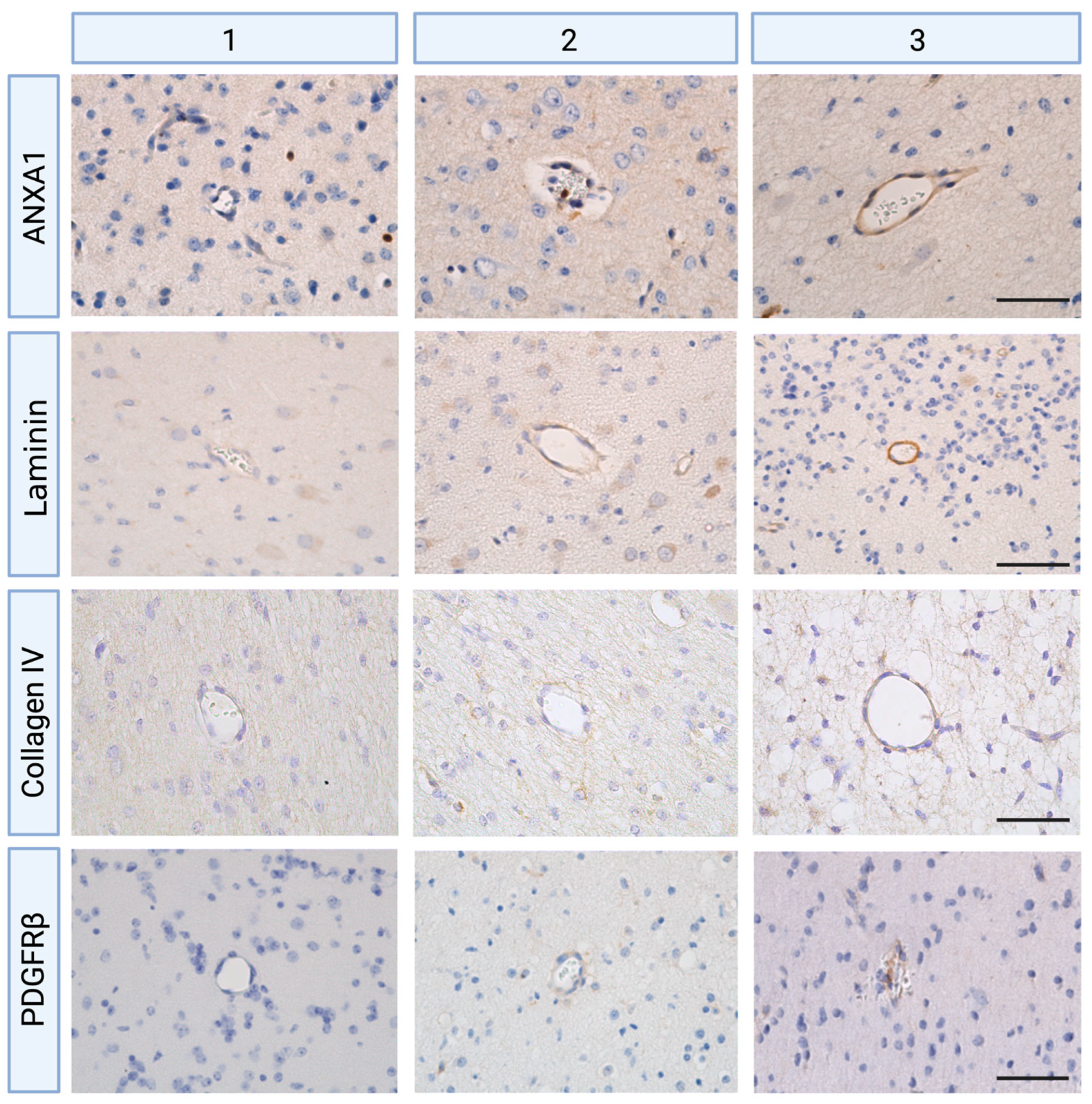
2.1. Global Hypoxia-Ischemia Is Associated with a Significant Depletion of ANXA1 Expression One Day after HI, Leading to Laminin Depletion Three Days Post-HI at the Neurovascular Unit (NVU) in the Ovine Fetus
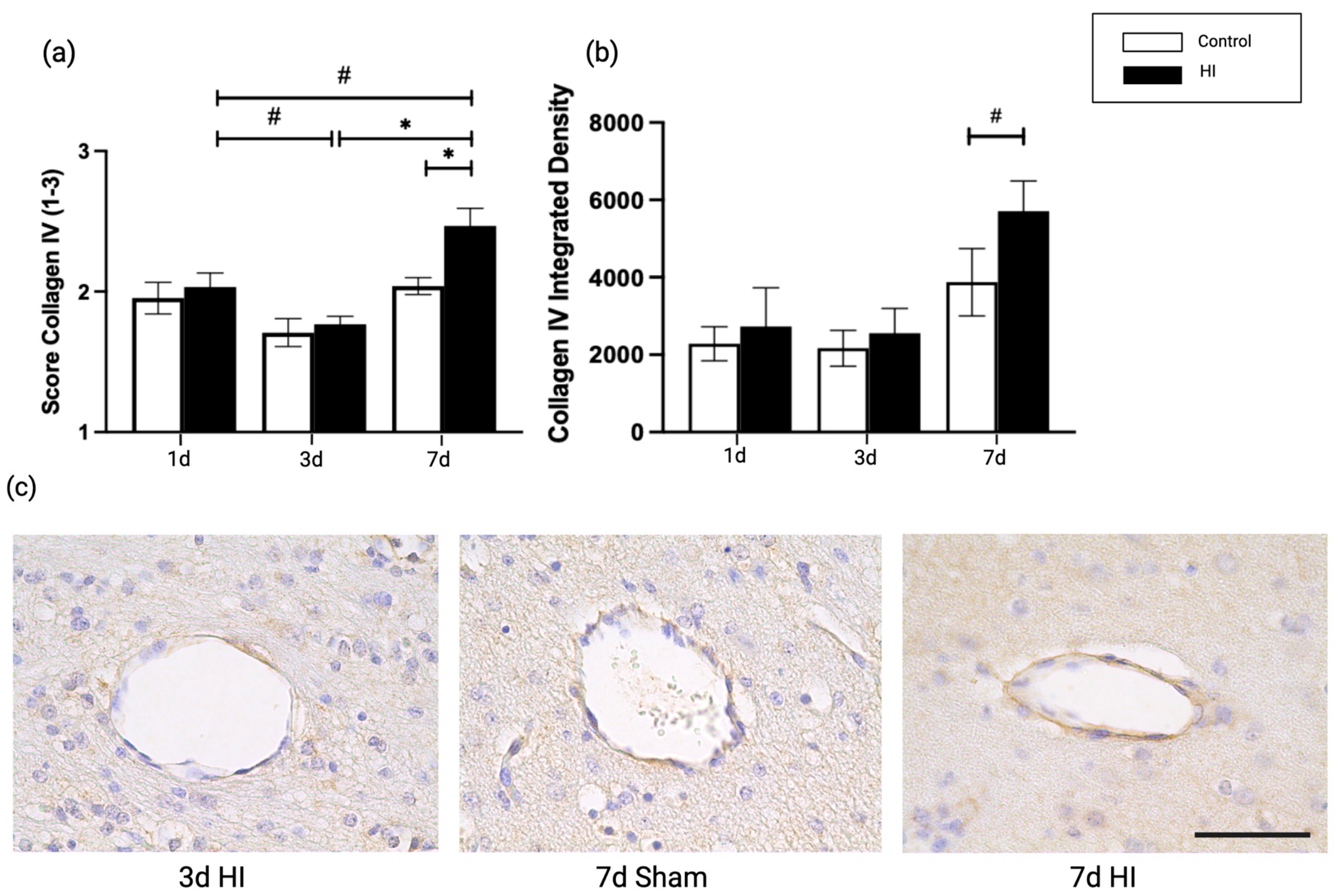
2.2. Global HI Induced an Increased Microvascular Collagen Type IV Expression at the NVU Seven Days Post-Reperfusion in the Ovine Fetus
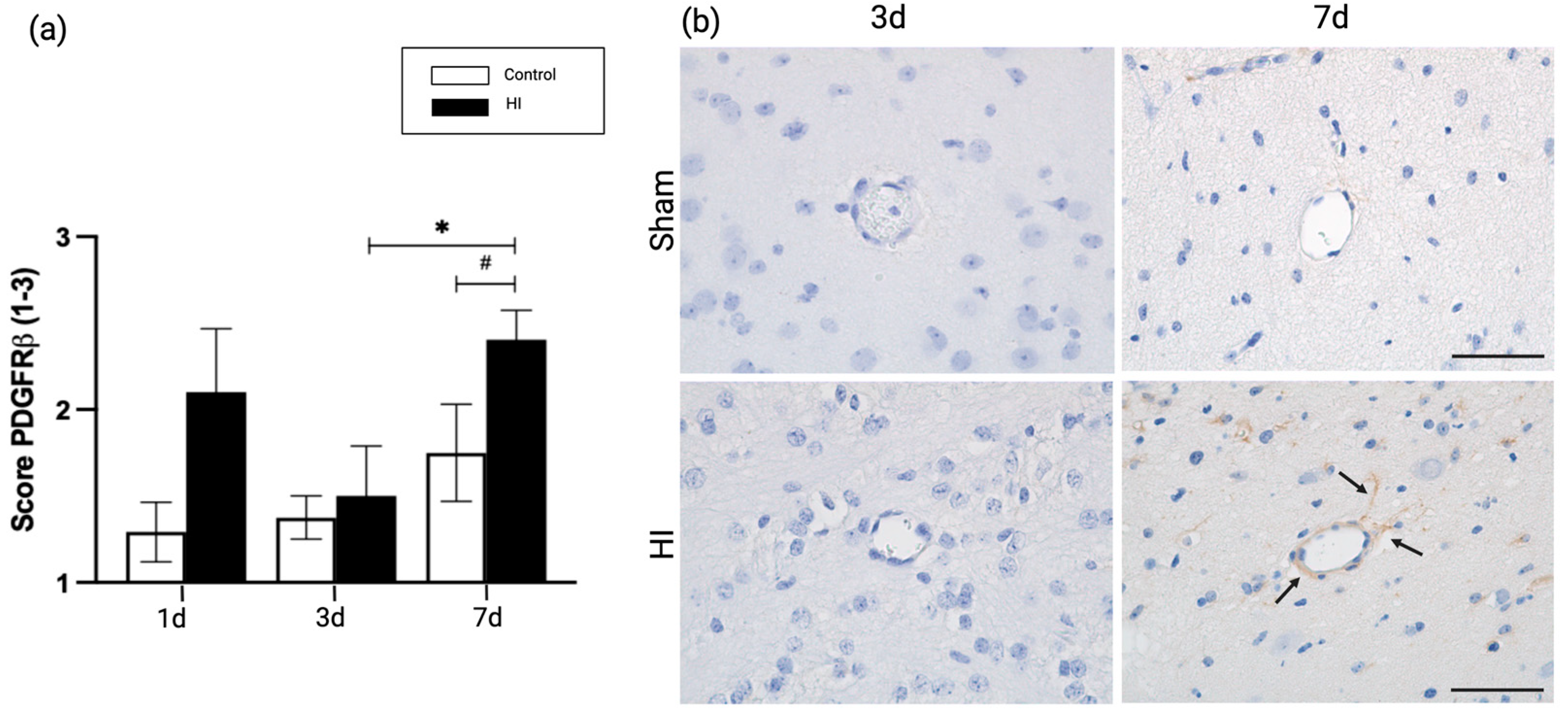
2.3. Global HI Induced Upregulation of Pericyte Coverage in the NVU Seven Days Post-Reperfusion in the Ovine Fetus
3. Discussion
4. Materials and Methods
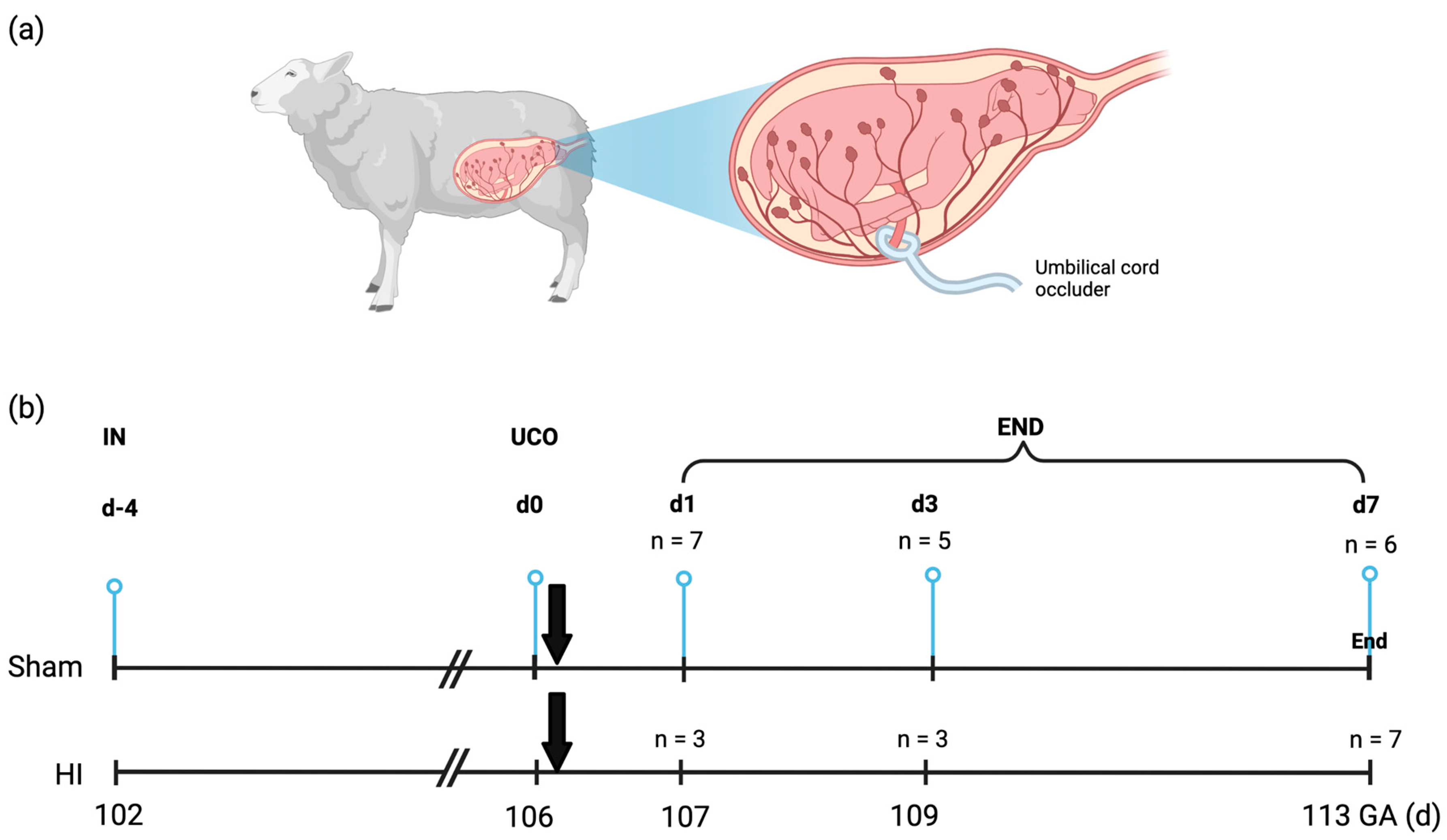
4.1. Experimental Design and Study Approval
4.2. Immunohistochemistry and Analysis
4.3. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Long, M.; Brandon, D.H. Induced Hypothermia for Neonates With Hypoxic-Ischemic Encephalopathy. J. Obstet. Gynecol. Neonatal Nurs. 2007, 36, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Allen, K.A.; Brandon, D.H. Hypoxic Ischemic Encephalopathy: Pathophysiology and Experimental Treatments. Newborn Infant Nurs. Rev. 2011, 11, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Ophelders, D.R.; Gussenhoven, R.; Klein, L.; Jellema, R.K.; Westerlaken, R.J.; Hütten, M.C.; Vermeulen, J.; Wassink, G.; Gunn, A.J.; Wolfs, T.G. Preterm brain injury, antenatal triggers, and therapeutics: Timing is key. Cells 2020, 9, 1871. [Google Scholar] [PubMed]
- Galinsky, R.; Lear, C.A.; Dean, J.M.; Wassink, G.; Dhillon, S.K.; Fraser, M.; Davidson, J.O.; Bennet, L.; Gunn, A.J. Complex interactions between hypoxia-ischemia and inflammation in preterm brain injury. Dev. Med. Child Neurol. 2018, 60, 126–133. [Google Scholar]
- Disdier, C.; Stonestreet, B.S. Hypoxic-ischemic-related cerebrovascular changes and potential therapeutic strategies in the neonatal brain. J. Neurosci. Res. 2020, 98, 1468–1484. [Google Scholar]
- Lee, W.L.A.; Michael-Titus, A.T.; Shah, D.K. Hypoxic-Ischaemic Encephalopathy and the Blood-Brain Barrier in Neonates. Dev. Neurosci. 2017, 39, 49–58. [Google Scholar] [CrossRef]
- Xiao, M.; Xiao, Z.J.; Yang, B.; Lan, Z.; Fang, F. Blood-brain barrier: More contributor to disruption of central nervous system homeostasis than victim in neurological disorders. Front. Neurosci. 2020, 14, 764. [Google Scholar]
- Iadecola, C. The neurovascular unit coming of age: A journey through neurovascular coupling in health and disease. Neuron 2017, 96, 17–42. [Google Scholar]
- Saunders, N.R.; Ek, C.J.; Habgood, M.D.; Dziegielewska, K.M. Barriers in the brain: A renaissance? Trends Neurosci. 2008, 31, 279–286. [Google Scholar] [CrossRef]
- Saunders, N.; Liddelow, S.; Dziegielewska, K. Barrier Mechanisms in the Developing Brain. Front. Pharm. 2012, 3, 46. [Google Scholar] [CrossRef]
- Abbott, N.J. Inflammatory mediators and modulation of blood-brain barrier permeability. Cell. Mol. Neurobiol. 2000, 20, 131–147. [Google Scholar] [CrossRef]
- Chakkarapani, A.A.; Aly, H.; Benders, M.; Cotten, C.M.; El-Dib, M.; Gressens, P.; Hagberg, H.; Sabir, H.; Wintermark, P.; Robertson, N.J. Therapies for neonatal encephalopathy: Targeting the latent, secondary and tertiary phases of evolving brain injury. Semin. Fetal Neonatal Med. 2021, 26, 101256. [Google Scholar] [CrossRef] [PubMed]
- Gussenhoven, R.; Klein, L.; Ophelders, D.R.; Habets, D.H.; Giebel, B.; Kramer, B.W.; Schurgers, L.J.; Reutelingsperger, C.P.; Wolfs, T.G. Annexin A1 as neuroprotective determinant for blood-brain barrier integrity in neonatal hypoxic-ischemic encephalopathy. J. Clin. Med. 2019, 8, 137. [Google Scholar] [CrossRef] [PubMed]
- Jellema, R.K.; Lima Passos, V.; Zwanenburg, A.; Ophelders, D.R.; De Munter, S.; Vanderlocht, J.; Germeraad, W.T.; Kuypers, E.; Collins, J.J.; Cleutjens, J.P.; et al. Cerebral inflammation and mobilization of the peripheral immune system following global hypoxia-ischemia in preterm sheep. J Neuroinflamm. 2013, 10, 13. [Google Scholar] [CrossRef]
- McArthur, S.; Cristante, E.; Paterno, M.; Christian, H.; Roncaroli, F.; Gillies, G.E.; Solito, E. Annexin A1: A central player in the anti-inflammatory and neuroprotective role of microglia. J. Immunol. 2010, 185, 6317–6328. [Google Scholar] [CrossRef]
- Cristante, E.; McArthur, S.; Mauro, C.; Maggioli, E.; Romero, I.A.; Wylezinska-Arridge, M.; Couraud, P.O.; Lopez-Tremoleda, J.; Christian, H.C.; Weksler, B.B.; et al. Identification of an essential endogenous regulator of blood-brain barrier integrity, and its pathological and therapeutic implications. Proc. Natl. Acad. Sci. USA 2013, 110, 832–841. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.H.; Solito, E.; Russo-Marie, F.; Flower, R.J.; Perretti, M. Promoting detachment of neutrophils adherent to murine postcapillary venules to control inflammation: Effect of lipocortin 1. Proc. Natl. Acad. Sci. USA 1998, 95, 14535–14539. [Google Scholar] [CrossRef]
- Hay, J.R.; Johnson, V.E.; Young, A.M.; Smith, D.H.; Stewart, W. Blood-Brain Barrier Disruption Is an Early Event That May Persist for Many Years After Traumatic Brain Injury in Humans. J Neuropathol. Exp. Neurol. 2015, 74, 1147–1157. [Google Scholar] [CrossRef]
- Ophelders, D.R.; Wolfs, T.G.; Jellema, R.K.; Zwanenburg, A.; Andriessen, P.; Delhaas, T.; Ludwig, A.K.; Radtke, S.; Peters, V.; Janssen, L.; et al. Mesenchymal Stromal Cell-Derived Extracellular Vesicles Protect the Fetal Brain After Hypoxia-Ischemia. Stem Cells Transl. Med. 2016, 5, 754–763. [Google Scholar] [CrossRef]
- Gautam, J.; Zhang, X.; Yao, Y. The role of pericytic laminin in blood brain barrier integrity maintenance. Sci. Rep. 2016, 6, 36450. [Google Scholar] [CrossRef]
- Bell, A.H.; Miller, S.L.; Castillo-Melendez, M.; Malhotra, A. The Neurovascular Unit: Effects of Brain Insults During the Perinatal Period. Front. Neurosci. 2019, 13, 1452. [Google Scholar] [CrossRef] [PubMed]
- Qian, R.G.; Glanville, R.W. Separation and characterization of two polypeptide chains from the 7S cross-linking domain of basement-membrane (type IV) collagen. Biochem. J. 1984, 222, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Bailey, A.J.; Sims, T.J.; Light, N. Cross-linking in type IV collagen. Biochem. J. 1984, 218, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Germain, S.; Monnot, C.; Muller, L.; Eichmann, A. Hypoxia-driven angiogenesis: Role of tip cells and extracellular matrix scaffolding. Curr. Opin. Hematol. 2010, 17, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Disdier, C.; Awa, F.; Chen, X.; Dhillon, S.K.; Galinsky, R.; Davidson, J.O.; Lear, C.A.; Bennet, L.; Gunn, A.J.; Stonestreet, B.S. Lipopolysaccharide-induced changes in the neurovascular unit in the preterm fetal sheep brain. J. Neuroinflamm. 2020, 17, 167. [Google Scholar] [CrossRef]
- Xiang, D.N.; Feng, Y.F.; Wang, J.; Zhang, X.; Shen, J.J.; Zou, R.; Yuan, Y.Z. Platelet-derived growth factor-BB promotes proliferation and migration of retinal microvascular pericytes by up-regulating the expression of C-X-C chemokine receptor types 4. Exp. Ther. Med. 2019, 18, 4022–4030. [Google Scholar] [CrossRef] [PubMed]
- Braun, A.; Xu, H.; Hu, F.; Kocherlakota, P.; Siegel, D.; Chander, P.; Ungvari, Z.; Csiszar, A.; Nedergaard, M.; Ballabh, P. Paucity of pericytes in germinal matrix vasculature of premature infants. J. Neurosci. 2007, 27, 12012–12024. [Google Scholar] [CrossRef]
- Lindahl, P.; Johansson, B.R.; Levéen, P.; Betsholtz, C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science 1997, 277, 242–245. [Google Scholar] [CrossRef]
- Tallquist, M.D.; French, W.J.; Soriano, P. Additive effects of PDGF receptor beta signaling pathways in vascular smooth muscle cell development. PLoS Biol. 2003, 1, E52. [Google Scholar] [CrossRef]
- Ek, C.J.; D’Angelo, B.; Baburamani, A.A.; Lehner, C.; Leverin, A.-L.; Smith, P.L.; Nilsson, H.; Svedin, P.; Hagberg, H.; Mallard, C. Brain Barrier Properties and Cerebral Blood Flow in Neonatal Mice Exposed to Cerebral Hypoxia-Ischemia. J. Cereb. Blood Flow Metab. 2015, 35, 818–827. [Google Scholar] [CrossRef]
- Ries, M.; Watts, H.; Mota, B.; Yanez Lopez, M.; Donat, C.; Baxan, N.; Pickering, J.; Chau, T.; Semmler, A.; Gurung, B. Annexin-A1 restores cerebrovascular integrity concomitant with reduced amyloid-β and tau pathology. Brain 2020, 144, 1526–1541. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, M.H.; Errede, M.; d’Amati, A.; Khan, N.Q.; Fanti, S.; Loiola, R.A.; McArthur, S.; Purvis, G.S.D.; O’Riordan, C.E.; Ferorelli, D.; et al. Impact of metabolic disorders on the structural, functional, and immunological integrity of the blood-brain barrier: Therapeutic avenues. FASEB J. 2022, 36, e22107. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.-C.; Chang, Y.-C.; Lin, Y.-C.; Sze, C.-I.; Huang, C.-C.; Ho, C.-J. Cerebral Microvascular Damage Occurs Early after Hypoxia–Ischemia via nNOS Activation in the Neonatal Brain. J. Cereb. Blood Flow Metab. 2014, 34, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Gautam, J.; Miner, J.H.; Yao, Y. Loss of Endothelial Laminin α5 Exacerbates Hemorrhagic Brain Injury. Transl. Stroke Res. 2019, 10, 705–718. [Google Scholar] [CrossRef] [PubMed]
- Knowland, D.; Arac, A.; Sekiguchi, K.J.; Hsu, M.; Lutz, S.E.; Perrino, J.; Steinberg, G.K.; Barres, B.A.; Nimmerjahn, A.; Agalliu, D. Stepwise recruitment of transcellular and paracellular pathways underlies blood-brain barrier breakdown in stroke. Neuron 2014, 82, 603–617. [Google Scholar] [CrossRef] [PubMed]
- Steiner, E.; Enzmann, G.U.; Lin, S.; Ghavampour, S.; Hannocks, M.-J.; Zuber, B.; Rüegg, M.A.; Sorokin, L.; Engelhardt, B. Loss of astrocyte polarization upon transient focal brain ischemia as a possible mechanism to counteract early edema formation. Glia 2012, 60, 1646–1659. [Google Scholar] [CrossRef]
- Zapata-Acevedo, J.F.; García-Pérez, V.; Cabezas-Pérez, R.; Losada-Barragán, M.; Vargas-Sánchez, K.; González-Reyes, R.E. Laminin as a Biomarker of Blood-Brain Barrier Disruption under Neuroinflammation: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 6788. [Google Scholar] [CrossRef]
- Leonard, M.O.; Godson, C.; Brady, H.R.; Taylor, C.T. Potentiation of Glucocorticoid Activity in Hypoxia through Induction of the Glucocorticoid Receptor1. J. Immunol. 2005, 174, 2250–2257. [Google Scholar] [CrossRef]
- Sawmynaden, P.; Perretti, M. Glucocorticoid upregulation of the annexin-A1 receptor in leukocytes. Biochem. Biophys. Res. Commun. 2006, 349, 1351–1355. [Google Scholar] [CrossRef]
- Perretti, M.; D’Acquisto, F. Annexin A1 and glucocorticoids as effectors of the resolution of inflammation. Nat. Rev. Immunol. 2009, 9, 62–70. [Google Scholar] [CrossRef]
- Rhen, T.; Cidlowski, J.A. Antiinflammatory action of glucocorticoids—New mechanisms for old drugs. N. Engl. J. Med. 2005, 353, 1711–1723. [Google Scholar] [CrossRef] [PubMed]
- Parente, L.; Solito, E. Annexin 1: More than an anti-phospholipase protein. Inflamm. Res. 2004, 53, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Wu, M.-F.; Zhu, L.-H.; Qiao, L.-X.; Zhao, R.-B.; Xia, Z.-K. Long non-coding RNA Snhg3 protects against hypoxia/ischemia-induced neonatal brain injury. Exp. Mol. Pathol. 2020, 112, 104343. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Teijeiro, S.; Menéndez, S.T.; Villaronga, M.Á.; Pena-Alonso, E.; Rodrigo, J.P.; Morgan, R.O.; Granda-Díaz, R.; Salom, C.; Fernandez, M.P.; García-Pedrero, J.M. Annexin A1 down-regulation in head and neck squamous cell carcinoma is mediated via transcriptional control with direct involvement of miR-196a/b. Sci. Rep. 2017, 7, 6790. [Google Scholar] [CrossRef]
- Kalluri, R. Basement membranes: Structure, assembly and role in tumour angiogenesis. Nat. Rev. Cancer 2003, 3, 422–433. [Google Scholar] [CrossRef]
- Timpl, R.; Fujiwara, S.; Dziadek, M.; Aumailley, M.; Weber, S.; Engel, J. Laminin, proteoglycan, nidogen and collagen IV: Structural models and molecular interactions. Ciba Found Symp. 1984, 108, 25–43. [Google Scholar] [CrossRef]
- Ancsin, J.B.; Kisilevsky, R. Laminin interactions important for basement membrane assembly are promoted by zinc and implicate laminin zinc finger-like sequences. J. Biol. Chem. 1996, 271, 6845–6851. [Google Scholar] [CrossRef]
- Kangwantas, K.; Pinteaux, E.; Penny, J. The extracellular matrix protein laminin-10 promotes blood–brain barrier repair after hypoxia and inflammation in vitro. J. Neuroinflamm. 2016, 13, 25. [Google Scholar] [CrossRef]
- Ulbrich, P.; Khoshneviszadeh, M.; Jandke, S.; Schreiber, S.; Dityatev, A. Interplay between perivascular and perineuronal extracellular matrix remodelling in neurological and psychiatric diseases. Eur. J. Neurosci. 2021, 53, 3811–3830. [Google Scholar] [CrossRef]
- Thomsen, M.S.; Routhe, L.J.; Moos, T. The vascular basement membrane in the healthy and pathological brain. J. Cereb. Blood Flow Metab. 2017, 37, 3300–3317. [Google Scholar] [CrossRef]
- Bergers, G.; Song, S. The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol. 2005, 7, 452–464. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.N.; Reynell, C.; Gesslein, B.; Hamilton, N.B.; Mishra, A.; Sutherland, B.A.; O’Farrell, F.M.; Buchan, A.M.; Lauritzen, M.; Attwell, D. Capillary pericytes regulate cerebral blood flow in health and disease. Nature 2014, 508, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Armulik, A.; Abramsson, A.; Betsholtz, C. Endothelial/Pericyte Interactions. Circ. Res. 2005, 97, 512–523. [Google Scholar] [CrossRef]
- Gautam, J.; Cao, Y.; Yao, Y. Pericytic Laminin Maintains Blood-Brain Barrier Integrity in an Age-Dependent Manner. Transl. Stroke Res. 2020, 11, 228–242. [Google Scholar] [CrossRef]
- Zhou, Y.-F.; Li, P.-C.; Wu, J.-H.; Haslam, J.A.; Mao, L.; Xia, Y.-P.; He, Q.-W.; Wang, X.-X.; Lei, H.; Lan, X.-L. Sema3E/PlexinD1 inhibition is a therapeutic strategy for improving cerebral perfusion and restoring functional loss after stroke in aged rats. Neurobiol. Aging 2018, 70, 102–116. [Google Scholar] [CrossRef] [PubMed]
- Di, Z.; Wu, X.; Xie, W.; Lin, X. Effect of Pericytes on Cerebral Microvasculature at Different Time Points of Stroke. Biomed Res. Int. 2021, 2021, 5281182. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, B.T.; Davis, T.P. The blood-brain barrier/neurovascular unit in health and disease. Pharm. Rev. 2005, 57, 173–185. [Google Scholar] [CrossRef]
- Franco, M.; Roswall, P.; Cortez, E.; Hanahan, D.; Pietras, K. Pericytes promote endothelial cell survival through induction of autocrine VEGF-A signaling and Bcl-w expression. Blood 2011, 118, 2906–2917. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Ayyadurai, S.; Zlokovic, B.V. Pericytes of the neurovascular unit: Key functions and signaling pathways. Nat. Neurosci. 2016, 19, 771–783. [Google Scholar] [CrossRef]
- Winkler, E.A.; Bell, R.D.; Zlokovic, B.V. Central nervous system pericytes in health and disease. Nat. Neurosci. 2011, 14, 1398–1405. [Google Scholar] [CrossRef]
- Xu, L.; Nirwane, A.; Yao, Y. Basement membrane and blood-brain barrier. Stroke Vasc. Neurol. 2019, 4, 78–82. [Google Scholar] [CrossRef]
- Tilling, T.; Korte, D.; Hoheisel, D.; Galla, H.J. Basement membrane proteins influence brain capillary endothelial barrier function in vitro. J. Neurochem. 1998, 71, 1151–1157. [Google Scholar] [CrossRef]
- Savettieri, G.; Di Liegro, I.; Catania, C.; Licata, L.; Pitarresi, G.L.; D’Agostino, S.; Schiera, G.; De Caro, V.; Giandalia, G.; Giannola, L.I.; et al. Neurons and ECM regulate occludin localization in brain endothelial cells. Neuroreport 2000, 11, 1081–1084. [Google Scholar] [CrossRef]
- Stanimirovic, D.B.; Friedman, A. Pathophysiology of the neurovascular unit: Disease cause or consequence? J. Cereb. Blood Flow Metab. 2012, 32, 1207–1221. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Zhang, J.; Fang, J.; Li, M.; Ding, H.; Zhang, W.; Chen, C. Dynamic inflammatory changes of the neurovascular units after ischemic stroke. Brain Res. Bull. 2022, 190, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Han, G.Y.; Song, L.; Ding, Z.; Wang, Q.; Yan, Y.; Huang, J.; Ma, C. The important double-edged role of astrocytes in neurovascular unit after ischemic stroke. Front. Aging Neurosci. 2022, 14, 196. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Song, Y.; He, T.; Wen, R.; Li, Y.; Chen, T.; Huang, S.; Wang, Y.; Tang, Y.; Shen, F. M2 microglial small extracellular vesicles reduce glial scar formation via the miR-124/STAT3 pathway after ischemic stroke in mice. Theranostics 2021, 11, 1232. [Google Scholar] [CrossRef]
- Baburamani, A.A.; Lo, C.; Castillo-Melendez, M.; Walker, D.W. Morphological evaluation of the cerebral blood vessels in the late gestation fetal sheep following hypoxia in utero. Microvasc. Res. 2013, 85, 1–9. [Google Scholar] [CrossRef]
- Nirwane, A.; Yao, Y. Cell-specific expression and function of laminin at the neurovascular unit. J. Cereb. Blood Flow Metab. 2022, 42, 1979–1999. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.Y.; van Bruggen, V.L.E.; Peutz-Kootstra, C.J.; Ophelders, D.R.M.G.; Jellema, R.K.; Reutelingsperger, C.P.M.; Rutten, B.P.F.; Wolfs, T.G.A.M. Time Dependent Changes in the Ovine Neurovascular Unit; A Potential Neuroprotective Role of Annexin A1 in Neonatal Hypoxic-Ischemic Encephalopathy. Int. J. Mol. Sci. 2023, 24, 5929. https://doi.org/10.3390/ijms24065929
Park HY, van Bruggen VLE, Peutz-Kootstra CJ, Ophelders DRMG, Jellema RK, Reutelingsperger CPM, Rutten BPF, Wolfs TGAM. Time Dependent Changes in the Ovine Neurovascular Unit; A Potential Neuroprotective Role of Annexin A1 in Neonatal Hypoxic-Ischemic Encephalopathy. International Journal of Molecular Sciences. 2023; 24(6):5929. https://doi.org/10.3390/ijms24065929
Chicago/Turabian StylePark, Hyun Young, Valéry L. E. van Bruggen, Carine J. Peutz-Kootstra, Daan R. M. G. Ophelders, Reint K. Jellema, Chris P. M. Reutelingsperger, Bart P. F. Rutten, and Tim G. A. M. Wolfs. 2023. "Time Dependent Changes in the Ovine Neurovascular Unit; A Potential Neuroprotective Role of Annexin A1 in Neonatal Hypoxic-Ischemic Encephalopathy" International Journal of Molecular Sciences 24, no. 6: 5929. https://doi.org/10.3390/ijms24065929
APA StylePark, H. Y., van Bruggen, V. L. E., Peutz-Kootstra, C. J., Ophelders, D. R. M. G., Jellema, R. K., Reutelingsperger, C. P. M., Rutten, B. P. F., & Wolfs, T. G. A. M. (2023). Time Dependent Changes in the Ovine Neurovascular Unit; A Potential Neuroprotective Role of Annexin A1 in Neonatal Hypoxic-Ischemic Encephalopathy. International Journal of Molecular Sciences, 24(6), 5929. https://doi.org/10.3390/ijms24065929






