Blockade of ß-Adrenergic Receptors by Nebivolol Enables Tumor Control Potential for Uveal Melanoma in 3D Tumor Spheroids and 2D Cultures
Abstract
1. Introduction
2. Results
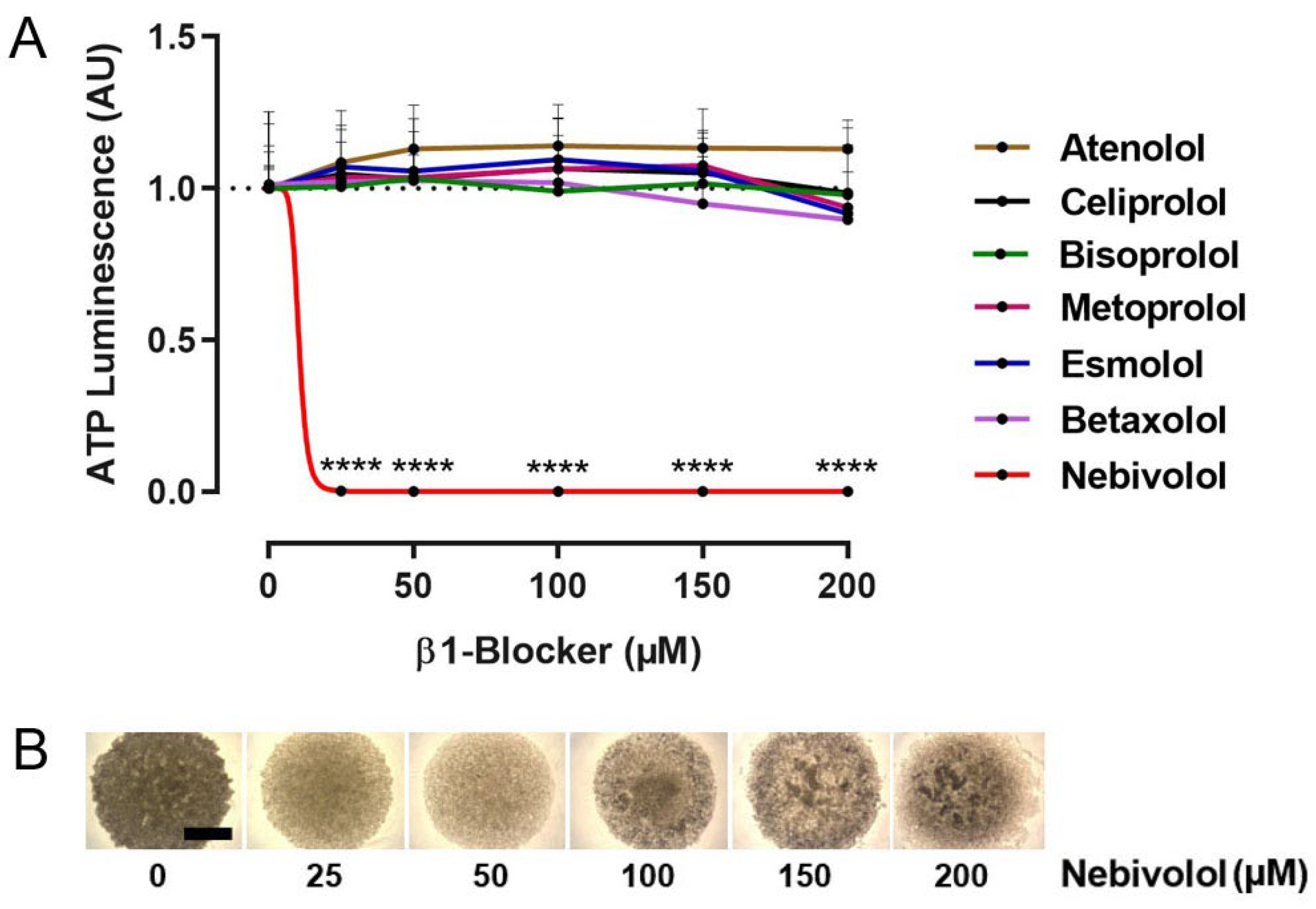
2.1. Identification of Nebivolol as a Potent Anti-Tumor β1-Blocker
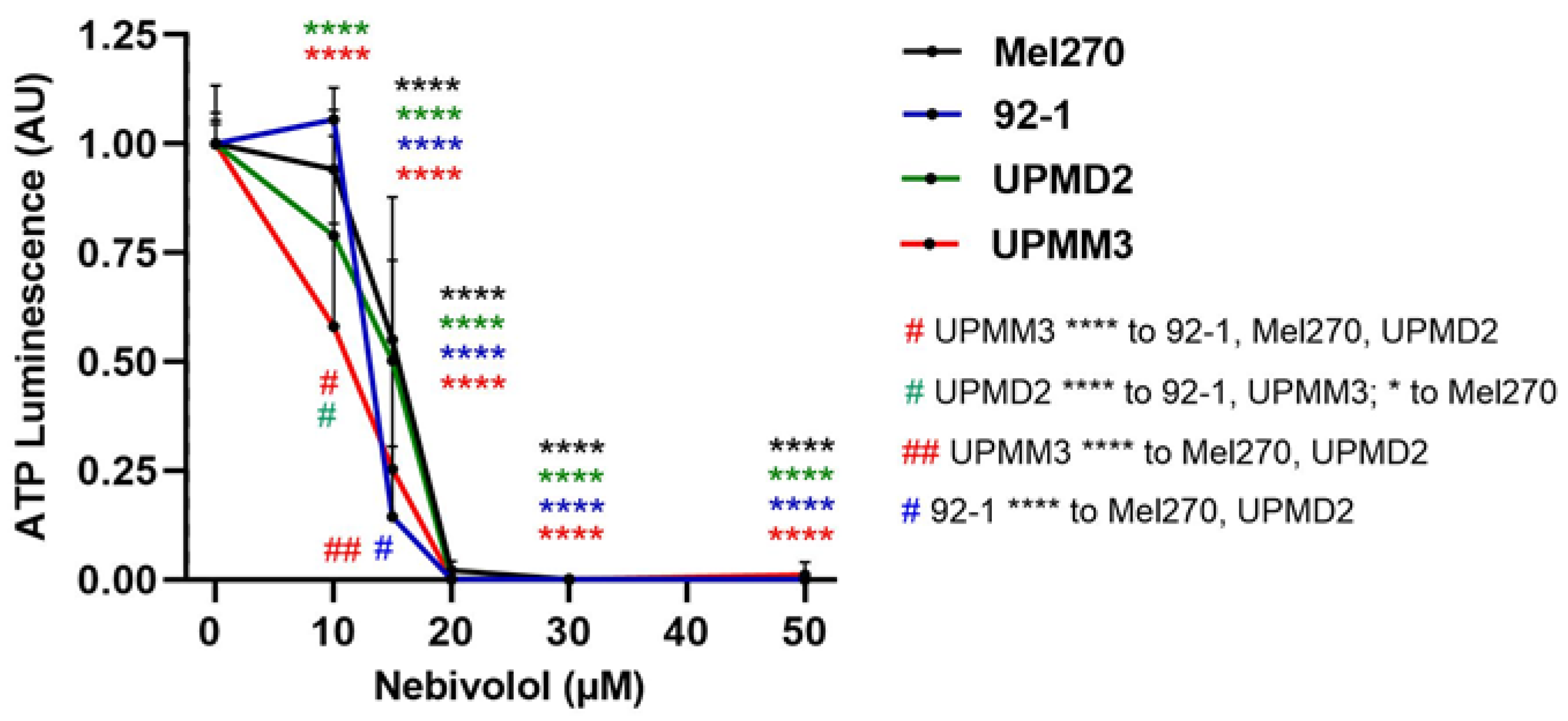
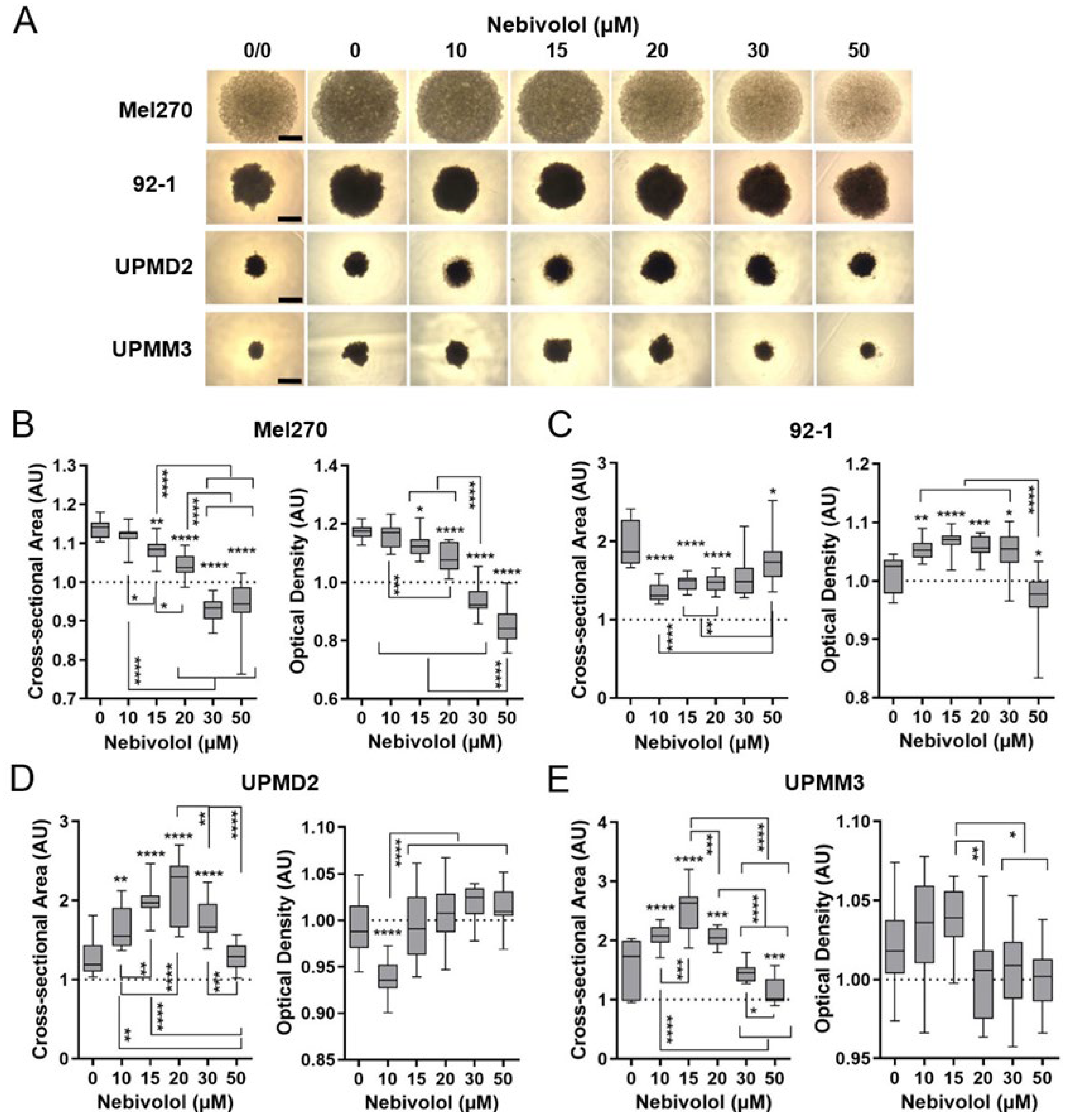
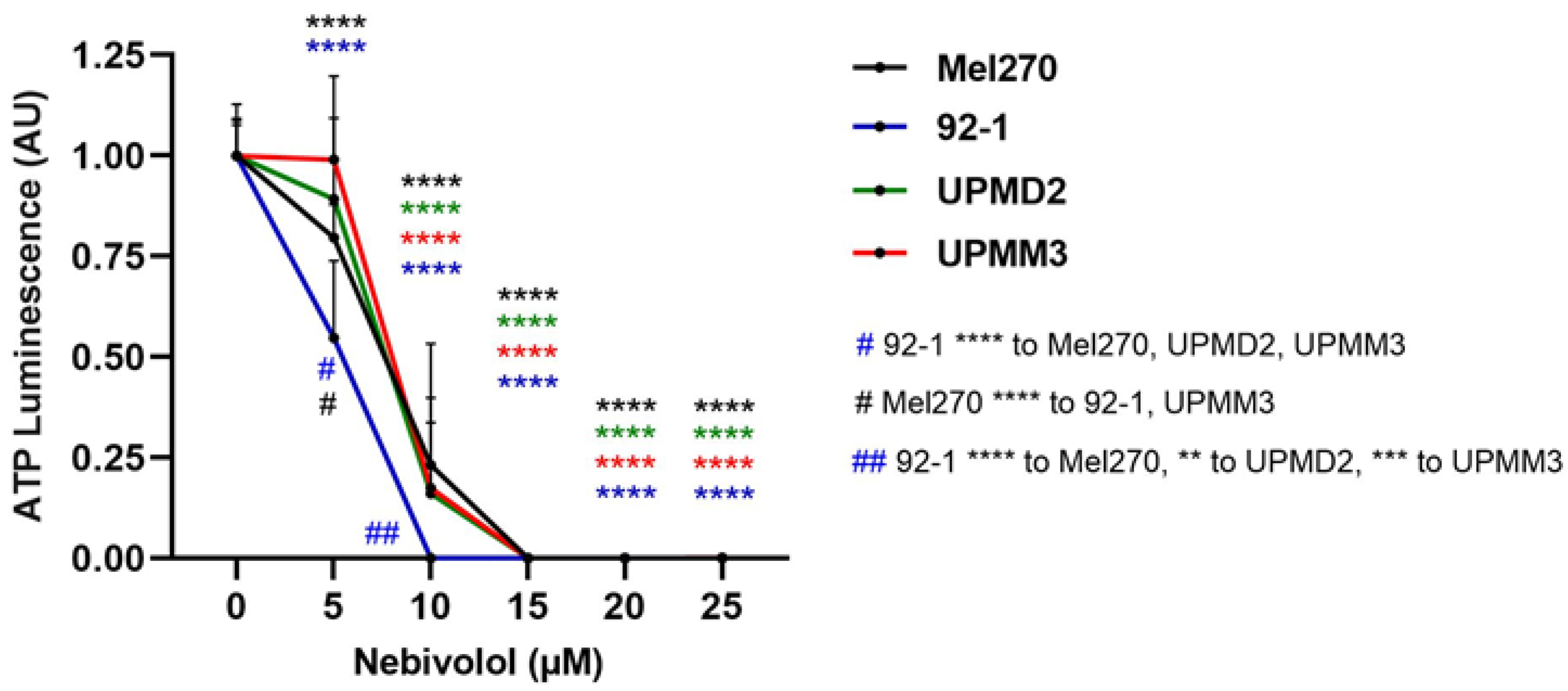
2.2. Comparison of Nebivolol Anti-Tumor Responses of Various 3D Tumor Spheroids
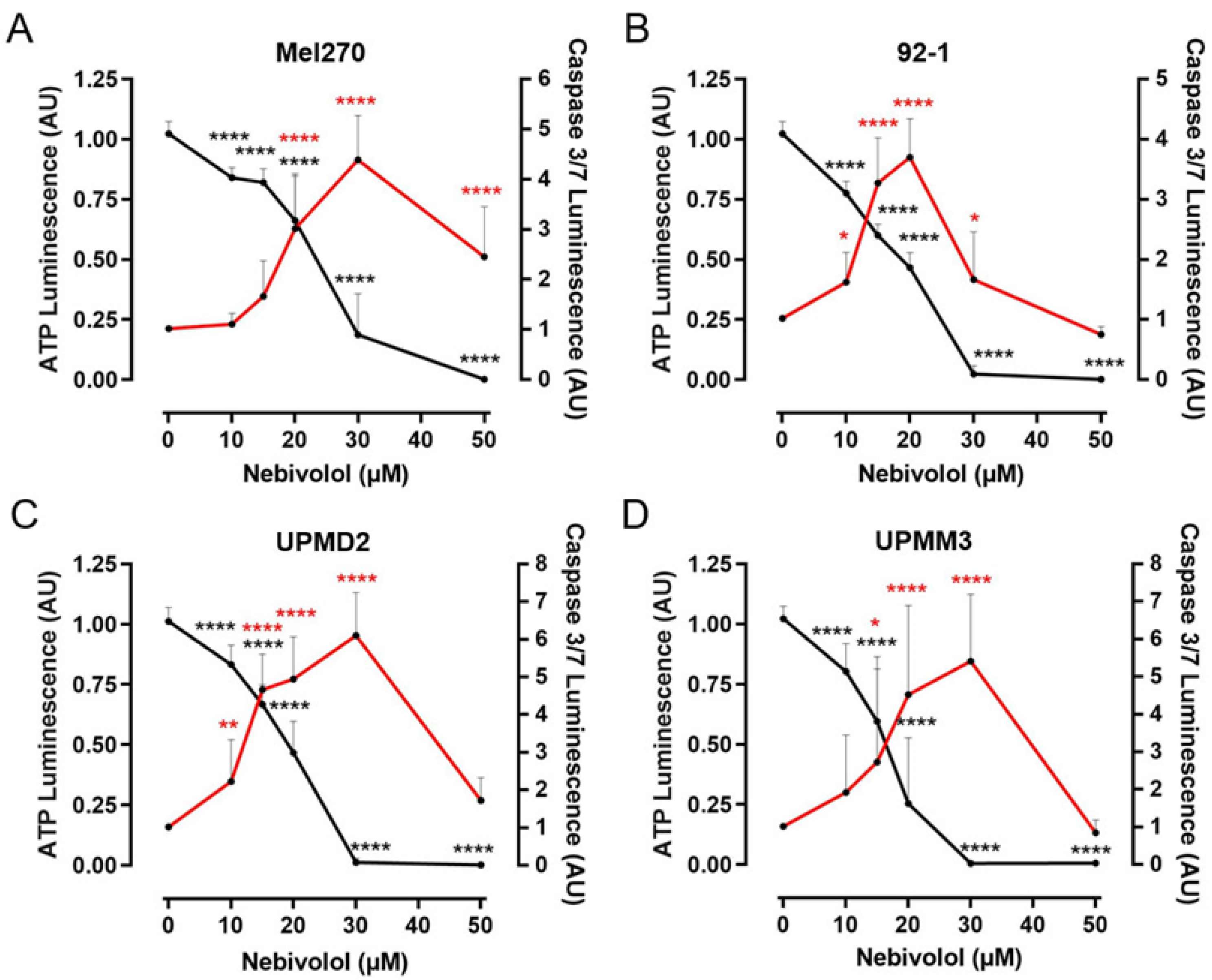
2.3. Comparison of Nebivolol Apoptotic Effects on Various 3D Tumor Spheroids
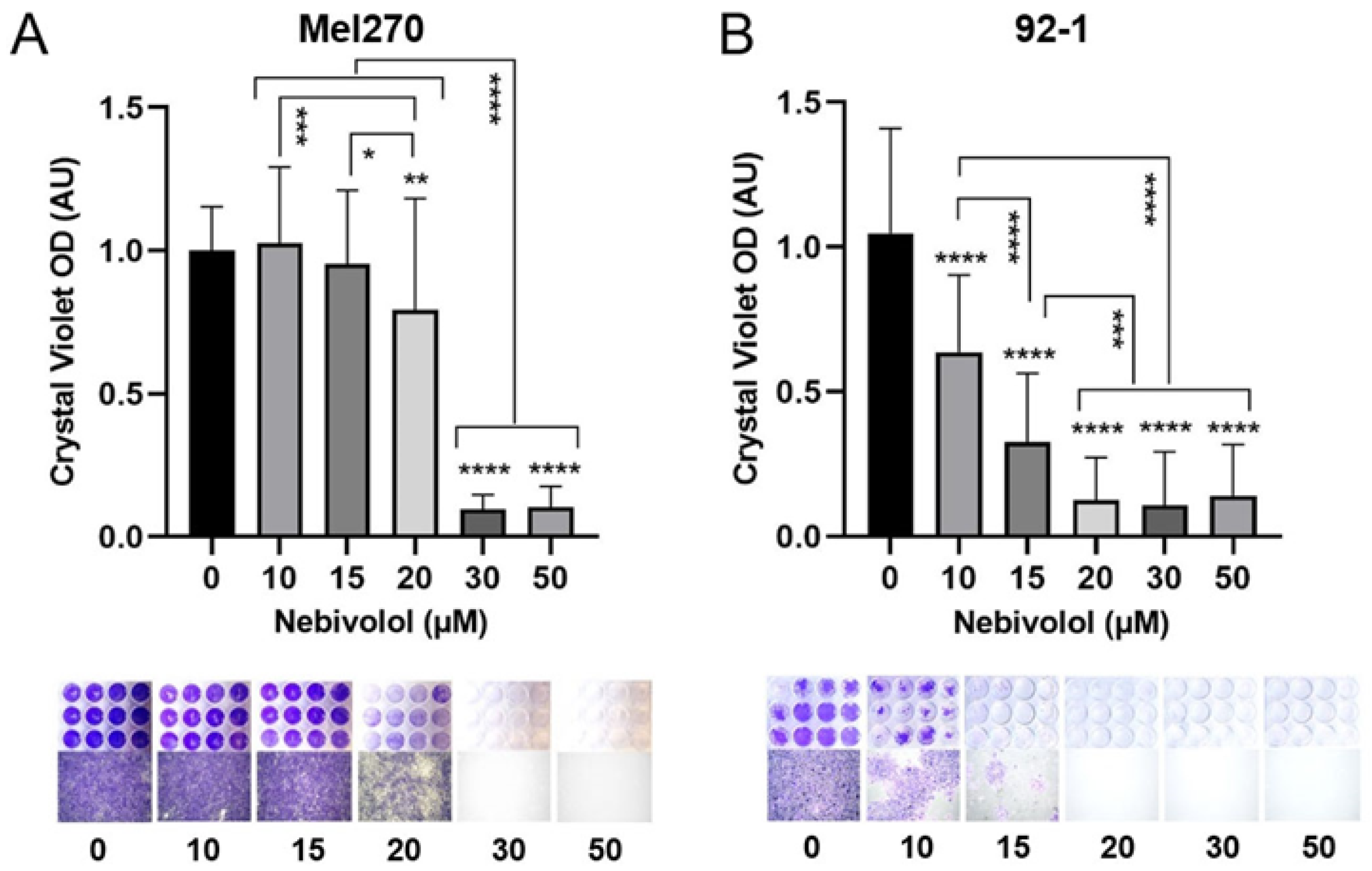
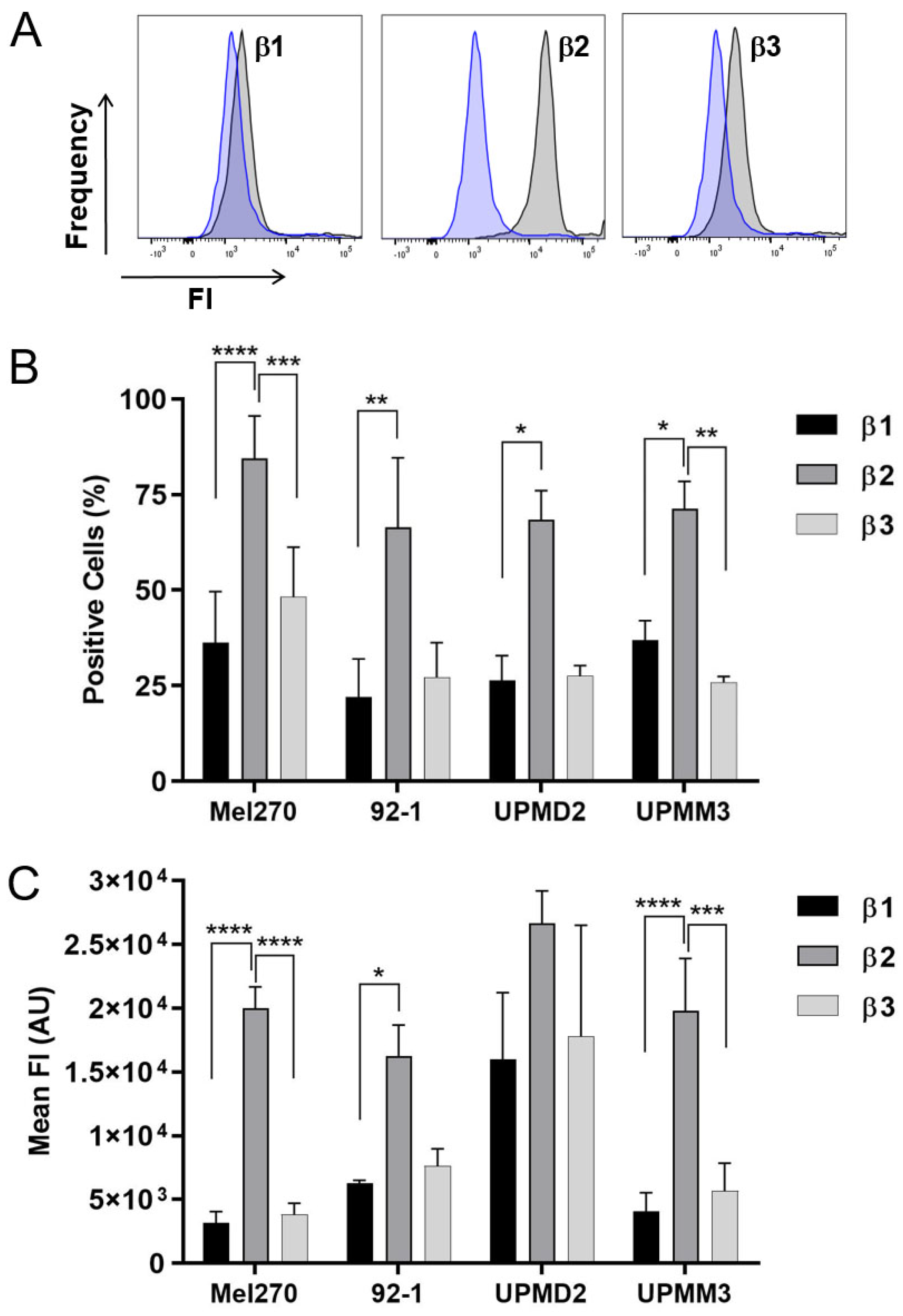
2.4. Anti-Tumor Responses of Various 2D Cell Lines and Adrenergic Receptor Levels
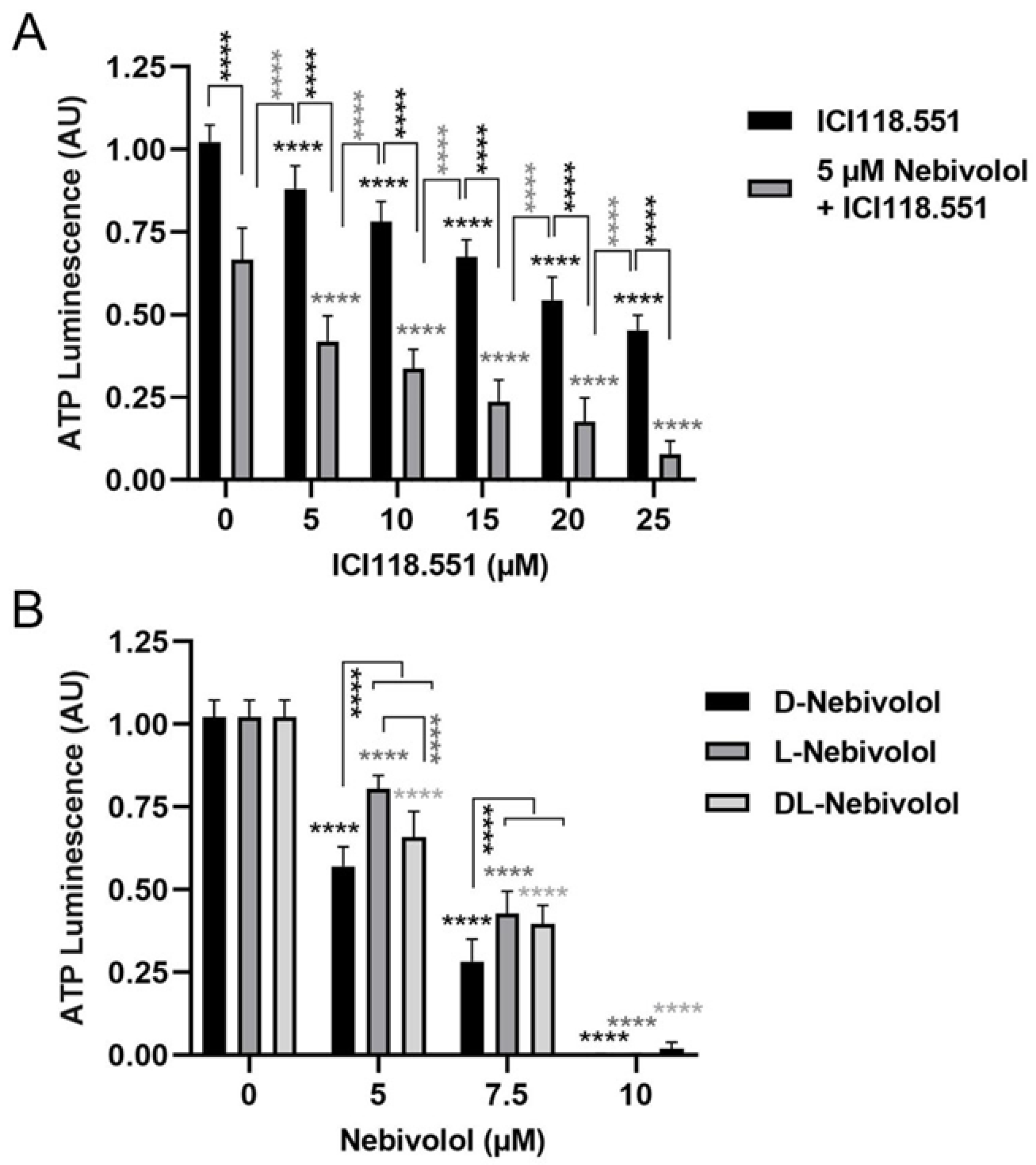
2.5. Effects of a Highly Selective ß2-Antagonist and Discrimination between the Different Nebivolol Enantiomers
3. Discussion
4. Materials and Methods
4.1. Characteristics of Cell Lines
4.2. Culture of Cell Lines and 3D Tumor Spheroids
4.3. Treatment with β-Blockers
4.4. 3D Spheroid Viability Measurement
4.5. Analysis of 3D Spheroid Apoptosis
4.6. Morphology, Growth, and Density Determination of 3D Spheroids
4.7. Long-Term Spheroid Cell Survival Assay
4.8. Cell Viability Analysis in 2D Cell Cultures
4.9. Detection of β-Adrenergic Receptors by Flow Cytometry
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Do Vale, G.T.; Ceron, C.S.; Gonzaga, N.A.; Simplicio, J.A.; Padovan, J.C. Three Generations of beta-blockers: History, Class Differences and Clinical Applicability. Curr. Hypertens. Rev. 2019, 15, 22–31. [Google Scholar] [CrossRef]
- Cole, S.W.; Sood, A.K. Molecular pathways: Beta-adrenergic signaling in cancer. Clin. Cancer Res. 2012, 18, 1201–1206. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Li, Z.; Lu, L.; Cho, C.H. beta-Adrenergic system, a backstage manipulator regulating tumour progression and drug target in cancer therapy. Semin. Cancer Biol. 2013, 23, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Armaiz-Pena, G.N.; Gonzalez-Villasana, V.; Nagaraja, A.S.; Rodriguez-Aguayo, C.; Sadaoui, N.C.; Stone, R.L.; Matsuo, K.; Dalton, H.J.; Previs, R.A.; Jennings, N.B.; et al. Adrenergic regulation of monocyte chemotactic protein 1 leads to enhanced macrophage recruitment and ovarian carcinoma growth. Oncotarget 2015, 6, 4266–4273. [Google Scholar] [CrossRef] [PubMed]
- Dal Monte, M.; Calvani, M.; Cammalleri, M.; Favre, C.; Filippi, L.; Bagnoli, P. beta-Adrenoceptors as drug targets in melanoma: Novel preclinical evidence for a role of β3-adrenoceptors. Br. J. Pharmacol. 2019, 176, 2496–2508. [Google Scholar] [CrossRef]
- Powe, D.G.; Voss, M.J.; Zanker, K.S.; Habashy, H.O.; Green, A.R.; Ellis, I.O.; Entschladen, F. Beta-blocker drug therapy reduces secondary cancer formation in breast cancer and improves cancer specific survival. Oncotarget 2010, 1, 628–638. [Google Scholar] [CrossRef]
- Rouhana, H.F.; Fruge, J.H.; Massengale, W.T. Beta blocker treatment for infantile hemangiomas. Dermatol. Online J. 2015, 21, 2. [Google Scholar] [CrossRef]
- Fumagalli, C.; Maurizi, N.; Marchionni, N.; Fornasari, D. beta-blockers: Their new life from hypertension to cancer and migraine. Pharmacol. Res. 2020, 151, 104587. [Google Scholar] [CrossRef]
- Frishman, W.H.; Saunders, E. beta-Adrenergic blockers. J. Clin. Hypertens. 2011, 13, 649–653. [Google Scholar] [CrossRef]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019, 18, 41–58. [Google Scholar] [CrossRef]
- Abdulkareem, N.M.; Bhat, R.; Powell, R.T.; Chikermane, S.; Yande, S.; Trinh, L.; Abdelnasser, H.Y.; Tabassum, M.; Ruiz, A.; Sobieski, M.; et al. Screening of GPCR drugs for repurposing in breast cancer. Front. Pharmacol. 2022, 13, 1049640. [Google Scholar] [CrossRef]
- Reiter, M.J. Cardiovascular drug class specificity: Beta-blockers. Prog. Cardiovasc. Dis. 2004, 47, 11–33. [Google Scholar] [CrossRef]
- Oliver, E.; Mayor, F., Jr.; D’Ocon, P. Beta-blockers: Historical Perspective and Mechanisms of Action. Rev. Esp. Cardiol. 2019, 72, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Van de Water, A.; Janssens, W.; Van Neuten, J.; Xhonneux, R.; De Cree, J.; Verhaegen, H.; Reneman, R.S.; Janssen, P.A. Pharmacological and hemodynamic profile of nebivolol, a chemically novel, potent, and selective beta 1-adrenergic antagonist. J. Cardiovasc. Pharmacol. 1988, 11, 552–563. [Google Scholar] [CrossRef]
- Cazzola, M.; Matera, M.G.; Ruggeri, P.; Sanduzzi, A.; Spicuzza, L.; Vatrella, A.; Girbino, G. Comparative effects of a two-week treatment with nebivolol and nifedipine in hypertensive patients suffering from COPD. Respiration 2004, 71, 159–164. [Google Scholar] [CrossRef]
- Wojciechowski, D.; Papademetriou, V. Beta-blockers in the management of hypertension: Focus on nebivolol. Expert Rev. Cardiovasc. Ther. 2008, 6, 471–479. [Google Scholar] [CrossRef]
- Munzel, T.; Gori, T. Nebivolol: The somewhat-different beta-adrenergic receptor blocker. J. Am. Coll. Cardiol. 2009, 54, 1491–1499. [Google Scholar] [CrossRef]
- Fonseca, V.A. Effects of beta-blockers on glucose and lipid metabolism. Curr. Med. Res. Opin. 2010, 26, 615–629. [Google Scholar] [CrossRef] [PubMed]
- Fongemie, J.; Felix-Getzik, E. A Review of Nebivolol Pharmacology and Clinical Evidence. Drugs 2015, 75, 1349–1371. [Google Scholar] [CrossRef] [PubMed]
- Traynor, K. New beta-blocker gets FDA approval. Am. J. Health Syst. Pharm. 2008, 65, 190. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.S.; Nagao, T.; Bond, R.A.; Janssens, W.J.; Vanhoutte, P.M. Nebivolol induces endothelium-dependent relaxations of canine coronary arteries. J. Cardiovasc. Pharmacol. 1991, 17, 964–969. [Google Scholar] [CrossRef] [PubMed]
- Ignarro, L.J. Different pharmacological properties of two enantiomers in a unique beta-blocker, nebivolol. Cardiovasc. Ther. 2008, 26, 115–134. [Google Scholar] [CrossRef] [PubMed]
- Niu, M.; Xu, J.; Liu, Y.; Li, Y.; He, T.; Ding, L.; He, Y.; Yi, Y.; Li, F.; Guo, R.; et al. FBXL2 counteracts Grp94 to destabilize EGFR and inhibit EGFR-driven NSCLC growth. Nat. Commun. 2021, 12, 5919. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Jiang, H.; Wang, Z.; Cai, L.Y.; Jiang, Y.C.; Xie, L.; Zhou, Y.; Zeng, X.; Ji, N.; Shen, Y.Q.; et al. Adrenergic Blockade by Nebivolol to Suppress Oral Squamous Cell Carcinoma Growth via Endoplasmic Reticulum Stress and Mitochondria Dysfunction. Front. Pharmacol. 2021, 12, 691998. [Google Scholar] [CrossRef]
- Kaliki, S.; Shields, C.L. Uveal melanoma: Relatively rare but deadly cancer. Eye 2017, 31, 241–257. [Google Scholar] [CrossRef] [PubMed]
- Naseripoor, M.; Azimi, F.; Mirshahi, R.; Khakpoor, G.; Poorhosseingholi, A.; Chaibakhsh, S. Global Incidence and Trend of Uveal Melanoma from 1943–2015: A Meta-Analysis. Asian Pac. J. Cancer Prev. 2022, 23, 1791–1801. [Google Scholar] [CrossRef]
- Chattopadhyay, C.; Kim, D.W.; Gombos, D.S.; Oba, J.; Qin, Y.; Williams, M.D.; Esmaeli, B.; Grimm, E.A.; Wargo, J.A.; Woodman, S.E.; et al. Uveal melanoma: From diagnosis to treatment and the science in between. Cancer 2016, 122, 2299–2312. [Google Scholar] [CrossRef]
- Jager, M.J.; Shields, C.L.; Cebulla, C.M.; Abdel-Rahman, M.H.; Grossniklaus, H.E.; Stern, M.H.; Carvajal, R.D.; Belfort, R.N.; Jia, R.; Shields, J.A.; et al. Uveal melanoma. Nat. Rev. Dis. Primers 2020, 6, 24. [Google Scholar] [CrossRef]
- Rossi, E.; Croce, M.; Reggiani, F.; Schinzari, G.; Ambrosio, M.; Gangemi, R.; Tortora, G.; Pfeffer, U.; Amaro, A. Uveal Melanoma Metastasis. Cancers 2021, 13, 5684. [Google Scholar] [CrossRef]
- Staby, K.M.; Gravdal, K.; Mork, S.J.; Heegaard, S.; Vintermyr, O.K.; Krohn, J. Prognostic impact of chromosomal aberrations and GNAQ, GNA11 and BAP1 mutations in uveal melanoma. Acta Ophthalmol. 2018, 96, 31–38. [Google Scholar] [CrossRef]
- Kujala, E.; Makitie, T.; Kivela, T. Very long-term prognosis of patients with malignant uveal melanoma. Investig. Ophthalmol. Vis. Sci. 2003, 44, 4651–4659. [Google Scholar] [CrossRef]
- Li, Y.; Shi, J.; Yang, J.; Ge, S.; Zhang, J.; Jia, R.; Fan, X. Uveal melanoma: Progress in molecular biology and therapeutics. Ther. Adv. Med. Oncol. 2020, 12, 1758835920965852. [Google Scholar] [CrossRef]
- Wei, A.Z.; Maniar, A.B.; Carvajal, R.D. New targeted and epigenetic therapeutic strategies for the treatment of uveal melanoma. Cancer Gene Ther. 2022, 29, 1819–1826. [Google Scholar] [CrossRef]
- Van der Kooij, M.K.; Speetjens, F.M.; van der Burg, S.H.; Kapiteijn, E. Uveal Versus Cutaneous Melanoma; Same Origin, Very Distinct Tumor Types. Cancers 2019, 11, 845. [Google Scholar] [CrossRef] [PubMed]
- Van Raamsdonk, C.D.; Griewank, K.G.; Crosby, M.B.; Garrido, M.C.; Vemula, S.; Wiesner, T.; Obenauf, A.C.; Wackernagel, W.; Green, G.; Bouvier, N.; et al. Mutations in GNA11 in uveal melanoma. N. Engl. J. Med. 2010, 363, 2191–2199. [Google Scholar] [CrossRef] [PubMed]
- Prescher, G.; Bornfeld, N.; Horsthemke, B.; Becher, R. Chromosomal aberrations defining uveal melanoma of poor prognosis. Lancet 1992, 339, 691–692. [Google Scholar] [CrossRef] [PubMed]
- Prescher, G.; Bornfeld, N.; Hirche, H.; Horsthemke, B.; Jockel, K.H.; Becher, R. Prognostic implications of monosomy 3 in uveal melanoma. Lancet 1996, 347, 1222–1225. [Google Scholar] [CrossRef] [PubMed]
- Harbour, J.W.; Onken, M.D.; Roberson, E.D.; Duan, S.; Cao, L.; Worley, L.A.; Council, M.L.; Matatall, K.A.; Helms, C.; Bowcock, A.M. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science 2010, 330, 1410–1413. [Google Scholar] [CrossRef]
- Hamadeh, F.; Medina, C.A.; Singh, A.D.; Reynolds, J.P.; Biscotti, C.V. Uveal melanoma: An analysis of cellular features and comparison to monosomy 3 status. Diagn. Cytopathol. 2016, 44, 377–383. [Google Scholar] [CrossRef]
- Bustamante, P.; Miyamoto, D.; Goyeneche, A.; de Alba Graue, P.G.; Jin, E.; Tsering, T.; Dias, A.B.; Burnier, M.N.; Burnier, J.V. Beta-blockers exert potent anti-tumor effects in cutaneous and uveal melanoma. Cancer Med. 2019, 8, 7265–7277. [Google Scholar] [CrossRef]
- Farhoumand, L.S.; Fiorentzis, M.; Kraemer, M.M.; Sak, A.; Stuschke, M.; Rassaf, T.; Hendgen-Cotta, U.; Bechrakis, N.E.; Berchner-Pfannschmidt, U. The Adrenergic Receptor Antagonist Carvedilol Elicits Anti-Tumor Responses in Uveal Melanoma 3D Tumor Spheroids and May Serve as Co-Adjuvant Therapy with Radiation. Cancers 2022, 14, 3097. [Google Scholar] [CrossRef] [PubMed]
- Fiorentzis, M.; Sokolenko, E.A.; Bechrakis, N.E.; Ting, S.; Schmid, K.W.; Sak, A.; Stuschke, M.; Seitz, B.; Berchner-Pfannschmidt, U. Electrochemotherapy with Bleomycin Enhances Radiosensitivity of Uveal Melanomas: First In Vitro Results in 3D Cultures of Primary Uveal Melanoma Cell Lines. Cancers 2021, 13, 3086. [Google Scholar] [CrossRef] [PubMed]
- Jager, M.J.; Magner, J.A.; Ksander, B.R.; Dubovy, S.R. Uveal Melanoma Cell Lines: Where do they come from? (An American Ophthalmological Society Thesis). Trans. Am. Ophthalmol. Soc. 2016, 114, T5. [Google Scholar] [PubMed]
- Pauwels, P.J.; Gommeren, W.; Van Lommen, G.; Janssen, P.A.; Leysen, J.E. The receptor binding profile of the new antihypertensive agent nebivolol and its stereoisomers compared with various beta-adrenergic blockers. Mol. Pharmacol. 1988, 34, 843–851. [Google Scholar] [PubMed]
- Desoize, B.; Gimonet, D.; Jardiller, J.C. Cell culture as spheroids: An approach to multicellular resistance. Anticancer Res. 1998, 18, 4147–4158. [Google Scholar] [PubMed]
- Kunz-Schughart, L.A.; Freyer, J.P.; Hofstaedter, F.; Ebner, R. The use of 3-D cultures for high-throughput screening: The multicellular spheroid model. J. Biomol. Screen. 2004, 9, 273–285. [Google Scholar] [CrossRef]
- Lin, R.Z.; Chang, H.Y. Recent advances in three-dimensional multicellular spheroid culture for biomedical research. Biotechnol. J. 2008, 3, 1172–1184. [Google Scholar] [CrossRef]
- Carvalho, M.P.; Costa, E.C.; Miguel, S.P.; Correia, I.J. Tumor spheroid assembly on hyaluronic acid-based structures: A review. Carbohydr. Polym. 2016, 150, 139–148. [Google Scholar] [CrossRef]
- Mergener, S.; Siveke, J.T.; Pena-Llopis, S. Monosomy 3 Is Linked to Resistance to MEK Inhibitors in Uveal Melanoma. Int. J. Mol. Sci. 2021, 22, 6727. [Google Scholar] [CrossRef]
- Nuevo-Tapioles, C.; Santacatterina, F.; Stamatakis, K.; Nunez de Arenas, C.; Gomez de Cedron, M.; Formentini, L.; Cuezva, J.M. Coordinate beta-adrenergic inhibition of mitochondrial activity and angiogenesis arrest tumor growth. Nat. Commun. 2020, 11, 3606. [Google Scholar] [CrossRef]
- Slee, E.A.; Harte, M.T.; Kluck, R.M.; Wolf, B.B.; Casiano, C.A.; Newmeyer, D.D.; Wang, H.G.; Reed, J.C.; Nicholson, D.W.; Alnemri, E.S.; et al. Ordering the cytochrome c-initiated caspase cascade: Hierarchical activation of caspases-2, -3, -6, -7, -8, and -10 in a caspase-9-dependent manner. J. Cell Biol. 1999, 144, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Brentnall, M.; Rodriguez-Menocal, L.; De Guevara, R.L.; Cepero, E.; Boise, L.H. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol. 2013, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Soni, I.V.; Hardy, J.A. Caspase-9 Activation of Procaspase-3 but Not Procaspase-6 Is Based on the Local Context of Cleavage Site Motifs and on Sequence. Biochemistry 2021, 60, 2824–2835. [Google Scholar] [CrossRef]
- Sokolenko, E.A.; Berchner-Pfannschmidt, U.; Ting, S.C.; Schmid, K.W.; Bechrakis, N.E.; Seitz, B.; Tsimpaki, T.; Kraemer, M.M.; Fiorentzis, M. Optimisation of the Chicken Chorioallantoic Membrane Assay in Uveal Melanoma Research. Pharmaceutics 2021, 14, 13. [Google Scholar] [CrossRef] [PubMed]
- Griewank, K.G.; Yu, X.; Khalili, J.; Sozen, M.M.; Stempke-Hale, K.; Bernatchez, C.; Wardell, S.; Bastian, B.C.; Woodman, S.E. Genetic and molecular characterization of uveal melanoma cell lines. Pigment Cell Melanoma Res. 2012, 25, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Verbik, D.J.; Murray, T.G.; Tran, J.M.; Ksander, B.R. Melanomas that develop within the eye inhibit lymphocyte proliferation. Int. J. Cancer 1997, 73, 470–478. [Google Scholar] [CrossRef]
- van den Aardweg, G.J.; Kilic, E.; de Klein, A.; Luyten, G.P. Dose fractionation effects in primary and metastatic human uveal melanoma cell lines. Investig. Ophthalmol. Vis. Sci. 2003, 44, 4660–4664. [Google Scholar] [CrossRef] [PubMed]
- De Waard-Siebinga, I.; Blom, D.J.; Griffioen, M.; Schrier, P.I.; Hoogendoorn, E.; Beverstock, G.; Danen, E.H.; Jager, M.J. Establishment and characterization of an uveal-melanoma cell line. Int. J. Cancer 1995, 62, 155–161. [Google Scholar] [CrossRef]
- Amirouchene-Angelozzi, N.; Nemati, F.; Gentien, D.; Nicolas, A.; Dumont, A.; Carita, G.; Camonis, J.; Desjardins, L.; Cassoux, N.; Piperno-Neumann, S.; et al. Establishment of novel cell lines recapitulating the genetic landscape of uveal melanoma and preclinical validation of mTOR as a therapeutic target. Mol. Oncol. 2014, 8, 1508–1520. [Google Scholar] [CrossRef]
- Nareyeck, G.; Zeschnigk, M.; Bornfeld, N.; Anastassiou, G. Novel cell lines derived by long-term culture of primary uveal melanomas. Ophthalmologica 2009, 223, 196–201. [Google Scholar] [CrossRef]
| Cell Line | Genetics | Morphology/Doubling Time | Prior Therapy | References |
|---|---|---|---|---|
| Mel270 | GNAQ Q209P, disomy-3 | Spindle/43 h | Irradiation | [43,56,57] |
| 92-1 | GNAQ Q209L, disomy-3, EIF1AX mutant | Epithelioid/38–58 h | Untreated | [43,57,58,59] |
| UPMD2 | GNA11 Q209L, disomy-3 | Epithelioid/150 h | Untreated | [49,55,60] |
| UPMM3 | GNAQ Q209P, monosomy-3, BAP1 mutant | Spindle and Epithelioid/100 h | Untreated | [49,55,60] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farhoumand, L.S.; Liu, H.; Tsimpaki, T.; Hendgen-Cotta, U.B.; Rassaf, T.; Bechrakis, N.E.; Fiorentzis, M.; Berchner-Pfannschmidt, U. Blockade of ß-Adrenergic Receptors by Nebivolol Enables Tumor Control Potential for Uveal Melanoma in 3D Tumor Spheroids and 2D Cultures. Int. J. Mol. Sci. 2023, 24, 5894. https://doi.org/10.3390/ijms24065894
Farhoumand LS, Liu H, Tsimpaki T, Hendgen-Cotta UB, Rassaf T, Bechrakis NE, Fiorentzis M, Berchner-Pfannschmidt U. Blockade of ß-Adrenergic Receptors by Nebivolol Enables Tumor Control Potential for Uveal Melanoma in 3D Tumor Spheroids and 2D Cultures. International Journal of Molecular Sciences. 2023; 24(6):5894. https://doi.org/10.3390/ijms24065894
Chicago/Turabian StyleFarhoumand, Lina S., Hongtao Liu, Theodora Tsimpaki, Ulrike B. Hendgen-Cotta, Tienush Rassaf, Nikolaos E. Bechrakis, Miltiadis Fiorentzis, and Utta Berchner-Pfannschmidt. 2023. "Blockade of ß-Adrenergic Receptors by Nebivolol Enables Tumor Control Potential for Uveal Melanoma in 3D Tumor Spheroids and 2D Cultures" International Journal of Molecular Sciences 24, no. 6: 5894. https://doi.org/10.3390/ijms24065894
APA StyleFarhoumand, L. S., Liu, H., Tsimpaki, T., Hendgen-Cotta, U. B., Rassaf, T., Bechrakis, N. E., Fiorentzis, M., & Berchner-Pfannschmidt, U. (2023). Blockade of ß-Adrenergic Receptors by Nebivolol Enables Tumor Control Potential for Uveal Melanoma in 3D Tumor Spheroids and 2D Cultures. International Journal of Molecular Sciences, 24(6), 5894. https://doi.org/10.3390/ijms24065894







