Three-Dimensional Bioprinting of an In Vitro Lung Model
Abstract
1. Introduction
2. Results
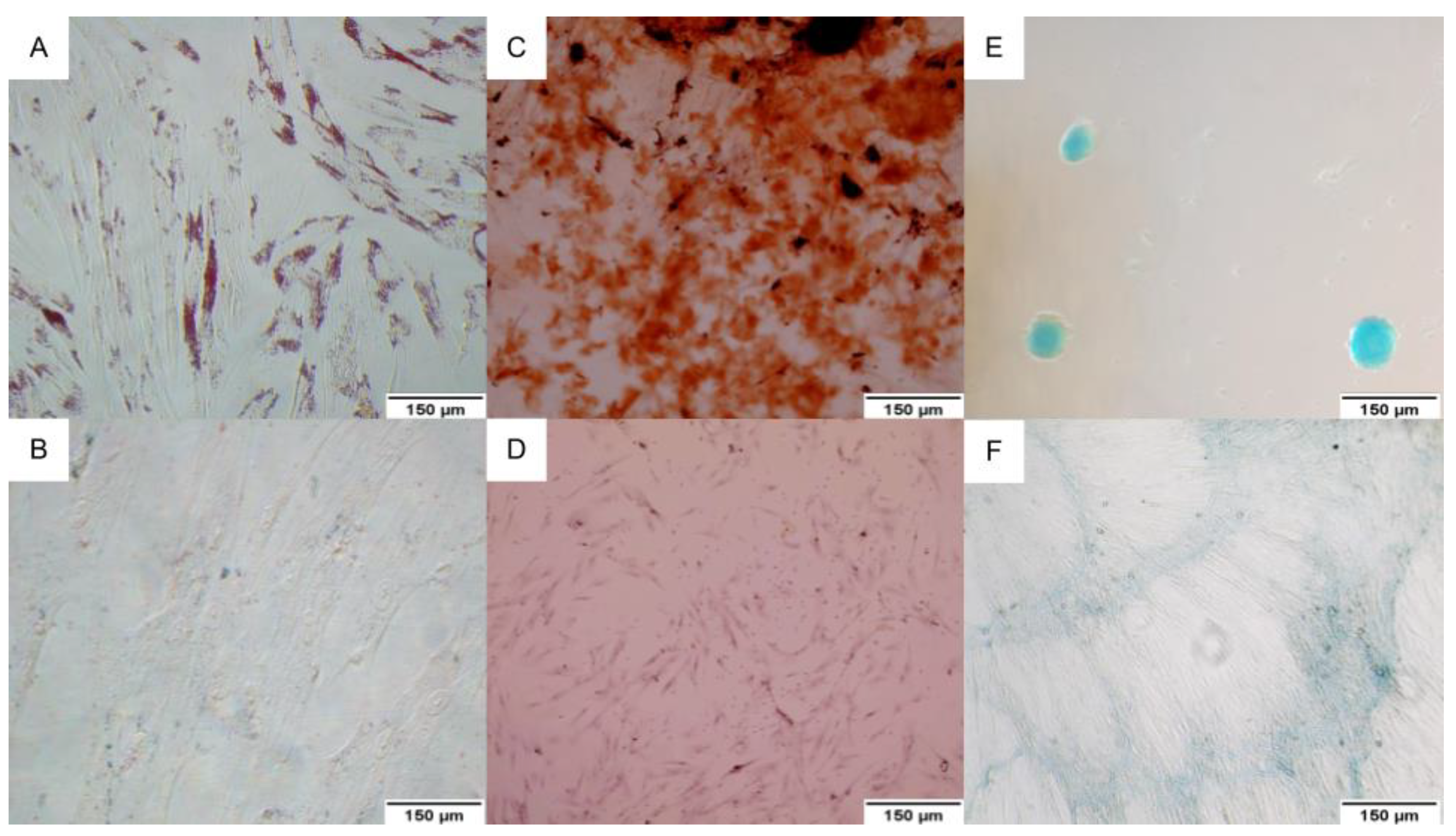
2.1. Mesenchymal Stem Cell Characterization
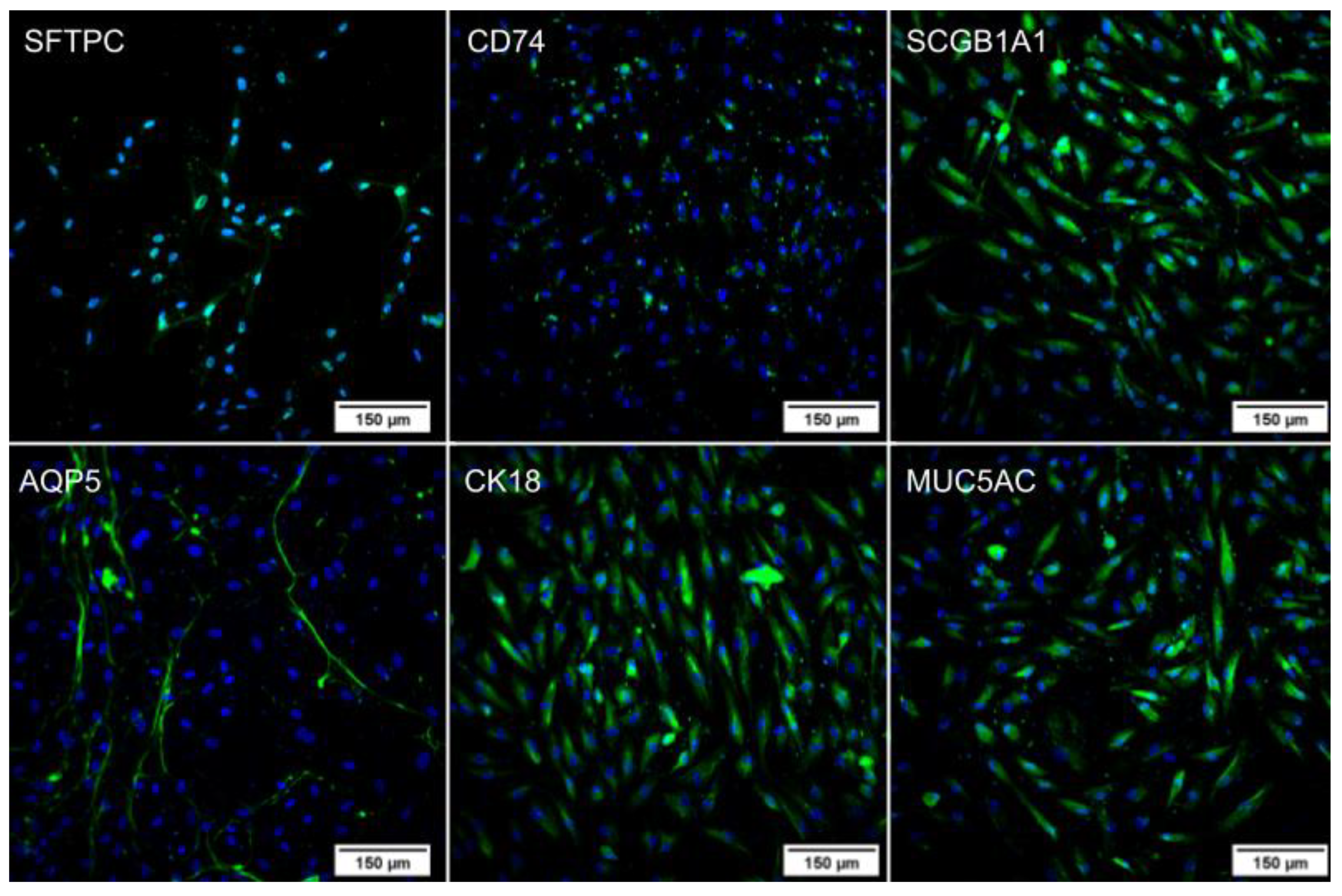
2.2. Pulmonary Differentiation
2.2.1. Immunocytochemistry
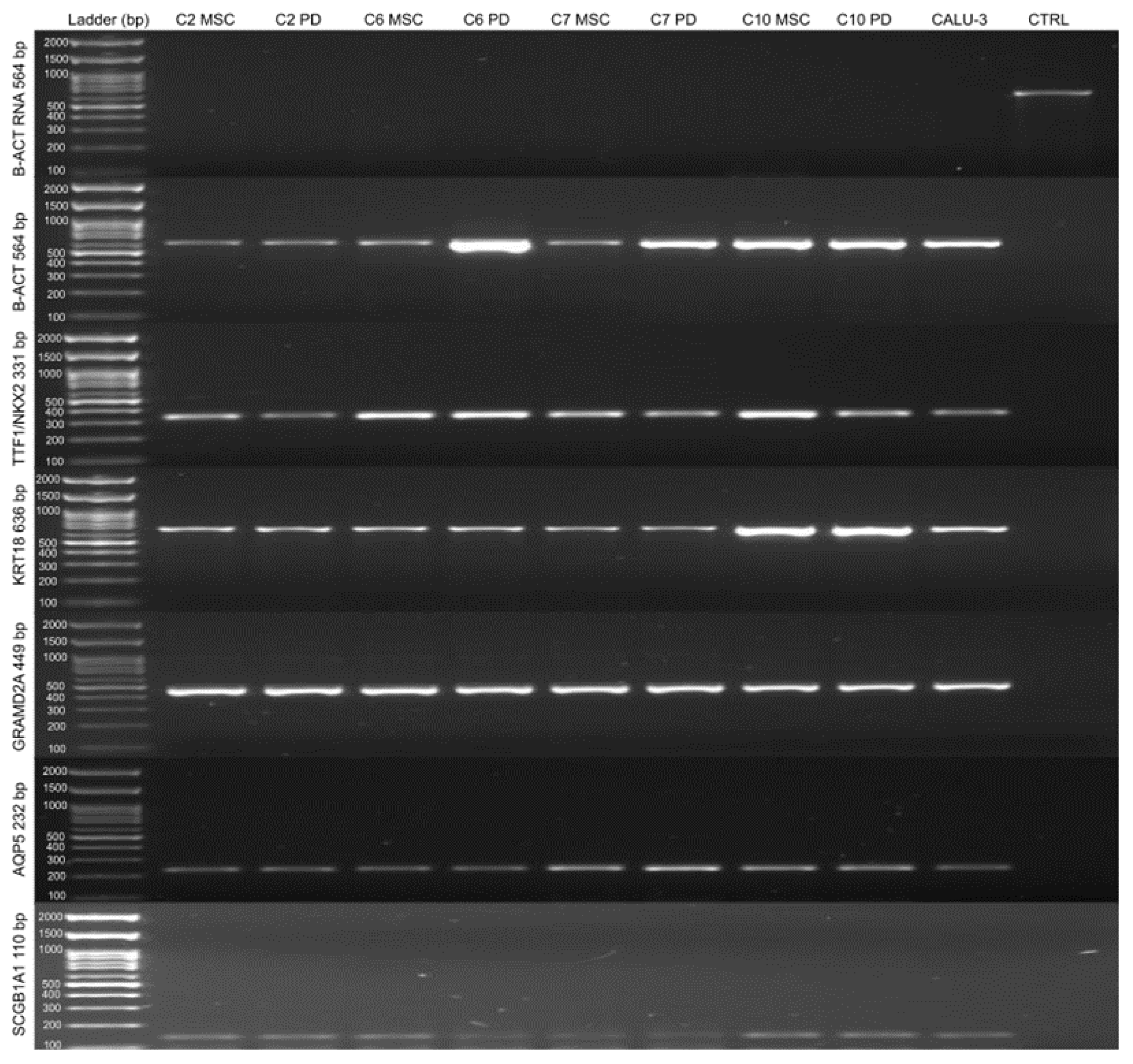
2.2.2. Qualitative Reverse Transcription–Polymerase Chain Reaction (RT-PCR)
2.3. Bioink
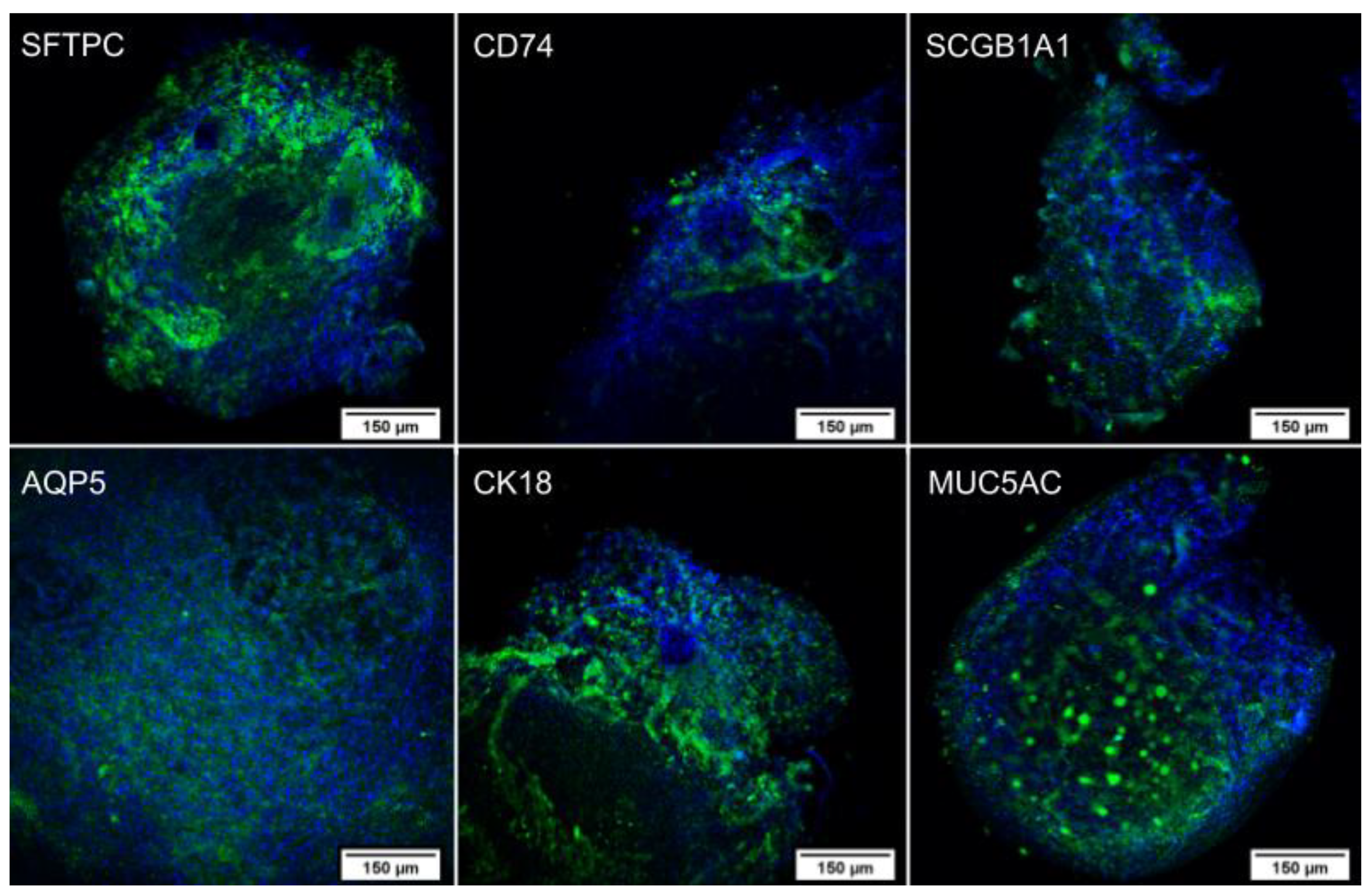
2.4. 3D Bioprinting
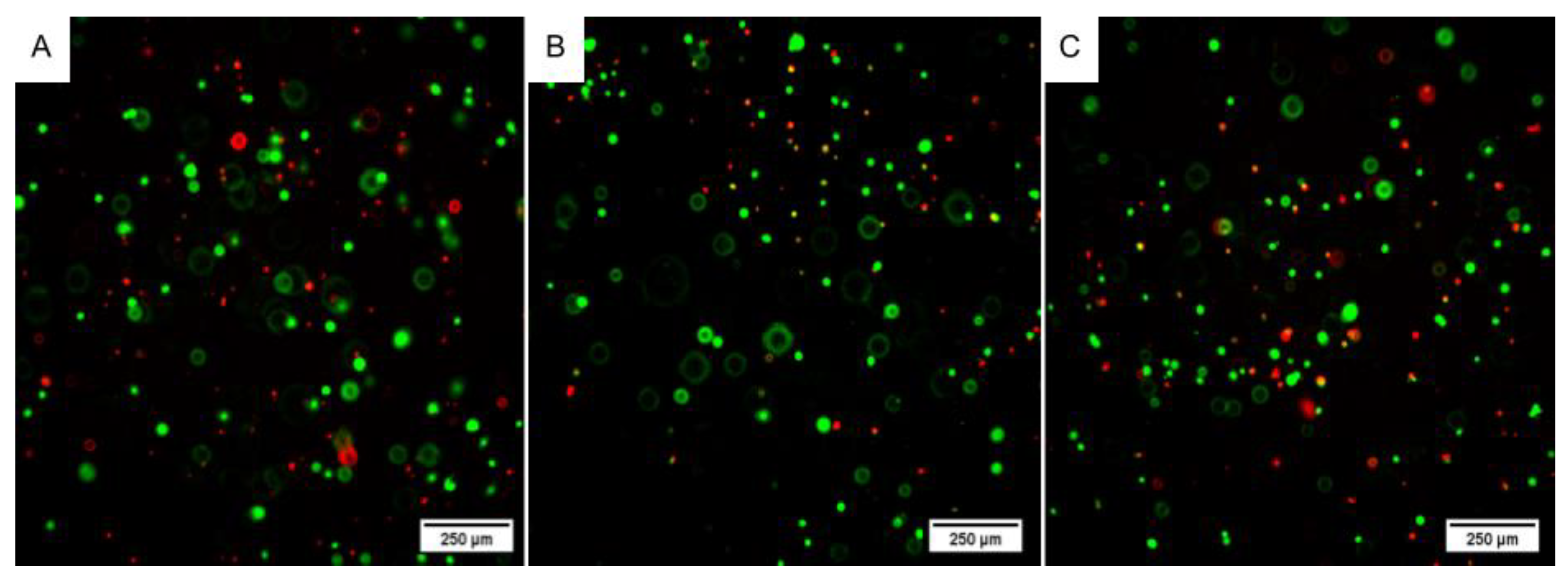
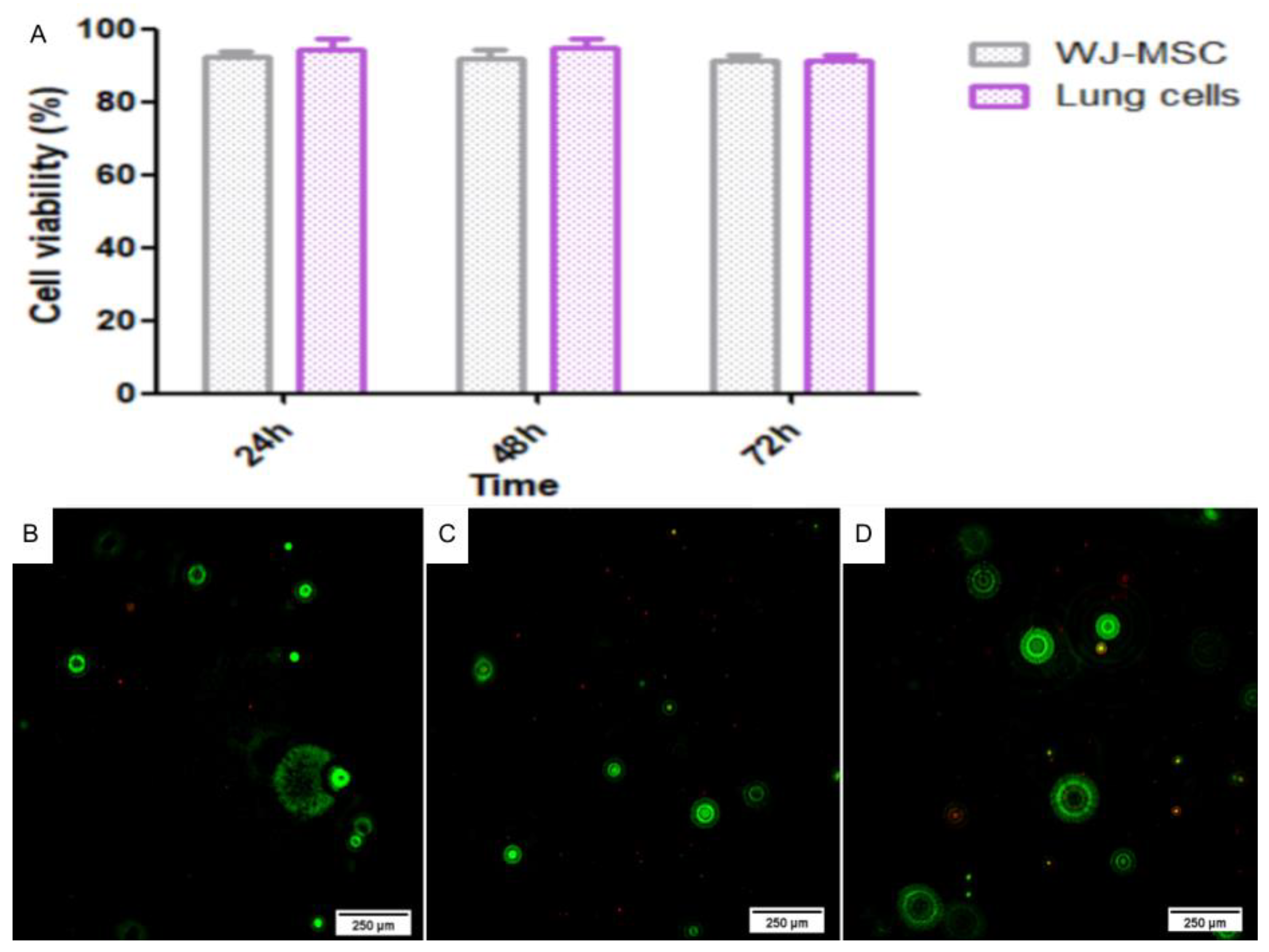
2.4.1. Live/Dead Assay
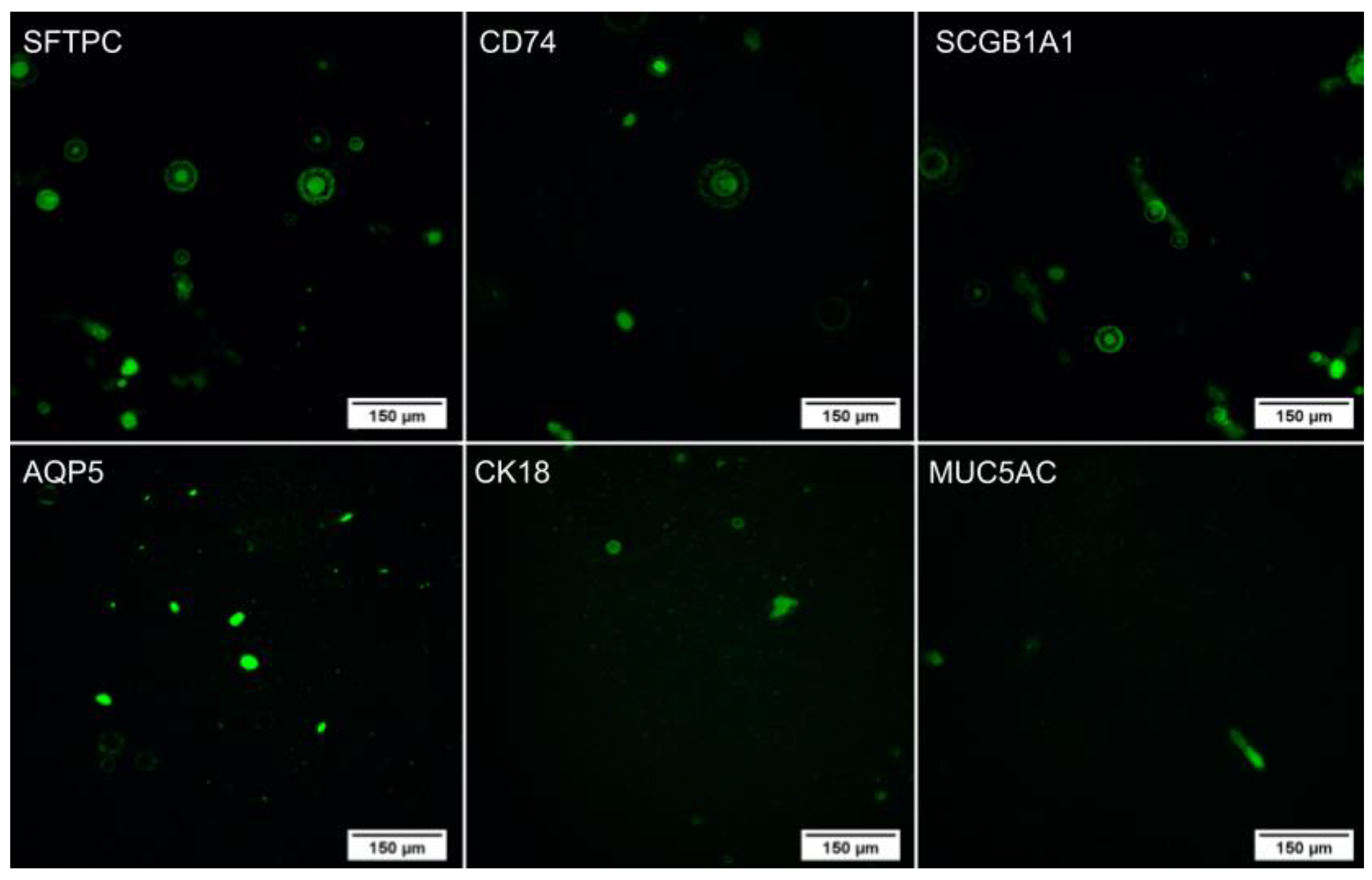
2.4.2. Immunocytochemistry
3. Discussion
4. Materials and Methods
4.1. Isolation and Culture of WJ-MSCs
4.2. Characterization of WJ-MSCs
4.2.1. Flow Cytometry
4.2.2. Trilineage Assay
4.3. Pulmonary Differentiation
4.4. Characterization of Pulmonary Cells
4.4.1. Immunocytochemistry
4.4.2. Qualitative Reverse Transcription–Polymerase Chain Reaction (RT-PCR)
4.5. 3D Bioprinting
4.6. Characterization of the 3D Lung Scaffold
4.6.1. Live/Dead Assay
4.6.2. Immunocytochemistry
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singhal, T. A Review of Coronavirus Disease-2019 (COVID-19). Indian J. Pediatr. 2020, 87, 281–286. [Google Scholar] [CrossRef]
- Harapan, H.; Itoh, N.; Yufika, A.; Winardi, W.; Keam, S.; Te, H.; Megawati, D.; Hayati, Z.; Wagner, A.L.; Mudatsir, M. Coronavirus Disease 2019 (COVID-19): A Literature Review. J. Infect. Public Health 2020, 13, 667–673. [Google Scholar] [CrossRef]
- Ciotti, M.; Ciccozzi, M.; Terrinoni, A.; Jiang, W.-C.; Wang, C.-B.; Bernardini, S. The COVID-19 Pandemic. Crit. Rev. Clin. Lab. Sci. 2020, 57, 365–388. [Google Scholar] [CrossRef]
- Muñoz-Fontela, C.; Dowling, W.E.; Funnell, S.G.P.; Gsell, P.-S.; Riveros-Balta, A.X.; Albrecht, R.A.; Andersen, H.; Baric, R.S.; Carroll, M.W.; Cavaleri, M.; et al. Animal Models for COVID-19. Nature 2020, 586, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Cleary, S.J.; Pitchford, S.C.; Amison, R.T.; Carrington, R.; Robaina Cabrera, C.L.; Magnen, M.; Looney, M.R.; Gray, E.; Page, C.P. Animal Models of Mechanisms of SARS-CoV-2 Infection and COVID-19 Pathology. Br. J. Pharmacol. 2020, 177, 4851–4865. [Google Scholar] [CrossRef]
- Hajar, R. Animal Testing and Medicine. Heart Views 2011, 12, 42. [Google Scholar] [CrossRef] [PubMed]
- van Meer, P.J.K.; Graham, M.L.; Schuurman, H.-J. The Safety, Efficacy and Regulatory Triangle in Drug Development: Impact for Animal Models and the Use of Animals. Eur. J. Pharmacol. 2015, 759, 3–13. [Google Scholar] [CrossRef]
- Fontana, F.; Figueiredo, P.; Martins, J.P.; Santos, H.A. Requirements for Animal Experiments: Problems and Challenges. Small 2021, 17, 2004182. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.A.; Vanbever, R. Preclinical Models for Pulmonary Drug Delivery. Expert Opin. Drug Deliv. 2009, 6, 1231–1245. [Google Scholar] [CrossRef] [PubMed]
- Lv, D.; Hu, Z.; Lu, L.; Lu, H.; Xu, X. Three-dimensional Cell Culture: A Powerful Tool in Tumor Research and Drug Discovery (Review). Oncol. Lett. 2017, 14, 6999–7010. [Google Scholar] [CrossRef]
- Berg, J.; Hiller, T.; Kissner, M.S.; Qazi, T.H.; Duda, G.N.; Hocke, A.C.; Hippenstiel, S.; Elomaa, L.; Weinhart, M.; Fahrenson, C.; et al. Optimization of Cell-Laden Bioinks for 3D Bioprinting and Efficient Infection with Influenza A Virus. Sci. Rep. 2018, 8, 13877. [Google Scholar] [CrossRef] [PubMed]
- Langhans, S.A. Three-Dimensional in Vitro Cell Culture Models in Drug Discovery and Drug Repositioning. Front. Pharmacol. 2018, 9, 6. [Google Scholar] [CrossRef]
- Ng, W.L.; Chua, C.K.; Shen, Y.-F. Print Me An Organ! Why We Are Not There Yet. Prog. Polym. Sci. 2019, 97, 101145. [Google Scholar] [CrossRef]
- Ng, W.L.; Ayi, T.C.; Liu, Y.-C.; Sing, S.L.; Yeong, W.Y.; Tan, B.-H. Fabrication and Characterization of 3D Bioprinted Triple-Layered Human Alveolar Lung Models. Int. J. Bioprinting 2021, 7, 332. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.J.; Dye, B.R.; Ferrer-Torres, D.; Hill, D.R.; Overeem, A.W.; Shea, L.D.; Spence, J.R. Generation of Lung Organoids from Human Pluripotent Stem Cells in Vitro. Nat. Protoc. 2019, 14, 518–540. [Google Scholar] [CrossRef] [PubMed]
- Mondal, A.; Gebeyehu, A.; Miranda, M.; Bahadur, D.; Patel, N.; Ramakrishnan, S.; Rishi, A.K.; Singh, M. Characterization and Printability of Sodium Alginate-Gelatin Hydrogel for Bioprinting NSCLC Co-Culture. Sci. Rep. 2019, 9, 19914. [Google Scholar] [CrossRef]
- Huang, S.X.L.; Green, M.D.; de Carvalho, A.T.; Mumau, M.; Chen, Y.-W.; D’Souza, S.L.; Snoeck, H.-W. The in Vitro Generation of Lung and Airway Progenitor Cells from Human Pluripotent Stem Cells. Nat. Protoc. 2015, 10, 413–425. [Google Scholar] [CrossRef]
- Marconett, C.N.; Zhou, B.; Sunohara, M.; Pouldar, T.M.; Wang, H.; Liu, Y.; Rieger, M.E.; Tran, E.; Flodby, P.; Siegmund, K.D.; et al. Cross-Species Transcriptome Profiling Identifies New Alveolar Epithelial Type I Cell–Specific Genes. Am. J. Respir. Cell Mol. Biol. 2017, 56, 310–321. [Google Scholar] [CrossRef]
- Tindle, C.; Fuller, M.; Fonseca, A.; Taheri, S.; Ibeawuchi, S.-R.; Beutler, N.; Katkar, G.D.; Claire, A.; Castillo, V.; Hernandez, M.; et al. Adult Stem Cell-Derived Complete Lung Organoid Models Emulate Lung Disease in COVID-19. eLife 2021, 10, e66417. [Google Scholar] [CrossRef]
- Kumar, P.; Vrana, N.E.; Ghaemmaghami, A.M. Prospects and Challenges in Engineering Functional Respiratory Epithelium for in Vitro and in Vivo Applications. Microphysiol. Syst. 2017, 1, 2. [Google Scholar] [CrossRef]
- Whitsett, J.A.; Zacharias, W.; Swarr, D.; Kalinichenko, V.V. Cell- and Tissue-Based Therapies for Lung Disease. In Principles of Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1253–1272. [Google Scholar] [CrossRef]
- Le Blanc, K.; Tammik, C.; Rosendahl, K.; Zetterberg, E.; Ringdén, O. HLA Expression and Immunologic Propertiesof Differentiated and Undifferentiated Mesenchymal Stem Cells. Exp. Hematol. 2003, 31, 890–896. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. The International Society for Cellular Therapy Position Statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Harichandan, A.; Bühring, H.-J. Prospective Isolation of Human MSC. Best Pract. Res. Clin. Haematol. 2011, 24, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Jacob, A.; Vedaie, M.; Roberts, D.A.; Thomas, D.C.; Villacorta-Martin, C.; Alysandratos, K.-D.; Hawkins, F.; Kotton, D.N. Derivation of Self-Renewing Lung Alveolar Epithelial Type II Cells from Human Pluripotent Stem Cells. Nat. Protoc. 2019, 14, 3303–3332. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, F.; Kramer, P.; Jacob, A.; Driver, I.; Thomas, D.C.; McCauley, K.B.; Skvir, N.; Crane, A.M.; Kurmann, A.A.; Hollenberg, A.N.; et al. Prospective Isolation of NKX2-1–Expressing Human Lung Progenitors Derived from Pluripotent Stem Cells. J. Clin. Investig. 2017, 127, 2277–2294. [Google Scholar] [CrossRef]
- Huang, S.X.L.; Islam, M.N.; O’Neill, J.; Hu, Z.; Yang, Y.-G.; Chen, Y.-W.; Mumau, M.; Green, M.D.; Vunjak-Novakovic, G.; Bhattacharya, J.; et al. Efficient Generation of Lung and Airway Epithelial Cells from Human Pluripotent Stem Cells. Nat. Biotechnol. 2014, 32, 84–91. [Google Scholar] [CrossRef]
- Isago, H.; Mitani, A.; Mikami, Y.; Horie, M.; Urushiyama, H.; Hamamoto, R.; Terasaki, Y.; Nagase, T. Epithelial Expression of YAP and TAZ Is Sequentially Required in Lung Development. Am. J. Respir. Cell Mol. Biol. 2020, 62, 256–266. [Google Scholar] [CrossRef]
- Warburton, D. YAP and TAZ in Lung Development: The Timing Is Important. Am. J. Respir. Cell Mol. Biol. 2020, 62, 141–142. [Google Scholar] [CrossRef]
- de Oliveira, N.B.; Irioda, A.C.; Stricker, P.E.F.; Mogharbel, B.F.; da Rosa, N.N.; Dziedzic, D.S.M.; de Carvalho, K.A.T. Natural Membrane Differentiates Human Adipose-Derived Mesenchymal Stem Cells to Neurospheres by Mechanotransduction Related to YAP and AMOT Proteins. Membranes 2021, 11, 687. [Google Scholar] [CrossRef]
- Calvert, B.A.; Ryan, A.L. Application of IPSC to Modelling of Respiratory Diseases. In Cell Biology and Translational Medicine, Volume 7; Turksen, K., Ed.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2019; Volume 1237, pp. 1–16. [Google Scholar] [CrossRef]
- Beers, M.F.; Moodley, Y. When Is an Alveolar Type 2 Cell an Alveolar Type 2 Cell? A Conundrum for Lung Stem Cell Biology and Regenerative Medicine. Am. J. Respir. Cell Mol. Biol. 2017, 57, 18–27. [Google Scholar] [CrossRef]
- Lee, D.F.; Salguero, F.J.; Grainger, D.; Francis, R.J.; MacLellan-Gibson, K.; Chambers, M.A. Isolation and Characterization of Alveolar Type II Pneumocytes from Adult Bovine Lung. Sci. Rep. 2018, 8, 11927. [Google Scholar] [CrossRef]
- Hasegawa, K.; Sato, A.; Tanimura, K.; Uemasu, K.; Hamakawa, Y.; Fuseya, Y.; Sato, S.; Muro, S.; Hirai, T. Fraction of MHCII and EpCAM Expression Characterizes Distal Lung Epithelial Cells for Alveolar Type 2 Cell Isolation. Respir. Res. 2017, 18, 150. [Google Scholar] [CrossRef]
- Marsh, L.M.; Cakarova, L.; Kwapiszewska, G.; von Wulffen, W.; Herold, S.; Seeger, W.; Lohmeyer, J. Surface Expression of CD74 by Type II Alveolar Epithelial Cells: A Potential Mechanism for Macrophage Migration Inhibitory Factor-Induced Epithelial Repair. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2009, 296, L442–L452. [Google Scholar] [CrossRef]
- Schröder, B. The Multifaceted Roles of the Invariant Chain CD74—More than Just a Chaperone. Biochim. Biophys. Acta BBA—Mol. Cell Res. 2016, 1863, 1269–1281. [Google Scholar] [CrossRef] [PubMed]
- Dickman, C.T.D.; Russo, V.; Thain, K.; Pan, S.; Beyer, S.T.; Walus, K.; Getsios, S.; Mohamed, T.; Wadsworth, S.J. Functional Characterization of 3D Contractile Smooth Muscle Tissues Generated Using a Unique Microfluidic 3D Bioprinting Technology. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2020, 34, 1652–1664. [Google Scholar] [CrossRef]
- Flodby, P.; Li, C.; Liu, Y.; Wang, H.; Rieger, M.E.; Minoo, P.; Crandall, E.D.; Ann, D.K.; Borok, Z.; Zhou, B. Cell-Specific Expression of Aquaporin-5 (Aqp5) in Alveolar Epithelium Is Directed by GATA6/Sp1 via Histone Acetylation. Sci. Rep. 2017, 7, 3473. [Google Scholar] [CrossRef] [PubMed]
- Hajj, R.; Baranek, T.; Le Naour, R.; Lesimple, P.; Puchelle, E.; Coraux, C. Basal Cells of the Human Adult Airway Surface Epithelium Retain Transit-Amplifying Cell Properties. Stem Cells 2007, 25, 139–148. [Google Scholar] [CrossRef]
- Noruddin, N.A.A.; Saim, A.B.; Chua, K.H.; Idrus, R. Human Nasal Turbinates as a Viable Source of Respiratory Epithelial Cells Using Co-Culture System Versus Dispase Dissociation Technique. Laryngoscope 2007, 117, 2139–2145. [Google Scholar] [CrossRef] [PubMed]
- Ricciardi, M.; Malpeli, G.; Bifari, F.; Bassi, G.; Pacelli, L.; Kamdje, A.H.N.; Chilosi, M.; Krampera, M. Comparison of Epithelial Differentiation and Immune Regulatory Properties of Mesenchymal Stromal Cells Derived from Human Lung and Bone Marrow. PLoS ONE 2012, 7, e35639. [Google Scholar] [CrossRef]
- Rock, J.R.; Randell, S.H.; Hogan, B.L.M. Airway Basal Stem Cells: A Perspective on Their Roles in Epithelial Homeostasis and Remodeling. Dis. Model. Mech. 2010, 3, 545–556. [Google Scholar] [CrossRef]
- Li, J.; Ye, Z. The Potential Role and Regulatory Mechanisms of MUC5AC in Chronic Obstructive Pulmonary Disease. Molecules 2020, 25, 4437. [Google Scholar] [CrossRef] [PubMed]
- Martinu, T.; Todd, J.L.; Gelman, A.E.; Guerra, S.; Palmer, S.M. Club Cell Secretory Protein in Lung Disease: Emerging Concepts and Potential Therapeutics. Annu. Rev. Med. 2023, 74, 427–441. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Dai, X.; Wang, X.; Li, X.; Diao, J.; Xu, T. Tumor-like Lung Cancer Model Based on 3D Bioprinting. 3 Biotech 2018, 8, 501. [Google Scholar] [CrossRef]
- Ozbolat, I.T.; Hospodiuk, M. Current Advances and Future Perspectives in Extrusion-Based Bioprinting. Biomaterials 2016, 76, 321–343. [Google Scholar] [CrossRef]
- Boularaoui, S.; Al Hussein, G.; Khan, K.A.; Christoforou, N.; Stefanini, C. An Overview of Extrusion-Based Bioprinting with a Focus on Induced Shear Stress and Its Effect on Cell Viability. Bioprinting 2020, 20, e00093. [Google Scholar] [CrossRef]
- Ramiah, P.; du Toit, L.C.; Choonara, Y.E.; Kondiah, P.P.D.; Pillay, V. Hydrogel-Based Bioinks for 3D Bioprinting in Tissue Regeneration. Front. Mater. 2020, 7, 76. [Google Scholar] [CrossRef]
- Baruffaldi, D.; Pirri, C.F.; Frascella, F. 3D Bioprinting of Cell-Laden Carbopol Bioinks. Bioprinting 2021, 22, e00135. [Google Scholar] [CrossRef]
- Irioda, A.C.; Cassilha, R.; Zocche, L.; Francisco, J.C.; Cunha, R.C.; Ferreira, P.E.; Guarita-Souza, L.C.; Ferreira, R.J.; Mogharbel, B.F.; Garikipati, V.N.S.; et al. Human Adipose-Derived Mesenchymal Stem Cells Cryopreservation and Thawing Decrease α 4-Integrin Expression. Stem Cells Int. 2016, 2016, 2562718. [Google Scholar] [CrossRef]
- Bunnell, B.; Flaat, M.; Gagliardi, C.; Patel, B.; Ripoll, C. Adipose-Derived Stem Cells: Isolation, Expansion and Differentiation. Methods 2008, 45, 115–120. [Google Scholar] [CrossRef]
- Han, Y.; Li, X.; Zhang, Y.; Han, Y.; Chang, F.; Ding, J. Mesenchymal Stem Cells for Regenerative Medicine. Cells 2019, 8, 886. [Google Scholar] [CrossRef]
- Stricker, P.E.F.; de Souza Dobuchak, D.; Irioda, A.C.; Mogharbel, B.F.; Franco, C.R.C.; de Souza Almeida Leite, J.R.; de Araújo, A.R.; Borges, F.A.; Herculano, R.D.; de Oliveira Graeff, C.F.; et al. Human Mesenchymal Stem Cells Seeded on the Natural Membrane to Neurospheres for Cholinergic-like Neurons. Membranes 2021, 11, 598. [Google Scholar] [CrossRef] [PubMed]
- Trzaska, K.A.; Rameshwar, P. Dopaminergic Neuronal Differentiation Protocol for Human Mesenchymal Stem Cells. In Mesenchymal Stem Cell Assays and Applications; Vemuri, M., Chase, L.G., Rao, M.S., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2011; Volume 698, pp. 295–303. [Google Scholar] [CrossRef]
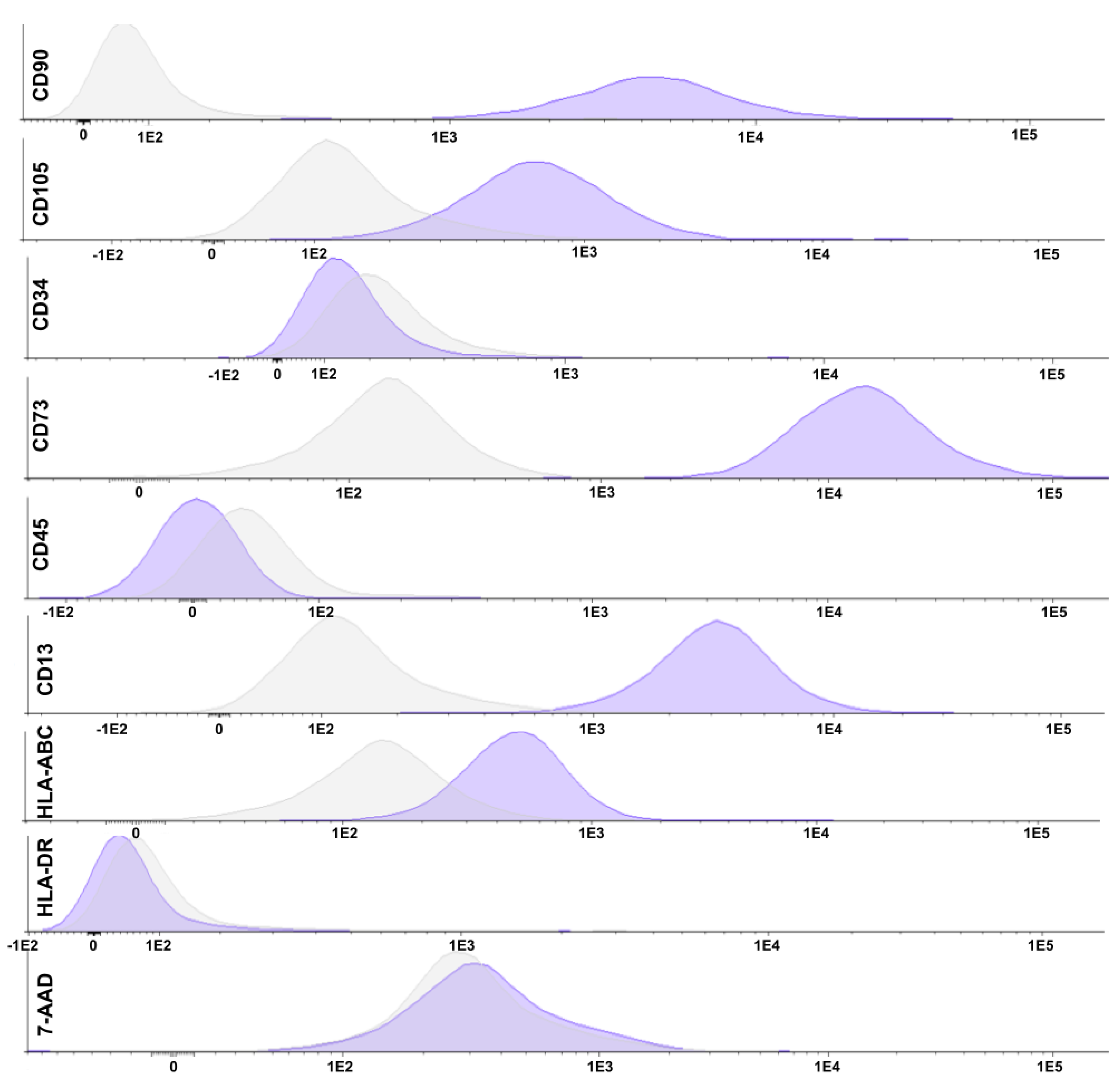

| Tube | Content |
|---|---|
| 1 | Cells without markers |
| 2 | Isotypic control |
| 3 | CD90 FITC/CD105 PE/7-AAD PERCP/CD34 PE-CY7/CD73 APC/CD45 APC-CY7 |
| 4 | HLA-DR FITC/CD13 PE/7-AAD PERCP/CD34 PE-CY7/HLA-ABC APC/CD45 APC-CY7 |
| Day | Medium |
|---|---|
| 1 | RPMI + 100 ng/mL activin A |
| 2 | RPMI + 0.2% (vol/vol) FBS + 100 ng/mL activin A |
| 3 | RPMI + 2% (vol/vol) FBS + 100 ng/mL activin A |
| 4 | RPMI + 2% (vol/vol) FBS + 100 ng/mL activin A |
| 5–10 (changing medium every day) | DMEM/F12 + 1× N2 + 1× B27 + 1× L-glutamine + 1× P/S + 10 mM HEPES buffer + 10 µM SB431542 + 200 ng/mL Noggin + 1 µM SAG + 500 ng/mL FGF4 + 2 µM CHIR99021 |
| 10–70 (changing medium twice a week) | DMEM/F12 + 1× N2 + 1× B27 + 1× L-glutamine + 1× P/S + 10 mM HEPES buffer + 1% (v/v) FBS e 500 ng/mL FGF10 |
| Antibody | Dilution | Code |
|---|---|---|
| AQP5 | 2 μg/mL | HPA065008 |
| CD74 | 1:100 | SAB521932 |
| KRT18 | 1:100 | C8541 |
| MUC5AC | 1:100 | M5293 |
| SCGB1A1 | 1: 100 | SAB1411381 |
| SFTPC | 1:100 | ZRB1496 |
| Anti-mouse secondary antibody with FITC | 10 μg/mL | F7512 |
| Anti-rabbit secondary antibody with FITC | 10 μg/mL | F0382 |
| Gene | Forward | Reverse | MW (pb) | AT (°C) |
|---|---|---|---|---|
| B-ACT | CTGGGACGACATGGAGAAAA | AAGGAAGGCTGGAAGAGTGC | 564 | 60 |
| TTF1/NKX2.1 | CGGCGCTTTCGGAGGGAATA | TGTAACACCTGCTTCCTCGTC | 331 | 62 |
| KRT18 | AAAGCCTGAGTCCTGTCCTT | CCAGCTGCAGTCGTGTGATA | 636 | 60 |
| GRAMD2A | ATGACCGCTTTAAGCCGGAG | ATGGCCAGTCCATTGGGAAG | 449 | 62 |
| AQP5 | TCCATTGGCCTGTCTGTCAC | CTTTGATGATGGCCACACGC | 232 | 58 |
| SCGB1A1 | TCCACCATGAAACTCGCTGT | AGGAGGGTTTCGATGACACG | 110 | 62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Rosa, N.N.; Appel, J.M.; Irioda, A.C.; Mogharbel, B.F.; de Oliveira, N.B.; Perussolo, M.C.; Stricker, P.E.F.; Rosa-Fernandes, L.; Marinho, C.R.F.; de Carvalho, K.A.T. Three-Dimensional Bioprinting of an In Vitro Lung Model. Int. J. Mol. Sci. 2023, 24, 5852. https://doi.org/10.3390/ijms24065852
da Rosa NN, Appel JM, Irioda AC, Mogharbel BF, de Oliveira NB, Perussolo MC, Stricker PEF, Rosa-Fernandes L, Marinho CRF, de Carvalho KAT. Three-Dimensional Bioprinting of an In Vitro Lung Model. International Journal of Molecular Sciences. 2023; 24(6):5852. https://doi.org/10.3390/ijms24065852
Chicago/Turabian Styleda Rosa, Nádia Nascimento, Julia Maurer Appel, Ana Carolina Irioda, Bassam Felipe Mogharbel, Nathalia Barth de Oliveira, Maiara Carolina Perussolo, Priscila Elias Ferreira Stricker, Lívia Rosa-Fernandes, Cláudio Romero Farias Marinho, and Katherine Athayde Teixeira de Carvalho. 2023. "Three-Dimensional Bioprinting of an In Vitro Lung Model" International Journal of Molecular Sciences 24, no. 6: 5852. https://doi.org/10.3390/ijms24065852
APA Styleda Rosa, N. N., Appel, J. M., Irioda, A. C., Mogharbel, B. F., de Oliveira, N. B., Perussolo, M. C., Stricker, P. E. F., Rosa-Fernandes, L., Marinho, C. R. F., & de Carvalho, K. A. T. (2023). Three-Dimensional Bioprinting of an In Vitro Lung Model. International Journal of Molecular Sciences, 24(6), 5852. https://doi.org/10.3390/ijms24065852








