Substituent-Guided Cluster Nuclearity for Tetranuclear Iron(III) Compounds with Flat {Fe4(μ3-O)2} Butterfly Core
Abstract
1. Introduction
2. Results and Discussion
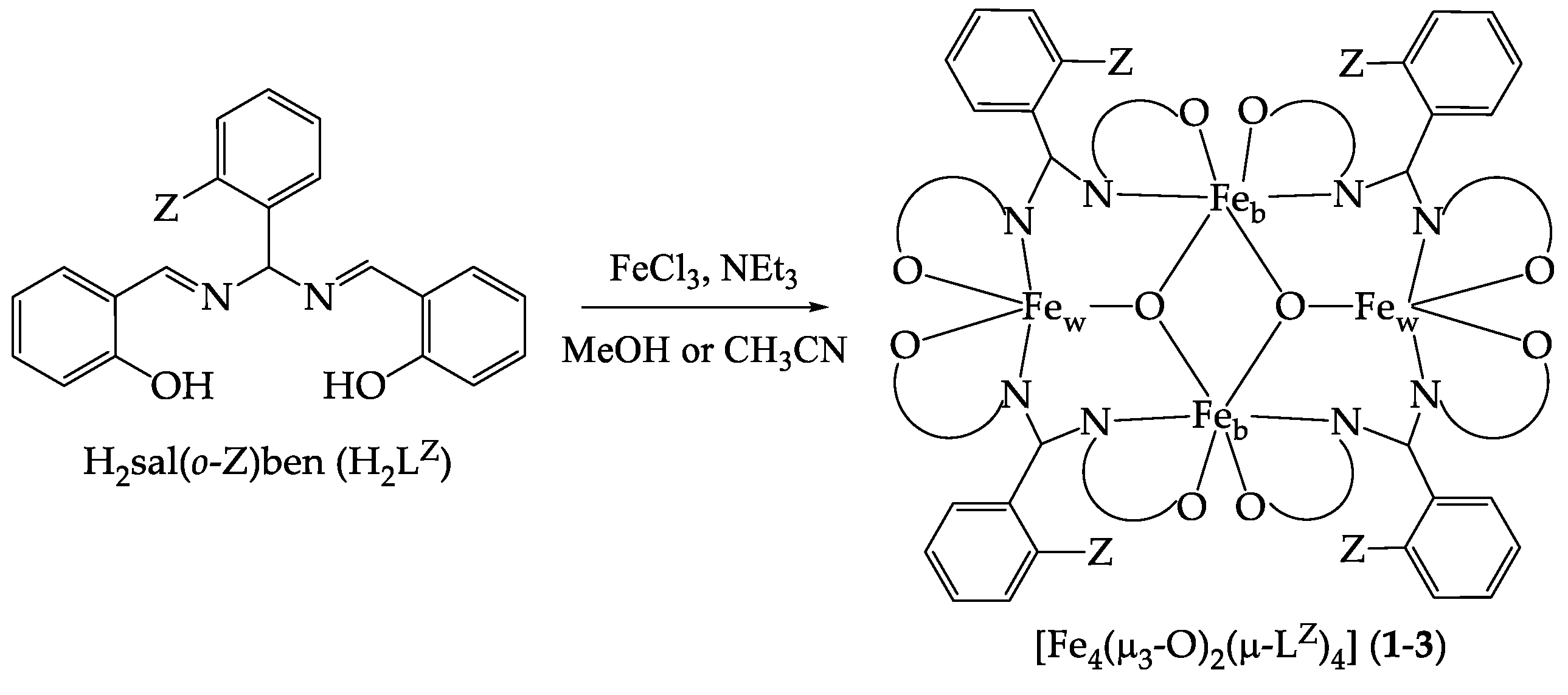
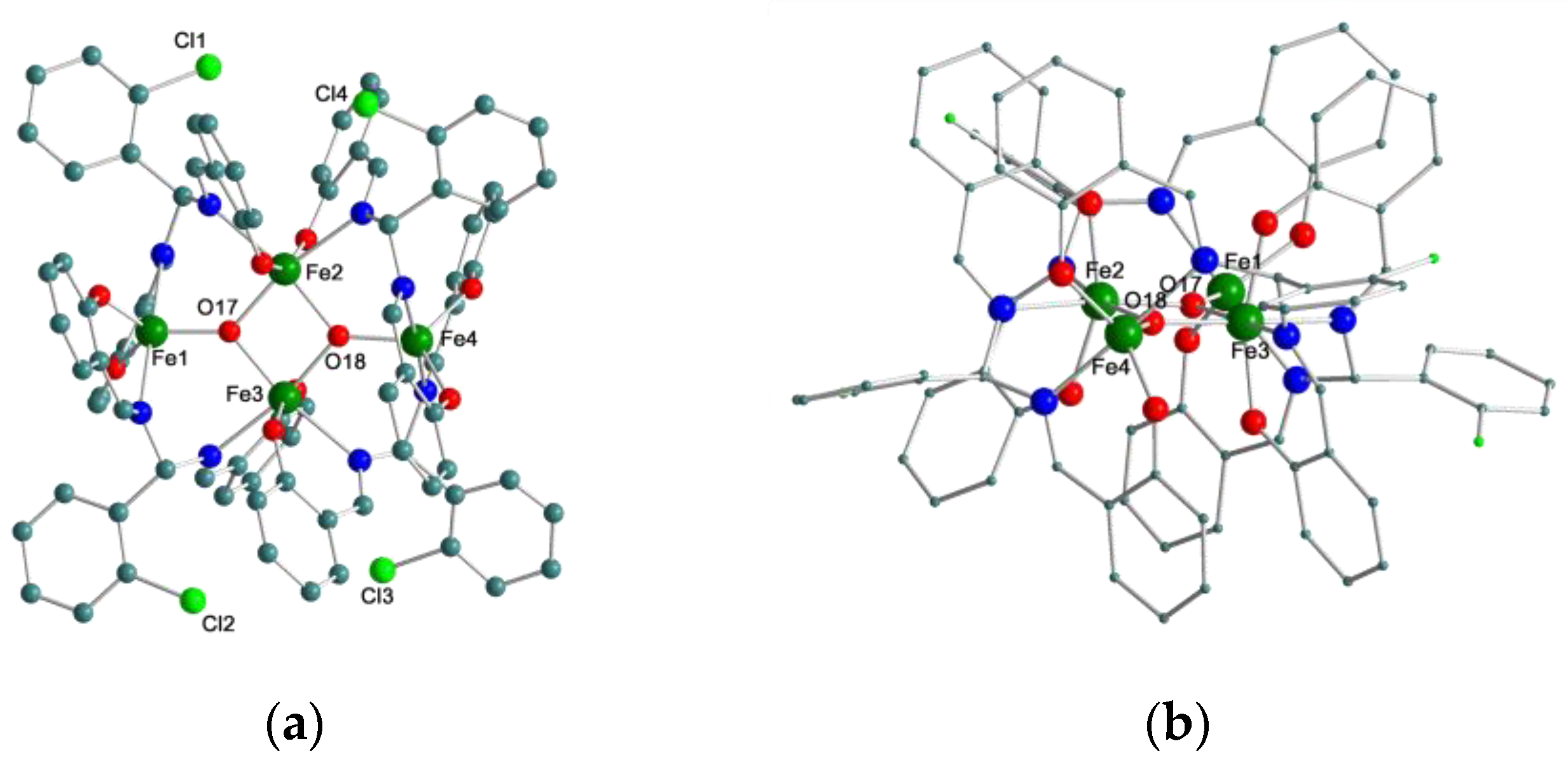
2.1. Synthesis and X-ray Crystal Structures
2.2. Optimized Geometries
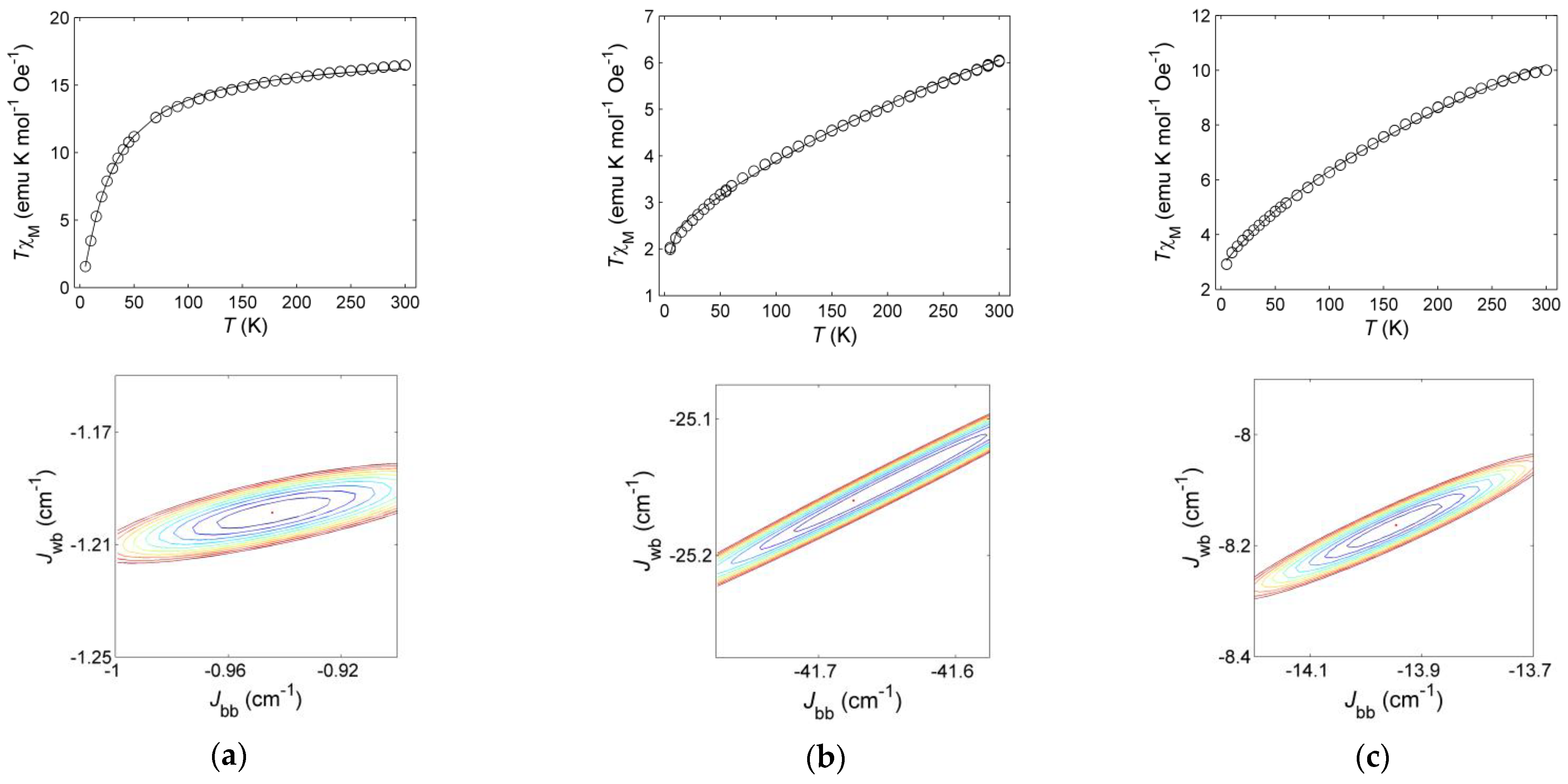
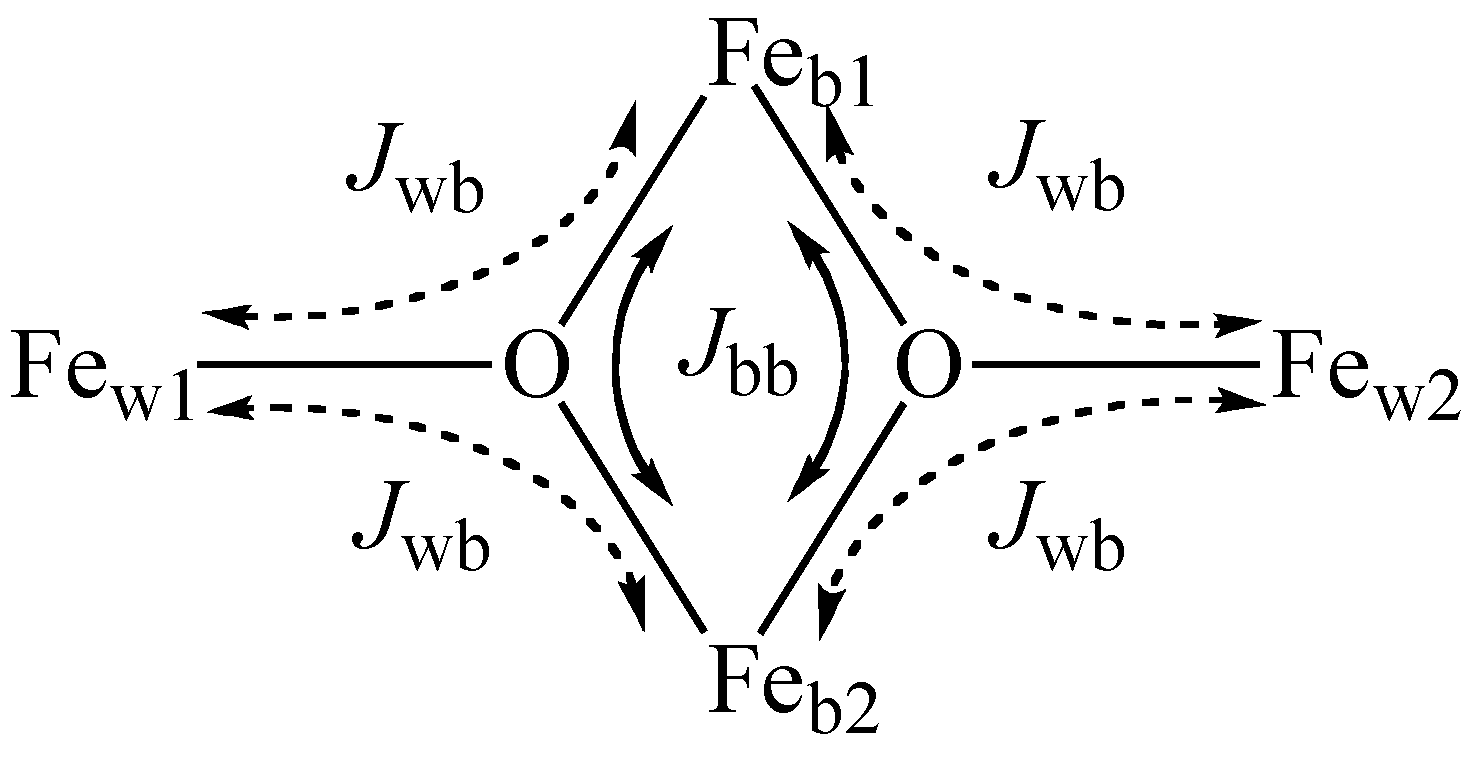
2.3. Magnetic Properties
2.4. Effect of the Substituents and QTAIM Analysis
3. Materials and Methods
3.1. General
3.2. Synthesis of [Fe4(μ3-O)2(μ-LNO2)4] (1)
3.3. Synthesis of [Fe4(μ3-O)2(μ-LCl)4] (2)
3.4. Synthesis of [Fe4(μ3-O)2(μ-LOMe)4] (3)
3.5. Crystal Structure Determination
3.6. Theoretical Calculations
3.7. Magnetic Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Magnetism: Molecules to Materials; Miller, J.S., Drillon, M., Eds.; Wiley-VCH: Weinheim, Germany; New York, NY, USA, 2001; ISBN 978-3-527-29772-6. [Google Scholar]
- Gatteschi, D.; Sessoli, R.; Villain, J. Molecular Nanomagnets; Oxford University Press: Oxford, UK, 2006; ISBN 978-0-19-856753-0. [Google Scholar]
- Spin-Crossover Materials: Properties and Applications; Halcrow, M.A., Ed.; Wiley: Chichester, UK, 2013; ISBN 978-1-119-99867-9. [Google Scholar]
- Miller, J.S. Organic- and Molecule-Based Magnets. Mater. Today 2014, 17, 224–235. [Google Scholar] [CrossRef]
- Oshio, H.; Hoshino, N.; Ito, T.; Nakano, M. Single-Molecule Magnets of Ferrous Cubes: Structurally Controlled Magnetic Anisotropy. J. Am. Chem. Soc. 2004, 126, 8805–8812. [Google Scholar] [CrossRef] [PubMed]
- Gregoli, L.; Danieli, C.; Barra, A.-L.; Neugebauer, P.; Pellegrino, G.; Poneti, G.; Sessoli, R.; Cornia, A. Magnetostructural Correlations in Tetrairon(III) Single-Molecule Magnets. Chem. Eur. J. 2009, 15, 6456–6467. [Google Scholar] [CrossRef] [PubMed]
- Murali, M.; Nayak, S.; Costa, J.S.; Ribas, J.; Mutikainen, I.; Turpeinen, U.; Clémancey, M.; Garcia-Serres, R.; Latour, J.-M.; Gamez, P.; et al. Discrete Tetrairon(III) Cluster Exhibiting a Square-Planar Fe4(μ4-O) Core: Structural and Magnetic Properties. Inorg. Chem. 2010, 49, 2427–2434. [Google Scholar] [CrossRef] [PubMed]
- Rigamonti, L.; Forni, A.; Sironi, M.; Ponti, A.; Ferretti, A.M.; Baschieri, C.; Pasini, A. Experimental and Theoretical Investigations on Magneto-Structural Correlation in Trinuclear Copper(II) Hydroxido Propellers. Polyhedron 2018, 145, 22–34. [Google Scholar] [CrossRef]
- Winpenny, R.E.P. Serendipitous Assembly of Polynuclear Cage Compounds. J. Chem. Soc. Dalton Trans. 2002, 1–10. [Google Scholar] [CrossRef]
- Ohta, S.; Yokozawa, S.; Ohki, Y.; Tatsumi, K. Oxido-Bridged Di-, Tri-, and Tetra-Nuclear Iron Complexes Bearing Bis(Trimethylsilyl)Amide and Thiolate Ligands. Inorg. Chem. 2012, 51, 2645–2651. [Google Scholar] [CrossRef] [PubMed]
- Botezat, O.; van Leusen, J.; Kögerler, P.; Baca, S.G. Tuning the Condensation Degree of {FeIIIn} Oxo Clusters via Ligand Metathesis, Temperature, and Solvents. Inorg. Chem. 2018, 57, 7904–7913. [Google Scholar] [CrossRef] [PubMed]
- Hale, A.R.; Lott, M.E.; Peralta, J.E.; Foguet-Albiol, D.; Abboud, K.A.; Christou, G. Magnetic Properties of High-Nuclearity Fex-Oxo(x = 7, 22, 24) Clusters Analyzed by a Multipronged Experimental, Computational, and Magnetostructural Correlation Approach. Inorg. Chem. 2022, 61, 11261–11276. [Google Scholar] [CrossRef] [PubMed]
- Nachtigall, O.; Kusserow, M.; Clérac, R.; Wernsdorfer, W.; Menzel, M.; Renz, F.; Mrozinski, J.; Spandl, J. [Fe19] “Super-Lindqvist” Aggregate and Large 3D Interpenetrating Coordination Polymer from Solvothermal Reactions of [Fe2(OtBu)6] with Ethanol. Angew. Chem. Int. Ed. 2015, 54, 10361–10364. [Google Scholar] [CrossRef] [PubMed]
- Cozzi, P.G. Metal–Salen Schiff Base Complexes in Catalysis: Practical Aspects. Chem. Soc. Rev. 2004, 33, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Rigamonti, L.; Demartin, F.; Forni, A.; Righetto, S.; Pasini, A. Copper(II) Complexes of Salen Analogues with Two Differently Substituted (Push−Pull) Salicylaldehyde Moieties. A Study on the Modulation of Electronic Asymmetry and Nonlinear Optical Properties. Inorg. Chem. 2006, 45, 10976–10989. [Google Scholar] [CrossRef] [PubMed]
- Rigamonti, L.; Forni, A.; Righetto, S.; Pasini, A. Push-Pull Unsymmetrical Substitution in Nickel(II) Complexes with Tetradentate N2O2 Schiff Base Ligands: Synthesis, Structures and Linear-Nonlinear Optical Studies. Dalton Trans. 2019, 48, 11217–11234. [Google Scholar] [CrossRef] [PubMed]
- Rigamonti, L.; Forni, A.; Cariati, E.; Malavasi, G.; Pasini, A. Solid-State Nonlinear Optical Properties of Mononuclear Copper(II) Complexes with Chiral Tridentate and Tetradentate Schiff Base Ligands. Materials 2019, 12, 3595. [Google Scholar] [CrossRef] [PubMed]
- Downing, R.S.; Urbach, F.L. Circular Dichroism of Square-Planar, Tetradentate Schiff Base Chelates of Copper(II). J. Am. Chem. Soc. 1969, 91, 5977–5983. [Google Scholar] [CrossRef]
- Kleij, A.W. Nonsymmetrical Salen Ligands and Their Complexes: Synthesis and Applications. Eur. J. Inorg. Chem. 2009, 2009, 193–205. [Google Scholar] [CrossRef]
- Mazzoni, R.; Roncaglia, F.; Rigamonti, L. When the Metal Makes the Difference: Template Syntheses of Tridentate and Tetradentate Salen-Type Schiff Base Ligands and Related Complexes. Crystals 2021, 11, 483. [Google Scholar] [CrossRef]
- Ashoka Sahadevan, S.; Cadoni, E.; Monni, N.; Sáenz de Pipaón, C.; Galan Mascaros, J.-R.; Abhervé, A.; Avarvari, N.; Marchiò, L.; Arca, M.; Mercuri, M.L. Structural Diversity in a New Series of Halogenated Quinolyl Salicylaldimides-Based FeIII Complexes Showing Solid-State Halogen-Bonding/Halogen···Halogen Interactions. Cryst. Growth Des. 2018, 18, 4187–4199. [Google Scholar] [CrossRef]
- Chowdhury, T.; Adhikary, A.; Roy, M.; Zangrando, E.; Samanta, D.; Das, D. Mapping of Solvent-Mediated Molecular Self-Assembly of Iron(III) Discrete Compounds: Exploring Their Magnetic Behavior and Phosphatase-Like Activity. Cryst. Growth Des. 2020, 20, 1254–1265. [Google Scholar] [CrossRef]
- Fik, M.A.; Czepa, W.; Kubicki, M.; Patroniak, V. Formation of Non-Covalent Porous Framework with Fe Ions and N2O Schiff Base Ligand: Structural and Thermal Studies. Supramol. Chem. 2017, 29, 643–650. [Google Scholar] [CrossRef]
- Pasini, A.; Demartin, F.; Piovesana, O.; Chiari, B.; Cinti, A.; Crispu, O. Novel Copper(II) Complexes of “Short” Salen Homologues. Structure and Magnetic Properties of the Tetranuclear Complex [Cu2(L2)2]2 [H2L2 = Phenyl-N,N ′-Bis(Salicylidene)Methanediamine]. J. Chem. Soc. Dalton Trans. 2000, 19, 3467–3472. [Google Scholar] [CrossRef]
- Chiari, B.; Cinti, A.; Crispu, O.; Demartin, F.; Pasini, A.; Piovesana, O. Binuclear Co(II)Co(II), Co(II)Co(III) and Co(III)Co(III) Complexes of “Short” Salen Homologues Derived from the Condensation of Salicylaldehyde and Methanediamine or Phenylmethanediamines. Synthesis, Structures and Magnetism. J. Chem. Soc. Dalton Trans. 2001, 24, 3611–3616. [Google Scholar] [CrossRef]
- Chiari, B.; Cinti, A.; Crispu, O.; Demartin, F.; Pasini, A.; Piovesana, O. New Pentanuclear Mixed Valence Co(ii)–Co(iii) Complexes of “Short” Salen Homologues. J. Chem. Soc. Dalton Trans. 2002, 4672–4677. [Google Scholar] [CrossRef]
- Rigamonti, L.; Zardi, P.; Carlino, S.; Demartin, F.; Castellano, C.; Pigani, L.; Ponti, A.; Ferretti, A.M.; Pasini, A. Selective Formation, Reactivity, Redox and Magnetic Properties of MnIII and FeIII Dinuclear Complexes with Shortened Salen-Type Schiff Base Ligands. Int. J. Mol. Sci. 2020, 21, 7882. [Google Scholar] [CrossRef] [PubMed]
- Rigamonti, L.; Forni, A. Effect of Crystal Packing and Coordinated Solvent Molecules on Metal-Ligand Bond Distances in Linear Trinuclear Nickel Compounds with Bridging Acetato and Schiff Base Ligands. Inorg. Chim. Acta 2018, 473, 216–222. [Google Scholar] [CrossRef]
- Rigamonti, L.; Forni, A.; Pievo, R.; Reedijk, J.; Pasini, A. Copper(II) Compounds with NNO Tridentate Schiff Base Ligands: Effect of Subtle Variations in Ligands on Complex Formation, Structures and Magnetic Properties. Inorg. Chim. Acta 2012, 387, 373–382. [Google Scholar] [CrossRef]
- Rigamonti, L.; Forni, A.; Pievo, R.; Reedijk, J.; Pasini, A. Synthesis, Crystal Structures and Magnetic Properties of Dinuclear Copper(II) Compounds with NNO Tridentate Schiff Base Ligands and Bridging Aliphatic Diamine and Aromatic Diimine Linkers. Dalton Trans. 2011, 40, 3381–3393. [Google Scholar] [CrossRef] [PubMed]
- Rigamonti, L.; Cinti, A.; Forni, A.; Pasini, A.; Piovesana, O. Copper(II) Complexes of Tridentate Schiff Bases of 5-Substituted Salicylaldehydes and Diamines—The Role of the Substituent and the Diamine in the Formation of Mono-, Di- and Trinuclear Species—Crystal Structures and Magnetic Properties. Eur. J. Inorg. Chem. 2008, 2008, 3633–3647. [Google Scholar] [CrossRef]
- Takajo, T.; Kambe, S.; Ando, W. Synthesis of N,N′-Bis[2-Hydroxybenzylidene]Arylmethanediamines. Synthesis 1984, 1984, 256–259. [Google Scholar] [CrossRef]
- Baca, S.G.; Filippova, I.G.; Keene, T.D.; Botezat, O.; Malaestean, I.L.; Stoeckli-Evans, H.; Kravtsov, V.C.; Chumacov, I.; Liu, S.; Decurtins, S. Iron(III)-Pivalate-Based Complexes with Tetranuclear {Fe4(μ3-O)2}8+ Cores and N-Donor Ligands: Formation of Cluster and Polymeric Architectures. Eur. J. Inorg. Chem. 2011, 2011, 356–367. [Google Scholar] [CrossRef]
- Overgaard, J.; Hibbs, D.E.; Rentschler, E.; Timco, G.A.; Larsen, F.K. Experimental and Theoretical Electron Density Distribution and Magnetic Properties of the Butterfly-like Complex [Fe4O2(O2CCMe3)8(NC5H4Me)2]·2CH3CN. Inorg. Chem. 2003, 42, 7593–7601. [Google Scholar] [CrossRef] [PubMed]
- Stamatatos, T.C.; Boudalis, A.K.; Sanakis, Y.; Raptopoulou, C.P. Reactivity and Structural and Physical Studies of Tetranuclear Iron(III) Clusters Containing the [Fe4(μ3-O)2]8+ “Butterfly” Core: An FeIII4 Cluster with an S = 1 Ground State. Inorg. Chem. 2006, 45, 7372–7381. [Google Scholar] [CrossRef] [PubMed]
- Gorun, S.M.; Lippard, S.J. Synthesis, Structure, and Characterization of the Tetranuclear Iron(III) Oxo Complex [Fe4O2(BICOH)2(BICO)2(O2CPh)4]Cl2. Inorg. Chem. 1988, 27, 149–156. [Google Scholar] [CrossRef]
- Pichon, C.; Dolbecq, A.; Mialane, P.; Marrot, J.; Rivière, E.; Goral, M.; Zynek, M.; McCormac, T.; Borshch, S.A.; Zueva, E.; et al. Fe2 and Fe4 Clusters Encapsulated in Vacant Polyoxotungstates: Hydrothermal Synthesis, Magnetic and Electrochemical Properties, and DFT Calculations. Chem. Eur. J. 2008, 14, 3189–3199. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Pressprich, M.; Coppens, P.; DeMarco, M.J. A New Tetranuclear Iron(III) Complex with an [Fe4O2] Core: Synthesis, Structure and Mössbauer Studies of [Fe4(μ3-O)2(μ-O2CCH3)6Cl2(3-Mepy)4].CH3CN (3-Mepy = 3-Methylpyridine). Acta Crystallogr. C Cryst. Struct. Commun. 1993, 49, 1255–1258. [Google Scholar] [CrossRef]
- Bacsa, J.; Zhao, H.; Dunbar, K.R. Two New Soluble Iron–Oxo Complexes: [Fe2(μ-O)(μ-O2CCF3)2(O2CCF3)2(C10H8N2)2] and [Fe4(μ3-O)2(μ-O2CCF3)6(O2CCF3)2(C10H8N2)2]·CF3CO2H. Acta Crystallogr. C Cryst. Struct. Commun. 2003, 59, m561–m564. [Google Scholar] [CrossRef] [PubMed]
- Cortés, P.; Atria, A.M.; Garland, M.T.; Baggio, R. Two Oxo Complexes with Tetranuclear [Fe4(μ3-O)2]8+ and Trinuclear [Fe3(μ3-O)]7+ Units. Acta Crystallogr. C Cryst. Struct. Commun. 2006, 62, m297–m302. [Google Scholar] [CrossRef] [PubMed]
- Mulyana, Y.; Nafady, A.; Mukherjee, A.; Bircher, R.; Moubaraki, B.; Murray, K.S.; Bond, A.M.; Abrahams, B.F.; Boskovic, C. New Family of Ferric Spin Clusters Incorporating Redox-Active Ortho-Dioxolene Ligands. Inorg. Chem. 2009, 48, 7765–7781. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, W.H.; Roth, M.E.; Lippard, S.J. Tetranuclear Iron-Oxo Complexes. Synthesis, Structure, and Properties of Species Containing the Nonplanar {Fe4O2}8+ Core and Seven Bridging Carboxylate Ligands. J. Am. Chem. Soc. 1987, 109, 6318–6326. [Google Scholar] [CrossRef]
- McCusker, J.K.; Vincent, J.B.; Schmitt, E.A.; Mino, M.L.; Shin, K.; Coggin, D.K.; Hagen, P.M.; Huffman, J.C.; Christou, G.; Hendrickson, D.N. Molecular Spin Frustration in the [Fe4O2]8+ Core: Synthesis, Structure, and Magnetochemistry of Tetranuclear Iron-Oxo Complex [Fe4O2(O2CR)7(Bpy)2](C1O4) (R = Me, Ph). J. Am. Chem. Soc. 1991, 113, 3012–3021. [Google Scholar] [CrossRef]
- Wemple, M.W.; Coggin, D.K.; Vincent, J.B.; McCusker, J.K.; Streib, W.E.; Huffman, J.C.; Hendrickson, D.N.; Christou, G. New Tetranuclear Metal Carboxylate Clusters with the [M4(μ3-O)2]8+ (M = MnIII or FeIII) Cores: Crystal Structures and Properties of [Mn4O2Cl2(O2CC6H3F2-3,5)6(Py)4], [Fe4O2Cl2(O2CMe)6(Bpy)2] and [NBun4][Fe4O2(O2CMe)7(Pic)2]. J. Chem. Soc., Dalton Trans. 1998, 4, 719–726. [Google Scholar] [CrossRef]
- Gkioni, C.; Boudalis, A.K.; Sanakis, Y.; Psycharis, V.; Raptopoulou, C.P. 2-Pyridyl Ketone Oximes in Iron(III) Carboxylate Chemistry: Synthesis, Structural and Physical Studies of Tetranuclear Clusters Containing the [Fe4(μ3-O)2]8+ ‘Butterfly’ Core. Polyhedron 2009, 28, 3221–3226. [Google Scholar] [CrossRef]
- Dutta, A.K.; Maji, S.K.; Dutta, S.; Robert Lucas, C.; Adhikary, B. Synthesis, Structural and Magnetic Properties of Oxo-, Chloroacetato-Bridged Tetra-Nuclear Iron(III) Complex. J. Mol. Struct. 2012, 1029, 68–74. [Google Scholar] [CrossRef]
- Dutta, A.K.; Maji, S.K.; Dutta, S.; Robert Lucas, C.; Adhikary, B. Syntheses, Crystal Structure, Spectroscopic, Redox and Magnetic Properties of Oxo- and Carboxylato-Bridged Polynuclear Iron(III) Complexes with Phenolate- and Pyridine-Substituted Benzimidazole Ligands. Polyhedron 2012, 44, 34–43. [Google Scholar] [CrossRef]
- Luo, Y.-H.; Chen, C.; Li, Y.-J.; Wang, J.-W.; Sun, B.-W. Protonation-Induced Color Change of an Amino Group Functionalized [Fe4(Μ3-O)2]8+ Cluster. Dyes Pigment. 2017, 143, 239–244. [Google Scholar] [CrossRef]
- Pal, C.K.; Mahato, S.; Yadav, H.R.; Shit, M.; Choudhury, A.R.; Biswas, B. Bio-Mimetic of Catecholase and Phosphatase Activity by a Tetra-Iron(III) Cluster. Polyhedron 2019, 174, 114156. [Google Scholar] [CrossRef]
- Lutsenko, I.A.; Kiskin, M.A.; Baravikov, D.E.; Nelyubina, Y.V.; Primakov, P.V.; Eremenko, I.L. Chemical Design of Heterometallic Carboxylate Structures with Fe3+ and Ag+ Ions as a Rational Synthetic Approach. Mendeleev Commun. 2021, 31, 628–630. [Google Scholar] [CrossRef]
- Dimiza, F.; Hatzidimitriou, A.G.; Sanakis, Y.; Papadopoulos, A.N.; Psomas, G. Trinuclear and Tetranuclear Iron(III) Complexes with Fenamates: Structure and Biological Profile. J. Inorg. Biochem. 2021, 218, 111410. [Google Scholar] [CrossRef] [PubMed]
- Halogen Bonding: Fundamentals and Applications; Metrangolo, P., Resnati, G., Arman, H.D., Eds.; Structure and Bonding; Springer: Berlin, Germany, 2008; ISBN 978-3-540-74329-3. [Google Scholar]
- Forni, A.; Pieraccini, S.; Rendine, S.; Gabas, F.; Sironi, M. Halogen-Bonding Interactions with π Systems: CCSD(T), MP2, and DFT Calculations. ChemPhysChem 2012, 13, 4224–4234. [Google Scholar] [CrossRef] [PubMed]
- Forni, A.; Pieraccini, S.; Rendine, S.; Sironi, M. Halogen Bonds with Benzene: An Assessment of DFT Functionals. J. Comput. Chem. 2014, 35, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Forni, A.; Pieraccini, S.; Franchini, D.; Sironi, M. Assessment of DFT Functionals for QTAIM Topological Analysis of Halogen Bonds with Benzene. J. Phys. Chem. A 2016, 120, 9071–9080. [Google Scholar] [CrossRef]
- Kahn, O. Molecular Magnetism; Wiley-VCH: Weinheim, Germany, 1993. [Google Scholar]
- Kambe, K. On the Paramagnetic Susceptibilities of Some Polynuclear Complex Salts. J. Phys. Soc. Jpn. 1950, 5, 48–51. [Google Scholar] [CrossRef]
- Press, W.H.; Teukolsky, S.A.; Vetterling, W.T.; Flannery, B.P. Numerical Recipes in C: The Art of Scientific Computing, 2nd ed.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 1992; ISBN 978-0-521-43108-8. [Google Scholar]
- O’Connor, C.J. Magnetochemistry-Advances in Theory and Experimentation. In Progress in Inorganic Chemistry; Lippard, S.J., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; pp. 203–283. ISBN 978-0-470-16630-7. [Google Scholar]
- Chaudhuri, P.; Rentschler, E.; Birkelbach, F.; Krebs, C.; Bill, E.; Weyhermüller, T.; Flörke, U. Ground Spin State Variation in Carboxylate-Bridged Tetranuclear [Fe2Mn2O2]8+ Cores and a Comparison with Their [Fe4O2]8+ and [Mn4O2]8+ Congeners. Eur. J. Inorg. Chem. 2003, 2003, 541–555. [Google Scholar] [CrossRef]
- Jerzykiewicz, L.B.; Utko, J.; Duczmal, M.; Starynowicz, P.; Sobota, P. Tetranuclear Manganese Complexes with [MnII4] and [MnII2MnIII2] Units: Syntheses, Structures, Magnetic Properties, and DFT Study. Eur. J. Inorg. Chem. 2010, 2010, 4492–4498. [Google Scholar] [CrossRef]
- Mitchell, K.J.; Abboud, K.A.; Christou, G. Magnetostructural Correlation for High-Nuclearity Iron(III)/Oxo Complexes and Application to Fe5, Fe6, and Fe8 Clusters. Inorg. Chem. 2016, 55, 6597–6608. [Google Scholar] [CrossRef]
- Carey, F.A.; Sundberg, R.J. Advanced Organic Chemistry, 5th ed.; Springer: New York, NY, USA, 2007; ISBN 978-0-387-44897-8. [Google Scholar]
- Takahata, Y.; Chong, D.P. Estimation of Hammett Sigma Constants of Substituted Benzenes through Accurate Density-Functional Calculation of Core-Electron Binding Energy Shifts. Int. J. Quantum Chem. 2005, 103, 509–515. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atoms in Molecules: A Quantum Theory; The International Series of Monographs on Chemistry; Oxford University Press: Oxford, UK; New York, NY, USA, 1994; ISBN 978-0-19-855865-1. [Google Scholar]
- Rigamonti, L.; Rusconi, M.; Forni, A.; Pasini, A. The Role of the Atomic Charges on the Ligands and Platinum(II) in Affecting the Cis and Trans Influences in [PtXL(PPh3)2]+ Complexes (X = NO3, Cl, Br, I; L = 4-Substituted Pyridines, Amines, PPh3). A 31P NMR and DFT Investigation. Dalton Trans. 2011, 40, 10162. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SADABS-2008/1—Bruker AXS Area Detector Scaling and Absorption Correction; Bruker AXS: Madison, WI, USA, 2008. [Google Scholar]
- Altomare, A.; Burla, M.C.; Camalli, M.; Cascarano, G.L.; Giacovazzo, C.; Guagliardi, A.; Moliterni, A.G.G.; Polidori, G.; Spagna, R. SIR 97: A New Tool for Crystal Structure Determination and Refinement. J. Appl. Crystallogr. 1999, 32, 115–119. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L.J. WinGX and ORTEP for Windows: An Update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision A.02; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Vosko, S.H.; Wilk, L.; Nusair, M. Accurate Spin-Dependent Electron Liquid Correlation Energies for Local Spin Density Calculations: A Critical Analysis. Can. J. Phys. 1980, 58, 1200–1211. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 Suite of Density Functionals for Main Group Thermochemistry, Thermochemical Kinetics, Noncovalent Interactions, Excited States, and Transition Elements: Two New Functionals and Systematic Testing of Four M06-Class Functionals and 12 Other Functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Todd, K.A. AIMAll (Version 12.05.09); TK Gristmill Software: Overland Park KS, USA, 2012. [Google Scholar]
- Bain, G.A.; Berry, J.F. Diamagnetic Corrections and Pascal’s Constants. J. Chem. Educ. 2008, 85, 532–536. [Google Scholar] [CrossRef]
| 1·1.5iPr2O 3 | 2·2iPr2O | 3 | |
|---|---|---|---|
| Fe1–O17 | 1.784(5), 1.783(5) | 1.7994(11) | – |
| 1.818 | 1.825 | 1.829 | |
| Fe4–O18 | 1.797(5), 1.789(5) | 1.8040(11) | – |
| 1.818 | 1.825 | 1.829 | |
| Fe2–O17 | 1.948(5), 1.979(5) | 1.9380(12) | – |
| 1.951 | 1.947 | 1.944 | |
| Fe2–O18 | 1.967(5), 1.964(5) | 1.9461(11) | – |
| 1.951 | 1.946 | 1.944 | |
| Fe3–O17 | 1.970(5), 1.948(5) | 1.9567(11) | – |
| 1.951 | 1.946 | 1.944 | |
| Fe3–O18 | 1.939(5), 1.967(5) | 1.9389(12) | – |
| 1.951 | 1.947 | 1.944 | |
| Fe1–O1 | 1.865(5), 1.865(5) | 1.9021(13) | – |
| 1.892 | 1.891 | 1.891 | |
| Fe1–O5 | 1.925(5), 1.925(5) | 1.8662(12) | – |
| 1.892 | 1.889 | 1.891 | |
| Fe1–N1 | 2.160(6), 2.165(7) | 2.1636(11) | – |
| 2.170 | 2.158 | 2.150 | |
| Fe1–N4 | 2.193(6), 2.171(6) | 2.1930(15) | – |
| 2.170 | 2.157 | 2.150 | |
| Fe2–O4 | 1.964(5), 1.924(5) | 1.9698(13) | – |
| 1.956 | 1.960 | 1.967 | |
| Fe2–O13 | 1.954(5), 1.915(6) | 1.9319(11) | – |
| 1.956 | 1.959 | 1.967 | |
| Fe2–N3 | 2.211(6), 2.198(7) | 2.2609(12) | – |
| 2.225 | 2.224 | 2.211 | |
| Fe2–N10 | 2.207(7), 2.233(6) | 2.2729(13) | – |
| 2.224 | 2.224 | 2.211 | |
| Fe1···Fe2 | 3.426(2), 3.427(2) | 3.4241(4) | – |
| 3.419 | 3.424 | 3.427 | |
| Fe1···Fe3 | 3.402(2), 3.406(2) | 3.3738(5) | – |
| 3.419 | 3.426 | 3.427 | |
| Fe4···Fe2 | 3.413(2), 3.415(2) | 3.4210(5) | – |
| 3.419 | 3.426 | 3.427 | |
| Fe4···Fe3 | 3.420(2), 3.428(2) | 3.3736(4) | – |
| 3.419 | 3.424 | 3.427 | |
| Fe1···Fe4 | 6.168(2), 6.172(2) | 6.1128(7) | – |
| 6.155 | 6.174 | 6.183 | |
| Fe2···Fe3 | 2.929(2), 2.937(2) | 2.9683(4) | – |
| 2.980 | 2.965 | 2.957 |
| 1 (NO2) | 2 (Cl) 1 | 3 (OMe) | |
|---|---|---|---|
| Jbb (cm–1) | –0.94 ± 0.06 | –41.7 ± 0.2 | –14.0 ± 0.3 |
| Jwb (cm–1) | –1.20 ± 0.02 | –25.2 ± 0.1 | –8.2 ± 0.1 |
| Jbb/Jwb | 0.79 ± 0.05 | 1.66 ± 0.01 | 1.71 ± 0.04 |
| Ground state | |5,5,0〉 | |3,5,2〉 | |3,5,2〉 |
| 1 (NO2) | 2 (Cl) | 3 (OMe) | |
|---|---|---|---|
| Feb | 1.899 | 1.898 | 1.897 |
| Few | 1.836 | 1.838 | 1.839 |
| μ3-O | –1.204 | –1.207 | –1.208 |
| Σ{Fe4(μ3-O)2} | 5.062 | 5.058 | 5.056 |
| ρBCP (e Å–3) | ∇2ρBCP (e Å–5) | GBCP (Hartree Å–3) | VBCP (Hartree Å–3) | HBCP (Hartree Å–3) | |
|---|---|---|---|---|---|
| Compound 1 | |||||
| Feb–O | 0.628 | 11.7 | 0.132 | –0.143 | –0.011 |
| Few–O | 0.889 | 16.9 | 0.210 | –0.245 | –0.035 |
| Compound 2 | |||||
| Feb–O | 0.636 | 11.9 | 0.134 | –0.146 | –0.012 |
| Few–O | 0.870 | 16.6 | 0.205 | –0.237 | –0.032 |
| Compound 3 | |||||
| Feb–O | 0.640 | 12.0 | 0.135 | –0.147 | –0.012 |
| Few–O | 0.863 | 16.5 | 0.202 | –0.234 | –0.032 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchi, L.; Carlino, S.; Castellano, C.; Demartin, F.; Forni, A.; Ferretti, A.M.; Ponti, A.; Pasini, A.; Rigamonti, L. Substituent-Guided Cluster Nuclearity for Tetranuclear Iron(III) Compounds with Flat {Fe4(μ3-O)2} Butterfly Core. Int. J. Mol. Sci. 2023, 24, 5808. https://doi.org/10.3390/ijms24065808
Marchi L, Carlino S, Castellano C, Demartin F, Forni A, Ferretti AM, Ponti A, Pasini A, Rigamonti L. Substituent-Guided Cluster Nuclearity for Tetranuclear Iron(III) Compounds with Flat {Fe4(μ3-O)2} Butterfly Core. International Journal of Molecular Sciences. 2023; 24(6):5808. https://doi.org/10.3390/ijms24065808
Chicago/Turabian StyleMarchi, Lorenzo, Stefano Carlino, Carlo Castellano, Francesco Demartin, Alessandra Forni, Anna M. Ferretti, Alessandro Ponti, Alessandro Pasini, and Luca Rigamonti. 2023. "Substituent-Guided Cluster Nuclearity for Tetranuclear Iron(III) Compounds with Flat {Fe4(μ3-O)2} Butterfly Core" International Journal of Molecular Sciences 24, no. 6: 5808. https://doi.org/10.3390/ijms24065808
APA StyleMarchi, L., Carlino, S., Castellano, C., Demartin, F., Forni, A., Ferretti, A. M., Ponti, A., Pasini, A., & Rigamonti, L. (2023). Substituent-Guided Cluster Nuclearity for Tetranuclear Iron(III) Compounds with Flat {Fe4(μ3-O)2} Butterfly Core. International Journal of Molecular Sciences, 24(6), 5808. https://doi.org/10.3390/ijms24065808









