Landscape of BCL2 Resistance Mutations in a Real-World Cohort of Patients with Relapsed/Refractory Chronic Lymphocytic Leukemia Treated with Venetoclax
Abstract
1. Introduction
2. Results
2.1. Molecular and Cytogenetic Characteristics of the Study Cohort
2.2. Sensitive Screening for BCL2 G101V and D103Y
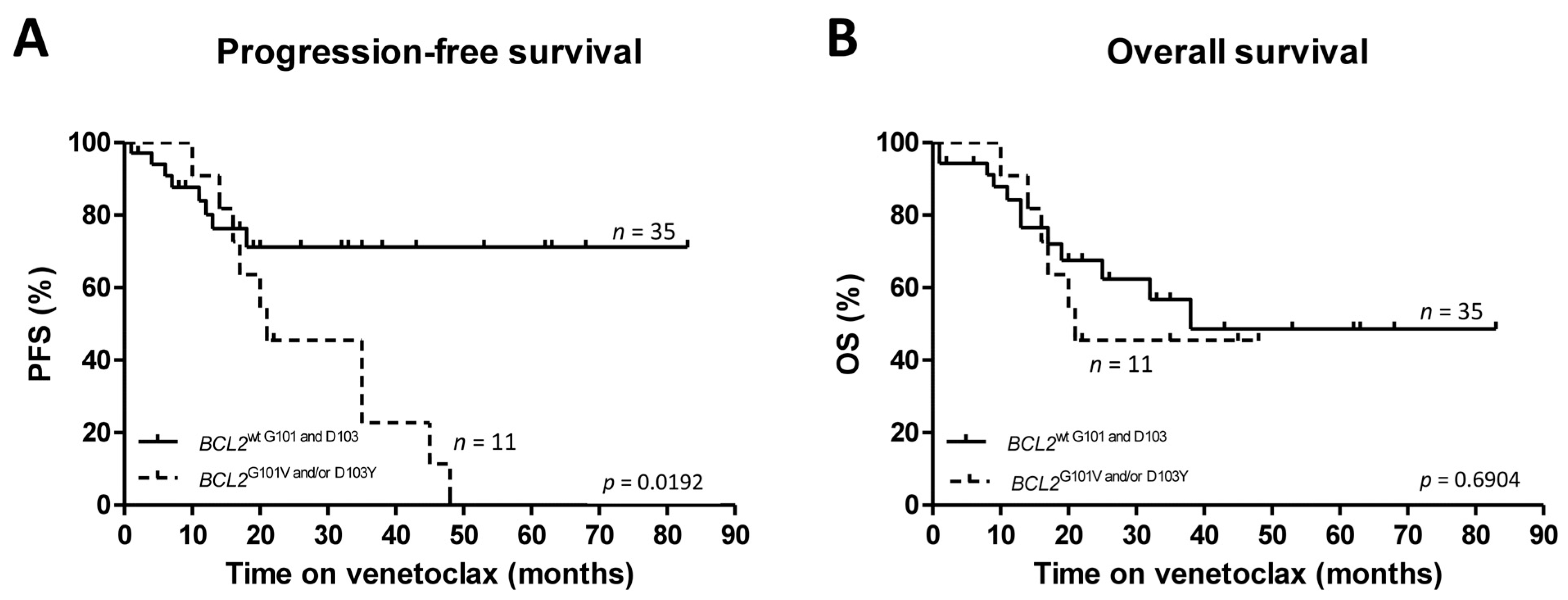
2.3. Management of Secondary Venetoclax Resistance in Patients with G101V and/or D103Y
2.4. Additional BCL2 Mutations Revealed by Targeted Next-Generation Sequencing
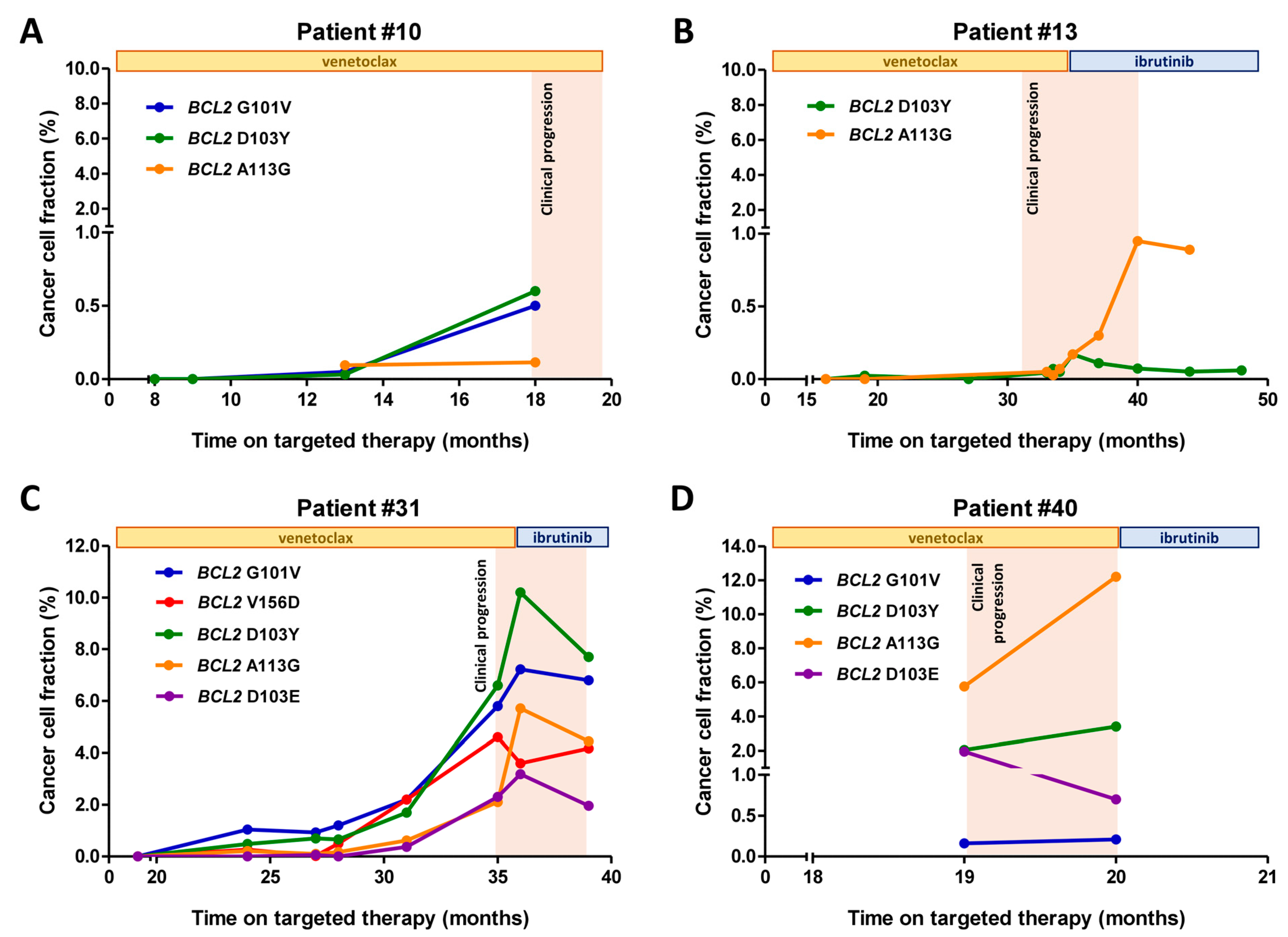
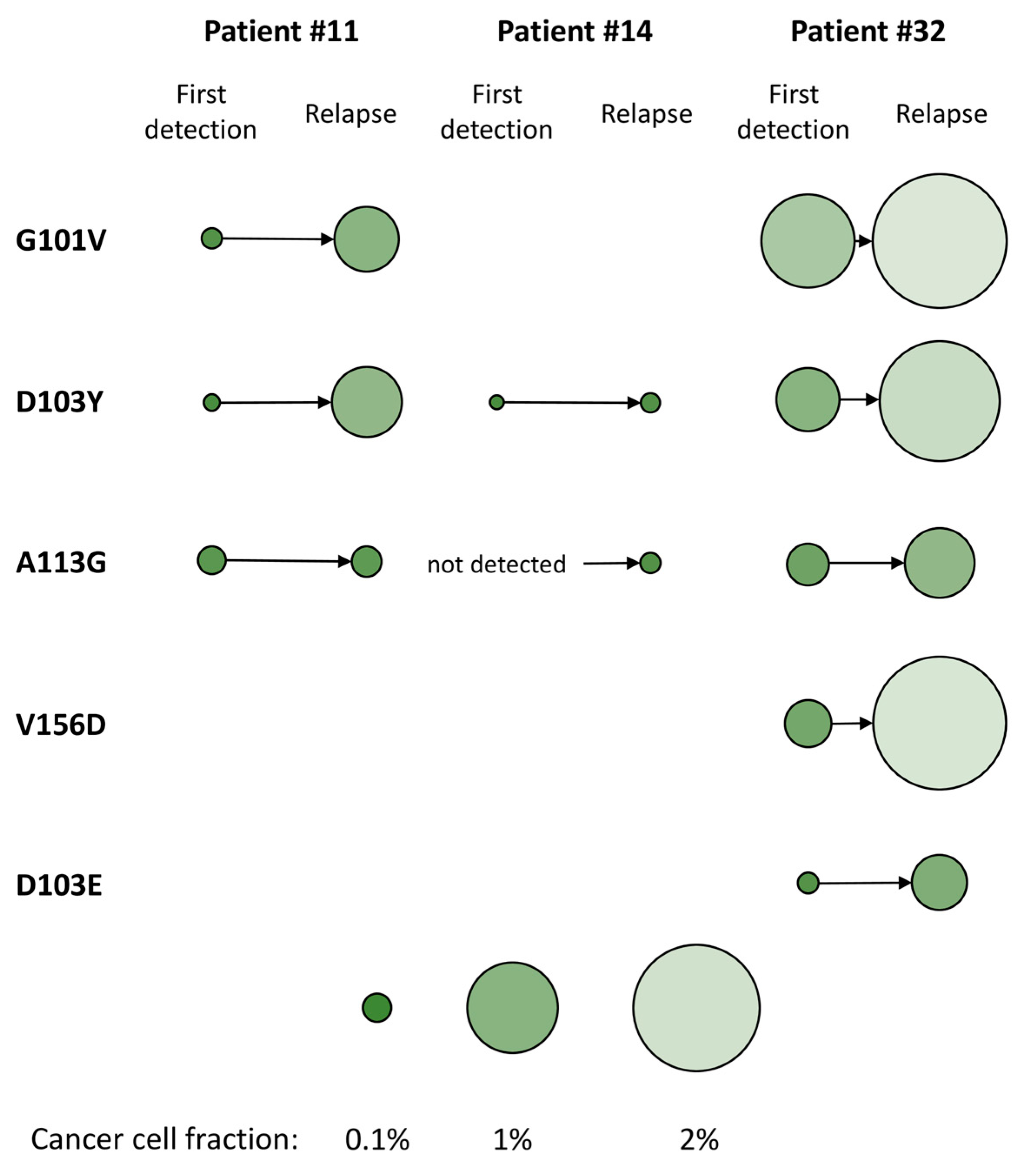
2.5. Time-Resolved Monitoring of Co-Occurring BCL2 Resistance Mutations
2.6. Secondary Venetoclax Resistance in Cases with Wild-Type BCL2
3. Discussion
4. Materials and Methods
4.1. Patient Samples
4.2. Droplet Digital PCR
4.3. Targeted Ultra-Deep Next-Generation Sequencing
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dohner, H.; Stilgenbauer, S.; Benner, A.; Leupolt, E.; Krober, A.; Bullinger, L.; Dohner, K.; Bentz, M.; Lichter, P. Genomic Aberrations and Survival in Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2000, 343, 1910–1916. [Google Scholar] [CrossRef] [PubMed]
- Byrd, J.C.; Furman, R.R.; Coutre, S.E.; Flinn, I.W.; Burger, J.A.; Blum, K.; Sharman, J.P.; Wierda, W.; Zhao, W.; NHeerema, A.; et al. Ibrutinib Treatment for First-Line and Relapsed/Refractory Chronic Lymphocytic Leukemia: Final Analysis of the Pivotal Phase Ib/Ii Pcyc-1102 Study. Clin. Cancer Res. 2020, 26, 3918–3927. [Google Scholar] [CrossRef]
- Stilgenbauer, S.; Eichhorst, B.; Schetelig, J.; Hillmen, P.; Seymour, J.F.; Coutre, S.; Jurczak, W.; Mulligan, S.P.; Schuh, A.; Assouline, S.; et al. Venetoclax for Patients with Chronic Lymphocytic Leukemia with 17p Deletion: Results from the Full Population of a Phase Ii Pivotal Trial. J. Clin. Oncol. 2018, 36, 1973–1980. [Google Scholar] [CrossRef]
- Seymour, J.F.; Kipps, T.J.; Eichhorst, B.F.; D’Rozario, J.; Owen, C.J.; Assouline, S.; Lamanna, N.; Robak, T.; de la Serna, J.; Jaeger, U.; et al. Enduring Undetectable Mrd and Updated Outcomes in Relapsed/Refractory Cll after Fixed-Duration Venetoclax-Rituximab. Blood 2022, 140, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.W.; Davids, M.S.; Pagel, J.M.; Kahl, B.S.; Puvvada, S.D.; Gerecitano, J.F.; Kipps, T.J.; Anderson, M.A.; Brown, J.R.; Gressick, L.; et al. Targeting Bcl2 with Venetoclax in Relapsed Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2016, 374, 311–322. [Google Scholar] [CrossRef]
- Seymour, J.F.; Kipps, T.J.; Eichhorst, B.; Hillmen, P.; D’Rozario, J.; Assouline, S.; Owen, C.; Gerecitano, J.; Robak, T.; De la Serna, J.; et al. Venetoclax-Rituximab in Relapsed or Refractory Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2018, 378, 1107–1120. [Google Scholar] [CrossRef]
- Fischer, K.; Al-Sawaf, O.; Bahlo, J.; Fink, A.-M.; Tandon, M.; Dixon, M.; Robrecht, S.; Warburton, S.; Humphrey, K.; Samoylova, O.; et al. Venetoclax and Obinutuzumab in Patients with Cll and Coexisting Conditions. N. Engl. J. Med. 2019, 380, 2225–2236. [Google Scholar] [CrossRef]
- Mato, A.R.; Tam, C.; Allan, J.; Brander, D.; Pagel, J.; Ujjani, C.; Hill, B.; Lamanna, N.; Lansigan, F.; Jacobs, R.; et al. Disease and Patient Characteristics, Patterns of Care, Toxicities, and Outcomes of Chronic Lymphocytic Leukemia (Cll) Patients Treated with Venetoclax: A Multicenter Study of 204 Patients. Blood 2017, 130, 4315. [Google Scholar]
- Al-Sawaf, O.; Zhang, C.; Tandon, M.; Sinha, A.; Fink, A.; Robrecht, S.; Samoylova, O.; Liberati, A.; Pinilla-Ibarz, J.; Opat, S.; et al. Venetoclax Plus Obinutuzumab Versus Chlorambucil Plus Obinutuzumab for Previously Untreated Chronic Lymphocytic Leukaemia (Cll14): Follow-up Results from a Multicentre, Open-Label, Randomised, Phase 3 Trial. Lancet Oncol. 2020, 21, 1188–1200. [Google Scholar] [CrossRef]
- Blombery, P. Mechanisms of Intrinsic and Acquired Resistance to Venetoclax in B-Cell Lymphoproliferative Disease. Leuk. Lymphoma 2019, 61, 257–262. [Google Scholar] [CrossRef]
- Anderson, M.A.; Tam, C.; Lew, T.E.; Juneja, S.; Juneja, M.; Westerman, D.; Wall, M.; Lade, S.; Gorelik, A.; Huang, D.C.S.; et al. Clinicopathological Features and Outcomes of Progression of Cll on the Bcl2 Inhibitor Venetoclax. Blood 2017, 129, 3362–3370. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, R.; Tian, L.; Anderson, M.; Flensburg, C.; Jarratt, A.; Garnham, A.; Jabbari, J.; Peng, H.; Lew, T.; Teh, C.; et al. Single-Cell Multiomics Reveal the Scale of Multi-Layered Adaptations Enabling Cll Relapse During Venetoclax Therapy. Blood 2022, 140, 2127–2141. [Google Scholar] [CrossRef] [PubMed]
- Blombery, P.; Anderson, M.A.; Gong, J.-N.; Thijssen, R.; Birkinshaw, R.W.; Thompson, E.R.; Teh, C.E.; Nguyen, T.; Xu, Z.; Flensburg, C.; et al. Acquisition of the Recurrent Gly101val Mutation in Bcl2 Confers Resistance to Venetoclax in Patients with Progressive Chronic Lymphocytic Leukemia. Cancer Discov. 2019, 9, 342–353. [Google Scholar] [CrossRef]
- Tausch, E.; Close, W.; Dolnik, A.; Bloehdorn, J.; Chyla, B.; Bullinger, L.; Döhner, H.; Mertens, D.; Stilgenbauer, S. Venetoclax Resistance and Acquired Bcl2 Mutations in Chronic Lymphocytic Leukemia. Haematologica 2019, 104, e434–e437. [Google Scholar] [CrossRef] [PubMed]
- Bojarczuk, K.; Sasi, B.K.; Gobessi, S.; Innocenti, I.; Pozzato, G.; Laurenti, L.; Efremov, D. Bcr Signaling Inhibitors Differ in Their Ability to Overcome Mcl-1-Mediated Resistance of Cll B Cells to Abt-199. Blood 2016, 127, 3192–3201. [Google Scholar] [CrossRef]
- Thomalla, D.; Beckmann, L.; Grimm, C.; Oliverio, M.; Meder, L.; Herling, C.D.; Nieper, P.; Feldmann, T.; Merkel, O.; Lorsy, E.; et al. Deregulation and Epigenetic Modification of Bcl2-Family Genes Cause Resistance to Venetoclax in Hematologic Malignancies. Blood 2022, 140, 2113–2126. [Google Scholar] [CrossRef]
- Herling, C.D.; Abedpour, N.; Weiss, J.; Schmitt, A.; Jachimowicz, R.D.; Merkel, O.; Cartolano, M.; Oberbeck, S.; Mayer, P.; Berg, V.; et al. Clonal Dynamics Towards the Development of Venetoclax Resistance in Chronic Lymphocytic Leukemia. Nat. Commun. 2018, 9, 727. [Google Scholar] [CrossRef]
- Guièze, R.; Liu, V.M.; Rosebrock, D.; Jourdain, A.A.; Hernández-Sánchez, M.; Zurita, A.M.; Sun, J.; Hacken, E.T.; Baranowski, K.; Thompson, P.A.; et al. Mitochondrial Reprogramming Underlies Resistance to Bcl-2 Inhibition in Lymphoid Malignancies. Cancer Cell 2019, 36, 369–384. [Google Scholar] [CrossRef]
- Fresquet, V.; Rieger, M.; Carolis, C.; García-Barchino, M.J.; Martinez-Climent, J.A. Acquired Mutations in Bcl2 Family Proteins Conferring Resistance to the Bh3 Mimetic Abt-199 in Lymphoma. Blood 2014, 123, 4111–4119. [Google Scholar] [CrossRef]
- Blombery, P.; Thompson, E.; Nguyen, T.; Birkinshaw, R.W.; Gong, J.-N.; Chen, X.; McBean, M.; Thijssen, R.; Conway, T.; Anderson, M.A.; et al. Multiple Bcl2 Mutations Cooccurring with Gly101val Emerge in Chronic Lymphocytic Leukemia Progression on Venetoclax. Blood 2020, 135, 773–777. [Google Scholar] [CrossRef]
- Lucas, F.; Larkin, K.; Gregory, C.T.; Orwick, S.; Doong, T.-J.; Lozanski, A.; Lozanski, G.; Misra, S.; Ngankeu, A.; Ozer, H.G.; et al. Novel Bcl2 Mutations in Venetoclax-Resistant, Ibrutinib-Resistant Cll Patients with Btk/Plcg2 Mutations. Blood 2020, 135, 2192–2195. [Google Scholar] [CrossRef] [PubMed]
- Blombery, P.; Thompson, E.; Nguyen, T.; Chen, X.; McBean, M.; Birkinshaw, R.; Czabotar, P.; Thijssen, R.; Anderson, M.; Seymour, J.; et al. Detection of Multiple Recurrent Novel Bcl2 Mutations Co-Occurring with Bcl2 Gly101val in Patients with Chronic Lymphocytic Leukemia on Long Term Venetoclax. Blood 2019, 134, 171. [Google Scholar] [CrossRef]
- Weiss, J.; Peifer, M.; Herling, C.D.; Frenzel, L.P.; Hallek, M. Acquisition of the Recurrent Gly101val Mutation in Bcl2 Confers Resistance to Venetoclax in Patients with Progressive Chronic Lymphocytic Leukemia (Comment to Tausch et al.). Haematologica 2019, 104, e540. [Google Scholar] [CrossRef]
- Hallek, M.; Cheson, B.D.; Catovsky, D.; Caligaris-Cappio, F.; Dighiero, G.; Döhner, H.; Hillmen, P.; Keating, M.; Montserrat, E.; Chiorazzi, N.; et al. Iwcll Guidelines for Diagnosis, Indications for Treatment, Response Assessment, and Supportive Management of Cll. Blood 2018, 131, 2745–2760. [Google Scholar] [CrossRef] [PubMed]
- Coutre, S.; Choi, M.; Furman, R.R.; Eradat, H.; Heffner, L.; Jones, J.A.; Chyla, B.; Zhou, L.; Agarwal, S.; Waskiewicz, T.; et al. Venetoclax for Patients with Chronic Lymphocytic Leukemia Who Progressed During or after Idelalisib Therapy. Blood 2018, 131, 1704–1711. [Google Scholar] [CrossRef]
- Jones, J.A.; Mato, A.; Wierda, W.; Davids, M.; Choi, M.; Cheson, B.; Furman, R.; Lamanna, N.; Barr, P.; Zhou, L.; et al. Venetoclax for Chronic Lymphocytic Leukaemia Progressing after Ibrutinib: An Interim Analysis of a Multicentre, Open-Label, Phase 2 Trial. Lancet Oncol. 2018, 19, 65–75. [Google Scholar] [CrossRef]
- Mato, A.R.; Thompson, M.; Allan, J.N.; Brander, D.M.; Pagel, J.M.; Ujjani, C.S.; Hill, B.T.; Lamanna, N.; Lansigan, F.; Jacobs, R.; et al. Real-World Outcomes and Management Strategies for Venetoclax-Treated Chronic Lymphocytic Leukemia Patients in the United States. Haematologica 2018, 103, 1511–1517. [Google Scholar] [CrossRef]
- Chyla, B.J.; Popovic, R.; Lu, C.; Dunbar, F.; Quarless, D.; Robinson, K.; Warder, S.; Jacobson, A.; Zhou, L.; Souers, A.; et al. Identification of Recurrent Genomic Alterations in the Apoptotic Machinery in Cll Patients Treated with Venetoclax Monotherapy. Am. J. Hematol. 2019, 134, 172. [Google Scholar] [CrossRef]
- Thompson, E.R.; Nguyen, T.; Kankanige, Y.; Markham, J.F.; Anderson, M.A.; Handunnetti, S.M.; Thijssen, R.; Yeh, P.S.; Tam, C.S.; Seymour, J.F.; et al. Single-Cell Sequencing Demonstrates Complex Resistance Landscape in Cll and Mcl Treated with Btk and Bcl2 Inhibitors. Blood Adv. 2022, 6, 503–508. [Google Scholar] [CrossRef]
- Souers, A.J.; Leverson, J.D.; Boghaert, E.R.; Ackler, S.L.; Catron, N.D.; Chen, J.; Dayton, B.D.; Ding, H.; Enschede, S.H.; Fairbrother, W.J.; et al. Abt-199, a Potent and Selective Bcl-2 Inhibitor, Achieves Antitumor Activity While Sparing Platelets. Nat. Med. 2013, 19, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Hallek, M.; Al-Sawaf, O. Chronic Lymphocytic Leukemia: 2022 Update on Diagnostic and Therapeutic Procedures. Am. J. Hematol. 2021, 96, 1679–1705. [Google Scholar] [CrossRef]
- Kater, A.P.; Wu, J.Q.; Kipps, T.; Eichhorst, B.; Hillmen, P.; D’Rozario, J.; Assouline, S.; Owen, C.; Robak, T.; de la Serna, J.; et al. Venetoclax Plus Rituximab in Relapsed Chronic Lymphocytic Leukemia: 4-Year Results and Evaluation of Impact of Genomic Complexity and Gene Mutations from the Murano Phase Iii Study. J. Clin. Oncol. 2020, 38, 4042–4054. [Google Scholar] [CrossRef]
- Hampel, P.J.; Rabe, K.G.; Call, T.G.; Ding, W.; Leis, J.F.; Kenderian, S.S.; Muchtar, E.; Wang, Y.; Koehler, A.B.; Parrondo, R.; et al. Combined Ibrutinib and Venetoclax for Treatment of Patients with Ibrutinib-Resistant or Double-Refractory Chronic Lymphocytic Leukaemia. Br. J. Haematol. 2022, 199, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Roeker, L.E.; Dreger, P.; Brown, J.R.; Lahoud, O.B.; Eyre, T.A.; Brander, D.M.; Skarbnik, A.; Coombs, C.C.; Kim, H.T.; Davids, M.; et al. Allogeneic Stem Cell Transplantation for Chronic Lymphocytic Leukemia in the Era of Novel Agents. Blood Adv. 2020, 4, 3977–3989. [Google Scholar] [CrossRef] [PubMed]
- Flinn, I.W.; Hillmen, P.; Montillo, M.; Nagy, Z.; Illes, A.; Etienne, G.; Delgado, J.; Kuss, B.J.; Tam, C.S.; Gasztonyi, Z.; et al. The Phase 3 Duo Trial: Duvelisib Vs Ofatumumab in Relapsed and Refractory Cll/Sll. Blood 2018, 132, 2446–2455. [Google Scholar] [CrossRef] [PubMed]
- Tannan, N.B.; Manzari, M.T.; Herviou, L.; Ferreira, M.D.S.; Hagen, C.J.; Kiguchi, H.; Manova-Todorova, K.; Seshan, V.E.; de Stanchina, E.; Heller, D.A.; et al. Tumor-Targeted Nanoparticles Improve the Therapeutic Index of Bcl2 and Mcl1 Dual Inhibition. Blood 2021, 137, 2057–2069. [Google Scholar] [CrossRef]
- Lin, V.S.; Lew, T.E.; Handunnetti, S.M.; Blombery, P.; Nguyen, T.; Westerman, D.A.; Kuss, B.J.; Tam, C.S.; Roberts, A.W.; Seymour, J.F.; et al. Btk Inhibitor Therapy Is Effective in Patients with Cll Resistant to Venetoclax. Blood 2020, 135, 2266–2270. [Google Scholar] [CrossRef]
- Brown, J.R.; Davids, M.S.; Chang, J.; Ma, S.; Biondo, J.; Mobasher, M.; Mun, Y.; Mato, A.R. Outcomes of Ibrutinib Therapy Given after Prior Venetoclax Therapy in Ibrutinib-Naïve Patients with Relapsed/Refractory (R/R) Chronic Lymphocytic Leukemia (Cll). Blood 2018, 132, 5556. [Google Scholar] [CrossRef]
- Greil, R.; Fraser, G.; Leber, B.; Marks, R.; Quaresmini, G.; Middeke, J.M.; Semenzato, G.; Schary, W.; Boyer, M.; Breuleux, M.; et al. Efficacy and Safety of Ibrutinib (Ibr) after Venetoclax (Ven) Treatment in Ibr-Naïve Patients with Relapsed/Refractory (R/R) Chronic Lymphocytic Leukemia (Cll): Follow-up of Patients from the Murano Study. Blood 2018, 132, 5548. [Google Scholar] [CrossRef]
- Lew, T.E.; Lin, V.S.; Cliff, E.R.S.; Blombery, P.; Thompson, E.R.; Handunnetti, S.M.; Westerman, D.A.; Kuss, B.J.; Tam, C.S.; Huang, D.C.S.; et al. Outcomes of Patients with Cll Sequentially Resistant to Both Bcl2 and Btk Inhibition. Blood Adv. 2021, 5, 4054–4058. [Google Scholar] [CrossRef]
- Scarfò, L.; Heltai, S.; Albi, E.; Scarano, E.; Schiattone, L.; Farina, L.; Moia, R.; Deodato, M.; Ferrario, A.; Motta, M.; et al. Minimal Residual Disease-Driven Treatment Intensification with Sequential Addition of Ibrutinib to Venetoclax in R/R Cll. Blood 2022, 140, 2348–2357. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.A.; Smith, V.M.; Jayne, S.; Drewes, C.; Bens, S.; Siebert, R.; Dyer, M.J.S.; Walter, H.S. Successful Retreatment with Venetoclax in a Patient with Chronic Lymphocytic Leukemia. Hemasphere 2022, 6, e752. [Google Scholar] [CrossRef] [PubMed]
- Hillmen, P.; Rawstron, A.C.; Brock, K.; Muñoz-Vicente, S.; Yates, F.J.; Bishop, R.; Boucher, R.; Macdonald, D.; Fegan, C.; McCaig, A.; et al. Ibrutinib Plus Venetoclax in Relapsed/Refractory Chronic Lymphocytic Leukemia: The Clarity Study. J. Clin. Oncol. 2019, 37, 2722–2729. [Google Scholar] [CrossRef]
- Niemann, C.U.; Levin, M.-D.; Dubois, J.; Kersting, S.; Enggaard, L.; Veldhuis, G.J.; Mous, R.; Mellink, C.H.M.; Dobber, J.A.; Poulsen, C.B.; et al. Venetoclax and Ibrutinib for Patients with Relapsed/Refractory Chronic Lymphocytic Leukemia. Blood 2021, 137, 1117–1120. [Google Scholar] [CrossRef] [PubMed]
- Bodor, C.; Kotmayer, L.; Laszlo, T.; Takacs, F.; Barna, G.; Kiss, R.; Sebestyen, E.; Nagy, T.; Hegyi, L.L.; Mikala, G.; et al. Screening and Monitoring of the Btk(C481s) Mutation in a Real-World Cohort of Patients with Relapsed/Refractory Chronic Lymphocytic Leukaemia During Ibrutinib Therapy. Br. J. Haematol. 2021, 194, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Rawstron, A.C.; Fazi, C.; Agathangelidis, A.; Villamor, N.; Letestu, R.; Nomdedeu, J.; Palacio, C.; Stehlikova, O.; Kreuzer, K.A.; Liptrot, S.; et al. A Complementary Role of Multiparameter Flow Cytometry and High-Throughput Sequencing for Minimal Residual Disease Detection in Chronic Lymphocytic Leukemia: An European Research Initiative on Cll Study. Leukemia 2015, 30, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Malcikova, J.; Tausch, E.; Rossi, D.; Sutton, L.A.; Soussi, T.; Zenz, T.; Kater, A.P.; Niemann, C.U.; Gonzalez, D.; Davi, F.; et al. Eric Recommendations for Tp53 Mutation Analysis in Chronic Lymphocytic Leukemia-Update on Methodological Approaches and Results Interpretation. Leukemia 2018, 32, 1070–1080. [Google Scholar] [CrossRef]
- Rosenquist, R.; Ghia, P.; Hadzidimitriou, A.; Sutton, L.A.; Agathangelidis, A.; Baliakas, P.; Darzentas, N.; Giudicelli, V.; Lefranc, M.P.; Langerak, A.W.; et al. Immunoglobulin Gene Sequence Analysis in Chronic Lymphocytic Leukemia: Updated Eric Recommendations. Leukemia 2017, 31, 1477–1481. [Google Scholar] [CrossRef]
- Tikkanen, T.; Leroy, B.; Fournier, J.; Risques, R.; Malcikova, J.; Soussi, T. Seshat: A Web Service for Accurate Annotation, Validation, and Analysis of Tp53 Variants Generated by Conventional and Next-Generation Sequencing. Hum. Mutat. 2018, 39, 925–933. [Google Scholar] [CrossRef]
- Bouaoun, L.; Sonkin, D.; Ardin, M.; Hollstein, M.; Byrnes, G.; Zavadil, J.; Olivier, M. Tp53 Variations in Human Cancers: New Lessons from the Iarc Tp53 Database and Genomics Data. Hum. Mutat. 2016, 37, 865–876. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotmayer, L.; László, T.; Mikala, G.; Kiss, R.; Lévay, L.; Hegyi, L.L.; Gróf, S.; Nagy, T.; Barna, G.; Farkas, P.; et al. Landscape of BCL2 Resistance Mutations in a Real-World Cohort of Patients with Relapsed/Refractory Chronic Lymphocytic Leukemia Treated with Venetoclax. Int. J. Mol. Sci. 2023, 24, 5802. https://doi.org/10.3390/ijms24065802
Kotmayer L, László T, Mikala G, Kiss R, Lévay L, Hegyi LL, Gróf S, Nagy T, Barna G, Farkas P, et al. Landscape of BCL2 Resistance Mutations in a Real-World Cohort of Patients with Relapsed/Refractory Chronic Lymphocytic Leukemia Treated with Venetoclax. International Journal of Molecular Sciences. 2023; 24(6):5802. https://doi.org/10.3390/ijms24065802
Chicago/Turabian StyleKotmayer, Lili, Tamás László, Gábor Mikala, Richárd Kiss, Luca Lévay, Lajos László Hegyi, Stefánia Gróf, Tibor Nagy, Gábor Barna, Péter Farkas, and et al. 2023. "Landscape of BCL2 Resistance Mutations in a Real-World Cohort of Patients with Relapsed/Refractory Chronic Lymphocytic Leukemia Treated with Venetoclax" International Journal of Molecular Sciences 24, no. 6: 5802. https://doi.org/10.3390/ijms24065802
APA StyleKotmayer, L., László, T., Mikala, G., Kiss, R., Lévay, L., Hegyi, L. L., Gróf, S., Nagy, T., Barna, G., Farkas, P., Weisinger, J., Nagy, Z., Balogh, A., Masszi, T., Demeter, J., Sulák, A., Kohl, Z., Alizadeh, H., Egyed, M., ... Alpár, D. (2023). Landscape of BCL2 Resistance Mutations in a Real-World Cohort of Patients with Relapsed/Refractory Chronic Lymphocytic Leukemia Treated with Venetoclax. International Journal of Molecular Sciences, 24(6), 5802. https://doi.org/10.3390/ijms24065802








