Radiation from UV-A to Red Light Induces ROS-Dependent Release of Neutrophil Extracellular Traps
Abstract
1. Introduction
2. Results
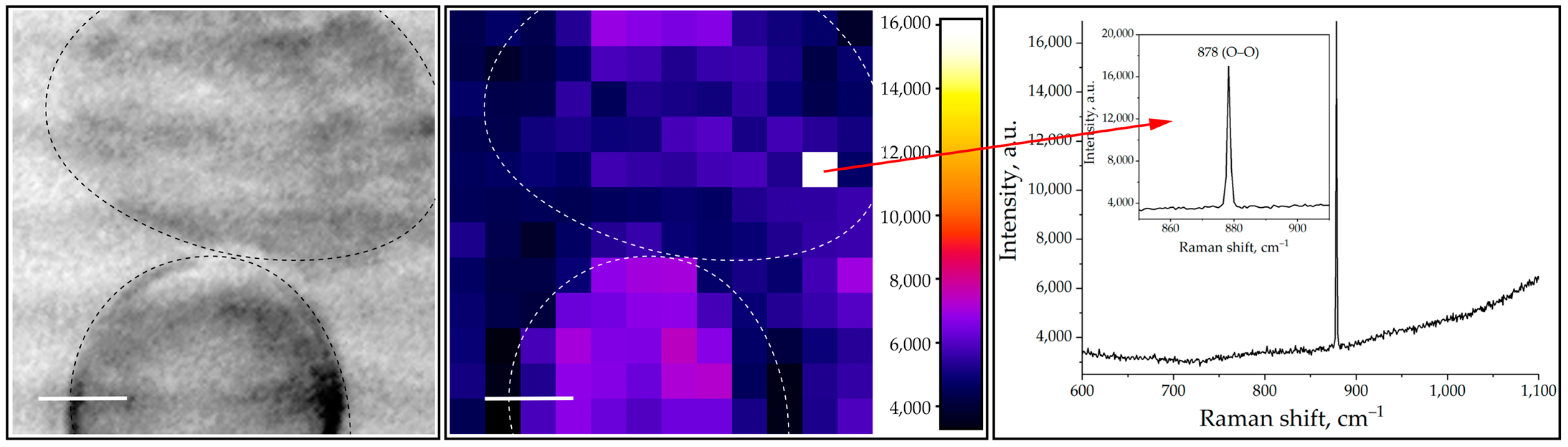
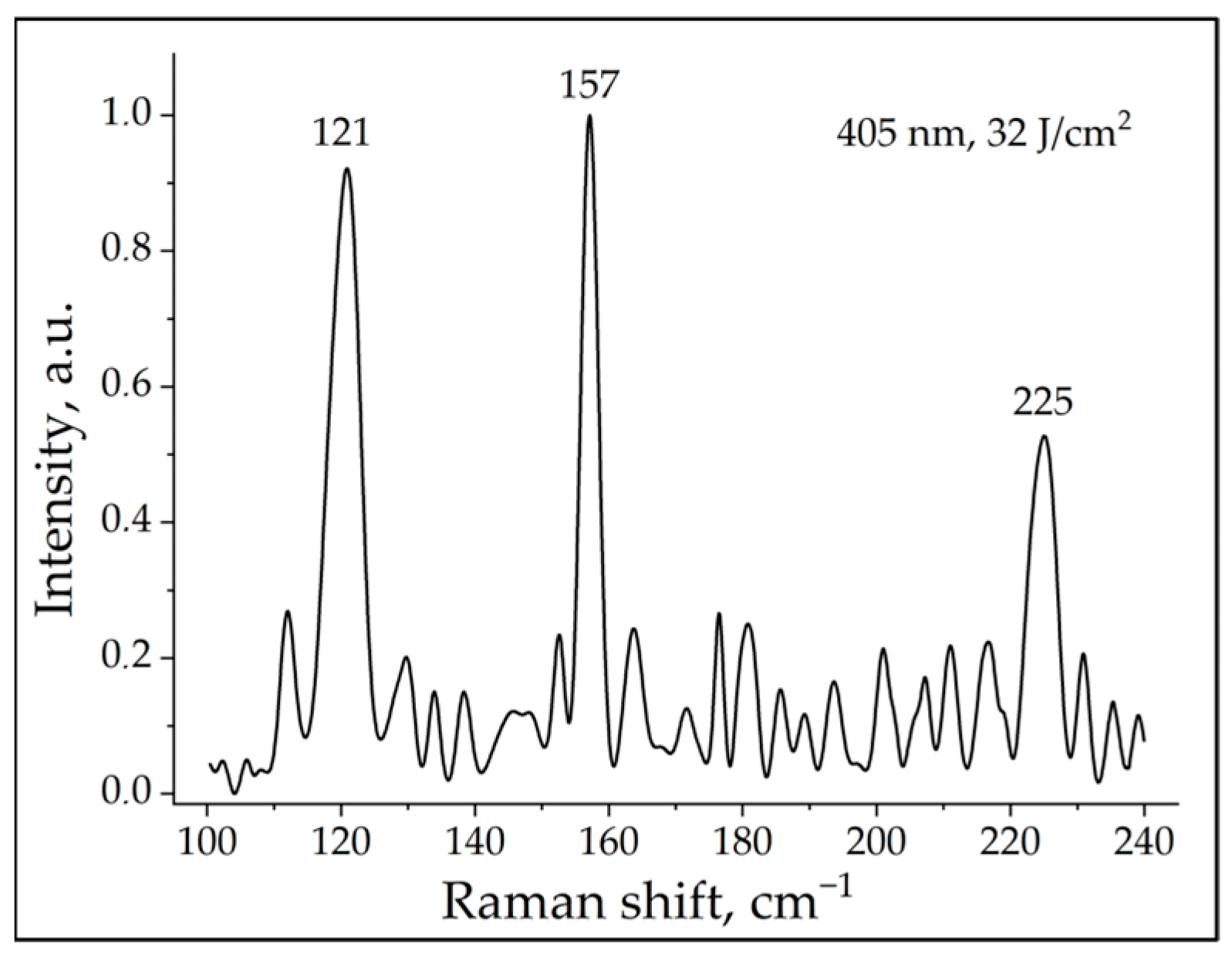
2.1. ROS Measurements with Raman Spectroscopy
2.2. NET Formation of Photoactivated Neutrophils Detected by Fluorescence Microscopy
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Isolation of Primary Human Neutrophils
4.3. Neutrophil Irradiation
4.4. Raman Spectroscopy
4.5. NETs Visualization with Fluorescent Microscopy
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Takei, H.; Araki, A.; Watanabe, H.; Ichinose, A.; Sendo, F. Rapid killing of human neutrophils by the potent activator phorbol 12-myristate 13-acetate (PMA) accompanied by changes different from typical apoptosis or necrosis. J. Leukoc. Biol. 1996, 59, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, T.A.; Abed, U.; Goosmann, C.; Hurwitz, R.; Schulze, I.; Wahn, V.; Weinrauch, Y.; Brinkmann, V.; Zychlinsky, A. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 2007, 176, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, B.E.; Grinstein, S. Unconventional roles of the NADPH oxidase: Signaling, ion homeostasis, and cell death. Sci. STKE 2007, 2007, pe11. [Google Scholar] [CrossRef] [PubMed]
- Pinegin, B.; Vorobjeva, N.; Pinegin, V. Neutrophil extracellular traps and their role in the development of chronic inflammation and autoimmunity. Autoimmun. Rev. 2015, 14, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Vorobjeva, N.V.; Pinegin, B.V. Neutrophil extracellular traps: Mechanisms of formation and role in health and disease. Biochemistry 2014, 79, 1286–1296. [Google Scholar] [CrossRef]
- Vorobjeva, N.V.; Chernyak, B.V. NETosis: Molecular Mechanisms, Role in Physiology and Pathology. Biochemistry 2020, 85, 1178–1190. [Google Scholar] [CrossRef]
- Metzler, K.D.; Goosmann, C.; Lubojemska, A.; Zychlinsky, A.; Papayannopoulos, V. A myeloperoxidase-containing complex regulates neutrophil elastase release and actin dynamics during NETosis. Cell Rep. 2014, 8, 883–896. [Google Scholar] [CrossRef]
- Neubert, E.; Bach, K.M.; Busse, J.; Bogeski, I.; Schön, M.P.; Kruss, S.; Erpenbeck, L. Blue and Long-Wave Ultraviolet Light Induce in vitro Neutrophil Extracellular Trap (NET) Formation. Front. Immunol. 2019, 10, 2428. [Google Scholar] [CrossRef]
- Zawrotniak, M.; Bartnicka, D.; Rapala-Kozik, M. UVA and UVB radiation induce the formation of neutrophil extracellular traps by human polymorphonuclear cells. J. Photochem. Photobiol. B 2019, 196, 111511. [Google Scholar] [CrossRef]
- Azzouz, D.; Palaniyar, N. ApoNETosis: Discovery of a novel form of neutrophil death with concomitant apoptosis and NETosis. Cell Death Dis. 2018, 9, 839. [Google Scholar] [CrossRef] [PubMed]
- Azzouz, D.; Khan, M.A.; Sweezey, N.; Palaniyar, N. Two-in-one: UV radiation simultaneously induces apoptosis and NETosis. Cell Death Discov. 2018, 4, 51. [Google Scholar] [CrossRef] [PubMed]
- Migliario, M.; Tonello, S.; Rocchetti, V.; Rizzi, M.; Renò, F. Near infrared laser irradiation induces NETosis via oxidative stress and autophagy. Lasers Med. Sci. 2018, 33, 1919–1924. [Google Scholar] [CrossRef] [PubMed]
- Cela, E.M.; Friedrich, A.; Paz, M.L.; Vanzulli, S.I.; Leoni, J.; González Maglio, D.H. Time-course study of different innate immune mediators produced by UV-irradiated skin: Comparative effects of short and daily versus a single harmful UV exposure. Immunology 2015, 145, 82–93. [Google Scholar] [CrossRef] [PubMed]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV radiation and the skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yang, C.; Jiang, G. Research progress on skin photoaging and oxidative stress. Postep. Dermatol. Alergol. 2021, 38, 931–936. [Google Scholar] [CrossRef] [PubMed]
- Niebauer, G.; Stockinger, L. On Senile Elastosis. Histochemical and Electron Microscopic Studies. Arch. Klin. Exp. Dermatol. 1965, 221, 122–143. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.J.; Choi, H.C.; Bata-Csorgo, Z.; Shao, Y.; Datta, S.; Wang, Z.Q.; Kang, S.; Voorhees, J.J. Ultraviolet irradiation increases matrix metalloproteinase-8 protein in human skin in vivo. J. Investig. Dermatol. 2001, 117, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Rijken, F.; Kiekens, R.C.; van den Worm, E.; Lee, P.L.; van Weelden, H.; Bruijnzeel, P.L. Pathophysiology of photoaging of human skin: Focus on neutrophils. Photochem. Photobiol. Sci. 2006, 5, 184–189. [Google Scholar] [CrossRef]
- Cho, S.; Lee, M.J.; Kim, M.S.; Lee, S.; Kim, Y.K.; Lee, D.H.; Lee, C.W.; Cho, K.H.; Chung, J.H. Infrared plus visible light and heat from natural sunlight participate in the expression of MMPs and type I procollagen as well as infiltration of inflammatory cell in human skin in vivo. J. Dermatol. Sci. 2008, 50, 123–133. [Google Scholar] [CrossRef]
- Skopelja-Gardner, S.; An, J.; Tai, J.; Tanaka, L.; Sun, X.; Hermanson, P.; Baum, R.; Kawasumi, M.; Green, R.; Gale, M., Jr.; et al. The early local and systemic Type I interferon responses to ultraviolet B light exposure are cGAS dependent. Sci. Rep. 2020, 10, 7908. [Google Scholar] [CrossRef] [PubMed]
- Skopelja-Gardner, S.; Tai, J.; Sun, X.; Tanaka, L.; Kuchenbecker, J.A.; Snyder, J.M.; Kubes, P.; Mustelin, T.; Elkon, K.B. Acute skin exposure to ultraviolet light triggers neutrophil-mediated kidney inflammation. Proc. Natl. Acad. Sci. USA 2021, 118, e2019097118. [Google Scholar] [CrossRef] [PubMed]
- Jakimiuk, K.; Gesek, J.; Atanasov, A.G.; Tomczyk, M. Flavonoids as inhibitors of human neutrophil elastase. J. Enzym. Inhib. Med. Chem. 2021, 36, 1016–1028. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.J. Tissue destruction by neutrophils. N. Engl. J. Med. 1989, 320, 365–376. [Google Scholar] [CrossRef]
- Chua, F.; Laurent, G.J. Neutrophil elastase: Mediator of extracellular matrix destruction and accumulation. Proc. Am. Thorac. Soc. 2006, 3, 424–427. [Google Scholar] [CrossRef]
- Shiraishi, M.; Nakaji, S.; Sugawara, K. The effects of UVA irradiation on human neutrophil function. Nihon Eiseigaku Zasshi 1996, 50, 1093–1102. (In Japanese) [Google Scholar] [CrossRef]
- Bohne, M.; Struy, H.; Gerber, A.; Gollnick, H. Protection against UVA damage and effects on neutrophil-derived reactive oxygen species by beta-carotene. Inflamm. Res. 1997, 46, 425–426. [Google Scholar] [CrossRef]
- Fischer, T.W.; Scholz, G.; Knöll, B.; Hipler, U.C.; Elsner, P. Melatonin reduces UV-induced reactive oxygen species in a dose-dependent manner in IL-3-stimulated leukocytes. J. Pineal Res. 2001, 31, 39–45. [Google Scholar] [CrossRef]
- Peloi, K.E.; Ratti, B.A.; Nakamura, C.V.; Neal, C.J.; Sakthivel, T.S.; Singh, S.; Seal, S.; de Oliveira Silva Lautenschlager, S. Engineered nanoceria modulate neutrophil oxidative response to low doses of UV-B radiation through the inhibition of reactive oxygen species production. J. Biomed. Mater. Res. A 2021, 109, 2570–2579. [Google Scholar] [CrossRef]
- Pillai, S.; Oresajo, C.; Hayward, J. Ultraviolet radiation and skin aging: Roles of reactive oxygen species, inflammation and protease activation, and strategies for prevention of inflammation-induced matrix degradation—A review. Int. J. Cosmet. Sci. 2005, 27, 17–34. [Google Scholar] [CrossRef]
- Estadt, S.N.; Maz, M.P.; Musai, J.; Kahlenberg, J.M. Mechanisms of Photosensitivity in Autoimmunity. J. Investig. Dermatol. 2022, 142, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Kaul, A.; Gordon, C.; Crow, M.K.; Touma, Z.; Urowitz, M.B.; van Vollenhoven, R.; Ruiz-Irastorza, G.; Hughes, G. Systemic lupus erythematosus. Nat. Rev. Dis. Prim. 2016, 2, 16039. [Google Scholar] [CrossRef] [PubMed]
- Dourmishev, L.; Meffert, H.; Piazena, H. Dermatomyositis: Comparative studies of cutaneous photosensitivity in lupus erythematosus and normal subjects. Photodermatol. Photoimmunol. Photomed. 2004, 20, 230–234. [Google Scholar] [CrossRef]
- Zhang, S.; Shu, X.; Tian, X.; Chen, F.; Lu, X.; Wang, G. Enhanced formation and impaired degradation of neutrophil extracellular traps in dermatomyositis and polymyositis: A potential contributor to interstitial lung disease complications. Clin. Exp. Immunol. 2014, 177, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.; Tony, H.P.; Bröcker, E.B.; Kneitz, C. Sun-induced life-threatening lupus nephritis. Ann. N. Y. Acad. Sci. 2007, 1108, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Barbhaiya, M.; Costenbader, K.H. Ultraviolet radiation and systemic lupus erythematosus. Lupus 2014, 23, 588–595. [Google Scholar] [CrossRef]
- Villanueva, E.; Yalavarthi, S.; Berthier, C.C.; Hodgin, J.B.; Khandpur, R.; Lin, A.M.; Rubin, C.J.; Zhao, W.; Olsen, S.H.; Klinker, M.; et al. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J. Immunol. 2011, 187, 538–552. [Google Scholar] [CrossRef] [PubMed]
- Denny, M.F.; Yalavarthi, S.; Zhao, W.; Thacker, S.G.; Anderson, M.; Sandy, A.R.; McCune, W.J.; Kaplan, M.J. A distinct subset of proinflammatory neutrophils isolated from patients with systemic lupus erythematosus induces vascular damage and synthesizes type I IFNs. J. Immunol. 2010, 184, 3284–3297. [Google Scholar] [CrossRef]
- Karu, T. Primary and secondary mechanisms of action of visible to near-IR radiation on cells. J. Photochem. Photobiol. B 1999, 49, 1–17. [Google Scholar] [CrossRef]
- Owusu, S.B.; Dupré-Crochet, S.; Bizouarn, T.; Houée-Levin, C.; Baciou, L. Accumulation of Cytochrome b558 at the Plasma Membrane: Hallmark of Oxidative Stress in Phagocytic Cells. Int. J. Mol. Sci. 2022, 23, 767. [Google Scholar] [CrossRef]
- Kohchi, C.; Inagawa, H.; Nishizawa, T.; Soma, G. ROS and innate immunity. Anticancer Res. 2009, 29, 817–821. [Google Scholar] [PubMed]
- Amulic, B.; Cazalet, C.; Hayes, G.L.; Metzler, K.D.; Zychlinsky, A. Neutrophil function: From mechanisms to disease. Annu. Rev. Immunol. 2012, 30, 459–489. [Google Scholar] [CrossRef] [PubMed]
- Paclet, M.H.; Laurans, S.; Dupré-Crochet, S. Regulation of Neutrophil NADPH Oxidase, NOX2: A Crucial Effector in Neutrophil Phenotype and Function. Front. Cell Dev. Biol. 2022, 10, 945749. [Google Scholar] [CrossRef] [PubMed]
- Simonian, M.A.; Babaian, M.A.; Simonian, G.M. Tsitokhromy b-558 iz syvorotki krovi i membran éritrotsitov. Vydelenie, ochistka i kratkie kharakteristiki [Cytochrome b-558 from blood serum and erythrocyte membranes. Isolation, purification, and brief characteristics]. Biokhimiia 1995, 60, 1977–1987. (In Russian) [Google Scholar] [PubMed]
- Shiro, Y.; Isogai, Y.; Nakamura, H.; Iizuka, T. Physiological functions and molecular structures of new types of hemoproteins. Prog. Biotechnol. 2002, 22, 189–204. [Google Scholar] [CrossRef]
- Reth, M. Hydrogen peroxide as second messenger in lymphocyte activation. Nat. Immunol. 2002, 3, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- Svobodová, A.; Vostálová, J. Solar radiation induced skin damage: Review of protective and preventive options. Int. J. Radiat. Biol. 2010, 86, 999–1030. [Google Scholar] [CrossRef] [PubMed]
- Gueymard, C.A.; Myers, D.; Emery, K. Proposed reference irradiance spectra for solar energy systems testing. Sol. Energy 2002, 73, 443–467. [Google Scholar] [CrossRef]
- Kripke, M.L.; Fisher, M.S. Immunologic parameters of ultraviolet carcinogenesis. J. Natl. Cancer Inst. 1976, 57, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Byrne, S.N.; MacDonald, L.J.; Chan, C.Y.; Halliday, G.M. Ultraviolet B suppresses immunity by inhibiting effector and memory T cells. Am. J. Pathol. 2008, 172, 993–1004. [Google Scholar] [CrossRef]
- Piskin, G.; Bos, J.D.; Teunissen, M.B. Neutrophils infiltrating ultraviolet B-irradiated normal human skin display high IL-10 expression. Arch. Dermatol. Res. 2005, 296, 339–342. [Google Scholar] [CrossRef] [PubMed]
- Freitas, M.; Porto, G.; Lima, J.L.; Fernandes, E. Isolation and activation of human neutrophils in vitro. The importance of the anticoagulant used during blood collection. Clin. Biochem. 2008, 41, 570–575. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arzumanyan, G.; Mamatkulov, K.; Arynbek, Y.; Zakrytnaya, D.; Jevremović, A.; Vorobjeva, N. Radiation from UV-A to Red Light Induces ROS-Dependent Release of Neutrophil Extracellular Traps. Int. J. Mol. Sci. 2023, 24, 5770. https://doi.org/10.3390/ijms24065770
Arzumanyan G, Mamatkulov K, Arynbek Y, Zakrytnaya D, Jevremović A, Vorobjeva N. Radiation from UV-A to Red Light Induces ROS-Dependent Release of Neutrophil Extracellular Traps. International Journal of Molecular Sciences. 2023; 24(6):5770. https://doi.org/10.3390/ijms24065770
Chicago/Turabian StyleArzumanyan, Grigory, Kahramon Mamatkulov, Yersultan Arynbek, Darya Zakrytnaya, Anka Jevremović, and Nina Vorobjeva. 2023. "Radiation from UV-A to Red Light Induces ROS-Dependent Release of Neutrophil Extracellular Traps" International Journal of Molecular Sciences 24, no. 6: 5770. https://doi.org/10.3390/ijms24065770
APA StyleArzumanyan, G., Mamatkulov, K., Arynbek, Y., Zakrytnaya, D., Jevremović, A., & Vorobjeva, N. (2023). Radiation from UV-A to Red Light Induces ROS-Dependent Release of Neutrophil Extracellular Traps. International Journal of Molecular Sciences, 24(6), 5770. https://doi.org/10.3390/ijms24065770






