Label-Free Surface-Enhanced Raman Spectroscopic Analysis of Proteins: Advances and Applications
Abstract
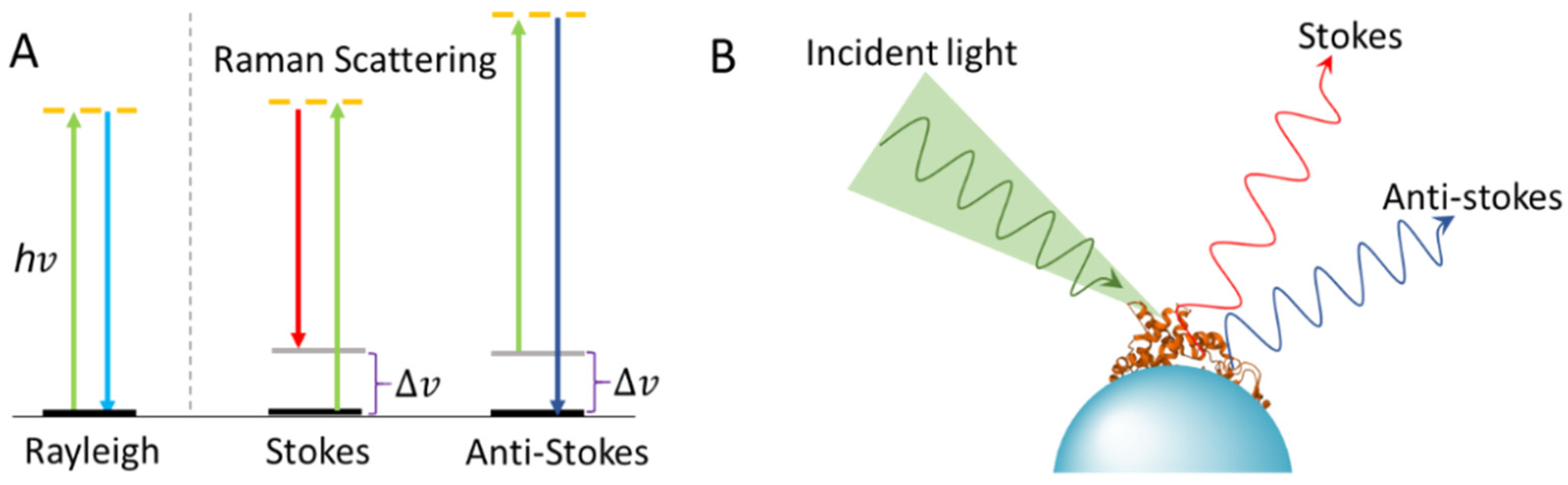
1. Introduction
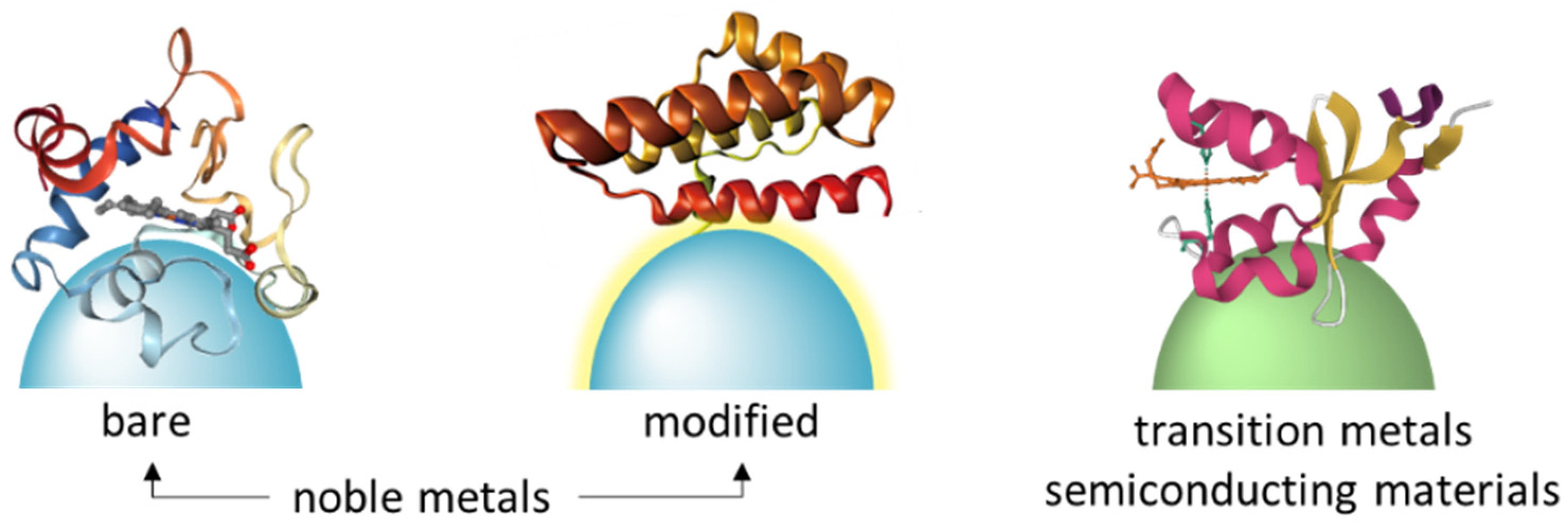
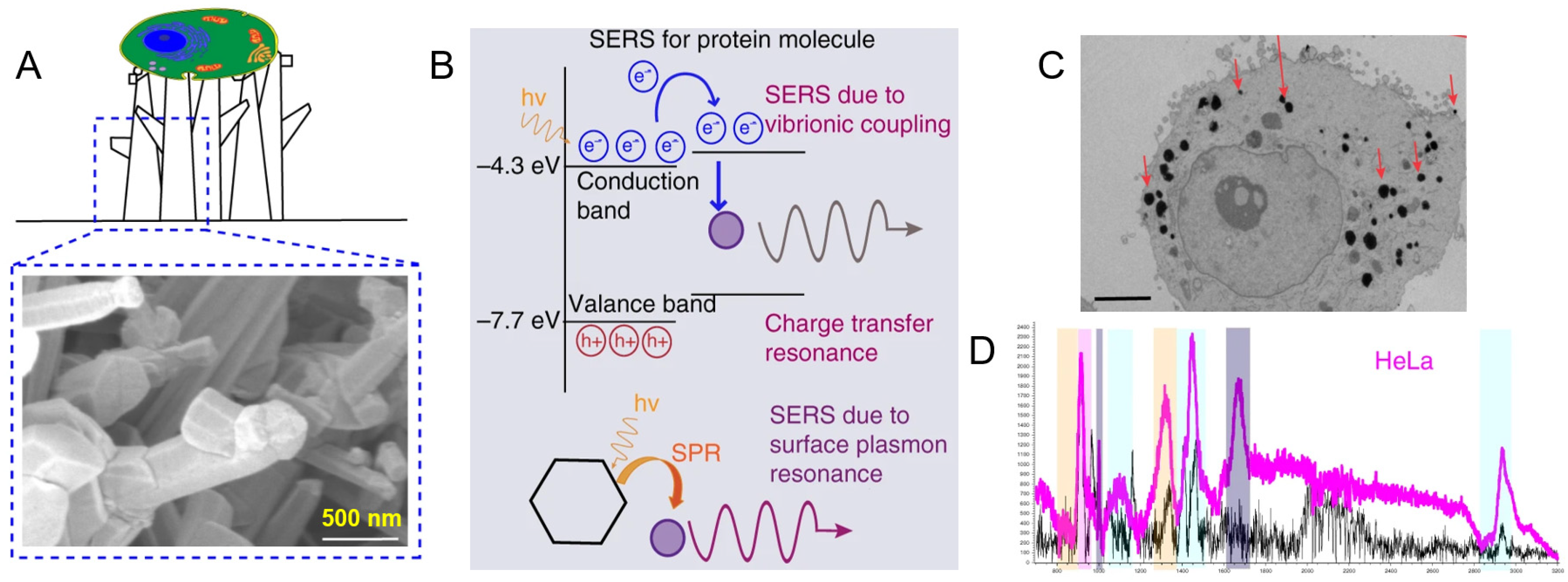
2. SERS-Active Materials for Protein Immobilization
3. SERRS of Proteins
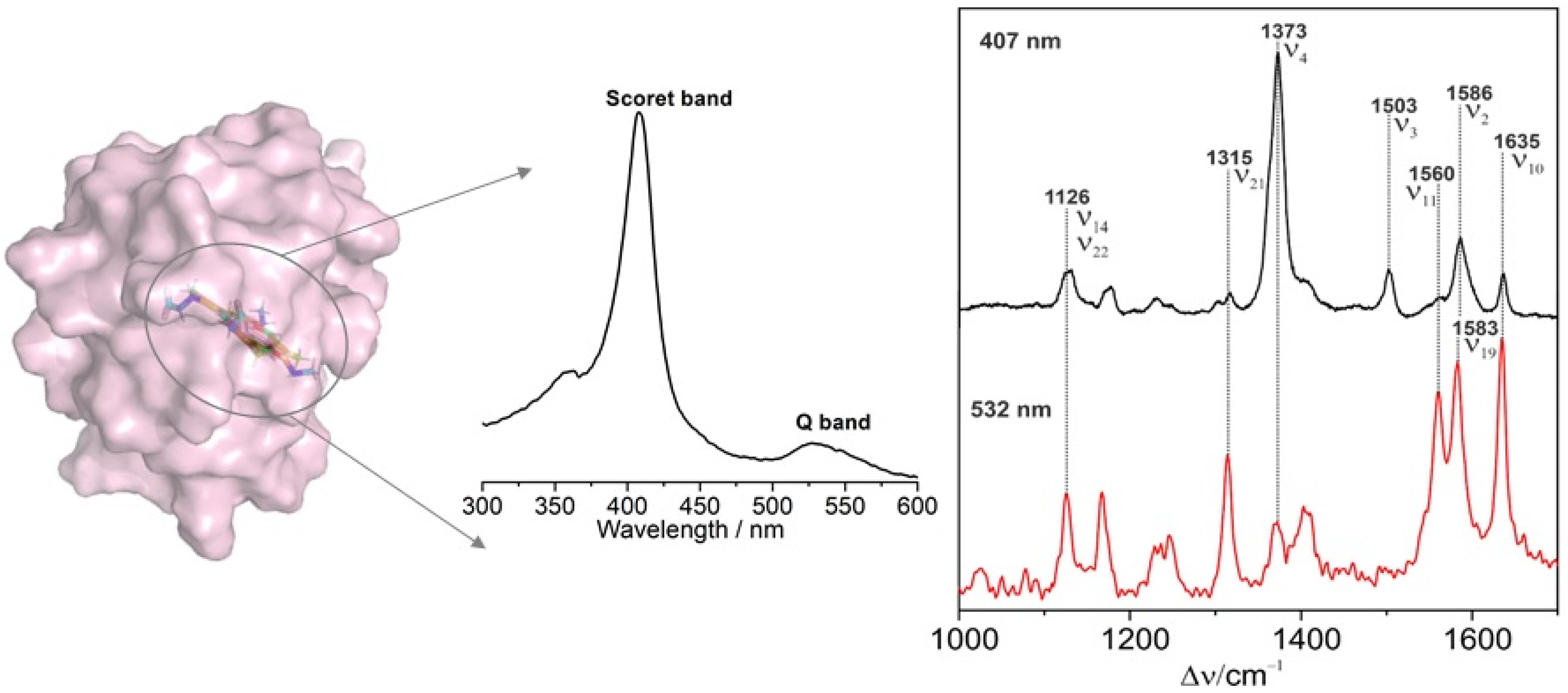
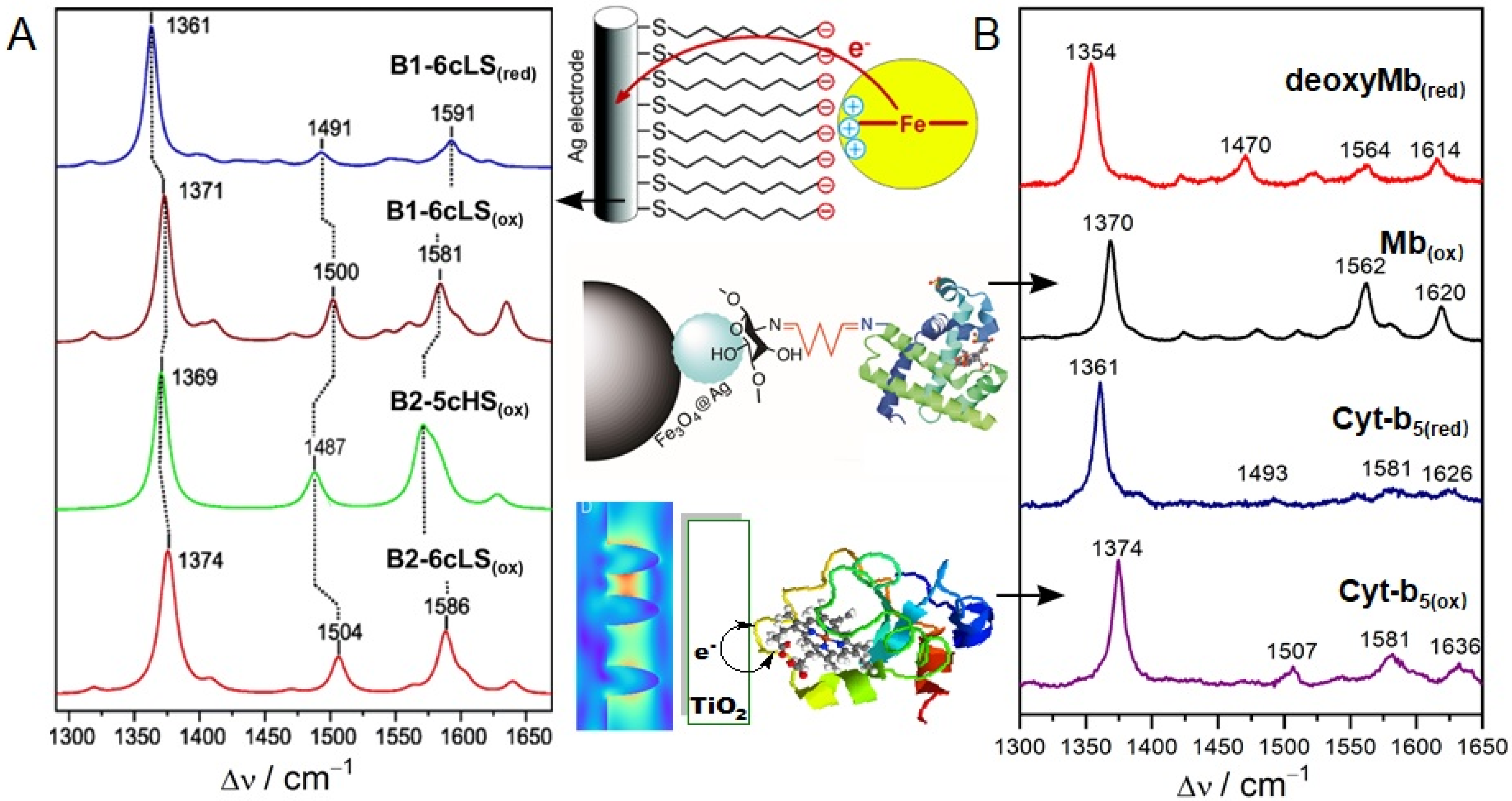
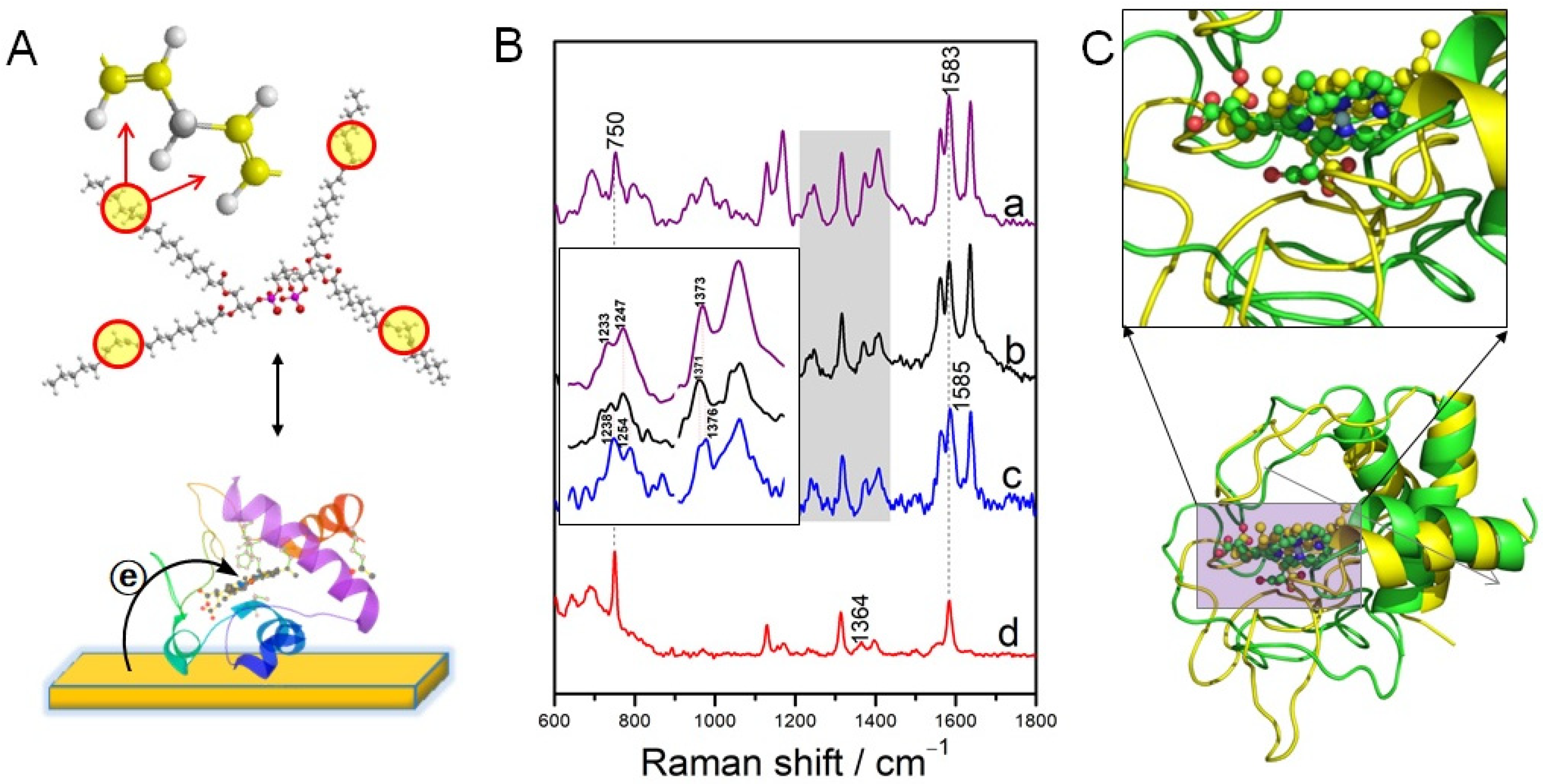
3.1. Hemoproteins
3.2. Flavoproteins
3.3. Other Proteins
4. SERS of Proteins
5. SERRS/SERS of Protein-Ligand Complex
6. Applications in Biology and Biomedicine
6.1. Protein Biomarkers
6.2. Proteins in Tissues
6.3. Intracellular and Extracellular Proteins
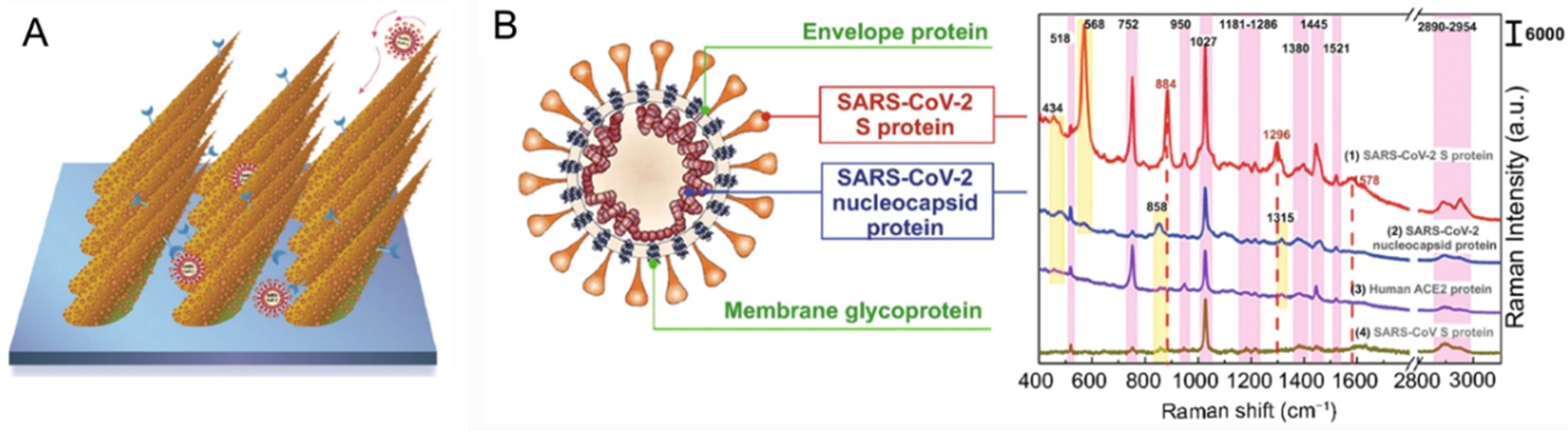
6.4. Proteins of Pathogens
7. SERS Spectral Reproducibility and Limitations
8. Conclusions and Outlook
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| SERS | Surface-Enhanced Raman Spectroscopy |
| SERRS | Surface-Enhanced Resonance Raman Spectroscopy |
| SPR | Surface Plasmon Resonance |
| CT | Charge Transfer |
| HOMO | Highest Occupied Molecular Orbital |
| LUMO | Lowest Unoccupied Molecular Orbital |
| Cyt-c | Cytochrome c |
| Cyt-b5 | Cytochrome-b5 |
| Mb | Myoglobin |
| FMN | Flavin Mononucleotide |
| FAD | Flavin Dinucleotide |
| Ag | Silver |
| Au | Gold |
| UV | Ultraviolet |
| CL | Cardiolipin |
References
- Keresztury, G. Raman Spectroscopy: Theory. In Handbook of Vibrational Spectroscopy; Chalmers, J.M., Griffiths, P., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006. [Google Scholar]
- Kneipp, K.; Moskovits, M.; Kneipp, H. Surface-Enhanced Raman Scattering: Physics and Applications; Springer: Berlin, Germany, 2006. [Google Scholar]
- Han, X.X.; Rodriguez, R.S.; Haynes, C.L.; Ozaki, Y.; Zhao, B. Surface-enhanced Raman spectroscopy. Nat. Rev. Methods Primers 2021, 1, 87. [Google Scholar] [CrossRef]
- Schlücker, S. Surface Enhanced Raman Spectroscopy: Analytical, Biophysical and Life Science Applications; Wiley-VCH: Weinheim, Germany, 2010. [Google Scholar]
- Lane, L.A.; Qian, X.; Nie, S. SERS Nanoparticles in Medicine: From Label-Free Detection to Spectroscopic Tagging. Chem. Rev. 2015, 115, 10489–10529. [Google Scholar] [CrossRef]
- Fleischmann, M.; Hendra, P.J.; McQuillan, A.J. Raman spectra of pyridine adsorbed at a silver electrode. Chem. Phys. Lett. 1974, 26, 163–166. [Google Scholar] [CrossRef]
- Jeanmaire, D.L.; Van Duyne, R.P. Surface raman spectroelectrochemistry: Part I. Heterocyclic, aromatic, and aliphatic amines adsorbed on the anodized silver electrode. J. Electroanal. Chem. 1977, 84, 1–20. [Google Scholar] [CrossRef]
- Albrecht, M.G.; Creighton, J.A. Anomalously intense Raman spectra of pyridine at a silver electrode. J. Am. Chem. Soc. 1977, 99, 5215–5217. [Google Scholar] [CrossRef]
- Moskovits, M. Surface-enhanced spectroscopy. Rev. Mod. Phys. 1985, 57, 783. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, H.; Han, X.X.; Zhao, B. Metal–semiconductor heterostructures for surface-enhanced Raman scattering: Synergistic contribution of plasmons and charge transfer. Mater. Horiz. 2021, 8, 370–382. [Google Scholar] [CrossRef]
- Kneipp, K.; Ozaki, Y.; Tian, Z.-Q. Recent Developments in Plasmon-Supported Raman Spectroscopy (45 Years of Enhanced Raman Signals); World Scientific Publishing Europe Ltd.: London, UK, 2017. [Google Scholar]
- Alessandri, I.; Lombardi, J.R. Enhanced Raman Scattering with Dielectrics. Chem. Rev. 2016, 116, 14921–14981. [Google Scholar] [CrossRef]
- Zhan, C.; Chen, X.-J.; Yi, J.; Li, J.-F.; Wu, D.-Y.; Tian, Z.-Q. From plasmon-enhanced molecular spectroscopy to plasmon-mediated chemical reactions. Nat. Rev. Chem. 2018, 2, 216–230. [Google Scholar] [CrossRef]
- Willets, K.A.; Van Duyne, R.P. Localized Surface Plasmon Resonance Spectroscopy and Sensing. Annu. Rev. Phys. Chem. 2007, 58, 267–297. [Google Scholar] [CrossRef]
- Nie, S.; Emory, S.R. Probing Single Molecules and Single Nanoparticles by Surface-Enhanced Raman Scattering. Science 1997, 275, 1102–1106. [Google Scholar] [CrossRef]
- Gersten, J.I.; Birke, R.L.; Lombardi, J.R. Theory of Enhance I Light Scattering from Molecules Adsorbed at the Metal-Solution Interface. Phys. Rev. Lett. 1979, 43, 147–150. [Google Scholar] [CrossRef]
- Han, X.X.; Ji, W.; Zhao, B.; Ozaki, Y. Semiconductor-enhanced Raman scattering: Active nanomaterials and applications. Nanoscale 2017, 9, 4847–4861. [Google Scholar] [CrossRef]
- Lombardi, J.R.; Birke, R.L. A Unified View of Surface-Enhanced Raman Scattering. Acc. Chem. Res. 2009, 42, 734–742. [Google Scholar] [CrossRef]
- Lombardi, J.R.; Birke, R.L. The theory of surface-enhanced Raman scattering. J. Chem. Phys. 2012, 136, 144704. [Google Scholar] [CrossRef]
- Lombardi, J.R.; Birke, R.L. Theory of Surface-Enhanced Raman Scattering in Semiconductors. J. Phys. Chem. C 2014, 118, 11120–11130. [Google Scholar] [CrossRef]
- Han, X.X.; Ozaki, Y.; Zhao, B. Label-free detection in biological applications of surface-enhanced Raman scattering. TrAC-Trends Anal. Chem. 2012, 38, 67–78. [Google Scholar] [CrossRef]
- Bell, S.E.J.; Sirimuthu, N.M.S. Quantitative surface-enhanced Raman spectroscopy. Chem. Soc. Rev. 2008, 37, 1012–1024. [Google Scholar] [CrossRef]
- Murgida, D.H.; Hildebrandt, P. Disentangling interfacial redox processes of proteins by SERR spectroscopy. Chem. Soc. Rev. 2008, 37, 937–945. [Google Scholar] [CrossRef]
- Zong, C.; Xu, M.; Xu, L.-J.; Wei, T.; Ma, X.; Zheng, X.-S.; Hu, R.; Ren, B. Surface-Enhanced Raman Spectroscopy for Bioanalysis: Reliability and Challenges. Chem. Rev. 2018, 118, 4946–4980. [Google Scholar] [CrossRef]
- Grabbe, E.S.; Buck, R.P. Surface-enhanced Raman spectroscopic investigation of human immunoglobulin G adsorbed on a silver electrode. J. Am. Chem. Soc. 1989, 111, 8362–8366. [Google Scholar] [CrossRef]
- Lee, N.S.; Hsieh, Y.Z.; Morris, M.D.; Schopfer, L.M. Reinterpretation of surface-enhanced resonance Raman scattering of flavoproteins on silver colloids. J. Am. Chem. Soc. 1987, 109, 1358–1363. [Google Scholar] [CrossRef]
- Xu, H.; Bjerneld, E.J.; Käll, M.; Börjesson, L. Spectroscopy of Single Hemoglobin Molecules by Surface Enhanced Raman Scattering. Phys. Rev. Lett. 1999, 83, 4357–4360. [Google Scholar] [CrossRef]
- Lee, P.C.; Meisel, D. Adsorption and surface-enhanced Raman of dyes on silver and gold sols. J. Phys. Chem. C 1982, 86, 3391–3395. [Google Scholar] [CrossRef]
- Drachev, V.P.; Thoreson, M.D.; Khaliullin, E.N.; Davisson, V.J.; Shalaev, V.M. Surface-Enhanced Raman Difference between Human Insulin and Insulin Lispro Detected with Adaptive Nanostructures. J. Phys. Chem. B 2004, 108, 18046–18052. [Google Scholar] [CrossRef]
- Han, X.X.; Kitahama, Y.; Itoh, T.; Wang, C.X.; Zhao, B.; Ozaki, Y. Protein-mediated sandwich strategy for surface-enhanced Raman scattering: Application to versatile protein detection. Anal. Chem. 2009, 81, 3350–3355. [Google Scholar] [CrossRef] [PubMed]
- Han, X.X.; Jia, H.Y.; Wang, Y.F.; Lu, Z.C.; Wang, C.X.; Xu, W.Q.; Zhao, B.; Ozaki, Y. Analytical technique for label-free multi-protein detection based on Western blot and surface-enhanced Raman scattering. Anal. Chem. 2008, 80, 2799–2804. [Google Scholar] [CrossRef] [PubMed]
- Han, X.X.; Zhao, B.; Ozaki, Y. Surface-enhanced Raman scattering for protein detection. Anal. Bioanal. Chem. 2009, 394, 1719–1727. [Google Scholar] [CrossRef]
- Han, X.X.; Köhler, C.; Kozuch, J.; Kuhlmann, U.; Paasche, L.; Sivanesan, A.; Weidinger, I.M.; Hildebrandt, P. Potential-Dependent Surface-Enhanced Resonance Raman Spectroscopy at Nanostructured TiO2: A Case Study on Cytochrome b5. Small 2013, 9, 4175–4181. [Google Scholar] [CrossRef]
- Zhu, J.; Jiang, M.; Ma, H.; Zhang, H.; Cheng, W.; Li, J.; Cai, L.; Han, X.X.; Zhao, B. Redox-State-Mediated Regulation of Cytochromec Release in Apoptosis Revealed by Surface-Enhanced Raman Scattering on Nickel Substrates. Angew. Chem. Int. Ed. 2019, 58, 16499–16503. [Google Scholar] [CrossRef]
- Li, J.; Cheng, W.; Wang, X.; Zhang, H.; Jin, J.; Ji, W.; Han, X.X.; Zhao, B. Electron Transfer of Cytochrome c on Surface-Enhanced Raman Scattering–Active Substrates: Material Dependence and Biocompatibility. Chem. Eur. J. 2017, 23, 9034–9038. [Google Scholar] [CrossRef] [PubMed]
- Sivanesan, A.; Ly, H.K.; Kozuch, J.; Sezer, M.; Kuhlmann, U.; Fischer, A.; Weidinger, I.M. Functionalized Ag nanoparticles with tunable optical properties for selective protein analysis. Chem. Commun. 2011, 47, 3553–3555. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Murgida, D.H.; Hildebrandt, P. Proton-Coupled Electron Transfer of Cytochrome c. J. Am. Chem. Soc. 2001, 123, 4062–4068. [Google Scholar] [CrossRef] [PubMed]
- Murgida, D.H.; Hildebrandt, P. Redox and redox-coupled processes of heme proteins and enzymes at electrochemical interfaces. Phys. Chem. Chem. Phys. 2005, 7, 3773–3784. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.-J.; Zong, C.; Zheng, X.-S.; Hu, P.; Feng, J.-M.; Ren, B. Label-Free Detection of Native Proteins by Surface-Enhanced Raman Spectroscopy Using Iodide-Modified Nanoparticles. Anal. Chem. 2014, 86, 2238–2245. [Google Scholar] [CrossRef] [PubMed]
- Han, X.X.; Schmidt, A.M.; Marten, G.; Fischer, A.; Weidinger, I.M.; Hildebrandt, P. Magnetic silver hybrid nanoparticles for surface-enhanced resonance Raman spectroscopic detection and decontamination of small toxic molecules. ACS Nano 2013, 7, 3212–3220. [Google Scholar] [CrossRef]
- Ma, H.; Tang, X.; Liu, Y.; Han, X.X.; He, C.; Lu, H.; Zhao, B. Surface-Enhanced Raman Scattering for Direct Protein Function Investigation: Controlled Immobilization and Orientation. Anal. Chem. 2019, 91, 8767–8771. [Google Scholar] [CrossRef]
- Ma, H.; Liu, S.; Liu, Y.; Zhu, J.; Han, X.X.; Ozaki, Y.; Zhao, B. In-situ fingerprinting phosphorylated proteins via surface-enhanced Raman spectroscopy: Single-site discrimination of Tau biomarkers in Alzheimer’s disease. Biosens. Bioelectron. 2021, 171, 112748. [Google Scholar] [CrossRef]
- Chen, L.; Tang, J.; Ma, H.; Jin, S.; Xue, X.; Han, X.X.; Jung, Y.M. High-efficiency charge transfer on SERS-active semiconducting K2Ti6O13 nanowires enables direct transition of photoinduced electrons to protein redox centers. Biosens. Bioelectron. 2021, 191, 113452. [Google Scholar] [CrossRef]
- Han, X.X.; Li, J.; Öner, I.H.; Zhao, B.; Leimkühler, S.; Hildebrandt, P.; Weidinger, I.M. Nickel electrodes as a cheap and versatile platform for studying structure and function of immobilized redox proteins. Anal. Chim. Acta 2016, 941, 35–40. [Google Scholar] [CrossRef]
- Habuchi, S.; Cotlet, M.; Gronheid, R.; Dirix, G.; Michiels, J.; Vanderleyden, J.; De Schryver, F.C.; Hofkens, J. Single-Molecule Surface Enhanced Resonance Raman Spectroscopy of the Enhanced Green Fluorescent Protein. J. Am. Chem. Soc. 2003, 125, 8446–8447. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Morris, I.K.; Singh, J.P.; Smith, K.M.; Spiro, T.G. Complete assignment of cytochrome c resonance Raman spectra via enzymic reconstitution with isotopically labeled hemes. J. Am. Chem. Soc. 1993, 115, 12446–12458. [Google Scholar] [CrossRef]
- Oellerich, S.; Wackerbarth, H.; Hildebrandt, P. Spectroscopic Characterization of Nonnative Conformational States of Cytochrome c. J. Phys. Chem. B 2002, 106, 6566–6580. [Google Scholar] [CrossRef]
- Zhang, H.; Kou, Y.; Li, J.; Chen, L.; Mao, Z.; Han, X.X.; Zhao, B.; Ozaki, Y. Nickel Nanowires Combined with Surface-Enhanced Raman Spectroscopy: Application in Label-Free Detection of Cytochrome c-Mediated Apoptosis. Anal. Chem. 2019, 91, 1213–1216. [Google Scholar] [CrossRef] [PubMed]
- Murgida, D.H.; Hildebrandt, P. Electron-Transfer Processes of Cytochrome c at Interfaces. New Insights by Surface-Enhanced Resonance Raman Spectroscopy. Acc. Chem. Res. 2004, 37, 854–861. [Google Scholar] [CrossRef]
- Xie, H.; Song, L.; Katz, S.; Zhu, J.; Liu, Y.; Tang, J.; Cai, L.; Hildebrandt, P.; Han, X.X. Electron transfer between cytochrome c and microsomal monooxygenase generates reactive oxygen species that accelerates apoptosis. Redox Biol. 2022, 53, 102340. [Google Scholar] [CrossRef]
- Kruse, F.; Nguyen, A.D.; Dragelj, J.; Heberle, J.; Hildebrandt, P.; Mroginski, M.A.; Weidinger, I.M. A Resonance Raman Marker Band Characterizes the Slow and Fast Form of Cytochrome c Oxidase. J. Am. Chem. Soc. 2021, 143, 2769–2776. [Google Scholar] [CrossRef]
- Copeland, R.A.; Fodor, S.P.A.; Spiro, T.G. Surface-enhanced Raman spectra of an active flavoenzyme: Glucose oxidase and riboflavin binding protein on silver particles. J. Am. Chem. Soc. 1984, 106, 3872–3874. [Google Scholar] [CrossRef]
- Copeland, R.A.; Spiro, T.G. Ultraviolet resonance Raman spectroscopy of flavin mononucleotide and flavin-adenine dinucleotide. J. Phys. Chem. 1986, 90, 6648–6654. [Google Scholar] [CrossRef]
- Xu, J.; Birke, R.L.; Lombardi, J.R. Surface-enhanced Raman spectroscopy from flavins adsorbed on a silver electrode: Observation of the unstable semiquinone intermediate. J. Am. Chem. Soc. 1987, 109, 5645–5649. [Google Scholar] [CrossRef]
- Gu, M.; Lu, H.P. Raman mode-selective spectroscopic imaging of coenzyme and enzyme redox states. J. Raman Spectros. 2016, 47, 801–807. [Google Scholar] [CrossRef]
- Oladepo, S.A.; Xiong, K.; Hong, Z.; Asher, S.A.; Handen, J.; Lednev, I.K. UV Resonance Raman Investigations of Peptide and Protein Structure and Dynamics. Chem. Rev. 2012, 112, 2604–2628. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, Z.; Beta, I.A.; Mikhonin, A.V.; Asher, S.A. UV−Resonance Raman Thermal Unfolding Study of Trp-Cage Shows That It Is Not a Simple Two-State Miniprotein. J. Am. Chem. Soc. 2005, 127, 10943–10950. [Google Scholar] [CrossRef] [PubMed]
- Chi, Z.; Asher, S.A. UV Raman Determination of the Environment and Solvent Exposure of Tyr and Trp Residues. J. Phys. Chem. B 1998, 102, 9595–9602. [Google Scholar] [CrossRef]
- Rygula, A.; Majzner, K.; Marzec, K.M.; Kaczor, A.; Pilarczyk, M.; Baranska, M. Raman spectroscopy of proteins: A review. J. Raman Spectrosc. 2013, 44, 1061–1076. [Google Scholar] [CrossRef]
- Tuma, R. Raman spectroscopy of proteins: From peptides to large assemblies. J. Raman Spectrosc. 2005, 36, 307–319. [Google Scholar] [CrossRef]
- Kitt, J.P.; Bryce, D.A.; Minteer, S.D.; Harris, J.M. Raman Spectroscopy Reveals Selective Interactions of Cytochrome c with Cardiolipin That Correlate with Membrane Permeability. J. Am. Chem. Soc. 2017, 139, 3851–3860. [Google Scholar] [CrossRef]
- Han, X.X.; Huang, G.G.; Zhao, B.; Ozaki, Y. Label-Free Highly Sensitive Detection of Proteins in Aqueous Solutions Using Surface-Enhanced Raman Scattering. Anal. Chem. 2009, 81, 3329–3333. [Google Scholar] [CrossRef]
- Bao, Y.; Li, Y.; Ling, L.; Xiang, X.; Han, X.; Zhao, B.; Guo, X. Label-Free and Highly Sensitive Detection of Native Proteins by Ag IANPs via Surface-Enhanced Raman Spectroscopy. Anal. Chem. 2020, 92, 14325–14329. [Google Scholar] [CrossRef]
- Ding, S.-Y.; You, E.-M.; Tian, Z.-Q.; Moskovits, M. Electromagnetic theories of surface-enhanced Raman spectroscopy. Chem. Soc. Rev. 2017, 46, 4042–4076. [Google Scholar] [CrossRef]
- Kovacs, G.J.; Loutfy, R.O.; Vincett, P.S.; Jennings, C.; Aroca, R. Distance dependence of SERS enhancement factor from Langmuir-Blodgett monolayers on metal island films: Evidence for the electromagnetic mechanism. Langmuir 1986, 2, 689–694. [Google Scholar] [CrossRef]
- Milazzo, L.; Tognaccini, L.; Howes, B.D.; Sinibaldi, F.; Piro, M.C.; Fittipaldi, M.; Baratto, M.C.; Pogni, R.; Santucci, R.; Smulevich, G. Unravelling the Non-Native Low-Spin State of the Cytochrome c–Cardiolipin Complex: Evidence of the Formation of a His-Ligated Species Only. Biochemistry 2017, 56, 1887–1898. [Google Scholar] [CrossRef] [PubMed]
- Oellerich, S.; Lecomte, S.; Paternostre, M.; Heimburg, T.; Hildebrandt, P. Peripheral and Integral Binding of Cytochrome c to Phospholipids Vesicles. J. Phys. Chem. B 2004, 108, 3871–3878. [Google Scholar] [CrossRef]
- Zheng, X.-S.; Jahn, I.J.; Weber, K.; Cialla-May, D.; Popp, J. Label-free SERS in biological and biomedical applications: Recent progress, current challenges and opportunities. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 197, 56–77. [Google Scholar] [CrossRef] [PubMed]
- El-Said, W.A.; Fouad, D.M.; El-Safty, S.A. Ultrasensitive label-free detection of cardiac biomarker myoglobin based on surface-enhanced Raman spectroscopy. Sens. Actuators B Chem. 2016, 228, 401–409. [Google Scholar] [CrossRef]
- Mu, Z.; Zhao, X.; Huang, Y.; Lu, M.; Gu, Z. Photonic Crystal Hydrogel Enhanced Plasmonic Staining for Multiplexed Protein Analysis. Small 2015, 11, 6036–6043. [Google Scholar] [CrossRef]
- Panikkanvalappil, S.R.; El-Sayed, M.A. Gold-Nanoparticle-Decorated Hybrid Mesoflowers: An Efficient Surface-Enhanced Raman Scattering Substrate for Ultra-Trace Detection of Prostate Specific Antigen. J. Phys. Chem. B 2014, 118, 14085–14091. [Google Scholar] [CrossRef]
- Lv, Y.; Qin, Y.; Svec, F.; Tan, T. Molecularly imprinted plasmonic nanosensor for selective SERS detection of protein biomarkers. Biosens. Bioelectron. 2016, 80, 433–441. [Google Scholar] [CrossRef]
- Chen, S.; Dong, L.; Yan, M.; Dai, Z.; Sun, C.; Li, X. Rapid and sensitive biomarker detection using molecular imprinting polymer hydrogel and surface-enhanced Raman scattering. R. Soc. Open Sci. 2018, 5, 171488. [Google Scholar] [CrossRef]
- Ouhibi, A.; Raouafi, A.; Lorrain, N.; Guendouz, M.; Raouafi, N.; Moadhen, A. Functionalized SERS substrate based on silicon nanowires for rapid detection of prostate specific antigen. Sens. Actuators B Chem. 2021, 330, 129352. [Google Scholar] [CrossRef]
- Hassanain, W.A.; Izake, E.L. Toward Label-Free SERS Detection of Proteins through Their Disulfide Bond Structure. SLAS Discov. 2020, 25, 87–94. [Google Scholar] [CrossRef]
- Chi, Z.; Chen, X.G.; Holtz, J.S.W.; Asher, S.A. UV Resonance Raman-Selective Amide Vibrational Enhancement: Quantitative Methodology for Determining Protein Secondary Structure. Biochemistry 1998, 37, 2854–2864. [Google Scholar] [CrossRef] [PubMed]
- Pachetti, M.; D’Amico, F.; Pascolo, L.; Pucciarelli, S.; Gessini, A.; Parisse, P.; Vaccari, L.; Masciovecchio, C. UV Resonance Raman explores protein structural modification upon fibrillation and ligand interaction. Biophys. J. 2021, 120, 4575–4589. [Google Scholar] [CrossRef] [PubMed]
- Ceja-Fdez, A.; Carriles, R.; González-Yebra, A.L.; Vivero-Escoto, J.; de la Rosa, E.; López-Luke, T. Imaging and SERS Study of the Au Nanoparticles Interaction with HPV and Carcinogenic Cervical Tissues. Molecules 2021, 26, 3758. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Du, Y.; Wei, N.; Li, Q.; Li, S.; Sun, T.; Xu, S.; Wang, H.; Man, X.; Han, B. SERS studies on normal epithelial and cancer cells derived from clinical breast cancer specimens. Spectrochim. Acta Part A 2020, 237, 118364. [Google Scholar] [CrossRef] [PubMed]
- Zacharovas, E.; Velička, M.; Platkevičius, G.; Čekauskas, A.; Želvys, A.N.; Niaura, G.; Šablinskas, V. Toward a SERS Diagnostic Tool for Discrimination between Cancerous and Normal Bladder Tissues via Analysis of the Extracellular Fluid. ACS Omega 2022, 7, 10539–10549. [Google Scholar] [CrossRef]
- Wallace, G.Q.; Masson, J.-F. From single cells to complex tissues in applications of surface-enhanced Raman scattering. Analyst 2020, 145, 7162–7185. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, J.; Zheng, T.; Tian, Y. Label-Free SERS Strategy for In Situ Monitoring and Real-Time Imaging of Aβ Aggregation Process in Live Neurons and Brain Tissues. Anal. Chem. 2020, 92, 5910–5920. [Google Scholar] [CrossRef]
- Zhang, C.; Cui, X.; Yang, J.; Shao, X.; Zhang, Y.; Liu, D. Stimulus-responsive surface-enhanced Raman scattering: A “Trojan horse” strategy for precision molecular diagnosis of cancer. Chem. Sci. 2020, 11, 6111–6120. [Google Scholar] [CrossRef]
- Lussier, F.; Thibault, V.; Charron, B.; Wallace, G.Q.; Masson, J.-F. Deep learning and artificial intelligence methods for Raman and surface-enhanced Raman scattering. TrAC-Trends Anal. Chem. 2020, 124, 115796. [Google Scholar] [CrossRef]
- Szekeres, G.P.; Kneipp, J. Different binding sites of serum albumins in the protein corona of gold nanoparticles. Analyst 2018, 143, 6061–6068. [Google Scholar] [CrossRef] [PubMed]
- Büchner, T.; Drescher, D.; Merk, V.; Traub, H.; Guttmann, P.; Werner, S.; Jakubowski, N.; Schneider, G.; Kneipp, J. Biomolecular environment, quantification, and intracellular interaction of multifunctional magnetic SERS nanoprobes. Analyst 2016, 141, 5096–5106. [Google Scholar] [CrossRef] [PubMed]
- Perozziello, G.; Candeloro, P.; De Grazia, A.; Esposito, F.; Allione, M.; Coluccio, M.L.; Tallerico, R.; Valpapuram, I.; Tirinato, L.; Das, G.; et al. Microfluidic device for continuous single cells analysis via Raman spectroscopy enhanced by integrated plasmonic nanodimers. Opt. Express 2016, 24, A180–A190. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Li, Z.; Chen, G.; Lin, D.; Huang, S.; Huang, Z.; Li, Y.; Lin, J.; Chen, R.; Zeng, H. Ultrasound-mediated method for rapid delivery of nano-particles into cells for intracellular surface-enhanced Raman spectroscopy and cancer cell screening. Nanotechnology 2015, 26, 065101. [Google Scholar] [CrossRef]
- Han, Y.; Wu, S.-R.; Tian, X.-D.; Zhang, Y. Optimizing the SERS Performance of 3D Substrates through Tunable 3D Plasmonic Coupling toward Label-Free Liver Cancer Cell Classification. ACS Appl. Mater. Interfaces 2020, 12, 28965–28974. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Zeng, Q.Y.; Li, L.F.; Qi, M.N.; Qi, Q.C.; Li, S.X.; Xu, J.F. Label-free rapid identification of tumor cells and blood cells with silver film SERS substrate. Opt. Express 2018, 26, 33044–33056. [Google Scholar] [CrossRef]
- McHugh, K.J.; Jing, L.; Behrens, A.M.; Jayawardena, S.; Tang, W.; Gao, M.; Langer, R.; Jaklenec, A. Biocompatible Semiconductor Quantum Dots as Cancer Imaging Agents. Adv. Mater. 2018, 30, 1706356. [Google Scholar] [CrossRef]
- Rosenthal, S.J.; Chang, J.C.; Kovtun, O.; McBride, J.R.; Tomlinson, I.D. Biocompatible Quantum Dots for Biological Applications. Chem. Biol. 2011, 18, 10–24. [Google Scholar] [CrossRef]
- Haldavnekar, R.; Venkatakrishnan, K.; Tan, B. Non plasmonic semiconductor quantum SERS probe as a pathway for in vitro cancer detection. Nat. Commun. 2018, 9, 3065. [Google Scholar] [CrossRef]
- Keshavarz, M.; Tan, B.; Venkatakrishnan, K. Label-Free SERS Quantum Semiconductor Probe for Molecular-Level and in Vitro Cellular Detection: A Noble-Metal-Free Methodology. ACS Appl. Mater. Interfaces 2018, 10, 34886–34904. [Google Scholar] [CrossRef]
- Zhou, X.; Hu, Z.; Yang, D.; Xie, S.; Jiang, Z.; Niessner, R.; Haisch, C.; Zhou, H.; Sun, P. Bacteria Detection: From Powerful SERS to Its Advanced Compatible Techniques. Adv. Sci. 2020, 7, 2001739. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Das, A.; Bi, L.; Choi, N.; Moon, J.-I.; Wu, Y.; Park, S.; Choo, J. Recent advances in surface-enhanced Raman scattering-based microdevices for point-of-care diagnosis of viruses and bacteria. Nanoscale 2020, 12, 21560–21570. [Google Scholar] [CrossRef]
- Leong, S.X.; Leong, Y.X.; Tan, E.X.; Sim, H.Y.F.; Koh, C.S.L.; Lee, Y.H.; Chong, C.; Ng, L.S.; Chen, J.R.T.; Pang, D.W.C.; et al. Noninvasive and Point-of-Care Surface-Enhanced Raman Scattering (SERS)-Based Breathalyzer for Mass Screening of Coronavirus Disease 2019 (COVID-19) under 5 min. ACS Nano 2022, 16, 2629–2639. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Premasiri, W.R.; Ziegler, L.D. Surface enhanced Raman spectroscopy of Chlamydia trachomatis and Neisseria gonorrhoeae for diagnostics, and extra-cellular metabolomics and biochemical monitoring. Sci. Rep. 2018, 8, 5163. [Google Scholar] [CrossRef] [PubMed]
- Mosier-Boss, P.A. Review on SERS of Bacteria. Biosensors 2017, 7, 51. [Google Scholar] [CrossRef]
- Zeiri, L.; Bronk, B.V.; Shabtai, Y.; Eichler, J.; Efrima, S. Surface-Enhanced Raman Spectroscopy as a Tool for Probing Specific Biochemical Components in Bacteria. Appl. Spectrosc. 2004, 58, 33–40. [Google Scholar] [CrossRef]
- Efrima, S.; Zeiri, L. Understanding SERS of bacteria. J. Raman Spectrosc. 2009, 40, 277–288. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, Y.; Jiang, X.; Sun, B.; Zhu, Y.; Wang, H.; Su, Y.; He, Y. Simultaneous Capture, Detection, and Inactivation of Bacteria as Enabled by a Surface-Enhanced Raman Scattering Multifunctional Chip. Angew. Chem. Int. Ed. 2015, 54, 5132–5136. [Google Scholar] [CrossRef]
- Wu, X.; Huang, Y.-W.; Park, B.; Tripp, R.A.; Zhao, Y. Differentiation and classification of bacteria using vancomycin functionalized silver nanorods array based surface-enhanced Raman spectroscopy and chemometric analysis. Talanta 2015, 139, 96–103. [Google Scholar] [CrossRef]
- Ho, C.-S.; Jean, N.; Hogan, C.A.; Blackmon, L.; Jeffrey, S.S.; Holodniy, M.; Banaei, N.; Saleh, A.A.E.; Ermon, S.; Dionne, J. Rapid identification of pathogenic bacteria using Raman spectroscopy and deep learning. Nat. Commun. 2019, 10, 4927. [Google Scholar] [CrossRef]
- Rho, E.; Kim, M.; Cho, S.H.; Choi, B.; Park, H.; Jang, H.; Jung, Y.S.; Jo, S. Separation-free bacterial identification in arbitrary media via deep neural network-based SERS analysis. Biosens. Bioelectron. 2022, 202, 113991. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.M.; Hashemi, S.A.; Rahmanian, V.; Kalashgrani, M.Y.; Gholami, A.; Omidifar, N.; Chiang, W.-H. Highly Sensitive Flexible SERS-Based Sensing Platform for Detection of COVID-19. Biosensors 2022, 12, 466. [Google Scholar] [CrossRef] [PubMed]
- Yue, W.; Xia, Z.; Zeng, Z.; Chen, Z.; Qiao, L.; Li, P.; He, Y.; Luo, X. In Situ Surface-Enhanced Raman Scattering Detection of a SARS-CoV-2 Biomarker Using Flexible and Transparent Polydimethylsiloxane Films with Embedded Au Nanoplates. ACS Appl. Nano Mater. 2022, 5, 12897–12906. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, S.; Wang, X.; Dong, T.; Wang, Y.; Li, D.; Gao, X.; Qu, Z.; Li, Y. A novel enhanced substrate for label-free detection of SARS-CoV-2 based on surface-enhanced Raman scattering. Sens. Actuators B Chem. 2022, 359, 131568. [Google Scholar] [CrossRef]
- Yang, Y.; Peng, Y.; Lin, C.; Long, L.; Hu, J.; He, J.; Zeng, H.; Huang, Z.; Li, Z.-Y.; Tanemura, M.; et al. Human ACE2-Functionalized Gold “Virus-Trap” Nanostructures for Accurate Capture of SARS-CoV-2 and Single-Virus SERS Detection. Nano-Micro Lett. 2021, 13, 109. [Google Scholar] [CrossRef]
- Ma, H.; Han, X.X.; Zhao, B. Enhanced Raman spectroscopic analysis of protein post-translational modifications. TrAC-Trends Anal. Chem. 2020, 131, 116019. [Google Scholar] [CrossRef]



| Frequencies | Assignments | |||||
|---|---|---|---|---|---|---|
| SERS | Raman | |||||
| Mb | Cyt-b5 | Cyt-c | Cyt-c | Cyt-b5 | Mb | |
| 1621 | 1630 | 1640 | 1635 | 1626 | 1620 | ν10 ν(CαCm)asym |
| 1565 | 1579 | 1585 | 1583 | 1581 | 1562 | ν2 ν(CβCβ) |
| 1567 | 1560 | ν11 ν(CβCβ) | ||||
| 1489 | 1492 | 1505 | 1500 | 1493 | 1479 | ν3 ν(CαCm)sym |
| 1406 | 1401 | ν29 ν(pyr quarter-ring) | ||||
| 1370 | 1374 | 1374 | 1371 | 1374 | 1370 | ν4 ν(pyr half-ring)sym |
| 1317 | 1314 | ν21 δ(CmH) | ||||
| 1170 | 1168 | ν30 ν(pyr half-ring)sym | ||||
| 1130 | 1129 | ν22 ν(pyr half-ring)sym | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, L.; Fang, G.; Tang, J.; Cheng, Q.; Han, X. Label-Free Surface-Enhanced Raman Spectroscopic Analysis of Proteins: Advances and Applications. Int. J. Mol. Sci. 2022, 23, 13868. https://doi.org/10.3390/ijms232213868
Cai L, Fang G, Tang J, Cheng Q, Han X. Label-Free Surface-Enhanced Raman Spectroscopic Analysis of Proteins: Advances and Applications. International Journal of Molecular Sciences. 2022; 23(22):13868. https://doi.org/10.3390/ijms232213868
Chicago/Turabian StyleCai, Linjun, Guilin Fang, Jinpin Tang, Qiaomei Cheng, and Xiaoxia Han. 2022. "Label-Free Surface-Enhanced Raman Spectroscopic Analysis of Proteins: Advances and Applications" International Journal of Molecular Sciences 23, no. 22: 13868. https://doi.org/10.3390/ijms232213868
APA StyleCai, L., Fang, G., Tang, J., Cheng, Q., & Han, X. (2022). Label-Free Surface-Enhanced Raman Spectroscopic Analysis of Proteins: Advances and Applications. International Journal of Molecular Sciences, 23(22), 13868. https://doi.org/10.3390/ijms232213868





