Synthesis and Evaluation on the Fungicidal Activity of S-Alkyl Substituted Thioglycolurils
Abstract
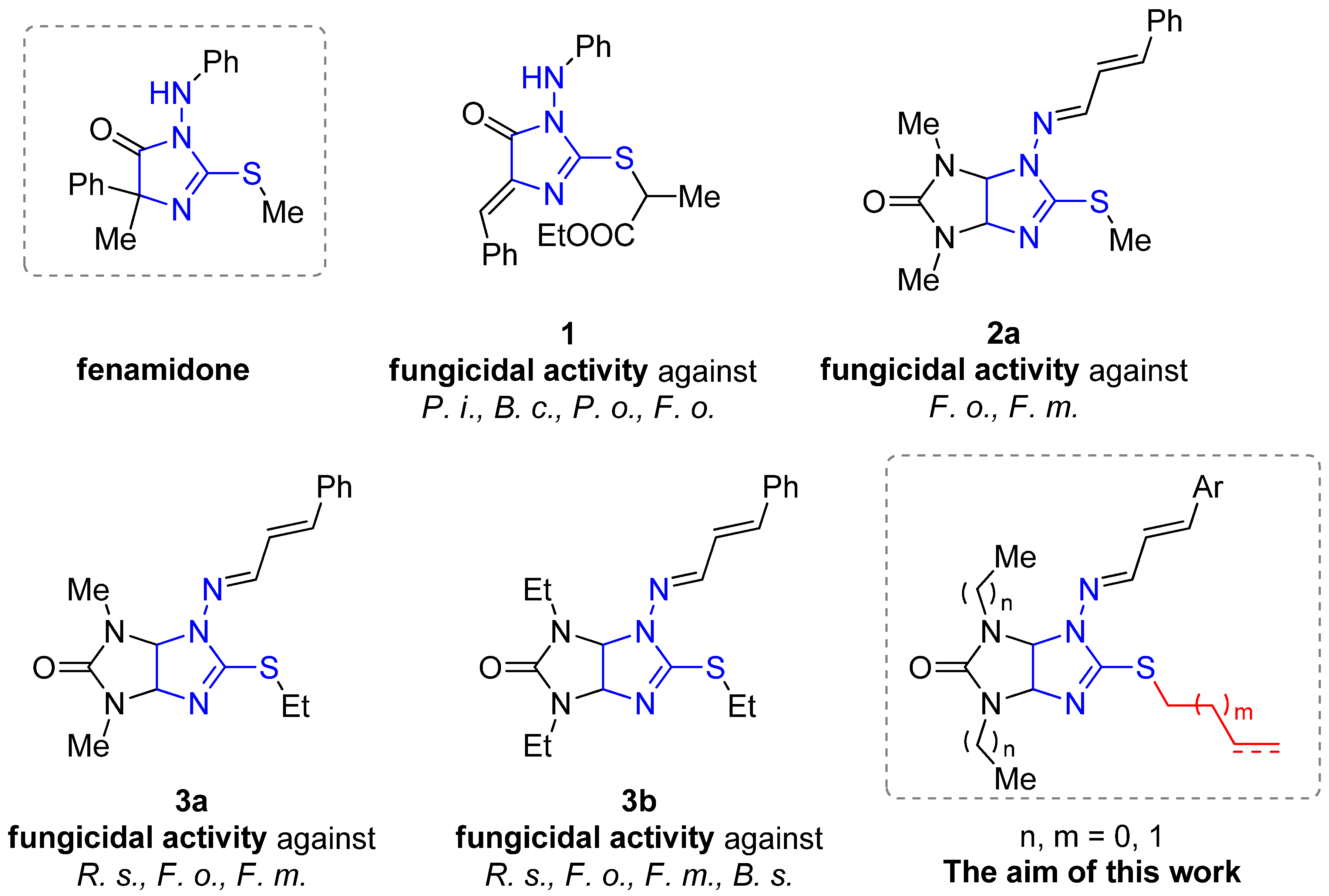
1. Introduction
2. Results and Discussion
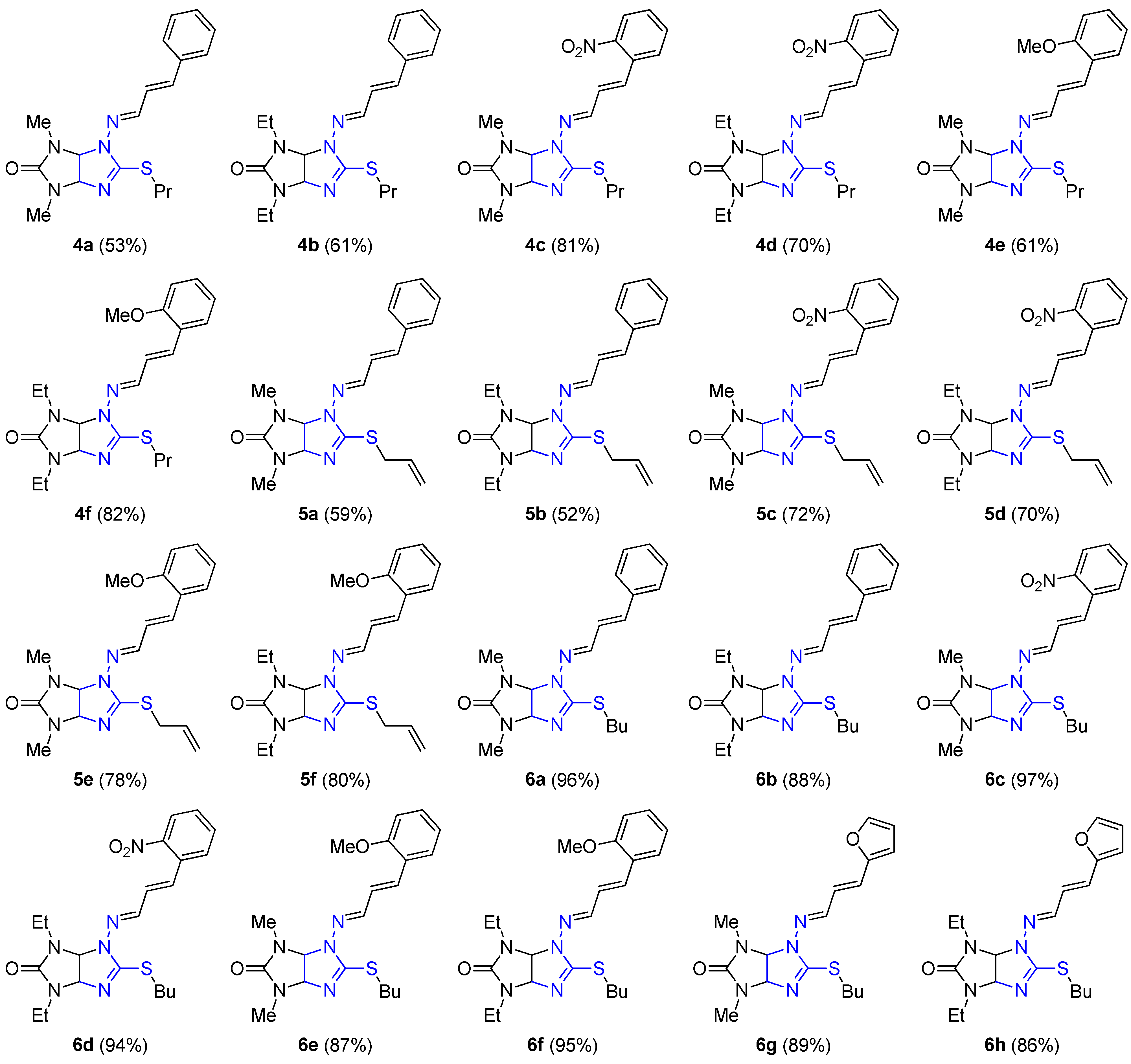
2.1. Chemistry
2.2. Fungicidal Activity Testing
2.3. Cytotoxicity Assay
3. Materials and Methods
3.1. Chemistry
3.1.1. General Information
3.1.2. General Procedure for the Synthesis of Alkylthio Derivatives of Thioglycoluril 4a–f, 5a–f, and 4a–f
3.2. Bioassays of Fungicidal Activities against Phytopathogenic Fungi
3.3. Antifungal Assay against C. albicans and C. neoformans
3.4. Cytotoxicity Assay by COADD
3.5. Hemolysis Assay
3.6. Cytotoxicity assay against 60 Cancer Cell Lines at the National Cancer Institute
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yin, W.; Cui, H.; Jiang, H.; Zhang, Y.; Liu, L.; Wu, T.; Sun, Y.; Zhao, L.; Su, X.; Zhao, D.; et al. Broadening antifungal spectrum and improving metabolic stability based on a scaffold strategy: Design, synthesis, and evaluation of novel 4-phenyl-4,5-dihydrooxazole derivatives as potent fungistatic and fungicidal reagents. Eur. J. Med. Chem. 2022, 227, 113955. [Google Scholar] [CrossRef]
- Brauer, V.S.; Rezende, C.P.; Pessoni, A.M.; De Paula, R.G.; Rangappa, K.S.; Nayaka, S.C.; Gupta, V.K.; Almeida, F. Antifungal Agents in Agriculture: Friends and Foes of Public Health. Biomolecules 2019, 9, 521. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Long, L.; Zhang, F.; Chen, Q.; Chen, C.; Yu, X.; Liu, Q.; Bao, J.; Long, Z. Antifungal activity, main active components and mechanism of Curcuma longa extract against Fusarium graminearum. PLoS ONE 2018, 13, e0194284. [Google Scholar] [CrossRef] [PubMed]
- Rauzan, B.M.; Lorsbach, B.A. Designing Sustainable Crop Protection Actives. In ACS Symposium Series; Rauzan, B.M., Lorsbach, B.A., Eds.; American Chemical Society: Washington, DC, USA, 2021; Volume 1390, pp. 1–9. ISBN 978-0-8412-9821-7. [Google Scholar]
- Wiederhold, N.P. Antifungal resistance: Current trends and future strategies to combat. Infect. Drug Res. 2017, 10, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Lopat’eva, E.R.; Budnikov, A.S.; Krylov, I.B.; Alekseenko, A.L.; Ilovaisky, A.I.; Glinushkin, A.P.; Terent’ev, A.O. 4-Disubstituted Pyrazolin-3-Ones—Novel Class of Fungicides against Phytopathogenic Fungi. Agrochemicals 2023, 2, 34–46. [Google Scholar] [CrossRef]
- Thind, T.S. Changing Trends in Discovery of New Fungicides: A Perspective. Indian Phytopathol. 2021, 74, 875–883. [Google Scholar] [CrossRef]
- Beliaeva, M.A.; Seebeck, F.P. Discovery and Characterization of the Metallopterin-Dependent Ergothioneine Synthase from Caldithrix abyssi. JACS Au 2022, 2, 2098–2107. [Google Scholar] [CrossRef]
- Zheng, X.; Ma, Z.; Zhang, D. Synthesis of Imidazole-Based Medicinal Molecules Utilizing the van Leusen Imidazole Synthesis. Pharmaceuticals 2020, 13, 37. [Google Scholar] [CrossRef]
- Rossi, R.; Ciofalo, M. An Updated Review on the Synthesis and Antibacterial Activity of Molecular Hybrids and Conjugates Bearing Imidazole Moiety. Molecules 2020, 25, 5133. [Google Scholar] [CrossRef]
- Ali, M.; Ali, S.; Khan, M.; Rashid, U.; Ahmad, M.; Khan, A.; Al-Harrasi, A.; Ullah, F.; Latif, A. Synthesis, biological activities, and molecular docking studies of 2-mercaptobenzimidazole based derivatives. Bioorg. Chem. 2018, 80, 472–479. [Google Scholar] [CrossRef]
- Siwach, A.; Verma, P.K. Synthesis and therapeutic potential of imidazole containing compounds. BMC Chem. 2021, 15, 12. [Google Scholar] [CrossRef]
- Kuptsova, A.O.; Vinogradova, E.E.; Kravchenko, A.N.; Gazieva, G.A. Methods for substitution of the thioxo group with the oxo group in imidazolidine-2-thione derivatives. Russ. Chem. Bull. 2022, 71, 885–904. [Google Scholar] [CrossRef]
- Cho, S.H.; Kim, S.-H.; Shin, D. Recent applications of hydantoin and thiohydantoin in medicinal chemistry. Eur. J. Med. Chem. 2019, 164, 517–545. [Google Scholar] [CrossRef] [PubMed]
- Devi, P.; Shahnaz, M.; Prasad, D.N. Recent Overview on Synthesis of 2-Mercaptobenzimidazole Derivatives and its Activities. J. Drug Deliv. Ther. 2022, 12, 203–207. [Google Scholar] [CrossRef]
- Imran, M.; Bawadekji, A.; Alotaibi, N. Synthesis and evaluation of antimicrobial properties of some azole derivatives. Tropical J. Pharm. Res. 2020, 19, 377–382. [Google Scholar] [CrossRef]
- FRAC. Fungicide Resistance Action Committee. Available online: http://www.frac.info/home (accessed on 28 October 2022).
- Porter, L.D.; Cummings, T.F.; Johnson, D.A. Effects of Soil-Applied Late Blight Foliar Fungicides on Infection of Potato Tubers by Phytophthora infestans. Plant Dis. 2006, 90, 964–968. [Google Scholar] [CrossRef]
- Ding, M.W.; Chen, Y.F.; Huang, N.Y. Synthesis and fungicidal activities of derivatives of 2-alkylthio-3-amino-4H-imidazol-4-one. Phosphorus Sulfur Silicon Relat. Elem. 2004, 179, 2287–2296. [Google Scholar] [CrossRef]
- Gazieva, G.A.; Anikina, L.V.; Nechaeva, T.V.; Pukhov, S.A.; Karpova, T.B.; Popkov, S.V.; Nelyubina, Y.V.; Kolotyrkina, N.G.; Kravchenko, A.N. Synthesis and biological evaluation of new substituted thioglycolurils, their analogues and derivatives. Eur. J. Med. Chem. 2017, 140, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Gazieva, G.A.; Nechaeva, T.V.; Kostikova, N.N.; Sigay, N.V.; Serkov, S.A.; Popkov, S.V. Synthesis, S-alkylation, and fungicidal activity of 4-(benzylideneamino)thioglycolurils. Russ. Chem. Bull. 2018, 67, 1059–1064. [Google Scholar] [CrossRef]
- Gazieva, G.A.; Kravchenko, A.N. Unexpected Formation of Thioglycolurils Precursors. J. Heterocycl. Chem. 2015, 52, 1858–1863. [Google Scholar] [CrossRef]
- Popkov, S.V.; Kovalenko, L.V.; Bobylev, M.M.; Molchanov, O.Y.; Krimer, M.Z.; Tashchi, V.P.; Putsykin, Y.G. The Synthesis and Fungicidal Activity of 2-Substituted 1-Azol-1-Ylmethyl-6-Arylidenecyclohexanols. Pestic. Sci. 1997, 49, 125–129. [Google Scholar] [CrossRef]
- Xia, D.; Cheng, X.; Liu, X.; Zhang, C.; Wang, Y.; Liu, Q.; Zeng, Q.; Huang, N.; Cheng, Y.; Lv, X. Discovery of Novel Pyrazole Carboxylate Derivatives Containing Thiazole as Potential Fungicides. J. Agric. Food Chem. 2021, 69, 8358–8365. [Google Scholar] [CrossRef]
- Obydennov, K.L.; Kalinina, T.A.; Galieva, N.A.; Beryozkina, T.V.; Zhang, Y.; Fan, Z.; Glukhareva, T.V.; Bakulev, V.A. Synthesis, Fungicidal Activity, and Molecular Docking of 2-Acylamino and 2-Thioacylamino Derivatives of 1H-Benzo[d]Imidazoles as Anti-Tubulin Agents. J. Agric. Food Chem. 2021, 69, 12048–12062. [Google Scholar] [CrossRef]
- Metodicheskie Rekomendatsii po Opredeleniyu Fungitsidnoi Aktivnosti Novykh Soedinenii. (Methodological Recommendations for Estimation of the Fungicidal Activities of Novel Compounds); NIITEKhIM: Cherkassy, Ukraine, 1984; p. 32. (In Russian)
- Blaskovich, M.A.T.; Zuegg, J.; Elliott, A.G.; Cooper, M.A. Helping Chemists Discover New Antibiotics. ACS Infect. Dis. 2015, 1, 285–287. [Google Scholar] [CrossRef]
- Hansford, K.A.; Blaskovich, M.A.T.; Cooper, M.A. Chemical philanthropy: A path forward for antibiotic discovery? Future Med. Chem. 2016, 8, 925–929. [Google Scholar] [CrossRef] [PubMed]
- Frolov, N.A.; Fedoseeva, K.A.; Hansford, K.A.; Vereshchagin, A.N. Novel Phenyl-Based Bis-quaternary Ammonium Compounds as Broad-Spectrum Biocides. ChemMedChem 2021, 16, 2954–2959. [Google Scholar] [CrossRef]
- Frei, A.; Zuegg, J.; Elliott, A.G.; Baker, M.; Braese, S.; Brown, C.; Chen, F.; Dowson, C.G.; Dujardin, G.; Jung, N.; et al. Metal complexes as a promising source for new antibiotics. Chem. Sci. 2020, 11, 2627–2639. [Google Scholar] [CrossRef] [PubMed]
- Zalevskaya, O.; Gur’eva, Y.; Kutchin, A.; Hansford, K.A. Antimicrobial and Antifungal Activities of Terpene-Derived Palladium Complexes. Antibiotics 2020, 9, 277. [Google Scholar] [CrossRef]
- Shoemaker, R.H. The NCI60 human tumour cell line anticancer drug screen. Nat. Rev. Cancer 2006, 6, 813. [Google Scholar] [CrossRef] [PubMed]
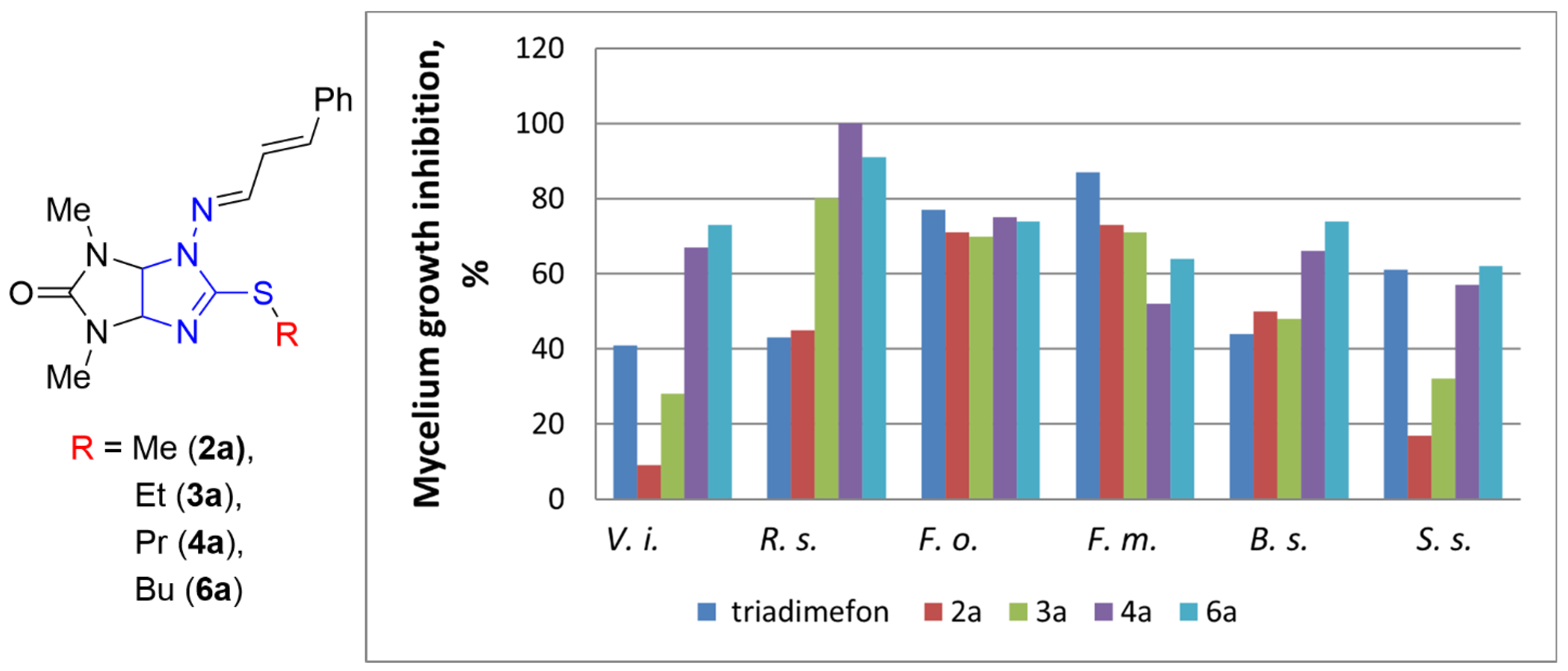
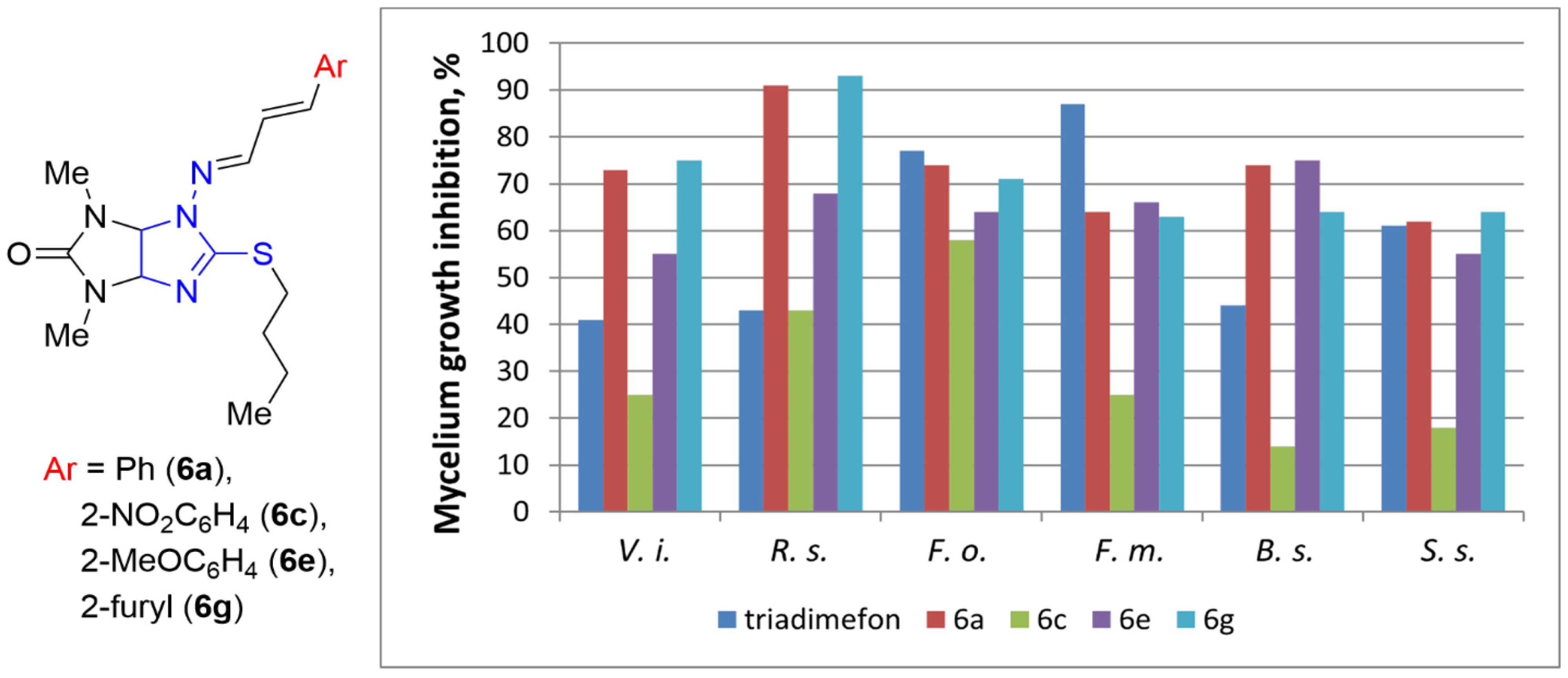
| Entry | 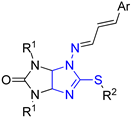 Compound | Mycelium Growth Inhibition, % (C = 30 μg mL−1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R1 | R2 | Ar | V. i. | R. s. | F. o. | F. m. | B. s. | S. s. | ||
| 1 | 4a | Me | Pr | Ph | 67 | 100 | 75 | 52 | 66 | 57 |
| 2 | 4b | Et | Pr | Ph | 44 | 58 | 43 | 57 | 57 | 23 |
| 3 | 4c | Me | Pr | 2-NO2C6H4 | 22 | 39 | 21 | 21 | 38 | 16 |
| 4 | 4d | Et | Pr | 2-NO2C6H4 | 33 | 34 | 29 | 38 | 46 | 7 |
| 5 | 4e | Me | Pr | 2-MeOC6H4 | 42 | 74 | 60 | 38 | 62 | 53 |
| 6 | 5c | Me | All | 2-NO2C6H4 | 22 | 34 | 16 | 23 | 38 | 7 |
| 7 | 5d | Et | All | 2-NO2C6H4 | 22 | 32 | 26 | 42 | 39 | 10 |
| 8 | 5e | Me | All | 2-MeOC6H4 | 47 | 43 | 49 | 47 | 52 | 26 |
| 9 | 5f | Et | All | 2-MeOC6H4 | 56 | 85 | 72 | 48 | 57 | 57 |
| 10 | 6a | Me | Bu | Ph | 73 | 91 | 74 | 64 | 74 | 62 |
| 11 | 6b | Et | Bu | Ph | 58 | 54 | 51 | 57 | 62 | 26 |
| 12 | 6c | Me | Bu | 2-NO2C6H4 | 25 | 43 | 58 | 25 | 14 | 18 |
| 13 | 6d | Et | Bu | 2-NO2C6H4 | 28 | 45 | 19 | 34 | 56 | 17 |
| 14 | 6e | Me | Bu | 2-MeOC6H4 | 55 | 68 | 64 | 66 | 75 | 55 |
| 15 | 6f | Et | Bu | 2-MeOC6H4 | 45 | 57 | 32 | 50 | 63 | 21 |
| 16 | 6g | Me | Bu | 2-furyl | 75 | 93 | 71 | 63 | 64 | 54 |
| 17 | 6h | Et | Bu | 2-furyl | 100 | 78 | 46 | 64 | 73 | 36 |
| 18 | Triadimefon | 41 | 43 | 77 | 87 | 44 | 61 | |||
| Entry | 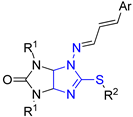 Compound | C. albicans Mycelium Growth Inhibition, % (C = 32 μg mL−1) | MIC C.a. | HC10 | CC50 | |||
|---|---|---|---|---|---|---|---|---|
| R1 | R2 | Ar | ||||||
| 1 | 2a | Me | Me | Ph | 11.46 | - | - | - |
| 2 | 2b | Et | Me | Ph | 7.44 | - | - | - |
| 3 | 2e | Me | Me | 2-MeOC6H4 | 2.69 | - | - | - |
| 4 | 3a | Me | Et | Ph | 99.38 | 8 | >32 | >32 |
| 5 | 3b | Et | Et | Ph | 98.74 | 32 | >32 | 12.05 |
| 6 | 3d | Et | Et | 2-NO2C6H4 | 0.78 | - | - | - |
| 7 | 3e | Me | Et | 2-MeOC6H4 | 98.45 | >32 | >32 | 13.52 |
| 8 | 3g | Me | Et | 2-furyl | 98.78 | 8 | >32 | >32 |
| 9 | 4a | Me | Pr | Ph | 11.42 | - | - | - |
| 10 | 4d | Me | Pr | 2-NO2C6H4 | 21.77 | - | - | - |
| 11 | 6b | Et | Bu | Ph | 5.64 | - | - | - |
| 12 | Flc a | 0.125 [31] | ||||||
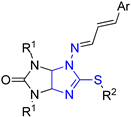 | 60 Cell Line Assay in One Dose 10 mM Concentration | ||||||
|---|---|---|---|---|---|---|---|
| Compound | R1 | R2 | Ar | Mean Growth, % | Range of Growth, % a | Most Sensitive Cell Line | Positive Antiproliferative Effect b |
| 4b | Et | Pr | Ph | 94.44 | 70.05–117.64 | UO-31 (renal cancer) | 0/60 |
| 5d | Et | All | 2-NO2C6H4 | 95.69 | 68.34–116.54 | T-47D (breast cancer) | 0/57 |
| 5e | Me | All | 2-MeOC6H4 | 95.93 | 68.68–124.64 | A498 (renal cancer) | 0/59 |
| 5f | Et | All | 2-MeOC6H4 | 71.22 | 2.79–122.21 | MDA-MB-435 (melanoma) | 7/60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vinogradova, E.E.; Alekseenko, A.L.; Popkov, S.V.; Kolotyrkina, N.G.; Kravchenko, A.N.; Gazieva, G.A. Synthesis and Evaluation on the Fungicidal Activity of S-Alkyl Substituted Thioglycolurils. Int. J. Mol. Sci. 2023, 24, 5756. https://doi.org/10.3390/ijms24065756
Vinogradova EE, Alekseenko AL, Popkov SV, Kolotyrkina NG, Kravchenko AN, Gazieva GA. Synthesis and Evaluation on the Fungicidal Activity of S-Alkyl Substituted Thioglycolurils. International Journal of Molecular Sciences. 2023; 24(6):5756. https://doi.org/10.3390/ijms24065756
Chicago/Turabian StyleVinogradova, Ekaterina E., Anna L. Alekseenko, Sergey V. Popkov, Natalya G. Kolotyrkina, Angelina N. Kravchenko, and Galina A. Gazieva. 2023. "Synthesis and Evaluation on the Fungicidal Activity of S-Alkyl Substituted Thioglycolurils" International Journal of Molecular Sciences 24, no. 6: 5756. https://doi.org/10.3390/ijms24065756
APA StyleVinogradova, E. E., Alekseenko, A. L., Popkov, S. V., Kolotyrkina, N. G., Kravchenko, A. N., & Gazieva, G. A. (2023). Synthesis and Evaluation on the Fungicidal Activity of S-Alkyl Substituted Thioglycolurils. International Journal of Molecular Sciences, 24(6), 5756. https://doi.org/10.3390/ijms24065756







