The Oxidized Lipoproteins In Vivo: Its Diversity and Behavior in the Human Circulation
Abstract
1. Introduction
2. OxLDL Prepared In Vitro
3. Detection of oxLDL In Vivo Using Immunological Methods
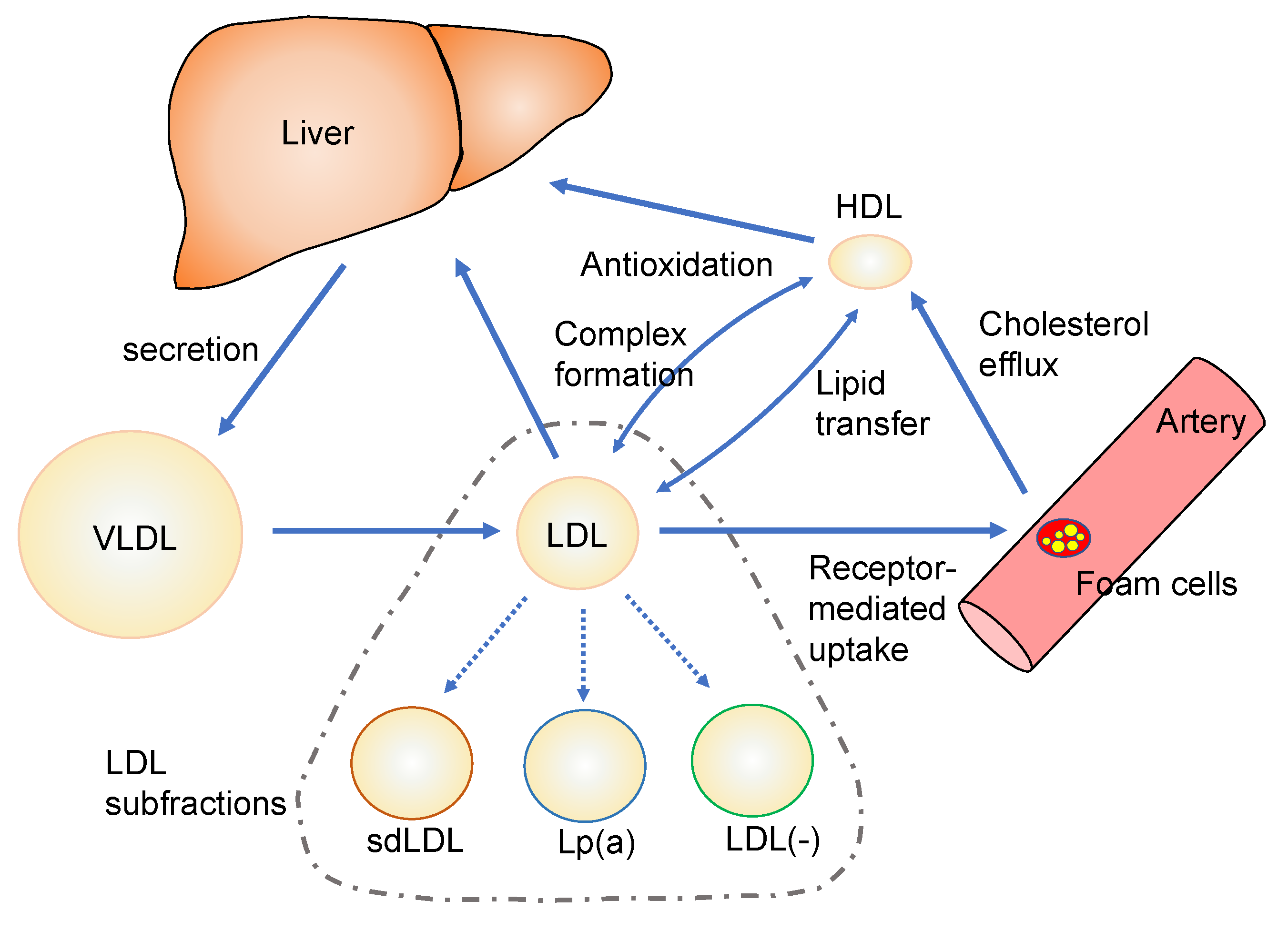
4. Characterization of Modified Lipoproteins and LDL Subfractions
4.1. Small Dense LDL
4.2. Lipoprotein(a)
4.3. Electronegative LDL
4.4. Oxidatevely Modified HDL
5. Possible Perspective of oxLDL Generation and Metabolism
- 1.
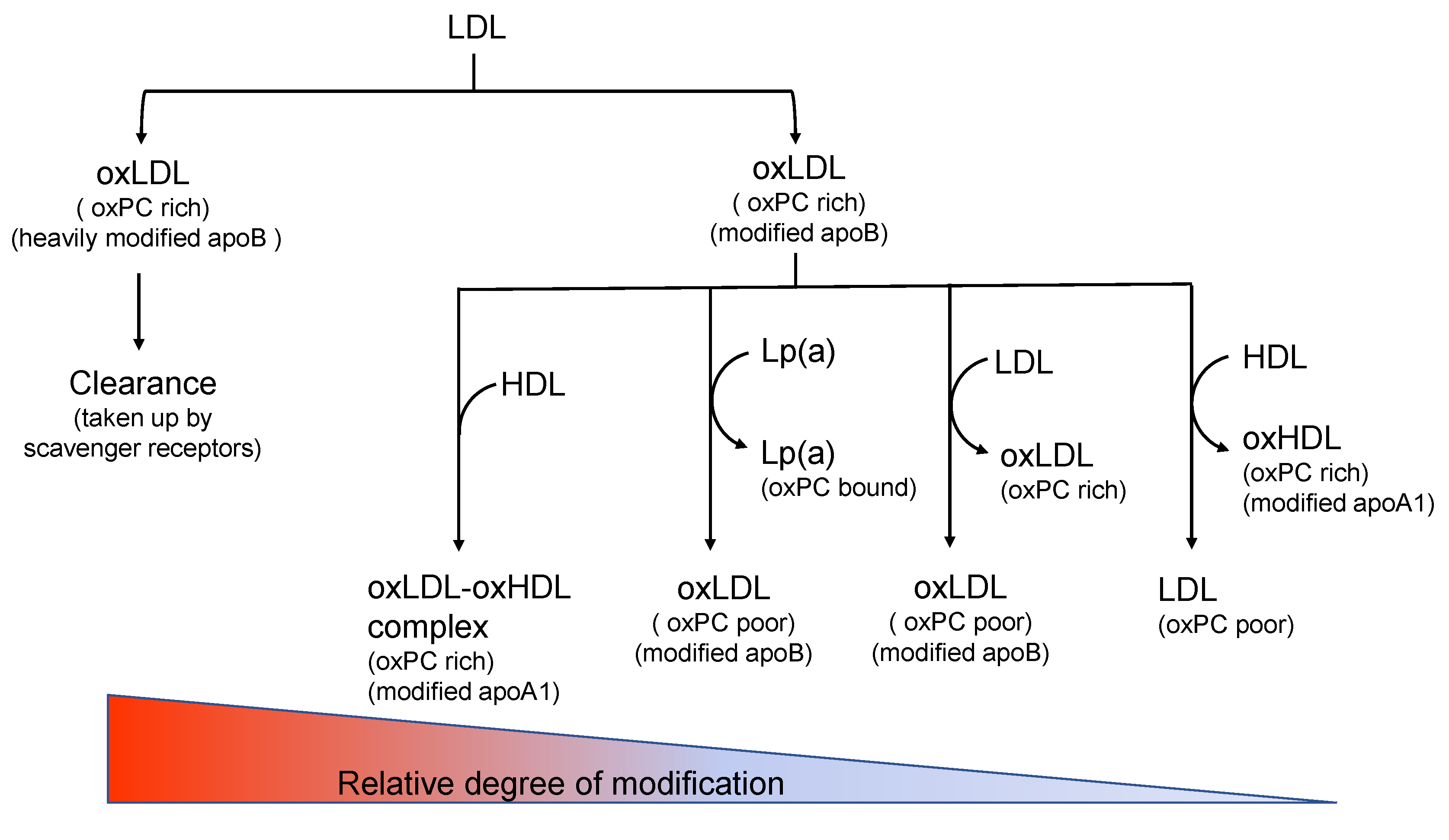
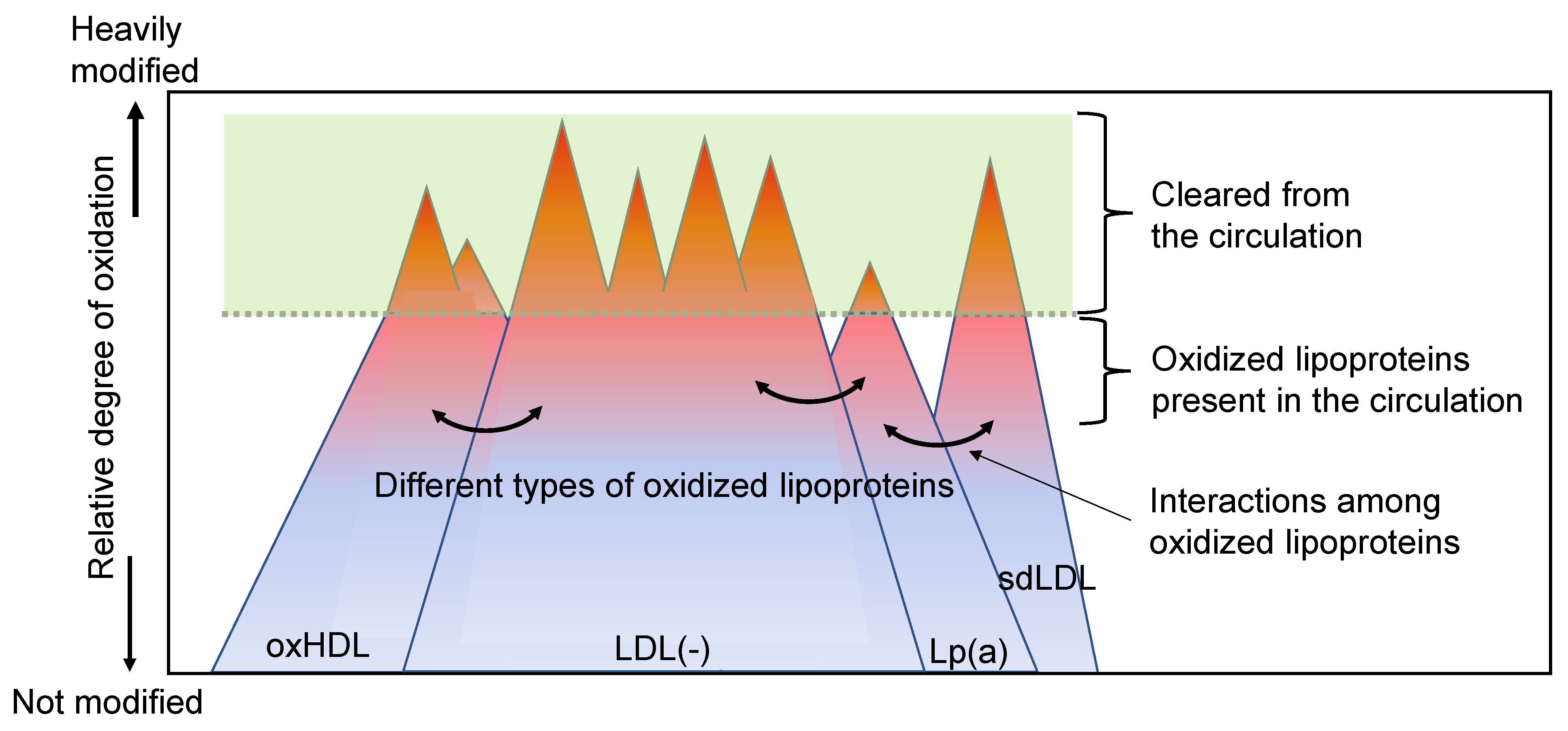
- In vivo oxLDLs can be heterogeneous and the extent of modifications can be gradual. In addition, the type of modifications can be multiple, and many different methods to generate in vivo oxLDLs may be possible.
- 2.
- LDLs are oxidatively modified in physiological circumstances, either in circulation or arterial tissues. Under the circumstances, other lipoproteins, including HDLs, are always present together with LDLs. Thus, oxLDLs could come into contact with other lipoproteins, and oxidized lipids could transfer from oxLDLs to other lipoproteins. In addition, the interaction of oxLDLs with various cells is also possible.
- 3.
- OxLDLs are cleared from circulation when they are heavily modified enough to bind to scavenger receptors. It is well known that heavily oxidized LDLs are rapidly cleared through liver Kupffer cells. Alternatively, macrophages in vascular tissues take up heavily modified LDLs and degrade them in the lysosomes. Thus, the concentration of heavily modified oxLDLs in circulation is substantially low. However, the oxLDL concentration could be variable depending on the types of modifications.
- 4.
- Oxidative modifications of LDLs and other lipoproteins occur constantly, and at the same time, the reduction of oxidized products and degradation of oxLDLs continues, and heavily modified LDLs are cleared. Thus, the oxLDL levels in circulation should be defined by the balance between the oxidative stress that produces oxLDLs and the protection, catabolism, and clearance of oxLDLs that decrease the oxLDL level; an increase in the plasma oxLDL could represent the imbalance between them.
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Faxon, D.P.; Fuster, V.; Libby, P.; Beckman, J.A.; Hiatt, W.R.; Thompson, R.W.; Topper, J.N.; Annex, B.H.; Rundback, J.H.; Fabunmi, R.P.; et al. American Heart Association. Atherosclerotic Vascular Disease Conference: Writing Group III: Pathophysiology. Circulation 2004, 109, 2617–2625. [Google Scholar] [CrossRef]
- Miller, Y.I.; Tsimikas, S. Oxidation-specific epitopes as targets for biotheranostic applications in humans: Biomarkers, molecular imaging and therapeutics. Curr. Opin. Lipidol. 2013, 24, 426–437. [Google Scholar] [CrossRef]
- Nofer, J.R.; Kehrel, B.; Fobker, M.; Levkau, B.; Assmann, G.; von Eckardstein, A. HDL and arteriosclerosis: Beyond reverse cholesterol transport. Atherosclerosis 2002, 161, 1–16. [Google Scholar] [CrossRef]
- Nagano, M.; Nakajima, H.; Toh, R.; Hirata, K.-I.; Ishida, T. Cardioprotective effects of high-density lipoprotein beyond its anti-atherogenic action. J. Atheroscler. Thromb. 2018, 25, 985–993. [Google Scholar] [CrossRef]
- Huxley, R.R.; Barzi, F.; Lam, T.H.; Czernichow, S.; Fang, X.; Welborn, T.; Shaw, J.; Ueshima, H.; Zimmet, P.; Jee, S.H.; et al. Isolated low levels of high-density lipoprotein cholesterol are associated with an increased risk of coronary heart disease: An individual participant data meta-analysis of 23 studies in the Asia-Pacific region. Circulation 2011, 124, 2056–2064. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Zhao, D.; Wang, M.; Zhao, F.; Han, X.; Qi, Y.; Liu, J. Association between circulating oxidized LDL and atherosclerotic cardiovascular disease: A meta-analysis of observational studies. Can. J. Cardiol. 2017, 33, 1624–1632. [Google Scholar] [CrossRef] [PubMed]
- Itabe, H.; Ueda, M. Measurement of plasma oxidized low-density lipoprotein and its clinical implications. J. Atheroscler. Thromb. 2007, 14, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pastorino, A.M.; Cominacini, L.; Maiorino, M.; Campagnola, M.; Garbin, U.; Davoli, A.; De Santis, A.; Lo Cascio, V. Effect of plasma on the degradation of hydroperoxides of unesterified linoleic acid and copper-peroxidized LDL. Free Radix Biol. Med. 1994, 16, 459–463. [Google Scholar] [CrossRef]
- Van Berkel, T.J.; De Rijke, Y.B.; Kruijt, J.K. Different fate in vivo of oxidatively modified low density lipoprotein and acetylated low density lipoprotein in rats. Recognition by various scavenger receptors on Kupffer and endothelial liver cells. J. Biol. Chem. 1991, 266, 2282–2289. [Google Scholar] [CrossRef]
- Berliner, J.A.; Territo, M.C.; Sevanian, A.; Ramin, S.; Kim, J.A.; Bamshad, B.; Esterson, M.; Fogelman, A.M. Minimally modified low density lipoprotein stimulates monocyte endothelial interactions. J. Clin. Investig. 1990, 85, 1260–1266. [Google Scholar] [CrossRef]
- Subbanagounder, G.; Watson, A.D.; Berliner, J.A. Bioactive products of phospholipid oxidation: Isolation, identification, measurement and activities. Free Radic. Biol. Med. 2000, 28, 1751–1761. [Google Scholar] [CrossRef]
- Itabe, H.; Mori, M.; Fujimoto, Y.; Higashi, Y.; Takano, T. Minimally modified LDL is an oxidized LDL enriched with oxidized phosphatidylcholines. J. Biochem. 2003, 134, 459–465. [Google Scholar] [CrossRef]
- Greaves, D.R.; Gordon, S. Recent insights into the biology of macrophage scavenger receptors. J. Lipid Res. 2005, 46, 11–20. [Google Scholar] [CrossRef]
- Chou, M.Y.; Hartvigsen, K.; Hansen, L.F.; Fogelstrand, L.; Shaw, P.X.; Boullier, A.; Binder, C.J.; Witztum, J.L. Oxidation-specific epitopes are important targets of innate immunity. J. Intern. Med. 2008, 263, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Hazen, S.L. Oxidized phospholipids as endogenous pattern recognition ligands in innate immunity. J. Biol. Chem. 2008, 283, 15527–15531. [Google Scholar] [CrossRef]
- Ehara, S.; Ueda, M.; Naruko, T.; Haze, K.; Itoh, A.; Otsuka, M.; Komatsu, R.; Matsuo, T.; Itabe, H.; Takano, T.; et al. Elevated levels of oxidized low density lipoprotein show a positive relationship with the severity of acute coronary syndromes. Circulation 2001, 103, 1955–1960. [Google Scholar] [CrossRef]
- Nishi, K.; Itabe, H.; Uno, M.; Kitazato, K.T.; Horiguchi, H.; Shinno, K.; Nagahiro, S. Oxidized LDL in carotid plaques and plasma associates with plaque instability. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1649–1654. [Google Scholar] [CrossRef] [PubMed]
- Bräsen, J.H.; Häkkinen, T.; Malle, E.; Beisiegel, U.; Ylä-Herttuala, S. Patterns of oxidized epitopes, but not NF-kB expression, change during atherogenesis in WHHL rabbits. Atherosclerosis 2003, 166, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Shiomi, M.; Yamada, S.; Matsukawa, A.; Itabe, H.; Ito, T. Invasion of atheromatous plaques into tunica media causes coronary outward remodeling in WHHLMI rabbits. Atherosclerosis 2008, 198, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Pennathur, S.; Bergt, C.; Shao, B.; Byun, J.; Kassim, S.Y.; Singh, P.; Green, P.S.; McDonald, T.O.; Brunzell, J.; Chait, A.; et al. Human atherosclerotic intima and blood of patients with established coronary artery disease contain high density lipoprotein damaged by reactive nitrogen species. J. Biol. Chem. 2004, 279, 42977–42983. [Google Scholar] [CrossRef] [PubMed]
- Itabe, H.; Suzuki, K.; Tsukamoto, Y.; Komatsu, R.; Ueda, M.; Mori, M.; Higashi, Y.; Takano, T. Lysosomal accumulation of oxidized phosphatidylcholine-apolipoprotein B complex in macrophages: Intracellular fate of oxidized low density lipoprotein. Biochim. Biophys. Acta 2000, 1487, 233–245. [Google Scholar] [CrossRef]
- Iwamoto, S.; Fujita, Y.; Kakino, A.; Yanagida, K.; Matsuda, H.; Yoshimoto, R.; Sawamura, T. An alternative protein standard to measure activity of LOX-1 ligand containing apoB (LAB)—Utilization of anti-LOX-1 single- chain antibody fused to apoB fragment. J. Atheroscler. Thromb. 2011, 18, 818–828. [Google Scholar] [CrossRef]
- Itabe, H.; Kato, R.; Sasabe, N.; Obama, T.; Yamamoto, M. Significance of oxidized low-density lipoprotein in body fluids as a marker related to diseased conditions. Curr. Med. Chem. 2019, 26, 1576–1593. [Google Scholar] [CrossRef]
- Austin, M.A.; King, M.C.; Vranizan, K.M.; Krauss, R.M. Atherogenic lipoprotein phenotype: A proposed genetic marker for coronary heart disease risk. Circulation 1990, 82, 495–506. [Google Scholar] [CrossRef]
- Hirano, T.; Ito, Y.; Koba, S.; Toyoda, M.; Ikejiri, A.; Saegusa, H.; Yamazaki, J.; Yoshino, G. Clinical significance of small dense low-density lipoprotein cholesterol levels determined by the simple precipitation method. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Varady, K.A.; Lamarche, B. Lipoprint adequately estimates LDL size distribution, but not absolute size, versus polyacrylamide gradient gel electrophoresis. Lipids 2011, 465, 1163–1167. [Google Scholar] [CrossRef] [PubMed]
- Ikezaki, H.; Lim, E.; Cupples, L.A.; Liu, C.-T.; Asztalos, B.F.; Schaefer, E.J. Small dense low-density lipoprotein cholesterol is the most atherogenic lipoprotein parameter in the prospective Framingham offspring study. J. Am. Heart Assoc. 2021, 10, e019140. [Google Scholar] [CrossRef]
- Hirano, T.; Ito, Y. Accuracy of small dense low-density lipoprotein-cholesterol concentration estimated via Sampson’s equation in healthy subjects and patients with diabetes. J. Atheroscler. Thromb, 2022; Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Tani, M.; Kawakami, A.; Mizuno, Y.; Imase, R.; Ito, Y.; Kondo, K.; Ishii, H.; Yoshida, M. Small dense LDL enhances THP-1 macrophage foam cell formation. J. Atheroscler. Thromb. 2011, 18, 698–704. [Google Scholar] [CrossRef]
- Medlow, P.; McEneny, J.; Murphy, M.H.; Trinick, T.; Duly, E.; Davison, G.W. Lipoprotein subfraction oxidation in acute exercise and ageing. Free Radic. Res. 2016, 50, 345–353. [Google Scholar] [CrossRef]
- Kondo, A.; Muranaka, Y.; Ohta, I.; Notsu, K.; Manabe, M.; Kotani, K.; Saito, K.; Maekawa, M.; Kanno, T. Relationship between triglyceride concentrations and LDL size evaluated by malondialdehyde-modified LDL. Clin. Chem. 2001, 47, 893–900. [Google Scholar] [CrossRef]
- Wang, L.; Tao, L.; Hao, L.; Stanley, T.H.; Huang, K.-H.; Lambert, J.D.; Kris-Etherton, P.M. A moderate-fat diet with one avocado per day increases plasma antioxidants and decreases the oxidation of small, dense LDL in adults with overweight and obesity: A randomized controlled trial. J. Nutr. 2020, 105, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, K.; Noureen, A.; Kronnenberg, F.; Utermann, G. Structure, function, and genetic s of lipoprotein(a). J. Lipid Res. 2016, 56, 1339–1359. [Google Scholar] [CrossRef]
- Mehta, A.; Jain, V.; Saeed, A.; Saseen, J.J.; Gulati, M.; Ballantyne, C.M.; Virani, S.S. Lipoprotein(a) and ethnicities. Atherosclerosis 2022, 349, 42–52. [Google Scholar] [CrossRef]
- Enkhmaa, B.; Anuurad, E.; Berglund, L. Lipoprotein (a): Impact by ethnicity and environmental and medical conditions. J. Lipid Res. 2016, 57, 1111–1125. [Google Scholar] [CrossRef] [PubMed]
- Coassin, S.; Kronenberg, F. Lipoprotein(a) beyond the kringle IV repeat polymorphism: The complexity of genetic variation in the LPA gene. Atherosclerosis 2022, 349, 17–35. [Google Scholar] [CrossRef] [PubMed]
- Tsimikas, S.; Mallat, Z.; Talmud, P.J.; Kastelein, J.J.P.; Wareham, N.J.; Sandhu, M.S.; Miller, E.R.; Benessiano, J.; Tedgui, A.; Witztum, J.L.; et al. Oxidation-specific biomarkers, lipoprotein(a), and risk of fatal and non-fatal coronary events. J. Am. Coll. Cardiol. 2010, 56, 946–955. [Google Scholar] [CrossRef]
- Leibundgut, G.; Scipione, C.; Yin, H.; Schneider, M.; Boffa, M.B.; Green, S.; Yang, X.; Dennis, E.; Witztum, J.L.; Koschinsky, M.L.; et al. Determinants of binding of oxidized phospholipids on apolipoprotein (a) and lipoprotein (a). J. Lipid Res. 2013, 54, 2815–2830. [Google Scholar] [CrossRef]
- Emerging Risk Factors Collaboration; Erqou, S.; Kaptoge, S.; Perry, P.L.; Di Angelantonio, E.; Thompson, A.; White, I.R.; Marcovina, S.M.; Collins, R.; Thompson, S.M.; et al. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA 2009, 302, 412–423. [Google Scholar] [CrossRef]
- Erqou, S.; Thompson, A.; Di Angelantonio, E.; Saleheen, D.; Kaptoge, S.; Marcovina, S.; Danesh, J. Apolipoprotein(a) isoforms and the risk of vascular disease systematic review of 40 studies involving 58,000 participants. J. Am. Coll. Cardiol. 2010, 55, 210–2167. [Google Scholar] [CrossRef]
- Forbes, C.A.; Quek, R.G.W.; Deshpande, S.; Worthy, G.; Wolff, R.; Stirk, L.; Kleijnen, J.; Gandra, S.R.; Djedjos, S.; Wong, N.D. The relationship between Lp(a) and CVD outcomes: A systematic review. Lipids Health Dis. 2016, 15, 95. [Google Scholar] [CrossRef]
- Mohammadi-Shemirani, P.; Chong, M.; Narula, S.; Perrot, N.; Conen, D.; Roberts, J.D.; Thériault, S.; Bossé, Y.; Lanktree, M.B.; Pigeyre, M.; et al. Elevated lipoprotein(a) and risk of atrial fibrillation: An observational and Mendelian randomization study. J. Am. Coll. Cardiol. 2022, 79, 1579–1590. [Google Scholar] [CrossRef]
- Kumar, P.; Swarnkar, P.; Misra, S.; Nath, M. Lipoprotein (a) level as a risk factor for stroke and its subtype: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 15660. [Google Scholar] [CrossRef] [PubMed]
- Genser, B.; Karen, C.D.; Siekmeier, R.; Stojakovic, T.; Grammer, T.; Maerz, W. Lipoprotein (a) and risk of cardiovascular disease--a systematic review and meta analysis of prospective studies. Clin. Lab. 2011, 57, 143–156. [Google Scholar]
- Smolders, B.; Lemmens, R.; Thijs, V. Lipoprotein (a) and stroke: A meta-analysis of observational studies. Stroke 2007, 38, 1959–1966. [Google Scholar] [CrossRef] [PubMed]
- Clarke, R.; Peden, J.F.; Hopewell, J.C.; Kyriakou, T.; Goel, A.; Heath, S.C.; Parish, S.; Barlera, S.; Franzosi, M.G.; Rust, S.; et al. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N. Engl. J. Med. 2009, 361, 2518–2528. [Google Scholar] [CrossRef] [PubMed]
- Klarin, D.; Lynch, J.; Aragam, K.; Chaffin, M.; Assimes, T.L.; Huang, J.; Lee, K.M.; Shao, Q.; Huffman, J.E.; Natarajan, P.; et al. Genome-wide association study of peripheral artery disease in the Million Veteran Program. Nat. Med. 2019, 25, 1274–1279. [Google Scholar] [CrossRef] [PubMed]
- van Zuydam, N.R.; Stiby, A.; Abdalla, M.; Austin, E.; Dahlström, E.H.; McLachlan, S.; Vlachopoulou, E.; Ahlqvist, E.; Liao, C.D.; Sandholm, N.; et al. Genome-wide association study of peripheral artery disease. Circ. Genom. Precis Med. 2021, 14, e002862. [Google Scholar] [CrossRef]
- Palmer, M.R.; Kim, D.S.; Crosslin, D.R.; Stanaway, I.B.; Rosenthal, E.A.; Carrell, D.S.; Cronkite, D.J.; Gordon, A.; Du, X.; Li, Y.K.; et al. Loci identified by a genome-wide association study of carotid artery stenosis in the eMERGE network. Genet. Epidemiol. 2021, 45, 4–15. [Google Scholar] [CrossRef]
- Levin, M.G.; Zuber, V.; Walker, V.M.; Klarin, D.; Lynch, J.; Malik, R.; Aday, A.W.; Bottolo, L.; Pradhan, A.D.; Dichgans, M.; et al. Prioritizing the role of major lipoproteins and subfractions as risk factors for peripheral artery disease. Circulation 2021, 144, 353–364. [Google Scholar] [CrossRef]
- Xia, J.; Guo, C.; Liu, K.; Xie, Y.; Cao, H.; Peng, W.; Sun, Y.; Liu, X.; Li, B.; Zhang, L. Association of lipoprotein (a) variants with risk of cardiovascular disease: A Mendelian randomization study. Lipids Health Dis. 2021, 20, 57. [Google Scholar] [CrossRef]
- Wang, S.; Zha, L.; Chen, J.; Du, D.; Liu, D.; Zhong, M.; Shang, R.; Sun, D.; Sun, C.; Jin, E. The relationship between lipoprotein(a) and risk of cardiovascular disease: A Mendelian randomization analysis. Eur. J. Med. Res. 2022, 27, 211. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.; Ference, B.A.; Staley, J.R.; Freitag, D.F.; Masonm, A.M.; Nielsen, S.F.; Willeit, P.; Young, R.; Surendran, P.; Karthikeyan, S.; et al. Association of LPA variants with risk of coronary disease and the Implications for lipoprotein(a)-lowering therapies: A Mendelian randomization analysis. JAMA Cardiol. 2018, 3, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Steffen, B.T.; Duprez, D.; Bertoni, A.G.; Guan, W.; Tsai, M.Y. Lp(a) [Lipoprotein(a)]-related risk of heart failure is evident in whites but not in other racial/ethnic groups. The multi-ethnic study of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 2498–2504. [Google Scholar] [CrossRef]
- Matsukura, M.; Ozaki, K.; Shigematsu, H.; Kudo, T.; Inoue, Y.; Kimura, H.; Hosaka, A.; Shigematsu, K.; Miyata, T.; Watanabe, T.; et al. Genome-wide association study of peripheral arterial disease in a Japanese population. PLoS ONE 2015, 10, e0139262. [Google Scholar] [CrossRef]
- Deshmukh, H.A.; Colhoun, H.M.; Johnson, T.; †McKeigue, P.M.; Betteridge, D.J.; Durrington, P.N.; Fuller, J.H.; Livingstone, S.; Charlton-Menys, V.; Neil, A.; et al. Genome-wide association study of genetic determinants of LDL-c response to atorvastatin therapy: Importance of Lp(a). J. Lipid Res. 2012, 53, 1000–1011. [Google Scholar] [CrossRef] [PubMed]
- de Boer, L.M.; Oorthuys, A.O.J.; Wiegman, A.; Langendam, M.W.; Kroon, J.; Spijker, R.; Zwinderman, A.H.; Hutten, B.A. Statin therapy and lipoprotein(a) levels: A systematic review and meta-analysis. Eur. J. Prev. Cardiol. 2022, 29, 779–792. [Google Scholar] [CrossRef] [PubMed]
- Raal, F.J.; Giugliano, R.P.; Sabatine, M.S.; Koren, M.J.; Blom, D.; Seidah, N.G.; Honarpour, N.; Lira, A.; Xue, A.; Chiruvolu, P.; et al. PCSK9 inhibition-mediated reduction in Lp(a) with evolocumab: An analysis of 10 clinical trials and the LDL receptor’s role. J. Lipid Res. 2016, 57, 1086–1096. [Google Scholar] [CrossRef] [PubMed]
- McCormick, S.P.A.; Schneider, W.J. Lipoprotein(a) catabolism: A case of multiple receptors. Pathology 2019, 51, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Cazzolato, G.; Avogaro, P.; Bittolo-Bon, G. Characterization of a more electronegatively charged LDL subfraction by ion exchange HPLC. Free Rad. Biol. Med. 1991, 11, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-H.; Jiang, T.; Yang, J.-H.; Jiang, W.; Lu, J.; Marathe, G.K.; Pownall, H.J.; Ballantyne, C.M.; McIntyre, T.M.; Henry, P.D.; et al. Low-density lipoprotein in hypercholesterolemic human plasma induces vascular endothelial cell apoptosis by inhibiting fibroblast growth factor 2 transcription. Circulation 2003, 107, 2102–2108. [Google Scholar] [CrossRef]
- Sánchez-Quesada, J.L.; Benítez, S.; Ordóñez-Llanos, J. Electronegative low-density lipoprotein. Curr. Opin. Lipidol. 2004, 15, 329–335. [Google Scholar] [CrossRef]
- Hodis, H.N.; Kramsch, D.M.; Avogaro, P.; Bittolo-Bon, G.; Cazzolato, G.; Hwang, J.; Peterson, H.; Sevanian, A. Biochemical and cytotoxic characteristics of an in vivo circulating oxidized low density lipoprotein (LDL−). J. Lipid Res. 1994, 35, 669–677. [Google Scholar] [CrossRef]
- Nyyssönen, N.; Kaikkonen, J.; Salonen, J.T. Characterization and determinants of an electronegatively charged low-density lipoprotein in human plasma. Scand. J. Clin. Lab. Investig. 1996, 56, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Sevanian, A.; Bittolo-Bon, G.; Cazzolato, G.; Hodis, H.; Hwang, J.; Zamburlini, A.; Maiorino, M.; Ursini, F. LDL- is a lipid hydroperoxide-enriched circulating lipoprotein. J. Lipid Res. 1997, 38, 419–428. [Google Scholar] [CrossRef]
- Benitez, S.; Camacho, M.; Bancells, C.; Vila, L.; Sánches-Quesada, J.L.; Ordóñez-Llanos, J. Wide proinflammatory effect of electronegative low-density lipoprotein on human endothelial cells assayed by a protein array. Biochim. Biophys. Acta 2006, 1761, 1014–1021. [Google Scholar] [CrossRef]
- Sánchez-Quesada, J.L.; Camacho, M.; Antón, R.; Benítez, S.; Vila, L.; Ordóñez-Llanos, J. Electronegative LDL of FH subjects: Chemical characterization and induction of chemokine release from human endothelial cells. Atherosclerosis 2003, 166, 261–270. [Google Scholar] [CrossRef]
- Sawada, N.; Obama, T.; Koba, S.; Takaki, T.; Iwamoto, S.; Aiuchi, T.; Kato, R.; Kikuchi, M.; Hamazaki, Y.; Itabe, H. Circulating oxidized LDL, increased in patients with acute myocardial infarction, is accompanied by heavily modified HDL. J. Lipid Res. 2020, 61, 816–829. [Google Scholar] [CrossRef] [PubMed]
- Hoff, H.F.; Gaubatz, J.W. Isolation, purification, and characterization of a lipoprotein containing apo B from the human aorta. Atherosclerosis 1982, 42, 273–297. [Google Scholar] [CrossRef] [PubMed]
- Chappy, B.; Myara, I.; Benoit, M.O.; Mazière, C.; Mazière, J.C.; Moatti, N. Characteristics of ten charge-differing subfractions isolated from human native low-density lipoproteins (LDL). No evidence of peroxidative modifications. Biochim. Biophys. Acta 1995, 1259, 261–270. [Google Scholar] [CrossRef]
- Zheng, L.; Nukuna, B.; Brennan, M.L.; Sun, M.; Goormastic, M.; Settle, M.; Schmitt, D.; Fu, X.; Thomson, L.; Fox, P.L.; et al. Apolipoprotein A-I is a selective target for myeloperoxidase-catalyzed oxidation and functional impairment in subjects with cardiovascular disease. J. Clin. Investig. 2004, 114, 529–541. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; DiDonato, J.A.; Levison, B.S.; Schmitt, D.; Li, L.; Wu, Y.; Buffa, J.; Kim, T.; Gerstenecker, G.S.; Gu, X.; et al. An abundant dysfunctional apolipoprotein A1 in human atheroma. Nat. Med. 2014, 20, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Podrez, E.A. Characterization of covalent modifications of HDL apoproteins by endogenous oxidized phospholipids. Free Radic. Biol. Med. 2018, 115, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Puig, N.; Montolio, L.; Camps-Renom, P.; Navarra, L.; Jiménez-Altayó, F.; Jiménez-Xarrié, E.; Sánchez-Quesada, J.L.; Benitez, S. Electronegative LDL promotes inflammation and triglyceride accumulation in macrophages. Cells 2020, 9, 583. [Google Scholar] [CrossRef] [PubMed]
- Ligi, D.; Benitez, S.; Croce, L.; Rivas-Urbina, A.; Puig, N.; Ordóñez-Llanos, J.; Mannello, F.; Sanchez-Quesada, J.L. Electronegative LDL induces MMP-9 and TIMP-1 release in monocytes through CD14 activation: Inhibitory effect of glycosaminoglycan sulodexide. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 3559–3567. [Google Scholar] [CrossRef]
- Chang, S.F.; Chang, P.Y.; Chou, Y.C.; Lu, S.C. Electronegative LDL induces M1 polarization of human macrophages through a LOX-1-dependent pathway. Inflammation 2020, 43, 1524–1535. [Google Scholar] [CrossRef]
- Chang, C.K.; Chen, P.K.; Lan, J.L.; Chang, S.H.; Hsieh, T.Y.; Liao, P.J.; Chen, C.H.; Chen, D.Y. Association of electronegative LDL with macrophage foam cell formation and CD11c expression in rheumatoid arthritis patients. Int. J. Mol. Sci. 2020, 21, 5883. [Google Scholar] [CrossRef]
- Puig, N.; Estruch, M.; Jin, L.; Sanchez-Quesada, J.L.; Benitez, S. The role of distinctive sphingolipids in the inflammatory and apoptotic effects of electronegative LDL on monocytes. Biomolecules 2019, 9, 300. [Google Scholar] [CrossRef]
- Faulin, T.D.E.S.; Kazuma, S.M.; Tripodi, G.L.; Cavalcante, M.F.; Wakasuqui, F.; Oliveira, C.L.P.; Degenhardt, M.F.S.; Michaloski, J.; Giordano, R.J.; Ketelhuth, D.F.J.; et al. Proinflammatory action of a new electronegative low-density lipoprotein epitope. Biomolecules 2019, 9, 386. [Google Scholar] [CrossRef]
- Tripodi, G.L.; Prieto, M.B.; Abdalla, D.S.P. Inflammasome activation in human macrophages induced by a LDL (−) mimetic peptide. Inflammation 2020, 43, 722–730. [Google Scholar] [CrossRef]
- Kakino, A.; Fujita, Y.; Ke, L.-Y.; Chan, H.-C.; Tsai, M.-H.; Dai, C.-Y.; Chen, C.-H.; Sawamura, T. Adiponectin forms a complex with atherogenic LDL and inhibits its downstream effects. J. Lipid Res. 2021, 62, 100001. [Google Scholar] [CrossRef] [PubMed]
- Ohinata, H.; Obama, T.; Makiyama, T.; Watanabe, Y.; Itabe, H. High-density lipoprotein suppresses neutrophil extracellular traps enhanced by oxidized low-density lipoprotein or oxidized phospholipids. Int. J. Mol. Sci. 2022, 23, 13992. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.S.; Chan, H.C.; Tsai, M.H.; Stancel, N.; Lee, H.C.; Cheng, K.H.; Tung, Y.C.; Chan, H.C.; Wang, C.Y.; Shin, S.J.; et al. Range of L5 LDL levels in healthy adults and L5’s predictive power in patients with hyperlipidemia or coronary artery disease. Sci. Rep. 2018, 8, 11866. [Google Scholar] [CrossRef]
- Chu, C.S.; Ke, L.Y.; Chan, H.C.; Chan, H.C.; Chen, C.C.; Cheng, K.H.; Lee, H.C.; Kuo, H.F.; Chang, C.T.; Chang, K.C.; et al. Four statin benefit groups defined by the 2013 ACC/AHA New Cholesterol Guideline are characterized by increased plasma level of electronegative low-density lipoprotein. Acta Cardiol. Sin. 2016, 32, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-T.; Shen, M.-Y.; Lee, A.-S.; Wang, C.-C.; Chen, W.-Y.; Chang, C.-M.; Chang, K.-C.; Stancel, N.; Chen, C.-H. Electronegative low-density lipoprotein increases the risk of ischemic lower-extremity peripheral artery disease in uremia patients on maintenance hemodialysis. Sci. Rep. 2017, 7, 4654. [Google Scholar] [CrossRef] [PubMed]
- Mello, A.P.Q.; da Silva, I.T.; Abdalla, D.S.P.; Damasceno, N.R.T. Electronegative low-density lipoprotein: Origin and impact on health and disease. Atherosclerosis 2011, 215, 257–265. [Google Scholar] [CrossRef]
- Cavalcante, M.F.; Adorne, M.D.; Turato, W.M.; Kemmerer, M.; Uchiyama, M.K.; Asbahr, A.C.C.; Alves, A.C.S.; Farsky, S.H.P.; Drewes, C.; Spatti, M.C.; et al. scFv-Anti-LDL(−)-metal-complex multi-wall functionalized-nanocapsules as a promising tool for the prevention of atherosclerosis progression. Front Med. 2021, 8, 652137. [Google Scholar] [CrossRef]
- Wang, X.S.; Shao, B.; Oda, M.N.; Heinecke, J.W.; Mahler, S.; Stocker, R. A sensitive and specific ELISA detects methionine sulfoxide-containing apolipoprotein A-I in HDL. J. Lipid Res. 2009, 50, 586–594. [Google Scholar] [CrossRef]
- Rasmiena, A.A.; Barlow, C.K.; Ng, T.W.; Tull, D.; Meikle, P.J. High density lipoprotein efficiently accepts surface but not internal oxidised lipids from oxidised low density lipoprotein. Biochim. Biophys. Acta 2016, 1861, 69–77. [Google Scholar] [CrossRef]
- Sangvanich, P.; Mackness, B.; Gaskell, S.J.; Durrington, P.; Mackness, M. The effect of high-density lipoproteins on the formation of lipid/protein conjugates during in vitro oxidation of low-density lipoprotein. Biochem. Biophys. Res. Commun. 2003, 300, 501–506. [Google Scholar] [CrossRef]
- Shao, B. Site-specific oxidation of apolipoprotein A-I impairs cholesterol export by ABCA1, a key cardioprotective function of HDL. Biochim. Biophys. Acta 2012, 1821, 490–501. [Google Scholar] [CrossRef]
- Gao, D.; Ashraf, M.Z.; Zhang, L.; Kar, N.; Byzova, T.V.; Podrez, E.A. Cross-linking modifications of HDL apoproteins by oxidized phospholipids: Structural characterization, in vivo detection, and functional implications. J. Biol. Chem. 2020, 295, 1973–1984. [Google Scholar] [CrossRef]
- Szapacs, M.E.; Kim, H.Y.H.; Porter, N.A.; Liebler, D.C. Identification of proteins adducted by lipid peroxidation products in plasma and modifications of apolipoprotein A1 with a novel biotinylated phospholipid probe. J. Proteome Res. 2008, 7, 4237–4246. [Google Scholar] [CrossRef] [PubMed]
- DiDonato, J.A.; Huang, Y.; Aulak, K.S.; Even-Or, O.; Gerstenecker, G.; Gogonea, V.; Wu, Y.; Fox, P.L.; Tang, W.H.W.; Plow, E.F.; et al. Function and distribution of apolipoprotein A1 in the artery wall are markedly distinct from those in plasma. Circulation 2013, 128, 1644–1655. [Google Scholar] [CrossRef]
- Britesa, F.; Martina, M.; Guillas, I.; Kontush, A. Antioxidative activity of high-density lipoprotein (HDL): Mechanistic insights into potential clinical benefit. BBA Clin. 2017, 8, 66–77. [Google Scholar] [CrossRef]
- Cukier, A.M.O.; Therond, P.; Didichenko, S.A.; Guillas, I.; Chapman, M.J.; Samuel, D.; Wrightg, S.D.; Kontush, A. Structure-function relationships in reconstituted HDL: Focus on antioxidative activity and cholesterol efflux capacity. BBA-Mol. Cell Biol. Lipids 2017, 1862, 890–900. [Google Scholar] [CrossRef] [PubMed]
- Sawada, N.; Obama, T.; Mizuno, M.; Fukuhara, K.; Iwamoto, S.; Aiuchi, T.; Makiyama, T.; Itabe, H. Transfer and enzyme-mediated metabolism of oxidized phosphatidylcholine and lysophosphatidylcholine between low- and high-density lipoproteins. Antioxidants 2020, 9, 1045. [Google Scholar] [CrossRef] [PubMed]
- Itabe, H.; Sawada, N.; Makiyama, T.; Obama, T. Structure and dynamics of oxidized lipoproteins in vivo: Roles of high-density lipoprotein. Biomedicines 2021, 9, 655. [Google Scholar] [CrossRef] [PubMed]
- Honda, H.; Ueda, M.; Kojima, S.; Mashiba, S.; Michihata, T.; Takahashi, K.; Shishido, K.; Akizawa, T. Oxidized high-density lipoprotein as a risk factor for cardiovascular events in prevalent hemodialysis patients. Atherosclerosis 2012, 220, 493–501. [Google Scholar] [CrossRef]
- Miki, T.; Miyoshi, T.; Kotani, K.; Kohno, K.; Asonuma, H.; Sakuragi, S.; Koyama, Y.; Nakamura, K.; Ito, H. Decrease in oxidized high-density lipoprotein is associated with slowed progression of coronary artery calcification: Subanalysis of a prospective multicenter study. Atherosclerosis 2019, 283, 1–6. [Google Scholar] [CrossRef]
- Janac, J.M.; Zeljkovic, A.; Jelic-Ivanovic, Z.D.; Dimitrijevic-Sreckovic, V.S.; Vekic, J.; Mijkovic, M.M.; Stefanovic, A.; Kotur-Stevuljevic, J.M.; Ivanisevic, J.M.; Spasojevic-Kalimanovska, V.V. Increased oxidized high-density lipoprotein/high-density lipoprotein-cholesterol ratio as a potential indicator of disturbed metabolic health in overweight and obese individuals. Lab. Med. 2020, 51, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Carnuta, M.S.; Stancu, V.S.; Toma, L.; Sanda, G.M.; Niculescu, L.S.; Deleanu, M.; Popescu, A.C.; Popescu, M.R.; Vlad, A.; Dimulescu, D.R.; et al. Dysfunctional high-density lipoproteins have distinct composition, diminished anti-infammatory potential and discriminate acute coronary syndrome from stable coronary artery disease patients. Sci. Rep. 2017, 7, 7295. [Google Scholar] [CrossRef] [PubMed]
- Li, H.M.; Mo, Z.W.; Peng, Y.M.; Li, Y.; Dai, W.P.; Yuan, H.Y.; Chang, F.J.; Wang, T.T.; Wang, M.; Hu, K.H.; et al. Angiogenic and antiangiogenic mechanisms of high density lipoprotein from healthy subjects and coronary artery diseases patients. Redox Biol. 2020, 36, 101642. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Itabe, H.; Obama, T. The Oxidized Lipoproteins In Vivo: Its Diversity and Behavior in the Human Circulation. Int. J. Mol. Sci. 2023, 24, 5747. https://doi.org/10.3390/ijms24065747
Itabe H, Obama T. The Oxidized Lipoproteins In Vivo: Its Diversity and Behavior in the Human Circulation. International Journal of Molecular Sciences. 2023; 24(6):5747. https://doi.org/10.3390/ijms24065747
Chicago/Turabian StyleItabe, Hiroyuki, and Takashi Obama. 2023. "The Oxidized Lipoproteins In Vivo: Its Diversity and Behavior in the Human Circulation" International Journal of Molecular Sciences 24, no. 6: 5747. https://doi.org/10.3390/ijms24065747
APA StyleItabe, H., & Obama, T. (2023). The Oxidized Lipoproteins In Vivo: Its Diversity and Behavior in the Human Circulation. International Journal of Molecular Sciences, 24(6), 5747. https://doi.org/10.3390/ijms24065747






