The Tryptophan and Kynurenine Pathway Involved in the Development of Immune-Related Diseases
Abstract
1. Introduction
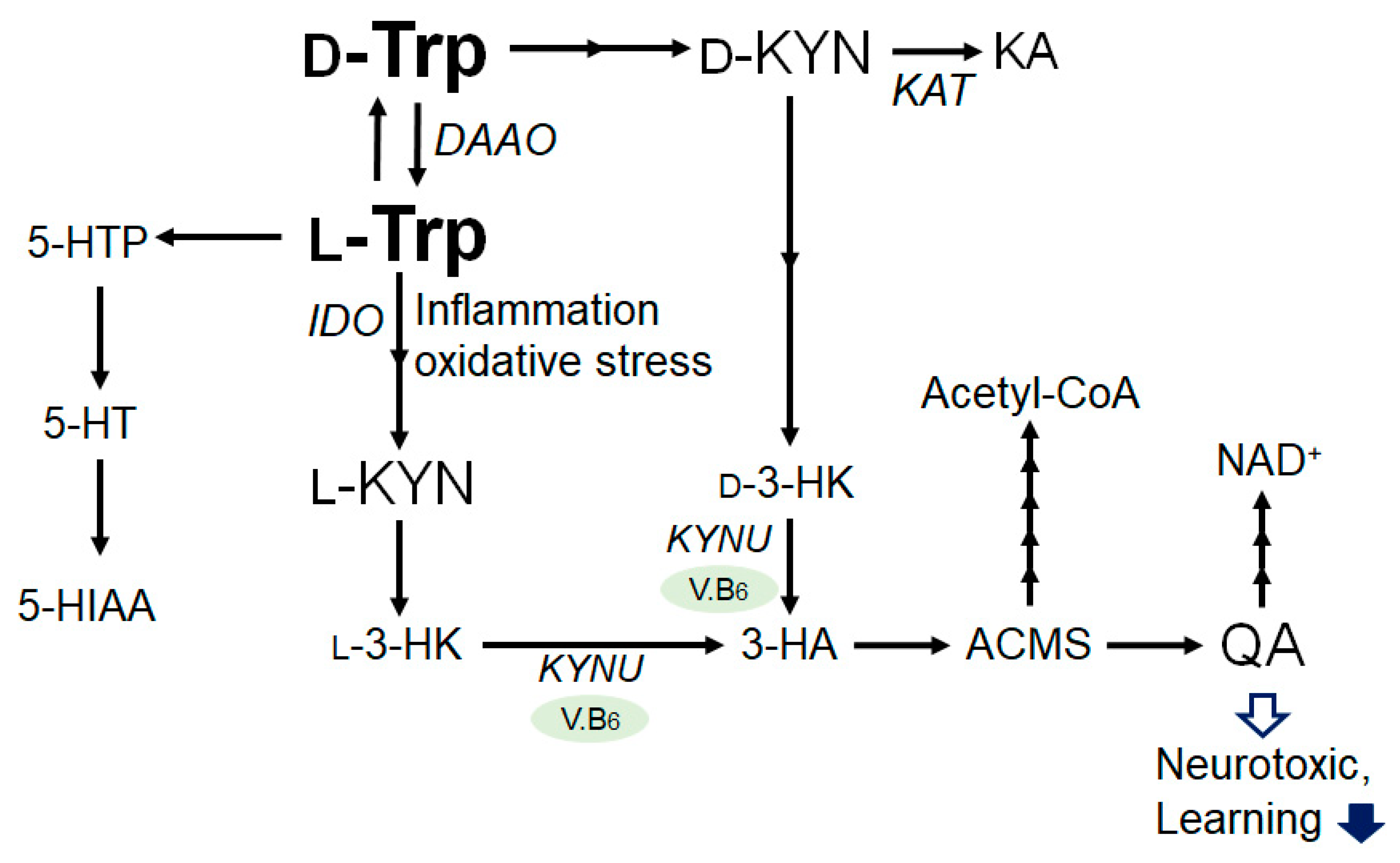
2. Tryptophan and Kynurenine Metabolic Pathway
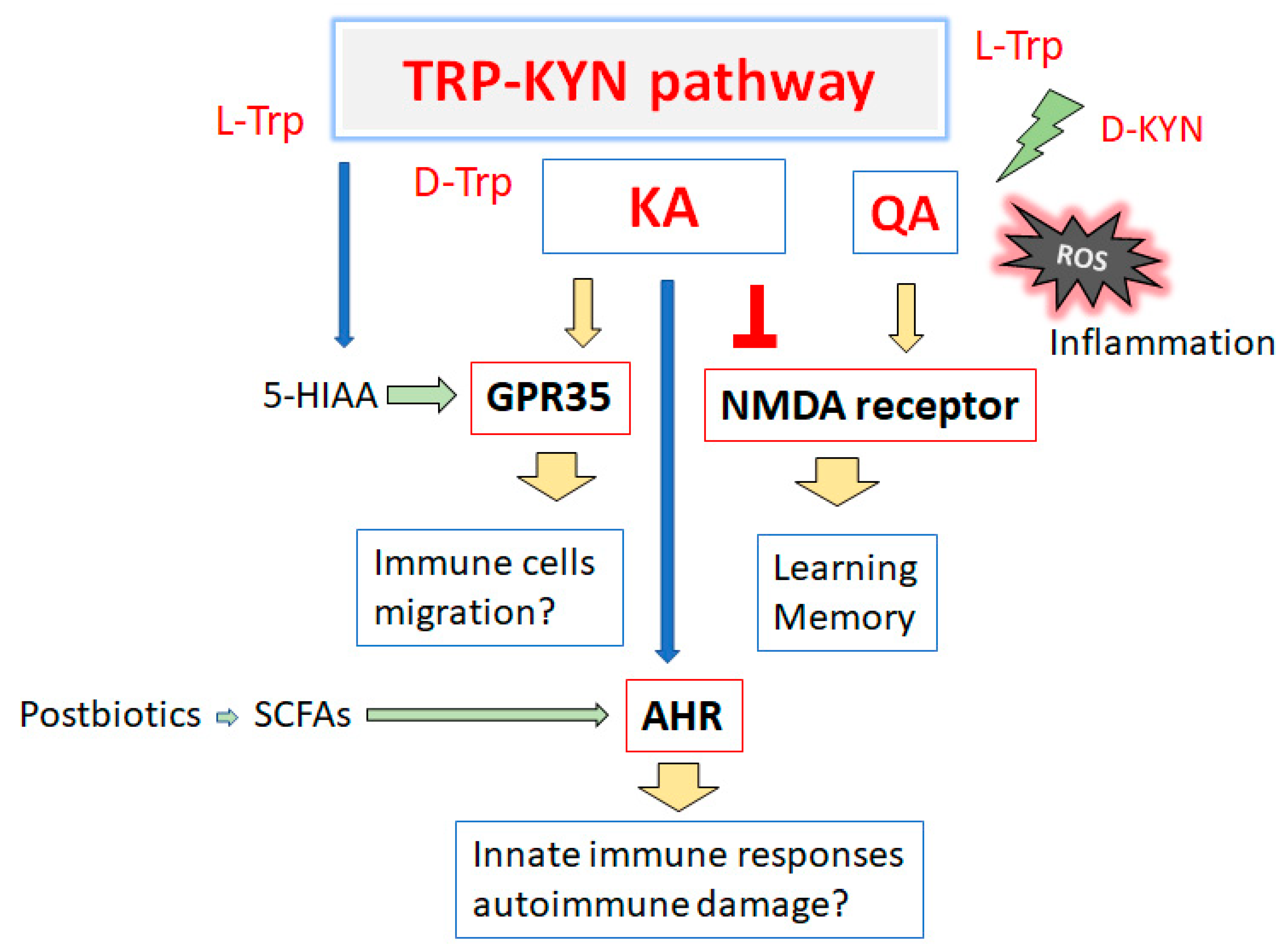
3. Connection between Kynurenine Pathway and Immunity
4. Several Immune-Related Diseases Associated with the Tryptophan and Kynurenine Pathway
4.1. Major Depressive and Bipolar Disorders
4.2. Cardiovascular Disease
4.3. Kynurenine Pathway in Acute Kidney Injury and Chronic Kidney Disease
4.4. Inflammatory Bowel Disease
4.5. Osteoporosis
4.6. Polycystic Ovary Syndrome
5. Kynurenine Metabolites Involved in Brain Memory System and Immunity
6. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AHR | Aryl hydrocarbon receptor |
| DAAO | D-amino acid oxidase |
| D-KYN | D-Kynurenine |
| D-Trp | D-Tryptophan |
| GPR35 | G protein-coupled receptor 35 |
| 5-HIAA | 5-Hydroxyindoleacetic acid |
| 5-HT | 5-hydroxytryptamine (Serotonin) |
| 5-HTP | 5-Hydroxytryptophan |
| IBD | Inflammatory bowel disease |
| IDO | Indoleamine 2,3-dioxygenase |
| IFN | Interferon |
| IL | Interleukin |
| KA | Kynurenic acid |
| KAT | Kynurenine aminotransferase |
| KYNU | Kynureninase |
| L-KYN | L-Kynurenine |
| L-Trp | L-Tryptophan |
| NAD | Nicotinamide adenine dinucleotide |
| NMDA | N-methyl-D-aspartate |
| NMDAR | N-methyl-D-aspartic acid receptor |
| QA | Quinolinic acid |
| QOL | Quality of life |
| ROS | Reactive oxygen species |
| SCFAs | Short-chain fatty acids |
| TNF | Tumor necrosis factor |
| Treg | T-regulatory cells |
| Th17 | T-helper 17 cells |
| UC | Ulcerative colitis |
| V.B6 | Vitamin B6 |
References
- Metghalchi, S.; Ponnuswamy, P.; Simon, T.; Haddad, Y.; Laurans, L.; Clément, M.; Dalloz, M.; Romain, M.; Esposito, B.; Koropoulis, V.; et al. Indoleamine 2,3-Dioxygenase Fine-Tunes Immune Homeostasis in Atherosclerosis and Colitis through Repression of Interleukin-10 Production. Cell Metab. 2015, 22, 460–471. [Google Scholar] [CrossRef]
- Strasser, B.; Becker, K.; Fuchs, D.; Gostner, J.M. Kynurenine pathway metabolism and immune activation: Peripheral measurements in psychiatric and co-morbid conditions. Neuropharmacology 2017, 112, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Sulo, G.; Vollset, S.E.; Nygård, O.; Midttun, Ø.; Ueland, P.M.; Eussen, S.J.; Pedersen, E.R.; Tell, G.S. Neopterin and kynurenine-tryptophan ratio as predictors of coronary events in older adults, the Hordaland Health Study. Int. J. Cardiol. 2013, 168, 1435–1440. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Guillemin, G.J. Kynurenine pathway metabolites in humans: Disease and healthy States. Int. J. Tryptophan Res. 2009, 2, 1–19. [Google Scholar] [CrossRef]
- Cervenka, I.; Agudelo, L.Z.; Ruas, J.L. Kynurenines: Tryptophan’s metabolites in exercise, inflammation, and mental health. Science 2017, 357, eaaf9794. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, S.M.; Clarke, G.; Borre, Y.E.; Dinan, T.G.; Cryan, J.F. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav. Brain Res. 2015, 277, 32–48. [Google Scholar] [CrossRef]
- Mauler, M.; Schanze, N.; Krauel, K.; Schoenichen, C.; Glatzki, F.; Poeschl, S.; Stallmann, D.; Mezger, J.; Gauchel, N.; Sharipova, D.; et al. Peripheral serotonin lacks effects on endothelial adhesion molecule expression in acute inflammation. J. Thromb. Haemost. 2022, 20, 222–229. [Google Scholar] [CrossRef]
- Zeitelhofer, M.; Adzemovic, M.Z.; Gomez-Cabrero, D.; Bergman, P.; Hochmeister, S.; N’diaye, M.; Paulson, A.; Ruhrmann, S.; Almgren, M.; Tegnér, J.N.; et al. Functional genomics analysis of vitamin D effects on CD4+ T cells in vivo in experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA 2017, 114, E1678–E1687. [Google Scholar] [CrossRef]
- Schiering, C.; Wincent, E.; Metidji, A.; Iseppon, A.; Li, Y.; Potocnik, A.J.; Omenetti, S.; Henderson, C.J.; Wolf, C.R.; Nebert, D.W.; et al. Feedback control of AHR signalling regulates intestinal immunity. Nature 2017, 542, 242–245. [Google Scholar] [CrossRef]
- Schaupp, L.; Muth, S.; Rogell, L.; Kofoed-Branzk, M.; Melchior, F.; Lienenklaus, S.; Ganal-Vonarburg, S.C.; Klein, M.; Guendel, F.; Hain, T.; et al. Microbiota-Induced Type I Interferons Instruct a Poised Basal State of Dendritic Cells. Cell 2020, 181, 1080–1096.e19. [Google Scholar] [CrossRef]
- Kappel, B.A.; De Angelis, L.; Heiser, M.; Ballanti, M.; Stoehr, R.; Goettsch, C.; Mavilio, M.; Artati, A.; Paoluzi, O.A.; Adamski, J.; et al. Cross-omics analysis revealed gut microbiome-related metabolic pathways underlying atherosclerosis development after antibiotics treatment. Mol. Metab. 2020, 36, 100976. [Google Scholar] [CrossRef] [PubMed]
- Taleb, S. Tryptophan Dietary Impacts Gut Barrier and Metabolic Diseases. Front. Immunol. 2019, 10, 2113. [Google Scholar] [CrossRef] [PubMed]
- Arnone, D.; Saraykar, S.; Salem, H.; Teixeira, A.L.; Dantzer, R.; Selvaraj, S. Role of Kynurenine pathway and its metabolites in mood disorders: A systematic review and meta-analysis of clinical studies. Neurosci. Biobehav. Rev. 2018, 92, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Le Floc’h, N.; Otten, W.; Merlot, E. Tryptophan metabolism, from nutrition to potential therapeutic applications. Amino Acids 2011, 41, 1195–1205. [Google Scholar] [CrossRef] [PubMed]
- Badawy, A.A. Kynurenine Pathway of Tryptophan Metabolism: Regulatory and Functional Aspects. Int. J. Tryptophan Res. 2017, 10, 1178646917691938. [Google Scholar] [CrossRef]
- Miyake, Y.; Tanaka, K.; Okubo, H.; Sasaki, S.; Arakawa, M. Tryptophan intake is related to a lower prevalence of depressive symptoms during pregnancy in Japan: Baseline data from the Kyushu Okinawa Maternal and Child Health Study. Eur. J. Nutr. 2022, 61, 4215–4222. [Google Scholar] [CrossRef]
- Badawy, A.A.; Evans, M. The regulation of rat liver tryptophan pyrrolase activity by reduced nicotinamide-adenine dinucleotide (phosphate). Experiments with glucose and nicotinamide. Biochem. J. 1976, 156, 381–390. [Google Scholar] [CrossRef]
- Christensen, M.H.E.; Fadnes, D.J.; Røst, T.H.; Pedersen, E.R.; Andersen, J.R.; Våge, V.; Ulvik, A.; Midttun, Ø.; Ueland, P.M.; Nygård, O.K.; et al. Inflammatory markers, the tryptophan-kynurenine pathway, and vitamin B status after bariatric surgery. PLoS ONE 2018, 13, e0192169. [Google Scholar] [CrossRef]
- Joisten, N.; Ruas, J.L.; Braidy, N.; Guillemin, G.J.; Zimmer, P. The kynurenine pathway in chronic diseases: A compensatory mechanism or a driving force? Trends Mol. Med. 2021, 27, 946–954. [Google Scholar] [CrossRef]
- Trombetti, S.; Sessa, R.; Catapano, R.; Rinaldi, L.; Lo Bianco, A.; Feliciello, A.; Izzo, P.; Grosso, M. Exploring the Leukemogenic Potential of GATA-1S, the Shorter Isoform of GATA-1: Novel Insights into Mechanisms Hampering Respiratory Chain Complex II Activity and Limiting Oxidative Phosphorylation Efficiency. Antioxidants 2021, 10, 1603. [Google Scholar] [CrossRef]
- Lanser, L.; Kink, P.; Egger, E.M.; Willenbacher, W.; Fuchs, D.; Weiss, G.; Kurz, K. Inflammation-Induced Tryptophan Breakdown is Related With Anemia, Fatigue, and Depression in Cancer. Front. Immunol. 2020, 11, 249. [Google Scholar] [CrossRef] [PubMed]
- Sforzini, L.; Nettis, M.A.; Mondelli, V.; Pariante, C.M. Inflammation in cancer and depression: A starring role for the kynurenine pathway. Psychopharmacology 2019, 236, 2997–3011. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.W.; Feng, G.S. Relationship between interferon-gamma, indoleamine 2,3-dioxygenase, and tryptophan catabolism. FASEB J. 1991, 5, 2516–2522. [Google Scholar] [CrossRef] [PubMed]
- Badawy, A.A.; Guillemin, G. The Plasma [Kynurenine]/[Tryptophan] Ratio and Indoleamine 2,3-Dioxygenase: Time for Appraisal. Int. J. Tryptophan Res. 2019, 12, 1178646919868978. [Google Scholar] [CrossRef]
- Stone, T.W. Kynurenic acid antagonists and kynurenine pathway inhibitors. Expert. Opin. Investig. Drugs 2001, 10, 633–645. [Google Scholar] [CrossRef]
- Baumgartner, R.; Forteza, M.J.; Ketelhuth, D.F.J. The interplay between cytokines and the Kynurenine pathway in inflammation and atherosclerosis. Cytokine 2019, 122, 154148. [Google Scholar] [CrossRef]
- Stone, T.W.; Clanchy, F.I.L.; Huang, Y.S.; Chiang, N.Y.; Darlington, L.G.; Williams, R.O. An integrated cytokine and kynurenine network as the basis of neuroimmune communication. Front. Neurosci. 2022, 16, 1002004. [Google Scholar] [CrossRef]
- O’Connor, J.C.; André, C.; Wang, Y.; Lawson, M.A.; Szegedi, S.S.; Lestage, J.; Castanon, N.; Kelley, K.W.; Dantzer, R. Interferon-gamma and tumor necrosis factor-alpha mediate the upregulation of indoleamine 2,3-dioxygenase and the induction of depressive-like behavior in mice in response to bacillus Calmette-Guerin. J. Neurosci. 2009, 29, 4200–4209. [Google Scholar] [CrossRef]
- Savitz, J. The kynurenine pathway: A finger in every pie. Mol. Psychiatry 2020, 25, 131–147. [Google Scholar] [CrossRef]
- Wirthgen, E.; Hoeflich, A.; Rebl, A.; Günther, J. Kynurenic Acid: The Janus-Faced Role of an Immunomodulatory Tryptophan Metabolite and Its Link to Pathological Conditions. Front. Immunol. 2018, 8, 1957. [Google Scholar] [CrossRef]
- Zulpaite, R.; Miknevicius, P.; Leber, B.; Strupas, K.; Stiegler, P.; Schemmer, P. Tryptophan Metabolism via Kynurenine Pathway: Role in Solid Organ Transplantation. Int. J. Mol. Sci. 2021, 22, 1921. [Google Scholar] [CrossRef] [PubMed]
- Platten, M.; Nollen, E.A.A.; Röhrig, U.F.; Fallarino, F.; Opitz, C.A. Tryptophan metabolism as a common therapeutic target in cancer.; neurodegeneration and beyond. Nat. Rev. Drug Discov. 2019, 18, 379–401. [Google Scholar] [PubMed]
- Gouasmi, R.; Ferraro-Peyret, C.; Nancey, S.; Coste, I.; Renno, T.; Chaveroux, C.; Aznar, N.; Ansieau, S. The Kynurenine Pathway and Cancer: Why Keep It Simple When You Can Make It Complicated. Cancers 2022, 14, 2793. [Google Scholar] [CrossRef]
- Schwarcz, R.; Bruno, J.P.; Muchowski, P.J.; Wu, H.Q. Kynurenines in the mammalian brain: When physiology meets pathology. Nat. Rev. Neurosci. 2012, 13, 465–477. [Google Scholar] [CrossRef]
- Polyzos, K.A.; Ketelhuth, D.F. The role of the kynurenine pathway of tryptophan metabolism in cardiovascular disease. An emerging field. Hamostaseologie 2015, 35, 128–136. [Google Scholar] [CrossRef]
- Al Saedi, A.; Sharma, S.; Summers, M.A.; Nurgali, K.; Duque, G. The multiple faces of tryptophan in bone biology. Exp. Gerontol. 2020, 129, 110778. [Google Scholar] [CrossRef]
- Chaves Filho, A.J.M.; Lima, C.N.C.; Vasconcelos, S.M.M.; de Lucena, D.F.; Maes, M.; Macedo, D. IDO chronic immune activation and tryptophan metabolic pathway: A potential pathophysiological link between depression and obesity. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 80, 234–249. [Google Scholar] [CrossRef]
- Agudelo, L.Z.; Ferreira, D.M.S.; Cervenka, I.; Bryzgalova, G.; Dadvar, S.; Jannig, P.R.; Pettersson-Klein, A.T.; Lakshmikanth, T.; Sustarsic, E.G.; Porsmyr-Palmertz, M.; et al. Kynurenic Acid and Gpr35 Regulate Adipose Tissue Energy Homeostasis and Inflammation. Cell Metab. 2018, 27, 378–392.e5. [Google Scholar] [CrossRef] [PubMed]
- Marx, W.; McGuinness, A.J.; Rocks, T.; Ruusunen, A.; Cleminson, J.; Walker, A.J.; Gomes-da-Costa, S.; Lane, M.; Sanches, M.; Diaz, A.P.; et al. The kynurenine pathway in major depressive disorder, bipolar disorder, and schizophrenia: A meta-analysis of 101 studies. Mol. Psychiatry 2021, 26, 4158–4178. [Google Scholar] [CrossRef] [PubMed]
- Messaoud, A.; Rym, M.; Wahiba, D.; Neffati, F.; Najjar, M.F.; Gobbi, G.; Manchia, M.; Valtorta, F.; Lotfi, G.; Comai, S. Investigation of the Relationship among Cortisol.; Pro-inflammatory Cytokines, and the Degradation of Tryptophan into Kynurenine in Patients with Major Depression and Suicidal Behavior. Curr. Top. Med. Chem. 2022, 22, 2119–2125. [Google Scholar]
- Solmi, M.; Suresh Sharma, M.; Osimo, E.F.; Fornaro, M.; Bortolato, B.; Croatto, G.; Miola, A.; Vieta, E.; Pariante, C.M.; Smith, L.; et al. Peripheral levels of C-reactive protein, tumor necrosis factor-α, interleukin-6, and interleukin-1β across the mood spectrum in bipolar disorder: A meta-analysis of mean differences and variability. Brain Behav. Immun. 2021, 97, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Vasupanrajit, A.; Jirakran, K.; Tunvirachaisakul, C.; Maes, M. Suicide attempts are associated with activated immune-inflammatory, nitro-oxidative, and neurotoxic pathways: A systematic review and meta-analysis. J. Affect Disord. 2021, 295, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Daiber, A.; Steven, S.; Weber, A.; Shuvaev, V.V.; Muzykantov, V.R.; Laher, I.; Li, H.; Lamas, S.; Münzel, T. Targeting vascular (endothelial) dysfunction. Br. J. Pharmacol. 2017, 174, 1591–1619. [Google Scholar] [CrossRef] [PubMed]
- Dahlem, C.; Kado, S.Y.; He, Y.; Bein, K.; Wu, D.; Haarmann-Stemmann, T.; Kado, N.Y.; Vogel, C.F.A. AHR Signaling Interacting with Nutritional Factors Regulating the Expression of Markers in Vascular Inflammation and Atherogenesis. Int. J. Mol. Sci. 2020, 21, 8287. [Google Scholar] [CrossRef]
- Wenzel, P.; Knorr, M.; Kossmann, S.; Stratmann, J.; Hausding, M.; Schuhmacher, S.; Karbach, S.H.; Schwenk, M.; Yogev, N.; Schulz, E.; et al. Lysozyme M-positive monocytes mediate angiotensin II-induced arterial hypertension and vascular dysfunction. Circulation 2011, 124, 1370–1381. [Google Scholar] [CrossRef]
- Paeslack, N.; Mimmler, M.; Becker, S.; Gao, Z.; Khuu, M.P.; Mann, A.; Malinarich, F.; Regen, T.; Reinhardt, C. Microbiota-derived tryptophan metabolites in vascular inflammation and cardiovascular disease. Amino Acids 2022, 54, 1339–1356. [Google Scholar] [CrossRef]
- Pedersen, E.R.; Tuseth, N.; Eussen, S.J.; Ueland, P.M.; Strand, E.; Svingen, G.F.; Midttun, Ø.; Meyer, K.; Mellgren, G.; Ulvik, A.; et al. Associations of plasma kynurenines with risk of acute myocardial infarction in patients with stable angina pectoris. Arter. Thromb. Vasc. Biol. 2015, 35, 455–462. [Google Scholar] [CrossRef]
- Hoste, E.A.J.; Kellum, J.A.; Selby, N.M.; Zarbock, A.; Palevsky, P.M.; Bagshaw, S.M.; Goldstein, S.L.; Cerdá, J.; Chawla, L.S. Global epidemiology and outcomes of acute kidney injury. Nat. Rev. Nephrol. 2018, 14, 607–625. [Google Scholar] [CrossRef]
- Sawhney, S.; Fraser, S.D. Epidemiology of AKI: Utilizing Large Databases to Determine the Burden of AKI. Adv. Chronic Kidney Dis. 2017, 24, 194–204. [Google Scholar] [CrossRef]
- Carney, E.F. The impact of chronic kidney disease on global health. Nat. Rev. Nephrol. 2020, 16, 251. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, A.; Binnie, M.; McGuire, K.; Webster, S.P.; Hughes, J.; Howie, S.E.M.; Mole, D.J. Kynurenine 3-monooxygenase is a critical regulator of renal ischemia-reperfusion injury. Exp. Mol. Med. 2019, 51, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Xiao, X.; Fogle, P.; Dong, Z. Changes in metabolic profiles during acute kidney injury and recovery following ischemia/reperfusion. PLoS ONE 2014, 9, e106647. [Google Scholar] [CrossRef] [PubMed]
- Volarevic, V.; Markovic, B.S.; Jankovic, M.G.; Djokovic, B.; Jovicic, N.; Harrell, C.R.; Fellabaum, C.; Djonov, V.; Arsenijevic, N.; Lukic, M.L. Galectin 3 protects from cisplatin-induced acute kidney injury by promoting TLR-2-dependent activation of IDO1/Kynurenine pathway in renal DCs. Theranostics 2019, 9, 5976–6001. [Google Scholar] [CrossRef]
- Poyan Mehr, A.; Tran, M.T.; Ralto, K.M.; Leaf, D.E.; Washco, V.; Messmer, J.; Lerner, A.; Kher, A.; Kim, S.H.; Khoury, C.C.; et al. De novo NAD+ biosynthetic impairment in acute kidney injury in humans. Nat. Med. 2018, 24, 1351–1359. [Google Scholar] [CrossRef] [PubMed]
- Nikolaus, S.; Schulte, B.; Al-Massad, N.; Thieme, F.; Schulte, D.M.; Bethge, J.; Rehman, A.; Tran, F.; Aden, K.; Häsler, R.; et al. Increased Tryptophan Metabolism Is Associated With Activity of Inflammatory Bowel Diseases. Gastroenterology 2017, 153, 1504–1516.e2. [Google Scholar] [CrossRef]
- Zhou, G.; Liu, H.; Wei, P.; He, Q.; Zhang, J.; Shi, Q.; Liu, T.; Liu, S. Amino acids-targeted metabolomics reveals novel diagnostic biomarkers for ulcerative colitis and Crohn’s disease. Amino Acids, 2023; online ahead of print. [Google Scholar] [CrossRef]
- Wnorowski, A.; Wnorowska, S.; Kurzepa, J.; Parada-Turska, J. Alterations in Kynurenine and NAD+ Salvage Pathways during the Successful Treatment of Inflammatory Bowel Disease Suggest HCAR3 and NNMT as Potential Drug Targets. Int. J. Mol. Sci. 2021, 22, 13497. [Google Scholar] [CrossRef]
- Zhong, W.; Gao, L.; Zhou, Z.; Lin, H.; Chen, C.; Huang, P.; Huang, W.; Zhou, C.; Huang, S.; Nie, L.; et al. Indoleamine 2,3-dioxygenase 1 deficiency attenuates CCl4-induced fibrosis through Th17 cells down-regulation and tryptophan 2,3-dioxygenase compensation. Oncotarget 2017, 8, 40486–40500. [Google Scholar] [CrossRef]
- Ogbechi, J.; Clanchy, F.I.; Huang, Y.S.; Topping, L.M.; Stone, T.W.; Williams, R.O. IDO activation, inflammation and musculoskeletal disease. Exp. Gerontol. 2020, 131, 110820. [Google Scholar] [CrossRef]
- Cheung, A.M.; Papaioannou, A.; Morin, S.; Osteoporosis Canada Scientific Advisory Council. Postmenopausal Osteoporosis. N. Engl. J. Med. 2016, 374, 2096. [Google Scholar]
- Chen, X.; Wang, Z.; Duan, N.; Zhu, G.; Schwarz, E.M.; Xie, C. Osteoblast-osteoclast interactions. Connect. Tissue Res. 2018, 59, 99–107. [Google Scholar] [CrossRef]
- Zhang, W.; Dang, K.; Huai, Y.; Qian, A. Osteoimmunology: The Regulatory Roles of T Lymphocytes in Osteoporosis. Front. Endocrinol. 2020, 11, 465. [Google Scholar] [CrossRef] [PubMed]
- Francisconi, C.F.; Vieira, A.E.; Azevedo, M.C.S.; Tabanez, A.P.; Fonseca, A.C.; Trombone, A.P.F.; Letra, A.; Silva, R.M.; Sfeir, C.S.; Little, S.R.; et al. RANKL Triggers Treg-Mediated Immunoregulation in Inflammatory Osteolysis. J. Dent. Res. 2018, 97, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Schlegel, M.P.; Afonso, M.S.; Brown, E.J.; Rahman, K.; Weinstock, A.; Sansbury, B.E.; Corr, E.M.; van Solingen, C.; Koelwyn, G.J.; et al. Regulatory T Cells License Macrophage Pro-Resolving Functions During Atherosclerosis Regression. Circ Res. 2020, 127, 335–353. [Google Scholar] [CrossRef]
- Mellor, A.L.; Munn, D.H. IDO expression by dendritic cells: Tolerance and tryptophan catabolism. Nat. Rev. Immunol. 2004, 4, 762–774. [Google Scholar] [CrossRef]
- Zara, C.; Severino, A.; Flego, D.; Ruggio, A.; Pedicino, D.; Giglio, A.F.; Trotta, F.; Lucci, C.; D’Amario, D.; Vinci, R.; et al. Indoleamine 2,3-Dioxygenase (IDO) Enzyme Links Innate Immunity and Altered T-Cell Differentiation in Non-ST Segment Elevation Acute Coronary Syndrome. Int. J. Mol. Sci. 2017, 19, 63. [Google Scholar] [CrossRef]
- Shorakae, S.; Ranasinha, S.; Abell, S.; Lambert, G.; Lambert, E.; de Courten, B.; Teede, H. Inter-related effects of insulin resistance, hyperandrogenism, sympathetic dysfunction and chronic inflammation in PCOS. Clin. Endocrinol. 2018, 89, 628–633. [Google Scholar] [CrossRef]
- Jovanovic, F.; Sudhakar, A.; Knezevic, N.N. The Kynurenine Pathway and Polycystic Ovary Syndrome: Inflammation as a Common Denominator. Int. J. Tryptophan Res. 2022, 15, 11786469221099214. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Mu, L.; Zhang, C.; Long, X.; Zhang, Y.; Li, R.; Zhao, Y.; Qiao, J. Abnormal Activation of Tryptophan-Kynurenine Pathway in Women With Polycystic Ovary Syndrome. Front. Endocrinol. 2022, 13, 877807. [Google Scholar] [CrossRef]
- Tsuji, A.; Nakata, C.; Sano, M.; Fukuwatari, T.; Shibata, K. L-tryptophan metabolism in pregnant mice fed a high L-tryptophan diet and the effect on maternal.; placental.; and fetal growth. Int. J. Tryptophan Res. 2013, 6, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Richard, D.M.; Dawes, M.A.; Mathias, C.W.; Acheson, A.; Hill-Kapturczak, N.; Dougherty, D. L-tryptophan: Basic metabolic functions, behavioral research and therapeutic indications. Int. J. Tryptophan Res. 2009, 2, 45–60. [Google Scholar] [CrossRef]
- Bacqué-Cazenave, J.; Bharatiya, R.; Barrière, G.; Delbecque, J.P.; Bouguiyoud, N.; Di Giovanni, G.; Cattaert, D.; De Deurwaerdère, P. Serotonin in Animal Cognition and Behavior. Int. J. Mol. Sci. 2020, 21, 1649. [Google Scholar] [CrossRef] [PubMed]
- Perkins, M.N.; Stone, T.W. Actions of kynurenic acid and quinolinic acid in the rat hippocampus in vivo. Exp. Neurol. 1985, 88, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Maddison, D.C.; Giorgini, F. The kynurenine pathway and neurodegenerative disease. Semin. Cell Dev. Biol. 2015, 40, 134–141. [Google Scholar] [CrossRef]
- Jo, W.K.; Zhang, Y.; Emrich, H.M.; Dietrich, D.E. Glia in the cytokine-mediated onset of depression: Fine tuning the immune response. Front. Cell Neurosci. 2015, 9, 268. [Google Scholar] [CrossRef]
- Rahman, A.; Rao, M.S.; Khan, K.M. Intraventricular infusion of quinolinic acid impairs spatial learning and memory in young rats: A novel mechanism of lead-induced neurotoxicity. J. Neuroinflamm. 2018, 15, 263. [Google Scholar] [CrossRef]
- Sanguino-Gómez, J.; Buurstede, J.C.; Abiega, O.; Fitzsimons, C.P.; Lucassen, P.J.; Eggen, B.J.L.; Lesuis, S.L.; Meijer, O.C.; Krugers, H.J. An emerging role for microglia in stress-effects on memory. Eur. J. Neurosci. 2022, 55, 2491–2518. [Google Scholar] [CrossRef]
- Lesuis, S.L.; Lucassen, P.J.; Krugers, H.J. Early life stress impairs fear memory and synaptic plasticity; a potential role for GluN2B. Neuropharmacology 2019, 149, 195–203. [Google Scholar] [CrossRef]
- Wakselman, S.; Béchade, C.; Roumier, A.; Bernard, D.; Triller, A.; Bessis, A. Developmental neuronal death in hippocampus requires the microglial CD11b integrin and DAP12 immunoreceptor. J. Neurosci. 2008, 28, 8138–8143. [Google Scholar] [CrossRef]
- Fukuwatari, T. Possibility of Amino Acid Treatment to Prevent the Psychiatric Disorders via Modulation of the Production of Tryptophan Metabolite Kynurenic Acid. Nutrients 2020, 12, 1403. [Google Scholar] [CrossRef]
- Nakazawa, K.; McHugh, T.J.; Wilson, M.A.; Tonegawa, S. NMDA receptors, place cells and hippocampal spatial memory. Nat. Rev. Neurosci. 2004, 5, 361–372. [Google Scholar] [CrossRef]
- Nomoto, M.; Ohkawa, N.; Inokuchi, K.; Oishi, N. Requirement for hippocampal CA3 NMDA receptors in artificial association of memory events stored in CA3 cell ensembles. Mol. Brain 2023, 16, 12. [Google Scholar] [CrossRef] [PubMed]
- Hossen, E.; Funahashi, Y.; Faruk, M.O.; Ahammad, R.U.; Amano, M.; Yamada, K.; Kaibuchi, K. Rho-Kinase/ROCK Phosphorylates PSD-93 Downstream of NMDARs to Orchestrate Synaptic Plasticity. Int. J. Mol. Sci. 2022, 24, 404. [Google Scholar] [CrossRef] [PubMed]
- Fernández, A.M.M.M.; Molla, H.M.; Thomases, D.R.; Tseng, K.Y. Prefrontal α7nAChR Signaling Differentially Modulates Afferent Drive and Trace Fear Conditioning Behavior in Adolescent and Adult Rats. J. Neurosci. 2021, 41, 1908–1916. [Google Scholar] [CrossRef] [PubMed]
- Sathyasaikumar, K.V.; Stachowski, E.K.; Wonodi, I.; Roberts, R.C.; Rassoulpour, A.; McMahon, R.P.; Schwarcz, R. Impaired kynurenine pathway metabolism in the prefrontal cortex of individuals with schizophrenia. Schizophr. Bull. 2011, 37, 1147–1156. [Google Scholar] [CrossRef]
- Beggiato, S.; Ieraci, A.; Zuccarini, M.; Di Iorio, P.; Schwarcz, R.; Ferraro, L. Alterations in rat prefrontal cortex kynurenic acid levels are involved in the enduring cognitive dysfunctions induced by tetrahydrocannabinol exposure during the adolescence. Front. Psychiatry 2022, 13, 996406. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Li, F. Potential Markers of Neurocognitive Disorders After Cardiac Surgery: A Bibliometric and Visual Analysis. Front Aging Neurosci. 2022, 14, 868158. [Google Scholar] [CrossRef] [PubMed]
- Hebbrecht, K.; Morrens, M.; Giltay, E.J.; van Nuijs, A.L.N.; Sabbe, B.; van den Ameele, S. The Role of Kynurenines in Cognitive Dysfunction in Bipolar Disorder. Neuropsychobiology 2022, 81, 184–191. [Google Scholar] [CrossRef]
- Kaya, B.; Melhem, H.; Niess, J.H. GPR35 in Intestinal Diseases: From Risk Gene to Function. Front. Immunol. 2021, 12, 717392. [Google Scholar] [CrossRef]
- Farooq, S.M.; Hou, Y.; Li, H.; O’Meara, M.; Wang, Y.; Li, C.; Wang, J.M. Disruption of GPR35 Exacerbates Dextran Sulfate Sodium-Induced Colitis in Mice. Dig. Dis. Sci. 2018, 63, 2910–2922. [Google Scholar] [CrossRef]
- De Giovanni, M.; Tam, H.; Valet, C.; Xu, Y.; Looney, M.R.; Cyster, J.G. GPR35 promotes neutrophil recruitment in response to serotonin metabolite 5-HIAA. Cell 2022, 185, 815–830.e19. [Google Scholar] [CrossRef] [PubMed]
- Sathyasaikumar, K.V.; Notarangelo, F.M.; Kelly, D.L.; Rowland, L.M.; Hare, S.M.; Chen, S.; Mo, C.; Buchanan, R.W.; Schwarcz, R. Tryptophan Challenge in Healthy Controls and People with Schizophrenia: Acute Effects on Plasma Levels of Kynurenine, Kynurenic Acid and 5-Hydroxyindoleacetic Acid. Pharmaceuticals 2022, 15, 1003. [Google Scholar] [CrossRef] [PubMed]
- Koduru, L.; Lakshmanan, M.; Hoon, S.; Lee, D.Y.; Lee, Y.K.; Ow, D.S. Systems Biology of Gut Microbiota-Human Receptor Interactions: Toward Anti-inflammatory Probiotics. Front. Microbiol. 2022, 13, 846555. [Google Scholar] [CrossRef] [PubMed]
- Modoux, M.; Rolhion, N.; Lefevre, J.H.; Oeuvray, C.; Nádvorník, P.; Illes, P.; Emond, P.; Parc, Y.; Mani, S.; Dvorak, Z.; et al. Butyrate acts through HDAC inhibition to enhance aryl hydrocarbon receptor activation by gut microbiota-derived ligands. Gut Microbes 2022, 14, 2105637. [Google Scholar] [CrossRef]
- Korecka, A.; Dona, A.; Lahiri, S.; Tett, A.J.; Al-Asmakh, M.; Braniste, V.; D’Arienzo, R.; Abbaspour, A.; Reichardt, N.; Fujii-Kuriyama, Y.; et al. Bidirectional communication between the Aryl hydrocarbon Receptor (AhR) and the microbiome tunes host metabolism. NPJ Biofilms Microbiomes 2016, 2, 16014. [Google Scholar] [CrossRef]
- Huang, F.C.; Huang, S.C. The Pivotal Role of Aryl Hydrocarbon Receptor-Regulated Tight Junction Proteins and Innate Immunity on the Synergistic Effects of Postbiotic Butyrate and Active Vitamin D3 to Defense against Microbial Invasion in Salmonella Colitis. Nutrients 2023, 15, 305. [Google Scholar] [CrossRef]
- Dean, J.W.; Helm, E.Y.; Fu, Z.; Xiong, L.; Sun, N.; Oliff, K.N.; Muehlbauer, M.; Avram, D.; Zhou, L. The aryl hydrocarbon receptor cell intrinsically promotes resident memory CD8+ T cell differentiation and function. Cell Rep. 2023, 42, 111963. [Google Scholar] [CrossRef]
- Frieser, D.; Pignata, A.; Khajavi, L.; Shlesinger, D.; Gonzalez-Fierro, C.; Nguyen, X.H.; Yermanos, A.; Merkler, D.; Höftberger, R.; Desestret, V.; et al. Tissue-resident CD8+ T cells drive compartmentalized and chronic autoimmune damage against CNS neurons. Sci. Transl. Med. 2022, 14, eabl6157. [Google Scholar] [CrossRef]
- Selvam, A.K.; Björnstedt, M. A Novel Assay Method to Determine the β-Elimination of Se-Methylselenocysteine to Monomethylselenol by Kynurenine Aminotransferase 1. Antioxidants 2020, 9, 139. [Google Scholar] [CrossRef]
- Pérez-de la Cruz, V.; Amori, L.; Sathyasaikumar, K.V.; Wang, X.D.; Notarangelo, F.M.; Wu, H.Q.; Schwarcz, R. Enzymatic transamination of D-kynurenine generates kynurenic acid in rat and human brain. J. Neurochem. 2012, 120, 1026–1035. [Google Scholar] [CrossRef]
- Blanco Ayala, T.; Lugo Huitrón, R.; Carmona Aparicio, L.; Ramírez Ortega, D.; González Esquivel, D.; Pedraza Chaverrí, J.; Pérez de la Cruz, G.; Ríos, C.; Schwarcz, R.; Pérez de la Cruz, V. Alternative kynurenic acid synthesis routes studied in the rat cerebellum. Front. Cell Neurosci. 2015, 9, 178. [Google Scholar] [CrossRef]
- Wang, X.D.; Horning, K.J.; Notarangelo, F.M.; Schwarcz, R. A method for the determination of D-kynurenine in biological tissues. Anal. Bioanal. Chem. 2013, 405, 9747–9754. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, M.; Osawa, Y.; Nakamoto, K.; Morita, N.; Yamamoto, Y.; Ando, T.; Tashita, C.; Nabeshima, T.; Saito, K. Kynurenine produced by indoleamine 2,3-dioxygenase 2 exacerbates acute liver injury by carbon tetrachloride in mice. Toxicology 2020, 438, 152458. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, A.S.; Murtaza, G.; Yan, D.; Irfan, M.; Xue, M.; Meng, Z.H.; Qu, F. Development of Molecularly Imprinted 2D Photonic Crystal Hydrogel Sensor for Detection of L-Kynurenine in Human Serum. Talanta 2020, 208, 120403. [Google Scholar] [CrossRef]
- Sharma, A.; Kazim, S.F.; Larson, C.S.; Ramakrishnan, A.; Gray, J.D.; McEwen, B.S.; Rosenberg, P.A.; Shen, L.; Pereira, A.C. Divergent roles of astrocytic versus neuronal EAAT2 deficiency on cognition and overlap with aging and Alzheimer’s molecular signatures. Proc. Natl. Acad. Sci. USA 2019, 116, 21800–21811. [Google Scholar] [CrossRef] [PubMed]
- Bowman, R.E.; Micik, R.; Gautreaux, C.; Fernandez, L.; Luine, V.N. Sex-dependent changes in anxiety, memory, and monoamines following one week of stress. Physiol. Behav. 2009, 97, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Koren, T.; Yifa, R.; Amer, M.; Krot, M.; Boshnak, N.; Ben-Shaanan, T.L.; Azulay-Debby, H.; Zalayat, I.; Avishai, E.; Hajjo, H.; et al. Insular cortex neurons encode and retrieve specific immune responses. Cell 2021, 184, 5902–5915.e17. [Google Scholar] [CrossRef]
- Gogolla, N. The brain remembers where and how inflammation struck. Cell 2021, 184, 5851–5853. [Google Scholar] [CrossRef]
- Sakaguchi, M.; Hayashi, Y. Catching the engram: Strategies to examine the memory trace. Mol. Brain 2012, 5, 32. [Google Scholar] [CrossRef]
- Wu, X.; Yu, X.; Yao, L.; Li, R. Bayesian network analysis revealed the connectivity difference of the default mode network from the resting-state to task-state. Front. Comput. Neurosci. 2014, 8, 118. [Google Scholar] [CrossRef]
- Wang, C.; Yue, H.; Hu, Z.; Shen, Y.; Ma, J.; Li, J.; Wang, X.D.; Wang, L.; Sun, B.; Shi, P.; et al. Microglia mediate forgetting via complement-dependent synaptic elimination. Science 2020, 367, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Deng, Y.S.; Dai, S.K.; Mi, T.W.; Li, R.Y.; Liu, P.P.; Liu, C.; He, B.D.; He, X.C.; Du, H.Z.; et al. Loss of microglial EED impairs synapse density, learning, and memory. Mol. Psychiatry 2022, 27, 2999–3009. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chang, L.; Wan, X.; Tan, Y.; Qu, Y.; Shan, J.; Yang, Y.; Ma, L.; Hashimoto, K. (R)-ketamine ameliorates demyelination and facilitates remyelination in cuprizone-treated mice: A role of gut-microbiota-brain axis. Neurobiol. Dis. 2022, 165, 105635. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Son, J.; Lee, D.; Tsai, J.; Wang, D.; Chocron, E.S.; Jeong, S.; Kittrell, P.; Murchison, C.F.; Kennedy, R.E.; et al. Gut- and oral-dysbiosis differentially impact spinal- and bulbar-onset ALS, predicting ALS severity and potentially determining the location of disease onset. BMC Neurol. 2022, 22, 62. [Google Scholar] [CrossRef]
- Trujillo-Del Río, C.; Tortajada-Pérez, J.; Gómez-Escribano, A.P.; Casterá, F.; Peiró, C.; Millán, J.M.; Herrero, M.J.; Vázquez-Manrique, R.P. Metformin to treat Huntington disease: A pleiotropic drug against a multi-system disorder. Mech. Ageing Dev. 2022, 204, 111670. [Google Scholar] [CrossRef]
- Yoshikawa, S.; Taniguchi, K.; Sawamura, H.; Ikeda, Y.; Tsuji, A.; Matsuda, S. A New Concept of Associations between Gut Microbiota, Immunity and Central Nervous System for the Innovative Treatment of Neurodegenerative Disorders. Metabolites 2022, 12, 1052. [Google Scholar] [CrossRef]
- Taniguchi, K.; Ikeda, Y.; Nagase, N.; Tsuji, A.; Kitagishi, Y.; Matsuda, S. Implications of Gut-Brain axis in the pathogenesis of Psychiatric disorders. AIMS Bioeng. 2021, 8, 243–256. [Google Scholar] [CrossRef]
- Matsuda, S.; Nakagawa, Y.; Kitagishi, Y.; Nakanishi, A.; Murai, T. Reactive Oxygen Species.; Superoxide Dimutases.; and PTEN-p53-AKT-MDM2 Signaling Loop Network in Mesenchymal Stem/Stromal Cells Regulation. Cells 2018, 7, 36. [Google Scholar] [CrossRef]
- Ikeda, Y.; Taniguchi, K.; Nagase, N.; Tsuji, A.; Kitagishi, Y.; Matsuda, S. Reactive oxygen species may influence on the crossroads of stemness.; senescence.; and carcinogenesis in a cell via the roles of APRO family proteins. Explor. Med. 2021, 2, 443–454. [Google Scholar] [CrossRef]
- Zhang, L.; Qian, Y.; Li, J.; Zhou, X.; Xu, H.; Yan, J.; Xiang, J.; Yuan, X.; Sun, B.; Sisodia, S.S.; et al. BAD-mediated neuronal apoptosis and neuroinflammation contribute to Alzheimer’s disease pathology. iScience 2021, 24, 102942. [Google Scholar] [CrossRef]
- Acosta, S.; Jernberg, J.; Sanberg, C.D.; Sanberg, P.R.; Small, B.J.; Gemma, C.; Bickford, P.C. NT-020, a natural therapeutic approach to optimize spatial memory performance and increase neural progenitor cell proliferation and decrease inflammation in the aged rat. Rejuvenation Res. 2010, 13, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Kynurenine pathway metabolism and the microbiota-gut-brain axis. Neuropharmacology 2017, 112, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Desbonnet, L.; Clarke, G.; Traplin, A.; O’Sullivan, O.; Crispie, F.; Moloney, R.D.; Cotter, P.D.; Dinan, T.G.; Cryan, J.F. Gut microbiota depletion from early adolescence in mice: Implications for brain and behaviour. Brain Behav. Immun. 2015, 48, 165–173. [Google Scholar] [CrossRef]
- Clarke, G.; Grenham, S.; Scully, P.; Fitzgerald, P.; Moloney, R.D.; Shanahan, F.; Dinan, T.G.; Cryan, J.F. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol. Psychiatry 2013, 18, 666–673. [Google Scholar] [CrossRef]
- Purton, T.; Staskova, L.; Lane, M.M.; Dawson, S.L.; West, M.; Firth, J.; Clarke, G.; Cryan, J.F.; Berk, M.; O’Neil, A.; et al. Prebiotic and probiotic supplementation and the tryptophan-kynurenine pathway: A systematic review and meta analysis. Neurosci. Biobehav. Rev. 2021, 123, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Rudzki, L.; Ostrowska, L.; Pawlak, D.; Małus, A.; Pawlak, K.; Waszkiewicz, N.; Szulc, A. Probiotic Lactobacillus Plantarum 299v decreases kynurenine concentration and improves cognitive functions in patients with major depression: A double-blind, randomized, placebo controlled study. Psychoneuroendocrinology 2019, 100, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Desbonnet, L.; Garrett, L.; Clarke, G.; Bienenstock, J.; Dinan, T.G. The probiotic Bifidobacteria infantis: An assessment of potential antidepressant properties in the rat. J. Psychiatr. Res. 2008, 43, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Valladares, R.; Bojilova, L.; Potts, A.H.; Cameron, E.; Gardner, C.; Lorca, G.; Gonzalez, C.F. Lactobacillus johnsonii inhibits indoleamine 2,3-dioxygenase and alters tryptophan metabolite levels in BioBreeding rats. FASEB J. 2013, 27, 1711–1720. [Google Scholar] [CrossRef]
- Asai, T.; Yoshikawa, S.; Ikeda, Y.; Taniguchi, K.; Sawamura, H.; Tsuji, A.; Matsuda, S. Encouraging Tactics with Genetically Modified Probiotics to Improve Immunity for the Prevention of Immune-Related Diseases including Cardio-Metabolic Disorders. Biomolecules 2022, 13, 10. [Google Scholar] [CrossRef]
- Yoshikawa, S.; Taniguchi, K.; Sawamura, H.; Ikeda, Y.; Tsuji, A.; Matsuda, S. Encouraging probiotics for the prevention and treatment of immune-related adverse events in novel immunotherapies against malignant glioma. Explor. Target. Antitumor Ther. 2022, 3, 817–827. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsuji, A.; Ikeda, Y.; Yoshikawa, S.; Taniguchi, K.; Sawamura, H.; Morikawa, S.; Nakashima, M.; Asai, T.; Matsuda, S. The Tryptophan and Kynurenine Pathway Involved in the Development of Immune-Related Diseases. Int. J. Mol. Sci. 2023, 24, 5742. https://doi.org/10.3390/ijms24065742
Tsuji A, Ikeda Y, Yoshikawa S, Taniguchi K, Sawamura H, Morikawa S, Nakashima M, Asai T, Matsuda S. The Tryptophan and Kynurenine Pathway Involved in the Development of Immune-Related Diseases. International Journal of Molecular Sciences. 2023; 24(6):5742. https://doi.org/10.3390/ijms24065742
Chicago/Turabian StyleTsuji, Ai, Yuka Ikeda, Sayuri Yoshikawa, Kurumi Taniguchi, Haruka Sawamura, Sae Morikawa, Moeka Nakashima, Tomoko Asai, and Satoru Matsuda. 2023. "The Tryptophan and Kynurenine Pathway Involved in the Development of Immune-Related Diseases" International Journal of Molecular Sciences 24, no. 6: 5742. https://doi.org/10.3390/ijms24065742
APA StyleTsuji, A., Ikeda, Y., Yoshikawa, S., Taniguchi, K., Sawamura, H., Morikawa, S., Nakashima, M., Asai, T., & Matsuda, S. (2023). The Tryptophan and Kynurenine Pathway Involved in the Development of Immune-Related Diseases. International Journal of Molecular Sciences, 24(6), 5742. https://doi.org/10.3390/ijms24065742





