A Comparative Study of the Inhibitory Action of Berberine Derivatives on the Recombinant Protein FtsZ of E. coli
Abstract
1. Introduction
2. Results
2.1. Chemistry
2.2. Molecular Docking Analysis
2.3. Inhibition of FtsZ Enzymatic Activity by Berberine Analogues
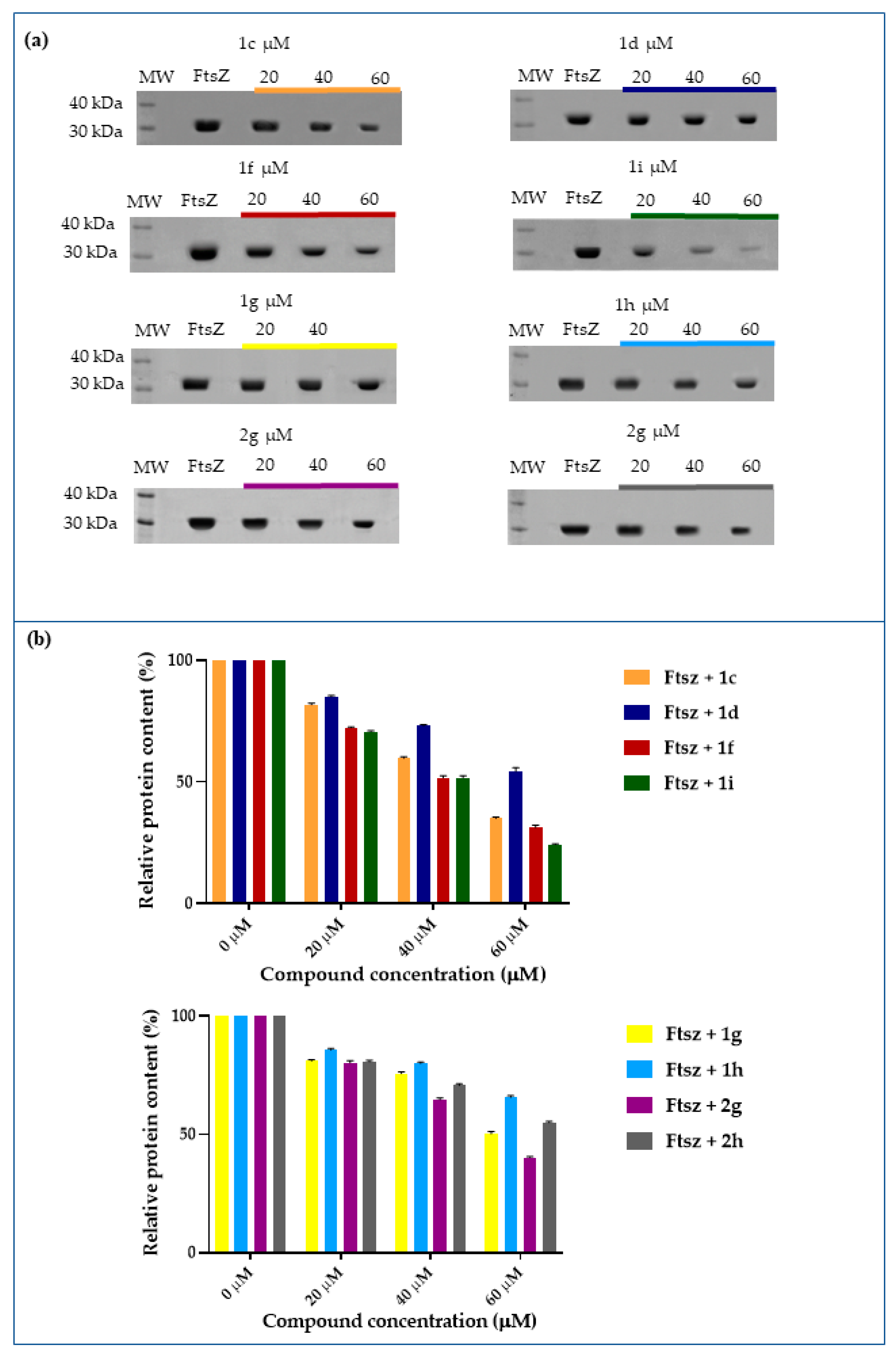
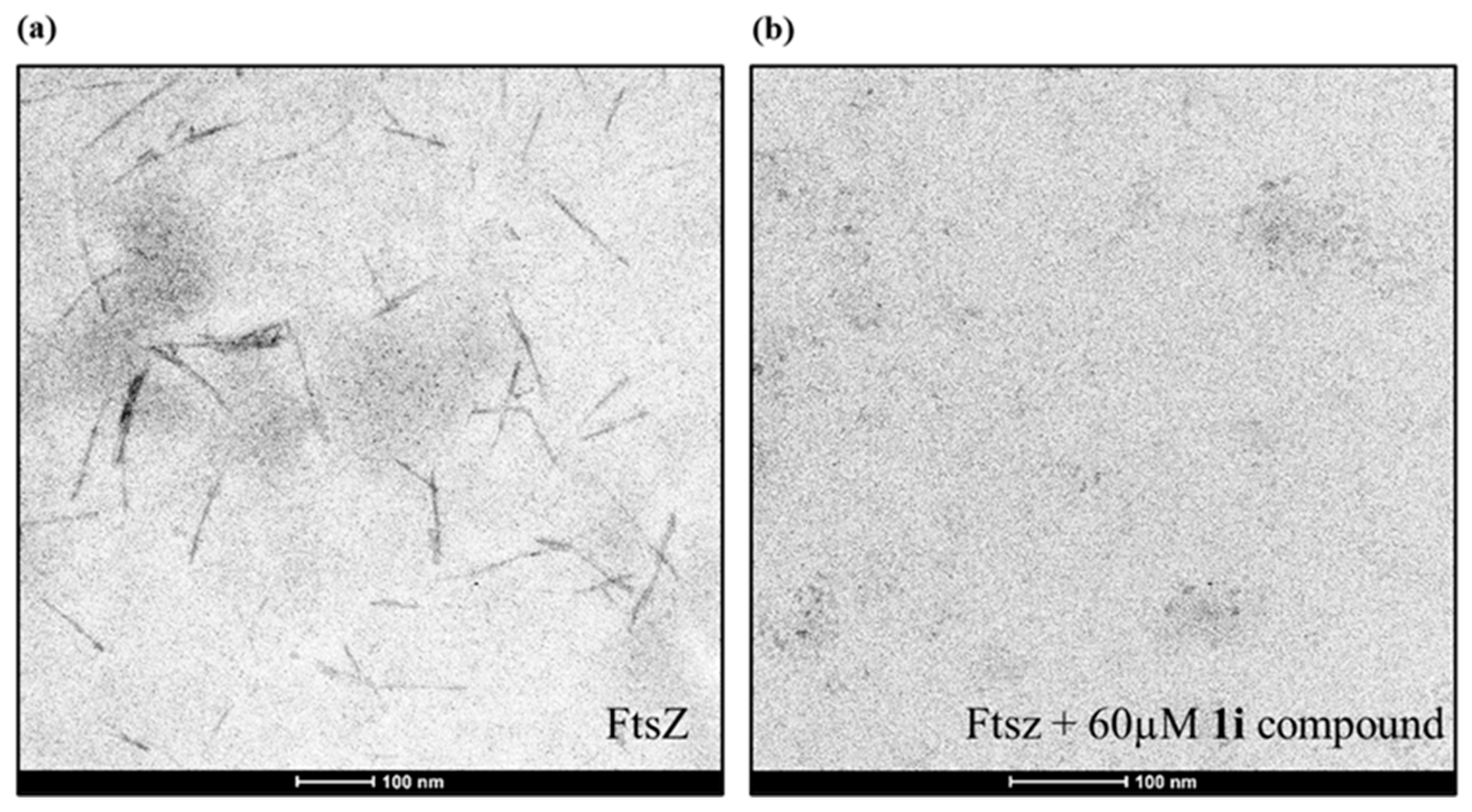
2.4. Inhibition of FtsZ Polymerization in the Presence of Berberine Analogues
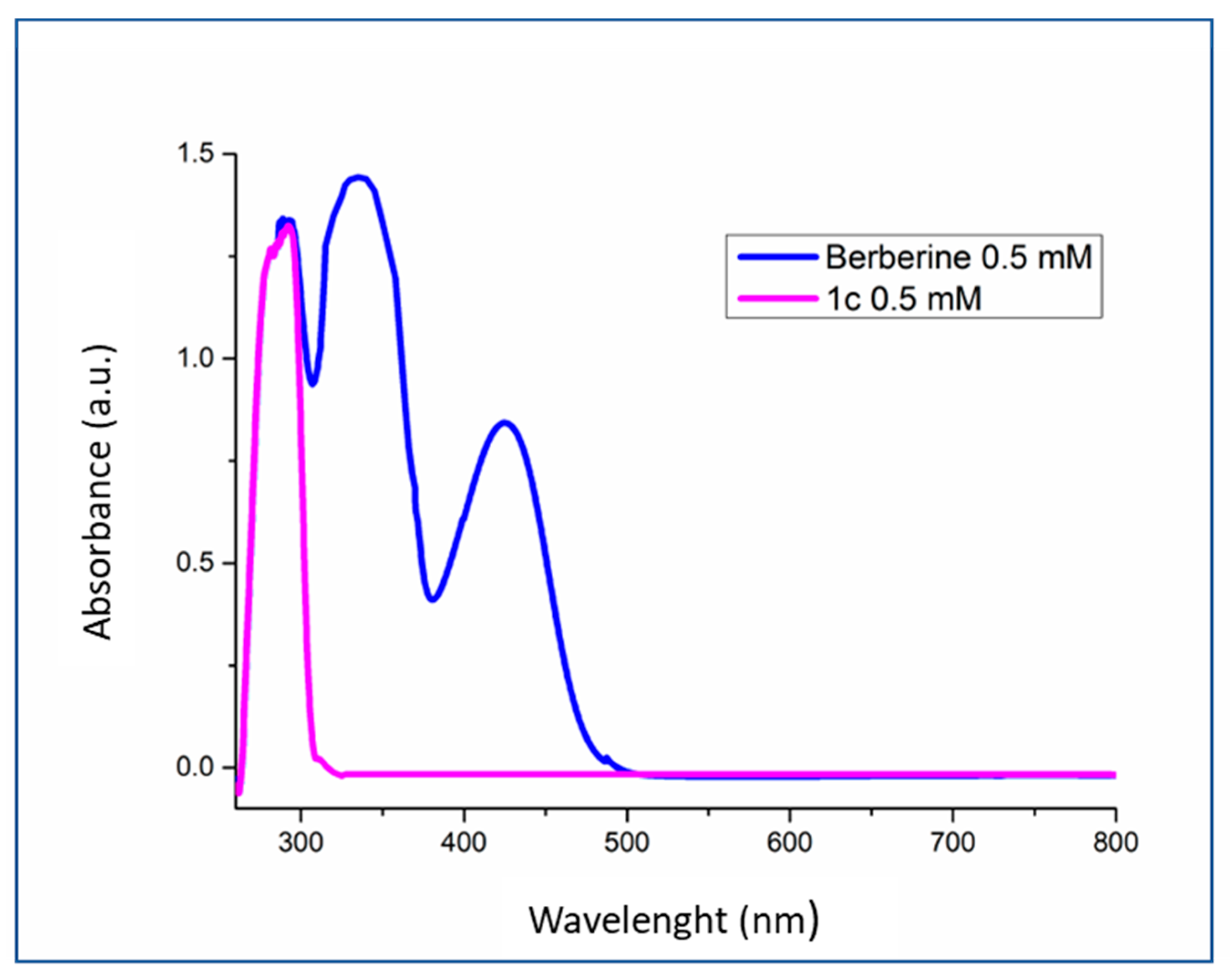
2.5. Binding of 1c Berberine Analogue to the FtsZ Recombinant Protein
2.6. Antimicrobial Activity of Berberine Analogues
3. Discussion
4. Materials and Methods
4.1. Chemistry
4.2. Molecular Docking Analysis
4.3. Experimental Assays
4.4. FtsZ Polymerization Assays
4.5. TEM Microscopy Analysis
4.6. Fluorescence Spectroscopy
4.7. Determination of the Minimum Inhibitory Concentration Values of Berberine Analogues
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frieri, M.; Kumar, K.; Boutin, A. Antibiotic resistance. J. Infect. Public Health 2017, 10, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Saha, M.; Sarkar, A. Review on Multiple Facets of Drug Resistance: A Rising Challenge in the 21st Century. J. Xenobiot. 2021, 11, 197–214. [Google Scholar] [CrossRef] [PubMed]
- Polo, A.B.; Fabri, R.L.; Apolônio, A.C.M. Searching for mechanisms of action of antimicrobials. Arch. Microbiol. 2020, 202, 2347–2354. [Google Scholar] [CrossRef] [PubMed]
- Ojima, I.; Kumar, K.; Awasthi, D.; Vineberg, J.G. Drug discovery targeting cell division proteins, microtubules and FtsZ. Bioorg. Med. Chem. 2014, 22, 5060–5077. [Google Scholar] [CrossRef]
- Sass, P.; Brötz-Oesterhelt, H. Bacterial cell division as a target for new antibiotics. Curr. Opin. Microbiol. 2013, 16, 522–530. [Google Scholar] [CrossRef]
- Silber, N.; Matos de Opitz, C.L.; Mayer, C.; Sass, P. Cell division protein FtsZ: From structure and mechanism to antibiotic target. Future Microbiol. 2020, 15, 801–831. [Google Scholar]
- De Benedetti, S.; Fisher, J.F.; Mobashery, S. Bacterial Cell Wall: Morphology and Biochemistry. In Practical Handbook of Microbiology; CRC Press: Boca Raton, FL, USA, 2021; pp. 167–204. [Google Scholar]
- Du, R.L.; Sun, N.; Fung, Y.H.; Zheng, Y.Y.; Chen, Y.W.; Chan, P.H.; Wong, W.L.; Wong, K.Y. Discovery of FtsZ inhibitors by virtual screening as antibacterial agents and study of the inhibition mechanism. RSC Med. Chem. 2022, 13, 79–89. [Google Scholar]
- Han, H.; Wang, Z.; Li, T.; Teng, D.; Mao, R.; Hao, Y.; Yang, N.; Wang, X.; Wang, J. Recent progress of bacterial FtsZ inhibitors with a focus on peptides. FEBS J. 2021, 288, 1091–1106. [Google Scholar] [CrossRef]
- Di Somma, A.; Canè, C.; Moretta, A.; Duilio, A. Interaction of Temporin-L Analogues with the E. coli FtsZ Protein. Antibiotics 2021, 10, 704. [Google Scholar]
- Casiraghi, A.; Suigo, L.; Valoti, E.; Straniero, V. Targeting bacterial cell division: A binding site-centered approach to the most promising inhibitors of the essential protein FtsZ. Antibiotics 2020, 9, 69. [Google Scholar] [CrossRef]
- Fang, Z.; Li, Y.; Zheng, Y.; Li, X.; Lu, Y.J.; Yan, S.C.; Wong, W.L.; Chan, K.F.; Wong, K.Y.; Sun, N. Antibacterial activity and mechanism of action of a thiophenyl substituted pyrimidine derivative. RSC Adv. 2019, 9, 10739–10744. [Google Scholar] [CrossRef] [PubMed]
- Khameneh, B.; Iranshahy, M.; Soheili, V.; Fazly Bazzaz, B.S. Review on plant antimicrobials: A mechanistic viewpoint. Antimicrob. Resist. Infect. Control 2019, 8, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Najeebullah, S.; Ali, M.; Shinwari, Z.K. Phytopharmacological and ethnomedicinal uses of the Genus Berberis (Berberidaceae): A review. Trop. J. Pharm. 2016, 15, 2047–2057. [Google Scholar] [CrossRef]
- Roselli, M.; Cavalluzzi, M.M.; Bruno, C.; Lovece, A.; Carocci, A.; Franchini, C.; Habtemariam, S.; Lentini, G. Synthesis and evaluation of berberine derivatives and analogs as potential antiacetylcholinesterase and antioxidant agents. Phytochem. Lett. 2016, 18, 150–156. [Google Scholar] [CrossRef]
- Mahady, G.B. Medicinal plants for the prevention and treatment of bacterial infections. Curr. Pharm Des. 2005, 11, 2405–2427. [Google Scholar] [CrossRef]
- Jin, J.L.; Hua, G.Q.; Meng, Z.; Gao, P.J. Antibacterial mechanisms of berberine and reasons for little resistance of bacteria. Chin. Herb. Med. 2010, 3, 27–35. [Google Scholar]
- Milani, G.; Cavalluzzi, M.; Solidoro, R.; Salvagno, L.; Quintieri, L.; Di Somma, A.; Rosato, A.; Corbo, F.; Franchini, C.; Duilio, A.; et al. Molecular Simplification of Natural Products: Synthesis, Antibacterial Activity, and Molecular Docking Studies of Berberine Open Models. Biomedicines 2021, 9, 452. [Google Scholar] [CrossRef]
- Wang, L.; Yang, X.; Li, X.; Stoika, R.; Wang, X.; Lin, H.; Ma, Y.; Wang, R.; Liu, K. Synthesis of hydrophobically modified berberine derivatives with high anticancer activity through modulation of the MAPK pathway. New J. Chem. 2020, 44, 14024–14034. [Google Scholar] [CrossRef]
- Carocci, A.; Catalano, A.; Turi, F.; Lovece, A.; Cavalluzzi, M.M.; Bruno, C.; Colabufo, N.A.; Contino, M.; Perrone, M.G.; Franchini, C.; et al. Stereoselective Modulation of P-Glycoprotein by Chiral Small Molecules. ChemMedChem 2016, 11, 93–101. [Google Scholar] [CrossRef]
- Di Somma, A.; Avitabile, C.; Cirillo, A.; Moretta, A.; Merlino, A.; Paduano, L.; Duilio, A.; Romanelli, A. The antimicrobial peptide Temporin L impairs E. coli cell division by interacting with FtsZ and the divisome complex. BBA General Sub. 2020, 1864, 129606. [Google Scholar] [CrossRef]
- Ropponen, H.K.; Richter, R.; Hirsch, A.K.; Lehr, C.M. Mastering the Gram-negative bacterial barrier–Chemical approaches to increase bacterial bioavailability of antibiotics. Adv. Drug Deliv. Rev. 2021, 172, 339–360. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, P.C.; Mau, J.L.; Huang, S.H. Antimicrobial effect of various combinations of plant extracts. Food Microbiol. 2001, 18, 35–43. [Google Scholar] [CrossRef]
- Qing, Z.X.; Zhao, H.; Tang, Q.; Mo, C.M.; Huang, P.; Cheng, P.; Yang, P.; Yang, X.Y.; Liu, X.B.; Zheng, Y.J.; et al. Systematic identification of flavonols, flavonol glycosides, triterpene and siraitic acid glycosides from Siraitia grosvenorii using high-performance liquid chromatography/quadrupole-time-of-flight mass spectrometry combined with a screening strategy. J. Pharm. Biomed. Anal. 2017, 138, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Qing, Z.-X.; Yang, P.; Tang, Q.; Cheng, P.; Liu, X.-B.; Zheng, Y.-J.; Liu, Y.-S.; Zeng, J.-G. Isoquinoline alkaloids and their antiviral, antibacterial, and antifungal activities and structure-activity relationship. Curr. Org. Chem. 2017, 21, 1920–1934. [Google Scholar] [CrossRef]
- Imenshahidi, M.; Hosseinzadeh, H. Berberis vulgaris and berberine: An update review. Phytother. Res. 2016, 30, 1745–1764. [Google Scholar] [CrossRef] [PubMed]
- Filli, M.S.; Ibrahim, A.A.; Kesse, S.; Aquib, M.; Boakye-Yiadom, K.O.; Farooq, M.A.; Zhang, Y.; Wang, B. Synthetic berberine derivatives as potential new drugs. Braz. J. Pharm. Sci. 2022, 58. [Google Scholar] [CrossRef]
- Domadia, P.N.; Bhunia, A.; Sivaraman, J.; Swarup, S.; Dasgupta, D. Berberine targets assembly of Escherichia coli cell division protein FtsZ. Biochemistry 2008, 47, 3225–3234. [Google Scholar] [CrossRef]
- ur Rahman, M.; Wang, P.; Wang, N.; Chen, Y. A key bacterial cytoskeletal cell division protein FtsZ as a novel therapeutic antibacterial drug target. Bosn. J. Basic Med. Sci. 2020, 20, 310. [Google Scholar]
- Tan, Q.; Awano, N.; Inouye, M. YeeV is an Escherichia coli toxin that inhibits cell division by targeting the cytoskeleton proteins, FtsZ and MreB. Mol. Microbiol. 2011, 79, 109–118. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Y. I-TASSER server: New development for protein structure and function predictions. Nucleic Acids Res. 2015, 43, W174–W181. [Google Scholar] [CrossRef] [PubMed]
- Schneidman-Duhovny, D.; Inbar, Y.; Nussinov, R.; Wolfson, H.J. PatchDock and SymmDock: Servers for rigid and symmetric docking. Nucleic Acids Res. 2005, 33 (Suppl. S2), W363–W367. [Google Scholar] [CrossRef] [PubMed]
- Mashiach, E.; Schneidman-Duhovny, D.; Andrusier, N.; Nussinov, R.; Wolfson, H.J. FireDock: A web server for fast interaction refinement in molecular docking. Nucleic Acids Res. 2008, 36 (Suppl. S2), W229–W232. [Google Scholar] [CrossRef] [PubMed]
- Salentin, S.; Schreiber, S.; Haupt, V.J.; Adasme, M.F.; Schroeder, M. PLIP: Fully automated protein–ligand interaction profiler. Nucleic Acids Res. 2015, 43, W443–W447. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Kaul, M.; Parhi, A.K.; Zhang, Y.; LaVoie, E.J.; Tuske, S.; Arnold, E.; Kerrigan, J.E.; Pilch, D.S. A bactericidal guanidinomethyl biaryl that alters the dynamics of bacterial FtsZ polymerization. J. Med. Chem. 2012, 55, 10160–10176. [Google Scholar] [CrossRef] [PubMed]
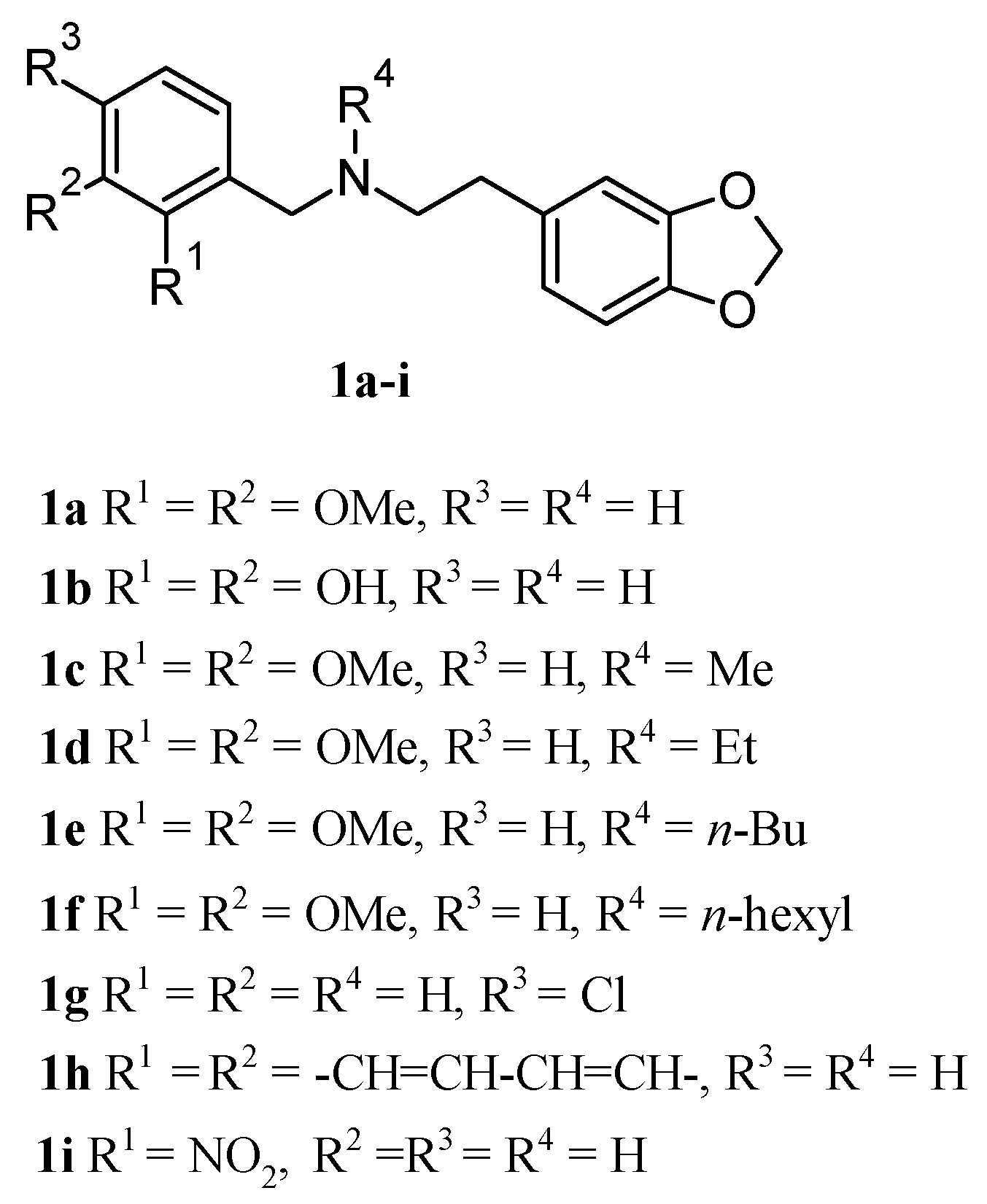
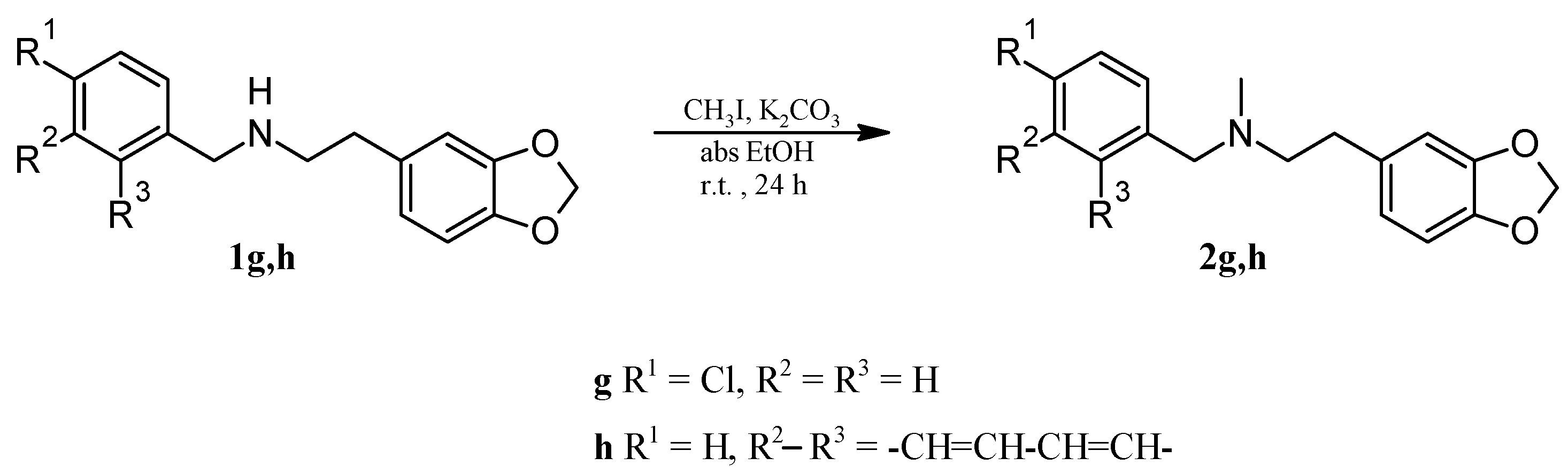
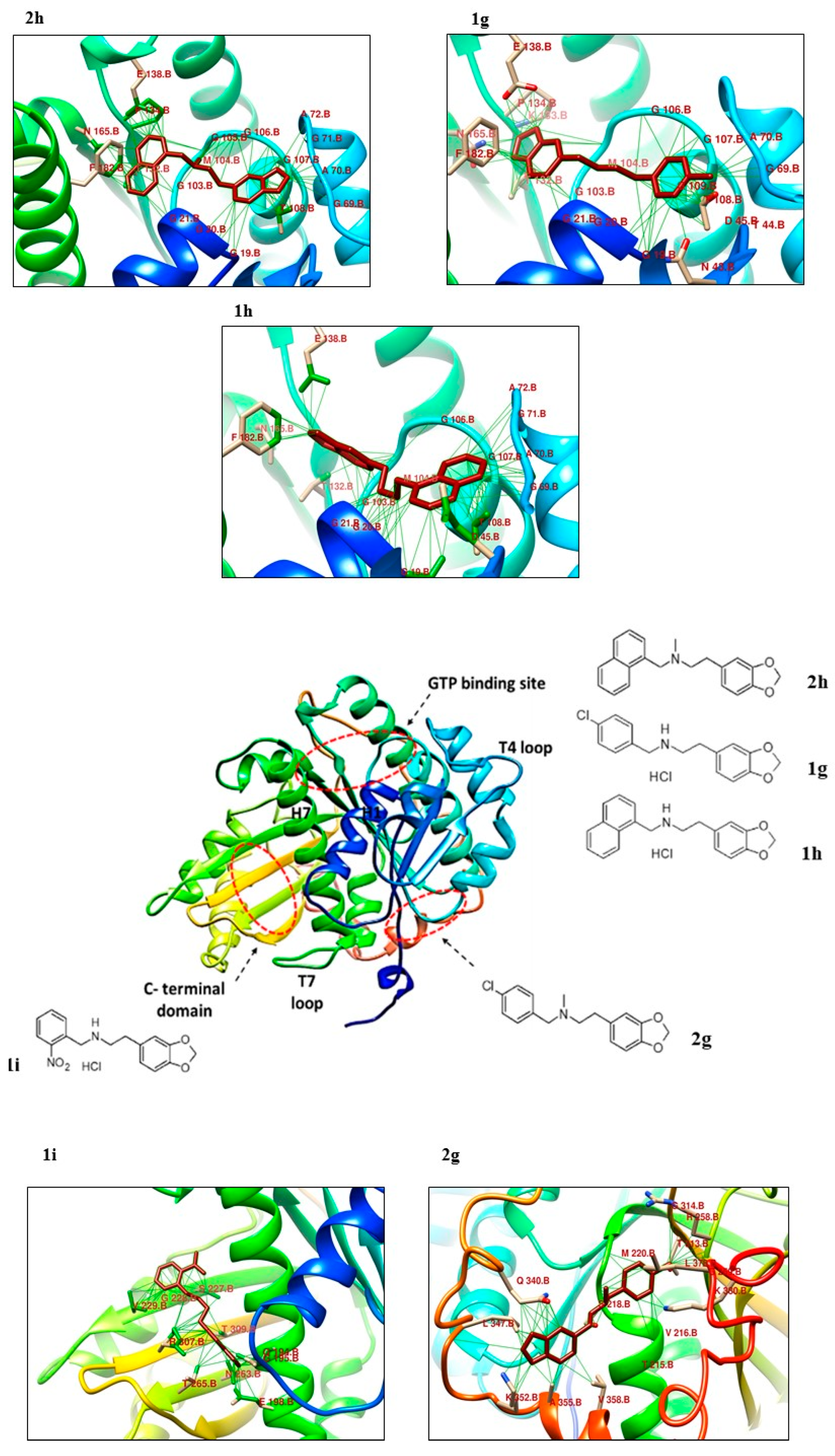
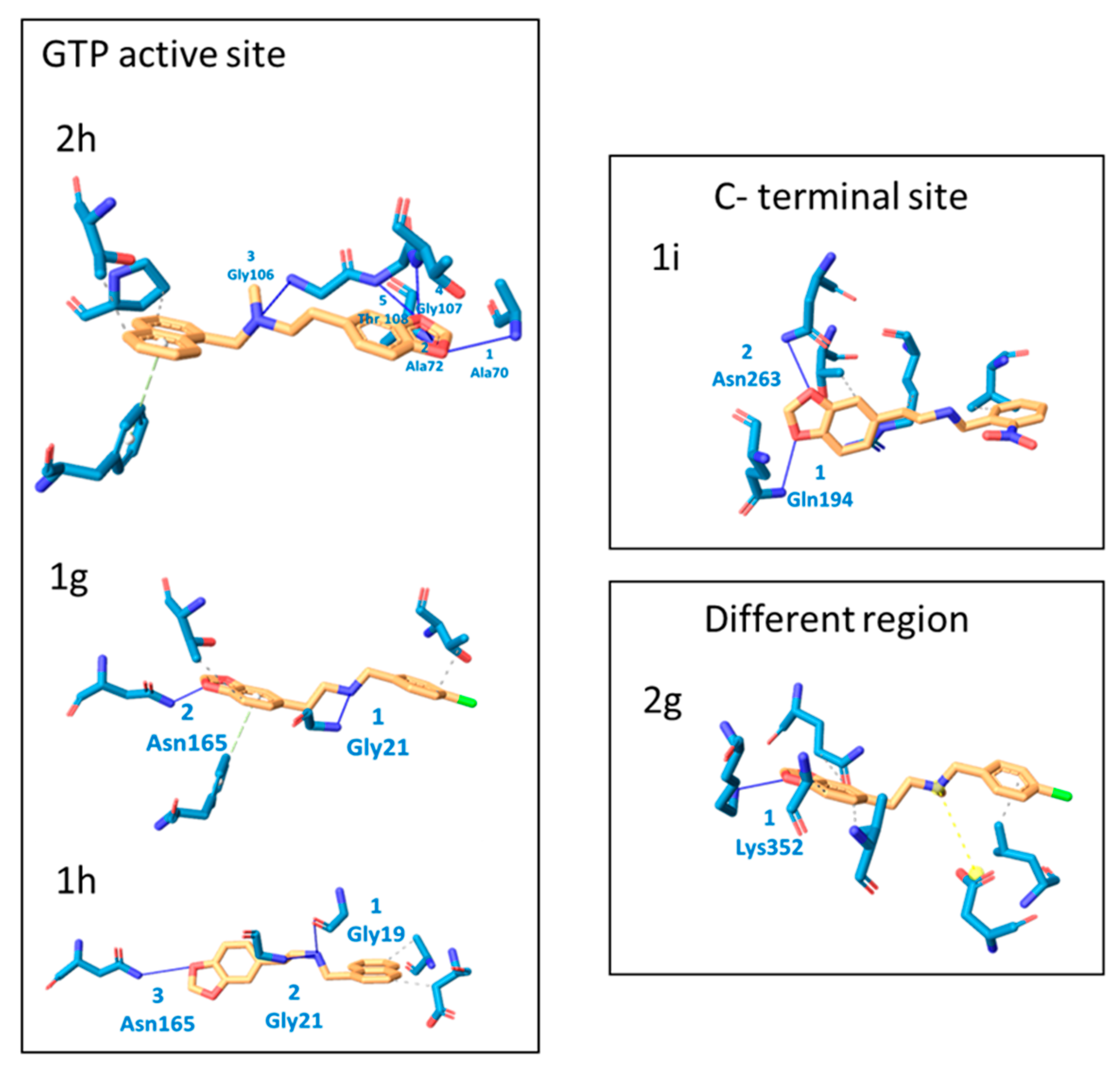

| Protein– Ligand | Interaction | DeltaG Value | |||
|---|---|---|---|---|---|
| Hydrophobic Interactions | Hydrogen Bonds | Non-Covalent Interactions | Π Stacking | ||
| 1g | 2 Thr108, Thr132 | 2 Gly21, Asn165 | 1 Phe182 | - | −7.52 |
| 1h | 2 Asp45, Ala70 | 3 Gly19, Gly21, Asn165 | - | - | −7.41 |
| 1i | 4 Val229, Val229, Thr265, Arg307 | 2 Gln194, Asn263 | - | - | −7.86 |
| 2g | 4 Gln340, Ala355, Val358, Leu 378 | 1 Lys 352 | 1 Asp 370 | - | −7.07 |
| 2h | 2 Thr132, Pro134 | 5 Ala70, Ala72, Gly106, Gly107, Thr108 | 1 Phe 182 | −7.45 | |
| Kinetic Parameters | ||
|---|---|---|
| KM (µM) for GTP | Vmax (Pi Released/min) | |
| GTP | 42 ± 4 | 0.63 ± 0.01 |
| 1c | 121 ± 5 | 0.54 ± 0.03 |
| 1d | 84 ± 6 | 0.58 ± 0.01 |
| 1f | 40 ± 5 | 0.35 ± 0.01 |
| 1i | 37 ± 6 | 0.35 ± 0.01 |
| 1g | 84 ± 9 | 0.60 ± 0.02 |
| 1h | 60 ± 6 | 0.59 ± 0.01 |
| 2g | 22 ± 4 | 0.39 ± 0.01 |
| 2h | 77 ± 8 | 0.58 ± 0.01 |
| Inhibition Constant | |
|---|---|
| Ki (µM) for Compound | |
| 1c | 25 |
| 1d | 44 |
| 1g | 41 |
| 1h | 119 |
| 2h | 51 |
 | ||||||
|---|---|---|---|---|---|---|
| Compound | R1 | R2 | R3 | R4 | MIC E. coli a | FtsZ Binding Site b |
| 1a | OMe | OMe | H | H | >512 | C-terminal |
| 1c | OMe | OMe | H | Me | 128 | GTP-binding pocket |
| 1d | OMe | OMe | H | Et | >512 | GTP-binding pocket |
| 1f | OMe | OMe | H | n-hexyl | >512 | C-terminal |
| 1g | H | H | Cl | H | 128 | GTP-binding pocket |
| 1h | CH=CH–CH=CH | H | H | 128 | GTP-binding pocket | |
| 1i | NO2 | H | H | H | 256 | C-terminal |
| 2g | H | H | Cl | Me | 128 | Other binding site |
| 2h | CH=CH–CH=CH | H | Me | >512 | GTP-binding pocket | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Somma, A.; Canè, C.; Rotondo, N.P.; Cavalluzzi, M.M.; Lentini, G.; Duilio, A. A Comparative Study of the Inhibitory Action of Berberine Derivatives on the Recombinant Protein FtsZ of E. coli. Int. J. Mol. Sci. 2023, 24, 5674. https://doi.org/10.3390/ijms24065674
Di Somma A, Canè C, Rotondo NP, Cavalluzzi MM, Lentini G, Duilio A. A Comparative Study of the Inhibitory Action of Berberine Derivatives on the Recombinant Protein FtsZ of E. coli. International Journal of Molecular Sciences. 2023; 24(6):5674. https://doi.org/10.3390/ijms24065674
Chicago/Turabian StyleDi Somma, Angela, Carolina Canè, Natalie Paola Rotondo, Maria Maddalena Cavalluzzi, Giovanni Lentini, and Angela Duilio. 2023. "A Comparative Study of the Inhibitory Action of Berberine Derivatives on the Recombinant Protein FtsZ of E. coli" International Journal of Molecular Sciences 24, no. 6: 5674. https://doi.org/10.3390/ijms24065674
APA StyleDi Somma, A., Canè, C., Rotondo, N. P., Cavalluzzi, M. M., Lentini, G., & Duilio, A. (2023). A Comparative Study of the Inhibitory Action of Berberine Derivatives on the Recombinant Protein FtsZ of E. coli. International Journal of Molecular Sciences, 24(6), 5674. https://doi.org/10.3390/ijms24065674











