Vertical Sleeve Gastrectomy Offers Protection against Disturbed Flow-Induced Atherosclerosis in High-Fat Diet-Fed Mice
Abstract
1. Introduction
2. Results
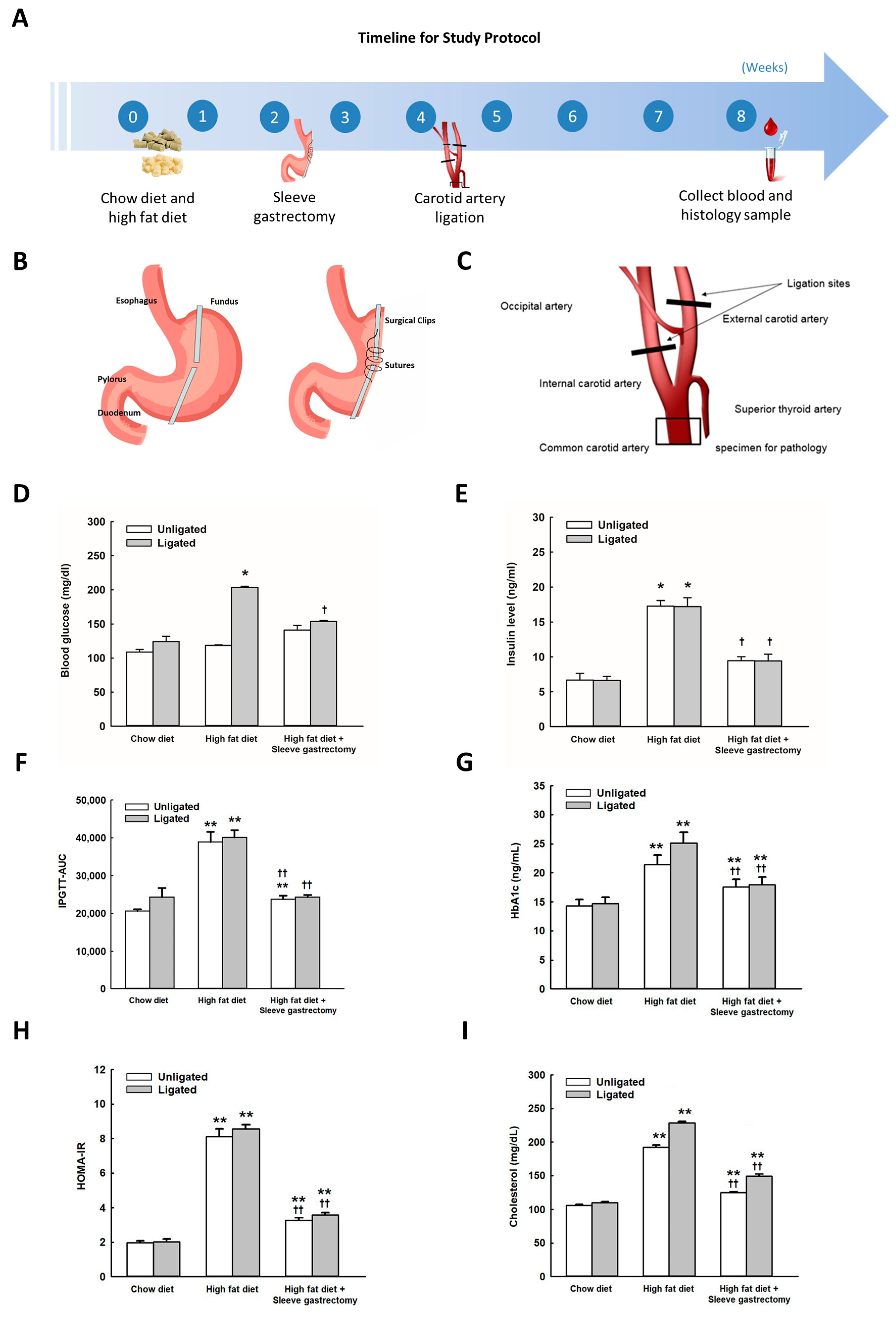
2.1. Effects of SG on Body Weight, Glucose Metabolism, Insulin Resistance, and Cholesterol in HFD-Fed Mice
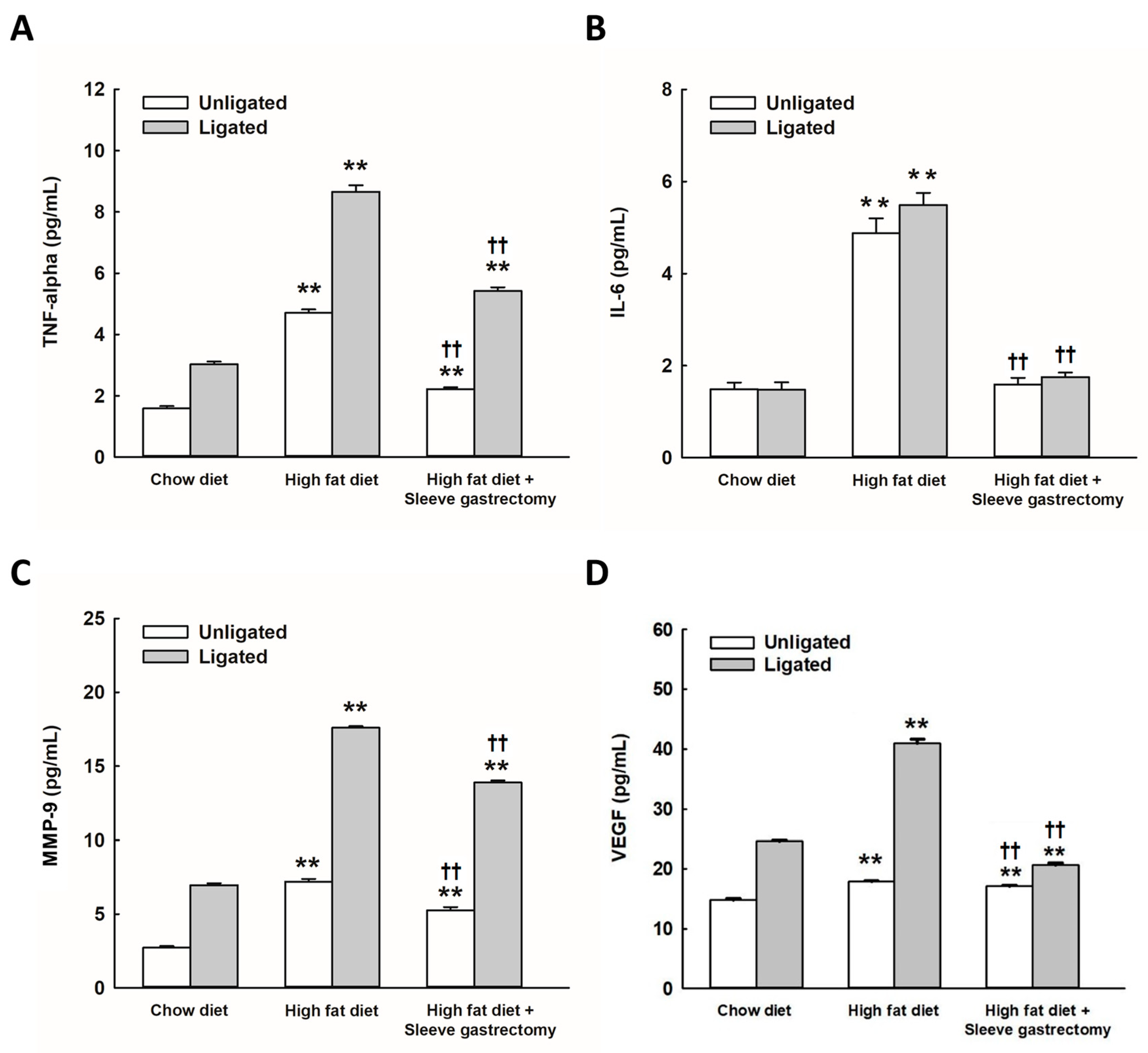
2.2. SG Alleviates HFD-Induced Circulating Inflammatory Cytokines
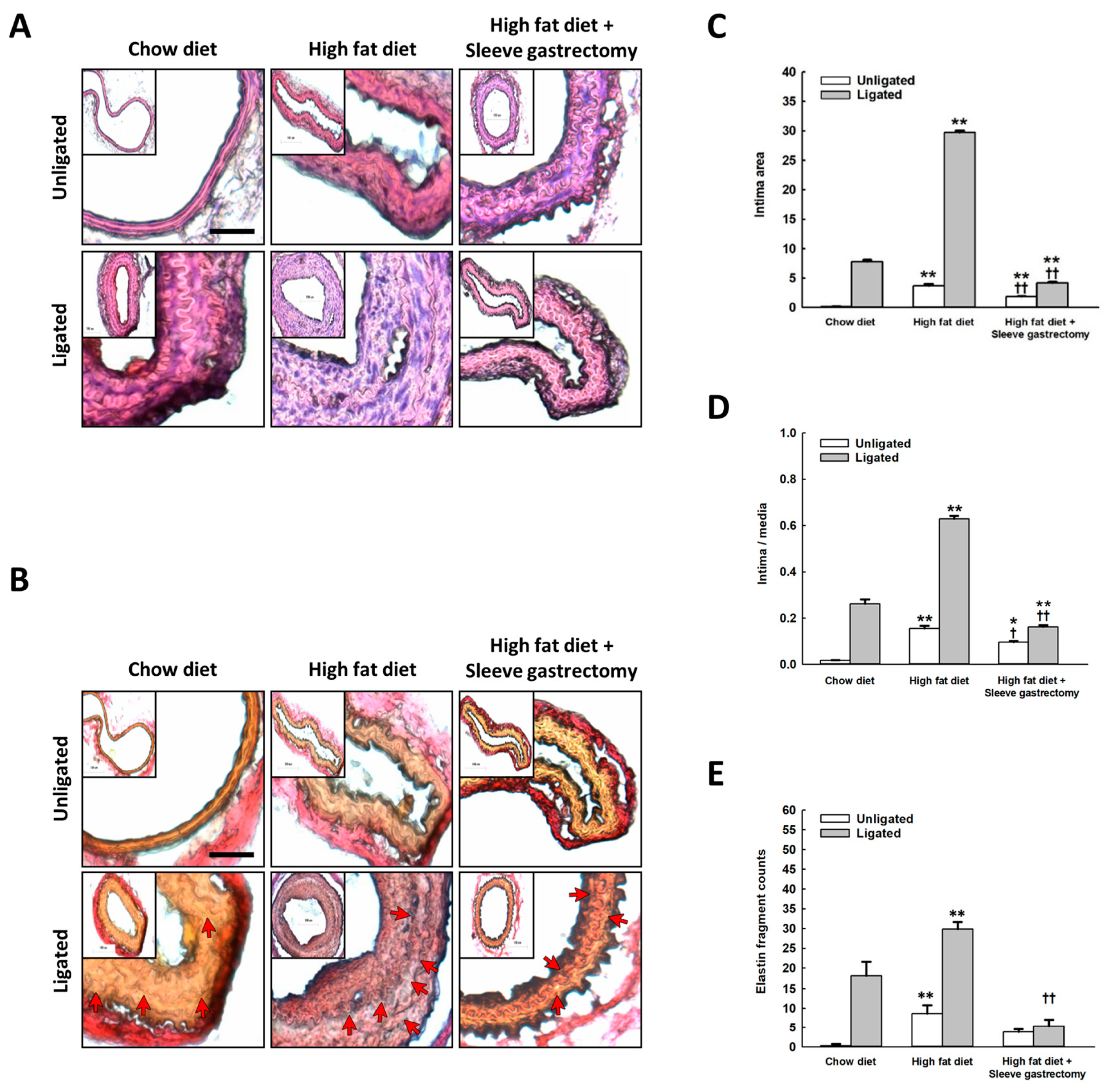
2.3. Effect of SG on Vascular Remodeling after Carotid Artery Ligation
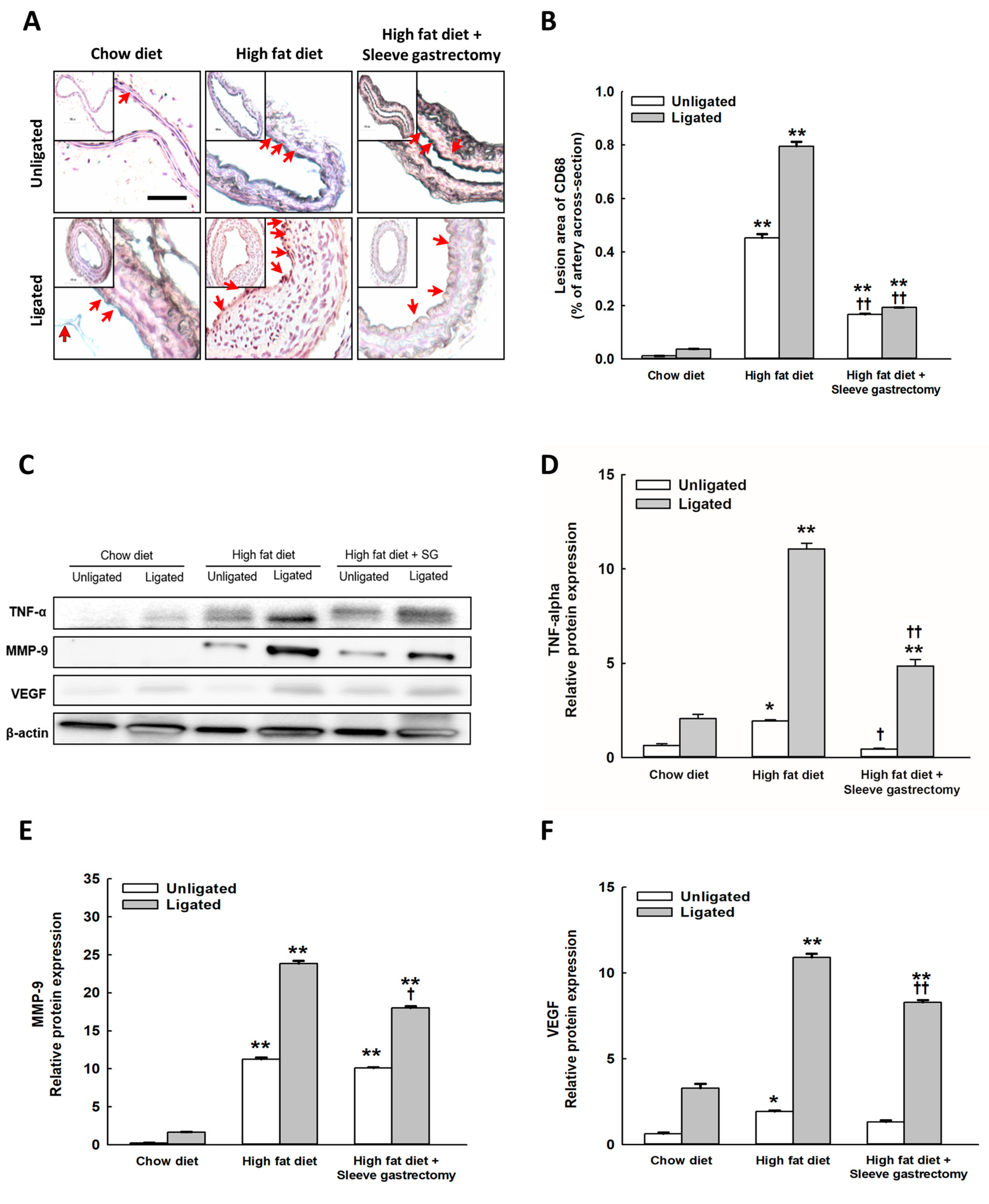
2.4. SG Decreased the Inflammatory Expression of CD68, and TNF-α, MMP-9, and VEGF Protein Expression after Carotid Ligation
2.5. HFD Restriction Had Less Protective Effect on Carotid-Ligated Vascular Remodeling Than SG Surgery
3. Discussion
4. Methods
4.1. Experimental Animals
4.2. Animal Grouping and Diets
4.3. SG
4.4. Partial Ligation of the Left Carotid Artery
4.5. Morphometric Analysis
4.6. Elastic Fragmentation Measurement
4.7. Postoperative Care
4.8. Intraperitoneal Glucose Tolerance Test (IPGTT)
4.9. Histology and Immunohistochemistry
4.10. Measurement of Inflammatory Cytokines
4.11. Western Blotting
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Flegal, K.M.; Carroll, M.D.; Kit, B.K.; Ogden, C.L. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA 2012, 307, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.S.; Kwon, S.C.; Wyatt, L.; Islam, N.; Trinh-Shevrin, C. Weighing in on the hidden Asian American obesity epidemic. Prev. Med. 2015, 73, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Cawley, J.; Meyerhoefer, C.; Biener, A.; Hammer, M.; Wintfeld, N. Savings in Medical Expenditures Associated with Reductions in Body Mass Index Among US Adults with Obesity, by Diabetes Status. Pharmacoeconomics 2015, 33, 707–722. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M. Obesity, metabolic syndrome, and cardiovascular disease. J. Clin. Endocrinol. Metab. 2004, 89, 2595–2600. [Google Scholar] [CrossRef]
- Hussain, H.T.; Parker, J.L.; Sharma, A.M. Clinical trial success rates of anti-obesity agents: The importance of combination therapies. Obes. Rev. 2015, 16, 707–714. [Google Scholar] [CrossRef]
- Khera, R.; Murad, M.H.; Chandar, A.K.; Dulai, P.S.; Wang, Z.; Prokop, L.J.; Loomba, R.; Camilleri, M.; Singh, S. Association of Pharmacological Treatments for Obesity With Weight Loss and Adverse Events: A Systematic Review and Meta-analysis. JAMA 2016, 315, 2424–2434. [Google Scholar] [CrossRef]
- Torres, A.; Rubio, M.A.; Ramos-Levi, A.M.; Sanchez-Pernaute, A. Cardiovascular Risk Factors After Single Anastomosis Duodeno-Ileal Bypass with Sleeve Gastrectomy (SADI-S): A New Effective Therapeutic Approach? Curr. Atheroscler. Rep. 2017, 19, 58. [Google Scholar] [CrossRef] [PubMed]
- Gerber, P.; Anderin, C.; Szabo, E.; Naslund, I.; Thorell, A. Impact of age on risk of complications after gastric bypass: A cohort study from the Scandinavian Obesity Surgery Registry (SOReg). Surg. Obes. Relat. Dis. 2018, 14, 437–442. [Google Scholar] [CrossRef]
- Abbatini, F.; Rizzello, M.; Casella, G.; Alessandri, G.; Capoccia, D.; Leonetti, F.; Basso, N. Long-term effects of laparoscopic sleeve gastrectomy, gastric bypass, and adjustable gastric banding on type 2 diabetes. Surg. Endosc. 2010, 24, 1005–1010. [Google Scholar] [CrossRef]
- Singhal, S.; Agarwal, D.; Kanojiya, R.; Arora, D.; Avesthi, A.; Kothari, A.J.I.S.J. Effect of laparoscopic sleeve gastrectomy on lipid profile of obese patients in complete nine month follow up. Int. Surg. J. 2016, 3, 42–46. [Google Scholar] [CrossRef]
- Fisher, D.P.; Johnson, E.; Haneuse, S.; Arterburn, D.; Coleman, K.J.; O′Connor, P.J.; O′Brien, R.; Bogart, A.; Theis, M.K.; Anau, J.; et al. Association Between Bariatric Surgery and Macrovascular Disease Outcomes in Patients With Type 2 Diabetes and Severe Obesity. JAMA 2018, 320, 1570–1582. [Google Scholar] [CrossRef]
- Lupoli, R.; Di Minno, M.N.; Guidone, C.; Cefalo, C.; Capaldo, B.; Riccardi, G.; Mingrone, G. Effects of bariatric surgery on markers of subclinical atherosclerosis and endothelial function: A meta-analysis of literature studies. Int. J. Obes. 2016, 40, 395–402. [Google Scholar] [CrossRef]
- Gokce, N.; Karki, S.; Dobyns, A.; Zizza, E.; Sroczynski, E.; Palmisano, J.N.; Mazzotta, C.; Hamburg, N.M.; Pernar, L.I.; Carmine, B.; et al. Association of Bariatric Surgery With Vascular Outcomes. JAMA Netw. Open 2021, 4, e2115267. [Google Scholar] [CrossRef] [PubMed]
- Miceli, G.; Basso, M.G.; Rizzo, G.; Pintus, C.; Tuttolomondo, A. The Role of the Coagulation System in Peripheral Arterial Disease: Interactions with the Arterial Wall and Its Vascular Microenvironment and Implications for Rational Therapies. Int. J. Mol. Sci. 2022, 23, 14914. [Google Scholar] [CrossRef]
- Wei, J.H.; Chou, R.H.; Huang, P.H.; Lee, W.J.; Chen, S.C.; Lin, S.J. Metabolic surgery ameliorates cardiovascular risk in obese diabetic patients: Influence of different surgical procedures. Surg. Obes. Relat. Dis. 2018, 14, 1832–1840. [Google Scholar] [CrossRef] [PubMed]
- Winzell, M.S.; Ahren, B. The high-fat diet-fed mouse: A model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes 2004, 53 (Suppl. S3), S215–S219. [Google Scholar] [CrossRef]
- Surwit, R.S.; Kuhn, C.M.; Cochrane, C.; McCubbin, J.A.; Feinglos, M.N. Diet-induced type II diabetes in C57BL/6J mice. Diabetes 1988, 37, 1163–1167. [Google Scholar] [CrossRef]
- Singla, P.; Bardoloi, A.; Parkash, A.A. Metabolic effects of obesity: A review. World J. Diabetes 2010, 1, 76–88. [Google Scholar] [CrossRef]
- Gumbau, V.; Bruna, M.; Canelles, E.; Guaita, M.; Mulas, C.; Basés, C.; Celma, I.; Puche, J.; Marcaida, G.; Oviedo, M. A prospective study on inflammatory parameters in obese patients after sleeve gastrectomy. Obes. Surg. 2014, 24, 903–908. [Google Scholar] [CrossRef]
- Salman, M.A.; Salman, A.A.; Nafea, M.A.; Sultan, A.; Anwar, H.W.; Ibrahim, A.H.; Awad, A.; Ahmed, R.A.; Seif El Nasr, S.M.; Abouelregal, T.E.; et al. Study of changes of obesity-related inflammatory cytokines after laparoscopic sleeve gastrectomy. ANZ J. Surg. 2019, 89, 1265–1269. [Google Scholar] [CrossRef] [PubMed]
- Lackey, D.E.; Olefsky, J.M. Regulation of metabolism by the innate immune system. Nat. Rev. Endocrinol. 2016, 12, 15–28. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Marso, S.P.; Zhou, Z.; Foroudi, F.; Topol, E.J.; Lincoff, A.M. Neointimal hyperplasia after arterial injury is increased in a rat model of non-insulin-dependent diabetes mellitus. Circulation 2001, 104, 815–819. [Google Scholar] [CrossRef]
- Zimmerman, M.A.; Selzman, C.H.; Reznikov, L.L.; Miller, S.A.; Raeburn, C.D.; Emmick, J.; Meng, X.; Harken, A.H. Lack of TNF-alpha attenuates intimal hyperplasia after mouse carotid artery injury. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 283, R505–R512. [Google Scholar] [CrossRef] [PubMed]
- Rectenwald, J.E.; Moldawer, L.L.; Huber, T.S.; Seeger, J.M.; Ozaki, C.K. Direct evidence for cytokine involvement in neointimal hyperplasia. Circulation 2000, 102, 1697–1702. [Google Scholar] [CrossRef]
- Saltiel, A.R.; Olefsky, J.M. Inflammatory mechanisms linking obesity and metabolic disease. J. Clin. Investig. 2017, 127, 1–4. [Google Scholar] [CrossRef]
- Lumeng, C.N.; Bodzin, J.L.; Saltiel, A.R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Investig. 2007, 117, 175–184. [Google Scholar] [CrossRef]
- Chen, J.; Wang, W.; Ni, Q.; Zhang, L.; Guo, X. Interleukin 6-regulated macrophage polarization controls atherosclerosis-associated vascular intimal hyperplasia. Front. Immunol. 2022, 13, 952164. [Google Scholar] [CrossRef]
- Shen, Y.; Liu, Y.; Zheng, S.Q.; Han, J.; Pei, E.L.; Li, Z.H.; Xie, X.Y.; Li, Z.Q.; Luo, M. Effects of Left Gastric Artery Ligation Versus Sleeve Gastrectomy on Obesity-Induced Adipose Tissue Macrophage Infiltration and Inflammation in Diet-Induced Obese Rats. Med. Sci. Monit. 2019, 25, 6719–6726. [Google Scholar] [CrossRef]
- Frikke-Schmidt, H.; Zamarron, B.F.; O′Rourke, R.W.; Sandoval, D.A.; Lumeng, C.N.; Seeley, R.J. Weight loss independent changes in adipose tissue macrophage and T cell populations after sleeve gastrectomy in mice. Mol. Metab. 2017, 6, 317–326. [Google Scholar] [CrossRef]
- Shiojima, I.; Walsh, K. The role of vascular endothelial growth factor in restenosis: The controversy continues. Circulation 2004, 110, 2283–2286. [Google Scholar] [CrossRef]
- Schreyer, S.A.; Wilson, D.L.; LeBoeuf, R.C. C57BL/6 mice fed high fat diets as models for diabetes-accelerated atherosclerosis. Atherosclerosis 1998, 136, 17–24. [Google Scholar] [CrossRef]
- Skovso, S. Modeling type 2 diabetes in rats using high fat diet and streptozotocin. J. Diabetes Investig. 2014, 5, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Heydemann, A. An Overview of Murine High Fat Diet as a Model for Type 2 Diabetes Mellitus. J. Diabetes Res. 2016, 2016, 2902351. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.S.; Chen, J.S.; Lin, L.Y.; Schmid-Schonbein, G.W.; Chien, S.; Huang, P.H.; Chen, J.W.; Lin, S.J. Inhibition of Serine Protease Activity Protects Against High Fat Diet-Induced Inflammation and Insulin Resistance. Sci. Rep. 2020, 10, 1725. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Park, N.Y.; Valacchi, G.; Lim, Y. Calorie restriction with a high-fat diet effectively attenuated inflammatory response and oxidative stress-related markers in obese tissues of the high diet fed rats. Mediat. Inflamm. 2012, 2012, 984643. [Google Scholar] [CrossRef]
- Grayson, B.E.; Hakala-Finch, A.P.; Kekulawala, M.; Laub, H.; Egan, A.E.; Ressler, I.B.; Woods, S.C.; Herman, J.P.; Seeley, R.J.; Benoit, S.C.; et al. Weight loss by calorie restriction versus bariatric surgery differentially regulates the hypothalamo-pituitary-adrenocortical axis in male rats. Stress 2014, 17, 484–493. [Google Scholar] [CrossRef]
- Seimon, R.V.; Hostland, N.; Silveira, S.L.; Gibson, A.A.; Sainsbury, A. Effects of energy restriction on activity of the hypothalamo-pituitary-adrenal axis in obese humans and rodents: Implications for diet-induced changes in body composition. Horm. Mol. Biol. Clin. Investig. 2013, 15, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.S.; Ghasemzadeh, N.; Eapen, D.J.; Sher, S.; Arshad, S.; Ko, Y.A.; Veledar, E.; Samady, H.; Zafari, A.M.; Sperling, L.; et al. Novel Biomarker of Oxidative Stress Is Associated With Risk of Death in Patients With Coronary Artery Disease. Circulation 2016, 133, 361–369. [Google Scholar] [CrossRef]
- Mitra, S.; Deshmukh, A.; Sachdeva, R.; Lu, J.; Mehta, J.L. Oxidized low-density lipoprotein and atherosclerosis implications in antioxidant therapy. Am. J. Med. Sci. 2011, 342, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Gomez, M.; Vila, J.; Elosua, R.; Molina, L.; Bruguera, J.; Sala, J.; Masia, R.; Covas, M.I.; Marrugat, J.; Fito, M. Relationship of lipid oxidation with subclinical atherosclerosis and 10-year coronary events in general population. Atherosclerosis 2014, 232, 134–140. [Google Scholar] [CrossRef]
- Leiva, E.; Wehinger, S.; Guzmán, L.; Orrego, R. Role of Oxidized LDL in Atherosclerosis. Hypercholesterolemia 2015, 55–78. [Google Scholar] [CrossRef]
- Hinagata, J.; Kakutani, M.; Fujii, T.; Naruko, T.; Inoue, N.; Fujita, Y.; Mehta, J.L.; Ueda, M.; Sawamura, T. Oxidized LDL receptor LOX-1 is involved in neointimal hyperplasia after balloon arterial injury in a rat model. Cardiovasc. Res. 2006, 69, 263–271. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; Delany, J.P.; Otto, A.D.; Kuller, L.; Vockley, J.; South-Paul, J.E.; Thomas, S.B.; Brown, J.; McTigue, K.; Hames, K.C.; et al. Effects of diet and physical activity interventions on weight loss and cardiometabolic risk factors in severely obese adults: A randomized trial. JAMA 2010, 304, 1795–1802. [Google Scholar] [CrossRef]
- Ravussin, E.; Redman, L.M.; Rochon, J.; Das, S.K.; Fontana, L.; Kraus, W.E.; Romashkan, S.; Williamson, D.A.; Meydani, S.N.; Villareal, D.T.; et al. A 2-Year Randomized Controlled Trial of Human Caloric Restriction: Feasibility and Effects on Predictors of Health Span and Longevity. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 1097–1104. [Google Scholar] [CrossRef] [PubMed]
- Martin, W.P.; Docherty, N.G.; Le Roux, C.W. Impact of bariatric surgery on cardiovascular and renal complications of diabetes: A focus on clinical outcomes and putative mechanisms. Expert. Rev. Endocrinol. Metab. 2018, 13, 251–262. [Google Scholar] [CrossRef]
- Alkharaiji, M.; Anyanwagu, U.; Donnelly, R.; Idris, I. Effect of Bariatric Surgery on Diagnosed Chronic Kidney Disease and Cardiovascular Events in Patients with Insulin-treated Type 2 Diabetes: A Retrospective Cohort Study from a Large UK Primary Care Database. Obes. Surg. 2020, 30, 1685–1695. [Google Scholar] [CrossRef] [PubMed]
- Rega-Kaun, G.; Kaun, C.; Ebenbauer, B.; Jaegersberger, G.; Prager, M.; Wojta, J.; Hohensinner, P.J. Bariatric surgery in morbidly obese individuals affects plasma levels of protein C and thrombomodulin. J. Thromb. Thrombolysis. 2019, 47, 51–56. [Google Scholar] [CrossRef]
- Giri, H.; Panicker, S.R.; Cai, X.; Biswas, I.; Weiler, H.; Rezaie, A.R. Thrombomodulin is essential for maintaining quiescence in vascular endothelial cells. Proc. Natl. Acad. Sci. USA 2021, 118, e2022248118. [Google Scholar] [CrossRef]
- Ait-Oufella, H.; Taleb, S.; Mallat, Z.; Tedgui, A. Recent advances on the role of cytokines in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 969–979. [Google Scholar] [CrossRef]
- Schlager, A.; Khalaileh, A.; Mintz, Y.; Abu Gazala, M.; Globerman, A.; Ilani, N.; Rivkind, A.I.; Salpeter, S.; Dor, Y.; Zamir, G. A mouse model for sleeve gastrectomy: Applications for diabetes research. Microsurgery 2011, 31, 66–71. [Google Scholar] [CrossRef]
- Wei, J.H.; Yeh, C.H.; Lee, W.J.; Lin, S.J.; Huang, P.H. Sleeve Gastrectomy in Mice using Surgical Clips. J. Vis. Exp. 2020, 165, 1–11. [Google Scholar] [CrossRef]
- Nam, D.; Ni, C.W.; Rezvan, A.; Suo, J.; Budzyn, K.; Llanos, A.; Harrison, D.G.; Giddens, D.P.; Jo, H. A model of disturbed flow-induced atherosclerosis in mouse carotid artery by partial ligation and a simple method of RNA isolation from carotid endothelium. J. Vis. Exp. 2010, 40, e1861. [Google Scholar] [CrossRef]
- Guyton, J.R.; Hartley, C.J. Flow restriction of one carotid artery in juvenile rats inhibits growth of arterial diameter. Am. J. Physiol.-Heart Circ. Physiol. 1985, 248, H540–H546. [Google Scholar] [CrossRef]
- Korshunov, V.A.; Berk, B.C. Flow-induced vascular remodeling in the mouse: A model for carotid intima-media thickening. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 2185–2191. [Google Scholar] [CrossRef]
- Chen, C.Y.; Tsai, H.Y.; Tsai, S.H.; Chu, P.H.; Huang, P.H.; Chen, J.W.; Lin, S.J. Deletion of the FHL2 gene attenuates intima-media thickening in a partially ligated carotid artery ligated mouse model. J. Cell. Mol. Med. 2020, 24, 160–173. [Google Scholar] [CrossRef] [PubMed]
- Pressler, J.W.; Haller, A.; Sorrell, J.; Wang, F.; Seeley, R.J.; Tso, P.; Sandoval, D.A. Vertical sleeve gastrectomy restores glucose homeostasis in apolipoprotein A-IV KO mice. Diabetes 2015, 64, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.M.; Campbell, F.M.; Drew, J.E.; Koch, C.; Hoggard, N.; Rees, W.D.; Kamolrat, T.; Thi Ngo, H.; Steffensen, I.L.; Gray, S.R.; et al. The development of diet-induced obesity and glucose intolerance in C57BL/6 mice on a high-fat diet consists of distinct phases. PLoS ONE 2014, 9, e106159. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, J.-H.; Lee, W.-J.; Luo, J.-L.; Huang, H.-L.; Wang, S.-C.; Chou, R.-H.; Huang, P.-H.; Lin, S.-J. Vertical Sleeve Gastrectomy Offers Protection against Disturbed Flow-Induced Atherosclerosis in High-Fat Diet-Fed Mice. Int. J. Mol. Sci. 2023, 24, 5669. https://doi.org/10.3390/ijms24065669
Wei J-H, Lee W-J, Luo J-L, Huang H-L, Wang S-C, Chou R-H, Huang P-H, Lin S-J. Vertical Sleeve Gastrectomy Offers Protection against Disturbed Flow-Induced Atherosclerosis in High-Fat Diet-Fed Mice. International Journal of Molecular Sciences. 2023; 24(6):5669. https://doi.org/10.3390/ijms24065669
Chicago/Turabian StyleWei, Jih-Hua, Wei-Jei Lee, Jing-Lin Luo, Hsin-Lei Huang, Shen-Chih Wang, Ruey-Hsing Chou, Po-Hsun Huang, and Shing-Jong Lin. 2023. "Vertical Sleeve Gastrectomy Offers Protection against Disturbed Flow-Induced Atherosclerosis in High-Fat Diet-Fed Mice" International Journal of Molecular Sciences 24, no. 6: 5669. https://doi.org/10.3390/ijms24065669
APA StyleWei, J.-H., Lee, W.-J., Luo, J.-L., Huang, H.-L., Wang, S.-C., Chou, R.-H., Huang, P.-H., & Lin, S.-J. (2023). Vertical Sleeve Gastrectomy Offers Protection against Disturbed Flow-Induced Atherosclerosis in High-Fat Diet-Fed Mice. International Journal of Molecular Sciences, 24(6), 5669. https://doi.org/10.3390/ijms24065669





